Abstract
Objective
To assess the risk of adverse events associated with heterologous primary (two dose) and booster (three dose) vaccine schedules for covid-19 with Oxford-AstraZeneca’s ChAdOx1-S priming followed by mRNA vaccines (Pfizer-BioNTech’s BNT162b2 or Moderna’s mRNA-1273) as compared with homologous mRNA vaccine schedules for covid-19.
Design
Nationwide cohort study.
Setting
Denmark, 1 January 2021 to 26 March 2022.
Participants
Adults aged 18-65 years who received a heterologous vaccine schedule of priming with ChAdOx1-S and one or two mRNA booster doses (with either the BNT162b2 or mRNA-1273 vaccine) were compared with adults who received a homologous BNT162b2 or mRNA-1273 vaccine schedule (ie, two dose v two dose, and three dose v three dose schedule).
Main outcome measures
The incidence of hospital contacts for a range of adverse cardiovascular and haemostatic events within 28 days after the second or third vaccine dose, comparing heterologous versus homologous vaccine schedules. Secondary outcomes included additional prioritised adverse events of special interest. Poisson regression was used to estimate incidence rate ratios with adjustment for selected covariates.
Results
Individuals who had had a heterologous primary vaccine (n=137 495) or a homologous vaccine (n=2 688 142) were identified, in addition to those who had had a heterologous booster (n=129 770) or a homologous booster (n=2 197 213). Adjusted incidence rate ratios of adverse cardiovascular and haemostatic events within 28 days for the heterologous primary and booster vaccine schedules in comparison with the homologous mRNA vaccine schedules were 1.22 (95% confidence interval 0.79 to 1.91) and 1.00 (0.58 to 1.72) for ischaemic cardiac events, 0.74 (0.40 to 1.34) and 0.72 (0.37 to 1.42) for cerebrovascular events, 1.12 (0.13 to 9.58) and 4.74 (0.94 to 24.01) for arterial thromboembolisms, 0.79 (0.45 to 1.38) and 1.09 (0.60 to 1.98) for venous thromboembolisms, 0.84 (0.18 to 3.96) and 1.04 (0.60 to 4.55) for myocarditis or pericarditis, 0.97 (0.45 to 2.10) and 0.89 (0.21 to 3.77) for thrombocytopenia and coagulative disorders, and 1.39 (1.01 to 1.91) and 1.02 (0.70 to 1.47) for other bleeding events, respectively. No associations with any of the outcomes were found when restricting to serious adverse events defined as stay in hospital for more than 24 h.
Conclusion
Heterologous primary and booster covid-19 vaccine schedules of ChAdOx1-S priming and mRNA booster doses as both second and third doses were not associated with increased risk of serious adverse events compared with homologous mRNA vaccine schedules. These results are reassuring but given the rarity of some of the adverse events, associations cannot be excluded.
Introduction
Heterologous vaccine schedules for covid-19—that is, use of different covid-19 vaccines as the first (priming) and second or third (booster) dose—have emerged as a subject of substantial public health interest. This interest has been mainly fuelled by safety concerns associated with the adenovirus vectored covid-19 vaccine ChAdOx1-S (Oxford-AstraZeneca).1 2 Concerns have led several countries, including Denmark, to halt ChAdOx1-S vaccination3 and to recommend heterologous mRNA booster strategies with either the BNT162b2 (Pfizer-BioNTech) or mRNA-1273 (Moderna) vaccine for individuals who had received priming immunisation with ChAdOx1-S.4 Also, because effective covid-19 vaccine roll-outs are crucial in managing the pandemic, more flexible heterologous schedules for covid-19 vaccines could mitigate against stalling of roll-outs due to shortages in vaccine supply.5 Additionally, research suggests that heterologous covid-19 vaccine schedules might provide at least similar immunogenicityas homologous schedules and have shown to produce strong antibody and T cell responses, including against covid-19 variants of concern.6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23
In a regulatory context, heterologous covid-19 vaccine schedules are considered off-label use and safety surveillance activities are of the most importance in regulatory decision making and used to guard patient safety. Data for the safety of heterologous covid-19 vaccine schedules, however, are mainly limited to published studies on immunogenicity or reactogenicity. Some of these studies reported similar reactogenicity profiles for both heterologous primary and booster vaccine schedules,13 14 15 16 whereas other studies suggested a tolerable short term increase in reactogenicity.18 19 20 21 22 23 24 25 Although no severe adverse events were found related to immunisation, these studies were not statistically powered to identify risks of the rare or serious adverse events of special interest to the covid-19 vaccines.19 26 27 28 Additionally, one observational study from Sweden29 and another from Spain,30 examining the effectiveness of heterologous primary vaccination schedules for covid-19, each reported rates of three different safety outcomes. A primary schedule was defined as including one priming vaccine dose and one booster dose. However, the incidences of the safety outcomes were very low in both studies and statistical analyses were not conducted. Moreover, no larger scale studies have examined the risk of these rare or serious adverse events for heterologous booster schedules. As such, analyses that adequately assess the safety of heterologous vaccine schedules for covid-19 are needed to inform the public, clinicians, and regulatory authorities.27 28 31
We used the Danish healthcare registers to investigate the risk of 19 adverse events of special interest to the covid-19 vaccines associated with heterologous primary and booster vaccine schedules of ChAdOx1-S priming and an mRNA booster dose or doses, as compared with homologous mRNA covid-19 vaccine schedules in a nationwide cohort.
Methods
Data sources
We constructed this nationwide cohort study by prospectively obtaining individual level data from different Danish national healthcare registers and cross linked the data by use of the unique civil personal registration number, which is assigned to all Danish citizens.32 Received vaccinations were obtained from the Danish Vaccination Register.33 Hospital contacts and diagnoses (recorded according to ICD-10 (international classification of diseases, 10th revision)) were identified from the National Patient Register.34 Demographic information about age, sex, migration, and vital status was gathered from the Danish Civil Registration System.35 Positive polymerase-chain-reaction (PCR) laboratory tests for SARS-CoV-2 were ascertained from the Danish Microbiology Database, which holds data for all microbiological test results in Denmark.36
The study was approved by the Danish Data Protection Agency. Ethical approval as well as informed consent is not required for register based research in Denmark. As a result of the national regulations of private data protection, cell counts of fewer than three (but not zero) could not be reported.
Study population and vaccination schedules
Eligibility criteria were age of 18-65 years (at first vaccination), Danish residency, no previous positive PCR test for SARS-CoV-2, and having received a primary (ie, a priming and one booster dose) covid-19 vaccine schedule during the study period of 1 January 2021 to 26 March 2022. We excluded individuals from analysis of an outcome event if an individual had a history of the specific outcome event during the six months before the index date.
A heterologous vaccine schedule was defined as having received a ChAdOx1-S vaccine as the priming immunisation and an mRNA vaccine as the booster dose or doses—that is, either BNT162b2 or mRNA-1273 as the second or third dose, or as both second and third dose. In Denmark, the heterologous second dose with an mRNA vaccine was offered around week 10-12 after the ChAdOx1-S priming dose. For the booster dose schedules comparison between homologous and heterologous vaccines (ie, three v three dose), we excluded individuals who received two different mRNA vaccines because these were few. A homologous vaccine schedule was defined as having received the same mRNA vaccine as both the priming and booster dose or doses, that is, either two or three doses of BNT162b2 or two or three doses of mRNA-1273.
Outcomes
We identified any incident of hospital contact where an outcome event was recorded within the first 28 days from the day after the second or third vaccine dose (booster) was administered (ie, the index date).In the main comparison, we assessed the associated risk of the outcomes with any heterologous schedules versus any homologous mRNA vaccine schedule. In a secondary comparison, the associated risk for the distinct heterologous vaccine schedule was compared with the homologous counterpart.
We included 19 different adverse safety outcomes of interest, which consisted of a range of main cardiovascular and haemostatic adverse events and additional adverse events, adapted from prioritised lists of adverse events of special interest for the covid-19 vaccines (see supplementary table 1 for definitions).26 37 38 The cardiovascular and haemostatic adverse events were: ischaemic cardiac events, cerebrovascular events (whereby infarction and intracranial bleeding events were also assessed separately), arterial thromboembolism, venous thromboembolism (cerebral venous thromboembolism and pulmonary embolism were also assessed separately), myocarditis or pericarditis events, thrombocytopenia and coagulative disorder events, and other bleeding events (ie, other than intracranial haemorrhages). Secondary outcomes included Guillain-Barré syndrome, Bell’s palsy, transverse myelitis, encephalomyelitis or encephalitis, narcolepsy, anaphylaxis, appendicitis, and all cause mortality. All outcomes were examined separately.
Statistical analysis
Follow-up started on the date of the respective booster dose (ie, second or third dose) and ended on the day of an outcome event, death, emigration, loss to follow-up, positive PCR test result for SARS-CoV-2, or end of data (26 March 2022), whichever occurred first. Cumulative incidence curves for the cardiovascular and haemostatic adverse events were estimated by the Kaplan-Meier estimator. The associations were assessed by incidence rate ratios (IRRs) and the corresponding 95% confidence intervals, computed by use of Poisson regression. The analyses were adjusted for calendar period (in monthly intervals), sex, age (defined by birth year; in 10 year intervals), region of residency (at time of the second dose), birth country, vaccine priority group (grouped as: at risk individuals, healthcare personnel, and the general population), any hospital contacts in the past six months, and comorbidities (five years previously; see supplementary table 1), as covariates. The vaccine priority groups were governmentally assigned and individuals were prioritised according to the risk of severe covid-19 (based on various risk factors such as severe illness and immunocompromised conditions (eg, use of immunosuppressant treatment)) as well as whether being healthcare workers. Statistical tests were two sided; estimates were considered statistically significant if the 95% confidence interval did not overlap with 1.00. Subgroup analyses were conducted according to sex and birth year (before 1975 v 1975 or after). We did not take multiple testing into account. All data management and statistical calculations were performed using R software, version 4.1.1 (R Foundation for Statistical Computing).
Sensitivity analyses included restricting the outcome definitions to events within 28 days where individuals stayed in hospital for more than 24 h (to increase the specificity and severity of the detected events), restricting the follow-up to two weeks (to explore the possibility of a more acute onset), and extending the follow-up from 28 days to 180 days after the index date (to explore for any associations with later onsets; receiving a booster dose was added as an additional censoring criterion for this analysis). In post hoc analyses, we categorised the outcome of other bleeding events according to the specific sites of bleeding for the primary schedules comparison. Additionally, we analysed the risk of Guillain-Barré syndrome and narcolepsy, where we extended the follow-up to 180 days after the index date.
Patient and public involvement
Owing to the urgency of the study question, funding restrictions, and privacy constrains, no patients or members of the public were formally involved in defining the research question, study design or outcome measures, or the conduct of the study.
Results
Population
Between 1 January 2021 and 26 March 2022, 2 825 637 individuals received a primary (two dose) vaccination schedule and 2 326 983 received a booster (three dose) schedule and were eligible for study inclusion (table 1 and supplementary fig 1). After receiving ChAdOx1-S as the priming immunisation (ie, first dose), 137 495 individuals received a heterologous primary vaccine schedule (ie, for the second dose, 88 429 received BNT162b2 and 49 066 received mRNA-1273) and 129 770 had a booster vaccine schedule (ie, received mRNA vaccines for the second and third doses). Among these heterologous vaccine recipients at the second dose, median age was 46.2 (interquartile range 34.3-55.5), 80% were women, and most (89%) were in the vaccine priority group for healthcare workers. The comparison groups receiving homologous mRNA vaccination included 2 688 142 individuals at primary schedule vaccination. A total of 2 260 232 individuals were primed and had their second vaccine with BNT162b2, and 427 910 were primed and had their second dose with mRNA-1273; 2 197 213 received the same mRNA vaccine as a third dose. Among these homologous vaccine recipients at second dose, median age was 44.3 (interquartile range 31.1-54.8), 49% were women, and most (91%) were in the vaccine priority group categorised as others (ie, the general population).
Table 1.
Baseline characteristics of study population receiving primary (two dose) and booster (three dose) vaccine schedules for covid-19, according to heterologous (ChAdOx1-S priming and mRNA booster dose(s)) and homologous (mRNA primary and booster vaccine) vaccination schedule
| |
Heterologous vaccination schedule | Homologous vaccination schedule | |||
|---|---|---|---|---|---|
| ChAdOx1-S, mRNA | ChAdOx1-S, mRNA, mRNA | mRNA, mRNA | mRNA, mRNA, mRNA | ||
| Total number vaccinated | 137 495 | 129 770 | 2 688 142 | 2 197 213 | |
| Vaccinated with BNT162b2 | 88 429 (64.3) | 83 443 (64.3) | 2 260 232 (84.1) | 1 889 694 (86.0) | |
| Vaccinated with mRNA-1273 | 49 066 (35.7) | 46 327 (35.7) | 427 910 (15.9) | 307 519 (14.0) | |
| Median age at vaccination (IQR) | 46.2 (34.3-55.5) | 47.2 (35.6-56.3) | 44.3 (31.1-54.8) | 47.4 (34.0-56.5) | |
| Sex | |||||
| Male | 27 430 (19.9) | 25 697 (19.8) | 1 371 119 (51.0) | 1 112 907 (50.7) | |
| Female | 110 065 (80.1) | 104 073 (80.2) | 1 317 023 (49.0) | 1 084 306 (49.3) | |
| Vaccine priority group | |||||
| Patients with increased risk | 75 (0.1) | 66 (0.1) | 39 444 (1.5) | 35 673 (1.6) | |
| Healthcare workers | 121 627 (88.5) | 114 970 (88.6) | 207 196 (7.7) | 175 703 (8.0) | |
| Others | 15 793 (11.5) | 14 734 (11.4) | 2 441 502 (90.8) | 1 985 837 (90.4) | |
| Birth year* | |||||
| Before 1965 | 30 755 (22.4) | 30 139 (23.2) | 547 443 (20.4) | 518 840 (23.6) | |
| 1965-74 | 37 084 (27.0) | 35 841 (27.6) | 663 163 (24.7) | 604 361 (27.5) | |
| 1975-84 | 30 121 (21.9) | 28 265 (21.8) | 529 154 (19.7) | 427 275 (19.4) | |
| 1985-94 | 23 899 (17.4) | 21 354 (16.5) | 509 404 (19.0) | 329 782 (15.0) | |
| After 1994 | 15 636 (11.4) | 14 171 (10.9) | 438 978 (16.3) | 316 955 (14.4) | |
| Any previous hospital contacts within six months | |||||
| No | 87 404 (63.6) | 82 493 (63.6) | 1 807 034 (67.2) | 1 468 129 (66.8) | |
| Yes | 50 091 (36.4) | 47 277 (36.4) | 881 108 (32.8) | 729 084 (33.2) | |
| Comorbidity history† | |||||
| No | 132 895 (96.7) | 125 357 (96.6) | 2 570 174 (95.6) | 2 091 617 (95.2) | |
| Yes | 4600 (3.3) | 4413 (3.4) | 117 968 (4.4) | 105 596 (4.8) | |
| Region of residency‡ | |||||
| Capital Region of Denmark | 39 606 (28.8) | 36 756 (28.3) | 842 595 (31.3) | 673 603 (30.7) | |
| Central Denmark Region | 31 762 (23.1) | 30 480 (23.5) | 636 008 (23.7) | 529 075 (24.1) | |
| North Denmark Region | 16 010 (11.6) | 15 337 (11.8) | 274 173 (10.2) | 225 530 (10.3) | |
| Region Zealand | 21 481 (15.6) | 20 117 (15.5) | 370 702 (13.8) | 304 645 (13.9) | |
| Region of Southern Denmark | 28 636 (20.8) | 27 080 (20.9) | 564 664 (21.0) | 464 360 (21.1) | |
| Birth country | |||||
| Denmark | 123 551 (89.9) | 117 471 (90.5) | 2 234 430 (83.1) | 1 885 243 (85.8) | |
| Non-western countries | 8109 (5.9) | 6908 (5.3) | 214 970 (8.0) | 130 145 (5.9) | |
| Western countries | 4956 (3.6) | 4560 (3.5) | 120 796 (4.5) | 86 527 (3.9) | |
| Unknown | 879 (0.6) | 831 (0.6) | 117 946 (4.4) | 95 298 (4.3) | |
Values are numbers (%) unless otherwise stated. Sums of percentages might not equal 100 due to rounding. mRNA vaccines include BNT162b2 and mRNA-1273. IQR=interquartile range.
Eg, birth year of 1975 corresponds to turning 46 years of age in year 2021.
Includes cardiac conditions, diabetes mellitus, cancer, cerebrovascular, and venous thromboembolism disorders (see supplementary table 1 for definitions).
Region of residency at the time of second vaccine dose.
Main analysis
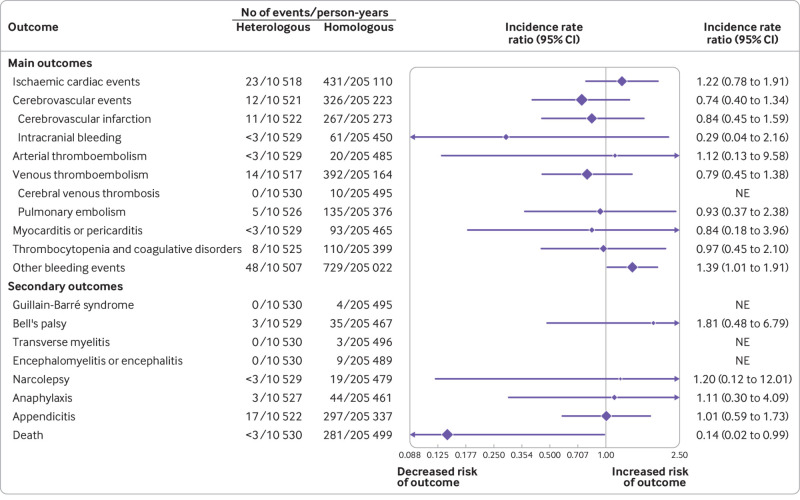
Overall, the risks of adverse outcomes were low for both heterologous and homologous vaccinated groups (fig 1). Compared with recipients of a homologous vaccine schedule, receiving a heterologous primary or booster vaccine schedule for covid-19 was not associated with an increased risk of hospital contact for most cardiovascular or haemostatic adverse events within 28 days after any booster dose (fig 2 and fig 3). However, for the primary vaccine schedules comparison (ie, comparing the two dose schedules), for any type of hospital contact with a diagnosis within the other bleeding events outcome category within 28 days, the lower95% confidence interval was 1.01 (IRR 1.39, 95% confidence interval 1.01 to 1.91), compared with 1.02 (0.70 to 1.47) for the booster schedules comparison (ie, comparing the three dose schedules). The number of cases of the secondary outcomes were generally low to none. No increased risk among heterologous vaccinated was found for the secondary outcomes where IRR could be examined.
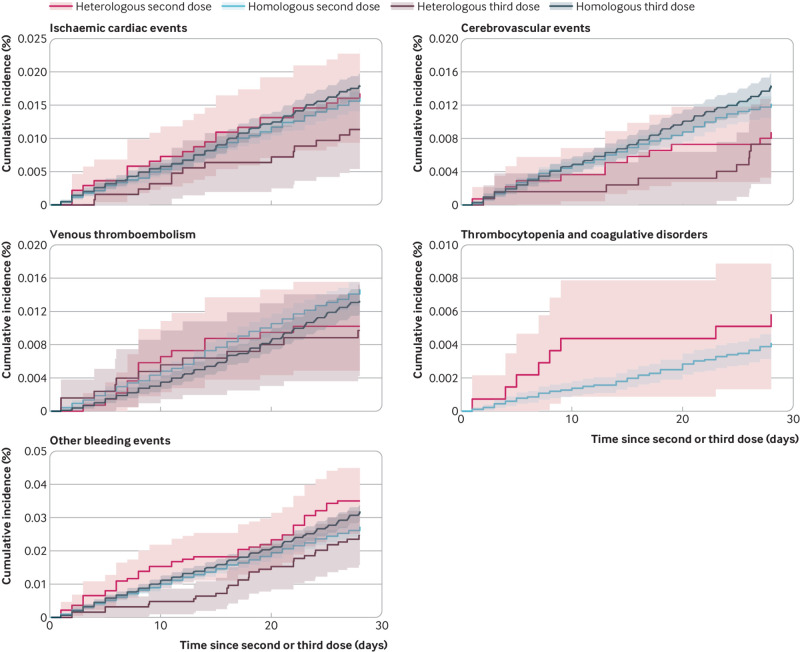
Fig 1.
Cumulative incidence curves of the cardiovascular and haemostatic adverse events for the heterologous and homologous primary and booster vaccine schedules. Cumulative incidence curves of the main outcomes within the first 28 days after the last day of vaccination for the heterologous vaccine schedules of ChAdOx1-S priming and mRNA booster dose or doses (ie, two or three doses) and homologous primary and booster mRNA vaccine schedules (ie, two or three doses) with the corresponding 95% confidence intervals (shaded areas). The number of events of arterial thromboembolisms and myocarditis or pericarditis, as well as for thrombocytopenia and coagulative disorder for the three dose comparison, were low to none, which is why cumulative incidence curves for these outcomes were not estimated. Other bleeding events include a composite of bleeding-related diagnoses other than intracranial haemorrhages
Fig 2.
Risk of adverse safety outcomes comparing heterologous primary vaccine schedules of ChAdOx1-S priming and an mRNA booster dose with homologous primary mRNA vaccine schedules. Incidence rate ratios (IRRs) for the outcomes within 28 days were adjusted for calendar period, sex, birth year (as a proxy for age), region of residency, birth country, vaccine priority group, hospital contact in the past six months, and comorbidities. Cerebrovascular infarction includes non-haemorrhagic strokes and transient ischaemic attacks. Other bleeding events include a composite of bleeding-related diagnoses other than intracranial haemorrhages. CI=confidence interval; NE=not estimated
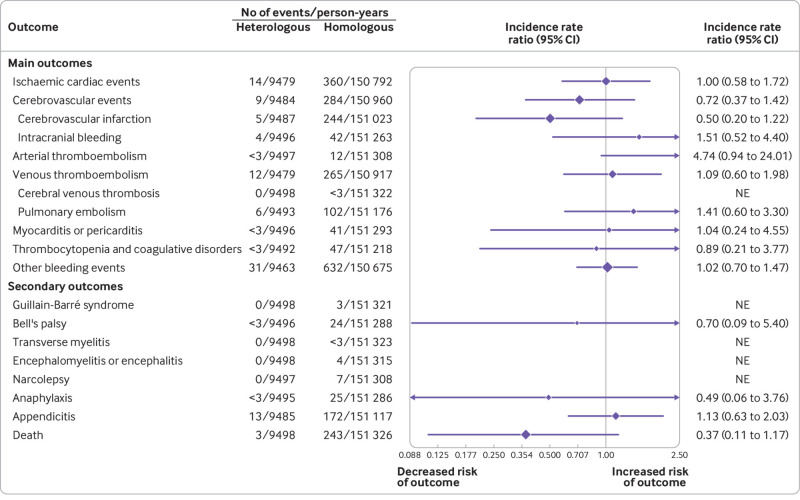
Fig 3.
Risk of adverse safety outcomes comparing heterologous booster vaccine schedules of ChAdOx1-S priming and two mRNA booster doses with homologous mRNA booster vaccine schedules. Incidence rate ratios (IRRs) for the outcomes within 28 days were adjusted for calendar period, sex, birth year (proxy for age), region of residency, birth country, vaccine priority group, hospital contact in the past six months, and comorbidities. Cerebrovascular infarction includes non-haemorrhagic strokes and transient ischaemic attacks. Other bleeding events includes a composite of bleeding-related diagnoses other than intracranial haemorrhages. CI=confidence interval; NE=not estimated
Secondary comparison according to individual mRNA vaccines
The analysis of the risk of any hospital contact with a cardiovascular or haemostatic event within 28 days in relation to the specific heterologous mRNA booster as compared with the respective homologous counterpart (in mRNA vaccine and schedule) showed similar findings to those of the main analysis (supplementary figs 2 and 3). Trends of the associations were similar for the outcome of other bleeding events across the two heterologous primary vaccinated groups (ie, the two dose schedules of ChAdOx1-S followed by BNT162b2 or mRNA-1273). But for the larger population sample of individuals who were vaccinated with heterologous BNT162b2, the lower 95% confidence interval was above 1.00(IRR 1.49, 95% confidence interval 1.02 to 2.17), whereas the IRR for the BNT162b2 heterologous booster schedule was 0.89 (0.55 to 1.46).
Subgroups analyses
The results of the subgroup analyses according to sex and age were overall similar to those of the main analysis (supplementary figs 4-7). As expected, given that 80% of the heterologous vaccinated individuals were women, the lower 95% confidence interval for the outcome of other bleeding events was similarly close to 1.00 among women who had a heterologous primary vaccination (IRR 1.46, 95% confidence interval 1.00 to 2.13), but not among men (IRR 1.16, 95% confidence interval 0.60 to 2.24); the IRR for women who received the heterologous booster was 1.04 (0.68 to 1.59) and for men was 0.83 (0.36 to 1.88). No increased risks were noted when subgrouping according to age (birth year before 1975, or 1975 and after) for both heterologous primary and booster vaccine schedules (supplemental figs 6-7).
Sensitivity analyses
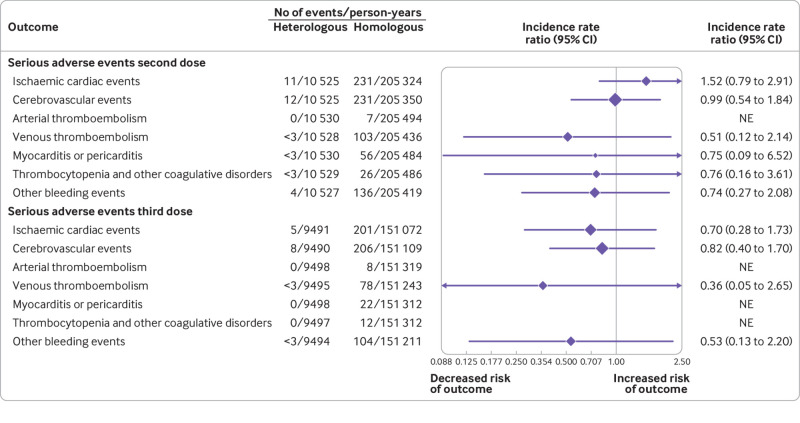
When restricting to serious adverse events only (ie, stay in hospital of >24 h within 28 days; fig 4), we observed no differences in the risks between heterologous and homologous vaccinations (including for other bleeding events: IRR 0.74, 95% confidence interval 0.27 to 2.08 for primary schedules and 0.53, 0.13 to 2.20 for booster schedules) and the number of cases were generally low. We also found no increased risk of the outcomes among heterologous vaccination schedules when restricting the follow-up to the first two weeks after the index date nor when extending the follow-up to 180 days (supplementary figs 8 and 9).
Fig 4.
Sensitivity analysis of the associated risk of serious adverse events with heterologous primary and booster vaccine schedules. Shows the results of the sensitivity analysis where restricting the outcome definitions to serious adverse events, defined as excluding brief hospital contacts of less than 24 h. Top panel shows the results of the primary (two dose) vaccine schedules comparison while the booster (three dose) schedules comparison is presented in the bottom panel. Incidence rate ratios (IRRs) were adjusted for calendar period, sex, birth year (proxy for age), region of residency, birth country, vaccine priority group, hospital contact in the last six months, and comorbidities. Other bleeding events includes a composite of bleeding-related diagnoses other than intracranial haemorrhages. CI=confidence interval; NE=not estimated
Post hoc analyses
As the prespecified analyses of primary vaccine schedules showed a lower 95% confidence interval of 1.01 for any type of hospital contact due to other bleeding events (this observation was not noted when restricting to serious events), we examined for a potential association in relation to a specific site of bleeding post hoc. This investigation showed no association to a specific site but a general non-differential insignificant increase in the estimates across all bleeding subtypes (supplementary table 2). Moreover, we extended the follow-up to 180 days for the secondary outcomes of Guillain-Barré syndrome and narcolepsy to allow for a longer onset period and delay between disease onset and diagnosis.39 40 Cases of these diseases were few to none and analyses did not show any significant results (supplementary figs 8 and 9).
Discussion
Principal findings
In this large, nationwide cohort study, we compared the safety of heterologous primary and booster covid-19 vaccine schedules with ChAdOx1-S priming and BNT162b2 or mRNA-1273 booster dose or doses against homologous mRNA vaccine schedules. Our findings are reassuring in that the number of cases were generally low and we found no differences in the risks of serious cardiovascular or haemostatic adverse events as well as the additional secondary outcome events. Among the 19 adverse safety outcomes examined, our main analysis was suggestive of a potential association between heterologous primary vaccine schedules and excess of any type of hospital contact due to other bleeding events. This observed signal disappeared when restricting to serious adverse events (ie, stay in hospital of >24 h).
Strengths and limitations of this study
Our study results should be evaluated in combination with potential weaknesses. A main limitation is that the outcome definitions relied on ICD-10 codes registered during hospital contacts. Although, the comparative design mitigates concerns of potential temporal systematic biases in the recording of ICD-10 codes, we cannot exclude biases in the registration of the outcomes due to potential differences in clinical safety awareness or healthcare seeking behaviour between the vaccinated groups. This type of ascertainment bias would not be expected to affect the validity of any signals found for serious and acute adverse events, but this bias is a particular concern for analyses of less well defined or less severe disorders. As such, our associations would be skewed towards an increased risk if the safety statements on the ChAdOx1-S vaccine issued by the medicinal regulatory authorities41 led to an increased clinical alertness, especially considering any symptoms potentially related to thrombogenic and haemostatic adverse events for the heterologous vaccinated individuals relative to the homologous mRNA vaccinated.
Similarly, due to the Danish covid-19 vaccination roll-out strategy, the heterologous vaccinated group consisted predominantly of healthcare and social services workers. A greater healthcare seeking behaviour for this vaccinated group (relative to the general population) would also lead to a falsely larger effect due to a more sensitive or earlier detection of less severe events. We believe these biases probably contributed to the findings of the category of other bleeding events in the prespecified analyses among individuals who received the heterologous primary vaccination schedule (ie, two doses) because alertness to potential side effects to the heterologous vaccine schedules would likely have been greater at this time. Although such biases are difficult to adequately quantify, this effect is indicated in our results because the trends of the estimates for other bleeding events were similar across all subgroup analyses and further supported by our post hoc analyses in which we found no risk of a specific bleeding subtype.
Furthermore, no signal for this outcome was observed among the population who received heterologous booster schedules (ie, three doses). This pattern largely argues against a true association but rather suggests a general biased increase in outcome detection. Of note, the composite outcome of other bleeding events has not been validated. Consequently, we do not believe that our study provides consistent evidence for an association between bleeding events and heterologous primary vaccine schedules. Nonetheless, this observation should ideally be evaluated in future studies of different data sources. Importantly, no other signals were found for the 19 adverse safety outcomes examined and the analyses, when restricting to hospital contacts with a duration of more than 24 h (in which the specificity and severity of the captured outcomes are increased), showed no significant differences in the risk of the serious adverse events between the vaccinated groups.
As such, our results could inform clinicians, patients, and medicinal regulatory authorities on the safety of the heterologous vaccine schedule of ChAdOx1-S priming and mRNA booster dose or doses with the BNT162b2 or mRNA-1273 vaccines. This study was based on cross linkage of data from several Danish healthcare registers, which allows for prospective and individual level ascertainment of health information registered during routine clinical care. The nationwide coverage of the Danish registers facilitated a large study population to assess the comparative safety in relation to the risk of rare adverse events. Because of the relative rarity of the individual events, however, the statistical power was limited for some of these analyses, leading to a low precision of the estimates. Based on the upper 95% confidence intervals of the main analysis, our results are inconsistent with a relative increased risk of more than twofold for seven of the 15 outcomes analysed. As this research is the first observational study, to our knowledge, to evaluate the safety of the heterologous primary and booster covid-19 vaccine schedules with a wide range of adverse outcome events, findings should be evaluated for supporting evidence in other independent populations.
Comparison with other studies
A Swedish study examined the effectiveness of heterologous ChAdOx1-S/mRNA primary (ie, two dose) vaccine schedules as compared with matched unvaccinated individuals but also reported rates of three safety outcomes; however, the crude number of cases for these safety events were low.29 The study identified two cases of other venous thromboembolisms (I82 code from ICD-10; ie, not pulmonary, cerebral, or deep venous thromboembolisms) among the heterologous vaccinated, no cases of arterial thromboembolisms (I74), and three cases of purpura and other haemorrhagic conditions (D69). No cases of any of these three safety outcomes were found among the matched unvaccinated individuals. A Spanish study compared 14 325 heterologous ChAdOx1-S and BNT162b primary schedule vaccinated with homologous ChAdOx1-S vaccinated (matched 1:1).30 Of the safety outcomes examined, the authors found one event of venous thromboembolism, one event of venous thromboembolism with thrombocytopenia, and no events of myocarditis or pericarditis among individuals vaccinated with a heterologous schedule (no events were reported in the comparative group). Similar to the Swedish study, the overall numbers were small so statistical testing was not possible. These methodological differences limit a direct comparison to our results.
Our study results have a high degree of generalisability; however, as per study design, individuals were not studied if they were younger than 18 years or older than 65 years, had a previous positive PCR test for SARS-CoV-2, or had a recent history of the outcome events of interest. Therefore, our results cannot be directly used to help evaluate the safety of heterologous covid-19 vaccine schedules within these specific and clinically important subgroups. Likewise, our study addressed the question of safety in regards to a heterologous mRNA booster dose or doses in individuals having received ChAdOx1-S priming and thus, might have limited applicability to other heterologous covid-19 vaccine schedules.
Conclusion
In this nationwide cohort study, we found no association between a heterologous covid-19 vaccine schedule of ChAdOx1-S priming and mRNA booster or boosters and the risk of the 19 analysed serious adverse events, as compared with homologous mRNA vaccine schedules. Further safety surveillance of heterologous primary and booster vaccine schedules for covid-19 is warranted. Nonetheless, these results could help to inform patients, clinicians, and regulatory authorities.
What is already known on this topic
Emerging findings suggest that heterologous vaccine schedules for covid-19 provide similar or better immunogenicity to that of homologous covid-19 vaccine schedules with a tolerable reactogenicity
Information on the safety of heterologous primary and booster covid-19 vaccine schedules are incomplete
What this study adds
Heterologous covid-19 vaccine schedules of ChAdOx1-S priming followed by an mRNA vaccine (BNT162b2 or mRNA-1273) plus any booster dose (ie, a second as well as a third dose) were compared with homologous mRNA vaccine schedules
Findings were not consistent with an increased risk of 19 adverse safety outcomes examined among heterologous vaccinated individuals as compared with homologous vaccinated, with no differences in the risks of events classified as serious
Heterologous covid-19 vaccine schedules of ChAdOx1-S priming and an mRNA booster dose or doses appear to have a similar safety profile to homologous primary and booster mRNA vaccine schedules
Web extra.
Extra material supplied by authors
Web appendix: Online appendix
Contributors: EMT and AH had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analyses. All authors conceived and designed the study; acquired, analysed, and interpreted the data; and critically revised the manuscript for important intellectual content. NA drafted the manuscript and EMT carried out the statistical analysis. NA and AH are the guarantors. AH supervised the study. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: AH is supported by an Ascending Investigator grant from the Lundbeck Foundation for vaccine safety research. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Competing interest: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
The lead author (the manuscript’s guarantor) affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: Studied participants were anonymised in the utilised data sources. The study results will be disseminated to the public and health professionals by a press release, and through social medias with layman’s terms.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
The study was approved by the Danish Data Protection Agency. Ethical approval as well as informed consent is not required for register-based research in Denmark.
Data availability statement
No additional data available.
References
- 1. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med 2021;384:2092-101. 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med 2021;384:2124-30. 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wise J. Covid-19: European countries suspend use of Oxford-AstraZeneca vaccine after reports of blood clots. BMJ 2021;372:n699. 10.1136/bmj.n699. [DOI] [PubMed] [Google Scholar]
- 4.European Centre for Disease Prevention and Control. Overview of EU/EEA country recommendations on COVID-19 vaccination with Vaxzevria, and a scoping review of evidence to guide decision-making. 2021.https://www.ecdc.europa.eu/en/publications-data/overview-eueea-country-recommendations-covid-19-vaccination-vaxzevria-and-scoping.
- 5. Mathieu E, Ritchie H, Ortiz-Ospina E, et al. A global database of COVID-19 vaccinations. Nat Hum Behav 2021;5:947-53. 10.1038/s41562-021-01122-8. [DOI] [PubMed] [Google Scholar]
- 6. Spencer AJ, McKay PF, Belij-Rammerstorfer S, et al. Heterologous vaccination regimens with self-amplifying RNA and adenoviral COVID vaccines induce robust immune responses in mice. Nat Commun 2021;12:2893. 10.1038/s41467-021-23173-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dimeglio C, Herin F, Da-Silva I, et al. Heterologous ChAdOx1-S/BNT162b2 vaccination: neutralizing antibody response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis 2022;74:1315-6. 10.1093/cid/ciab705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tenbusch M, Schumacher S, Vogel E, et al. DZIF-VACCELERATE-CoVaKo study team . Heterologous prime-boost vaccination with ChAdOx1 nCoV-19 and BNT162b2. Lancet Infect Dis 2021;21:1212-3. 10.1016/S1473-3099(21)00420-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vallée A, Vasse M, Mazaux L, et al. An immunogenicity report for the comparison between heterologous and homologous prime-boost schedules with ChAdOx1-S and BNT162b2 Vaccines. J Clin Med 2021;10:3817. 10.3390/jcm10173817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barros-Martins J, Hammerschmidt SI, Cossmann A, et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat Med 2021;27:1525-9. 10.1038/s41591-021-01449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Behrens GM, Cossmann A, Stankov MV, et al. SARS-CoV-2 delta variant neutralisation after heterologous ChAdOx1-S/BNT162b2 vaccination. Lancet 2021;398:1041-2. 10.1016/S0140-6736(21)01891-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hammerschmidt SI, Bosnjak B, Bernhardt G, et al. Neutralization of the SARS-CoV-2 Delta variant after heterologous and homologous BNT162b2 or ChAdOx1 nCoV-19 vaccination. Cell Mol Immunol 2021;18:2455-6. 10.1038/s41423-021-00755-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Normark J, Vikström L, Gwon Y-D, et al. Heterologous ChAdOx1 nCoV-19 and mRNA-1273 Vaccination. N Engl J Med 2021;385:1049-51. 10.1056/NEJMc2110716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hillus D, Schwarz T, Tober-Lau P, et al. EICOV/COVIM Study Group . Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: a prospective cohort study. Lancet Respir Med 2021;9:1255-65. 10.1016/S2213-2600(21)00357-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Groß R, Zanoni M, Seidel A, et al. Heterologous ChAdOx1 nCoV-19 and BNT162b2 prime-boost vaccination elicits potent neutralizing antibody responses and T cell reactivity against prevalent SARS-CoV-2 variants. EBioMedicine 2022;75:103761. 10.1016/j.ebiom.2021.103761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schmidt T, Klemis V, Schub D, et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat Med 2021;27:1530-5. 10.1038/s41591-021-01464-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu X, Shaw RH, Stuart ASV, et al. Com-COV Study Group . Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet 2021;398:856-69. 10.1016/S0140-6736(21)01694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Borobia AM, Carcas AJ, Pérez-Olmeda M, et al. CombiVacS Study Group . Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet 2021;398:121-30. 10.1016/S0140-6736(21)01420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stuart ASV, Shaw RH, Liu X, et al. Com-COV2 Study Group . Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating mRNA, viral-vector, and protein-adjuvant vaccines in the UK (Com-COV2): a single-blind, randomised, phase 2, non-inferiority trial. Lancet 2022;399:36-49. 10.1016/S0140-6736(21)02718-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sablerolles RSG, Rietdijk WJR, Goorhuis A, et al. SWITCH Research Group . Immunogenicity and reactogenicity of vaccine boosters after Ad26.COV2.S Priming. N Engl J Med 2022;386:951-63. 10.1056/NEJMoa2116747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Munro APS, Janani L, Cornelius V, et al. COV-BOOST study group . Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet 2021;398:2258-76. 10.1016/S0140-6736(21)02717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Atmar RL, Lyke KE, Deming ME, et al. DMID 21-0012 Study Group . Homologous and Heterologous Covid-19 Booster Vaccinations. N Engl J Med 2022;386:1046-57. 10.1056/NEJMoa2116414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Costa Clemens SA, Weckx L, Clemens R, et al. RHH-001 study team . Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study. Lancet 2022;399:521-9. 10.1016/S0140-6736(22)00094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shaw RH, Stuart A, Greenland M, Liu X, Nguyen Van-Tam JS, Snape MD, Com-COV Study Group . Heterologous prime-boost COVID-19 vaccination: initial reactogenicity data. Lancet 2021;397:2043-6. 10.1016/S0140-6736(21)01115-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Powell AA, Power L, Westrop S, et al. Real-world data shows increased reactogenicity in adults after heterologous compared to homologous prime-boost COVID-19 vaccination, March-June 2021, England. Euro Surveill 2021;26:2100634. 10.2807/1560-7917.ES.2021.26.28.2100634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li X, Ostropolets A, Makadia R, et al. Characterising the background incidence rates of adverse events of special interest for covid-19 vaccines in eight countries: multinational network cohort study. BMJ 2021;373. 10.1136/bmj.n1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Duarte-Salles T, Prieto-Alhambra D. Heterologous vaccine regimens against COVID-19. Lancet 2021;398:94-5. 10.1016/S0140-6736(21)01442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Belda-Iniesta C. Optimising SARS-CoV-2 vaccination schedules. Lancet 2021;398:819-21. 10.1016/S0140-6736(21)01729-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nordström P, Ballin M, Nordström A. Effectiveness of heterologous ChAdOx1 nCoV-19 and mRNA prime-boost vaccination against symptomatic Covid-19 infection in Sweden: A nationwide cohort study. Lancet Reg Health Eur 2021;11:100249. 10.1016/j.lanepe.2021.100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hermosilla E, Coma E, Xie J, et al. Comparative effectiveness and safety of homologous two-dose ChAdOx1 versus heterologous vaccination with ChAdOx1 and BNT162b2. Nat Commun 2022;13:1639. 10.1038/s41467-022-29301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Higdon MM, Wahl B, Jones CB, et al. A systematic review of coronavirus disease 2019 vaccine efficacy and effectiveness against severe acute respiratory syndrome coronavirus 2 infection and disease. Open Forum Infect Dis 2022;9:ofac138. 10.1093/ofid/ofac138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schmidt M, Schmidt SAJ, Adelborg K, et al. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol 2019;11:563-91. 10.2147/CLEP.S179083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Grove Krause T, Jakobsen S, Haarh M, Mølbak K. The Danish vaccination register. Euro Surveill 2012;17:20155. 10.2807/ese.17.17.20155-en. [DOI] [PubMed] [Google Scholar]
- 34. Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health 2011;39(Suppl):30-3. 10.1177/1403494811401482. [DOI] [PubMed] [Google Scholar]
- 35. Pedersen CB. The Danish Civil Registration System. Scand J Public Health 2011;39(Suppl):22-5. 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- 36. Voldstedlund M, Haarh M, Mølbak K, MiBa Board of Representatives . The Danish Microbiology Database (MiBa) 2010 to 2013. Euro Surveill 2014;19:20667. 10.2807/1560-7917.ES2014.19.1.20667. [DOI] [PubMed] [Google Scholar]
- 37. Center for Biologics Evaluation and Research Office of Biostatistics and Epidemiology . CBER surveillance program background rates of adverse events of special interest for covid-19 vaccine safety monitoring protocol. 2021. https://www.bestinitiative.org/wp-content/uploads/2021/02/C19-Vaccine-Safety-AESI-Background-Rate-Protocol-FINAL-2020.pdf
- 38. Law B, Sturkenboom M. Priority list of adverse events of special interest: COVID-19. 2020. https://brightoncollaboration.us/wp-content/uploads/2020/06/SPEAC_D2.3_V2.0_COVID-19_20200525_public.pdf
- 39. Chao C, Jacobsen SJ. Evaluation of autoimmune safety signal in observational vaccine safety studies. Hum Vaccin Immunother 2012;8:1302-4. 10.4161/hv.21268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sarkanen T, Alakuijala A, Julkunen I, Partinen M. Narcolepsy Associated with Pandemrix Vaccine. Curr Neurol Neurosci Rep 2018;18:43. 10.1007/s11910-018-0851-5. [DOI] [PubMed] [Google Scholar]
- 41.European Medicines Agency. COVID-19 Vaccine AstraZeneca: PRAC investigating cases of thromboembolic events - vaccine’s benefits currently still outweigh risks Update. European Medicines Agency. 2021. https://www.ema.europa.eu/en/news/covid-19-vaccine-astrazeneca-prac-investigating-cases-thromboembolic-events-vaccines-benefits
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Online appendix
Data Availability Statement
No additional data available.