Abstract
Introduction:
A marked increase in hospitalizations for severe, injection-related infections (SIRI) has been associated with the opioid epidemic. Outpatient parenteral antibiotic therapy (OPAT) is typically not offered to persons with opioid use disorder (OUD) and SIRI, though increasing evidence suggests it may be feasible and safe. This study evaluates the efficacy and cost-effectiveness of an integrated care model combining Buprenorphine treatment of OUD with OPAT for SIRI (B-OPAT) compared with treatment as usual on key OUD, infectious disease, and health economic outcomes. B-OPAT expands and incorporates key elements of established clinical models, including inpatient initiation of buprenorphine for OUD, inpatient infectious disease consultation for SIRI, office-based treatment of OUD, and OPAT, and includes more frequent clinical outpatient visits than standard OPAT. A qualitative evaluation is included to contextualize effectiveness outcomes and identify barriers and facilitators to intervention adoption and implementation.
Methods:
B-OPAT is a single-site, randomized, parallel-group, superiority trial recruiting 90 adult inpatients hospitalized with OUD and SIRI who require at least 2 weeks of intravenous (IV) antibiotic therapy. After screening, eligible participants are randomized 1:1 to either discharge once medically stable to an integrated outpatient treatment care model combining Buprenorphine and OPAT (B-OPAT) or to Treatment As Usual (TAU). The primary outcome measure is the proportion of urine samples negative for illicit opioids in the 12 weeks after discharge from the hospital. Key secondary OUD outcomes include self-reported number of days of illicit opioid abstinence and 12-week retention in buprenorphine treatment. The infection outcomes are completion of recommended IV antibiotic therapy, peripherally inserted central catheter (PICC) complications, and readmission related to primary SIRI.
Conclusions:
The B-OPAT study will help address the important question of whether it is clinically effective and cost-effective to discharge persons with OUD and SIRI to an integrated outpatient care model combining OUD treatment with OPAT relative to TAU (Clinicaltrials.gov Identifier: NCT04677114).
Keywords: Buprenorphine, endocarditis, opioid use disorder, vascular access devices
Introduction
The opioid epidemic continues with devastating consequences including more than 100,000 overdose deaths in 2021, 1 and increasing incidence of severe infectious complications of opioid use disorder (OUD) and injection drug use (IDU), such as infectious endocarditis (IE) and osteomyelitis.2,3 The resulting marked increase in hospitalizations for these severe, injection-related infections (SIRI) has made abundantly clear that there are major gaps in the evidence base to guide clinical care, particularly in the transition out of the hospital. While patients with these infections who do not have substance use disorder (SUD) are frequently able to be enrolled in an outpatient parenteral antibiotic therapy (OPAT) program and discharged to complete antibiotics via a peripherally inserted central catheter (PICC), 4 OPAT is typically not offered to patients with OUD and SIRI due to concerns of ongoing IDU and PICC complications.5,6 However, OPAT is associated with decreased risk of hospital-acquired infections and with improved patient satisfaction. 7 Increasing observational evidence suggests that OPAT may be appropriate for patient populations with SUD as well,8,9 but there is an urgent need for prospective clinical trials. Furthermore, IDU has been identified as a risk factor for IE 10 and vertebral osteomyelitis; 11 however, clinical practice guidelines for these infections do not include specific recommendations for incorporating evidence-based OUD treatment. Therefore, demonstration of a successful, integrated, outpatient model incorporating buprenorphine treatment and OPAT may change current clinical care and OPAT guidelines.
We previously conducted a pilot, proof-of-concept, randomized study to establish the safety and feasibility of discharging hospitalized patients with OUD and SIRI to complete intravenous (IV) antibiotics in an integrated, outpatient model combining buprenorphine treatment with OPAT.12,13 In that study, 20 participants were enrolled and randomized, 10 to early discharge to complete antibiotics as outpatients via OPAT, and 10 to complete antibiotics as inpatients per usual care. Both groups received buprenorphine induction in the hospital and continued outpatient buprenorphine treatment for 12 weeks after discharge. In the early discharge OPAT group, participants also received home delivery of IV antibiotics, instruction for self-administering antibiotics, weekly PICC dressing changes, and a follow-up infectious disease visit. All participants (100%) completed the recommended course of IV antibiotics, with the early discharge OPAT group completing a mean of 20.1 (±11.1) days of IV antibiotics as outpatients. The mean length of hospital stay in the early discharge group was 22.4 (±7.8) days versus 45.9 (±7.8) in the usual care group (p < 0.001). The 12-week retention in outpatient treatment after hospital discharge was similar in both groups. The proportion of urine samples negative for illicit opioids was significantly greater in the early discharge group compared with usual care (p = 0.049). 13 This pilot safety and feasibility study provided preliminary data and a care model framework for the clinical trial described here and was the first prospective randomized controlled trial (RCT) on OPAT in people who inject drugs (PWID).
This study evaluates the efficacy and cost-effectiveness of the B-OPAT integrated care model for treatment of OUD and SIRI compared with treatment as usual on key OUD, infectious disease, and health economic outcomes. A qualitative evaluation is included to contextualize effectiveness outcomes from provider and patient perspectives and identify barriers and facilitators to intervention adoption and implementation. We describe the design, protocol, outcomes measures, and analytic considerations for this ongoing RCT.
Methods
Study design
B-OPAT is a single-site, randomized, parallel-group, superiority trial recruiting adult (18 years or older) inpatients hospitalized with OUD and SIRI who require at least 2 weeks of IV antibiotic therapy. Eligible participants are carefully screened and, if qualified, randomized 1:1 to either an integrated addiction and infectious disease outpatient treatment care model combining Buprenorphine pharmacotherapy for OUD and OPAT for SIRI (B-OPAT) or to Treatment As Usual (TAU).
The total duration of study participation in both groups is from informed consent to 24 weeks post-hospital discharge. The active study intervention period is 12 weeks post-hospital discharge, followed by a 3-month follow-up period. The inpatient study participation time will vary depending on group assignment and clinical course (e.g. time to medical readiness for discharge may take longer for IE than for bacteremia), but is estimated at ~2 weeks for B-OPAT and ~5 weeks for TAU.
Research questions
The primary objective of the clinical trial is to evaluate the integrated outpatient care model, B-OPAT, compared with TAU on key OUD and infection outcomes. For OUD outcomes, it is hypothesized that (1) the proportion of urine samples negative for illicit opioids in the 12 weeks after discharge from the hospital will be greater in the B-OPAT group compared with TAU (primary outcome); (2) self-reported number of days of illicit opioid abstinence will be greater in the B-OPAT group compared with TAU; (3) self-reported number of days without injection use of any drug will be greater in the B-OPAT group; and (4) 12-week retention in buprenorphine treatment will be higher in the B-OPAT group. For infection outcomes, it is hypothesized that (1) completion of recommended IV antibiotic therapy will be similar between the two groups; (2) discharge against medical advice will be lower in the B-OPAT group compared with TAU; (3) PICC-line complications will be similar in the two groups; and (4) readmission related to primary SIRI will be similar in the two groups.
The goal of the health economic evaluation is to calculate the cost of implementing and running B-OPAT and determine the relative value of the model from the healthcare sector and societal perspectives. 14 The healthcare sector includes all direct healthcare costs incurred on behalf of the participant, including out-of-pocket expenditures, while the societal perspective includes all direct and indirect costs incurred by society on behalf of the participant. It is hypothesized that reduced utilization of high-cost healthcare and criminal-legal resources and increased productivity, time abstinent from opioids, and quality-adjusted life years (QALYs) will result in the integrated outpatient care model being cost-effective compared with TAU from both perspectives, at traditionally accepted value thresholds.
Study setting
The B-OPAT study is being conducted at the University of Kentucky (UK), in Lexington, KY, USA. Participants are recruited and enrolled while hospitalized at two UK Hospitals, which are tertiary referral centers for the region. The integrated outpatient B-OPAT care model occurs at the UK First Bridge Clinic, which provides low-threshold OUD treatment within the UK Center on Drug and Alcohol Research (CDAR). Home infusion services are provided by a company that serves many healthcare systems in the area. Post-discharge infectious disease care occurs at the UK Bluegrass Care Clinic.
Study inclusion/exclusion criteria
Inclusion criteria include adults aged ⩾18 years with OUD and SIRI (IE by Duke’s criteria, 15 osteomyelitis, septic arthritis, bacteremia, severe skin and soft tissue infections requiring IV antibiotic therapy), providing informed consent, accepting of buprenorphine treatment, anticipated to be discharged home after medically stabilized, and have ⩾5 days of IV antibiotics remaining at the time of medical readiness for discharge (as defined by the primary clinical team). Five days of IV antibiotic therapy remaining was chosen as a minimum amount of time for exposure to home IV antibiotics via PICC if randomized to the B-OPAT arm. Individuals for whom injection behavior cannot be confirmed (either by exam or history) may enroll if they are otherwise eligible.
Exclusion criteria include stroke or cerebral mycotic aneurysms preventing aortic or mitral valve surgery, fungal IE, patients who require inpatient physical rehabilitation, current pregnancy, hypersensitivity or allergy to buprenorphine, class III or IV heart failure, end-stage liver disease, end-stage renal disease, other screening laboratory, medical, and/or psychiatric condition that may prevent the volunteer from safely participating in the study in the opinion of the investigator (e.g. benzodiazepine or alcohol dependence requiring medically supervised withdrawal), self-report of desire to inject into PICC line, pending legal action that could interfere with study participation, unstable home environment precluding safe outpatient administration of IV antibiotics, and living more than an approximately 60-min drive from Lexington, KY, given the intensive outpatient component to the intervention.
Experimental and control groups
Integrated Outpatient Care Model (B-OPAT)
The experimental B-OPAT integrated care model expands and incorporates key elements of established clinical models. While hospitalized, B-OPAT participants receive standard clinical care, including infectious disease consultation for treatment of SIRI, addiction medicine consultation for inpatient initiation of buprenorphine for OUD, 16 and overdose education and naloxone distribution. Consultation from other clinical services occurs as indicated (e.g. cardiothoracic surgery, orthopedics). Once the antibiotic plan is finalized and the participants are medically stable, OPAT services are arranged per standard practice, with the exception being that if home health is not available, then the PICC dressing changes and blood draws for laboratory monitoring occur at the First Bridge Clinic. B-OPAT participants are then discharged with the PICC in place to complete IV antibiotics at home via enhanced OPAT, which includes office-based buprenorphine treatment for OUD 17 and more frequent clinical outpatient visits than would typically be recommended in standard OPAT. 4
Patients are eligible to continue study participation if they choose to transition from sublingual to extended-release buprenorphine (Sublocade®) at discharge. The outpatient visit frequency in B-OPAT is less than the initial thrice-weekly visits in the pilot study, 13 which adopted a conservative approach as there were no previous randomized trials of OPAT in persons with OUD and IDU at that time. As the pilot study demonstrated safety, this study modestly decreased clinic visit requirements (detailed in section ‘Recruitment, screening, and informed consent’) to be more manageable for patients and clinical providers, and likely more realistic for potential future real-world implementation.
TAU
TAU is designed to reflect the current standard approach to treatment of SIRI in US hospitals, with the added component of evidence-based treatment of OUD with buprenorphine. While hospitalized, participants in TAU also receive infectious disease consultation for treatment of SIRI, addiction medicine consultation for inpatient initiation of buprenorphine for OUD, 16 and consultation from other clinical services as indicated. Participants in TAU are also likely to have PICCs placed as hospitalized patients with SIRI typically receive them to facilitate IV medication administration and blood draws. In TAU, the primary medical team determines where participants will complete antibiotics, and patients receive follow-up appointments for continued buprenorphine treatment and infectious disease care per standard clinical care. Participants in TAU are able to receive continued buprenorphine treatment after discharge at the First Bridge Clinic if they choose to, or at another location if that is their preference. Patients with SIRI typically are asked to stay in the hospital for the duration of IV antibiotic therapy, but some may be discharged to complete IV antibiotics in a skilled nursing facility, residential addiction treatment setting, or may be discharged without a PICC on partial oral or long-acting injectable lipoglycopeptide antibiotic therapy.18,19 The clinical landscape of OUD and SIRI is changing rapidly, and this inclusive approach to the TAU arm provides greater external validity and generalizability.
Sample size and power calculation
Sample size estimates were generated for the primary outcome (illicit opioid negative urine samples over 12 weeks, missing considered positive), and for the key secondary outcome of treatment retention based on the pilot study. 13 Estimates were premised with power = 0.8, alpha = 0.05, 20 and demonstrated medium effect sizes with a sample ranging from 66 to 94 subjects. Thus, the planned sample size of 90 (45 per group) ensures adequate power to detect a between-group difference with medium effect size, and while allowing for possible additional attrition due to SIRI mortality of 5–10% (~4–9 participants).
Stratification and randomization
Randomization occurs in the hospital for those meeting all eligibility criteria and providing informed consent. They are randomized (n = 45 per group) to TAU or B-OPAT. Subjects are stratified prior to randomization on the clinical SIRI subgroup, current stimulant use disorder (yes/no), and sex (male/female). The clinical SIRI stratification variable has three subgroups: (1) low-risk IE [native tricuspid valve (TV) managed without surgery], (2) high-risk IE (native TV managed surgically, native aortic valve, native mitral valve, and any prosthetic valve), and (3) non-IE (osteomyelitis, septic arthritis, bacteremia). It is important to stratify on the SIRI subgroup because of significant variation in morbidity and mortality risk associated with these infections. In-hospital IE mortality is estimated at 20% and 25–30% at 6 months. 21 Expected in-hospital mortality for persons with IDU and isolated TV IE not requiring surgery, however, is lower at 5%. 22 Mortality rates in osteomyelitis are less well-defined in the literature, but are meaningfully lower than in IE, for example, 15% 5-year mortality estimates in vertebral osteomyelitis. 23 The second stratification criterion is current stimulant use disorder because it was present in 50% of participants in the preliminary study 13 and complicates OUD treatment.
Study procedures
Recruitment, screening, and informed consent
Potential participants are identified via electronic chart review of patients admitted to the inpatient services at the UK Hospitals. Patients also may be referred to the study by the hospital clinical teams, infectious disease consult service, and/or the addiction consult services. A study clinician conducts an initial review of the electronic health record (EHR) for potential eligibility, identifying the presence of a qualifying SIRI, and absence of immediately disqualifying health conditions. Research staff then approach potential participants to introduce the study and offer initial study screening. Potentially eligible participants provide consent for in-person screening, which confirms the presence of OUD by DSM 5 criteria, willingness to accept buprenorphine treatment, and other eligibility criteria. Participants meeting screening eligibility then are offered full study informed consent and completion of screening assessments (see Table 1 for screening and initial assessment measures). Randomization then occurs once the participant is approaching medical stability.
Table 1.
Schedule of research assessments for both study arms.
| Inpatient | Post-hospital discharge week | Follow-up month | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial | Weekly | 1–3 | 4 | 5–7 | 8 | 9–11 | 12 | 4 | 5 | 6 | |
| Addiction Severity Index – Lite | x | x | x | x | x | x | x | ||||
| Mini Neuropsychiatric Interview v. 5.0 | x | ||||||||||
| Patient Health Questionnaire (PHQ-9) | x | x | x | x | x | x | x | ||||
| General Anxiety Disorders (GAD-7) | x | x | x | x | x | x | x | ||||
| Brief Trauma Questionnaire | x | ||||||||||
| Primary Care – PTSD | x | x | x | x | x | x | x | ||||
| Brief Pain Inventory | x | x | x | x | x | x | x | x | |||
| Subjective Opioid Withdrawal Scale | x | x | x | x | x | x | x | x | x | x | x |
| Clinical Opiate Withdrawal Scale | x | x | x | x | x | x | x | x | x | x | x |
| Visual Analog Scale (VAS) a | x | x | x | x | x | x | x | x | x | x | x |
| Timeline Followback (TLFB) | x | x | x | x | x | x | x | x | x | x | x |
| TLFB-antibiotics b | x | x | x | x | |||||||
| PROPr | x | x | x | x | x | x | x | ||||
| Non-study Medical and Other Services | x | x | x | x | x | x | x | ||||
| Criminal and Legal Activities Form | x | x | x | x | x | x | x | ||||
| Urine drug testing | x | x | x | x | x | x | x | x | x | x | x |
| Chart review | x | x | x | x | x | x | x | ||||
| PICC check | x | x | x | x | x | ||||||
| Concommitant medications | x | x | x | x | x | x | x | x | x | x | x |
| Safety/AE check | x | x | x | x | x | x | x | x | x | x | x |
AE, adverse event; PICC, peripherally inserted central catheter; PTSD, post-traumatic spectrum disorder.
VAS items: opioid withdrawal, desire to use opioids, desire to inject into PICC (if present), desire to inject substances other than opioids.
TLFB-antibiotics will be included in B-OPAT, and in TAU if subjects finish IV antibiotics outside the hospital.
Integrated Outpatient Care Model (B-OPAT)
After randomization, the discharge transition and arrangement of OPAT requires coordination between the clinical and research teams. The research team notifies the primary team (most frequently hospital medicine), infectious disease, and addiction medicine of the randomization to B-OPAT. The infectious disease OPAT team (part of standard clinical services offered by the UK Division of Infectious Diseases) provides final antibiotic recommendations including laboratory monitoring, follow-up appointment, and patient education regarding self-administration of IV antibiotics. The primary hospital team confirms medical readiness for discharge, coordinates other needed follow-up (e.g. with relevant surgical specialties), provides the antibiotic prescription, and refers to home infusion and home health services. The home infusion company provides additional education to the patient regarding care of the PICC and antibiotic infusion and arranges delivery of antibiotics and PICC care supplies to the patient’s home. The addiction consult team coordinates the post-discharge appointment within 1 week of discharge at the First Bridge Clinic and provides the buprenorphine prescription until that appointment or an injection of XR-buprenorphine prior to discharge. The participant is discharged at the time of medical stability as determined by the primary clinical team.
After hospital discharge and while the PICC is in place, participants attend appointments twice weekly at the First Bridge Clinic, located at the UK CDAR for continued buprenorphine treatment. Clinician visits include medication management supported by motivational interviewing, and participants receive short buprenorphine prescriptions until the next scheduled visit (to leverage the positive reinforcement attributes of buprenorphine in relieving opioid craving and withdrawal, thereby maximizing treatment retention and adherence). Patients receiving XR-buprenorphine are asked to attend the same frequency of appointments. Peer recovery support and counseling services are also on-site and available.
The weekly PICC dressing changes and labs for clinical monitoring of antibiotic therapy are done as part of these clinical appointments. The OPAT team follows the antibiotic results per standard clinical practice and coordinates with the home infusion company if antibiotic adjustments are needed. Participants also attend a follow-up appointment with an infectious disease specialist within 4 weeks after discharge as part of standard clinical follow-up of SIRI. At the end of OPAT, the PICC is removed by a trained member of the First Bridge clinical team (clinician or nurse), or at the infectious disease appointment. After the PICC is removed, clinician visits continue once weekly for continued buprenorphine treatment until 12 weeks post-discharge. Visits may be more frequent if clinically indicated. After week 12, participants are referred to standard care and may continue in the First Bridge Clinic if desired. All clinical care visits are billed to insurance in B-OPAT and TAU. Kentucky is a Medicaid expansion state, and the most common payor expected in this study population is Medicaid. Research follow-up visits continue monthly for another 3 months.
TAU
Participants randomized to TAU will complete IV antibiotics in the setting determined by the primary clinical team. Participants will be referred to continued outpatient buprenorphine treatment by the addiction consult service and follow-up with infectious disease will be arranged per usual clinical practice. Post-discharge research assessments occur at the same frequency and for the same duration as the B-OPAT group.
Research assessments and outcome measures
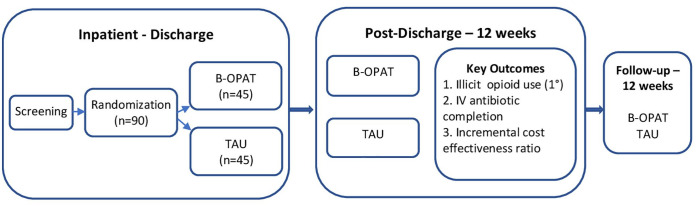
The screening and initial assessments take place in the hospital to establish the presence of SUD and comorbid psychiatric symptoms and disorders, self-reported drug use, health-related quality of life (HRQoL), and other key elements. Details of the SIRI diagnosis, medical comorbidities, surgical procedures, and PICC line insertion and complications during the hospitalization are collected from the EHR. Research assessments then occur weekly until discharge. After discharge, research assessments occur weekly for the 12-week study intervention, and then monthly for 3 follow-up months. Post-discharge research assessments are coordinated with clinical visits to minimize participant burden. The list and frequency of research assessments are summarized in Table 1 and Figure 1 illustrates the study schema.
Figure 1.
Study schema.
Primary and key secondary outcome measures
The primary outcome of illicit opioid use over the 12-week post-discharge study period is measured using urine test results (positive or negative for illicit opioids) at each weekly research visit. The proportion of urine tests negative for illicit opioids is then calculated. Illicit opioid use was chosen as the primary outcome because it is most representative of the clinical efficacy of an OUD treatment model, as well as being highly relevant to the perceived risk of managing PICCs in the outpatient setting and the risk of future injection-related infections. Self-reported number of days of illicit opioid use and of injection use of any drug are measured using Timeline Followback (TLFB). Retention in outpatient buprenorphine treatment is defined as number of days from discharge until the end of the last outpatient buprenorphine prescription per medical chart review.
For the infection outcomes, IV antibiotic completion is assessed via self-report and chart review. The utilization of healthcare resources, including readmission related to the primary SIRI, is measured using medical records, adverse event monitoring, and self-report using time-anchoring methodology via the Non-study Medical and Other Services (NMOS) form. 24 Healthcare resources include inpatient, outpatient, and emergency department visits, medications for OUD, residential and outpatient SUD treatment days, and mental health treatment visits. PICC complications are monitored via chart review, self-report, and weekly visual assessments by a study clinician or nurse and are defined as the rate of complications (phlebitis, catheter-associated bloodstream infection, venous thrombosis, hematoma, mechanical complication)25–27 per number of PICC days inpatient and outpatient.
Safety measures
Safety measures collected during study visits (see Table 1) include (1) adverse events assessments, (2) prior and concomitant medications assessments, (3) healthcare utilization, (4) evaluation of the PICC and any complications, and (4) adherence to and complications from recommended antibiotic regimens.
Health economic outcomes
The primary economic outcome is the incremental cost-effectiveness ratio (ICER), calculated as the incremental mean cost of B-OPAT relative to TAU, divided by the incremental mean effectiveness of the two arms. The primary measure of effectiveness for the economic evaluation will be the QALY, calculated from the participant’s self-reported values on the Patient-Reported Outcomes Measurement Information System (PROMIS)-Preference (PROPr) instrument. The QALY is a measure that weights the amount of time a person spends in a particular health state, with the HRQoL associated with that state, and is recommended as the primary effectiveness measure in economic evaluation studies due to its ability to be compared across interventions and disorders, 14 the importance of HRQoL as a measure of patient well-being,28,29 and the fact that generally accepted value thresholds exist for QALYs, unlike clinical measures.30,31 PROPr measures HRQoL across the following domains: cognitive function, depression, anxiety, fatigue, pain interference, pain intensity, physical function, sleep, and ability to participate in social roles and activities. 32 PROPr has five levels for each domain, ranging from ‘no problems’ to ‘extreme problems’. Responses will be used to generate a global health utility index value that represents the general US population’s preference for the participant’s current health state. 33 This value is then used to calculate QALYs.34–38
The secondary measure of effectiveness for the economic evaluation will be Opioid-Free Years, which will be operationalized as the predicted proportion of the year that the participant was not using opioids. Time free from opioid use is an important measure of treatment effectiveness for clinical stakeholders, and calculating cost-per-Opioid Free Year enables comparisons with existing economic evaluations that have utilized similar effectiveness measures.35,39
Qualitative interviews
Fifteen participants from each group (N = 30) are randomly selected to complete two in-depth qualitative interviews at 4 and 12 weeks post-discharge, to (1) assess facilitators and barriers to intervention effectiveness during hospitalization and the outpatient transition, and (2) gather patient perspectives on the most salient elements of the treatment experience and care model that impacted observed outcomes. The interviews gather information on patient satisfaction with the experimental B-OPAT intervention, identify targets that might increase retention, and explore substantial barriers and potential adaptations to enhance acceptability by patients. The in-depth interviews also provide rich data on the following: when patients experienced the most difficulties during hospitalization and the outpatient transition, factors that supported treatment engagement throughout the study period, as well as patient perspectives and priorities related to outcomes. Qualitative interviews are coordinated with clinical visits to minimize participant burden.
In addition, one-time in-depth qualitative interviews are conducted among a sample of key healthcare provider stakeholders and organizational leaders (N = 15) to elicit critical information from participating health care providers and leadership on the multi-level barriers to implementing the experimental intervention, be they provider- or systems-level issues. Qualitative interviews with stakeholders are designed to assess attitudes and experiences of delivering care to patients with OUD and SIRI, structural or organizational barriers to implementing the treatment model, and challenges for scalability. Stakeholders are asked about contextual, organizational, and environmental factors salient to intervention implementation, including current capacities and clinical operations and workflows, training needs for staff, and other factors that could potentially impede successful adoption of the intervention.
Participant incentives
Participants are paid $25 for initial screening and informed consent, $50 for the full screening assessment, $25 for each short outpatient research assessment (weeks 1–3 of each outpatient month), $50 for each longer monthly outpatient research assessment, $50 for each monthly follow-up visit, and $250 as a bonus if all research assessments are completed. The subset of enrolled participants randomly invited to complete two qualitative interviews receive $25 per interview.
Analytic plan
Primary and key secondary analyses
All primary and secondary analyses will be conducted with the intent-to-treat (ITT) population, which will consist of all randomized subjects. Between-group comparisons on single initial assessment and baseline variables will be conducted using t tests for continuous variables and chi-square tests for dichotomous variables to detect whether group adjustments are needed through the use of covariates. Adverse events will be described descriptively for each treatment group. Generalized estimating equation (GEE) analyses with an exchangeable correlation structure will be used to assess differences between groups on the primary outcome of negative opioid urine samples in the 12-week post-hospital discharge period. Missing values for the primary outcome will be considered positive. GEE results are reported as odds ratios (OR), indicating the likelihood that the B-OPAT group had different outcomes than the TAU group, and 95% CIs surrounding the OR. 40 In addition, other secondary or exploratory analysis will be conducted, such as a cumulative distribution function (CDF) of percent opioid negative samples across the same 3-month period will be calculated for each subject and will be analyzed using Wilcoxon rank-sum test.
Assessing group differences for the secondary outcomes, such as completion of recommended IV antibiotic therapy, self-reported number of days of illicit opioid abstinence, self-reported number of days without injection use of any drug, and buprenorphine treatment retention, will be analyzed with GEE when outcomes are dichotomous and analyzed in Proc Mixed with an autoregressive covariance structure when outcomes are continuous (Singer 1998). These analyses will permit an examination for effects of assignment to treatment group, time effects associated with the course of treatment, and group × time interactions. All models will be run in SAS (SAS Institute, Inc., Cary, NC, USA), and statistical significance will be set at p < 0.05.
Attrition is expected over the 12-week outpatient study. Inspection of missing data and correlates of missingness will be examined upon study completion. Data sets containing missing values will be analyzed by missing data statistical methodology (e.g. GEE, Proc Mixed).41–43 The primary outcome of negative opioid urine samples will be analyzed with missing values as positive as a more conservative approach than missing values as missing. We will also conduct sensitivity analyses, including Missing Not-At-Random (MNAR) approaches, to examine the robustness of our outcomes under varying assumptions.
Health economic analysis
The economic analyses will be conducted using well-established guidelines,14,44,45 from the healthcare sector and societal perspectives. First, we will conduct a microcosting analysis to identify resources necessary to both implement and sustain B-OPAT, and estimate the costs associated with those resources. Second, we will estimate the economic value of B-OPAT relative to TAU, measured via four ICERs, one for each stakeholder perspective, and covering two time points: 12 weeks post-hospital discharge (intervention period) and 24 weeks post-hospital discharge (intervention plus follow-up).
The resource costing method will be used to estimate participant-level costs. This method involves determining a per-unit price weight (adjusted for inflation) that reflects ‘real-world’ costs faced by a chosen stakeholder, for each relevant resource, and multiplying it by the number of resource units utilized.14,45–47 We will model the monthly person period for all relevant costs and outcomes, over the 12-week (post-discharge) intervention and the subsequent 3-month follow-up periods. Individual, multivariable generalized linear mixed models (GLMMs) will be estimated to predict the mean value for each resource and effectiveness measure, at each time period, by study arm. The GLMM is well-suited for prospective, ITT economic evaluations, as it allows for the assessment of the mean and variance functions that best fit the observed data (economic data are often heavily skewed), and uses all available data for each participant, regardless of whether or not it is complete. 45 Final predicted mean values will be estimated via the statistical method of recycled predictions. We will then test for cost-offsets between arms, for each relevant resource, as well as for mean total costs. 45 Tests will be conducted using standard errors derived from nonparametric bootstrapping techniques within the multivariable framework, to account for sampling uncertainty.
Finally, ICERs will be calculated and their uncertainty assessed through confidence intervals and acceptability curves, both of which also rely on values obtained from the nonparametric bootstrapping. The cost-effectiveness acceptability curve displays the probability that the intervention is cost-effective relative to TAU across a range of willingness-to-pay thresholds (i.e. cost-per-QALY and cost-per-Opioid-Free Year), for a given time period. 45 Sensitivity analyses will be performed to account for uncertainty pertaining to assumptions or parameter estimates applied in the original analyses. 14
Oversight of data and safety
The study was approved by the University of Kentucky Institutional Review Board (IRB) (IRB ID: 60903), which also provides oversight of participant safety. An independent Data and Safety Monitoring Board provides additional oversight by examining accumulating data to assure protection of participant safety and recommending to the sponsor [National Institute on Drug Abuse (NIDA)] whether there is support for continuation of the trial, evidence that study procedures should be changed, or whether the study should be halted for reasons concerning participant safety.
Current status of the trial
The study began recruitment in February 2021, and approximately 30% of planned enrollment has occurred to date. Participant enrollment is expected to continue through November 2023.
Summary
There is increasing recognition that integrating evidence-based treatment for OUD in general medical settings is critical to improving outcomes, including for SIRI 48 and other infections associated with SUDs such as HIV 49 and hepatitis C. 50 For patients with OUD hospitalized with SIRI, there is an urgent need for research to define and evaluate integrated clinical care models that provide alternatives to prolonged hospitalizations to complete IV antibiotic courses. PWID have historically been excluded from OPAT studies; the prevailing assumption has been that it is not safe to offer OPAT to PWID due to the potential for substance use through the PICC and risk of incomplete antibiotic treatment. The B-OPAT study will help address the important question of whether it is clinically effective and cost-effective to discharge persons with OUD and SIRI to an integrated outpatient care model combining OUD treatment with OPAT relative to treatment as usual.
The clinical activities in the inpatient and outpatient settings are not individually experimental, but are explicitly coordinated and integrated in the investigational B-OPAT model, improving the external validity and likelihood of implementation in other settings. Although housing insecurity is common in this patient population, a stable home environment is an inclusion criterion because a home address is needed to deliver antibiotics, refrigeration is often needed for the antibiotics, and patients must be able to maintain personal hygiene. Even though safe housing is a typical requirement for OPAT, this requirement may limit generalizability of the study results. The randomized controlled design, while challenging to undertake in an active, and frequently changing clinical environment like the hospital, is critical to reduce bias. Patients hospitalized with OUD and SIRI frequently experience stigma.51,52 The blanket assumption that OPAT is ‘not safe’ for PWID is a stigmatized perspective that persons with SUDs are either not capable of or not willing to make positive choices with respect to their medical care. The inclusion of in-depth qualitative interviews with participants, care providers, and hospital administration will provide additional insights into these, and other issues important to the implementation and adoption of the B-OPAT model.
In keeping with the innovative, integrated design of the B-OPAT care model, this study includes a unique multidimensional assessment of the patient sample enrolled and their clinical care, including outcomes on OUD, infectious diseases, and an economic analysis from the healthcare and societal perspectives. Demonstration of a successful, cost-effective, integrated, outpatient model incorporating buprenorphine treatment and OPAT that improves OUD and infectious disease outcomes, and is more acceptable to patients, may change current clinical care and OPAT guidelines.
Supplemental Material
Supplemental material, sj-doc-1-tai-10.1177_20499361221108005 for Design and protocol of the Buprenorphine plus Outpatient Parenteral Antimicrobial Therapy (B-OPAT) study: a randomized clinical trial of integrated outpatient treatment of opioid use disorder and severe, injection-related infections by Laura C. Fanucchi, Sean M. Murphy, Hilary Surratt, Shashi N. Kapadia, Sharon L. Walsh, James A. Grubbs, Alice C. Thornton, Paul Nuzzo and Michelle R. Lofwall in Therapeutic Advances in Infectious Disease
Declarations
Ethics approval and consent to participate: The study was approved by the University of Kentucky (UK) Institutional Review Board (IRB) (IRB ID: 60903). A HIPAA (Health Insurance Portability and Accountability Act) waiver of informed consent was received by the UK IRB to conduct the initial chart review. A Prisoner Research Certification was obtained from the US Department of Health and Human Services, Office for Human Research Protections allowing prisoners to be included in the study. This study does not recruit participants who are currently incarcerated, but if a participant becomes incarcerated after enrollment, they may continue study participation. Participants are asked to provide written informed consent before enrolment.
Consent for publication: Not applicable.
Author contributions: Laura C. Fanucchi: Conceptualization; Funding acquisition; Methodology; Project administration; Writing – original draft.
Sean M. Murphy: Investigation; Methodology; Writing – review & editing.
Hilary Surratt: Investigation; Methodology; Writing – review & editing.
Shashi N. Kapadia: Investigation; Methodology; Writing – review & editing.
Sharon L. Walsh: Methodology; Writing – review & editing.
James A. Grubbs: Methodology; Writing – review & editing.
Alice C. Thornton: Methodology; Writing – review & editing.
Paul Nuzzo: Methodology; Project administration; Writing – review & editing.
Michelle R. Lofwall: Conceptualization; Methodology; Project administration; Writing – review & editing.
ORCID iD: Laura C. Fanucchi  https://orcid.org/0000-0003-0582-2399
https://orcid.org/0000-0003-0582-2399
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is funded by the National Institute on Drug Abuse (NIDA), National Institutes of Health by grant R01DA048892 (Laura Fanucchi and Michelle Lofwall, MPI). The investigators are responsible for study design, data collection, management, analysis, and interpretation of data, and writing and submission of reports.
Conflict of interest statement: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: In the last 3 years, Dr Lofwall has received consulting fees from Titan Pharmaceuticals and Camurus. Camurus has funded her travel to a scientific conference and conference registration. Dr Walsh is on scientific advisory boards for Titan Pharmaceuticals, Opiant Pharmaceuticals, Astra Zeneca, Pocket Naloxone, the Addiction Policy Forum, and Yale University; has provided consultation to Brainsway, Cerevel, Otsuka, Arbor Pharmaceuticals, and Summit Biosciences; has presented on a sponsored panel for Camurus; and has received study drug from Vanda Pharmaceuticals and Braeburn Pharmaceuticals. The remaining authors have no industry conflicts to disclose; see ICMJE disclosure forms for details.
Availability of data and materials: Not applicable.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Laura C. Fanucchi, Division of Infectious Diseases, College of Medicine, University of Kentucky, 845 Angliana Ave., Lexington, KY, 40508, USA; Center on Drug and Alcohol Research, College of Medicine, University of Kentucky, Lexington, KY, USA.
Sean M. Murphy, Department of Population Health Sciences, Weill Cornell Medicine, New York, NY, USA
Hilary Surratt, Center on Drug and Alcohol Research, College of Medicine, University of Kentucky, Lexington, KY, USA; Department of Behavioral Science, College of Medicine, University of Kentucky, Lexington, KY, USA.
Shashi N. Kapadia, Department of Population Health Sciences, Weill Cornell Medicine, New York, NY, USA Division of Infectious Diseases, Weill Cornell Medicine, New York, NY, USA.
Sharon L. Walsh, Center on Drug and Alcohol Research, College of Medicine, University of Kentucky, Lexington, KY, USA Departments of Behavioral Science and Psychiatry, College of Medicine, University of Kentucky, Lexington, KY, USA; Department of Pharmaceutical Sciences, College of Pharmacy, University of Kentucky, Lexington, KY, USA.
James A. Grubbs, Division of Infectious Diseases, College of Medicine, University of Kentucky, Lexington, KY, USA
Alice C. Thornton, Division of Infectious Diseases, College of Medicine, University of Kentucky, Lexington, KY, USA
Paul Nuzzo, Center on Drug and Alcohol Research, College of Medicine, University of Kentucky, Lexington, KY, USA.
Michelle R. Lofwall, Center on Drug and Alcohol Research, College of Medicine, University of Kentucky, Lexington, KY, USA Departments of Behavioral Science and Psychiatry, College of Medicine, University of Kentucky, Lexington, KY, USA.
References
- 1. U.S. overdose deaths in 2021 increased half as much as in 2020 – but are still up 15%. Centers for Disease Control National Center for Health Statistics, 2022, https://www.cdc.gov/nchs/pressroom/nchs_press_releases/2022/202205.htm (accessed 23 May 2022). [Google Scholar]
- 2. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002–12. Health Affair 2016; 35: 832–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gordon RJ, Lowy FD. Bacterial infections in drug users. N Engl J Med 2005; 353: 1945–1954. [DOI] [PubMed] [Google Scholar]
- 4. Norris AH, Shrestha NK, Allison GM, et al. 2018 infectious diseases Society of America Clinical Practice Guideline for the Management of Outpatient Parenteral Antimicrobial Therapy. Clin Infect Dis 2019; 68: e1–e35. [DOI] [PubMed] [Google Scholar]
- 5. Fanucchi L, Leedy N, Li J, et al. Perceptions and practices of physicians regarding outpatient parenteral antibiotic therapy in persons who inject drugs. J Hosp Med 2016; 11: 581–582. [DOI] [PubMed] [Google Scholar]
- 6. Mitchell ED, Czoski Murray C, Meads D, et al. Clinical and cost-effectiveness, safety and acceptability of community intravenous antibiotic service models: CIVAS systematic review. BMJ Open 2017; 7: e013560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seaton RA, Barr DA. Outpatient parenteral antibiotic therapy: principles and practice. Eur J Intern Med 2013; 24: 617–623. [DOI] [PubMed] [Google Scholar]
- 8. D’Couto HT, Robbins GK, Ard KL, et al. Outcomes according to discharge location for persons who inject drugs receiving outpatient parenteral antimicrobial therapy. Open Forum Infect Dis 2018; 5: ofy056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Suzuki J, Johnson J, Montgomery M, et al. Outpatient parenteral antimicrobial therapy among people who inject drugs: a review of the literature. Open Forum Infect Dis 2018; 5: ofy194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications – a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132: 1435–1486. [DOI] [PubMed] [Google Scholar]
- 11. Berbari EF, Kanj SS, Kowalski TJ, et al. 2015 Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis 2015; 61: e26–e46. [DOI] [PubMed] [Google Scholar]
- 12. Fanucchi LC, Walsh SL, Thornton AC, et al. Integrated outpatient treatment of opioid use disorder and injection-related infections: a description of a new care model. Prev Med 2019; 128: 105760. [DOI] [PubMed] [Google Scholar]
- 13. Fanucchi LC, Walsh SL, Thornton AC, et al. Outpatient parenteral antimicrobial therapy plus buprenorphine for opioid use disorder and severe injection-related infections. Clin Infect Dis 2019; 70: 1226–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Neumann PJ, Sanders GD, Russell LB, et al. Cost-effectiveness in health and medicine. 2nd ed. Oxford: Oxford University Press, 2017. [Google Scholar]
- 15. Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000; 30: 633–638. [DOI] [PubMed] [Google Scholar]
- 16. Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med 2014; 174: 1369–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fiellin DA, Schottenfeld RS, Cutter CJ, et al. Primary care-based buprenorphine taper vs maintenance therapy for prescription opioid dependence: a randomized clinical trial. JAMA Intern Med 2014; 174: 1947–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hurley H, Sikka M, Jenkins T, et al. Outpatient antimicrobial treatment for people who inject drugs. Infect Dis Clin North Am 2020; 34: 525–538. [DOI] [PubMed] [Google Scholar]
- 19. Appa A, Barocas JA. Can I safely discharge a patient with a substance use disorder home with a peripherally inserted central catheter? NEJM Evid 2022; 1: 2100012. [DOI] [PubMed] [Google Scholar]
- 20. Cohen J. Statistical power analysis for behavioral sciences. New York: Lawrence Erlbaum Associates, 1988. [Google Scholar]
- 21. Cahill TJ, Prendergast BD. Infective endocarditis. Lancet 2016; 387: 882–893. [DOI] [PubMed] [Google Scholar]
- 22. Moss R, Munt B. Injection drug use and right sided endocarditis. Heart 2003; 89: 577–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kokabu T, Takahata M, Ishiguro N, et al. Long-term prognosis of hematogenous vertebral osteomyelitis: mortality, quality of life, and pain. J Orthop Sci 2017; 22: 822–827. [DOI] [PubMed] [Google Scholar]
- 24. Bhandari A, Wagner T. Self-reported utilization of health care services: improving measurement and accuracy. Med Care Res Rev 2006; 63: 217–235. [DOI] [PubMed] [Google Scholar]
- 25. Grau D, Clarivet B, Lotthé A, et al. Complications with peripherally inserted central catheters (PICCs) used in hospitalized patients and outpatients: a prospective cohort study. Antimicrob Resist Infect Control 2017; 6: 18–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Safdar N, Maki DG. Risk of catheter-related bloodstream infection with peripherally inserted central venous catheters used in hospitalized patients. Chest 2005; 128: 489–495. [DOI] [PubMed] [Google Scholar]
- 27. Valbousquet Schneider L, Jr, Duron S, Arnaud FX, et al. Evaluation of PICC complications in orthopedic inpatients with bone infection for long-term intravenous antibiotics therapy. J Vasc Access 2015; 16: 299–308. [DOI] [PubMed] [Google Scholar]
- 28. Bray JW, Aden B, Eggman AA, et al. Quality of life as an outcome of opioid use disorder treatment: a systematic review. J Subst Abuse Treat 2017; 76: 88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. U.S. Department of Health and Human Services. Healthy people 2020, https://www.healthypeople.gov/2020/topics-objectives/topic/health-related-quality-of-life-well-being (accessed 21 June 2022). [DOI] [PubMed]
- 30. Braithwaite RS, Meltzer DO, King JT, Jr. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule. Med Care 2008; 46: 349–356. [DOI] [PubMed] [Google Scholar]
- 31. Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness: the curious resilience of the $50,000-per-QALY threshold. N Engl J Med 2014; 371: 796–797. [DOI] [PubMed] [Google Scholar]
- 32. Hanmer J, Cella D, Feeny D, et al. Selection of key health domains from PROMIS((R)) for a generic preference-based scoring system. Qual Life Res 2017; 26: 3377–3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dewitt B, Feeny D, Fischhoff B, et al. Estimation of a preference-based summary score for the patient-reported outcomes measurement information system: the PROMIS((R))-preference (PROPr) scoring system. Med Decis Making 2018; 38: 683–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Murphy SM, Campbell AN, Ghitza UE, et al. Cost-effectiveness of an internet-delivered treatment for substance abuse: data from a multisite randomized controlled trial. Drug Alcohol Depend 2016; 161: 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Murphy SM, Polsky D. Economic evaluations of opioid use disorder interventions: a systematic review. Pharmacoeconomics 2016; 34: 863–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Murphy SM, Polsky D, Lee JD, et al. Cost-effectiveness of extended release naltrexone to prevent relapse among criminal-justice-involved persons with a history of opioid use disorder. Addiction 2017; 112: 1440–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Polsky D, Glick HA, Yang J, et al. Cost-effectiveness of extended buprenorphine–naloxone treatment for opioid-dependent youth: data from a randomized trial. Addiction 2010; 105: 1616–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Murphy SM, McCollister KE, Leff JA, et al. Cost-effectiveness of extended-release naltrexone versus buprenorphine-naloxone to prevent opioid relapse among individuals initiating treatment in an inpatient detoxification setting. Ann Intern Med 2019; 170: 90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Onuoha EN, Leff JA, Schackman BR, et al. Economic evaluations of pharmacologic treatment for opioid use disorder: a systematic literature review. Value Health 2021; 24: 1068–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics 1988; 44: 1049–1060. [PubMed] [Google Scholar]
- 41. Gibbons RD, Hedeker D, Elkin I, et al. Some conceptual and statistical issues in analysis of longitudinal psychiatric data: application to the NIMH treatment of Depression Collaborative Research Program dataset. Arch Gen Psychiatry 1993; 50: 739–750. [DOI] [PubMed] [Google Scholar]
- 42. Kreft I, De Leeuw J. Introducing multilevel modeling. Thousand Oaks, CA: SAGE, 1998. [Google Scholar]
- 43. Diggle PJ, Liang K, Zeger SL. Analysis of longitudinal data. Oxford: Oxford University Press, 1996. [Google Scholar]
- 44. Drummond MF, Schulpher MJ, Claxton K, et al. Methods for the economic evaluation of health care programmes. 4th ed. Oxford: Oxford University Press, 2015. [Google Scholar]
- 45. Glick HA, Doshi JA, Sonnad SS, et al. Economic evaluation in clinical trials. Oxford: Oxford University Press, 2014. [Google Scholar]
- 46. Drummond MF, Davies L. Economic analysis alongside clinical trials. Int J Technol Assess Health Care 1991; 7: 561–573. [DOI] [PubMed] [Google Scholar]
- 47. Brady T, Robinson B. Medicare hospital prospective payment system: how DRG rates are calculated and updated. Inspections OoEa 2001, https://oig.hhs.gov/oei/reports/oei-09-00-00200.pdf (accessed 21 September 2017).
- 48. Kimmel SD, Walley AY, Li Y, et al. Association of treatment with medications for opioid use disorder with mortality after hospitalization for injection drug use: associated infective endocarditis. JAMA Network Open 2020; 3: e2016228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fanucchi L, Springer SA, Korthuis PT. Medications for treatment of opioid use disorder among persons living with HIV. Curr HIV/AIDS Rep 2019; 16: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rosenthal ES, Silk R, Mathur P, et al. Concurrent initiation of hepatitis C and opioid use disorder treatment in people who inject drugs. Clin Infect Dis 2020; 71: 1715–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bearnot B, Mitton JA, Hayden M, et al. Experiences of care among individuals with opioid use disorder-associated endocarditis and their healthcare providers: results from a qualitative study. J Subst Abuse Treat 2019; 102: 16–22. [DOI] [PubMed] [Google Scholar]
- 52. Priest KC, Englander H, McCarty D. Hospital policies for opioid use disorder treatment: a policy content analysis and environmental scan checklist. Gen Hosp Psychiatry 2021; 70: 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-doc-1-tai-10.1177_20499361221108005 for Design and protocol of the Buprenorphine plus Outpatient Parenteral Antimicrobial Therapy (B-OPAT) study: a randomized clinical trial of integrated outpatient treatment of opioid use disorder and severe, injection-related infections by Laura C. Fanucchi, Sean M. Murphy, Hilary Surratt, Shashi N. Kapadia, Sharon L. Walsh, James A. Grubbs, Alice C. Thornton, Paul Nuzzo and Michelle R. Lofwall in Therapeutic Advances in Infectious Disease