Abstract
The killing of Listeria monocytogenes following exposure to low pH, organic acids, and osmotic stress was enhanced by the addition of 5% (vol/vol) ethanol. At pH 3, for example, the presence of this agent stimulated killing by more than 3 log units in 40 min of exposure. The rate of cell death at pH 3.0 was dependent on the concentration of ethanol. Thus, while the presence 10% (vol/vol) ethanol at pH 3.0 stimulated killing by more than 3 log units in just 5 min, addition of 1.25% (vol/vol) ethanol resulted in less than 1 log unit of killing in 10 min. The ability of 5% (vol/vol) ethanol to stimulate killing at low pH and at elevated osmolarity was also dependent on the amplitude of the imposed stress, and an increase in the pH from 3.0 to 4.0 or a decrease in the sodium chloride concentration from 25 to 2.5% led to a marked reduction in the effectiveness of 5% (vol/vol) ethanol as an augmentative agent. Combinations of organic acids, low pH, and ethanol proved to be particularly effective bactericidal treatments; the most potent combination was pH 3.0, 50 mM formate, and 5 % (vol/vol) ethanol, which resulted in 5 log units of killing in just 4 min. Ethanol-enhanced killing correlated with damage to the bacterial cytoplasmic membrane.
The gram-positive bacterium Listeria monocytogenes is recognized as a food-borne pathogen with significance for humans (11), and major outbreaks of infection have been linked to the consumption of contaminated coleslaw (24), cheeses (14), and pasteurized milk (12). Today, L. monocytogenes is a major concern to manufacturers worldwide due to the high mortality rate of listeriosis in susceptible populations and to the resistance of the pathogen to a number of food preservation practices. In particular, the ability of the organism to grow at refrigeration temperatures (30) and on dry surfaces (31) and its ability to tolerate acidic conditions (1, 2, 7) make it well adapted to food environments which normally restrict bacterial growth. Consequently, control of this bacterium is a significant challenge for the food manufacturer.
Acidification of foods with short-chain organic acids, either by fermentation or by deliberate addition, is an important and widespread mechanism for controlling food-borne pathogens in a variety of foods. However, a number of studies have demonstrated that L. monocytogenes is more acid tolerant than most food-borne pathogens, although the sensitivity of the organism to organic acids varies with the nature of the acidulant used (28). An additional consideration relevant to the survival of this pathogen in foods is that fact that acid tolerance can be enhanced by exposing the organism to moderately acidic conditions (8, 17), a factor which can further reduce the effectiveness of acid-based preservation systems against L. monocytogenes.
The innate resistance of L. monocytogenes to many of the food preservation systems that are effective against other food-borne pathogens has prompted research aimed at developing combination systems for more effective control of this pathogen (20). Recently, it has been shown that Escherichia coli O157:H7 strains can be effectively killed by combination treatments involving low pH and ethanol and that death can be correlated with the ability of ethanol to disrupt the capacity of the cell for pH homeostasis (15). Ethanol has been widely used as a disinfectant in the medical field for many years, and it is generally accepted that the alcohol generated during preparation of fermented foods and drinks has a preservative function against microorganisms (25). The beneficial effects of deliberate addition of low concentrations of ethanol to prolong the shelf lives of packaged foods have also been recognized (25, 27). Much of this work, however, has focused on the use of ethanol as an agent for preventing microbial growth (27) rather than as an antimicrobial agent as described by Jordan et al. (15).
In this study we assessed the influence of ethanol on the sensitivity of L. monocytogenes to acidic pH values and a number of short-chain organic acids. In addition, we tried to determine whether the presence of ethanol sensitized L. monocytogenes to other environmental stresses, including osmotic upshock and downshock.
MATERIALS AND METHODS
Bacterial strain and storage conditions.
L. monocytogenes NCTC 7973, obtained from the National Collection of Type Cultures (Colindale, London, United Kingdom), was stored at −70°C in Microbank cryovials (Pro-Lab Diagnostics, Wirral, United Kingdom). Before use, L. monocytogenes NCTC 7973 was cultured at 37°C on TSA-YE and maintained as colonies for up to 2 weeks at 4°C; TSA-YE was tryptone soya agar (Oxoid Ltd., Basingstoke, United Kingdom) supplemented with 0.6% (wt/vol) yeast extract (Oxoid Ltd.).
Growth conditions and viable counts.
For starter cultures, colonies from TSA-YE plates were inoculated aseptically into 10 ml of tryptone soya broth (Oxoid Ltd.) supplemented with 0.6% (wt/vol) yeast extract (TSB-YE) at pH 7 and grown at 37°C for 24 h. To prepare stationary-phase cultures, these preparations were diluted 1:100 in 50 ml of TSB-YE (pH 7) in 250-ml baffled flasks and grown with shaking (150 rpm) in an orbital incubator at 37°C for 28 h. (The pH of the medium at the end of growth was determined to be between 5.75 and 5.85.) Routinely, viable counts were obtained by using serial dilutions in maximum recovery diluent (MRD)(Oxoid Ltd.). Dilutions were plated onto TSA-YE plates, which were incubated at 37°C for approximately 36 h to allow colonies to form. It should be noted that the plate incubation conditions were not optimized to recover injured cells, and consequently, injured but viable cells may not have been recovered.
Survival at low pH in the presence or absence of ethanol.
L. monocytogenes was grown to the stationary phase, diluted 1:100 in 10 ml of TSB-YE acidified to either pH 3.0 or 4.0 with HCl, and briefly vortexed. Organic acids and/or ethanol was added as required. Organic acids were prepared in deionized water by using a free acid and the salt of the acid to give the required undissociated acid concentration. The pH was adjusted with hydrochloric acid or sodium hydroxide, as appropriate. The pKa values of the different acids were taken to be 4.74 for acetate (6), 4.17 for l-ascorbate (6), 4.20 for benzoate (9), 3.13 for citrate (6), 3.75 for formate (6), 3.86 for dl-lactate (6), 3.46 for dl-malate, (9), 4.87 for propionate (9), and 4.76 for sorbate (6). When an acid had several acidic functions, the lowest pKa corresponding to the major acidic function was chosen. These values were used to calculate undissociated acid concentrations at designated pH values by using the Henderson-Hasselbalch equation. It should be noted that because of poor solubilities in water, benzoate and sorbate were used at lower concentrations (10 mM) than the other organic acids. All challenges were carried out at 37°C. Cells were recovered in and diluted in 100 mM sodium phosphate buffer (pH 7) and were enumerated on TSA-YE plates as described above.
Survival during osmotic stress in the presence or absence of ethanol.
L. monocytogenes was grown to the stationary phase and diluted 1:100 in 10 ml of TSB-YE (pH 7) supplemented with sodium chloride (NaCl), sucrose, or glycerol to generate elevated increased osmolarity, and 5% (vol/vol) ethanol was added as required. The solutions were then vortexed and kept at 37°C. Cells were recovered in MRD and enumerated on TSA-YE plates as described above. To generate hypoosmotic stress, L. monocytogenes cells were grown to the stationary phase, diluted 1:2,000 in either 10 ml of TSB-YE or 10 ml of deionized water at pH 7 supplemented with 5% (vol/vol) ethanol as required, and vortexed. All challenge solutions were buffered to pH 7.0 by using 5 mM HEPES. Cells were recovered from challenge solutions in MRD and enumerated as described above.
Fluorescence measurements.
Stationary-phase cultures of L. monocytogenes were diluted 1:10 in TSB-YE (pH 7) with and without 5% (vol/vol) ethanol, kept at 37°C for different times, and assessed for permeability to fluorescent dyes as described previously (4, 23). In separate experiments propidium iodide (2.9 μM) and ethidium bromide (100 μM) were added to samples taken from the incubation mixtures. After 10 min of incubation in the presence of the dyes, the samples were centrifuged for 5 min at 13,000 × g and washed twice in MRD. Fluorescence was measured with a luminescence spectrometer (model LS-5B; Perkin-Elmer); the excitation wavelength was set at 493 nm for ethidium bromide and at 495 nm for propidium iodide, and the emission wavelengths were set at 610 and 615 nm, respectively. The slit width was 10 nm. Fluorescence data for cell suspensions were normalized by using optical density at 600 nm and were expressed as percentages of the value obtained for cells permeabilized by heating at 80°C for 10 min. Fluorescence values obtained for cells which were not stained with either ethidium bromide or propidium iodide were subtracted from all experimental values.
RESULTS
Augmentation of killing of L. monocytogenes by combinations of lactate, ethanol, and low pH.
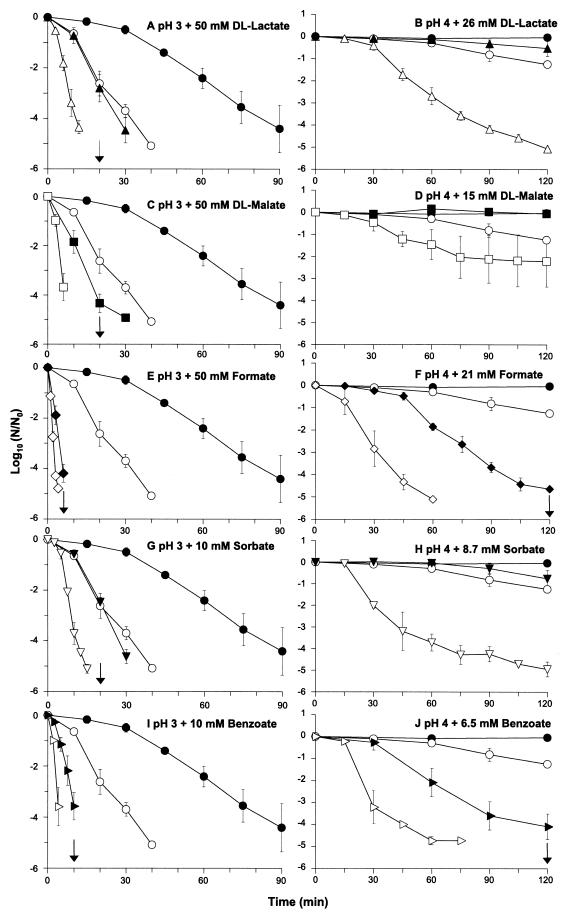
Addition of either ethanol or lactate dramatically reduced the viability of stationary-phase cells of L. monocytogenes when they were exposed to pH 3.0 (Fig. 1A). The rates of inactivation observed with the two compounds were similar, and in the presence of either agent viability decreased by approximately 4 log units in 30 min, compared with the less-than-1-log-unit decrease in viability that was observed when cells were incubated at pH 3.0 alone. A combination of the two agents proved to be even more bactericidal and reduced the viability by more than 4 log units in just 12 min. When either ethanol or lactate was used alone, 12 min of exposure resulted in less than 1 log unit of killing, indicating that the two agents act synergistically.
FIG. 1.
Augmentation of killing of L. monocytogenes at pH 3 and 4 by ethanol combined with various organic acids. Cells grown to the stationary phase (approximately 3 × 109 CFU ml−1) were diluted 1:100 in challenge media at pH 3 or 4 containing either no organic acid (● and ○), dl-lactate (▴ and ▵), dl-malate (■ and □), formate (⧫ and ◊), sorbate (▾ and ▿), or benzoate (▸ and ▹), and viability was assessed. Cells were incubated in the presence (open symbols) or in the absence (solid symbols) of 5% ethanol. For each acid the concentration added at pH 3.0 and 4.0 was the same, and the values shown represent the amounts of undissociated acid. The data are means, and the error bars indicate standard deviations for experiments performed at least in triplicate. The arrows indicate sampling times when no survivors were detected for ethanol combined with organic acids. The limit of detection was 250 CFU ml−1.
Inactivation of L. monocytogenes by combinations of organic acids, low pH, and ethanol.
Next, we investigated whether ethanol also augmented killing by various organic acids at pH 3.0. For every organic acid tested, a combination of 5% ethanol and the acid resulted in a dramatic decline in viability that was always greater than the decline observed with either agent alone (Table 1 and Fig. 1). At pH 3.0 in the absence of ethanol, citrate, ascorbate, propionate, and acetate were the least effective bactericidal agents, and the most effective compounds were formate, benzoate, malate, lactate, and sorbate, in that order. These compounds were also the most effective bactericidal organic acids when ethanol was present, and 4-log-unit killing occurred in 3, 4, 6, 10, and 12 min with formate, benzoate, malate, sorbate, and lactate, respectively (Fig. 1). When benzoate and formate were used alone, they were highly effective at killing L. monocytogenes at pH 3. Nevertheless, in all cases addition of ethanol resulted in shorter killing times.
TABLE 1.
Tolerance of stationary phase L. monocytogenes to organic acids and/or ethanol at pH 3 and 4
| Acid | % Survivala
|
|||
|---|---|---|---|---|
| pH 3
|
pH 4
|
|||
| Without ethanol | With ethanol (5% vol/vol) | Without ethanol | With ethanol (5% vol/vol) | |
| None | 72 ± 1.2 | 0.89 ± 0.20 | 85 ± 22 | 44 ± 30 |
| Citrate (50 mM)b | 93 ± 4.6 | 0.15 ± 0.10 | 11 ± 5 | 0.29 ± 0.20 |
| l-Ascorbate (50 mM) | 31 ± 8 | 0.14 ± 0.02 | 0.76 ± 0.80 | 6.2 ± 3.4 |
| Propionate (50 mM) | 7.7 ± 3.6 | 0.004 ± 0.004 | 57 ± 28 | 7.7 ± 3.7 |
| Acetate (50 mM) | 5.7 ± 3.7 | 0.045 ± 0.038 | 63 ± 26 | 13 ± 11 |
| dl-Lactate (50 mM) | 0.27 ± 0.25 | NSc | 9.3 ± 3.8 | 1.0 ± 0.3 |
| dl-Malate (50 mM) | 0.015 ± 0.012 | NS | 0.35 ± 0.11 | 0.042 ± 0.028 |
| Formate (50 mM) | NS | NS | 0.13 ± 0.05 | 0.002 ± 0.001 |
| Sorbate (10 MM) | 0.36 ± 0.12 | NS | 5.9 ± 2.1 | 0.056 ± 0.041 |
| Benzoate (10 mM) | NS | NS | 0.040 ± 0.015 | NS |
Survival is expressed as a percentage of the colony counts obtained at time zero. Values were determined after 20 min of exposure at pH 3.0 or after 1 h of exposure at pH 4. The data are means ± standard deviations for experiments performed at least in triplicate. The limit of detection was 250 CFU ml−1.
The values in parentheses are the concentrations of undissociated acids at pH 3 or 4. The total concentration of acid added was different for each pH.
NS, no survivors detected.
Increasing the pH from 3 to 4 resulted in a marked reduction in the effectiveness of ethanol when it was added alone (Fig. 1 and Table 1). Thus, while exposure to ethanol at pH 3.0 led to a 5-log unit reduction in the number of cells in 40 min, when the experiment was repeated by using pH 4.0 less than a 1-log unit reduction in viability was observed at the same time. The increase from pH 3.0 to 4.0 also led to a reduction in the ability of ethanol to stimulate cell death when it was used in combination with organic acids. Nevertheless, with one exception, the rate of cell death in the presence of organic acids was always substantially greater in the presence of ethanol. For example, when cells were exposed to lactate and incubated at pH 4.0, little decrease in viability was evident, but when ethanol was present, a 5-log unit reduction in viability occurred in 120 min (Fig. 1). For comparison, at pH 3.0 the same combination of acid and ethanol brought about an equivalent reduction in viability in just 12 min. When cells were exposed to ascorbate at pH 4.0, the extent of cell death was eight-fold less in the presence of ethanol than in the absence of ethanol (Table 1), and in this situation ethanol actually antagonized the killing effect of the organic acid. This finding differs markedly from the situation observed at pH 3.0, when the presence of ethanol increased the effectiveness of ascorbate.
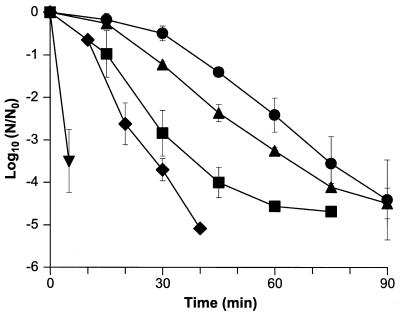
Dependence of cell death at pH 3.0 on the concentration of ethanol.
The rate of cell death at pH 3.0 showed a clear dependence on the concentration of ethanol present (Fig. 2). Thus, while cell death in the presence of 10% ethanol was stimulated by more than 3 log units following 5 min of exposure, in the presence of 1.25% ethanol cell death was enhanced by less than 1 log unit after the same time. The bactericidal effect of ethanol was also observed to be dependent on the pH of the medium, and when the experiment was repeated at pH 7.0, exposure to 1.25 to 10% ethanol did not result in any significant loss in viability over the 90-min experiment (unpublished data).
FIG. 2.
Effect of ethanol concentration on the tolerance of L. monocytogenes to pH 3.0. Cells were grown to the stationary phase (approximately 3 × 109 CFU ml−1) and diluted 1:100 in challenge media at pH 3.0, and viability was assessed in the presence of 0% (●), 1.25% (▴), 2.5% (■), 5% (⧫), or 10% (▾) ethanol. The data are means, and the error bars indicate standard deviations for experiments performed in triplicate. The limit of detection was 250 CFU ml−1.
Influence of ethanol on survival of L. monocytogenes during osmotic stress.
To determine whether ethanol also sensitized L. monocytogenes to stresses other than low pH, survival of L. monocytogenes during both hyperosmotic stress and hypoosmotic stress was monitored in the absence or presence of this compound.
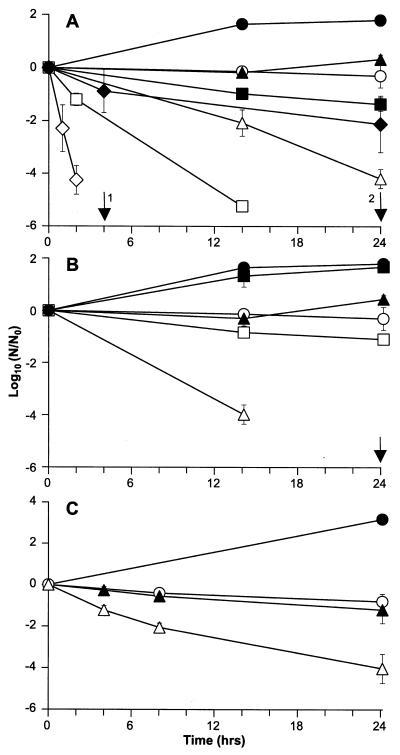
Initially, hyperosmotic stress was generated by exposing cells to different concentrations of NaCl. In the absence of ethanol only exposure to 15 and 25% NaCl resulted in a measurable decline in viability, but this decline was generally less than 2 log units of killing over a 24-h period. While the presence of ethanol had no effect on the viability of cells exposed to 2.5% NaCl (unpublished data) at higher osmolarities, addition of this alcohol resulted in increases in the rate of cell death, so that for NaCl concentrations of 8, 15, and 25%, 4 to 5-log unit reductions in the numbers of cells occurred in 24, 14, and 2 h, respectively (Fig. 3A).
FIG. 3.
Effect of ethanol on resistance of L. monocytogenes to hyperosmotic shock and hypoosmotic shock. (A) Cells were grown to the stationary phase (approximately 2 × 109 CFU ml−1) and transferred to media containing no added NaCl (● and ○), 8% NaCl (▴ and ▵), 15% NaCl (■ and □), or 25% NaCl (⧫ and ◊), and viability was assessed in the presence (open symbols) or in the absence (solid symbols) of 5% ethanol. The arrows indicate sampling times when no CFU were detected for ethanol combined with 25 and 15% NaCl (arrows 1 and 2, respectively). (B) Cells were transferred to media containing no added solute (● and ○), 47.2% (wt/vol) sucrose (▴ and ▵), or 17.8% (wt/vol) glycerol (■ and □), and viability was assessed in the presence (open symbols) or in the absence (solid symbols) of 5% ethanol. The arrow indicates a sampling time when no CFU were detected for ethanol combined with sucrose. (C) Cells were diluted 1:2,000 in 5 mM HEPES (pH 7.0) (▴ and ▵) or TSB-YE containing 5 mM HEPES (pH 7.0) (● and ○), and viability was assessed in the presence (open symbols) or in the absence (solid symbols) of 5% ethanol. The data are means, and the error bars indicate standard deviations for experiments performed in triplicate. The limit of detection was 250 CFU ml−1.
Next, we sought to establish whether ethanol also increased the sensitivity of cells of L. monocytogenes to elevated osmolarity when the stress was generated with nonionic osmolytes (Fig. 3B). Nonionic osmolytes were used at an osmolality equivalent to that of 7.5% NaCl. While exposure of L. monocytogenes to 47% sucrose alone did not lead to a significant loss of viability of L. monocytogenes over a 24-h period, a combination of sucrose and ethanol resulted in 4 log units of killing in 14 h. This rate of killing was higher than that observed for cells exposed to ethanol and 8% NaCl, when the same reduction in viability occurred only after 24 h (Fig. 3A). Exposure of the bacterial cells to 17.8% glycerol, which diffuses freely across the inner membrane and therefore does not generate osmotic stress, resulted in no loss of viability over a 24-h period. In this case, however, addition ethanol had a much less marked effect on viability, and in the presence of this agent only a 1 log unit of killing had occurred after 24 h (Fig. 3B).
To generate rapid osmotic downshock, cells were diluted in 5 mM HEPES (pH 7.0). As determined above, exposure to ethanol also compromised the ability of cells to resist the stresses that arose during survival in the presence of 5 mM HEPES (pH 7.0). While the viability of L. monocytogenes in this low-ionic-strength environment did not decline significantly over a 24-h period, inclusion of ethanol in the buffer resulted in a 4-log unit decline in the number of cells within 24 h (Fig. 3C).
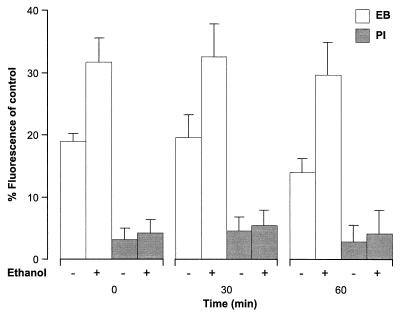
Effect of sublethal concentrations of ethanol on cell permeability as monitored with fluorescent dyes.
To determine whether exposure to ethanol caused changes in the permeability of the membrane of L. monocytogenes and whether this could have been responsible for the sensitizing effect of ethanol, cells were exposed to 5% ethanol and the permeation of the fluorescent dyes ethidium bromide and propidium iodide was assessed (Fig. 4). A one-way analysis of variance of the data demonstrated that exposure of cells to ethanol resulted in a significant (P < 0.01) increase in uptake of ethidium bromide at each time but did not significantly alter the permeability of cells to propidium iodide. Viability remained unaffected by exposure to 5% ethanol (unpublished data).
FIG. 4.
Uptake of fluorescent dyes by cells of L. monocytogenes exposed to ethanol. Cells grown to the stationary phase (approximately 3 × 109 CFU ml−1) were diluted 1:10 in TSB-YE alone (−) or TSB-YE with 5% ethanol (+). Samples were removed at intervals and subsequently stained with ethidium bromide (EB) (open bars) or propidium iodide (PI) (shaded bars). Fluorescence was expressed as a percentage of the value obtained with control cells heated at 80°C for 10 min, which was assumed to be 100%. The data are means, and the error bars indicate standard deviations for experiments performed in triplicate.
DISCUSSION
The sensitivity of E. coli O157:H7 strains to low pH can be increased by combination treatments using low pH, lactate, and ethanol (15). Here, we sought to establish whether similar treatments potentiate cell death in L. monocytogenes and whether they could form the basis of novel methods for controlling this pathogen.
In the absence of ethanol, exposure of L. monocytogenes cells to pH 3.0 led to a significant decline in viability over a 90-min period. However, addition of 5% ethanol brought about a dramatic increase in the rate of inactivation of L. monocytogenes exposed to pH 3.0. For comparison, the time taken to induce 4 log units of killing at pH 3.0 in the absence of ethanol was 90 min, while in the presence of this agent 5 log units of killing occurred in just 40 min.
Stationary-phase cells were chosen to assess the sensitivity of L. monocytogenes to the combination treatments because in general this is the cell form that is most resistant to acid (8). However, it is well established that L. monocytogenes cells can adapt to become tolerant to acid conditions during growth at mildly acidic pH values. However, cells which had been habituated at pH 5.0 were also sensitive to killing by lactate and ethanol, and a combination of these two agents reduced the viability of these cells by 4 log units in less than 12 min (unpublished data).
A number of studies have demonstrated the inhibitory activity of organic acids against L. monocytogenes and have shown that the effects are mainly related to the amount of undissociated acid (1, 2, 5, 10, 13, 16, 21, 32, 33). Thus, it was not surprising that addition of various organic acids to cells exposed to pH 3.0 led to a marked decrease in the survival of L. monocytogenes at this pH. Citrate was found to be the least effective acid for inducing cell death, while the most effective agents were formate, benzoate, malate, lactate, and sorbate, in that order. In all cases addition of ethanol in combination with the organic acid led to a further increase in cell death. For the less effective compounds, such as malate, lactate, and sorbate, it was clear that the organic acids and ethanol act synergistically to bring about cell death. When used alone at pH 3.0, benzoate and formate were highly effective bactericidal agents. Nevertheless, in both cases addition of ethanol resulted in shorter killing times. The most effective bactericidal combination, 5% ethanol and 50 mM formate, resulted in 5 log units of killing in just 4 min.
As has been observed previously for E. coli O157:H7 (15), the killing process mediated by ethanol was highly dependent on the pH of the media, and increasing the pH from 3 to 4 resulted in a marked reduction in the effectiveness of the agents. However, in all but one situation, addition of ethanol always led to a significant increase in cell death. Intriguingly, addition of ethanol to cells exposed to pH 4.0 and ascorbate actually led to a reduction in the effectiveness of the organic acid in L. monocytogenes killing, and this may have reflected a different bactericidal mechanism for ascorbate than for the other organic acids used. In this context, the presence of ascorbate can lead to intracellular production of H2O2 (18). If the toxicity of ascorbate for L. monocytogenes is in part due to production of H2O2, then the antagonistic effect of ethanol may result from the fact that exposure to ethanol can induce resistance to H2O2 and oxidative stress in L. monocytogenes (19).
Sensitization of L. monocytogenes cells by ethanol is not a phenomenon that is particularly associated with pH stress since we have demonstrated that ethanol also potentiates the death of cells when they are exposed to both hyper- and hypoosmotic stresses. When the osmolarity of the environment was raised by the presence of both ionic (NaCl) and nonionic (sucrose) solutes or was reduced by exposure to water, the presence of ethanol resulted in a significant reduction in the number of cells. However, when the osmolarity of the medium was raised by adding glycerol, the sensitizing effect of ethanol was much less. While exposure to elevated concentrations of sucrose and NaCl and osmotic downshock result in changes in the distribution of solutes across the bacterial inner membrane as a consequence of osmotic stress and osmoregulatory mechanisms, glycerol, which moves freely across the cell membrane, does not generate hyperosmotic stress and, therefore, does not induce such changes. Thus, it is possible that ethanol sensitizes cells to osmotic stress by altering the permeability of the cell membrane, thereby interfering with the distribution of solutes within the cytoplasm during osmotic stress. A similar mechanism may explain the observation that the effectiveness of ethanol as a growth-inhibiting agent for Staphylococcus aureus is dependent on water activity (26). The fact that ethanol also sensitizes cells to acid stress and to organic acids is also consistent with the concept that this agent alters membrane permeability. In this respect, any compound which increases the permeability of this barrier and which consequently is able to increase the passage of protons or organic acids into the cytoplasm should lead to disruption in the ability of the cell to maintain pH homeostasis and thus decrease resistance to this stress.
To ascertain whether exposure to ethanol did indeed alter membrane permeability, the ability of ethanol to affect the distribution of the fluorescent stains ethidium bromide and propidium iodide was assessed. Both these dyes have been used to assess injured cells in microbiological populations. As both dyes are normally excluded from the cytoplasm by the inner membrane, accumulation of these compounds in the cytoplasm is a good measure of impairment of the barrier function of the cell envelope (4, 22, 23, 29). Exposure of L. monocytogenes to ethanol clearly increased the permeability of the cells to ethidium bromide but not their permeability to propidium iodide. Since propidium iodide has a higher molecular weight (668.4) than ethidium bromide (394.3), this finding may have been a reflection of a change in membrane permeability which was sufficient to allow passage of only the smaller dye. Nevertheless, although the change in the permeability of the bacterial inner membrane may have been small in terms of the size of the compounds which could pass through it, such an alteration in permeability could have allowed increased passage of protons, organic acids, and osmotic solutes into and out of the cytoplasm depending on the relative concentration gradients. Since the change in ethidium bromide uptake occurred immediately and before any appreciable increase in the rate of cell death during acid or osmotic stress could have taken place, the initial change in the distribution of solutes either was reversible during recovery or did not immediately cause cell death. Ultimately though, ethanol increases the rate of cell death during exposure to such inimical conditions, and the observations presented here suggest that ethanol sensitizes cells to pH stress and osmotic stress by increasing the permeability of the membrane barrier.
Two previous studies have described the effects of ethanol on L. monocytogenes. In the first study, Oh and Marshall (20) demonstrated that while 5% ethanol strongly inhibited growth of this pathogen, a combination of ethanol and lactic acid did not increase the inhibitory effect of ethanol. This observation clearly does not support our observations. However, since the study of Oh and Marshall (20) was carried out at pH 7.0, the lack of interaction between ethanol and lactic acid reported by these authors was probably a consequence of the marked dependence of the killing effect on pH, as reported here. Another previous study raised the concern that exposure to sublethal environmental stresses may protect L. monocytogenes against lethal preservation factors (19). Intriguingly, one of the treatments shown to induce this stress hardening was exposure of the bacterial cells to 5% ethanol, which was shown to actually increase the resistance of L. monocytogenes to acidic pH and 25% NaCl. At first glance, these observations also appear to be in conflict with those reported here. However, while Lou and Yousef (19) exposed cells to 5% ethanol to induce resistance, the ethanol was removed prior to exposure to the stress treatments, and thus the protocol which these authors used differs significantly from that used in this study, in which ethanol was present during exposure of cells to the stress treatments. Thus, if ethanol is actually present during exposure to stress, the mechanism of sensitization described in this study seems to override any stress hardening activity attributed to this compound.
We demonstrated that ethanol can enhance the rate of inactivation of L. monocytogenes during exposure to low pH, organic acids, and osmotic stress and that sensitization of the cells can be correlated with membrane damage. It is possible, therefore, that some of the combination treatments involving ethanol described in this paper or modifications of them may be useful for control of L. monocytogenes as bactericidal treatments. Ethanol is widely used as a disinfectant in medicine and has also been used as an antimicrobial agent in food, and low concentrations of ethanol have been used to prolong the shelf lives of packaged foods (25, 27). Ethanol is present naturally in a wide range of foods and beverages and also is a permitted solvent for flavorings and colors used in a number of products. Consequently, on toxicological grounds there appears to be no reason why ethanol should not be acceptable as a food preservative (25). Indeed, it has “generally regarded as safe” status in the United States as a food ingredient (3). Thus, ethanol in combination with organic acids and low pH could be used to reduce the viability of L. monocytogenes in foods since some of the combinations examined here were particularly effective at reducing the viability of L. monocytogenes in short time periods. For example, some of the treatments described here could be used as washes to inactivate this pathogen on contaminated surfaces either on raw materials or on processing equipment. While combinations of ethanol with formate and benzoate were the most effective treatments for reducing the viability of L. monocytogenes, lactic acid may be a more appropriate choice for food treatments since the lack of acute and chronic toxicity of this compound has led to its widespread use as a food preservative and decontaminating agent.
ACKNOWLEDGMENTS
C.B was supported by a Biotechnology and Biological Sciences Research Council (United Kingdom) postgraduate studentship.
We are grateful to B. M. Mackey and S. L. Jordan for technical advice.
REFERENCES
- 1.Ahamad N, Marth E H. Behavior of Listeria monocytogenes at 7, 13, 21 and 35°C in tryptose broth acidified with acetic, citric, or lactic acid. J Food Prot. 1989;52:688–695. doi: 10.4315/0362-028X-52.10.688. [DOI] [PubMed] [Google Scholar]
- 2.Ahamad N, Marth E H. Acid-injury of Listeria monocytogenes. J Food Prot. 1990;53:26–29. doi: 10.4315/0362-028X-53.1.26. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. Fed. Reg. 1974;39:34185. [Google Scholar]
- 4.Benito A, Ventoura G, Casadei M, Robinson T, Mackey B. Variation in resistance of natural isolates of Escherichia coli O157 to high hydrostatic pressure, mild heat, and other stresses. Appl Environ Microbiol. 1999;65:1564–1569. doi: 10.1128/aem.65.4.1564-1569.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchanan R L, Golden M H. Interactions between pH and malic acid concentration on the inactivation of Listeria monocytogenes. J Food Safe. 1998;18:37–48. [Google Scholar]
- 6.Budavari S, O'Neil M J, Smith A, Heckelman P E, Kinneary J F, editors. The Merck index. 12th ed. Whitehouse Station, N.J: Merck Research Laboratories Division of Merck & Co. Inc.; 1996. [Google Scholar]
- 7.Conner D E, Scott V N, Bernard D T. Growth inhibition and survival of Listeria monocytogenes as affected by acidic conditions. J Food Prot. 1990;53:652–655. doi: 10.4315/0362-028X-53.8.652. [DOI] [PubMed] [Google Scholar]
- 8.Davis M J, Coote P J, O'Byrne C P. Acid tolerance in Listeria monocytogenes: the adaptive acid tolerance response (ATR) and growth-phase-dependent acid resistance. Microbiology. 1996;142:2975–2982. doi: 10.1099/13500872-142-10-2975. [DOI] [PubMed] [Google Scholar]
- 9.Dawson R M C, Elliot D C, Elliot W H, Jones K M. Data for biochemical research. 3rd ed. Oxford, United Kingdom: Oxford Science Publications, Clarendon Press; 1986. [Google Scholar]
- 10.El-Shenawy M A, Marth E H. Inhibition and inactivation of Listeria monocytogenes by sorbic acid. J Food Prot. 1988;51:842–847. doi: 10.4315/0362-028X-51.11.842. [DOI] [PubMed] [Google Scholar]
- 11.Farber J M, Peterkin P I. Listeria monocytogenes, a foodborne pathogen. Microbiol Rev. 1991;55:476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleming D W, Cochi S L, MacDonald K L, Brondum J, Hayes P S, Plikaytis B D, Holmes M B, Audurier A, Broome C V, Reingold A L. Pasteurized milk as vehicle of infection in an outbreak of listeriosis. N Engl J Med. 1985;312:404–407. doi: 10.1056/NEJM198502143120704. [DOI] [PubMed] [Google Scholar]
- 13.Ita P S, Hutkins R W. Intracellular pH and survival of Listeria monocytogenes ScottA in tryptic soy broth containing acetic, lactic, citric, and hydrochloric acids. J Food Prot. 1991;54:15–19. doi: 10.4315/0362-028X-54.1.15. [DOI] [PubMed] [Google Scholar]
- 14.James S M, Fanning S L, Agree B A, Hall B, Parker E, Vogt J, Run G, Williams J, Lieb L, Salminen C, Prendergast T, Werner S B, Chin J. Listeriosis outbreak associated with Mexican-style cheese. California Morb Mortal Wkly Rep. 1985;34:357–359. [PubMed] [Google Scholar]
- 15.Jordan S L, Glover J, Malcom L, Thomson-Carter F M, Booth I R, Park S F. Augmentation of killing of Escherichia coli O157 by combinations of lactate, ethanol, and low-pH conditions. Appl Environ Microbiol. 1999;65:1308–1311. doi: 10.1128/aem.65.3.1308-1311.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kouassi Y, Shelef L A. Metabolic activities of Listeria monocytogenes in the presence of sodium propionate, acetate, lactate and citrate. J Appl Bacteriol. 1996;81:147–153. doi: 10.1111/j.1365-2672.1996.tb04492.x. [DOI] [PubMed] [Google Scholar]
- 17.Kroll R G, Patchett R A. Induced acid tolerance in Listeria monocytogenes. Lett Appl Microbiol. 1992;14:224–227. [Google Scholar]
- 18.Loewen P C, Switala J, Triggs-Raine B L. Catalases HPI and HPII in Escherichia coli are induced independently. Arch Biochem Biophys. 1985;243:144–149. doi: 10.1016/0003-9861(85)90782-9. [DOI] [PubMed] [Google Scholar]
- 19.Lou Y, Yousef A E. Adaptation to sublethal environmental stresses protects Listeria monocytogenes against lethal preservation factors. Appl Environ Microbiol. 1997;63:1252–1255. doi: 10.1128/aem.63.4.1252-1255.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oh D-H, Marshall D L. Antimicrobial activity of ethanol, glycerol monolaurate or lactic acid against Listeria monocytogenes. Int J Food Microbiol. 1993;20:239–246. doi: 10.1016/0168-1605(93)90168-g. [DOI] [PubMed] [Google Scholar]
- 21.Östling C E, Lindgren S E. Inhibition of enterobacteria and Listeria growth by lactic, acetic and formic acids. J Appl Bacteriol. 1993;75:18–24. doi: 10.1111/j.1365-2672.1993.tb03402.x. [DOI] [PubMed] [Google Scholar]
- 22.Puchkov E O, Melkozernov A N. Fluorimetric assessment of Pseudomonas fluorescens viability after freeze-thawing using ethidium bromide. Lett Appl Microbiol. 1995;21:368–372. [Google Scholar]
- 23.Robinson T P, Ocio M J, Lyn F, Kaloti A, Mackey B M. The use of ethidium bromide to assess a novel injury/recovery phenomenon in Listeria monocytogenes in inhibitory NaCl conditions. Lett Appl Microbiol. 1997;25:367–370. doi: 10.1046/j.1472-765x.1997.00244.x. [DOI] [PubMed] [Google Scholar]
- 24.Schlech W F, Lavigne P M, Bortolussi R A, Allen A C, Haldane E V, Wort A J, Hightower A W, Johnson S E, King S H, Nicholls E S, Broome C V. Epidemic listeriosis: evidence for transmission by food. N Engl J Med. 1983;308:203–206. doi: 10.1056/NEJM198301273080407. [DOI] [PubMed] [Google Scholar]
- 25.Seiler D A L, Russell N J. Ethanol as a food preservative. In: Russell N J, Gould G W, editors. Food preservatives. Glasgow, Scotland: Blackie; 1991. pp. 153–171. [Google Scholar]
- 26.Shapiro M, Nelson D A, Labuza T P. Ethanol inhibition of Staphylococcus aureus at limited water activity. J Food Sci. 1978;43:1467–1469. [Google Scholar]
- 27.Shibasaki I. Food preservation with a nontraditional antimicrobial agent. J Food Safe. 1982;4:35–58. [Google Scholar]
- 28.Sorrells K M, Enigl D C, Hatfield J R. Effect of pH, acidulant, time and temperature on the growth and survival of Listeria monocytogenes. J Food Prot. 1989;52:571–573. doi: 10.4315/0362-028X-52.8.571. [DOI] [PubMed] [Google Scholar]
- 29.Votyakovka T V, Kaprelyants A S, Kell D B. Influence of viable cells on the resuscitation of dormant cells in Micrococcus luteus cultures held in an extended stationary phase: the population effect. Appl Environ Microbiol. 1994;60:3284–3291. doi: 10.1128/aem.60.9.3284-3291.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker S J, Archer P, Banks J G. Growth of Listeria monocytogenes at refrigeration temperatures. J Appl Bacteriol. 1990;68:157–162. doi: 10.1111/j.1365-2672.1990.tb02561.x. [DOI] [PubMed] [Google Scholar]
- 31.Wong A C L. Biofilms in food processing environments. J Dairy Sci. 1998;81:2765–2770. doi: 10.3168/jds.s0022-0302(98)75834-5. [DOI] [PubMed] [Google Scholar]
- 32.Young K M, Foegeding P M. Acetic, lactic and citric acids and pH inhibition of Listeria monocytogenes Scott A and the effect on intracellular pH. J Appl Bacteriol. 1993;74:515–520. [PubMed] [Google Scholar]
- 33.Yousef A E, El-Shenawy M A, Marth E H. Inactivation and injury of Listeria monocytogenes in a minimal medium as affected by benzoic acid and incubation temperature. J Food Sci. 1989;54:650–652. [Google Scholar]