Heart failure (HF) is a well-recognized global public health problem with a diverse natural history and negative quality-of-life effects.1 The definition of HF has also changed, covering an increasingly broad clinical condition and phenotypic spectrum of patients.2 Previous projections suggested a substantial increase in HF prevalence by the year 2030 for patients of all ages3 and increasing trends of predicted risk for HF development.4 However, it is unknown to what extent the evolution of definition criteria, availability of effective prevention strategies, improved survival rates, aging of populations, and changes in epidemiology of cardiovascular risk factors and coronary heart disease (CHD) over the last 20 years have affected HF prevalence in the United States. We examined secular trends in a serial cross-sectional study cohort of the US National Health and Nutrition Examination Survey (NHANES).5
We considered adults in NHANES between 1999 and 2018 with available information on HF diagnosis and the relevant medical conditions of CHD and myocardial infarction at each 2-year survey cycle. The information of interest was self-reported according to predefined questionnaires.3 We gathered information on age and sex and investigated the secular change in HF prevalence by calculating age- and sex-adjusted prevalence rates of HF for each 2-year survey cycle. We calculated the prevalence of HF for each NHANES cycle using survey-weighted methods.6 Linear and restricted cubic spline meta-regression models were used to examine the secular trends over time (using survey cycles) while controlling for CHD prevalence as the main cause of HF in adults. Myocardial infarction was not considered in the models because of multicollinearity with the CHD variable. All analyses were conducted with R, version 4.0.2 (R Foundation, Vienna, Austria).
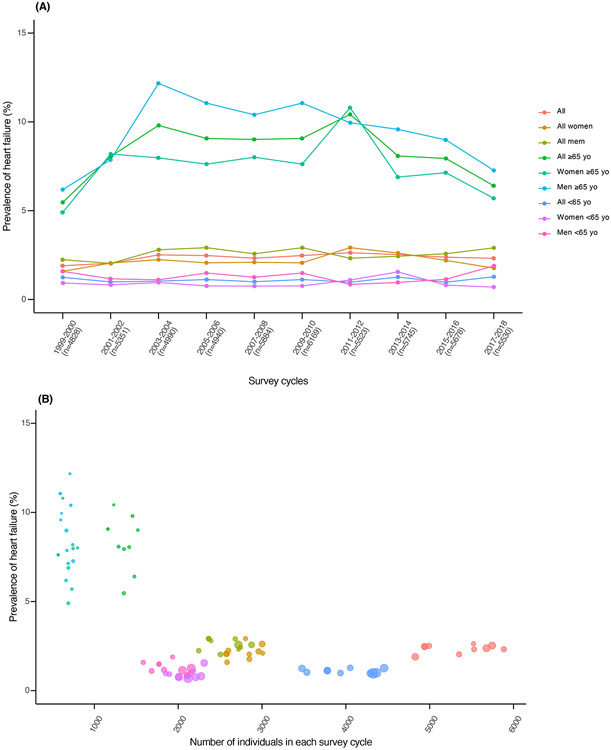
An unweighted total of 53,409 subjects (27,802 women, 25,607 men) over 10 survey cycles with available information on previously medically diagnosed HF were included in our analysis. Overall, 1,834 NHANES participants across all survey cycles reported HF (832 women, 1,002 men) Table 1. displays the range of prevalence estimates in subgroups. The HF prevalence remained relatively stable over the 20-year period and ranged from 1.9% to 2.6%, 1.6% to 2.9%, and 2.0% to 2.9% for all subjects, women, and men, respectively, without evident secular trend (Table 1, Figure 1). In ≥65-year-old subjects, the HF prevalence was considerably higher, with a wider range of estimates of 5.5% to 10.4%, 4.7% to 10.8%, and 6.2% to 12.2% for all subjects, women, and men, respectively. The HF prevalence increased sharply during the survey cycles from 1999 to 2004 in all subjects (5.5% to 9.8%), women (4.9% to 8.0%), and men (6.2% to 12.2%), with p <0.05 for all changes in the slope (Table 1). After 2004, the same subgroups followed a similar pattern without pronounced variation in prevalence estimates, but with a trend toward lower values. By 2017 to 2018, the prevalence decreased to 6.4%, 5.7%, and 7.3%, respectively (Figure 1). The meta-regression model indicated stable HF prevalence over 20 years for younger subjects (<65-years-old). The sample sizes in each cycle were different, and the precision of HF prevalence estimates was not homogeneously distributed along the entire range of subgroup sizes (Figure 1). The higher prevalence estimate variability per cycle pertained to the older subjects for whom small-sized subgroups and less precise estimates were available.
Table 1.
Range of prevalence estimates of heart failure among sex-/age-subgroups across all survey cycles 1999–2018 and meta-regression estimates for the time of cycle-survey
| Prevalence of heart failure (%) | Meta-regression | |||||||
|---|---|---|---|---|---|---|---|---|
| Linear model | Restricted cubic splines | |||||||
| Number of individuals / Number of cases |
Range (min – max) |
Median (IQR) | Estimate (95%CI) | P value | Splines | Estimate (95%CI) | P value | |
| All individuals | 53409 / 1834 | 1.9 – 2.6 | 2.4 (2.3 – 2.5) | 0.04 (−0.02 – 0.10) | 0.187 | 1 | 0.13 (−0.04 – 0.31) | 0.129 |
| 2 | −0.10 (−1.58 – 1.39) | 0.898 | ||||||
| 3 | −0.03 (−4.06 – 4.00) | 0.989 | ||||||
| All women | 27802 / 832 | 1.6 – 2.9 | 2.1 (2.1 – 2.2) | 0.02 (−0.05 – 0.10) | 0.548 | 1 | 0.13 (−0.12 – 0.38) | 0.305 |
| 2 | 0.31 (−1.49 – 2.10) | 0.736 | ||||||
| 3 | −1.27 (−6.01 – 3.48) | 0.602 | ||||||
| All men | 25607 / 1002 | 2.0 – 2.9 | 2.6 (2.4 – 2.9) | 0.03 (−0.04 – 0.09) | 0.395 | 1 | 0.11 (−0.12 – 0.34) | 0.330 |
| 2 | −0.15 (−2.16 – 1.87) | 0.886 | ||||||
| 3 | 0.20 (−5.28 – 5.68) | 0.942 | ||||||
| All ≥65 yo | 13414 / 1219 | 5.5 – 10.4 | 8.5 (8.0 – 9.1) | −0.06 (−0.33 – 0.22) | 0.675 | 1 | 0.74 (0.02 – 1.45) | 0.044 |
| 2 | −1.74 (−7.90 – 4.41) | 0.579 | ||||||
| 3 | 2.80 (−13.8 – 19.4) | 0.741 | ||||||
| Women ≥65 yo | 6844 / 569 | 4.7 – 10.8 | 7.6 (7.0 – 8.0) | 0.03 (−0.31 – 0.38) | 0.850 | 1 | 0.88 (0.06 – 1.71) | 0.035 |
| 2 | −2.28 (−9.45 – 4.89) | 0.532 | ||||||
| 3 | 4.09 (−15.2 – 23.4) | 0.678 | ||||||
| Men ≥65 yo | 6570 / 650 | 6.2 – 12.2 | 9.8 (8.1 – 10.9) | −0.11 (−0.51 – 0.30) | 0.599 | 1 | 1.13 (0.01 – 2.25) | 0.048 |
| 2 | −1.91 (−11.1 – 7.29) | 0.684 | ||||||
| 3 | 2.47 (−22.2 – 27.1) | 0.844 | ||||||
| All <65 yo | 39995 / 615 | 1.0 – 1.3 | 1.1 (1.0 – 1.2) | 0.01 (−0.04 – 0.06) | 0.666 | 1 | −0.02 (−0.16 – 0.11) | 0.723 |
| 2 | 0.48 (−0.64 – 1.61) | 0.402 | ||||||
| 3 | −1.31 (−4.42 – 1.80) | 0.408 | ||||||
| Women <65 yo | 20958 / 263 | 0.7 – 1.6 | 0.8 (0.8 – 0.9) | 0.00 (−0.07 – 0.06) | 0.973 | 1 | −0.02 (−0.24 – 0.20) | 0.875 |
| 2 | 0.81 (−0.68 – 2.31) | 0.287 | ||||||
| 3 | −2.34 (−6.31 – 1.64) | 0.249 | ||||||
| Men <65 yo | 19037 / 352 | 0.8 – 1.9 | 1.2 (1.1 – 1.5) | 0.04 (−0.04 – 0.12) | 0.377 | 1 | 0.01 (−0.17 – 0.19) | 0.892 |
| 2 | 0.01 (−1.83 – 1.84) | 0.996 | ||||||
| 3 | 0.05 (−5.02 – 5.11) | 0.986 | ||||||
Figure 1.
Heart failure prevalence in NHANES over a 20-year period. (A) Changes in prevalence of heart failure stratified by sex and age in NHANES cohort from 1999 to 2018. In parentheses is indicated the total number of subjects in each survey cycle. (B) Distribution of heart failure prevalence and number of subjects included in each survey cycle; the area of each cycle is proportional to the precision (inverse of the variance) of the prevalence estimate in each cycle and subgroup. The colors correspond to the groups indicated in (A).
Overall, despite population aging and increasing broadness of HF definition over time, we found a relatively stable HF prevalence in NHANES over the 20-year period (1999 to 2018), with a change of <5% in prevalence across all cycles and subgroups. A sharp increase in HF prevalence was observed in older subjects in between 1999-2004, which was diminished in the subsequent years. This analysis is limited to self-reported medical conditions, which prevented us from further distinguishing HF phenotypes and separately examining potential differential trends in the prevalence of those phenotypes. Although our findings do not validate previous projections from 2013,3 they are in concordance with age- and sex-standardized estimates of HF prevalence in other countries with increasing absolute HF burden, such as the United Kingdom.7 The advantage of the prevalence estimates presented here includes the representativeness of the US NHANES. The present analysis calls into question whether the observed relatively stable HF prevalence pattern is the result of the evolved HF definition, population aging, and improved control of cardiovascular risk factors evident at the population level in the United States.8,9
Disclosures
Dr. Bhatt discloses the following relationships—advisory board: Bayer, Boehringer Ingelheim, Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Janssen, Level Ex, Medscape Cardiology, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, Stasys; board of directors: Boston, Virginia Research Institute, DRS.LINQ (stock options), Society of Cardiovascular Patient Care, TobeSoft; chair: Inaugural Chair, American Heart Association Quality Oversight Committee; data monitoring committees: Acesion Pharma, Assistance Publique-Hôpitaux de Paris, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (Chair, PEITHO trial), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, Performance 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo; for the ABILITY-DM trial, funded by Concept Medical), Novartis, Population Health Research Institute; Rutgers University (for the NIH-funded MINT Trial); honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol-Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; REDUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and United States national coleader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CaRT Research and Publications Committee (Chair); research funding: Abbott, Afimmune, Aker Biomarine, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Bristol-Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Lilly, Medtronic, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Owkin, Pfizer, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, 89Bio; royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); site co-investigator: Abbott, Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Philips, Svelte; trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, Takeda. Dr. Patel reports research funding from the National Institutes of Health (project No. 1R01ES032470, R01AI127250). Dr. Siontis has nothing relevant to disclose.
Data are publicly available and code can be provided by the authors upon request. All authors had access to the data and a role in writing the manuscript. The National Center for Health Statistics Research Ethics Review Board approved NHANES.
References
- 1.Nayak A, Hicks AJ, Morris AA. Understanding the complexity of heart failure risk and treatment in black patients. Circ Heart Fail 2020;13(8):e007264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy SP, Ibrahim NE, Januzzi JL Jr.. Heart failure with reduced ejection fraction: a review. JAMA 2020;324(5):488–504. [DOI] [PubMed] [Google Scholar]
- 3.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Piña IL, Trogdon JG, American Heart Association Advocacy Coordinating Committee, Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention, Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013;6(3):606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glynn PA, Ning H, Bavishi A, Freaney PM, Shah S, Yancy CW, Lloyd-Jones DM, Khan SS. Heart failure risk distribution and trends in the United States population, NHANES 1999–2016. Am J Med 2021;134(3):e153–e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. National health and nutrition examination survey. Available at: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm. Accessed February 9, 2022. [Google Scholar]
- 6.Lumley T Analysis of Complex Survey Samples version 4.1-1. J Stat Soft 2021;9(8). [Google Scholar]
- 7.Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, Allison M, Hemingway H, Cleland JG, McMurray JJV, Rahimi K. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet 2018;391(10120):572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He J, Zhu Z, Bundy JD, Dorans KS, Chen J, Hamm LL. Trends in cardiovascular risk factors in US adults by race and ethnicity and socioeconomic status, 1999–2018. JAMA 2021;326(13):1286–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horwich TB, Fonarow GC. Prevention of heart failure. JAMA Cardiol 2017;2(1):116. 10.1016/j.amjcard.2022.02.037 [DOI] [PubMed] [Google Scholar]