Abstract
Background: The advantage of prophylactic cavotricuspid isthmus (CTI) ablation for AF patients without documented atrial flutter is still unclear. The present study aimed to evaluate the role of prophylactic CTI ablation in this population. Methods: A systematic review and meta-analysis study was conducted. The overall effects estimation was conducted using random effects models. The pooled effects were presented as the risk difference and standardised mean difference for dichotomous and continuous outcomes, respectively. Results: A total of 1,476 patients from four studies were included. The risk of atrial tachyarrhythmias following a successful catheter ablation procedure was greater in the pulmonary vein isolation + CTI ablation group than pulmonary vein isolation alone group (34.8% versus 28.2%; risk difference 0.08; 95% CI [0.00–0.17]; p=0.04). Prophylactic CTI ablation was associated with a higher recurrent AF rate (33.8% versus 27.1%; risk difference 0.07; 95% CI [0.01–0.13]; p=0.02). Additional prophylactic CTI ablation to pulmonary vein isolation significantly increased the radio frequency application time (standardised mean difference 0.52; 95% CI [0.04–1.01]; p=0.03). Conclusion: This study suggested that prophylactic CTI ablation was an ineffective and inefficient approach in AF without documented typical atrial flutter patients.
Keywords: AF, atrial flutter, atrial tachyarrhythmias, cavotricuspid isthmus ablation, pulmonary vein isolation
AF and atrial flutter (AFL) commonly coexist, and reveal a strong clinical interrelationship.[1] The presence of AFL is documented in 20.6% of AF patients.[2] Pulmonary vein (PV) firing during AF episodes makes an essential contribution to initiating typical AFL.[3] In contrast, AF induced by pacing protocol during typical AFL ablation is a strong predictor for AF.[4]
Complete PV isolation (PVI) by single-shot or point-by-point catheter ablation approach is the cornerstone of catheter ablation in AF patients.[5] Compared with antiarrhythmic drugs (AADs), catheter ablation significantly reduces the AF recurrence rate, provides better symptom control and improves the quality of life in AF patients.[6,7]
In AF patients with left ventricular ejection fraction (LVEF) ≤35%, catheter ablation gives additional benefit in reducing hospitalisation due to worsening heart failure and all-cause mortality.[8] However, the main issue is the high rate of atrial tachyarrhythmias (ATAs) following the catheter ablation procedure.[9,10] Substrate modification, linear ablation and stepwise catheter ablation approaches have been conducted with a view to improving clinical outcomes.[11–19]
Ablation of the cavotricuspid isthmus (CTI) is well known as the therapeutic strategy for typical AFL.[20] This approach has also been proposed as an additional ablation procedure to PVI for AF patients in improving ATAs-free survival. A prior meta-analysis of randomised controlled trials (RCTs) revealed that prophylactic PVI during CTI ablation successfully improved the 1-year ATAs-free survival rate.[21] However, several previous studies about prophylaxis CTI ablation during PVI in AF patients demonstrated conflicting results.[14,22–24] This systematic review and meta-analysis study aimed to evaluate the advantage of prophylactic CTI ablation in AF patients without documented AFL.
Methods
Literature Search
According to the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidance, we conducted a systematic review and meta-analysis study.[25] Until January 2021, articles comparing PVI + prophylactic CTI ablation versus PVI alone in AF patients without documented AFL were identified from the electronic scientific databases including ClinicalTrials.gov, ScienceDirect, PubMed, Cochrane and ProQuest. We used the following keywords: ‘atrial fibrillation’ OR ‘AF’ OR ‘AFib’ AND ‘catheter ablation’ OR ‘ablation’ AND ‘pulmonary vein isolation’ OR ‘PVI’ AND ‘cavotricuspid isthmus’ OR ‘CTI’. We also manually looked for potentially relevant articles from other sources, such as the reference lists of all eligible articles or Google Scholar. The literature search was conducted by two investigators.
Eligibility Criteria
We used the following inclusion criteria: original research articles comparing PVI + prophylactic CTI ablation versus PVI alone in AF patients without documented AFL; catheter ablation purposed for rhythm control; written in English; availability of the data about ATAs, AF and AFL during the follow-up period; and availability of the procedural aspect data, including all-procedural complications, fluoroscopy time, procedure time or radiofrequency (RF) application time. We also excluded articles with the following criteria: duplications; treatment group and control group were incomparable; documented AFL prior to catheter ablation; outcomes of interest were not reported; using the data from similar studies; unavailability of full text; cross-sectional study; meta-analysis; review article; editorial; or case reports.
Endpoints
In this meta-analysis, the study endpoints were divided into clinical and procedural endpoints. Clinical endpoints involved ATAs, recurrent AF and new-onset AFL during the follow-up period. The atrial tachyarrhythmias were defined as documented AF, AFL and/or atrial tachycardia episodes by ECG or Holter monitor with duration ≥30 seconds after a 3-month blanking period. The procedural time, fluoroscopy time, RF application time and all-procedural complications were the procedural endpoints in this study.
Study Quality Assessment
For cohort studies, the study quality was evaluated using the Newcastle–Ottawa scale. It consisted of three domains with the highest score of 9. A cohort study was categorised as a good-quality study if it had: three or four stars in the selection domain; one or two stars in the comparability domain; and two or three stars in the outcome domain.[26]
The quality assessment of RCTs was performed using version 2 of the Cochrane risk of bias tool in randomised trials.[27] Two investigators performed the study quality assessment. The disagreement between both investigators was resolved through discussion and the third investigator's second opinion.
Data Extraction
Two investigators conducted the data extraction process. Essential information about the following was extracted from each study: the name of the first author; year of publication; study design; centres involved; country; number of patients; type of arrhythmia; blanking period duration; follow-up period duration; and arrhythmia detection methods. We also extracted data about patient baseline characteristics from each study, including: demographic (sex and age); AF (paroxysmal AF proportion, AF duration and CHA2DS2-VASc score); comorbidities (heart failure, hypertension, stroke, coronary artery disease and diabetes); and echocardiographic parameters (LVEF, left atrial diameter and left atrial volume index).
We also identified important information about: the incidence of ATAs, AF or AFL following the catheter ablation procedure; the procedural endpoints involved in procedural time, fluoroscopy time or RF application time; and all-procedural complications. We demonstrated the numerical data as the mean and SD. The mean and SD could also be estimated from the median and interquartile range using the Tiejun Tong group formula.[28–30] The number and percentage represented categorical data.
Statistical Analysis
The statistical analysis was performed by two investigators using Review Manager (RevMan) version 5.3 (Cochrane). This process was conducted based on the standard guideline direction.[31] We used Q-statistics to investigate heterogeneity among studies. The heterogeneity was identified if the p-value for heterogeneity was <0.1 or the I2 statistic was >50%.[32–35] Meta-analyses were conducted using random effects models to anticipate clinical and methodological diversity among the included studies.[34] The pooled effects were presented as a risk difference (RD), and standardised mean difference (SMD) for dichotomous and continuous outcomes. We also estimated the 95% CI of each pooled effect. A p-value <0.05 was considered statistically significant. The funnel plot was used to estimate the presence of the publication bias.[33],
Results
Study Selection Process
In the initial search phase, we successfully identified a total of 141 articles from electronic scientific databases (ClinicalTrials.gov [n=6], ScienceDirect [n=66], PubMed [n=21], Cochrane [n=5] and ProQuest [n=43]), and 12 articles from the reference lists of eligible articles. After removal of duplicates, we had 89 articles. In the beginning, 74 articles were excluded due to being unrelated to our systematic review and meta-analysis. In the next phase, we also excluded 10 articles because of the following: included AFL patients (n=3), meta-analysis (n=1), review (n=3) and unavailability of full text (n=4). Finally, four good-quality studies, including two cohort studies and two RCTs, were involved in this systematic review and meta-analysis.[14,22–24] The flow diagram of the study selection process is shown in Figure 1.
Figure 1: Flow Diagram of the Study Selection Process.
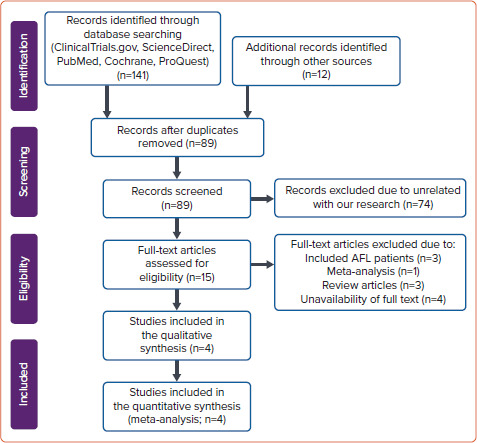
AFL = atrial flutter.
Baseline Characteristics
We summarised the characteristics of the studies in Supplementary Material Table 1. The study from Kim et al. only involved paroxysmal AF patients.[22] However, other studies also included another type of AF, such as persistent AF or long-standing persistent AF.[14,23,24] 3D mapping systems, such as CARTO or EnSite NavX, were used in four studies.[14,22–24] The blanking period duration in all studies was 3 months, and the shortest follow-up period was 12 months. AADs were given to some of the patients during the follow-up period.[14,22–24] However, the data about the usage rates of AADs during follow-up were available in only two studies. The usage rates of AADs were 18.9% and 47.8% in the studies by Pontoppidan et al. and Mesquita et al., respectively. No significant difference was found in the AADs usage rate between both groups.[14,24] Besides standard 12-lead ECG recording, all studies used ambulatory monitor systems, such as the Holter monitor or event recorder, to detect arrhythmia.[14][22–24]
A total of 1,476 AF patients from four studies were included in this systematic review and meta-analysis (724 patients in the PVI + prophylactic CTI ablation group and 752 patients in the PVI alone group).[14,22–24] The mean age of all study participants was 58.5 ± 10.8 years, and 68.7% of them were men. The CHA2DS2-VASc scores were available in three studies, with an overall mean value of 1.7 ± 2.9.[22–24] Comorbid conditions, including heart failure, hypertension, stroke, coronary artery disease and diabetes, were found in 7.3%, 45.8%, 4.7%, 4.7% and 10% of study participants, respectively. The data about LVEF and left atrial diameter were reported in three studies.[14,22,23] The overall mean value of the LVEF and left atrial diameter were 64.0% ± 20.7% and 41.3 mm ± 39.1 mm, respectively.
Only a study by Mesquita et al. provided left atrial volume index data. The mean left atrial volume index in the PVI + prophylactic CTI ablation group and PVI alone group were 57.3 ± 3.5 ml/m2 and 55.3 ± 3.0 ml/m2, respectively.[24] Patient characteristics in each study are summarised in Supplementary Material Table 2.
Study Quality, Heterogeneity and Publication Bias
Based on version 2 of the Cochrane risk of bias tool in randomised trials assessment, two RCTs involved in this meta-analysis were not at high risk of bias. According to the Newcastle–Ottawa scale, two cohort studies were classified as good-quality studies (Figure 2). Heterogeneity was present in the meta-analysis of atrial tachyarrhythmias (I2]=57%, p-value for heterogeneity=0.07), new-onset atrial flutter (I2]=61%, p-value for heterogeneity=0.11), procedure time (I2]=97%, p-value for heterogeneity <0.01), fluoroscopy time (I2]=99%, p-value for heterogeneity <0.01) and radiofrequency application time (I2]=79%, p-value for heterogeneity=0.03). This heterogeneity could be caused by the various AF types, comorbid conditions, atrial size, operator experience and follow-up duration. However, we did not find the heterogeneity in the meta-analysis of recurrent atrial fibrillation (I2]=31%, p-value for heterogeneity=0.23) and procedural complications (I2]=42%, p-value for heterogeneity=0.19). Because two cohort studies and two RCTs were involved in this systematic review and meta-analysis study, the pooled effects were conducted using random effects models to anticipate clinical and methodological diversity among included studies (Figures 3 and 4).[14,22–24] As shown in the funnel plot (Figure 5), publication biases were not found in the analysis of atrial tachyarrhythmias, recurrent atrial fibrillation, new-onset atrial flutter, procedural complications and RF application time.
Figure 2: Study Quality Assessment.
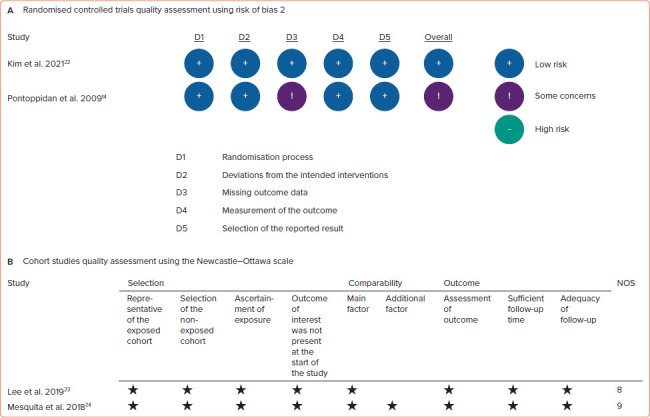
A: Risk of bias 2 for randomised controlled trials; B: Newcastle–Ottawa scale for cohort studies. NOS = Newcastle–Ottawa scale.
Figure 3: Forest Plot of the Clinical Endpoints.
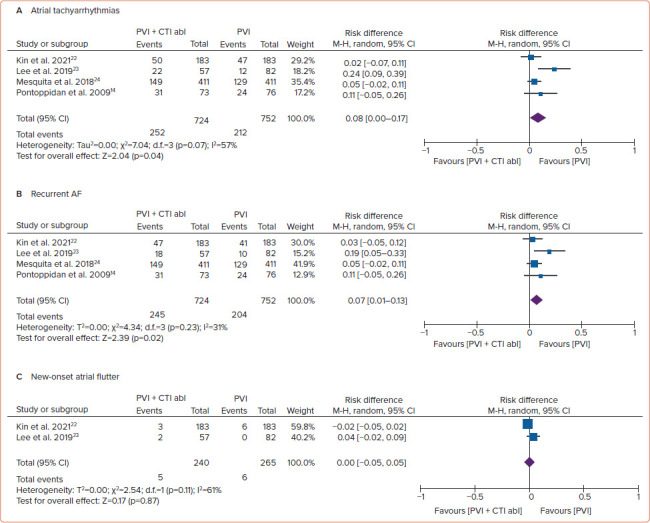
A: Atrial tachyarrhythmias; B: Recurrent AF; C: New-onset atrial flutter following single catheter ablation. abl = ablation; CTI = cavotricuspid isthmus; M-H = Mantel–Haenszel; PVI = pulmonary vein isolation.
Figure 4: Forest Plot of the Procedural Endpoints.
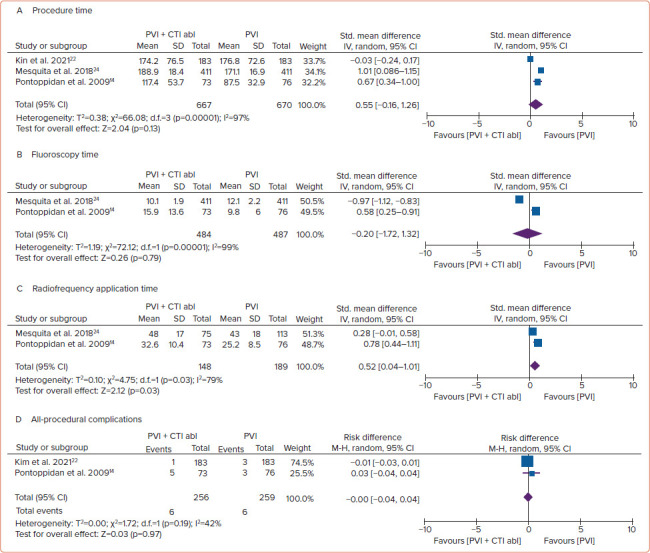
A: Procedure times; B: Fluoroscopy times; C: Radiofrequency application time; D: All-procedural complications. Abl = ablation; CTI = cavotricuspid isthmus; IV = inverse variance; M-H = Mantel–Haenszel; PVI = pulmonary vein isolation; Std. = standard.
Figure 5: Funnel Plots.
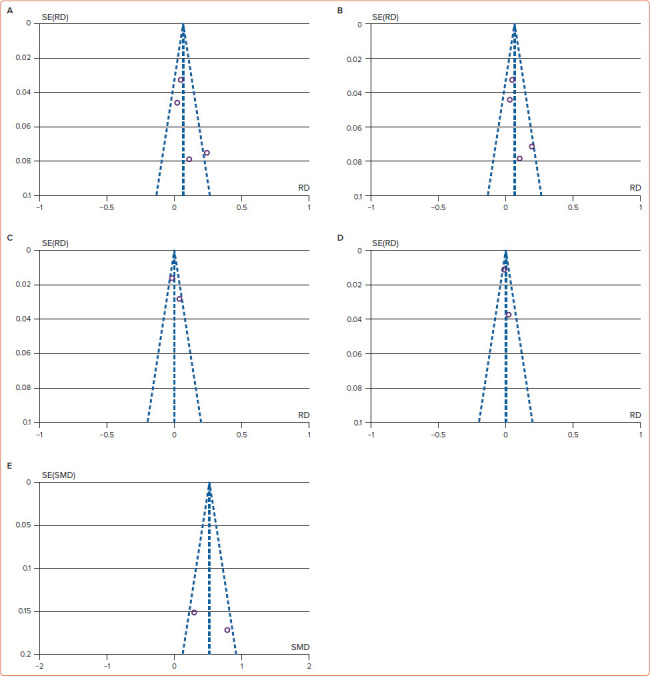
A: Atrial tachyarrhythmias; B: Recurrent AF; C: New-onset atrial flutter; D: All procedural complications; and E: Radiofrequency application time show no publication bias.
Clinical Endpoints
In the analysis of clinical endpoints, the risk of ATAs following a successful catheter ablation procedure was higher in the PVI + CTI ablation group than PVI alone group (34.8% versus 28.2%; RD 0.08; 95% CI [0.00–0.17]; p=0.04). We also conducted subgroup analysis for recurrent AF and new-onset AFL following the catheter ablation procedure. Additional CTI ablation was associated with a greater recurrent AF rate (33.8% versus 27.1%; RD 0.07; 95% CI [0.01–0.13]; p=0.02). However, both groups revealed a non-significant difference in new-onset AFL (2.1% versus 2.3%; RD 0.00; 95% CI [−0.05, 0.05]; p=0.87) during the follow-up period (Figure 3).
Procedural Endpoints
The procedure time (SMD 0.55; 95% CI [–0.16, 1.26]; p=0.13) and fluoroscopy time (SMD –0.20; 95% CI [–1.72, 1.32 min]; p=0.79) between both groups were not significantly different. However, additional prophylactic CTI ablation to PVI significantly increased RF application time (SMD 0.52; 95% [CI 0.04–1.01]; p=0.03). Both groups had a similar rate of all-procedural complications (2.3% versus 2.3%; RD 0.00; 95% CI [−0.04, 0.04]; p=0.97; Figure 3). We did not perform a subgroup analysis of the specific procedural complication, because only two studies reported it.[14,22] Moreover, the number of specific complications in each study was too low. Therefore, the subgroup analysis was not possible (Figure 4).
Discussion
Main Results and the Current Recommendation
We tried to assess whether prophylactic CTI ablation could improve outcomes in AF patients without documented AFL. Our main result was that PVI + prophylactic CTI ablation was not superior to PVI alone in reducing ATAs, recurrent AF and new-onset AFL following a successful catheter ablation procedure. Moreover, prophylactic CTI ablation was associated with a higher risk of ATAs and recurrent AF. Prophylactic CTI ablation also did not increase all-procedural complications. However, it was associated with longer RF application time.
The current guideline strongly recommends PVI as a rhythm control strategy for paroxysmal AF and persistent AF.[5] On the other side, CTI ablation is recommended for recurrent and symptomatic CTI-dependent AFL.[20] The rates of ATAs following those procedures are still high. In AF patients undergoing PVI ablation, 29.4–42% of patients developed ATAs during the follow-up period.[36–38] However, in AFL patients, the ATAs can be found in 16.7–50% of patients after CTI ablation.[39][40]
The Link Between Atrial Flutter and AF
A long time ago, AFL and AF were thought to be different arrhythmias, driven by a single macroreentrant arrhythmia circuit and multiple re-entrant wave fronts in both atria. However, in the real clinical setting, approximately 50% of AFL patients suffer from AF during long-term follow-up.[41] The studies from Watson et al. and Waldo et al. provided new insight into understanding the mechanism of AF and AFL. Those studies observed that a transitional period of AF typically preceded the AFL onset.[42,43] The transition from an AF to AFL mechanism was described in an animal study by Ortiz et al. That study revealed that the conversion from AF to AFL was due to: the formation of a long line of the functional block; the presence of stable re-entry circuits; and the re-emergence of the slow conduction area. In contrast, the conversion from AFL to AF following these several mechanisms caused the following: a decrease in the cycle length; disappearance of the slow conducting area; a decrease in the length of the functional block line; and an unstable re-entry circuit with the very short cycle length with various appearance, shape and location.[44]
Three years later, a human study by Roithinger et al. documented the existence of the stereotypical pattern of the subendocardial organisation during the transformation from AF to AFL.[45] In 2001, Hsieh et al. reported that several ectopic beats initiated the spontaneous transformation from typical AFL to AF. It could be eliminated by performing catheter ablation to those ectopic foci.[46] Therefore, it is thought that a better outcome will be achieved by performing prophylactic PVI in AFL patients undergoing CTI ablation or conducting prophylactic CTI ablation in AF patients undergoing PVI.
Prophylactic Cavotricuspid Isthmus Ablation During Pulmonary Vein Isolation
Several studies were conducted to assess the advantage of prophylactic CTI ablation in AF patients without documented AFL.[14,22–24] An RCT by Pontoppidan et al. revealed that prophylactic CTI ablation failed to reduce the recurrent AF or new-onset AFL.[14] An RCT with a longer follow-up duration by Kim et al. also showed similar results.[22] A cohort study with a large number of patients by Mesquita et al. also demonstrated that prophylactic CTI ablation failed to increase AF-free survival.[24] The high rate of post-catheter ablation atypical AFL in the PVI alone group could be due to a macroreentrant circuit resulting from atrial remodelling. A retrospective study from Lee et al. showed that additional CTI ablation was associated with higher ATAs after the catheter ablation procedure. However, in that study, the higher ATAs in the prophylactic CTI ablation group could be caused by left atrial remodelling. In that group, patients had a larger left atrial size (39.67 ± 5.07 mm versus 35.95 ± 5.56 mm; p<0.01). A study from Lee et al. gave us the important lesson that left atrial remodelling or left atrial size plays a major role in the development of ATAs.[23]
To the best of our knowledge, our study is the first systematic review and meta-analysis study that quantified the efficacy and safety profile of prophylactic CTI ablation in AF patients without documented AFL. A previous meta-analysis study by Romero et al. compared PVI + CTI ablation with PVI alone in AF patients.[47] However, that meta-analysis included two RCTs that involved AF patients with documented coexisting AFL.[48,49] In our meta-analysis, we did not only exclude the studies from Wazni et al. and Mohanty et al., but we also added a study from Kim et al.[48,49,22] We provided data about procedural aspects, including procedure time, fluoroscopy time, RF application time and all-procedural complications, that were not provided by the prior meta-analysis study by Romero et al.[47]
Our study revealed that prophylactic CTI ablation could not reduce the risk of ATAs during the follow-up period. Our result supported the prior study by Romero et al. (RR 1.29; 95% CI [0.93–1.79]; p=0.13).[47] In our meta-analysis, we also found that prophylactic CTI ablation failed to reduce recurrent AF. This finding could be influenced by the results of the study by Lee et al.[23] In that study, the left atrial size in both groups was significantly different. That could be a significant confounder. A larger atrial size was associated with a higher AF recurrence rate following PVI.[50,51] Our meta-analysis demonstrated that prophylactic CTI ablation did not reduce new-onset AFL in AF without documented typical AFL patients. In both groups, the most AF ablation was PVI.[14,22–24] Additional ablation strategies, such as linear ablation, complex fractionated atrial electrogram ablation and superior vena cava isolation, were conducted if PVI was not effective.[14,23] A prior study from Ipek et al. showed that linear ablation for AF was correlated with a higher left AFL incidence following AF ablation.[52] In contrast, CTI ablation is the well-known treatment of choice for CTI-dependent AFL, and it is crucial to differentiate between CTI-dependent and non-CTI-dependent AFL.[20] In this meta-analysis, data about the specific types of AFL were only available in a study by Lee et al.[23] Therefore, we could not conduct a subgroup analysis.
As expected, prophylactic CTI ablation required a longer RF application time. However, the procedure time, fluoroscopy time and procedural complications between both groups were not significantly different. Our results suggested that conducting prophylactic CTI ablation in AF patients without documented AFL was ineffective and inefficient. Our results also supported the AF catheter ablation strategy in most of the centres. The first-time AF catheter ablation strategy is high-power, short-duration PVI only.[53,54] If any atrial tachyarrhythmia is documented during the follow-up period, additional linear ablation can be considered.
Limitations
Special attention is required in interpreting the results of our study due to the presence of several limitations. First, even though only studies that assessed prophylactic CTI ablation in AF patients without documented AF were included, most of those studies were small studies with a limited number of participants. Second, almost all studies in this meta-analysis involved paroxysmal AF and persistent AF patients. We did not conduct the subgroup analysis based on AF subtypes because: only four studies were included in this meta-analysis; the study from Kim et al. only involved paroxysmal AF patients; other studies included paroxysmal AF and persistent AF, and/or long-standing persistent AF; and we could not access the patients' individual-level data.[14,22–24] Third, the unequal baseline characteristics, especially the left atrial size, could be a confounder, because atrial remodelling plays an essential role as the substrate for ATAs. Fourth, the differences in the mapping system, catheter ablation technology, ablation strategy, operator experience, follow-up period duration, the physicians' preference-directed AADs usage during the follow-up period and arrhythmia detection method were other issues that could affect the outcomes.
Conclusion
In this meta-analysis, conducting prophylactic CTI ablation during PVI in AF patients without documented AFL did not reduce the risk of ATAs, recurrent AF and new-onset AFL in AF patients. Moreover, prophylactic CTI ablation was associated with a higher risk of ATAs and recurrent AF. In this population, conducting prophylactic CTI ablation during PVI prolonged RF application time. Our study suggested that prophylactic CTI ablation was an ineffective and inefficient approach for AF patients without documented AFL. RCTs with better methods, larger scale and longer follow-up durations are required to obtain better evidence.
Clinical Perspective
Pulmonary vein isolation (PVI) is the cornerstone of catheter ablation in AF patients.
Prophylactic cavotricuspid isthmus (CTI) ablation during PVI in AF patients without documented atrial flutter (AFL) fails to reduce the risk of ATAs, recurrent AF and new-onset AFL.
Prophylactic CTI ablation during PVI in AF patients without documented AFL prolongs radiofrequency application time.
Prophylactic CTI ablation during PVI in AF patients without documented AFL does not increase complication rates.
Supplementary Material
References
- 1.Waldo AL, Feld GK. Inter-relationships of atrial fibrillation and atrial flutter: mechanisms and clinical implications. J Am Coll Cardiol. 2008;51:779–86. doi: 10.1016/j.jacc.2007.08.066. [DOI] [PubMed] [Google Scholar]
- 2.Stempfel S, Aeschbacher S, Blum S et al. Symptoms and quality of life in patients with coexistent atrial fibrillation and atrial flutter. Int J Cardiol Heart Vasc. 2020;29:100556. doi: 10.1016/j.ijcha.2020.100556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaneshiro T, Yoshida K, Sekiguchi Y et al. Crucial role of pulmonary vein firing as an initiator of typical atrial flutter: evidence of a close relationship between atrial fibrillation and typical atrial flutter. J Arrhythm. 2017;33:86–91. doi: 10.1016/j.joa.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romero J, Diaz JC, Di Biase L et al. Atrial fibrillation inducibility during cavotricuspid isthmus-dependent atrial flutter ablation as a predictor of clinical atrial fibrillation. A meta-analysis. J Interv Card Electrophysiol. 2017;48:307–15. doi: 10.1007/s10840-016-0211-9. [DOI] [PubMed] [Google Scholar]
- 5.Hindricks G, Potpara T, Dagres N et al. ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 6.Mark DB, Anstrom KJ, Sheng S et al. Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1275–85. doi: 10.1001/jama.2019.0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Packer DL, Mark DB, Robb RA et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261–74. doi: 10.1001/jama.2019.0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marrouche NF, Brachmann J, Andresen D et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378:417–27. doi: 10.1056/NEJMoa1707855. [DOI] [PubMed] [Google Scholar]
- 9.Balk EM, Garlitski AC, Alsheikh-Ali AA et al. Predictors of atrial fibrillation recurrence after radiofrequency catheter ablation: a systematic review. J Cardiovasc Electrophysiol. 2010;21:1208–16. doi: 10.1111/j.1540-8167.2010.01798.x. [DOI] [PubMed] [Google Scholar]
- 10.Ganesan AN, Shipp NJ, Brooks AG et al. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. J Am Heart Assoc. 2013;2:e004549. doi: 10.1161/JAHA.112.004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaïs P, Hocini M, Hsu LF et al. Technique and results of linear ablation at the mitral isthmus. Circulation. 2004;110:2996–3002. doi: 10.1161/01.CIR.0000146917.75041.58. [DOI] [PubMed] [Google Scholar]
- 12.Di Biase L, Elayi CS, Fahmy TS et al. Atrial fibrillation ablation strategies for paroxysmal patients: randomized comparison between different techniques. Circ Arrhythm Electrophysiol. 2009;2:113–9. doi: 10.1161/CIRCEP.108.798447. [DOI] [PubMed] [Google Scholar]
- 13.Oral H, Chugh A, Yoshida K et al. A randomized assessment of the incremental role of ablation of complex fractionated atrial electrograms after antral pulmonary vein isolation for long-lasting persistent atrial fibrillation. J Am Coll Cardiol. 2009;53:782–9. doi: 10.1016/j.jacc.2008.10.054. [DOI] [PubMed] [Google Scholar]
- 14.Pontoppidan J, Nielsen JC, Poulsen SH et al. Prophylactic cavotricuspid isthmus block during atrial fibrillation ablation in patients without atrial flutter: a randomised controlled trial. Heart. 2009;95:994–9. doi: 10.1136/hrt.2008.153965. [DOI] [PubMed] [Google Scholar]
- 15.Rolf S, Kircher S, Arya A et al. Tailored atrial substrate modification based on low-voltage areas in catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2014;7:825–33. doi: 10.1161/CIRCEP.113.001251. [DOI] [PubMed] [Google Scholar]
- 16.Dong JZ, Sang CH, Yu RH et al. Prospective randomized comparison between a fixed ‘2C3L’ approach vs. stepwise approach for catheter ablation of persistent atrial fibrillation. Europace. 2015;17:1798–806. doi: 10.1093/europace/euv067. [DOI] [PubMed] [Google Scholar]
- 17.Schreiber D, Rostock T, Fröhlich M et al. Five-year follow-up after catheter ablation of persistent atrial fibrillation using the stepwise approach and prognostic factors for success. Circ Arrhythm Electrophysiol. 2015;8:308–17. doi: 10.1161/CIRCEP.114.001672. [DOI] [PubMed] [Google Scholar]
- 18.Verma A, Jiang CY, Betts TR et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812–22. doi: 10.1056/NEJMoa1408288. [DOI] [PubMed] [Google Scholar]
- 19.Waranugraha Y, Rizal A, Setiawan D, Aziz IJ. Additional complex fractionated atrial electrogram ablation does not improve the outcomes of non-paroxysmal atrial fibrillation: a systematic review and meta-analysis of randomized controlled trials. Indian Heart J. 2021;73:63–73. doi: 10.1016/j.ihj.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brugada J, Katritsis DG, Arbelo E et al. 2019 ESC guidelines for the management of patients with supraventricular tachycardia: the Task Force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC). Eur Heart J. 2020;41:655–720. doi: 10.1093/eurheartj/ehz467. [DOI] [PubMed] [Google Scholar]
- 21.Koerber SM, Turagam MK, Gautam S et al. Prophylactic pulmonary vein isolation during cavotricuspid isthmus ablation for atrial flutter: a meta-analysis. Pacing Clin Electrophysiol. 2019;42:493–8. doi: 10.1111/pace.13637. [DOI] [PubMed] [Google Scholar]
- 22.Kim SH, Oh YS, Choi Y et al. Long-term efficacy of prophylactic cavotricuspid isthmus ablation during atrial fibrillation ablation in patients without typical atrial flutter: a prospective, multicentre, randomized trial. Korean Circ J. 2021;51:58–64. doi: 10.4070/kcj.2020.0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee WC, Fang HY, Chen HC et al. Additional cavotricuspid isthmus block ablation may not improve the outcome of atrial fibrillation ablation. Pacing Clin Electrophysiol. 2019;42:1421–8. doi: 10.1111/pace.13799. [DOI] [PubMed] [Google Scholar]
- 24.Mesquita J, Ferreira AM, Cavaco D et al. Impact of prophylactic cavotricuspid isthmus ablation in atrial fibrillation recurrence after a first pulmonary vein isolation procedure. Int J Cardiol. 2018;259:82–7. doi: 10.1016/j.ijcard.2018.01.025. [DOI] [PubMed] [Google Scholar]
- 25.Liberati A, Altman DG, Tetzlaff J et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wells G, Shea B, O'Connell D The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2000. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 22 February 2021)
- 27.Sterne JAC, Savović J, Page MJ et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 28.Tiejun Tong group. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. http://www.math.hkbu.edu.hk/~tongt/papers/median2mean.html (accessed 26 February 2021) [DOI] [PMC free article] [PubMed]
- 29.Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27:1785–805. doi: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 30.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cleophas TJ, Zwinderman AH. Modern meta-analysis: review and update of methodologies. Cham, Switzerland: Springer International Publishing, 2017. [DOI]
- 32.Fletcher J. What is heterogeneity and is it important? BMJ. 2007;334:94–6. doi: 10.1136/bmj.39057.406644.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waranugraha Y, Rizal A, Setiawan D, Aziz IJ. The benefit of atrioventricular junction ablation for permanent atrial fibrillation and heart failure patients receiving cardiac resynchronization therapy: an updated systematic review and meta-analysis. Indian Pacing Electrophysiol J. 2021;21:101–11. doi: 10.1016/j.ipej.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson RD, Ariyarathna N, Lee G et al. Catheter ablation versus medical therapy for treatment of ventricular tachycardia associated with structural heart disease: systematic review and meta-analysis of randomized controlled trials and comparison with observational studies. Heart Rhythm. 2019;16:1484–91. doi: 10.1016/j.hrthm.2019.05.026. [DOI] [PubMed] [Google Scholar]
- 35.Waranugraha Y, Rizal A, Syaban MFR et al. Direct comparison of non-vitamin K antagonist oral anticoagulant versus warfarin for stroke prevention in non-valvular atrial fibrillation: a systematic review and meta-analysis of real-world evidences. Egypt Heart J. 2021;73:70. doi: 10.1186/s43044-021-00194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voskoboinik A, Moskovitch JT, Harel N et al. Revisiting pulmonary vein isolation alone for persistent atrial fibrillation: a systematic review and meta-analysis. Heart Rhythm. 2017;14:661–7. doi: 10.1016/j.hrthm.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 37.Cheng WH, Lo LW, Lin YJ et al. Ten-year ablation outcomes of patients with paroxysmal atrial fibrillation undergoing pulmonary vein isolation. Heart Rhythm. 2019;16:1327–33. doi: 10.1016/j.hrthm.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 38.Sau A, Howard JP, Al-Aidarous S et al. Meta-analysis of randomized controlled trials of atrial fibrillation ablation with pulmonary vein isolation versus without. JACC Clin Electrophysiol. 2019;5:968–76. doi: 10.1016/j.jacep.2019.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Celikyurt U, Knecht S, Kuehne M et al. Incidence of new-onset atrial fibrillation after cavotricuspid isthmus ablation for atrial flutter. Europace. 2017;19:1776–80. doi: 10.1093/europace/euw343. [DOI] [PubMed] [Google Scholar]
- 40.Kwon HJ, Lee SS, Park YJ et al. Effectiveness and safety of high-power and short-duration ablation for cavotricuspid isthmus ablation in atrial flutter. Pacing Clin Electrophysiol. 2020;43:941–6. doi: 10.1111/pace.14019. [DOI] [PubMed] [Google Scholar]
- 41.Cosío FG. Atrial flutter, typical and atypical: a review. Arrhythm Electrophysiol Rev. 2017;6:55–62. doi: 10.15420/aer.2017.5.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watson RM, Josephson ME. Atrial flutter. I. Electrophysiologic substrates and modes of initiation and termination. Am J Cardiol. 1980;45:732–41. doi: 10.1016/0002-9149(80)90115-0. [DOI] [PubMed] [Google Scholar]
- 43.Waldo AL, Cooper TB. Spontaneous onset of type I atrial flutter in patients. J Am Coll Cardiol. 1996;28:707–12. doi: 10.1016/0735-1097(96)00223-9. [DOI] [PubMed] [Google Scholar]
- 44.Ortiz J, Niwano S, Abe H et al. Mapping the conversion of atrial flutter to atrial fibrillation and atrial fibrillation to atrial flutter. Insights into mechanisms. Circ Res. 1994;74:882–94. doi: 10.1161/01.RES.74.5.882. [DOI] [PubMed] [Google Scholar]
- 45.Roithinger FX, Karch MR, Steiner PR et al. Relationship between atrial fibrillation and typical atrial flutter in humans: activation sequence changes during spontaneous conversion. Circulation. 1997;96:3484–91. doi: 10.1161/01.CIR.96.10.3484. [DOI] [PubMed] [Google Scholar]
- 46.Hsieh MH, Tai CT, Tsai CF et al. Mechanism of spontaneous transition from typical atrial flutter to atrial fibrillation: role of ectopic atrial fibrillation foci. Pacing Clin Electrophysiol. 2001;24:46–52. doi: 10.1046/j.1460-9592.2001.00046.x. [DOI] [PubMed] [Google Scholar]
- 47.Romero J, Patel K, Briceno D et al. Cavotricuspid isthmus line in patients undergoing catheter ablation of atrial fibrillation with or without history of typical atrial flutter: a meta-analysis. J Cardiovasc Electrophysiol. 2020;31:1987–95. doi: 10.1111/jce.14614. [DOI] [PubMed] [Google Scholar]
- 48.Wazni O, Marrouche NF, Martin DO et al. Randomized study comparing combined pulmonary vein-left atrial junction disconnection and cavotricuspid isthmus ablation versus pulmonary vein-left atrial junction disconnection alone in patients presenting with typical atrial flutter and atrial fibrillation. Circulation. 2003;108:2479–83. doi: 10.1161/01.CIR.0000101684.88679.AB. [DOI] [PubMed] [Google Scholar]
- 49.Mohanty S, Mohanty P, Di Biase L et al. Results from a single-blind, randomized study comparing the impact of different ablation approaches on long-term procedure outcome in coexistent atrial fibrillation and flutter (APPROVAL). Circulation. 2013;127:1853–60. doi: 10.1161/CIRCULATIONAHA.113.001855. [DOI] [PubMed] [Google Scholar]
- 50.Akutsu Y, Kaneko K, Kodama Y et al. Association between left and right atrial remodeling with atrial fibrillation recurrence after pulmonary vein catheter ablation in patients with paroxysmal atrial fibrillation: a pilot study. Circ Cardiovasc Imaging. 2011;4:524–31. doi: 10.1161/CIRCIMAGING.110.962761. [DOI] [PubMed] [Google Scholar]
- 51.Zhuang J, Wang Y, Tang K et al. Association between left atrial size and atrial fibrillation recurrence after single circumferential pulmonary vein isolation: a systematic review and meta-analysis of observational studies. Europace. 2012;14:638–45. doi: 10.1093/europace/eur364. [DOI] [PubMed] [Google Scholar]
- 52.Ipek GE, Marine JE, Habibi M et al. Association of left atrial function with incident atypical atrial flutter after atrial fibrillation ablation. Heart Rhythm. 2016;13:391–8. doi: 10.1016/j.hrthm.2015.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kottmaier M, Popa M, Bourier F et al. Safety and outcome of very high-power short-duration ablation using 70 W for pulmonary vein isolation in patients with paroxysmal atrial fibrillation. Europace. 2020;22:388–93. doi: 10.1093/europace/euz342. [DOI] [PubMed] [Google Scholar]
- 54.Waranugraha Y, Rizal A, Firdaus AJ et al. The superiority of high-power short-duration radiofrequency catheter ablation strategy for atrial fibrillation treatment: a systematic review and meta-analysis study. J Arrhythm. 2021;37:975–89. doi: 10.1002/joa3.12590. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


