Key Points
Question
Were there changes in the rates of in-hospital adverse events between 2010 and 2019?
Findings
In this serial cross-sectional study of 244 542 adult patients hospitalized in 3156 US hospitals from 2010 to 2019, there were statistically significant decreases in the annual rates of in-hospital adverse events for admissions for acute myocardial infarction (annual adjusted relative risk [RR], 0.94), heart failure (RR, 0.95), pneumonia (RR, 0.94), major surgical procedures (RR, 0.93), and all other conditions (RR, 0.97).
Meaning
The rates of adverse events in hospitalized patients significantly declined for patients with acute myocardial infarction, heart failure, pneumonia, and major surgical procedures between 2010 and 2019 and significantly declined for patients with all other conditions between 2012 and 2019.
Abstract
Importance
Patient safety is a US national priority, yet lacks a comprehensive assessment of progress over the past decade.
Objective
To determine the change in the rate of adverse events in hospitalized patients.
Design, Setting, and Participants
This serial cross-sectional study used data from the Medicare Patient Safety Monitoring System from 2010 to 2019 to assess in-hospital adverse events in patients. The study included 244 542 adult patients hospitalized in 3156 US acute care hospitals across 4 condition groups from 2010 through 2019: acute myocardial infarction (17%), heart failure (17%), pneumonia (21%), and major surgical procedures (22%); and patients hospitalized from 2012 through 2019 for all other conditions (22%).
Exposures
Adults aged 18 years or older hospitalized during each included calendar year.
Main Outcomes and Measures
Information on adverse events (abstracted from medical records) included 21 measures across 4 adverse event domains: adverse drug events, hospital-acquired infections, adverse events after a procedure, and general adverse events (hospital-acquired pressure ulcers and falls). The outcomes were the total change over time for the observed and risk-adjusted adverse event rates in the subpopulations.
Results
The study sample included 190 286 hospital discharges combined in the 4 condition-based groups of acute myocardial infarction, heart failure, pneumonia, and major surgical procedures (mean age, 68.0 [SD, 15.9] years; 52.6% were female) and 54 256 hospital discharges for the group including all other conditions (mean age, 57.7 [SD, 20.7] years; 59.8% were female) from 3156 acute care hospitals across the US. From 2010 to 2019, the total change was from 218 to 139 adverse events per 1000 discharges for acute myocardial infarction, from 168 to 116 adverse events per 1000 discharges for heart failure, from 195 to 119 adverse events per 1000 discharges for pneumonia, and from 204 to 130 adverse events per 1000 discharges for major surgical procedures. From 2012 to 2019, the rate of adverse events for all other conditions remained unchanged at 70 adverse events per 1000 discharges. After adjustment for patient and hospital characteristics, the annual change represented by relative risk in all adverse events per 1000 discharges was 0.94 (95% CI, 0.93-0.94) for acute myocardial infarction, 0.95 (95% CI, 0.94-0.96) for heart failure, 0.94 (95% CI, 0.93-0.95) for pneumonia, 0.93 (95% CI, 0.92-0.94) for major surgical procedures, and 0.97 (95% CI, 0.96-0.99) for all other conditions. The risk-adjusted adverse event rates declined significantly in all patient groups for adverse drug events, hospital-acquired infections, and general adverse events. For patients in the major surgical procedures group, the risk-adjusted rates of events after a procedure declined significantly.
Conclusions and Relevance
In the US between 2010 and 2019, there was a significant decrease in the rates of adverse events abstracted from medical records for patients admitted for acute myocardial infarction, heart failure, pneumonia, and major surgical procedures and there was a significant decrease in the adjusted rates of adverse events between 2012 and 2019 for all other conditions. Further research is needed to understand the extent to which these trends represent a change in patient safety.
This serial cross-sectional study used data from the Medicare Patient Safety Monitoring System for 2010 to 2019 to determine the change in the rate of adverse events in hospitalized patients.
Introduction
The Institute of Medicine published a report1 in 1999 that drew national attention to patient safety in the US. Since the report’s publication, many organizations developed, promoted, and implemented interventions to improve patient safety.2 Despite these efforts, national evidence evaluating patient safety progress is sparse.3,4 Prior publications5,6,7 and federal reports8 have suggested some progress for specific patient groups or specific types of adverse events. However, there is a need for a comprehensive and methodologically rigorous evaluation of patient safety trends in US hospitals.
The Medicare Patient Safety Monitoring System (MPSMS) was designed to measure important and often preventable adverse events among hospital inpatients. The MPSMS uses expert abstractors, leveraging software guidance, to abstract9 21 specific patient-safety adverse events (Box) for hospital stays across all states, Washington, DC, and Puerto Rico.10 A previous study reported that from 2005 to 2011, the MPSMS adverse events declined significantly among Medicare patients with a principal diagnosis of acute myocardial infarction or heart failure, but not among those with a diagnosis of pneumonia or among those who underwent a major surgical procedure during their inpatient stay.7 The current study assessed changes in adverse event rates for patient safety between 2010 and 2019 for acute myocardial infarction, heart failure, pneumonia, and major surgical procedures (the 4 patient groups previously studied) and a fifth group encompassing all other conditions.
Box. The 21 Medicare Patient Safety Monitoring System Measures of Adverse Events Across 4 Adverse Event Domains.
-
Adverse drug events
Associated with digoxin
Associated with hypoglycemic agents
Associated with heparin
Associated with low-molecular-weight heparin and factor Xa inhibitors
Associated with warfarin
-
Hospital-acquired infections
Central line–associated blood stream infections
Postoperative pneumonia
Hospital-acquired antibiotic-associated Clostridioides difficile
Physician-diagnosed catheter-associated urinary tract infections
Hospital-acquired methicillin-resistant Staphylococcus aureus
Hospital-acquired vancomycin-resistant Enterococcus
Ventilator-associated pneumonia
-
Adverse events after a procedure
Associated with a hip joint replacement
Associated with a knee joint replacement
Mechanical complications associated with central lines
Postoperative venous thromboembolic events
Postoperative cardiac events (cardiac and noncardiac surgeries)
Associated with femoral artery puncture for catheter angiographic procedures
Contrast nephropathy associated with catheter angiography
-
General adverse events
Hospital-acquired pressure ulcers
Inpatient falls
Methods
Study Sample
The Yale University institutional review board reviewed the study protocol and granted a waiver of informed consent to use the de-identified database.
The MPSMS study sample was initially the sample of records the Centers for Medicare & Medicaid Services (CMS) selected to validate hospital submissions to the CMS Hospital Inpatient Quality Reporting Program from 2010 through 2019. All acute care hospitals subject to the CMS Inpatient Prospective Payment System submitted data to the CMS Clinical Data Warehouse on chart-abstracted measures included in the Hospital Inpatient Quality Reporting and Hospital Value-Based Purchasing programs of the CMS. The CMS required randomly selected hospitals to submit validation samples for these programs. The number of hospitals sampled and medical records required per hospital by the CMS varied by year (additional information appears in the eText and in eTable 1 in the Supplement).
The sample included only medical records for the 4 conditions (acute myocardial infarction, heart failure, pneumonia, and major surgical procedures) included in the CMS Hospital Inpatient Quality Reporting Program and in the CMS Surgical Care Improvement Project11 between 2010 and 2011. Starting in 2012, these measures were expanded to cover all conditions. For this study, a subset of these medical records was used. Specifically, to measure trends for the 4 original conditions after 2011, the medical records of patients admitted for 1 of the 4 conditions were randomly sampled. In addition, medical records exclusive of these diagnoses and conditions were used to create a random sample for the all other conditions group. The sample size was set by several considerations, including the availability of funds to support chart abstraction (see eText in the Supplement for further information).
The medical records abstracted for the MPSMS were categorized for patients aged 18 years or older who had been discharged from an acute care hospital with a principal diagnosis of (1) acute myocardial infarction, (2) heart failure, (3) pneumonia, (4) major surgical procedure, and (5) all other conditions. Data were available on discharges for the 4 conditions that occurred between January 1, 2010, and September 30, 2019, and on discharges for all other conditions that occurred between January 1, 2012, and December 31, 2019. The cohorts for the 4 conditions were defined by the CMS Hospital Inpatient Quality Reporting Program quality measure specifications for all years.12,13 The number of MPSMS records for each of the 5 groups varied across years due to changes in the quality reporting programs and evolution of the sample over time to be more representative of the national distribution of hospital admissions.
Hospitals randomly selected for the CMS validation sample contributed approximately equal numbers of randomly selected medical records to the MPSMS regardless of their size. In 2010, 33 678 medical records were selected from 1385 hospitals, and in 2019, 10 199 medical records were selected from 804 hospitals (data for all years appear in eTable 2 in the Supplement).
The medical records were abstracted at the CMS Clinical Data Abstraction Center using MPSMS algorithms.7 Based on 80 monthly record repeated abstractions, the agreement between the abstraction and the repeated abstraction ranged from 94% to 99% for MPSMS data elements. Additional information on the sample appears in the eText in the Supplement. In addition, Agency for Healthcare Research and Quality National Inpatient Sample data14 for 2010 to 2017 were used to compare in-hospital mortality and length of stay with the MPSMS data (eTable 3 in the Supplement).
Patient and Hospital Characteristics
The patient characteristics included demographics (age, sex, and race and ethnicity), comorbidities (heart failure, obesity, coronary artery disease, kidney disease, cerebrovascular disease, chronic obstructive pulmonary disease, cancer, diabetes), and smoking status (Table). Race and ethnicity information is included because this data set has been used to conduct disparity studies for the Agency for Healthcare Research and Quality. Patients were categorized as American Indian/Alaska Native, Asian, Hispanic, Native Hawaiian/Other Pacific Islander, non-Hispanic Black, non-Hispanic White, and other (includes multiracial and any identified race and ethnicity not included in the aforementioned categories).
Table. Patient and Hospital Characteristics.
| Patient and hospital characteristics by condition groups, No. (%)a | ||||||
|---|---|---|---|---|---|---|
| Combined group of 4 conditions (acute MI, heart failure, pneumonia, and major surgical procedures) (n = 190 286) | All other conditions group (n = 54 256) | |||||
| 2012 (n = 27 256) |
2016 (n = 20 784) |
2019 (n = 4060) |
2012 (n = 9532) |
2016 (n = 7114) |
2019 (n = 6139) |
|
| Patient characteristics | ||||||
| Age, mean (SD), y | 67.7 (16.1) | 67.9 (15.8) | 67.1 (15.4) | 56.9 (20.6) | 58.2 (20.7) | 59.1 (20.2) |
| Age group, y | ||||||
| <65 | 10 781 (39.6) | 8107 (39.0) | 1610 (39.7) | 5728 (60.1) | 4024 (56.6) | 3347 (54.5) |
| 65-74 | 6087 (22.3) | 4997 (24.0) | 1083 (26.7) | 1552 (16.3) | 1275 (17.9) | 1232 (20.1) |
| 75-84 | 5979 (21.9) | 4429 (21.3) | 869 (21.4) | 1347 (14.1) | 1049 (14.7) | 947 (15.4) |
| ≥85 | 4409 (16.2) | 3251 (15.6) | 498 (12.3) | 905 (9.5) | 766 (10.8) | 613 (10.0) |
| Sex | ||||||
| Female | 14 731 (54.0) | 10 748 (51.7) | 2132 (52.5) | 5794 (60.8) | 4189 (58.9) | 3623 (59.0) |
| Male | 12 525 (46.0) | 10 036 (48.3) | 1928 (47.5) | 3738 (39.2) | 2925 (41.1) | 2516 (41.0) |
| Race and ethnicity | ||||||
| American Indian/Alaska Native | 250 (0.9) | 177 (0.9) | 46 (1.1) | 125 (1.3) | 86 (1.2) | 125 (2.0) |
| Asian | 471 (1.7) | 311 (1.5) | 64 (1.6) | 154 (1.6) | 156 (2.2) | 124 (2.0) |
| Hispanic | 1466 (5.4) | 1169 (5.6) | 218 (5.4) | 630 (6.6) | 512 (7.2) | 433 (7.1) |
| Native Hawaiian/Other Pacific Islander | 36 (0.1) | 44 (0.2) | 10 (0.2) | 12 (0.1) | 8 (0.1) | 9 (0.1) |
| Non-Hispanic Black | 3313 (12.2) | 2594 (12.5) | 551 (13.6) | 1182 (12.4) | 833 (11.7) | 853 (13.9) |
| Non-Hispanic White | 21 142 (77.6) | 16 029 (77.1) | 3031 (74.7) | 7185 (75.4) | 5326 (74.9) | 4397 (71.6) |
| Otherb | 578 (2.1) | 460 (2.2) | 140 (3.4) | 244 (2.6) | 193 (2.7) | 198 (3.2) |
| Risk factors | ||||||
| Obesity | 7578 (27.8) | 7718 (37.1) | 1655 (40.8) | 2276 (23.9) | 2197 (30.9) | 2152 (35.1) |
| Smoking | 6781 (24.9) | 5896 (28.4) | 1231 (30.3) | 2595 (27.2) | 2175 (30.6) | 1953 (31.8) |
| Use of corticosteroids | 2100 (7.7) | 1481 (7.1) | 273 (6.7) | 525 (5.5) | 474 (6.7) | 396 (6.5) |
| Comorbidities | ||||||
| Coronary artery disease | 12 794 (46.9) | 9646 (46.4) | 1647 (40.6) | 2063 (21.6) | 1588 (22.3) | 1348 (22.0) |
| Congestive heart failure | 10 379 (38.1) | 9094 (43.8) | 1412 (34.8) | 1224 (12.8) | 1181 (16.6) | 1064 (17.3) |
| Diabetes | 9479 (34.8) | 7775 (37.4) | 1371 (33.8) | 2548 (26.7) | 2038 (28.6) | 1864 (30.4) |
| Kidney disease | 7510 (27.6) | 6673 (32.1) | 1238 (30.5) | 1499 (15.7) | 1568 (22.0) | 1622 (26.4) |
| COPD | 8361 (30.7) | 6386 (30.7) | 1045 (25.7) | 1699 (17.8) | 1439 (20.2) | 1224 (19.9) |
| Cancer | 5432 (19.9) | 3912 (18.8) | 802 (19.8) | 1445 (15.2) | 1240 (17.4) | 1006 (16.4) |
| Cerebrovascular disease | 4193 (15.4) | 3159 (15.2) | 558 (13.7) | 1239 (13.0) | 1034 (14.5) | 892 (14.5) |
| Outcomes | ||||||
| Length of stay, mean (SD), d | 5.1 (5.2) | 4.5 (4.9) | 4.7 (5.4) | 3.8 (4.3) | 3.9 (4.4) | 4.1 (5.1) |
| In-hospital mortality | 1246 (4.6) | 735 (3.5) | 111 (2.7) | 111 (1.2) | 160 (2.2) | 106 (1.7) |
| Hospital characteristics | ||||||
| Accredited by the Joint Commission | 22 249 (81.6) | 16 465 (79.2) | 3300 (81.3) | 7218 (75.7) | 5533 (77.8) | 4826 (78.6) |
| Private not-for-profit | 16 353 (60.0) | 12 790 (61.5) | 2695 (66.4) | 4962 (52.1) | 4466 (62.8) | 3737 (60.9) |
| Treatment rates | ||||||
| PCI | 11 525 (42.3) | 10 199 (49.1) | 2324 (57.2) | 3095 (32.5) | 3162 (44.4) | 2653 (43.2) |
| CABG surgery | 9021 (33.1) | 6803 (32.7) | 1640 (40.4) | 2409 (25.3) | 2195 (30.9) | 1847 (30.1) |
| Rural location | 8532 (31.3) | 6457 (31.1) | 1179 (29.0) | 2993 (31.4) | 2321 (32.6) | 1787 (29.1) |
| Large teaching | 2216 (8.1) | 1830 (8.8) | 340 (8.4) | 560 (5.9) | 378 (5.3) | 429 (7.0) |
| No. of beds, median (IQR) | 155.0 (80.0-277.0) | 142.0 (78.0-269.0) | 169.0 (91.0-322.0) | 113.0 (45.0-228.0) | 144.0 (69.0-264.0) | 149.0 (72.0-312.0) |
Abbreviations: CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Some of the data are expressed as mean (SD) or median (IQR) as indicated in the rows. The data for every year from 2010 to 2019 appear in eTables 4-5 in the Supplement.
Includes multiracial and any race and ethnicity not included in the listed categories.
Race and ethnicity were self-reported during the hospital admission process and may have been collected as an open-ended question or as a fixed category question. Using the International Classification of Diseases, Ninth Revision, and the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, diagnosis codes, Elixhauser-specific comorbidities for each patient were calculated.15
Hospital characteristics (obtained from the annual survey database of the American Hospital Association16 for 2010-2017) included teaching status (teaching vs nonteaching), Joint Commission certification status (yes vs no), geographic location (urban vs rural), ownership (private not-for-profit vs other), number of beds (continuous), and treatment rates for coronary artery bypass graft surgery (yes vs no) and percutaneous coronary intervention (yes vs no). Publicly available data sources (including CMS hospital performance data) were used for hospitals with missing characteristics (87 of 3156 hospitals [2.8%]) to obtain the status (ie, teaching hospital, Joint Commission certification, ownership) and information on procedures (coronary artery bypass graft surgery and percutaneous coronary intervention). Missing information on the number of beds (1%) was imputed using multiple imputation with 10 imputations.17
In-hospital Adverse Events and Outcomes
The MPSMS data, including 21 in-hospital adverse event measures in 4 domains (adverse drug events, hospital-acquired infections, adverse events after a procedure, and general adverse events [hospital-acquired pressure ulcers and inpatient falls]), are described elsewhere7 and appear in the Box. The prespecified primary outcomes were 3 composite outcomes of the 21 measures: (1) the rate of occurrence of adverse events for which patients were at risk, (2) the proportion of patients with 1 or more adverse events, and (3) the number of adverse events per 1000 discharges. If the same adverse event could be counted in more than 1 measure (eg, postoperative pneumonia and ventilator-associated pneumonia), only 1 occurrence was included in the number of adverse events per 1000 discharges. In addition, in-hospital mortality and hospital length of stay were measured.
Statistical Analysis
We fitted mixed-effects models with a Poisson link function to evaluate the trends in the number of adverse events per 1000 hospitalizations, the rate of occurrence of adverse events, and the rate of patients with 1 or more adverse events. We used the modified Poisson method of Zou18 for the 2 latter outcomes. All models were fitted with state-specific random intercepts to account for within- and between-state variations and adjusted for patient and hospital characteristics as described above. The models included an ordinal time variable that ranged from 0 to 9 and corresponded to the years as time = 0 for 2010 and time = 9 for 2019. The incidence rate ratio for the time variable was used to represent the annual trend in relative risk (RR) for adverse events. The models were fitted for 2010 to 2019 for each of the 4 conditions (acute myocardial infarction, heart failure, pneumonia, and major surgical procedures) separately and for 2012 to 2019 for all other conditions.
We repeated the mixed-effects models, focusing solely on each of the 4 adverse event domains (adverse drug events, hospital-acquired infections, adverse events after a procedure, and general adverse events). Because the sampling approach for the MPSMS changed slightly between the 2010-2014 and 2015-2019 periods (details appear in the eText in the Supplement), a secondary analysis was conducted by repeating the analysis for the 2010-2014 and 2015-2019 periods separately. During the 2010-2014 period, the CMS funded the federal Partnership for Patients initiative.19 During the 2015-2019 period, the CMS Quality Improvement Organization Program adopted the goals to “sustain and expand current national reductions in patient harm and 30-day readmissions for the Medicare program.”20
To evaluate the trend in the older population, allowing a more direct comparison with the previous study covering 2005 to 2011,7 the analyses were repeated and limited to patients aged 65 years or older. Because it is unlikely that all comorbidities had been captured during the medical record abstraction process, we repeated all the analyses and included an Elixhauser-specific comorbidity score in addition to the abstracted comorbidities. We stratified the data to investigate trends in the patient subpopulations, including region, age, sex, and race and ethnicity for the 5 groups (acute myocardial infarction, heart failure, pneumonia, major surgical procedures, and all other conditions) and the 4 adverse event domains (adverse drug events, hospital-acquired infections, adverse events after a procedure, and general adverse events). In addition, we calculated the in-hospital mortality rate and the hospital length of stay for patients with 0 adverse events or with 1 or more adverse events occurring during their stay.
All analyses were conducted using SAS version 9.4 (SAS Institute Inc). All statistical testing was 2-sided at a significance level of .05. Because of the potential for type I error due to the large number of statistical comparisons, the findings should be interpreted as exploratory.
Results
Study Sample and Patient Characteristics
The patient characteristics for the combined group of the 4 conditions (acute myocardial infarction, heart failure, pneumonia, and major surgical procedures) and the all other conditions group appear in the Table and in eTable 2 in the Supplement. The study sample included 244 542 adult patients who accounted for 190 286 discharges in the combined group of 4 conditions (mean age, 68.0 years [SD, 15.9 years]; 52.6% were female) and 54 256 adult patients in the all other conditions group (mean age, 57.7 years [SD, 20.7 years]; 59.8% were female) from 3156 acute care hospitals across the US. The patients were hospitalized from 2010 through 2019 for acute myocardial infarction (17%), heart failure (17%), pneumonia (21%), and major surgical procedures (22%) and from 2012 through 2019 for all other conditions (22%).
With few exceptions, age, race and ethnicity, patient characteristics, and comorbidities across the 2010-2019 period remained generally stable in the combined group of the 4 conditions and individually in the 4 condition groups (eTables 4-9 in the Supplement). Notable exceptions include the increase in obesity seen in all 5 condition groups and the increased prevalence of kidney disease in the major surgical procedures group and in the all other conditions group. The median number of times a patient was eligible for the 21 MPSMS measures of adverse events (Box) was stable during the study years (eTables 10-14 in the Supplement).
Trends in Adverse Events
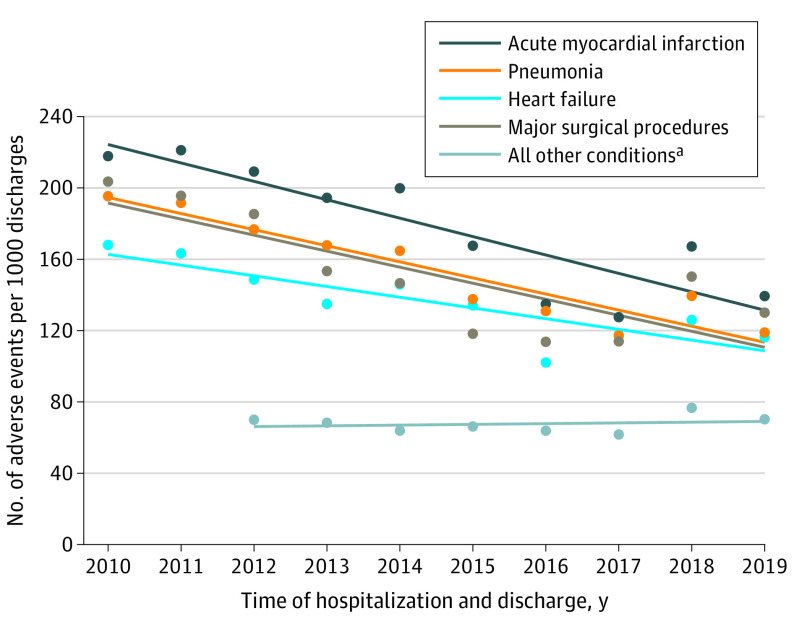
The observed adverse event rates declined significantly across 4 of the 5 condition groups, with variation in the rate of decline (Figure 1 and eTable 15 in the Supplement and Figure 2). For the acute myocardial infarction group, the number of observed adverse events declined significantly from 218 per 1000 discharges in 2010 to 139 per 1000 discharges in 2019 (absolute difference, 79 [95% CI, 46 to 111] per 1000 discharges). For the heart failure group, the number of observed adverse events declined significantly from 168 per 1000 discharges in 2010 to 116 per 1000 discharges in 2019 (absolute difference, 52 [95% CI, 26 to 77] per 1000 discharges). For the pneumonia group, the number of observed adverse events declined significantly from 195 per 1000 discharges in 2010 to 119 per 1000 discharges in 2019 (absolute difference, 76 [95% CI, 52 to 101] per 1000 discharges). For the major surgical procedures group, the number of observed adverse events declined significantly from 204 per 1000 discharges in 2010 to 130 per 1000 discharges in 2019 (absolute difference, 74 [95% CI, 55 to 92] per 1000 discharges). For the all other conditions group, there was no change in the number of adverse events; there were 70 adverse events per 1000 discharges in 2012 and 70 adverse events per 1000 discharges in 2019 (absolute difference, 0 [95% CI, −8 to 9] per 1000 discharges).
Figure 1. Observed Adverse Event Rates by the 4 Medicare Patient Safety Monitoring System Adverse Event Domains.
The circles denote the observed values and the lines represent the trends over time. Additional information appears in eTable 15 in the Supplement.
aData were not available for 2010 and 2011.
Figure 2. Total Change in the Observed Rates for All 21 Medicare Patient Safety Monitoring System Adverse Events.
The circles denote observed values and the lines represent the trends over time.
aData were not available for 2010 and 2011.
Figure 3 shows the results after adjustment for patient and hospital characteristics. Compared with the baseline year of 2010 (RR, 1.00), the RR for adverse events per 1000 discharges for 2019 was 0.59 (95% CI, 0.47-0.74) for the acute myocardial infarction group, 0.73 (95% CI, 0.59-0.90) for the heart failure group, 0.64 (95% CI, 0.52-0.78) for the pneumonia group, and 0.59 (95% CI, 0.51-0.69) for the major surgical procedures group. Compared with the baseline year of 2012 (RR, 1.00), the RR for adverse events per 1000 discharges for 2019 was 0.82 (95% CI, 0.73-0.92) for the all other conditions group.
Figure 3. Adjusted Relative Risks for the Number of Adverse Events per 1000 Discharges by the 5 Condition Groups.
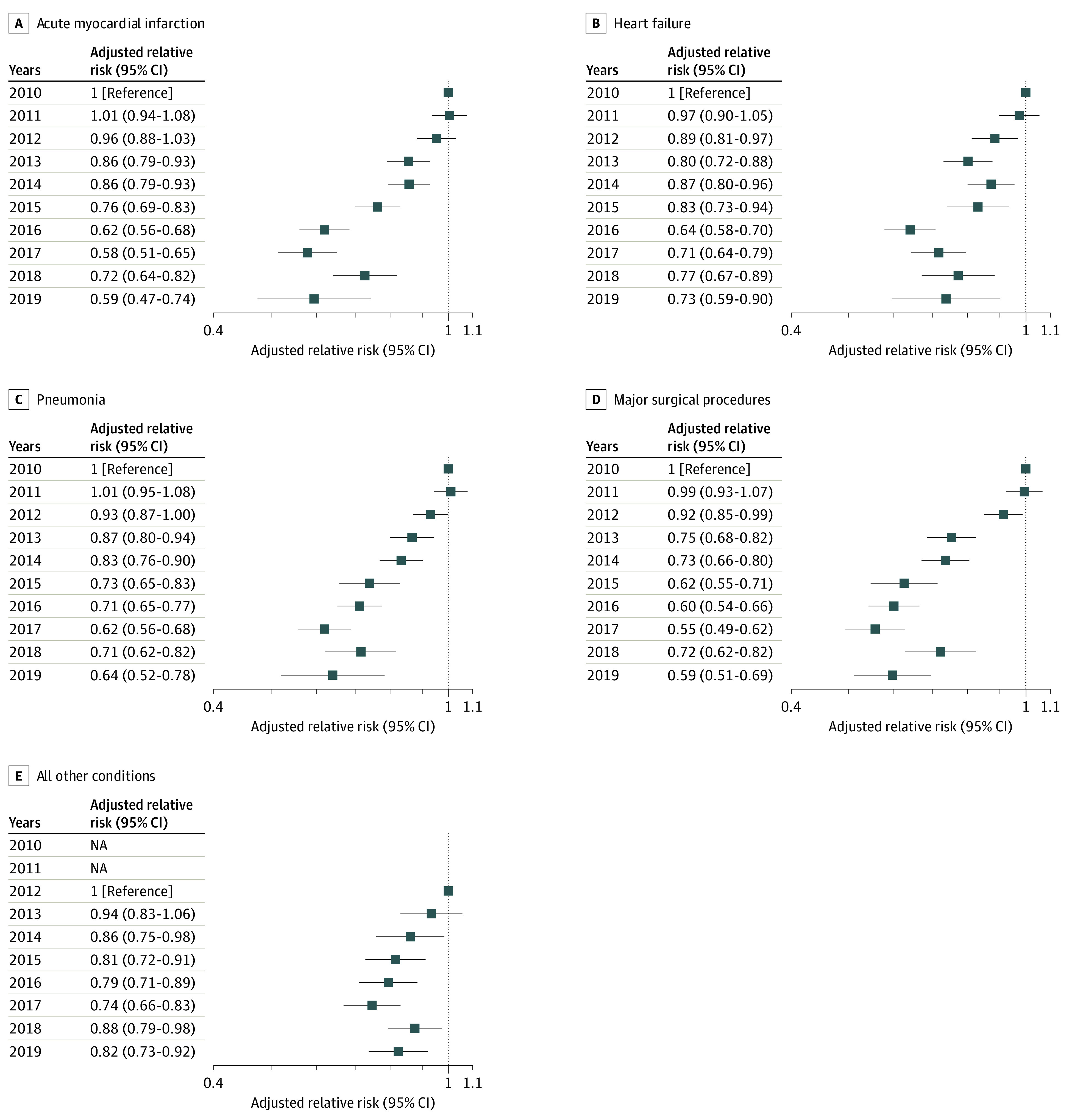
The relative risks were adjusted for age, sex, and race and ethnicity; Medicare Patient Safety Monitoring System–specific comorbidities; and hospital characteristics. For acute myocardial infarction, heart failure, pneumonia, and major surgical procedures, the reference year was 2010; for all other conditions, the reference year was 2012 and data from 2010 and 2011 were not available (NA). The x-axis is on a log scale.
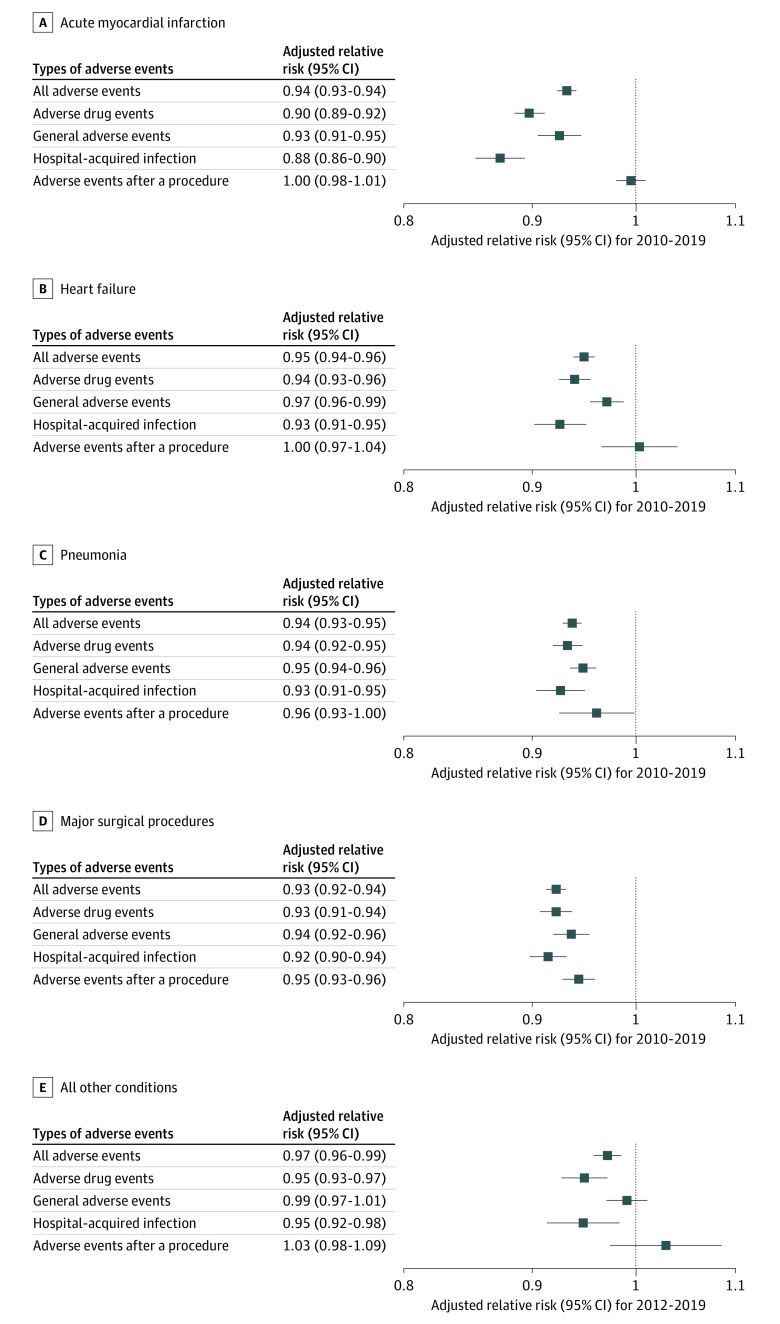
After adjustment for patient and hospital characteristics, the annual change represented by RR in all adverse events per 1000 discharges was 0.94 (95% CI, 0.93-0.94) for the acute myocardial infarction group, 0.95 (95% CI, 0.94-0.96) for the heart failure group, 0.94 (95% CI, 0.93-0.95) for the pneumonia group, 0.93 (95% CI, 0.92-0.94) for the major surgical procedures group, and 0.97 (95% CI, 0.96-0.99) for the all other conditions group (Figure 4 and eTable 16 in the Supplement).
Figure 4. Risk-Adjusted Annual Trends by the 5 Condition Groups and the 4 Medicare Patient Safety Monitoring System Adverse Event Domains.
The relative risks were adjusted for age, sex, and race and ethnicity; Medicare Patient Safety Monitoring System–specific comorbidities; and hospital characteristics. Additional information appears in eTable 16 in the Supplement.
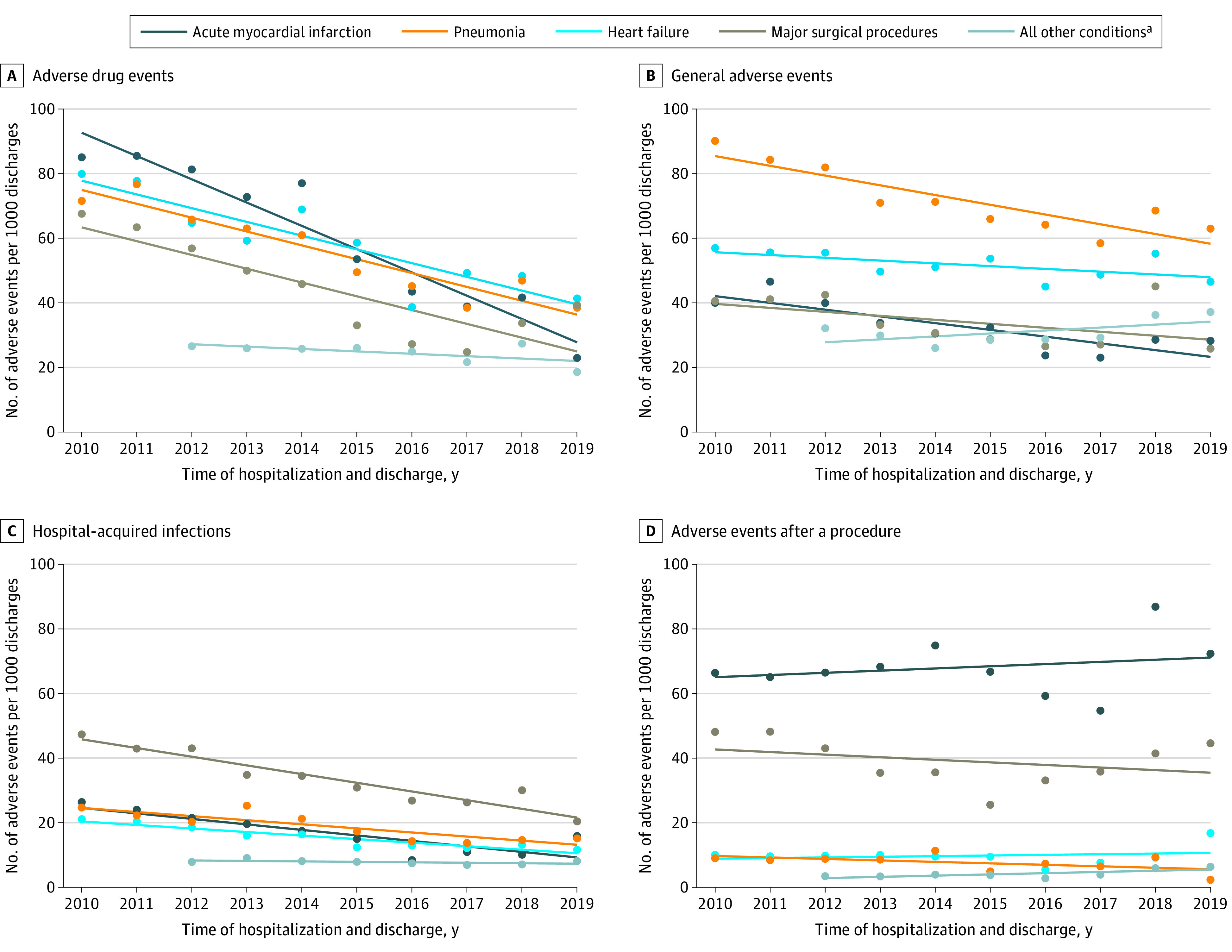
The annual trends for the occurrence rate of adverse events for which patients were at risk and the proportion of patients with 1 or more adverse events appear in eFigures 1A and 1B in the Supplement. The adjusted occurrence of adverse events and the percentage of patients experiencing 1 or more adverse events per hospitalization for each year appear in eFigures 2A and 2B in the Supplement. In addition to showing the data for all adverse events, Figure 4 shows the findings for the 4 adverse event domains (adverse drug events, hospital-acquired infections, adverse events after a procedure, and general adverse events).
In addition to the declines seen for all adverse events, declines also occurred in the adverse drug event and hospital-acquired infection domains for all 5 condition groups (acute myocardial infarction, heart failure, pneumonia, major surgical procedures, and all other conditions). Declines also occurred for the general adverse events domain for 4 of the 5 condition groups (ie, the 4 condition-specific groups but not the all other conditions group). For adverse events after a procedure, declines were seen in the major surgical procedures group but not in the other 4 condition groups. There were no statistically significant increases in any of the 4 adverse event domains for any of the 4 condition-specific groups or the all other conditions group for the full study period. These findings were similar in the analyses limited to patients aged 65 years or older (eFigure 3 in the Supplement) and when the adjustments included Elixhauser-specific comorbidities (eFigure 4 in the Supplement).
The 2012 observed adverse event rates were much lower in the all other conditions group than in the 4 condition-specific groups (Figure 1 and eTable 15 in Supplement and Figure 2). The patients in the all other conditions group were at risk for fewer adverse events (the summary and detailed rates of all 21 MPSMS adverse event measures for the 5 condition groups for 2010-2019 and 2012-2019 appear in eTables 10-14 in the Supplement).
The adjusted data for the periods of 2010 to 2014 and 2014 to 2019 appear in eFigure 5 in the Supplement. Fewer statistically significant reductions were seen in the 5 condition groups and 4 adverse event domains. There was 1 statistically significant increase (in the general adverse events domain for the all other conditions group). The observed change in the number of adverse events by age, race and ethnicity, and sex appears in eFigure 6 in the Supplement. These data indicate the adverse event rate and differences by age group; there were larger improvements in the observed data for older patients than for younger patients and there were few apparent differences by race and ethnicity or sex. The observed and risk-adjusted data for the change in adverse events by US region (Midwest, Northeast, South, and West) appear in eFigures 7 and 8 in the Supplement; few differences are evident.
Differences in Mortality and Length of Stay Among Patients With vs Without Adverse Events
For the 4 condition-specific groups (acute myocardial infarction, heart failure, pneumonia, and major surgical procedures), overall observed in-hospital mortality declined from 4.6% in 2010 to 2.7% in 2019 (absolute difference, 1.9% [95% CI, 1.3%-2.4%). The overall observed in-hospital mortality increased for the all other conditions group from 1.2% in 2012 to 2.2% in 2016 and was 1.7% in 2019 (absolute difference, 0.6% [95% CI, 0.2%-1.0%]; Table and eTables 10-14 in the Supplement). There were observed differences for in-hospital mortality and hospital length of stay between patients experiencing vs not experiencing each of the 21 MPSMS adverse events and these differences persisted over the study period (eTables 17-18 in the Supplement).
From 2010 to 2019, hospital length of stay in the 4 condition-specific groups declined slightly, whereas length of stay increased slightly in the all other conditions group. Patients with adverse events had substantially higher mortality rates and longer lengths of stay. The in-hospital mortality was 10.2% for patients with 1 or more MPSMS adverse events in the combined group for the 4 conditions vs 6.7% for the all other conditions group, whereas patients without adverse events had mortality rates of 3.4% and 1.4%, respectively (eTable 17 in the Supplement).
For patients with at least 1 adverse event in the combined group for the 4 conditions, the hospital length of stay was 9.9 days (SD, 9.1 days) vs 4.1 days (SD, 3.6 days) for patients without any adverse events. For patients with at least 1 adverse event in the all other conditions group, the hospital length of stay was 9.1 days (SD, 9.6 days) vs 3.6 days (SD, 3.5 days) for patients without adverse events (eTable 18 in the Supplement).
Discussion
There was a significant decrease in the adjusted adverse event rates abstracted from medical records in the US between 2010 and 2019 for patients admitted to the hospital for acute myocardial infarction, heart failure, pneumonia, and major surgical procedures and a significant decrease between 2012 and 2019 for patients admitted to the hospital for all other conditions. The adjusted annual reductions in adverse events varied year to year and by patient group.
To our knowledge, compared with other studies,5,21 the current study represents the largest and most comprehensive evaluation to date of adverse events among hospitalized patients in the US, which included a large majority of US acute care hospitals and all US states. Although it cannot be certain that the trends represent improvement in patient safety, some factors support that this may be the case. For example, the yearly estimates are based on a national patient safety measurement system (the MPSMS) that was specifically designed to monitor adverse events. The basis for the estimates is a highly structured and reproducible medical record abstraction process conducted at a central location by specialists in this work, and the definitions and measurement protocols were consistent over the study period.
The study period also saw major patient safety improvement efforts (eg, the Partnership for Patients program22; CMS programs that focus on acute myocardial infarction, heart failure, and pneumonia; and the Surgical Care Improvement Project23 that integrates safety and payment policy and publicly reported outcomes24). New technologies to support safety also were implemented25,26 along with new initiatives to increase person and family engagement in safety efforts.27
Even though these efforts may have contributed to the improvements measured in this study, other factors such as spread of safer processes of care may also have played a role. Advances in care not directly attributable to patient safety efforts also may have contributed to the improvements (eg, the widespread adoption of minimally invasive surgical techniques).
There were variations in the reductions occurring in the 4 different MPSMS adverse event domains (adverse drug events, hospital-acquired infections, adverse events after a procedure, and general adverse events) and in the 21 specific adverse event measures. For example, reductions in adverse drug events were generally larger than the combined general adverse events of hospital-acquired pressure ulcers and inpatient falls. This difference may be due to improvements in information technology that promote patient safety,28 and due to specific efforts to lower the rates of adverse drugs events.29 The only increase in adverse event rates was in the general adverse events domain for the all other conditions patient group in 2014 to 2019, and this finding may indicate a special need for new initiatives related to prevention of pressure ulcers and inpatient falls.
The statistically significant reduction of adverse events from 2010 to 2019 for the major surgical procedures group in all 4 adverse event domains contrasts with the results from the previous MPSMS longitudinal study,7 which covered the period from 2005 to 2011 and included only Medicare fee-for-service patients aged 65 years or older. These new findings were also observed when the data were limited to patients aged 65 years or older. Similarly, even though there were no statistically significant reductions in the previous study for patients with pneumonia or undergoing major surgical procedures, significant reductions were observed in the current study. The 2010-2019 results for the acute myocardial infarction and heart failure groups showed additional statistically significant improvements beyond those seen previously.
The lower overall rate of decline in adverse event rates in the all other conditions group, compared with the acute myocardial infarction, heart failure, pneumonia, and major surgical procedures groups, might be due to the quality improvement efforts targeted at the latter 4 conditions,30,31 whereas similar interventions did not occur for most of the conditions represented in the all other conditions group. Furthermore, the baseline adverse event rates were much lower in the all other conditions group, potentially leaving less opportunity to achieve improvement. The patients in the all other conditions group were also younger than the patients in the 4 condition-specific groups, and similarly, younger patients in those 4 groups also had lower adverse event rates and showed smaller reductions in their observed adverse events.
Limitations
This study has several limitations. First, it included a limited set of hospital-based adverse events and other adverse events may be important to evaluate.
Second, because each annual sample includes a fixed number of records per hospital, patients from smaller hospitals were overrepresented. The adjustment methods include hospital size and many other hospital and patient characteristics, but may still be distorted by this overrepresentation and may not fully account for all differences in hospitals and patients over time.
Third, there may have been an effect of medical record documentation changes on some of these trends. Fourth, the analyses did not account for variability in the prevalence of patients at risk for different adverse events.
Conclusions
In the US between 2010 and 2019, there was a significant decrease in the rates of adverse events abstracted from medical records for patients admitted for acute myocardial infarction, heart failure, pneumonia, and major surgical procedures and there was a significant decrease in the adjusted rates of adverse events between 2012 and 2019 for all other conditions. Further research is needed to understand the extent to which these trends represent a change in patient safety.
eText. Details Regarding the MPSMS Sample during the Period of Study
eTable 1. MPSMS Historical Sampling and Exclusions
eTable 2. Percent of Overall Sample Represented by Each Condition, 2010-2019
eTable 3. Summary National Inpatient Mortality and Length-of-Stay Data from HCUPnet (https://hcupnet.ahrq.gov/#setup), 2010-2017
eTable 4. Patient Characteristics, Hospital Characteristics, and Outcomes for Combined AMI, HF, Pneumonia, and Surgical Care (Four Conditions) Patients, 2010-2019
eTable 5. Patient Characteristics, Hospital Characteristics, and Outcomes for All-Other Conditions Patients, 2012-2019
eTable 6. Patient Characteristics, Hospital Characteristics, and Outcomes for AMI patients, 2010-2019
eTable 7. Patient Characteristics, Hospital Characteristics, and Outcomes for HF patients, 2010-2019
eTable 8. Patient Characteristics, Hospital Characteristics, and Outcomes for Pneumonia patients, 2010-2019
eTable 9. Patient Characteristics, Hospital Characteristics, and Outcomes for SCIP patients, 2010-2019
eTable 10. MPSMS Adverse Event and Exposure Data for AMI patients, 2010-2019
eTable 11. MPSMS Adverse Event and Exposure Data for HF patients, 2010-2019
eTable 12. MPSMS Adverse Event and Exposure Data for PNE patients, 2010-2019
eTable 13. MPSMS Adverse Event and Exposure Data for SCIP patients, 2010-2019
eTable 14. MPSMS Adverse Event and Exposure Data for All-Other Conditions patients, 2012-2019
eTable 15. Table Containing the Data in Figure 1
eTable 16. Table Containing the Data in Figure 4
eTable 17. Observed In-hospital Mortality Data for Patients Experiencing and not Experiencing each of the 21 MPSMS Adverse Events
eTable 18. Observed Length of Stay (LOS) Data for Patients Experiencing and not Experiencing each of the 21 MPSMS Adverse Events
eFigure 1A. Risk-Adjusted Annual Trend in the Rate of Occurrence of Adverse Events for Which Patients Were at Risk for Five Patient Groups for All Adverse Events and for Four Adverse Event Domains, 2010-2019 (2012-2019 for All-other Conditions)
eFigure 1B. Risk-Adjusted Annual Trend in the Proportion of Patients with One or More Adverse Events for Five Patient Groups for All Adverse Events and for Four Adverse Event Domains, 2010-2019 (2012-2019 for All-other Conditions)
eFigure 2A. Adjusted Annual Changes in Adverse Events by Year and Four Conditions (Referred to 2010)
eFigure 2B. Adjusted Annual Changes in Adverse Events by Year (Referred to 2012, All-Other Conditions)
eFigure 3. Patients 65 years old and over only: Risk-Adjusted Annual Change in Adverse Events by Clinical Domains and Overall, 2010-2019 (2012-2019 for “All-Other Conditions”)
eFigure 4. Risk-Adjusted Annual Change in Adverse Events by Clinical Domains and Overall (including Elixhauser comorbidities (2012-2019 for “All-Other Conditions”)
eFigure 5. Risk-Adjusted Annual Change by Clinical Domains and Overall (2010-2014 and 2014 to 2019)
eFigure 6. Observed Change in Number of Adverse Events per 1,000 Discharges by Age, Race, and Sex (gender), for the Five Patient Demographical Groups
eFigure 7. Observed Change in Number of Adverse Events per 1,000 Discharges by Region of the United States (as defined by US Census Bureau) and by Patient Groups
eFigure 8. Risk-Adjusted Annual Change in Adverse Events by Regions of the United States and Condition
References
- 1.Institute of Medicine . To Err Is Human: Building a Safer Health System. Published November 1999. Accessed June 17, 2022. https://nap.nationalacademies.org/resource/9728/To-Err-is-Human-1999--report-brief.pdf
- 2.Agency for Healthcare Research and Quality . Making Healthcare Safer III. Published April 2020. Accessed March 1, 2021. https://www.ahrq.gov/research/findings/making-healthcare-safer/mhs3/index.html
- 3.Shojania KG, Thomas EJ. Trends in adverse events over time: why are we not improving? BMJ Qual Saf. 2013;22(4):273-277. doi: 10.1136/bmjqs-2013-001935 [DOI] [PubMed] [Google Scholar]
- 4.Bates DW, Singh H. Two decades since To Err Is Human: an assessment of progress and emerging priorities in patient safety. Health Aff (Millwood). 2018;37(11):1736-1743. doi: 10.1377/hlthaff.2018.0738 [DOI] [PubMed] [Google Scholar]
- 5.Landrigan CP, Parry GJ, Bones CB, Hackbarth AD, Goldmann DA, Sharek PJ. Temporal trends in rates of patient harm resulting from medical care. N Engl J Med. 2010;363(22):2124-2134. Published correction appears in N Engl J Med. 2010;363(26):2573. doi: 10.1056/NEJMsa1004404 [DOI] [PubMed] [Google Scholar]
- 6.Baines RJ, Langelaan M, de Bruijne MC, et al. Changes in adverse event rates in hospitals over time: a longitudinal retrospective patient record review study. BMJ Qual Saf. 2013;22(4):290-298. doi: 10.1136/bmjqs-2012-001126 [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Eldridge N, Metersky ML, et al. National trends in patient safety for four common conditions, 2005-2011. N Engl J Med. 2014;370(4):341-351. doi: 10.1056/NEJMsa1300991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agency for Healthcare Research and Quality . AHRQ National Scorecard on Hospital-Acquired Conditions. Published July 2020. Accessed April 22,2022. https://www.ahrq.gov/hai/pfp/index.html
- 9.Hunt DR, Verzier N, Abend SL, et al. Fundamentals of Medicare patient safety surveillance: intent, relevance, and transparency. In: Henriksen K, Battles JB, Marks ES, et al. , eds. Advances in Patient Safety: From Research to Implementation (Volume 2: Concepts and Methodology). Agency for Healthcare Research and Quality; 2005. [PubMed] [Google Scholar]
- 10.Classen DC, Munier W, Verzier N, et al. Measuring patient safety: the Medicare patient safety monitoring system (past, present, and future). J Patient Saf. 2021;17(3):e234-e240. doi: 10.1097/PTS.0000000000000322 [DOI] [PubMed] [Google Scholar]
- 11.Bratzler DW, Hunt DR. The Surgical Infection Prevention and Surgical Care Improvement Projects: national initiatives to improve outcomes for patients having surgery. Clin Infect Dis. 2006;43(3):322-330. doi: 10.1086/505220 [DOI] [PubMed] [Google Scholar]
- 12.Centers for Medicare & Medicaid Services; Joint Commission . Specifications manual for national hospital inpatient quality measures: discharges 10-01-15 (4Q15) through 06-30-16 (2Q16): version 5.0b. Accessed December 4, 2019. https://www.qualitynet.org/files/5d0d3a18764be766b0103e9f?filename=HIQR_SpecMan_5_0b_Oct2015_ZIP.zip
- 13.Centers for Medicare & Medicaid Services; Joint Commission . Specifications manual for national hospital inpatient quality measures version 4.4b. Accessed December 4, 2019. https://www.qualitynet.org/files/5d0d3763764be766b01014aa?filename=HIQR_SpecsMan_v4_4a_Jan2015.zip
- 14.Agency for Healthcare Research and Quality . HCUPnet: Healthcare Cost and Utilization Project: free health care statistics. Accessed June 16, 2020. https://hcupnet.ahrq.gov/#setup
- 15.Agency for Healthcare Research and Quality . Clinical Classifications Software (CCS) for ICD-9-CM. Accessed April 22, 2022. https://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp
- 16.American Hospital Association . AHA data and insights: hospital quick search. Accessed April 14, 2021. https://www.ahadata.com/
- 17.Sterne JAC, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702-706. doi: 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 19.Centers for Medicare & Medicaid Services . CMS Innovation Center: Partnership for Patients initiative. Accessed June 15, 2021. https://innovation.cms.gov/innovation-models/partnership-for-patients
- 20.Centers for Medicare & Medicaid Services . Partnership for Patients and the Hospital Improvement Innovation Networks: continuing forward momentum on reducing patient harm. Accessed June 15, 2021. https://www.cms.gov/newsroom/fact-sheets/partnership-patients-and-hospital-improvement-innovation-networks-continuing-forward-momentum
- 21.US Department of Health and Human Services, Office of Inspector General . Adverse events in hospitals: national incidence among Medicare beneficiaries. Published November 2010. Accessed June 1, 2020. https://oig.hhs.gov/oei/reports/oei-06-09-00090.pdf
- 22.Hackbarth AD, Munier WB, Eldridge N, et al. An overview of measurement activities in the Partnership for Patients. J Patient Saf. 2014;10(3):125-132. doi: 10.1097/PTS.0000000000000071 [DOI] [PubMed] [Google Scholar]
- 23.Centers for Medicare & Medicaid Services . Hospital Value-Based Purchasing Program. Accessed March 1, 2021. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/HVBP/Hospital-Value-Based-Purchasing
- 24.Centers for Medicare & Medicaid Services . Find and compare nursing homes, hospitals, and other providers near you. Accessed April 22, 2022. https://www.medicare.gov/care-compare
- 25.Aggarwal R, Mytton OT, Greaves F, Vincent C. Technology as applied to patient safety: an overview. Qual Saf Health Care. 2010;19(suppl 2):i3-i8. doi: 10.1136/qshc.2010.040501 [DOI] [PubMed] [Google Scholar]
- 26.Furukawa MF, Eldridge N, Wang Y, Metersky M. Electronic health record adoption and rates of in-hospital adverse events. J Patient Saf. 2020;16(2):137-142. doi: 10.1097/PTS.0000000000000257 [DOI] [PubMed] [Google Scholar]
- 27.Institute for Healthcare Improvement . Safety is personal: partnering with patients and families for the safest care. Accessed June 17, 2020. http://www.ihi.org/resources/Pages/Publications/Safety-Is-Personal-Partnering-with-Patients-and-Families-for-the-Safest-Care.aspx
- 28.Zlabek JA, Wickus JW, Mathiason MA. Early cost and safety benefits of an inpatient electronic health record. J Am Med Inform Assoc. 2011;18(2):169-172. doi: 10.1136/jamia.2010.007229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Medicare & Medicaid Innovation . Project evaluation activity in support of Partnership for Patients: interim evaluation report. Accessed April 22, 2022. https://downloads.cms.gov/files/cmmi/pfp-interimevalrpt.pdf
- 30.Centers for Medicare & Medicaid Services . Hospital Inpatient Quality Reporting Program. Accessed March 1, 2021. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/HospitalRHQDAPU
- 31.Centers for Medicare & Medicaid Services QualityNet . Hospital-Acquired Condition Reduction Program (HACRP). Accessed June 17, 2020. https://www.qualitynet.org/inpatient/hac
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eText. Details Regarding the MPSMS Sample during the Period of Study
eTable 1. MPSMS Historical Sampling and Exclusions
eTable 2. Percent of Overall Sample Represented by Each Condition, 2010-2019
eTable 3. Summary National Inpatient Mortality and Length-of-Stay Data from HCUPnet (https://hcupnet.ahrq.gov/#setup), 2010-2017
eTable 4. Patient Characteristics, Hospital Characteristics, and Outcomes for Combined AMI, HF, Pneumonia, and Surgical Care (Four Conditions) Patients, 2010-2019
eTable 5. Patient Characteristics, Hospital Characteristics, and Outcomes for All-Other Conditions Patients, 2012-2019
eTable 6. Patient Characteristics, Hospital Characteristics, and Outcomes for AMI patients, 2010-2019
eTable 7. Patient Characteristics, Hospital Characteristics, and Outcomes for HF patients, 2010-2019
eTable 8. Patient Characteristics, Hospital Characteristics, and Outcomes for Pneumonia patients, 2010-2019
eTable 9. Patient Characteristics, Hospital Characteristics, and Outcomes for SCIP patients, 2010-2019
eTable 10. MPSMS Adverse Event and Exposure Data for AMI patients, 2010-2019
eTable 11. MPSMS Adverse Event and Exposure Data for HF patients, 2010-2019
eTable 12. MPSMS Adverse Event and Exposure Data for PNE patients, 2010-2019
eTable 13. MPSMS Adverse Event and Exposure Data for SCIP patients, 2010-2019
eTable 14. MPSMS Adverse Event and Exposure Data for All-Other Conditions patients, 2012-2019
eTable 15. Table Containing the Data in Figure 1
eTable 16. Table Containing the Data in Figure 4
eTable 17. Observed In-hospital Mortality Data for Patients Experiencing and not Experiencing each of the 21 MPSMS Adverse Events
eTable 18. Observed Length of Stay (LOS) Data for Patients Experiencing and not Experiencing each of the 21 MPSMS Adverse Events
eFigure 1A. Risk-Adjusted Annual Trend in the Rate of Occurrence of Adverse Events for Which Patients Were at Risk for Five Patient Groups for All Adverse Events and for Four Adverse Event Domains, 2010-2019 (2012-2019 for All-other Conditions)
eFigure 1B. Risk-Adjusted Annual Trend in the Proportion of Patients with One or More Adverse Events for Five Patient Groups for All Adverse Events and for Four Adverse Event Domains, 2010-2019 (2012-2019 for All-other Conditions)
eFigure 2A. Adjusted Annual Changes in Adverse Events by Year and Four Conditions (Referred to 2010)
eFigure 2B. Adjusted Annual Changes in Adverse Events by Year (Referred to 2012, All-Other Conditions)
eFigure 3. Patients 65 years old and over only: Risk-Adjusted Annual Change in Adverse Events by Clinical Domains and Overall, 2010-2019 (2012-2019 for “All-Other Conditions”)
eFigure 4. Risk-Adjusted Annual Change in Adverse Events by Clinical Domains and Overall (including Elixhauser comorbidities (2012-2019 for “All-Other Conditions”)
eFigure 5. Risk-Adjusted Annual Change by Clinical Domains and Overall (2010-2014 and 2014 to 2019)
eFigure 6. Observed Change in Number of Adverse Events per 1,000 Discharges by Age, Race, and Sex (gender), for the Five Patient Demographical Groups
eFigure 7. Observed Change in Number of Adverse Events per 1,000 Discharges by Region of the United States (as defined by US Census Bureau) and by Patient Groups
eFigure 8. Risk-Adjusted Annual Change in Adverse Events by Regions of the United States and Condition