Abstract
Uterine fibroids are benign monoclonal neoplasms of the myometrium, representing the most common tumors in women worldwide. To date, no long-term or noninvasive treatment option exists for hormone-dependent uterine fibroids, due to the limited knowledge about the molecular mechanisms underlying the initiation and development of uterine fibroids. This paper comprehensively summarizes the recent research advances on uterine fibroids, focusing on risk factors, development origin, pathogenetic mechanisms, and treatment options. Additionally, we describe the current treatment interventions for uterine fibroids. Finally, future perspectives on uterine fibroids studies are summarized. Deeper mechanistic insights into tumor etiology and the complexity of uterine fibroids can contribute to the progress of newer targeted therapies.
Keywords: uterine fibroids, developmental origin, genetic instability, reprogramming, epigenetics pathways, novel treatment, future directions
Graphical Abstract
Graphical Abstract.
ESSENTIAL POINTS.
Developmental exposure to EDCs in early life reprograms myometrial stem cells, thus increasing the risk of uterine fibroids development.
Several risk factors such as age, race, obesity, parity, hypertension, vitamin D deficiency, and diet in late life can trigger uterine fibroids pathogenesis.
Pathogenic exon 2 mutations in MED12 promote uterine fibroids formation and disrupt CDK8/19 kinase activity.
Several vital pathways and mechanisms such as sex hormones, ECM, Wnt/β-catenin, TGF-β, growth factors, epigenetic and epitranscriptomic regulation, YAP/TAZ, Rho/ROCK, and DNA damage repair pathways contribute to the development of uterine fibroids.
Fertility therapy is highly needed for the treatment of patients with uterine fibroids.
Uterine fibroid lesions were initially known as the “uterine stone.” In the second century AD, they were called scleromas. The term fibroid was first introduced in the 1860s. Uterine fibroids are the most common pelvic tumors among women of reproductive age, affecting more than 70% of women worldwide, particularly women of color (1-3). Uterine fibroids are heterogeneous in composition and size among women and within the same individual, and vary in number between individuals (4-14). In addition, the fibroid pseudocapsule presents as a fibro-neurovascular structure surrounding a uterine fibroid, separating it from normal peripheral myometrium (15-18). Although benign, uterine fibroids are associated with significant morbidity; they are the primary indication for hysterectomy, and a major source of gynecologic and reproductive dysfunction, ranging from menorrhagia and pelvic pain to infertility, recurrent miscarriage, and preterm labor (19, 20). Accordingly, the annual USA health care costs associated with uterine fibroids have been estimated at ~$34 billion (21). Uterine fibroids thus represent significant societal health and financial burden.
Epidemiology, Risk/Protective Factors, Driver Mutations, and Tumor-Initiating Stem Cells
Risk Factors and Epidemiology
The prevalence of uterine fibroids is increasing in some populations, such as in African American women (22). However, its reported incidence is likely to be an underestimation, as many tumors are asymptomatic or slightly symptomatic and therefore remain undiagnosed (1). In addition, approximately only 25% to 30% of women report the clinical symptoms of uterine fibroids (5).
The most important and frequently reported risk factor for uterine fibroids is race, disproportionately impacting African American women (Figs. 1 and 2). Other risk factors include older age, premenopausal state, nonparity, family history of uterine fibroids, hypertension, food additives, and frequent consumption of soybean milk. On the other hand, protective factors for uterine fibroids include combined oral contraception or injectable medroxyprogesterone acetate in the depot form, smoking in women of low mass, and parity (1). Other important risk factors include obesity (23-25), vitamin D deficiency (26-28), excessive vitamin E levels (29), altered reproductive tract microbiome (30), exposure to endocrine-disrupting chemicals (eg, organophosphate esters and plasticizers) (31, 32), and various early-life adverse environmental exposures (33). Individual and environmental risk factors associated with tobacco smoking and alcohol abuse can also contribute to the formation of uterine fibroids (34, 35). More risk factors are associated with a higher probability of uterine fibroid formation and development (1, 23).
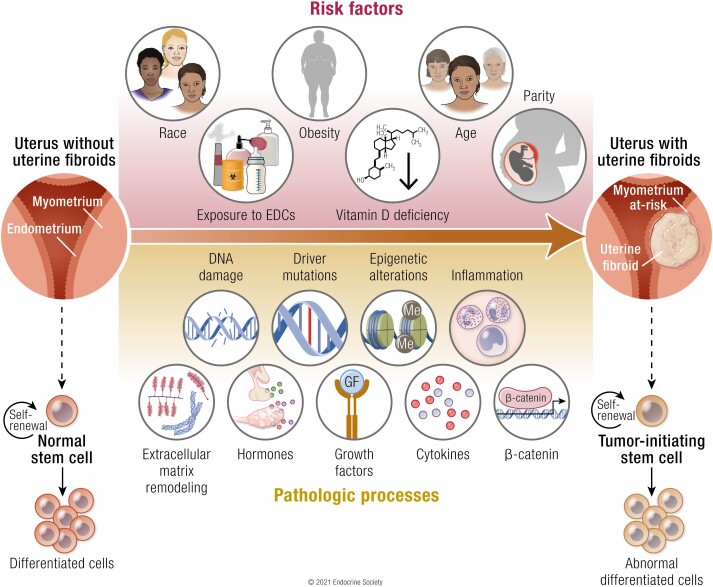
Figure 1.
Developmental origin of fibroids from myometrial stem cells. Intrauterine and early-life adverse environmental exposure to endocrine-disrupting chemicals may act as the early hit to induce normal myometrial stem cells’ reprogramming by hijacking epigenomic plasticity. The plasticity of the developing epigenome is susceptible to epigenomic changes in myometrial stem cells following later-life adverse exposures, thereby leading to mutations and their transformation into tumor-initiating stem cells. The development and growth of fibroids are mainly characterized by abnormal cell proliferation, inhibited apoptosis, DNA instability, excessive deposition of ECM, and other critical biological pathways. Abbreviations: ECM, extracellular matrix; MED12, RNA polymerase II transcriptional mediator complex subunit 12; ncRNAs, non-coding RNA.
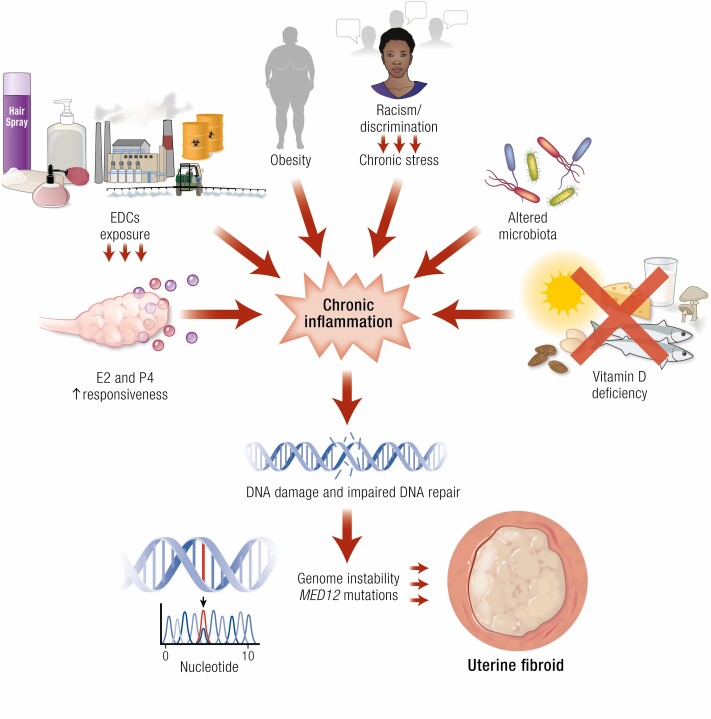
Figure 2.
Risk factors for uterine fibroids that mainly affect inflammation, DNA damage pathways, and genetic instability. External and internal factors, such as EDC exposure, hyper-responsiveness to sex steroid hormones, obesity, vitamin D deficiency, and altered reproductive tract microbiome, contribute to chronic systemic inflammation. The inflammatory environment, EDC exposure, and vitamin D deficiency promote DNA damage and the accumulation of mutations. Consequently, these genetic events may activate the pathways involved in cell proliferation, the inhibition of apoptosis, and ECM remodeling, ultimately leading to the development and growth of fibroids. Abbreviations: E2, estrogen; EDCs, endocrine-disrupting chemicals; MED12, RNA polymerase II transcriptional mediator complex subunit 12; P4, progesterone.
These points require some additional comments. Epidemiologists understand that they must study women from the community to eliminate bias and have a prospective study design with a large sample size and low loss to follow-up to enable the measurement of age-specific incidence and other risk factor-related pathogenesis of uterine fibroids (36). Improvement of awareness and education for uterine fibroids in the community will help to better understand the risk factors of this diseases. Notably, data from uterine fibroid research in underrepresented groups are lacking (37). On the other hand, epidemiological studies may reflect both the natural and false effects of a selected factor on the investigated outcome. Findings may be subject to different explanations because they may occur due to random errors, biases, or confounding, which may produce false results. These factors need to be considered at both the design and analysis stage of a study to minimize them. Notably, the same instruments for health outcomes evaluation in exposed and unexposed groups should be applied to avoid misclassification or bias. Studies without including confounding variables from the onset or without matching by age, race, and other factors should always be treated with caution (38).
Age
Increasing age is a significant risk factor for uterine fibroids, especially among women at the premenopausal stage and those ≥ 40 years of age (24, 39, 40). For instance, 60% of African American women aged 35–49 years reported uterine fibroids, whereas 80% of those aged ≥ 50 have uterine fibroids. Among White women, 40% of those aged ≤ 35 years and 70% aged ≥ 50 years developed uterine fibroids (3). These tumors have not been detected in prepubertal girls, and only sporadic cases have been reported in adolescents. However, the factor(s) involved in their development at such an early age is unknown. Due to the slight difference in biochemical pathways, uterine fibroids in young women do not exhibit typical uterine fibroid biology. In several cases, adolescent patients had a translocation between chromosomes 12 and 14, which is a confirmed risk factor for uterine fibroids (41, 42). Women at the menopausal stage have shrunk uterine fibroid lesions and decreased sex hormones. Notably, the use of hormonal replacement therapy may cause these lesions to regrow and may induce the first clinical symptoms of uterine fibroids (43).
Race and ethnicity
Populations of different races/ethnicities vary in the risk of developing uterine fibroids. The United States Census recognized 5 racial categories (White or European; Black or African American; Asian American; American Indian/Alaska Native; and Native Hawaiian/Pacific Islander) as well as people of 2 or more races (https://www.census.gov/topics/population/race/about.html. Accessed July, 2021). In addition, the Census Bureau also classified Hispanic or Non-Hispanic as ethnicity. Medical records and self-report were used and demonstrated that Black women, the largest racial minority in the United States, are most likely out of any racial category to develop uterine fibroids (3, 5, 44-46). The severity of uterine fibroid–derived symptoms also tended to be greater among African American women (47). Uterine fibroids are 3 times more common in African American women and 2 times more common in Hispanic women compared with White women.(3, 46). The more common occurrence of uterine fibroids in African American women may be attributed to higher concentrations of steroid hormones in African American women and may also be due to gene polymorphism, including the catechol-Ο-methyltransferase (COMT) encoding gene (48). However, the etiology of the increased incidence of uterine fibroids in African American women has not been fully elucidated. Additionally, the relationship between the higher incidence of uterine fibroids and more severe manifestations of disease may be due to vitamin D deficiency in African American women (49, 50). African American women are diagnosed with vitamin D deficiency at a rate of 5 to 10 times more than that of White women. It is thought that the limited absorption of ultraviolet (UV) radiation, which is essential for vitamin D metabolism, may be the reasoning for this discovery (50).
Furthermore, the African American population experiences higher levels of racial discrimination (51, 52) and there are multiple ways by which perceived racism can affect health (53). A positive association between self-reported experiences of racial discrimination and the incidence of uterine fibroids was demonstrated in a large follow-up study of the cohort of the Black Women’s Health Study (54). In this sense, Vines et al have found an association with the presence of uterine fibroids among the African American women in the high-stress intensity group (55).
Discrimination is thought to negatively influence physical wellbeing through the stress response (56, 57). The hypothesis that stress led to uterine fibroid pathogenesis could be explained by the fact that disturbance of the hypothalamic–pituitary–adrenal axis and the subsequent release of stress biomarkers such as cortisol and epinephrine (58) have been linked with increased uterine fibroid risk (59). In addition, stress also may provoke fluctuations in estrogen and progesterone hormone levels (60, 61), both important in uterine fibroid development. Furthermore, it is also biologically plausible that the higher uterine fibroid risk observed in African American women is associated with the systemic inflammation provoked by stress-related factors (Fig. 2) (62). To date, studies on the role of stress in uterine fibroid development among women of various race/ethnic groups are limited. In this sense, more studies that examine perceived racism as a chronic stressor linked to occurrence of uterine fibroids are needed to fully understand these dependencies.
Obesity
Obesity is directly related to increased energy consumption and reduced physical activity (63). Currently, obesity is the fifth leading cause of death (64). Several studies have found obesity as a significant risk factor for uterine fibroids development (23, 65), which has been attributed to the metabolic functions of adipose tissues. Adipose tissues produce and release various cytokines and growth factors involved in regulating diverse physiological and pathological processes, including immunity and inflammation (66). Adrenal androgens are mostly metabolized by aromatase in adipose tissues to estrogens (67-69). Obesity and particularly excess visceral fat may be complemented with the reduced production of the sex hormone–binding globulin (SHBG), which binds circulating hormones, disrupting the hormonal activity toward sensitive tissues, and thereby influencing the delicate hormonal balance in the body (70).
Each kilogram of excessive body weight is correlated with an increased risk of uterine fibroids development (71, 72). A study conducted in the United States found that women diagnosed with uterine fibroids are heavier than those without uterine fibroids (72). Moreover, an increase in the body mass index (BMI) by one unit (23), higher waist-to-hip ratios, and body fat percentage exceeding 30% (73) increase the risk for uterine fibroids. Abdominal visceral fat also enhances this risk (65). A recent meta-analysis of 22 studies, including 325 899 participants, and 19 593 cases, found a positive association between obesity and the risk or prevalence of fibroids (74).
Obesity is most prevalent among African Americans compared with other racial and ethnic populations in the United States, contributing to the higher risk of developing uterine fibroids in the African American population (25). Uterine fibroids occur more frequently in obese postmenopausal women and those who have undergone hormonal replacement therapy (75). Furthermore, obese women diagnosed with type 2 diabetes are more likely to develop uterine fibroids (75), and this observation has been related to elevated concentrations of insulin-like growth factor (IGF-1) (76). Insulin resistance plays a role in the development of uterine fibroids in obese women.
Parity
Main epidemiological studies demonstrated an inverse association between parity and uterine fibroids, suggestive of a protective effect (77). Nulliparous women are more commonly affected by uterine fibroids than multiparous women (44). Each subsequent child may lower the risk of this pathology (74). These study analyses were based on USA data, which need further investigation related to the difference in race and ethnicity in other countries. Steroid hormone exposure during pregnancy and dramatic remodeling of the uterine tissues after each pregnancy may be attributable to a decrease in uterine fibroid formation (77, 78).
Hypertension
There is a direct correlation between arterial hypertension and uterine fibroids (44, 79, 80). Increased diastolic blood pressure is associated with a higher risk of uterine fibroids, regardless of use of antihypertensive drugs (79). Women suffering from hypertension are 5 times more likely to develop uterine fibroids (81), and earlier diagnosis of hypertension is a significant factor. The formation of lesions is attributed to the chronic destruction of the myometrium due to increased blood flow and cytokines secreted by injured myometrial cells (79).
Vitamin D deficiency and diet
Vitamin D is a collective term for fat-soluble steroid compounds with pleiotropic solid influence in the human body (82, 83, 84) Vitamin D is synthesized in the human skin from 7-dehydrocholesterol upon exposure to sunlight. Then, it is transported by the vitamin D-binding protein to the liver and kidneys, where it is converted to 25-hydroxyvitamin D [25(OH)D] and 1,25-dihydroxyvitamin D [1,25(OH)D] (83), respectively, and ultimately carried to the target tissues (85).
Age, race, health, and even clothing affect the rate at which vitamin D is produced in the skin (86). Endogenous vitamin D production from sun exposure is influenced by climate, namely, reduced and/or inefficient sunlight absorption may cause vitamin D deficiency (86). The synthesis of vitamin D decreases with age (82, 86). Of note, individuals with darker skin pigmentation and complexion need longer sun exposure to produce adequate amounts of vitamin D (87). Approximately 80% of African American women have vitamin D deficiency, compared with only 20% of Caucasian women (88). The higher risk of vitamin D deficiency in African Americans has been attributed to due to darker skin pigmentation and decreased access to solar radiation, resulting in increased risk for uterine fibroids (89).
Adequate vitamin D can also be ensured through diet or supplementation (90). The most stable form in circulating blood, 25(OH)D, is used to assess vitamin D levels in individuals (91). However, different organizations have different classifications for 25(OH)D levels. According to the Endocrine Society, vitamin D deficiency is defined as 25(OH)D serum concentrations ≤20 ng/mL; insufficient, between 21 and 29 ng/mL; and sufficient, ≥30 ng/mL (92). Meanwhile, the United States Institute of Medicine (IOM) defines the sufficient 25(OH)D serum level as ≥20 ng/mL (93). (94)
Some experts consider low 25(OH)D serum concentrations as a marker of poor health (90). Conversely, increased concentrations of vitamin D have been associated with reduced prolonged menstruation cycle (95), infertility, hyperandrogenism, insulin resistance, and polycystic ovary syndrome (PCOS) (96). Furthermore, abnormal vitamin D levels tend to change the maternal-fetal vascular system and may cause abnormal pregnancy development, dysregulated metabolism, and disrupted placental function (97).
The role of vitamin D in the pathogenesis of uterine fibroids has been investigated (26). Three main studies demonstrated that vitamin D levels are much lower in the sera of uterine fibroid patients, suggesting the vitamin D may be linked to the pathogenesis of uterine fibroids. (98-100).
Lifestyle factors, such as diet and level of physical activity influence the formation of uterine fibroids. Women consuming more green vegetables, fruit, and fish than red meat are less commonly diagnosed with uterine fibroids (27, 49, 101). Of note, African American women consume lesser amounts of fruits, vegetables, vitamins, and minerals compared with White women (102, 103). Diets rich in citrus fruits markedly reduced the risk of uterine fibroids (104). (88, 105, 106).
Protective Factors
The use of oral and injectable contraceptives can reduce the risk of developing uterine fibroids (44). Hormonal contraception protected women from developing clinical symptoms of uterine fibroids (107). However, using oral contraceptives at adolescence may be considered a risk factor for developing symptomatic uterine fibroids later in life, whereas using them after adolescence reduces the risk (44, 108-110). Contraceptives increase estrogen and progesterone concentrations in the body, indicating that mechanisms other than hormonal levels are involved in the development of uterine fibroids (5).
Some substances of plant origin can prevent cell division and formation of fibrosis while modulating hypercritical pathways involved in the development of uterine fibroids (111). The use of phytochemicals in the prevention and treatment of uterine fibroids has been investigated and showed promising options (111-113). However, some substances that had been considered potentially helpful have been associated with adverse effects. For example, elevated vitamin E concentration in the serum may be an risk factor for uterine fibroids in Caucasian women. Vitamin E can function as a ligand for estrogen receptors (ERs) due to its structural determinants (29).
In addition, the consumption of milk and other dairy products may influence the development of uterine fibroids. An increased risk of uterine fibroids was associated with consumption of milk or soybeans (114). Other prospective cohort studies have yielded controversial results. One study reported no clear association with overall dairy consumption, whereas another study found that yogurt consumption and calcium intake from foods reduced the risk of uterine fibroid development (115). Moreover, some of the risk factors are described in the environmental exposure section.
Uterine Fibroids Driver Mutations
Within the past decade, the application of rapidly advanced genomic technologies, including high-throughput sequencing methodologies, has led to the identification of recurrent and largely mutually exclusive genetic alterations (so-called drivers) responsible for the formation of uterine fibroids. Among these, somatic mutations in the Xq13 gene encoding the RNA Polymerase II (Pol II) mediator subunit MED12 are the most prevalent, occurring in 45–90% cases of uterine fibroids depending upon patient ethnicity (8, 116-128). A proportionally smaller fraction of uterine fibroids has been attributed to genetic alterations leading to the overexpression of HMGA2, disruption of the COL4A5-COL4A6 locus, and biallelic loss of FH encoding the tricarboxylic acid (TCA) cycle enzyme fumarate hydratase (117, 129). Additionally, recurrent deletions and rearrangements involving chromosomes 6p21, 7q22, 22q, and 1p have been observed in patients with uterine fibroids. However, these mutations generally co-occur with other genetic alterations, suggesting that they represent secondary driver events restricted to a subpopulation of tumor cells (121, 130-133). Altogether, the identification of different fibroids driver mutations has permitted the genetic stratification of these tumors into at least 4 molecular subtypes (129, 134, 135). Interestingly, transcriptome-wide gene expression profiling studies of different uterine fibroid subtypes have revealed that distinct driver mutations are generally characterized by unique gene expression signatures, indicative of distinct pathways to tumorigenesis. This suggests that MED12 mutation–positive and MED12 mutation–negative uterine fibroids are likely unrelated by driver mutations occurring in a common MED12-dependent pathway (129, 134). There are 4 main driver mutations discovered in uterine fibroids.
MED12
High-frequency MED12 mutations have been observed in tumors from women of diverse racial and ethnic origins, including those of North American, European, African, Asian, and Middle Eastern descent, thus implicating MED12 as a dominant universal driver of uterine fibroids (8, 118-128). Nonetheless, data from a recent meta-analysis indicates that MED12 mutations occur more frequently in women of African as opposed to non-African descent (136). Regarding uterine fibroid–linked mutations in MED12, are all located within exons 1 or 2, and most are missense mutations with a smaller proportion corresponding to small in-frame deletions and insertions (118, 129, 137). Exon 2 mutations are far more frequent than those in exon 1, with the latter accounting for ~1% to 2% of pathogenic alterations reported in uterine fibroids (10, 129). Although missense mutations in exon 2 are distributed throughout the coding sequence, most are clustered in codons 36, 43, and 44, suggesting the importance of their corresponding and evolutionarily highly conserved amino acid residues (7, 8, 118, 121, 128). Notably, in addition to uterine fibroids, MED12 exon 2 mutations are also found at similarly high frequency (~80%) in breast fibroepithelial tumors, and to a lesser extent (~5%) of chronic lymphocytic leukemias (138-144).
In addition to their high frequency occurrence, several additional findings suggest that MED12 mutations are true drivers of fibrotic transformation. First, predominant monoallelic expression of mutant MED12 has been observed almost uniformly in MED12 mutation–positive uterine fibroids, indicating a pathogenic requirement for a functionally altered MED12 allele (7, 129, 137). Second, targeted expression of a MED12 mutant transgene (c. 131G>A; p.G44D) in the uterine mesenchyme of mice was sufficient to induce uterine fibroid formation, providing direct genetic proof of disease causality (145).
Combined molecular and clinical analyses have been applied to identify relationships between MED12 mutation status and tumor characteristics as well as patient clinical variables. These analyses have consistently revealed that MED12 mutations are associated with smaller tumor size, conventional tumor histology, and increased tumor number within the uterus (7, 123, 146-152). While most of these studies have been underpowered to detect associations with additional clinical features, a comparatively larger analysis including 750 fibroid tumors from 244 hysterectomy patients confirmed these associations and additionally found MED12 mutations to be positively correlated with subserosal (compared to intramural) location and inversely correlated with parity (10). No associations were observed between MED12 mutations and patient infertility, smoke consumption, BMI, history of pelvic inflammatory disease and chlamydia, hypertension, thyroid disorder, diabetes, oral contraceptive use, or family history of uterine fibroids (10). The observation that MED12 mutation–positive uterine fibroids are associated with a subserous location was subsequently confirmed in another large retrospective study that included 361 tumors from 234 myomectomy patients whose median age of 34 years also revealed that the MED12 mutation frequency in uterine fibroids from fertile-age women is comparable to that found in perimenopausal women (153). Altogether, these analyses support the relevance of MED12 driver mutations in the pathogenesis and clinical presentation of uterine fibroids.
The noted association between MED12 mutations and smaller tumor size has been variously ascribed to underlying study bias (ie, early clinical intervention in response to the combinatorial burden of multiple co-existing MED12-mutant tumors) or inherent biological differences in the growth properties of MED12 mutation–positive and MED12 mutation–negative tumors in situ. While the underlying basis for this association remains unknown, the notion that MED12 mutation–positive and MED12 mutation–negative tumors might exhibit unique growth features is supported by studies showing a clear distinction in the ability of primary cells from either tumor type to survive monolayer culture in vitro. Thus, while primary cells from MED12 mutation–negative uterine fibroids were shown capable of survival and maintenance for many passages under normal culture conditions, those derived from MED12 mutation–positive tumors were shown to be rapidly lost within the first several passages (154). Interestingly, while passaging of cells was noted to accelerate the loss of MED12–mutated cells from cultures, cell loss was nonetheless still observed in confluent cells absent passaging, revealing an apparent requirement for a niche-derived soluble factor(s) or matrix component(s) that is lacking in vitro (155). These novel findings reveal inherently unique growth requirements, and possible therapeutic vulnerabilities, for cells from MED12 mutation–positive uterine fibroids, and further suggest that alternative models will be required to overcome what currently constitutes a significant barrier to mechanistic studies concerning the molecular basis of MED12 in the pathogenesis of uterine fibroids.
An extenuating factor in rapid loss of MED12-mutant cells from culture may relate to the recent observation that MED12 mutation–positive uterine fibroids, compared with MED12 mutation–negative tumors, exhibit apparently greater cellular heterogeneity. In this regard, prior studies have revealed that uterine fibroids, while clonally derived, are nonetheless heterogeneous in their cellular composition, consisting predominantly of smooth muscle cells and fibroblasts, along with smaller numbers of vascular smooth muscle cells, vascular endothelial cells, and immune cells (156, 157). Significantly, recent work has shown that MED12-mutant tumors, compared with MED12-WT (HMGA-overexpressing) tumors, harbor significantly more collagen-producing tumor-associated fibroblasts (TAFs) that also contribute significantly to excessive levels of extracellular matrix (ECM) observed in MED12-mutant tumors (158). Notably, only smooth muscle cells, but not TAFs, carry MED12 mutations, suggesting antecedent divergence from a common progenitor before cell type–specific mutation acquisition or, alternatively, an extratumoral origin for TAFs. Interestingly, this work also showed that within MED12-mutant tumors, smooth muscle cells grow in response to progesterone, which has no effect on TAFs that instead grow in response to estrogen (158). The observation that MED12 mutation–positive uterine fibroids comprise similar ratios of smooth muscle cells and TAFs that respond differently to steroid hormones could explain the intriguing observation that estrogen alone can attenuate regression, but not promote growth, of progesterone-dependent MED12-mutant tumor xenografts (159). The high ratio of TAFs could also explain the rapid disappearance of MED12-mutant smooth muscle cells from primary cultures of uterine fibroids. Thus, growth-deficient MED12-mutant cells could be overwhelmed by TAFs, for which standard culture conditions were originally optimized. Ultimately, the number of heterogenous cell types within MED12 mutation–positive uterine fibroids and the degree to which they are clonally related remains to be firmly established, and newer technologies, including single-cell RNA sequencing, could help to resolve these outstanding issues.
The molecular basis by which pathogenic mutations in MED12 drive uterine fibroid formation is presently unclear, but dysregulation of RNA Pol II-driven gene expression is implicated. Mediator is a conserved multiprotein interface found between gene-specific transcription factors and Pol II (137) and channels regulatory signals from activator and repressor proteins to affect changes in gene expression programs that control diverse physiological processes, including cell growth, homeostasis, development, and differentiation. Structurally, Mediator is comprised of a 26-subunit core that binds tightly to Pol II in the so-called holo-enzyme (137). MED12, MED13, CycC, and CDK8 (or its paralog CDK19) comprise a 4-subunit “kinase” module that variably associates with the core Mediator (137).(137). Notably, the kinase module is a major ingress of signal transduction through the Mediator, and MED12-dependent CDK8 activation is required for the nuclear transduction of signals initiated by multiple oncogenic pathways, with which MED12 is biochemically and genetically linked (Fig. 3) (137). Furthermore, MED12 is a target of oncogenic mutation in colon, prostate, and renal cell carcinomas (119, 160, 161). However, these mutations predominantly occur in the MED12 C-terminus and thus lie distant from fibroids-linked mutations that cluster in the N-terminus, suggesting distinct tumorigenic mechanisms (162).
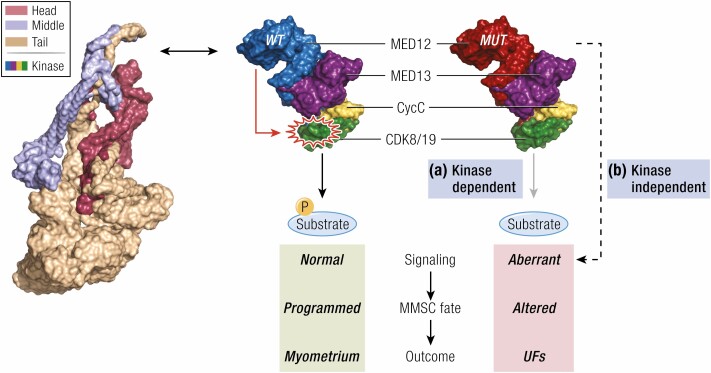
Figure 3.
Role of MED12 mutation in the pathogenesis of fibroids. Two mutually compatible models are demonstrating that fibroids driver mutations in MED12 trigger myometrial stem cell transformation and fibroids formation through altered signaling. In the first model (A), MED12 mutations in exon 2 disrupt the CDK8 T-loop conformation to affect Mediator kinase activity and the phosphorylation of downstream targets, including those that control myometrial stem cell fate and/or function. In the second model (B), MED12 mutations alter gene expression programs that control myometrial stem cell fate and/or function through kinase-independent mechanisms, such as MED12 interactions with transcriptional regulatory proteins (173). The 2 models are not mutually exclusive, and both scenarios could contribute to fibroids pathogenesis. Shown here is the 4-subunit Mediator kinase module comprising MED13, MED12, CycC, and CDK8/19 that variably associates with a core Mediator, which is collectively composed of 26 different subunits arranged into 3 structurally defined domains, ie, Head, Middle, and Tail. The structure of the core Mediator is from Clark et al (137), whereas that of the kinase module is from Li et al (167). Abbreviations: CDK8/19, cyclin-dependent kinase 8/19; CycC, cyclin C; MED12/13, RNA polymerase II transcriptional mediator complex subunit 12/13; MMSC, myometrial stem cell; UFs, uterine fibroids.
Uterine fibroid–linked mutations in MED12 are all located within exons 1 or 2, most of which are missense mutations, and a smaller proportion include in-frame deletions and insertions (118, 129, 137). Particularly, those occurring in exon 2 are far more frequent than those in exon 1, with the latter accounting for ~6% of pathogenic alterations reported in uterine fibroids (129). Although missense mutations in exon 2 are distributed throughout the coding sequence, most are clustered in codons 36, 43, and 44, suggesting the importance of their corresponding and evolutionary highly conserved amino acid residues (7, 8, 118, 121, 128).
Within the Mediator kinase module, MED12 is known to activate CycC-CDK8, and the mechanistic basis has recently been clarified (128, 163-167). Thus, MED12 binds directly to CDK8, leading to structural reconfiguration and stabilization of the CDK8 activation (T)-loop in a manner critically dependent upon MED12 residues recurrently mutated in uterine fibroids (167). These observations suggest that uterine fibroid driver mutations in MED12 could alter T-loop conformation and disrupt CDK8 kinase activity (Fig. 3) (167). Indeed, pathogenic exon 2 mutations in MED12 have been confirmed to disrupt CDK8/19 kinase activity both in vitro and in clinically relevant patients with uterine fibroids (128, 129, 165, 166). Collectively, these studies reveal a common molecular defect associated with uterine fibroid–linked mutations in MED12 and implicate the aberrant Mediator-associated CDK8/19 kinase activity in the pathogenesis of uterine fibroids. Mechanistically, Mediator kinase activity has been implicated in diverse cellular processes ranging from controlling transcription factor half-life and RNA Pol II activity to regulating chromatin chemistry and functional status (137, 168, 169). Accordingly, its disruption as a direct consequence of uterine fibroid mutations in MED12 could have broad implications for the dysregulation of gene expression programs that collectively contribute to tumor formation. Nonetheless, MED12 has also been shown to regulate transcription in a CDK8-independent manner (170-173), although this function is largely mediated through the MED12 C-terminus that lies spatially distant from N-terminal residues mutated in uterine fibroids (Fig. 3). Because MED12 mutations have been linked to pathways directly implicated in uterine fibroid pathology, including the Wnt/β-catenin, protein kinase B/mammalian target of rapamycin (AKT/mTOR), progesterone receptor, focal adhesion, extracellular matrix, angiogenic, and HIF1α pathways, among others (7, 134, 137, 174-176), the relative contribution of CDK8 to MED12-dependent regulation of these pathways and the extent to which altered Mediator kinase activity contributes to their dysregulation will be an important area of future investigation.
Finally, the molecular basis for the high-frequency occurrence of MED12 exon 2 mutations in uterine fibroids is not presently understood. Either of 2 alternative scenarios can be posited. First, high-frequency MED12 exon 2 mutations might simply reflect the selection of clustered mutations among disparate others arising randomly throughout the MED12 gene through errors of replication, particularly if these mutations similarly impact an important biological function of MED12 in the myometrium. As described previously, all uterine fibroid driver mutations in MED12 disrupt Mediator-associated CDK8/19 kinase activity, and it is perhaps notable that Mediator kinase has been implicated in the control of stem cell plasticity and fate determination. For example, a developmentally programmed reduction in CDK8 expression is associated with naïve pluripotency during animal development in vivo, and chemical inhibition of CDK8/19 was recently shown sufficient to revert primed pluripotent stem cells to a naïve pluripotent state in vitro (177, 178). Furthermore, CDK8 has been implicated in cancer stem cell self-renewal and tumorigenicity in colon and brain cancer (179, 180). Finally, within the uterus, it was recently shown that Mediator kinase subunits are enriched in myometrial stem cells (MMSCs), and further, that chemical inhibition of CDK8/19 in MMSCs led to reduced phosphorylation of stem cell-enriched transcription factors and altered expression of myogenic genes. Thus, it seems possible that MED12 exon 2 mutations, through disruption of Mediator kinase activity, could provide a selective advantage to myometrial stem/progenitor cells by altering their growth and/or differentiative trajectory, leading to the formation of uterine fibroid stem cells that, in turn, seed and sustain monoclonal tumor growth.
An alternative hypothesis to explain the high-frequency occurrence of MED12 exon 2 mutations invokes a sequence- or structure-specific basis for clustered mutagenesis through error-prone repair of site-specific DNA damage. Replication fork arrest as occurs, for example, on repeat and satellite sequences or noncanonical B-DNA, is often processed through DNA double-strand break intermediates, which are prone to erroneous repair and punctual mutagenesis (or chromosomal rearrangements) (181). Accordingly, such error-prone sequences, should they reside in the vicinity of MED12 exon 2, might favor the occurrence of high-frequency somatic mutations found in uterine fibroids. However, the genomic sequence within and flanking MED12 exon 2 is characterized by neither particularly high GC content nor repeat motifs characteristic of replication-resistant DNA, perhaps arguing against replication-dependent site-specific mutagenesis as a basis for the high-frequency occurrence of MED12 mutations. Nonetheless, it was recently noted that this region does harbor a 16-bp sequence with significant homology to a putative terminator-based hairpin sequence within the tRNA gene cluster of Staphylococcusaureus, a common component of the uterine microbiota (182). On this basis, it was hypothesized that MED12 hot-spot mutations arise through site-specific mutagenesis brought on by replication-dependent processing of aberrant R-loops produced by insertion of S. aureus RNA with the homologous DNA sequence in MED12 (182). Although speculative, this intriguing hypothesis nonetheless does invoke a direct link between host and microbiome that together form a complex interrelationship prone to homeostatic disruption and the development of human disease (183, 184). While the genic basis for high-frequency MED12 mutations in uterine fibroids thus remains obscure, it is nonetheless clear that once incurred, these mutations interact with additional environmental components including hormonal, angiogenic, and growth regulatory factors to drive tumor progression.
HMGA2
The high mobility group A (HMGA) family includes related HMGA1 and HMAG2 non-histone chromosomal proteins that regulate transcription by altering chromatin structure. The HMGA non-histone proteins bind to the AT-rich enhancers or promoters’ minor groove and introduce structural alterations in chromatin. One of the most commonly observed cytogenetic abnormalities (8%-10%) in uterine fibroids is a translocation involving chromosomes 12 and 14, which disrupts a putative regulatory sequence typically 5′ of the HMGA2 gene (185, 186). In addition, the expression levels of HMGA2 are elevated in uterine fibroids compared to myometrium with 12q15 rearrangements (187, 188). Uterine fibroids with HMGA2 aberrations displayed significant upregulation of proto-oncogene pleomorphic adenoma gene 1 (PLAG1), suggesting that HMGA2 triggered the pathogenesis of uterine fibroids through PLAG1 activation (189).
FH
Mutations in fumarate hydratase (FH) on chromosome 1 in band q42 were found in uterine fibroids (190, 191). Heterozygous germline mutations in the FH gene caused a syndrome known as hereditary leiomyomatosis and renal cell carcinoma (HLRCC) (192). FH deficiency, accounting for up to 1.6% of uterine fibroids, alters the expression profiles of fibroids, most strikingly increasing the expression of genes involved in glycolysis (132) as well as activating nuclear factor erythroid 2 related factor 2 (NRF2) target genes (189).
COL4A5/COL4A6
Similar to the FH deficiency subtype, COL4A5/COL4A6 deletions are a rare subtype constituting about 2% of uterine fibroids (193). Integrated data analysis reveals insulin receptor substrate-4 (IRS4), a gene located adjacent to COL4A5, as the most uniquely expressed gene in this uterine fibroid subtype (189).
Additionally, a small number of mutually exclusive drive mutations were recently identified. Germline mutations in SRCAP members YEATS4 and ZNHIT1 predispose women to uterine fibroids. The fibroids bearing these mutations exhibited defective deposition of the histone variant H2A.Z (194). Moreover, an integrative computational approach (decomposition and classification of genomic tensors) can discriminate normal and uterine fibroid subtype (195), suggesting that the inclusion of epigenetic features can help better understand the state and complexity of uterine fibroids.
Conversion of Myometrial Stem Cells to Uterine Fibroid Stem Cells
The human myometrium is the muscular wall of the uterus that is formed by an intricate network of smooth muscle fibers dispersed throughout an extracellular matrix of connective tissue. This process contributes to the normal tonicity of the uterus. Increasing evidence supports the hypothesis that uterine fibroids originate from stem cells in the myometrium, although the specific cell of origin has not yet been identified (196, 197). Stem cells derived from the myometrium and uterine fibroids have been isolated, and tumor-initiating cells in fibroids have been identified (198-201). Moreover, the markers used to enrich putative mesenchymal stem cells are similarly enriched for MMSCs from myometrium and uterine fibroids (202). Notably, MED12 mutations are only found in uterine fibroid stem cells and not in MMSCs (203). In addition, distinct MED12 mutations have been detected in different uterine fibroid tumors derived from the same uterus (7), indicating that the emergence of each mutation might be an independent event. The prevailing model for fibroid pathogenesis invokes the genetic transformation of a single MMSC into a tumor-initiating cell that seeds and sustains clonal tumor growth through endocrine, autocrine, and paracrine growth factors and hormone receptor signaling (204).
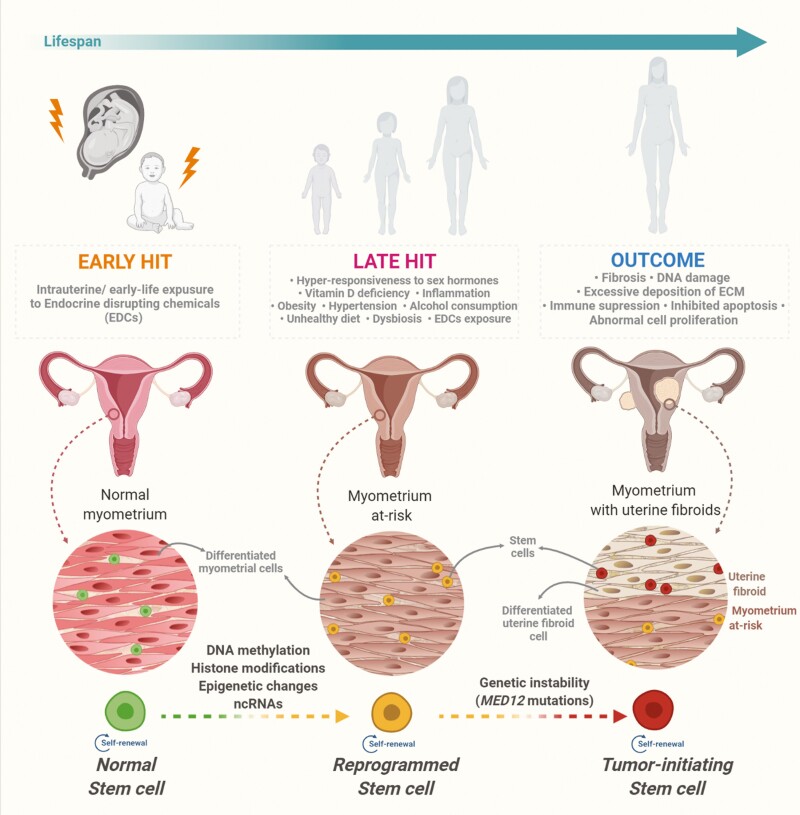
Several factors have been proposed as the origin of tumor-initiating cells, including genomic instability, inflammatory microenvironment, cell fusion, lateral gene transfer, and developmental environmental insult (205, 206). The adverse effect of developmental environmental insult may cause the deregulation of multiple developmental processes, including the disruption of stem cell niche, developmental reprogramming, and altered stem cell characteristics. Somatic stem/progenitor cells from various hormone-supported tissues remained susceptible to endocrine-disrupting chemicals (EDCs) (206, 207). In uterine fibroids, developmental exposure to EDCs impaired the biological characteristics of MMSCs in an Eker rat model with Tsc2 mutation. This model spontaneously develops uterine fibroids with 63% incidence. However, early-life exposure to EDCs, such as diethylstilbestrol (DES), increased the penetrance of the Tsc2 mutation, resulting in 100% incidence (208).
The impact of environmental exposure to MMSCs that increase susceptibility to uterine fibroid development have been investigated using the same model. The more MMSCs in the DES-exposed myometrium have been observed than those exposed to vehicle. In addition, MMSCs from 5-month-old DES rats exhibited increased proliferation rates compared to MMSCs from age-matched control rats (206). These results suggest that developmental exposure to EDCs targets MMSCs and alters their characteristics, which may underlie reprogramming of epigenome and initiation of hormone-dependent uterine fibroid pathogenesis (Fig. 1).
Early and late epigenome and environment interactions can potentially impact uterine function and increase the risk of uterine fibroids (Fig. 1) by shaping the developing epigenome of target genes (209). This epigenomic reprogramming may remain transcriptionally and phenotypically silent until triggered by a later life event, such as exposure to risk factors. For example, during a critical developmental window of the liver, exposure to BPA induced epigenomic reprogramming at specific genes and chromatin states in the neonatal liver to accelerate acquiring an adult epigenetic signature. Although it persists until adulthood, much of this reprogramming remained transcriptionally silent until a later-life challenge with a Western-style diet high in fat, fructose, and cholesterol, which disrupted metabolic function and significantly elevated serum cholesterol and lipid levels (209). Further studies on stem cells from reproductive organs may contribute to a better understanding of the genome-environment interaction leading to reproductive diseases, including uterine fibroids.
The occurrence of MED12 driver mutations and how they interact synergistically with other implicated pathways in uterine fibroids remain largely unknown. Therefore, the further investigation is highly needed to elucidate the mechanism of interplay between hormones, environments, DNA repair system, and other factors in the occurring of MED12 mutations.
Environmental Exposure and Pathogenesis of Uterine Fibroids
Direct, Intensive, and Adverse Environmental Exposure
Air pollution
Air pollution is one of the leading causes of death. Exposure to air pollutants affects vital cellular mechanisms and is intimately linked with the etiology of many chronic diseases such as chronic obstructive pulmonary disease and asthma (210-212). Particulate matter (PM) is a class of pollutants that comprises a complex combination of small-sized particles and gaseous components, such as organic chemicals, smoke, soot, sulfates, nitrates, acidic components, dust particles, and soil. The United States Environmental Protection Agency considers PM the pollutant category with the most significant impact factor on human health (213-215). Air pollution, including PM2.5, results in infertility, menstrual irregularity, and endometriosis (216). In addition, chronic exposure to PM2.5 is associated with the incidence of clinically symptomatic uterine fibroids (216). A 10-year cohort-based case-control study that included 11 028 Taiwanese women diagnosed with uterine fibroids suggested that exposure to PM2.5 and O3 may increase the risk of developing uterine fibroids (217). However, only limited research has investigated the relationship between air pollution and uterine fibroid development; therefore, more studies are needed to confirm these findings in other populations.
Alcohol consumption
Heavy alcohol consumption is a risk factor for uterine fibroids (35). The Nurses’ Health Study II revealed the positive association between current alcohol consumption and risk of uterine fibroids (35, 218). The Black Women Health Study concluded that uterine fibroids risk among African American women is positively correlated with past and current alcohol intake (219). In a study involving 133 000 female teachers and school administrators, drinking at least 20 g of alcohol per day was significantly associated with an increased risk for uterine fibroids (220). Another cross-section study on premenopausal Japanese women supported this causal risk factor for uterine fibroids and reported that mean alcohol intake is significantly higher among women with fibroids than those without (221). Moreover, a study of 1146 premenopausal African American and Caucasian women showed that current alcohol intake in Caucasian women is associated with an increased risk of uterine fibroids compared to African Americans and nondrinkers. Although no correlation between alcohol intake and uterine fibroid risk in African Americans was found, the relationship of current and past drinking history and uterine fibroid size was generally similar among African American and White women (35).
Although the underlying mechanism is largely unknown, several studies have proposed that alcohol intake increases the levels of steroid hormones in premenopausal women (222-224). Alcohol intake also altered the growth factors and cytokines, which play a critical role in uterine fibroid pathogenesis. Moreover, alcohol-induced DNA damage might be a contributor. Acetaldehyde, an endogenous and alcohol-derived metabolite, caused DNA damage, particularly double-stranded breaks, that, despite the activation of recombination repair, resulted in chromosomal rearrangements in stem cells (225). Other studies have reported alcohol-induced mitochondrial DNA damage in lung, brain, and breast cancers (226-228). More studies are needed to explore alcohol-induced DNA damage in uterine fibroids.
Cigarette smoking
The effect of smoking on uterine fibroids remains controversial (229). An inverse correlation between smoking and uterine fibroids risk was reported (220, 230, 231). However, this association was not found in other case-control and prospective cohort studies (2, 232, 233). Early studies found that estrone and estradiol levels were reduced in smokers relative to nonsmokers, and cigarette smoking altered the hepatic metabolism of estrogen, thus resulting in lower circulating levels of activated estrogen (2). However, the components of cigarette smoke may also exert estrogen-related effects on the uterus to promote cell proliferation (2).
Developmental Exposures
Epidemiological studies and endocrine-disrupting chemical effects
Various niche factors act on stem cells during development to alter gene expression and induce their proliferation or differentiation for fetus development. During development and tissue maintenance, the highly plastic state of stem/progenitor cells permits the required flexibility for proper tissue formation and repair. Unfortunately, this plasticity also provides an opportunity for aberrant cellular reprogramming via epigenetic mechanisms due to inappropriate exposures to toxins (234). Developmental adverse exposure can lead to persistent, life-long effects and result in various diseases (235-237).
EDCs interfere with the body’s endocrine system to produce adverse developmental, reproductive, neurological, and immune effects (198, 238, 239). An increasing number of studies have shown that endocrine disruptors may pose a serious disease risk during development (240). According to epidemiological and experimental studies, EDCs increased the risk of tumorigenesis, especially in organs susceptible to endocrine regulation. For example, upon exposure to estrogen and progesterone, differentiated myometrial cells secreted wingless-type (WNT) ligands that induced the nuclear translocation of β-catenin in stem/progenitor cells from uterine fibroids. The activation of the β-catenin pathway ultimately enhanced the growth and proliferation of these stem/progenitor cells (241).
EDCs can exhibit nonmonotonic dose–response curves and produce a pathophysiologic effect even at low doses. Numerous EDCs can interact with nuclear receptors to exert their actions in target cells and tissues (242-244). For example, the binding of EDCs to nuclear receptors can alter hormonal functions by mimicking the naturally occurring hormones in the body, thereby blocking the binding of endogenous hormones, or by interfering with the production or regulation of hormones and/or their receptors. An EDC may interact with more than one receptor, and multiple EDCs can interact with the same receptor, highlighting the complexity of the response of animals and humans to environmental EDC exposures. Notably, EDCs exposure can increase the risk of uterine fibroids (Figs. 2 and 4). Two extensive prospective studies reported a positive association between developmental exposure to DES, a synthetic and nonsteroid estrogen, and uterine fibroids risk. (245, 246). In the Nurses’ Health Study II (n = 11 831), prenatal exposure to DES increased the risk of uterine fibroids by 13% in women older than 35 years (246), and exposure during the first trimester of gestation increases the risk by 21%. Large fibroids were more commonly found in those exposed to prenatal DES in the second National Institute of Environmental Health Sciences (NIEHS) uterine fibroid study. In a subset of the NIEHS sister study, the main factors associated with increased risk of uterine fibroids included DES exposure, maternal or gestational diabetes, and monozygotic twins, having risk ratios of 2.02, 1.54, and 1.94, respectively. However, another prospective cohort study, which employed medical records to document exposure, reported no association between prenatal DES exposure and uterine fibroids. Many other EDCs, including parabens, environmental phenols, alternate plasticizers, organophosphate esters, tributyltin, and phthalates, have been associated with uterine fibroids outcomes and their related processes. Phthalates have received increasing attention as they are tightly linked to uterine fibroid prevalence and severity (31, 247-249) (Figs. 1 and 2).
Figure 4.
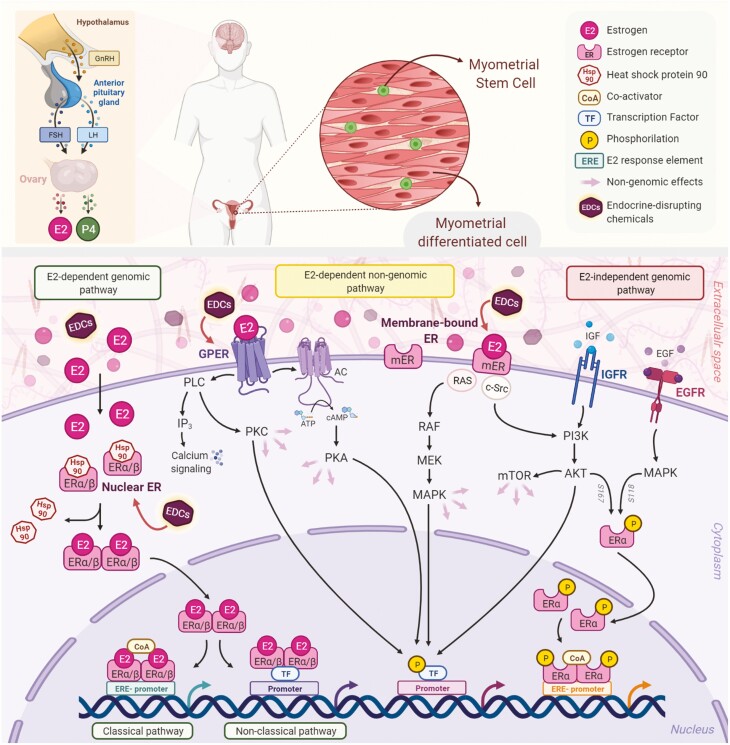
Estrogen receptor-mediated signaling pathways in the myometrium. The biosynthesis of natural E2 occurs in the ovary downstream the actions of the LH and the FSH, which are regulated by the GnRH. E2 mediates its biological response through several pathways, which can be classified as genomic and nongenomic. There are 3 main mechanisms of genomic regulation mediated by ER. Firstly, in the classical pathway, E2 ligands passively enter the cells by diffusion. ERα and ERβ are localized in the cytosol and are attached to the chaperon Hsp90, which is released after binding with estrogen. The estrogen-bound receptors form dimers that enter the nucleus and bind to the ERE, specific DNA sequences of the promoters of target genes affecting their transcription. Secondly, the nonclassical pathway involves binding the E2-bound ER to TFs that are already bound to the DNA. The third mechanism is hormone-independent. The ER can regulate E2 responses by activating the signaling of growth factors via the phosphorylation of different serine (118/167) residues on the receptor. In addition to upregulating gene expression, E2 exerts its nongenomic rapid biological actions by interaction with membrane receptors. GPER, a membrane-integrated 7-transmembrane receptor, activates heterotrimeric G-proteins after binding with estrogen to elicit various nongenomic responses, such as calcium signaling, PKC, and cAMP/PKA pathways. Bound-membrane ERs (ERα, ERβ, ER36, and ER46) also activate cytosolic signalings, such as PI3K/Akt and MAPK. In addition, the activation of kinases results in the phosphorylation of specific transcription factors that regulate gene expression. EDCs are exogenous, manufactured chemicals, such as genistein, bisphenol A, and phthalates that mimic natural estrogen molecular and cellular responses, thereby altering the functions of the endocrine system. These chemicals are associated with the developmental origin of fibroids and their pathogenesis. Abbreviations: AC, adenylyl cyclase; AKT, protein kinase B; cAMP, cyclic AMP; CoA, coactivator; E2, estrogen; EDCs, endocrine-disrupting chemicals; EGFR, epidermal growth factor receptor; ER, estrogen receptor; ERE, estrogen-responsive elements; FSH, follicle-stimulating hormone; GnRH, gonadotropin-releasing hormone; GPER, G-protein coupled estrogen receptor 1; Hsp90, heat shock protein 90; IGFR, insulin-like growth factor 1 receptor; IP3, inositol trisphosphate; LH, luteinizing hormone; MAPK, mitogen-activated protein kinase; MEK, mitogen-activated protein kinase kinase; mER, membrane-bound estrogen receptor; mTOR, mammalian target of rapamycin; PI3K, phosphoinositol-3-kinase; PKA, protein kinase A; PKC, protein kinase; PLC, phospholipase C; Raf, Rapidly Accelerated Fibrosarcoma Kinase; Ras, Ras GTPase; TFs, transcription factors.
Experimental studies using animal models
Diverse animal species and techniques have been used for the in vivo investigation of uterine fibroid pathophysiology. These animal models include the xenotransplantation of human uterine fibroid tissues (250-252) or cells (253, 254) in mice, the implementation of genetically modified mice (Tsc2 knockout (255), GPR10 overexpression (256), β-catenin overexpression (257)), and the utilization of species that spontaneously develop uterine fibroids, such as the guinea pig (258), the potbellied pig (257), and the Eker rat. The latter carries a germline mutation in the tuberous sclerosis complex-2 (Tsc2) tumor suppressor gene and develop uterine fibroids with a frequency of about 65% by 16 months of age (259). Although the Eker rat model is the most widely used in vivo animal model to study uterine fibroids, this animal model has some limitations. For example, mutations in the Tsc gene have not been linked to the disease in humans. In addition, the developing uterine fibroids show relatively small amounts of collagenous connective tissue stroma (260), unlike the human uterine fibroids, which present a high amount of abnormally formed cross-linked collagen (261). Finally, Eker rats develop both benign and malignant smooth muscle tumors (260). However, studies in the Eker rat animal model provide a great opportunity to reveal links between early-life exposure to EDCs and the origin and development of uterine fibroids. Upon neonatal exposure to EDCs, Eker rats developed increased susceptibility to spontaneous uterine fibroids, multiplicity, and tumor size with age (208, 262-264), whereas those without the Tsc2 mutation did not develop any tumors. These studies suggest that developmental exposure to EDCs increases the penetrance of the Tsc2 mutation (208). In addition, the window of susceptibility to environmental exposures coincided with critical periods of myometrial development (208). Exposure to DES during postnatal day (PND) 3–5 or 10–12 increased tumor incidence from 63% to 95% and 100%, respectively, in Eker rats carrying germline TSC2 mutation. During this time, estrogen protection of the developing uterus is disrupted (265) as DES and other xenoestrogens do not bind circulating steroid hormone–binding proteins, such as alpha-feto protein A. A later exposure at PND 17–19 did not result in increased uterine fibroid incidence. Overall, developmental exposure to EDCs during a critical time window of uterus development increases uterine fibroid risk later in life (Figs 1 & 2).
Molecular mechanism underlying developmental EDC exposure–induced risks of uterine fibroids
During development, various niche factors act on stem cells to alter gene expression, therefore altering the signaling pathway and modulating its biological characteristics for the development of the fetus. The adverse developmental exposure can lead to persistent, life-long effects and result various diseases via pathological reprogramming (208, 209, 234, 240, 266).
Early-life exposures to 3 EDCs (ie, DES, genistein, and BPA) have been investigated to detect their effect on estrogen signaling, which plays a role in triggering fibroids formation in an animal model (267-269). All 3 EDCs act as ER ligands and induce ER-mediated gene transcription. However, only DES and genistein induced nongenomic ER signaling to activate phosphoinositide-3-kinase (PI3K)/AKT in the developing uterus. The histone methyltransferase enhancer of zeste homolog 2 (EZH2) is phosphorylated by activated PI3K/AKT signaling to repress EZH2 activity and reduce the levels of histone 3 lysine 27 trimethyl (H3K27me3). Significantly, decreased H3K27 methylation via developmental exposure correlated with the promoting effect of xenoestrogens on uterine fibroids.
In addition to EZH2, altered DNA methylation patterns due to environmental exposure have been reported in animal studies. Neonatal exposure induced the reprogramming of DNA methylation in animals exposed to DES during PND 1–5 compared with PND 17 (prepubertal), 21, and 30 (postpuberty) (270). Furthermore, neonatal DES exposure reprogrammed LTF, an estrogen-responsive gene. At PND 21 and 30, the promoter upstream of the estrogen response element was demethylated in animals exposed to DES during PND 1–5. Importantly, this postpubertal DES-induced demethylation was dependent on ovarian hormones, as evidenced by the absence of this demethylation in DES-exposed ovariectomized mice (270). Another animal study showed that neonatal DES exposure–induced metabolic changes last until adulthood, suggesting a permanent effect on energy metabolism in the uterus (271). Thus, developmental exposure to EDCs causes uterine diseases via epigenomic reprogramming. However, studies on the mechanism of epigenetic reprogramming by EDCs and its influence on fibroids development are limited. Additional mechanistic studies to elucidate the epigenetic biomarkers/signatures specific to EDCs can contribute to the development of precision medicine.
The reprograming of MMSCs, the cell origin of uterine fibroids, was recently identified following early-life exposure in the Eker rat (PND 10-12) to EDC. MMSCs isolated from prefibroid-stage tissue were analyzed using omics methods and showed altered biological pathways, including estrogen signaling (272) and inflammatory pathways (273, 274). The reprogramming of estrogen pathways is driven by activated mixed-lineage leukemia protein-1 (275). In addition, DNA hypomethylation is involved in regulating estrogen and estrogen-responsive genes in MMSCs (274, 276). In summary, EDC exposure epigenetically targeted MMSCs, imparting a hormonal imprint on key signaling pathways, thus resulting in an increased risk of uterine fibroids in a hormone-dependent manner (274) (Figs. 1, 2, and 4).
Due to some limitations using animal models, the use of 3-dimensional (3D) models has attracted more attention in uterine fibroids research (277-279), particularly using myometrial stem cells instead of differentiated myometrial cells (280). The 3D model provides a more biomimetic cell culture environment than 2D substrates, with the advantage of more closely mimicking in vivo tissue architecture. MMSC-material interactions in 3D with topographical cues may provide an effective means to regulate many fibroid-related biological events, including differentiation, epigenetic state, or cell reprogramming, and rapidly advance our understanding of how the environment impacts risk for this disease as well as the tumor process via conversion of MMSCs to tumor-initiating cells.
Notably, so far, very few studies have attempted to disentangle the effects of early-life exposures concomitantly with late-life exposure on the pathogenesis of uterine fibroids. Thus, mechanistic insights into fibroid pathogenesis through the integration of risk exposures, genetic, epigenome, and MMSC biology will better understand the onset of fibroids.
Key Pathways Contributing to Uterine Fibroids Formation
Estrogen and Progesterone
Classically, uterine fibroids were considered estrogen-dependent tumors, based on their association with reproductive age (281, 282). The estrogen signaling pathway as a major impactful pathway in uterine fibroids comprises genomic (direct and indirect effects of gene expression) and nongenomic factors, including the Ras-Raf-MEK (MAPK/ERK kinase)-mitogen-activated protein kinase (MAPK) and PI3K-phosphatidylinositol-3,4,5-trisphosphate (PIP3)-Akt-mTOR) pathways (the Ras-Raf-MEK-MAPK and PI3K-PIP3-Akt-mTOR pathways, respectively) (Figs. 4 and 5).
Figure 5.
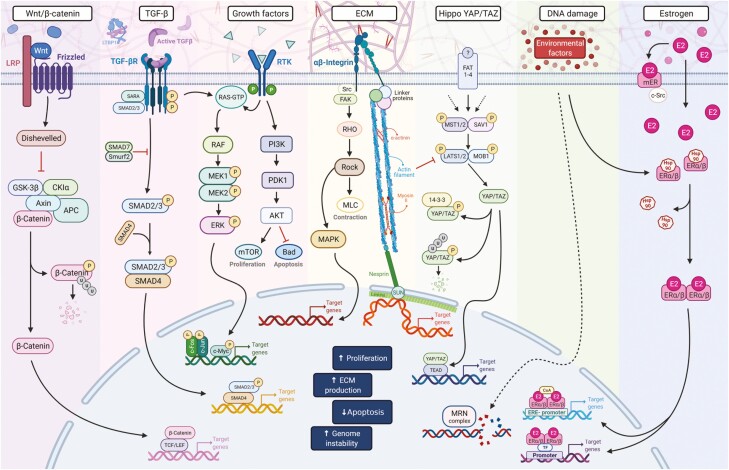
Critical pathways in uterine fibroids pathogenesis. The WNT/β-catenin, TGF-β, growth factor–regulated signaling, ECM, estrogen signaling, YAP/TAZ, Rho/ROCK, and DNA damage repair pathways play essential roles in fibroids formation and development. In addition, the crosstalk and interaction among these pathways may initiate and trigger uterine fibroids pathogenesis. Abbreviations: AKT, protein kinase B; APC, adenomatous polyposis coli; Bad, BCL2 associated agonist of cell death; CK1α, casein kinase 1 alpha; E2, Estrogen; ECM, extracellular matrix; ERE, estrogen-responsive elements; ERK, extracellular-signal-regulated kinase; ERα/β, estrogen receptor alpha/beta; FAK, focal adhesion kinase; GSK-3β, glycogen synthase kinase 3 beta; Hsp90, heat shock protein 90; LRP, lipoprotein receptor-related protein; MAPK, mitogen-activated protein kinase; MEK, mitogen-activated protein kinase kinase; mER, membrane-bound estrogen; MLC, Myosin regulatory light chain 2; MRN, Mre11-Rad50-Nbs1 complex; mTOR, mechanistic target of rapamycin; P, phosphorylated site; PDK1, 3-phosphoinositide-dependent protein kinase 1; PI3K, phosphatidylinositol 3-kinase; RAF, Rapidly Accelerated Fibrosarcoma kinase; RHO, Ras-homologous; RTK, receptor tyrosine kinases; SMAD, mothers against DPP (decapentaplegic); Src, proto-oncogene tyrosine-protein kinase; TF, transcription factor; TGFβ, transforming growth factor beta; TGFβR, transforming growth factor beta receptor; Wnt, Wingless-related integration site; YAP, Yes-associated protein; TAZ, transcriptional coactivator with PDZ-binding domain.
Several aberrations in ERs and signaling pathways are implicated in uterine fibroid pathobiology (283). Recently, another role for estrogen has been identified, ie, estrogen-induced, progesterone receptor expression and allowing progesterone receptor ligands to act on their target cells (284). Uterine fibroid cells have been shown to increase the expression of progesterone receptors in response to estradiol (285). Progesterone and progesterone receptors play a key role in uterine fibroid growth and development (286, 287). Increased proliferation of uterine fibroid cells in vitro was observed upon exposure to both estradiol and progesterone (288). Finally, a uterine fibroid xenograft animal model showed that steroids, including estradiol and progesterone, are required for tumor growth (289), supported by selective progesterone receptor modulators (SPRMs) (290).
Extracellular Matrix and Growth Factors
Excessive ECM accumulation and aberrant remodeling are crucial for fibrotic diseases, including uterine fibroids (Fig. 5). Uterine fibroid cells are characterized by abundant disorganized ECM deposition, which contributes to the formation of the bulk structure of the tumor. The large amounts of glycosaminoglycans and highly cross-linked interstitial collagens present in uterine fibroids (291) underlie the increased stiffness of the ECM. It is proposed that this ECM-rich rigid structure is the cause of the abnormal bleeding and pelvic pain (292). Moreover, ECM stiffness greatly impacts how cells sense physical forces and translate them into biochemical and biological responses, a molecular process collectively known as mechanotransduction. A clear example of proteins that respond to mechanical signals is β-catenin (293, 294), a protein known to be involved in the pathobiology of uterine fibroids (294). It has been proposed that uterine fibroids can be divided into 4 stages based on the extracellular collagenous matrix content (295, 296). Excessive collagen production by the transformed myocyte and its accumulation in the ECM results in decreased microvessel density, followed by myocyte death due to extreme deprivation of nutrients and oxygen (297). At the same time, changes in the ECM stiffness may activate mechanotransduction pathways that contribute to the myocyte phenotypic transformation. Interestingly, recent studies have linked mechanotransduction, nuclear rupture, and subsequent DNA damage in other diseases (298, 299). Unraveling the interactions among different mechanobiology pathways in the uterine fibroid’s context would eventually help to comprehend better the origin and development of these tumors.
ECM accumulation is also affected by growth factors (transforming growth factor [TGF]-β, activin-A, and platelet-derived growth factor ), cytokines (tumor necrosis factor-α [TNF-α]), steroid hormones (estrogen and progesterone) (300), and microRNAs (particularly the miR-29 family, including miR-200c and miR-93/106b) (301-303). Interestingly, ECM acts as a reservoir of profibrotic growth factors and enhances their activity by increasing their stability and prolonging signaling duration. Therefore, a better understanding of ECM composition and metabolism in uterine fibroids is critical for developing new therapeutics for uterine fibroids. At present, several classes of drugs, including gonadotropin-releasing hormone (GnRH) agonist (leuprolide acetate), GnRH antagonists, SPRMs (eg, ulipristal acetate), and natural compounds (vitamin D), targeting the ECM have been studied as treatment options for uterine fibroids (303, 304). In this sense, a local collagenase injection from Clostridium histolyticum, which specifically cleavage interstitial collagens, has been proposed as an alternative treatment for uterine fibroids. Notably, a phase I clinical trial (NCT02889848) has been completed, and it demonstrated the safety and tolerability of this treatment. Furthermore, direct injection of collagenase from C. histolyticum significantly reduced the stiffness of uterine fibroid tissue (305), which is fundamental to continued tumor growth throughout the activation of mechanotransduction pathways. Therefore, such mechanotransduction pathways may be disturbed after the reduction of fibroid stiffness, leading to ECM remodeling and finally to reduced fibroid size.
DNA Damage and Repair
DNA damage in the uterus
Women are exposed to several exogenous (eg, EDCs) and endogenous factors that can impart pathophysiological alterations in internal organs, including the uterus. Endogenous factors include regular menses and hormonal changes that induce DNA damage through oxidative stress. Fluctuations in circulating estrogen and progesterone levels during the menstrual cycle can lead to increased tumor susceptibility in women, including breast cancer. Additionally, DNA damage and repair, and apoptosis occur cyclically in the normal myometrium during the follicular phase. Smooth muscle cells proliferate in the luteal phase, which may be a vulnerable period for DNA damage. These damages need to be properly repaired; otherwise, the hormonal imbalance can lead to diseases. For example, repeated incidents of damage to myometrial cells affect DNA repair activity, which could predispose the uterine environment to chronic inflammation, thereby creating an ideal environment for uterine fibroid development. However, the biological mechanisms involved in uterine fibroid progression remain unknown. This section will discuss studies on DNA lesions in uterine fibroids (306-308) (Fig. 5).
Defective DNA damage response pathways
Although the mechanistic basis underlying genomic instability in uterine fibroids remains to be established, defects in DNA damage response and repair gene expression programs are heavily involved. Several DNA repair genes in uterine fibroid tumors are downregulated compared with adjacent matched myometrium from the same women, suggesting that impaired DNA repair capacity is linked to the genomic integrity and subsequent initiation/propagation of uterine fibroids. In addition, the expression of DNA repair-related genes RAD51 and BRCA1, which are involved in double-stranded break (DSB) homologous recombination (HR), were deregulated in uterine fibroid tumors (309, 310).
Interestingly, human uterine fibroid stem cells have accumulated DNA damage and reduced DNA repair gene expression and signaling, suggesting that human fibroid stem cells have impaired DNA repair capacity compared with normal myometrium stem cells. This compromised DNA repair system may contribute to promote mutagenesis, as well as the growth and propagation of uterine fibroids (311). In addition, DNA damage was significantly increased in uterine fibroids relative to MMSCs, as shown by increased mean percentage DNA in the tail of alkaline comet assay. Moreover, uterine fibroid stem cells had decreased expression of total DNA repair-related proteins belonging to DSB repair, specifically HR, and differential phosphorylation in comparison to adjacent MMSCs, indicating altered DNA damage response and increased DNA damage as evidenced by increased phosphorylation of histone H2A.X at serine 139 (ie, γ-H2AX) as a response to DNA DSB formation (311).
A germline mutation in Tsc2 predisposes to uterine fibroids in Eker rats, which is attributed to “second hits” in the normal allele of this gene. The risk for developing these tumors is significantly increased by early-life exposure to EDCs, suggesting that the early drivers for these tumors modulate increased uterine fibroid penetrance. Analyses of DNA repair capacity using gene and protein expression and DNA repair function in MMSCs derived from adult rats exposed to DES during uterine development were conducted. Adult MMSCs isolated from developmentally exposed rats showed decreased DNA end-joining ability, increased DNA damage, and impaired ability to repair DNA double-strand breaks compared with those from age-matched, vehicle-exposed rats, suggesting that early-life developmental EDC exposure alters the power of MMSCs to repair and reverse DNA damage, thereby providing a driver for the acquisition of mutations that may promote the development of these tumors in adulthood (312).
Other types of DNA repair, including nucleotide excision repair, which removes bulky DNA adducts caused by polycyclic aromatic hydrocarbons, and base-excision repair of oxidative DNA damage (313), should be explored in the context of uterine fibroids (Fig. 5).
Wnt/β-Catenin Signaling Pathway
The Wnt/β-catenin signaling pathway is involved in various physiological events, including development, tissue renewal, cell proliferation and differentiation, and several types of tumorigenesis (314, 315). This pathway has also been recently investigated in uterine fibroid formation (241, 316, 317) (Fig. 5). However, contrasting results on the β-catenin expression in uterine fibroids have been reported: 1 study detected upregulated expression in uterine fibroids (318), whereas others detected no difference between uterine fibroids and myometrium (319). Recently, the mislocalization of β-catenin has been causally linked to uterine fibroids phenotype, wherein fibroids expressed higher levels of nuclear β-catenin than normal myometrium tissues. Moreover, estrogen activated β-catenin nuclear translocation and enhanced β-catenin responsive gene expression in human uterine fibroids cells via the ER (320). Thus, a paracrine role for the WNT/β-catenin pathway that enables mature myometrium or fibroid cells to send mitogenic signals to neighboring tissue stem cells in response to estrogen and progesterone has been proposed, thus leading to the growth of uterine fibroids (241).
The use of vitamin D3 has been associated with the inhibition of the Wnt/β-catenin pathway and decreased uterine fibroid cell proliferation (321, 322). β-catenin inhibitors, such as ICG-001, cordycepin, and XAV939, and histone deacetylase (HDAC) inhibitors (HDACi), including apicidin and HDACi VIII, exhibited antiproliferative effects on uterine fibroid cells by suppressing the β-catenin signaling pathway. Additionally, HDACi exposure induced cell cycle arrest and apoptosis of uterine fibroid cells, thus representing a promising epigenetic-based therapy for uterine fibroids (320). Recently, the MED12 somatic mutation has been revealed to modulate oncogenic Wnt4/β-catenin and mTOR signaling pathways in uterine fibroids (174).
Compounds targeting Wnt/β-catenin signaling and other vital pathways are summarized in Table 2.
Table 2.
Compounds targeting key pathways involved in uterine fibroids pathogenesis
| Compound | Family | Molecular effects | References |
|---|---|---|---|
| Leuprolide acetate, Goserelin | GnRH agonist | ECM and TGF-β inhibition, tumor shrinkage | (445-448) |
| Ulipristal acetate | SPRM | Apoptosis induction, proliferation inhibition, ECM inhibition | (290) |
| Vitamin D | Natural compound | DNA repair induction, Wnt/β-catenin pathway inhibition, TGF-β-induced inhibition of ECM production, anti-inflammatory | (370, 395) |
| EGCG (green tea) | Natural compound | COMT suppression, apoptosis induction, proliferation inhibition | (449, 450) |
| Tranilast | Anti-allergic compound | ECM inhibition, inhibit proliferation, cell cycle arrest, induction of miR-29c and miR-200c expression | (451-453) |
| Curcumin | Natural compound | Inhibition of ECM production, inhibition of UF proliferation, anti-inflammatory effect | (454, 455) |
| 2-methoxyestradiol | Estradiol metabolite | PI3K/Akt/mTOR inhibition, ECM inhibition | (456) |
| Letrozole | Aromatase inhibitor | Apoptosis induction, inhibition of proliferation | (457) |
| All-trans retinoic acid | Active metabolite of vitamin A | TGF-β-induced inhibition of ECM production | (458) |
| Methyl jasmonate | Natural compound | Wnt/β-catenin pathway inhibition, apoptosis induction, inhibition of proliferation | (459) |
| Apicidin | Class I HDAC inhibitor | Inhibition of Wnt/β-catenin pathway, apoptosis induction, inhibition of proliferation | (320) |
| HDACi VIII | HDAC 6 inhibitor | Inhibition of Wnt/β-catenin pathway, apoptosis induction, inhibition of proliferation | (320) |
| ICG-001 | β-catenin inhibitor | Inhibition of Wnt/β-catenin pathway, inhibition of proliferation | (320) |
| Resveratrol | Natural compound | Inhibition of ECM production, apoptosis induction | (460) |
| Collagenase | Proteolytic enzyme | ECM degradation | (431, 461) |
| Simvastatin | Statin drug | ECM, apoptosis, ER-α palmitoylation and degradation | (462-466) |
| Verteporfin | YAP inhibitor | Anti-proliferation, -fibrosis and -mechanotransduction | (461) |
| Nintedanib | YAP inhibitor | Anti-fibrotic effect | (461) |
| 5-Aza | Hypomethylation drug | Fibroid stem cell differentiation and PGR signaling | (467, 468) |
Abbreviations: Akt, protein kinase B; COMT, Catechol-O-methyltransferase; ECM, extracellular matrix; EGCG, epigallocatechin gallate; GnRH, gonadotropin-releasing hormone; HDAC, histone deacetylase; miR, microRNA; mTOR, mechanistic target of rapamycin; PI3K, phosphoinositide 3-kinase; SPRM, selective progesterone receptor modulator; TGF-β, transforming growth factor beta; PGR, progesterone receptor; UF, uterine fibroids.
YAP/TAZ Pathway
ECM accumulation and aberrant cell proliferation are essential components in uterine fibroid pathogenesis. An important regulatory mechanism that connects mechanical stimuli to cellular proliferation is the Hippo pathway, including the effector proteins Yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding domain (TAZ) as main mediators. YAP/TAZ has been involved in fibrotic diseases such as lung fibrosis (323) as well as in hormone-regulated organs such as ovary (324) with aberrant activation that resulted in tumorigenesis (325). A recent study has shown that YAP/TAZ nuclear localization in situ and confluent cells was higher in uterine fibroid cells compared with normal myometrium and associated tissue stiffness was higher in uterine fibroids compared with normal myometrium. Moreover, exposing fibroid cells to verteporfin (a YAP inhibitor) reduced cell survival and reduced fibronectin deposition (326). Furthermore, a recent study showed that the antifibrotic drug nintedanib inhibited YAP and produced antifibrotic effects (327). The same study showed that in vivo injection of collagenase into uterine fibroids led to a reduction in Hippo/YAP signaling and crucial genes and pathways involved in fibroid growth (327) (Table 2). More studies are encouraged to further explore the role of the Hippo/YAP/TAZ pathway in fibroid pathogenesis with subsequent identification of novel targeted therapeutic strategies.
Rho/ROCK Signaling Pathway
Studies showed that mechanotransduction activates the Ras homolog family (Rho), which interacts with its downstream target Rho-kinase (ROCK) and activates the ERK/p38 MAPK-signaling cascade, and resulting in increased cell proliferation, decreased apoptosis, and upregulation of genes involved in ECM composition and remodeling (303). RhoA expression was found to be higher in uterine fibroid compared with myometrial cells (328). Interestingly, inhibition of integrin-β1 caused a decrease in active RhoA expression in uterine fibroid cells (301), while Fasudil, a ROCK inhibitor, relaxed the contraction of uterine fibroid cells in 3D collagen gels (278). It might be interesting to pursue potential antifibrotic effects of these pathway inhibitors in further studies.
Epigenetic Regulation
Tumorigenesis cannot be exclusively explained by genetic changes, as epigenetic processes are also involved (329, 330). All epigenetics involved in regulation of gene activities, including DNA methylation, histone modification, non-coding RNA, and heterochromatinization, are disturbed in the pathogenomics of uterine fibroids (331, 332).
Numerous data demonstrated aberrant alterations of genome methylation/demethylation in uterine fibroid cells (175, 333). In addition, unsupervised clustering of results from DNA methylation analysis can segregate normal myometrium from uterine fibroids and classify uterine fibroids into subtypes according to MED12 mutation or the activation of HMGA2 or HMGA1. Moreover, HOXA13 encoding the class of transcription factors called homeobox genes was identified to be hypomethylated and upregulated in fibroids compared to myometria. Abnormal HOXA13 expression was considered an essential factor contributing to the homeotic transformation into a more cervical phenotype which was linked to the development of uterine fibroids (135). MicroRNAs play a significant role in the epigenetic control of uterine fibroid development. Significant alteration in the synthesis profile of regulatory microRNAs of the families let7, miR-21, miR-93, miR-106b, miR-29, and miR-200 has been observed (334, 335). More recently, studies on the role of long non-coding RNAs in the pathogenesis of uterine fibroids have been investigated, further demonstrating the critical role of RNA network in uterine fibroids (332, 336-338). Epigenetic changes in the uterine fibroid genome can activate critical transduction signaling pathways, such as Wnt/β-catenin and Wnt/MAPK (320, 339). More studies focusing on the epigenetic regulation of pathways involved in uterine fibroid pathogenesis can provide potential therapies against this disease.
Inflammation
Inflammation is a protective response from the immune system against foreign agents and involves immune cells and the release of molecular mediators. However, prolonged inflammatory status leaves the body in a constant state of alert and subsequently transforms into chronic inflammation. Chronic inflammation contributes to various conditions, including gynecologic diseases and cancer (340, 341).
Tumor-extrinsic chronic systemic inflammation is caused by many factors, including obesity, tobacco smoking, autoimmune diseases, environmental chemical exposure, vitamin D deficiency, and excessive alcohol consumption (342, 343). In addition, a normal cell can be transformed into a tumorous cell when repeatedly subjected to DNA damage during prolonged inflammatory status, thus triggering tumor-initiating mutations via impaired DNA repair pathways (Fig. 2). Consequently, these genetic events may induce the proliferation, recruitment, and activation of inflammatory cells and increase the production of reactive oxygen species and cytokines, contributing to tumor-intrinsic inflammation (344).
Some epidemiological studies have explored the relationship between uterine fibroids and self-reported diagnosis of reproductive tract infection (RTIs) and inflammation (233, 345, 346). History of pelvic inflammatory disease and chlamydia infection, and intrauterine device use have been positive associated with uterine fibroid risk (233). In contrast, no association between uterine fibroids and self-reported diagnosis of pelvic inflammatory disease in African American and Caucasian women was found. However, a positive correlation with a self-reported history of chlamydia infection in Caucasian women and trichomonas, syphilis, and bacterial vaginosis in African American women has been reported (345). There is no significant association between self-reported RTIs and uterine fibroids. However, adverse associations of chlamydia infection and pelvic inflammatory disease with multiple fibroids have been reported (346). More studies using serology and biochemical characterization of past infections are needed to clarify the associations between RTIs and uterine fibroids. Interestingly, multiple associations between the higher occurrence of uterine fibroid inflammatory mediator gene polymorphisms, including interleukin (IL)-1β (347, 348), IL-4 (349), TNF-α (349, 350), IL-6 (351), IL-10 (348), and IL-12b receptor (352), have been reported.
A chronically active inflammatory immune system is suggested to be involved in uterine fibroid formation (341, 353-355). It is hypothesized that in the uterus of a woman with a chronically inflammatory immune profile (due to chronic low-grade systemic inflammation) suffering a series of insults, including intrauterine infection, injury, and menses, the immune system response is exacerbated, thereby directly or indirectly inducing cell proliferation and fibrosis, which are implicated in the formation and growth of uterine fibroids (354). Numerous studies using molecular strategies and focusing on inflammatory mediators in the context of uterine fibroids have been conducted. They detected the expression of a significant number of cytokines and chemokines with proinflammatory and profibrotic features in uterine fibroids (Table 1). Future research employing high-throughput methods and bioinformatic analysis should be conducted.
Table 1.
Inflammatory factors involved in uterine fibroids pathophysiology
| Factor | Biological relevance | Results in UF studies | References |
|---|---|---|---|
| IL-8 | Chemoattracts neutrophils | Lower levels of IL-8 (IHC/ELISA) and its receptor (IHC) in UF than in the surrounding myometrium | (436) |
| IL-4 | Induces the differentiation of naïve helper T cells into Th2 cells | UF progenitor cells had higher mRNA levels (qRT-PCR) and secreted higher levels of these factors (ELISA) than myometrial progenitor cells | (437) |
| IL-5 | Promote the growth, differentiation, and activation of eosinophils | ||
| IL-10 | Anti-inflammatory cytokine | ||
| IL-13 | Profibrotic cytokine responsible for Th2 responses | ||
| IL-6 | Proinflammatory cytokine | UF progenitor cells had lower mRNA (qRT-PCR) and secreted levels of these cytokines (ELISA) than myometrial progenitor cells | |
| IL-12 | Promotes the development of Th1 responses | ||
| IL-17A | Proinflammatory cytokine | ||
| IL-33 | Important in innate and adaptive immunity and contributes to tissue homeostasis | Serum IL-33 (ELISA) levels were elevated in women with UFs and positively correlated with the number, volume, and mass of UFs | (438) |
| GM-CSF | Hematopoietic growth factor | Smooth muscle cells isolated from UF patients expressed higher GM-CSF mRNA (RT-PCR) and protein (IHC) levels than myometrial smooth muscle cells | (353, 439) |
| Higher levels in the conditioned media of UF stem cells than those of the adjacent myometrium | (357) | ||
| G-CSF | Hematopoietic growth factor | UF progenitor cells presented lower mRNA levels (qRT-PCR) and secreted lower levels of this factor (ELISA) than myometrial progenitor cells | (437) |
| Higher levels in the conditioned media (ELISA) of UF stem cells than that of the adjacent myometrium | (357) | ||
| TNF-α | Pleiotropic cytokine that plays a major role in the cell cycle and controls apoptosis | Increased protein levels (IHC-TMA and WB) in UFs compared with adjacent myometrium tissues | (440) |
| TNF-α serum levels (ELISA) were elevated in women with clinically symptomatic UF | (441) | ||
| CXCL12 | Chemoattracts lymphocytes and macrophages; plays an important role in angiogenesis | Increased CXCL12 and decreased CXCR4, its receptor, mRNA levels (qRT-PCR) in the UF and myometrium of women with UF compared with those without. Increased CXCL12 protein secretion (ELISA) in cultured UF cells compared to adjacent and normal myometrial | (442) |
| MCP-1 | Chemoattracts monocytes/macrophages | Lower mRNA levels (Northern blot) in UFs compared to the adjacent myometrium | (443) |
| MIP-1α (CCL3) | Chemoattractant for monocytes/macrophages | Lower mRNA levels (RT-PCR) in UF tissues than the adjacent myometrium | (444) |
| MIP-1β (CCL4) | |||
| CCR5 | Chemokine receptor | ||
|
Eotaxin
(CCL11) |
Potent chemoattractant for eosinophils | Lower mRNA levels (RT-PCR) in UF tissues than the adjacent myometrium | (444) |
| Higher levels in the conditioned media (ELISA) UF SCs than that of adjacent myometrium SCs | (357) | ||
| INF-γ | Proinflammatory cytokine | UF progenitor cells had lower mRNA levels (qRT-PCR) and secreted lower levels of this factor (ELISA) than myometrial progenitor cells | (437) |
| Upregulated (IHC-TMA) in UFs compared to adjacent myometrial tissues. Higher levels in the conditioned media (ELISA) of UF SCs than that of adjacent myometrium SCs | (357) | ||
| NFkB | Transcription factor with a key role in regulating the immune response | Upregulated (IHC-TMA) in UFs compared to adjacent myometrial tissues | |
| IL-1β | Proinflammatory properties | ||
| TSLP | Involved in the maturation of T cells |
Abbreviations: CCR5, C-C chemokine receptor type 5; CXCL12, C-X-C Motif Chemokine Ligand 12; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; IL, interleukin; INF-γ, interferon gamma; MCP-1, monocyte chemoattractant protein-1; MIP-1α/β, macrophage inflammatory protein 1 alpha/beta; NFkB, nuclear factor kappa B; PD-L1, programmed death-ligand 1; SCs, stem cells; TMA, tissue microarray; TNF-α, tumor necrosis factor alpha; TSLP, thymic stromal lymphopoietin; UF, uterine fibroid.
The characterization of infiltrating and circulating immune cells in women with uterine fibroids has received increasing attention. More tissue-CD68-positive macrophages inside the uterine fibroids and in the surrounding myometrium have been found than in distant myometrium tissues (at least 1.5 cm from the border of fibroids). However, no differences in the number of CD45-positive leukocytes and MCT-positive mast cells have been observed (355). A recent study in a Chinese population demonstrated that the numbers of circulating CD4+CD8+ T cells, regulatory T (Treg, CD4+) cells, and T follicular helper (Tfh) cells was notably increased in patients with uterine fibroids relative to the healthy controls, whereas the numbers of natural killer (NK) and delta gamma (γδ) T cells (CD4− CD8−) was reduced (356). Furthermore, the levels of immune check-point PD-L1 proteins were increased in uterine fibroids compared to adjacent myometrial tissues (357). Interestingly, uterine fibroids demonstrated decreased PD-L1 expression and cytotoxic T-cell infiltration compared with malignant leiomyosarcomas, indicating that the immune system behavior spectrum varies among different uterine smooth muscle tumors (358).
The expression of growth factors and cytokines is partly regulated by ovarian steroid hormones (359), critical in uterine fibroids formation and growth (197). The relationship between uterine fibroid development and early-life exposure to xenoestrogen in the context of inflammation was recently revealed in an Eker rat animal model (360). The immune cells and secreted cytokines and chemokines may influence uterine fibroid development and the tumor microenvironment. The myometrial and systemic immune profiles of women with fibroids should be investigated better to understand the role of inflammation linking to epigenome (33) in uterine fibroid pathogenesis.
Role of Vitamin D Pathway
Vitamin D forms a complex with a specific receptor called the vitamin D receptor (VDR) to mediate its pleiotropic functions via steroid transcriptional mechanisms (361). VDR modulates the expression of various genes in a tissue-specific manner. Vitamin D exhibits antiproliferative and pro-apoptotic activities and induces cell differentiation in different diseases (82, 362), including musculoskeletal, infectious, cardiovascular, metabolic, autoimmune, neurocognitive diseases, and cancers. The role and mechanism of vitamin D action in uterine fibroids have been investigated in the past decade. In 2011, the antifibrotic effect of vitamin D was reported, showing that vitamin D is associated with reduced expression of TGF-β3-induced ECM proteins, including fibronectin and collagen type 1 in uterine fibroid cells, which were otherwise overexpressed (363). Vitamin D also inhibited the growth and proliferation of uterine fibroid cells by downregulating proliferating cell nuclear antigen (PCNA), cyclin-dependent kinase 1 (CDK1), and B-cell lymphoma 2, and inhibiting the expression and activity of catechol-O-methyltransferase (COMT) (364). In addition, TGF-β3, a vital factor in the pathogenesis of uterine fibroids, was inhibited by increased concentrations of vitamin D (365). In the Eker rat uterine fibroid model, the administration of vitamin D decreased the size of uterine fibroids (366). In 2013, the regulatory role of vitamin D in the expression and activity of matrix metalloproteinases (MMPs), which are involved in ECM deposition, was revealed (99). Later, active vitamin D was reported to function as a potent anti-estrogen and antiprogesterone agent (367).
Furthermore, the administration of vitamin D reduced the levels of Wnt/β-catenin, leading to the downregulation of mTOR signaling expression in uterine fibroids with MED12 somatic mutations (321). The same study reported that vitamin D suppresses the expression of FEN1, an enzyme involved in DNA damage repair, in a concentration-dependent manner (321). In 2018, low serum vitamin D levels in animals were associated with increased expression of sex steroid receptors and proliferation-related genes, fibrosis, and enhanced inflammation. Vitamin D-deficient diet enhanced DNA damage in murine myometrium (368). Importantly, vitamin D also exhibits anti-inflammatory functions, and the inflammation-induced pathology of uterine fibroids has been described (369). Vitamin D suppressed the uterine fibroid phenotype by targeting different DNA repair networks (370). Recently it was reported that long-lasting and high-dose treatment with vitamin D induced significant lesion volume reduction through reduced cell proliferation via the inhibition of TGF-β3 expression and inducing apoptosis (371). Italian researchers found that vitamin D in women acted as an antiproliferative compound against small uterine fibroids (372) by arresting cell growth and inhibiting the Wnt/β-catenin pathway (322). Vitamin D effectively reduces proliferation and extracellular matrix formation in different molecular subtypes of uterine fibroids (371). In the first randomized study trial, the supplementation of 50 000 IU of vitamin D for 12 weeks inhibited lesion growth, whereas the volume of uterine fibroids in the placebo group increased (373).
Overall, the relationship between vitamin D and uterine fibroid metabolism has been characterized. Recently, our research team proposed a preliminary clinical instruction of 25(OH)D measurements and vitamin D supplementation that may be useful for clinicians involved in treating patients with uterine fibroids (374).
Role of the Microbiome
Foreign microorganisms colonizing our body include bacteria, yeasts, fungi, protozoa, archaea, and viruses; these are collectively referred to as the “microbiome” (375). The cells comprising the microbiome and the host are considered as a single ecological and biological unit, the so-called human halobiont.
Intestinal estrogen metabolism
Gut microbes shape the complex micro-ecological system of the human body (376, 377). Many human intestinal microbes influence the physiological functions of the host and have profound effects on the synthesis and secretion of hormones, trace elements, growth factors, and immune system function. The intestinal flora and the host have a mutually beneficial relationship and are interdependent (377). The intestinal flora can be modified via hormone interaction both in vitro and in vivo, thus affecting the body’s biological equilibrium (378). Recently, whether the intestinal flora can control the levels of estrogen and its metabolites has been explored. The enzyme glucuronidase in the intestinal flora improved estrogen reabsorption (377, 379, 380). Several bacteria in the intestinal flora can metabolize estrogen, and they are referred to as the estrobolome. High bacterial enzyme activity of the estrobolome elevates the level of free estrogen in the enterohepatic circulation by promoting an endogenous hormonal environment, leading to an increase in hormone levels which may have a direct and indirect impact on the risk of uterine fibroids (381).
The abundance of the intestinal flora closely correlates to systemic estrogen. Many bacteria at the family and species levels control estrogen content, particularly Clostridium and Pneumococcus exhibit the most significant effect on estrogen metabolism (377, 382). Reduced estrogen levels have been related to decreased intestinal flora diversity, including the phylum Bacteroidetes, and increased abundance of the phylum Firmicutes and species diversity of the phylum Proteobacteria (377, 383). In addition, it has been shown that elevated levels of estrogen and its metabolites have related to the abundance of several taxa in the class Clostridia, including the order Clostridiales and the family Ruminococcaceae (384).
Wang et al have studied the effect of hysterectomy on the intestinal flora diversity, ie, the number of different species present, in patients with uterine fibroids (377). The top 3 dominant bacteria, Bacteroidetes, Proteobacteria, and Firmicutes, were the same in preoperative and postoperative patients at the phylum level. However, the enrichment of Bacteroidetes in postoperative patients was significantly lower than in preoperative patients, whereas the abundance of Proteobacteria was significantly higher. Although at the class level, Gammaproteobacteria, Bacteroidia, and Clostridia predominated in both groups, the author found a statistically higher abundance of Gammaproteobacteria (Proteobacteria) in patients after the surgery. Specifically, at the order level, the higher average abundance observed was in Enterobacteriales (377). Wang’s study reported that estrogen levels in the body could alter the intestinal flora; however, the underlying mechanism of this regulation remains unknown (377). Deeper analysis may help to understand whether gut microbiota could influence the risk of uterine fibroids through effects on endogenous estrogens.
Female reproductive tract sterility and uterine fibroid microbiome
For many years, the female reproductive tract was considered a sterile organ (30, 385, 386). Culture-based technologies were usually utilized to detect bacterial residence; however, they have limited applications as many microorganisms are difficult to culture in vitro. With the launch of the Human Microbiome Project in 2007, modern sequencing technologies using taxonomy-associated marker genes, such as the 16s rRNA gene or whole-genome sequences, have been used to distinguish bacteria at the species level (387). In addition, body sites that were historically supposed to be sterile are colonized by microorganisms (386, 388). Specifically, the Human Microbiome Project reported that the uterine cavity harbors a unique microbiome (30, 386, 389). However, the specific function of the uterine microbiome remains unknown (385, 389).
A pilot study evaluated the distribution of bacteria in African American women with uterine fibroids. The authors evaluated the bacterial diversity on the endometrium, adjacent myometrium, and uterine fibroids and compared them with samples from healthy controls. A greater diversity was found in the uterine fibroid patient samples compared with controls. In addition, uterine fibroid patients showed a significantly increased abundance of Bacteroidetes, Firmicutes, and Actinobacteria in the endometrium. At the general level, uterine fibroid tissues were significantly enriched in Clostridium, Allobaculum, Anaerostipes, Odoribacter, Turicibacter, Oscillospira, Ruminococcus, and Coprococcus, which are commonly associated with the gut environment. These results suggest that the systemic distribution of the gut bacteria extends to the uterus of patients with uterine fibroids, following dysbiosis or gut-barrier impairment (390). Further studies are needed to better understand whether the uterine microbiome play a role in the pathogenesis of uterine fibroids.
Conducting investigations using human tissue is critical for deciphering many normal and pathogenic processes and developing disease diagnosis techniques and future therapies. However, it could be a challenge to state the significance of the findings since uterine fibroid samples are obtained at a specific time point, at which limited information about biological samples is available. For example, whether the uterine fibroids are growing or shrinking at the time of sample collection is a relevant question. In addition, the heterogeneity of uterine fibroids also represent structural properties and collagen content changes (391), genetic (9), epigenetic (194, 392), and cell type variations. Furthermore, due to the paracrine and mechanical effect of uterine fibroids, the manner of collecting the myometrium is a significant factor. Notably, the biology of myometrium from the uterus with fibroids or without fibroids differs (393, 394). For these reasons and more, uterine fibroid studies need to be carefully designed and should take these factors into consideration. When working with samples, it is crucial to consider multiple factors, including race, age, BMI, menstrual cycle phase, driver mutations, to draw concise conclusions.
Integration of Multiple Pathways in Uterine Fibroids
It is important to emphasize that during the initiation and development of uterine fibroids, many biological events coincide, and multiple abnormal pathways interact, contributing to the pathogenesis of uterine fibroids. For instance, estrogen signaling activated the β-catenin pathway via the estrogen/receptor axis, therefore induced β-catenin nuclear translocation and enhanced β-catenin responsive gene expression in human uterine fibroids cells. Vitamin D3 has been associated with inhibiting the Wnt/ β-catenin, proinflammatory pathways, and ECM deposition in uterine fibroid cells (395) and restored the DNA repair capacity in both uterine fibroid cells and at-risk MMSCs (370, 396). Diet-induced vitamin D deficiency triggered inflammation and DNA damage in murine myometrium (368). Moreover, VDR-knockdown in normal human myometrial cells increased DNA damage and inhibited DNA repair capacity (370). These studies suggested the interaction of the vitamin D3/receptor pathway with β-catenin and DNA repair pathway. Activator protein 1 (AP1) is a family of transcription factors consisting of FOS and JUN members that form homodimers or heterodimers involved in many biological processes, including differentiation, proliferation, apoptosis, and fibrotic diseases. Recent studies demonstrated that AP1 signaling promotes ECM deposition (397), and ECM activated β-catenin signaling in uterine fibroids (317). In addition, uterine fibroids expressed higher Class I HDAC enzyme levels than normal myometrial tissues, associated with activated β-catenin signaling. (320). Notably, MED12 mutations have been linked to multiple pathways directly implicated in uterine fibroid pathology as described (7, 134, 137, 174, 175). Considering that diverse pathways play a role in the pathogenesis of uterine fibroids (Fig. 5), additional studies will be necessary to identify the molecular mechanism underlying the interaction network and signaling in uterine fibroids.
Malignant Transformation of Uterine Fibroids
Uterine leiomyosarcoma is a rare, aggressive, malignant tumor, which originates from the smooth muscle layer in the uterus and shares many common clinical grounds with uterine fibroids (398). Approximately 6 out of every 1 000 000 women are diagnosed with uterine leiomyosarcoma yearly. Unfortunately, its prognosis is poor, with the lowest survival rates among soft-tissue sarcomas. Uterine leiomyosarcoma represents ~1% of all uterine malignancies, and the average age of patients ranges from 40 to 50 years. Uterine leiomyosarcoma mainly metastasizes to the lungs, liver, brain, kidney, and bones (399).
Characteristics Common Between Uterine Fibroids and Leiomyosarcoma
Notably, uterine leiomyosarcomas and uterine fibroids share several common clinicopathological features that complicate their differential diagnosis. First, both tumors arise from the myometrium as focal masses within the uterine wall. Second, abnormal uterine bleeding, pelvic pain/pressure, and a pelvic mass are the primary presenting signs and symptoms for both uterine leiomyosarcoma and uterine fibroids, making it difficult to differentiate between them (400-403). Third, uterine leiomyosarcoma and fibroids share morphological and molecular characteristics that cannot be differentiated through current clinical diagnostic tests. Finally, it is said that the Black women are at higher risk for both uterine fibroids and leiomyosarcoma (404, 405). Because suspected uterine fibroids are often conservatively managed or with minimally invasive treatments, the misdiagnosis of leiomyosarcoma for a benign uterine fibroid could potentially result in significant treatment delays, increasing patient morbidity and mortality (406).
Malignant Transformation of Uterine Fibroids to Leiomyosarcomas
It has been debated whether uterine fibroids and uterine leiomyosarcoma are part of a disease continuum. Genetic studies have demonstrated that uterine leiomyosarcomas arise de novo and may be unrelated to benign fibroids. Uterine leiomyosarcoma typically has complex karyotypes and aneuploids, while uterine fibroids have characteristic rearrangements, many of which are shared by other benign neoplasms. Intermediate forms between these 2 patterns had not been described. However, recent microarray data identified a rare subset of uterine fibroids with deletions of chromosome 1 that have transcriptional profiles that cluster with those of uterine leiomyosarcoma, suggesting that some rare leiomyosarcomas may arise from a specific subset of uterine fibroids (407). Moreover, the observation that up to 4% to 30% of uterine leiomyosarcomas harbor exon 2 mutations in the established fibroids driver gene MED12 suggests that a subset of malignant leiomyosarcomas may derive from benign uterine fibroids (119, 408, 409). The discrepancy in the frequency of uterine fibroids and uterine leiomyosarcoma lies in the fact that only rare histological and karyotypic variants of fibroids may be responsive to malignant progression (410). Notably, a large number of random chromosomal aberrations in uterine leiomyosarcoma suggests that genomic instability is involved in the pathogenesis of these tumors, and such instability hinders efforts to identify the primary change(s) that may now be discovered through studies of variant uterine fibroids (411).
Novel therapeutic advances for the aggressive type of uterine cancer leiomyosarcoma are hindered by the overall lack of knowledge about the functional consequences of the complex genomic and pathway alterations found in patients and limited characterization of tumor biology. The employment of new models together with advanced technologies such as single-cell sequencing, mass cytometry, and other high-throughput approaches will ultimately help to characterize the mechanisms of its pathogenesis, resistance to conventional treatments and develop novel options for treating the patients with this aggressive disease.
Future Perspectives
Despite substantial progress in our understanding of the pathobiology of uterine fibroids, several gaps need to be addressed (Fig. 6).
Figure 6.
Future research prospects. Studies elucidating the interplay among genes, epigenome, and epitranscriptome in the context of stem cell biology, microbiome, and the interaction between the endometrium and uterine fibroids can advance the knowledge on the pathogenesis of uterine fibroids and are expected to contribute to developing novel therapeutic approaches for the treatment of patients with uterine fibroids. Abbreviations: ALKBH5, ALKB homolog 5; FTO, fat mass and obesity-associated protein; HMB: heavy menstrual bleeding; IGFBPs, insulin-like growth factor binding protein-3; METTL3,14, methyltransferase like 3 and 14; UFs, uterine fibroids; YTHDF1/YTHDC1, YTH domain-containing protein.
Transcriptome and Epigenome Analysis
The early-life environment dramatically affects the functions of developing organs and increases disease susceptibility across the lifespan. In a rat model, early-life exposure to DES affected several signaling pathways, including estrogen signaling, and reprogrammed histone mark H3K4me3 and DNA methylation patterns in MMSCs derived from the developing myometrium, thus accelerating the acquisition of an adult epigenomic signature. Furthermore, this epigenomic reprogramming persisted long after the initial exposure. These findings demonstrate the importance of the epigenome, that is, early interactions between the gene and environment reprogram the epigenome, and interactions in adulthood accelerate or activate restricted epigenetic reprogramming to increase genome instability and initiate fibroids pathogenesis. Omics studies also demonstrated that uterine fibroids contain disrupted epigenome linked with genetic stability (135, 412). Notably, the myometrium of uterine fibroids patients has a distinct transcriptomic pattern compared with non-diseased myometrium (394), and cells from diseased myometrium responded differently to progesterone (413). These studies suggest that adjacent myometrium from the uterus with uterine fibroids should not be considered as normal tissue.
To further elucidate the role of interaction among the genes, epigenome, and environment in process of uterine fibroid pathogenesis, the following issues should be addressed. First, the genetic landscapes of normal, at-risk, and uterine fibroids stem cells should be characterized using genome-wide omics methods to better understand the genome, epigenome, and epitranscriptome. Second, the other insults that cause the abnormal reprogramming of MMSCs should be identified. Lastly, the interaction between early hits in early life and late hits in adult life should be explored in the context of uterine fibroid development.
Recently, the rapid development of single-cell multi-omics approaches is transforming our understanding of disease initiation and progression. Therefore, they can be utilized to assess the transcriptome, epigenome, spatial profiling, and proteome of uterine fibroids, contributing to increased knowledge on the cell state, ontogeny, epigenome state, phenotype, and function of uterine fibroids (Fig. 6).
Epitranscriptomics
The epitranscriptome refers to the complete ensemble of chemical modifications affecting the RNA transcripts (coding and non-coding RNAs), and epitranscriptomics is an emerging field in molecular medicine with vast potential. To date, more than 160 different chemical modifications in RNA have been identified in living organisms. N6-methyladenosine (m6A) is the most pervasive, abundant, and conserved in eukaryotic mRNAs, occurring in ~25% of transcripts genome-wide, and it is enriched near stop codons, 5′- and 3′-untranslated regions, and within long internal exons (414, 415). m6A is co-transcriptionally incorporated by so-called writers, including the METTL3-METTL14 core methyltransferase complex and associated proteins, such as RBM15, WTPA, and VIRMA, that confer target mRNA specificity, eliminated by demethylases FTO and ALKBH5 (erasers), and recognized by readers, including the YTH protein family (Fig. 6). m6A-bound readers ultimately determine the posttranscriptional fate of methylated mRNAs by modulating cellular activities that control RNA stability, processing, and translation. m6A is thus a pervasive regulator of gene expression and a key determinant of cell fate and function. Accordingly, the disruption of its homeostasis has been implicated in several pathological conditions, including cancer. Thus far, epitranscriptomic studies on uterine fibroids are mainly unknown. However, several key m6A regulators were recently revealed to be dysregulated in uterine fibroids, suggesting that epitranscriptomics play an important role in uterine fibroid pathogenesis (416, 417). However, more studies are needed to explore the function of the RNA methylation machinery and m6A in uterine fibroid development. Recently, a potent and selective catalytic inhibitor of METTL3 was developed and can reduce acute myeloid leukemia growth and increase differentiation and apoptosis, suggesting that targeting METTL3 as a potential therapeutic strategy against diseases such as acute myeloid leukemia. Therefore, investigations on epitranscriptomic targeting uterine fibroids should be conducted.
Endometrium Receptivity and Heavy Menstrual Bleeding
Uterine fibroids cause female reproductive disorders, including heavy menstrual bleeding and poor receptivity and implantation, leading to infertility and affecting millions of women globally. These disorders indicate endometrial dysfunctions and are related to the presence of adjacent uterine fibroids. Remarkably, the degree of dysfunction is associated with the location and size of the uterine fibroids (418). However, how fibroids affect endometrial functions remains unclear.
Uterine fibroid-caused expansion in the endometrial surface area generally results in more significant menstrual bleeding and transformations in the shape of uterine cells, thus eventually affecting gene expression and function. Furthermore, recent studies demonstrated that uterine fibroids could actively influence the adjacent endometrium and the entire uterine cavity (419). Therefore, elucidating the mechanism underlying the effect of uterine fibroids on the endometrium is necessary. In addition, the impact of uterine fibroids on endometrium function via exosome is a promising area to be explored (266, 420) (Fig. 6).
Microbiome
The impact of alterations in the endometrial microbiome on uterine fibroid pathogenesis has not been investigated. The paradigm that the uterus is a sterile environment remains highly controversial. The vagina contains trillions of bacterial cells, whereas the uterus and the Fallopian tubes are generally considered sterile. As alterations in the gut microbiota can lead to several pathologies, such as inflammation, autoimmune diseases, and obesity, it is proposed that the uterine microbiota may be altered in patients with uterine fibroids. A diverse microbiota has been detected in fibroids relative to other uterine tissues and those from healthy controls. In addition, uterine fibroids may associate with local and systemic inflammation that may promote the translocation of the gut microbiota into the endometrium. Future studies should explore the composition and identify the role of the microbiome in uterine fibroids. How bioactive metabolites from the microbiome provide the constituents of the fibroids microenvironment and affect the surrounding tissues should also be investigated (Fig. 6).
Leiomyosarcoma
Although the current standard practice has been used to differentiate aggressive leiomyosarcoma from benign uterine fibroids, further studies are needed to explore early diagnosis tools to distinguish these 2 uterine tumors. Currently, several approaches are employed in basic research, which may lead to future clinical practice options.
Promoter-based imaging system
Uterine leiomyosarcoma is most often discovered by chance when a woman has a hysterectomy performed for uterine fibroids. To differentiate uterine leiomyosarcomas from fibroids with imaging, a molecular bio-imaging probe for noninvasive differentiation approach was recently established in a preclinical animal model (398). The diagnostic strategy was to use the cancer-specific enhanced survivin expression in malignant vs normal/benign cells to test its promoter driving potential of a downstream reporter gene that will detect cancer cells once activated. Because of solid promoter activity, tumor specificity, and capacity for clinical translation, survivin promoter-driven biological signal may represent a practical, new system to facilitate early leiomyosarcomas diagnosis. One study reported that adenovirus (Ad-SUR-LUC) was injected into the animals and paired the imaging reporter gene with a complementary imaging agent in a system that can be used to measure bioluminescence driven by the surviving promoter. This approach could distinguish leiomyosarcoma lesions from normal uterine tissue or benign uterine fibroid lesions with good accuracy. In this regard, this system can impact the management of suspicious uterine masses, a current major challenge in clinical gynecology.
Genetic and transcriptome profiling
In recent years, high-throughput sequencing technologies have provided unprecedented opportunities to depict the development of diseases at multiple molecular levels. The integration and analysis of these multi-omics datasets allow us to yield a better understanding and a clearer picture of the under-studied systems (421, 422). Through integrated comparative genomic and transcriptomic analysis, differential genetic targets between uterine leiomyosarcoma and fibroids have been identified (423, 424). In leiomyosarcomas, genetic mutation burden exhibited higher copy number variations, single nucleotide variants, small insertions/deletions, and gene fusions compared with uterine fibroids. Comparative genomic hybridization (CGH) array analysis reveals a chromosomal and genomic complexity starting from uterine fibroids, with few chromosomal alterations, to uterine leiomyosarcoma, which harbors many intrachromosomal damages, and chromothripsis as evidence of their genomic complexity (116, 424, 425). In addition, a differential transcriptomic profile was observed for uterine leiomyosarcomas (423). These novel genetic and transcriptional targets may be potential diagnostic markers to differentiate uterine leiomyosarcoma from fibroids (423, 426).
Aberrant specific pathways
The Hedgehog pathway is one of the key regulators involved in many biological events. Malfunction of this pathway is associated with various diseases, including several types of female cancers (427). An initial study demonstrated that elevated expression of SMO and GLI 1, the key members of the Hedgehog pathway, was observed in leiomyosarcoma relative to normal myometrium and uterine fibroid tumors. In addition, overexpression of Hedgehog proteins was correlated with poor prognosis in leiomyosarcomas patients (428). A subsequent study demonstrated that the expression profile of Hedgehog signaling pathway markers and the response to Hedgehog pharmacological inhibition differed between leiomyosarcoma cells and fibroids cells (429). The expression of crucial Hedgehog members SMO and GLIs 1, 2, and 3 was upregulated in leiomyosarcoma cells, increasing the nuclear levels of GLI proteins. Treatment with LDE225 (SMO inhibitor) and Gant61 (GLI inhibitor) resulted in a significant reduction in GLI protein levels in leiomyosarcoma concomitantly with a decrease in leiomyosarcoma cell proliferation, migration, and invasion (429).
Additionally, the expression of DNMT members (1, 3a, and 3b) was upregulated in leiomyosarcoma compared to normal uterine smooth muscle cells. Treatment of leiomyosarcoma cells with DNMT inhibitor (5-Aza-dC) decreased the expression of SMO and GLI1 as well as nuclear translocation of GL1 and 2 concomitantly with a decrease in leiomyosarcoma cell proliferation, migration, and increase in leiomyosarcoma cell apoptosis (429). These studies showed that the Hedgehog pathway and DNA methylation network and their interaction might play an essential role in uterine leiomyosarcoma development.
Targeted Therapeutics
Targeted therapy exclusively focuses on uterine fibroid cells to shrink tumor lesions with minimal insult to surrounding tissues, not interfering with systemic hormones or fertility. Targeted therapies have been proposed via the local injection of collagenase from Clostridium histolyticum and gene therapy to deliver designed viral vectors. Collagenase can dissolve disorganized extracellular collagen fibers in uterine fibroids, and proof-of-principle studies have significantly reduced uterine fibroid size (430, 431). Moreover, a phase I clinical trial with 15 women demonstrated the safety and tolerability of collagenase derived from C. histolyticum (NCT02889848). Localized targeted strategy via modified adenovirus vectors (432, 433) resulted in reduced tumor size and showed the absence of adenovirus in surrounding tissues, uterine fibroid-targeting specificity, and good safety profile. Adenovirus expressing dominantly negative ERs has also been used, and inhibition of fibroid growth in nude mice was observed (434). Magnetic nanoparticles can enhance the effectiveness of gene therapy against both differentiated human fibroid cells and tumor-initiating stem cells (435). Using localized nonsurgical adenovirus-based alternative for the treatment of uterine fibroids, the combination of viral-based gene delivery and nanotechnology led to more efficient targeting of fibroid tumors, the lower viral dose required, and consequently, an overall safer profile (435). Novel targeted therapies against uterine fibroids with better efficacy profile are needed, especially for African American women.
In summary, considerable progress has been made in the past decade to study the interplay of steroid hormones, risk factors, stem cells, genetics, and epigenetics that contribute to the developmental origin and pathogenesis of uterine fibroids. Deeper mechanistic insights into uterine fibroids’ etiology and complexity will lead to long-term, fertility-friendly, and effective drugs for preventing patients with this common tumor.
Acknowledgments
We would like to thank Dr. Darlene Dixon for critically reviewing our manuscript and Ms. Jinda Sekhon for editing our manuscript. Figures were created using BioRender.
Financial Support: This work was supported in part by National Institutes of Health (grant numbers: RO1 ES028615, RO1 HD094378, U54 MD007602, RO1 087417, RO1 HD094380, and HD106285).
Author Contributions: Q.Y. and A.A. conceived the manuscript. M.V.B., T.B., and Q.Y. contributed to the figures. M.V.B., M.A., and Q.Y. contributed to the tables. All authors wrote sections of the manuscript.
Glossary
Abbreviations
- 25(OH)D
25-hydroxyvitamin D
- AKT
protein kinase B
- BMI
body mass index
- DES
diethylstilbestrol
- DSB
double-stranded break
- ECM
extracellular matrix
- EDC
endocrine-disrupting chemical
- ER
estrogen receptor
- EZH2
enhancer of zeste homolog 2
- FH
fumarate hydratase
- GnRH
gonadotropin-releasing hormone
- HDAC
histone deacetylase
- HDACi
histone deacetylase inhibitor
- HMGA
high mobility group A
- IGF-1
insulin-like growth factor 1
- IL
interleukin
- MAPK
mitogen-activated protein kinase
- MED12
RNA polymerase II transcriptional mediator complex subunit 12
- MMSCs
myometrial stem cells
- mTOR
mammalian target of rapamycin
- PI3K
phosphoinositide-3-kinase
- RTI
reproductive tract infection
- SPRM
selective progesterone receptor modulator
- TAF
tumor-associated fibroblast
- TAZ
transcriptional coactivator with PDZ-binding domain
- TGF-β
transforming growth factor β
- TNF-α
tumor necrosis factor-α
- VDR
vitamin D receptor
- YAP
Yes-associated protein
Contributor Information
Qiwei Yang, Department of Obstetrics and Gynecology, University of Chicago, Chicago, IL 60637, USA.
Michal Ciebiera, Second Department of Obstetrics and Gynecology, Center of Postgraduate Medical Education, ul. Cegłowska 80, 01-809, Warsaw, Poland.
Maria Victoria Bariani, Department of Obstetrics and Gynecology, University of Chicago, Chicago, IL 60637, USA.
Mohamed Ali, Clinical Pharmacy Department, Faculty of Pharmacy, Ain Shams University, Cairo 11566, Egypt.
Hoda Elkafas, Department of Anesthesiology, University of Illinois at Chicago, Chicago, IL 60612, USA; Department of Pharmacology and Toxicology, Egyptian Drug Authority, formerly National Organization for Drug Control and Research, Cairo 35521, Egypt.
Thomas G Boyer, Department of Molecular Medicine, Institute of Biotechnology, University of Texas Health Science Center at San Antonio, San Antonio, TX, 78229-3900, USA.
Ayman Al-Hendy, Department of Obstetrics and Gynecology, University of Chicago, Chicago, IL 60637, USA.
Additional Information
Disclosures: Dr. Ayman Al-Hendy is a consultant for Abb-vie, Myovant, and OBS-EVA. No conflicts are declared for the remaining authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Al-Hendy A, Myers ER, Stewart E. Uterine fibroids: burden and unmet medical need. Semin Reprod Med. 2017;35(6):473-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wise LA, Laughlin-Tommaso SK. Epidemiology of uterine fibroids: from menarche to menopause. Clin Obstet Gynecol. 2016;59(1):2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100-107. [DOI] [PubMed] [Google Scholar]
- 4. Jayes FL, Liu B, Feng L, Aviles-Espinoza N, Leikin S, Leppert PC. Evidence of biomechanical and collagen heterogeneity in uterine fibroids. Plos One. 2019;14(4):e0215646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stewart EA, Laughlin-Tommaso SK, Catherino WH, Lalitkumar S, Gupta D, Vollenhoven B. Uterine fibroids. Nat Rev Dis Primers. 2016;2:16043. [DOI] [PubMed] [Google Scholar]
- 6. Hodge JC, Quade BJ, Rubin MA, Stewart EA, Dal Cin P, Morton CC. Molecular and cytogenetic characterization of plexiform leiomyomata provide further evidence for genetic heterogeneity underlying uterine fibroids. Am J Pathol. 2008;172(5):1403-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mäkinen N, Mehine M, Tolvanen J, et al. MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science. 2011;334(6053):252-255. [DOI] [PubMed] [Google Scholar]
- 8. McGuire MM, Yatsenko A, Hoffner L, Jones M, Surti U, Rajkovic A. Whole exome sequencing in a random sample of North American women with leiomyomas identifies MED12 mutations in majority of uterine leiomyomas. Plos One. 2012;7(3):e33251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yatsenko SA, Mittal P, Wood-Trageser MA, et al. Highly heterogeneous genomic landscape of uterine leiomyomas by whole exome sequencing and genome-wide arrays. Fertil Steril. 2017;107(2):457-466.e9. [DOI] [PubMed] [Google Scholar]
- 10. Heinonen HR, Pasanen A, Heikinheimo O, et al. Multiple clinical characteristics separate MED12-mutation-positive and -negative uterine leiomyomas. Sci Rep. 2017;7(1):1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jamaluddin MFB, Nahar P, Tanwar PS. Proteomic characterization of the extracellular matrix of human uterine fibroids. Endocrinology. 2018;159(7):2656-2669. [DOI] [PubMed] [Google Scholar]
- 12. Jamaluddin MFB, Ko YA, Kumar M, et al. Proteomic profiling of human uterine fibroids reveals upregulation of the extracellular matrix protein periostin. Endocrinology. 2018;159(2):1106-1118. [DOI] [PubMed] [Google Scholar]
- 13. Heinonen HR, Mehine M, Mäkinen N, et al. Global metabolomic profiling of uterine leiomyomas. Br J Cancer. 2017;117(12):1855-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Holdsworth-Carson SJ, Zhao D, Cann L, Bittinger S, Nowell CJ, Rogers PA. Differences in the cellular composition of small versus large uterine fibroids. Reproduction. 2016;152(5):467-480. [DOI] [PubMed] [Google Scholar]
- 15. Tinelli A, Malvasi A, Hurst BS, et al. Surgical management of neurovascular bundle in uterine fibroid pseudocapsule. JSLS. 2012;16(1):119-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tinelli A, Mynbaev OA, Sparic R, et al. Angiogenesis and vascularization of uterine leiomyoma: clinical value of pseudocapsule containing peptides and neurotransmitters. Curr Protein Pept Sci. 2017;18(2):129-139. [DOI] [PubMed] [Google Scholar]
- 17. Tinelli A, Kosmas I, Mynbaev OA, et al. Submucous fibroids, fertility, and possible correlation to pseudocapsule thickness in reproductive surgery. Biomed Res Int. 2018;2018:2804830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tinelli A, Favilli A, Lasmar RB, et al. The importance of pseudocapsule preservation during hysteroscopic myomectomy. Eur J Obstet Gynecol Reprod Biol. 2019;243:179-184. [DOI] [PubMed] [Google Scholar]
- 19. Stewart EA. Uterine fibroids. Lancet. 2001;357(9252):293-298. [DOI] [PubMed] [Google Scholar]
- 20. Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science. 2005;308(5728):1589-1592. [DOI] [PubMed] [Google Scholar]
- 21. Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol. 2012;206(3):211.e1-211.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee M, Chung YJ, Kim HK, et al. Estimated prevalence and incidence of uterine leiomyoma, and its treatment trend in South Korean women for 12 years: a national population-based study. J Womens Health (Larchmt). 2021;30(7):1038-1046. [DOI] [PubMed] [Google Scholar]
- 23. Ciebiera M, Włodarczyk M, Słabuszewska-Jóźwiak A, Nowicka G, Jakiel G. Influence of vitamin D and transforming growth factor β3 serum concentrations, obesity, and family history on the risk for uterine fibroids. Fertil Steril. 2016;106(7):1787-1792. [DOI] [PubMed] [Google Scholar]
- 24. Faerstein E, Szklo M, Rosenshein N. Risk factors for uterine leiomyoma: a practice-based case-control study. I. African-American heritage, reproductive history, body size, and smoking. Am J Epidemiol. 2001;153(1):1-10. [DOI] [PubMed] [Google Scholar]
- 25. Wise LA, Palmer JR, Spiegelman D, et al. Influence of body size and body fat distribution on risk of uterine leiomyomata in U.S. black women. Epidemiology. 2005;16(3):346-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ciebiera M, Wlodarczyk M, Ciebiera M, Zareba K, Lukaszuk K, Jakiel G. Vitamin D and uterine fibroids-review of the literature and novel concepts. Int J Mol Sci. 2018;19(7). doi: 10.3390/ijms19072051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sabry M, Halder SK, Allah AS, Roshdy E, Rajaratnam V, Al-Hendy A. Serum vitamin D3 level inversely correlates with uterine fibroid volume in different ethnic groups: a cross-sectional observational study. Int J Womens Health. 2013;5:93-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mohammadi R, Tabrizi R, Hessami K, et al. Correlation of low serum vitamin-D with uterine leiomyoma: a systematic review and meta-analysis. Reprod Biol Endocrinol. 2020;18(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ciebiera M, Szymańska-Majchrzak J, Sentkowska A, et al. Alpha-tocopherol serum levels are increased in caucasian women with uterine fibroids: a pilot study. Biomed Res Int. 2018;2018:6793726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baker JM, Chase DM, Herbst-Kralovetz MM. Uterine microbiota: residents, tourists, or invaders? Front Immunol. 2018;9:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee G, Kim S, Bastiaensen M, et al. Exposure to organophosphate esters, phthalates, and alternative plasticizers in association with uterine fibroids. Environ Res. 2020;189:109874. [DOI] [PubMed] [Google Scholar]
- 32. Bariani MV, Rangaswamy R, Siblini H, Yang Q, Al-Hendy A, Zota AR. The role of endocrine-disrupting chemicals in uterine fibroid pathogenesis. Curr Opin Endocrinol Diabetes Obes. 2020;27(6):380-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang Q, Ali M, El Andaloussi A, Al-Hendy A. The emerging spectrum of early life exposure-related inflammation and epigenetic therapy. Cancer Stud Mol Med. 2018;4(1):13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wong JY, Chang PY, Gold EB, Johnson WO, Lee JS. Environmental tobacco smoke and risk of late-diagnosis incident fibroids in the Study of Women’s Health across the Nation (SWAN). Fertil Steril. 2016;106(5):1157-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Takala H, Yang Q, El Razek AMA, Ali M, Al-Hendy A. Alcohol consumption and risk of uterine fibroids. Curr Mol Med. 2020;20(4):247-258. [DOI] [PubMed] [Google Scholar]
- 36. Baird DD, Patchel SA, Saldana TM, et al. Uterine fibroid incidence and growth in an ultrasound-based, prospective study of young African Americans. Am J Obstet Gynecol. 2020;223(3):402.e1-402.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marsh EE, Al-Hendy A, Kappus D, Galitsky A, Stewart EA, Kerolous M. Burden, prevalence, and treatment of uterine fibroids: a survey of U.S. Women. J Womens Health (Larchmt). 2018;27(11):1359-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jager KJ, Tripepi G, Chesnaye NC, Dekker FW, Zoccali C, Stel VS. Where to look for the most frequent biases? Nephrology (Carlton). 2020;25(6):435-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90(6):967-973. [DOI] [PubMed] [Google Scholar]
- 40. Wise LA, Palmer JR, Stewart EA, Rosenberg L. Age-specific incidence rates for self-reported uterine leiomyomata in the Black Women’s Health Study. Obstet Gynecol. 2005;105(3):563-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wright KN, Laufer MR. Leiomyomas in adolescents. Fertil Steril. 2011;95(7):2434.e15-2434.e17. [DOI] [PubMed] [Google Scholar]
- 42. Moroni RM, Vieira CS, Ferriani RA, Reis RM, Nogueira AA, Brito LG. Presentation and treatment of uterine leiomyoma in adolescence: a systematic review. BMC Womens Health. 2015;15:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang CH, Lee JN, Hsu SC, Kuo CH, Tsai EM. Effect of hormone replacement therapy on uterine fibroids in postmenopausal women–a 3-year study. Maturitas. 2002;43(1):35-39. [DOI] [PubMed] [Google Scholar]
- 44. Stewart EA, Cookson CL, Gandolfo RA, Schulze-Rath R. Epidemiology of uterine fibroids: a systematic review. BJOG. 2017;124(10):1501-1512. [DOI] [PubMed] [Google Scholar]
- 45. Baird DD, Patchel SA, Saldana TM, et al. Uterine fibroid incidence and growth in an ultrasound-based, prospective study of young African Americans. Am J Obstet Gynecol. 2020;223(3):402.e1-402.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Eltoukhi HM, Modi MN, Weston M, Armstrong AY, Stewart EA. The health disparities of uterine fibroid tumors for African American women: a public health issue. Am J Obstet Gynecol. 2014;210(3):194-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stewart EA, Nicholson WK, Bradley L, Borah BJ. The burden of uterine fibroids for African-American women: results of a national survey. J Womens Health (Larchmt). 2013;22(10):807-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Al-Hendy A, Salama SA. Catechol-O-methyltransferase polymorphism is associated with increased uterine leiomyoma risk in different ethnic groups. J Soc Gynecol Investig. 2006;13(2):136-144. [DOI] [PubMed] [Google Scholar]
- 49. Baird DD, Hill MC, Schectman JM, Hollis BW. Vitamin D and the risk of uterine fibroids. Epidemiology. 2013;24(3):447-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brakta S, Diamond JS, Al-Hendy A, Diamond MP, Halder SK. Role of vitamin D in uterine fibroid biology. Fertil Steril. 2015;104(3):698-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health. 2006;96(5):826-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Van Dyke ME, Baumhofer NK, Slopen N, et al. Pervasive discrimination and allostatic load in African American and white adults. Psychosom Med. 2020;82(3):316-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Williams DR, Mohammed SA. Discrimination and racial disparities in health: evidence and needed research. J Behav Med. 2009;32(1):20-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wise LA, Palmer JR, Cozier YC, Hunt MO, Stewart EA, Rosenberg L. Perceived racial discrimination and risk of uterine leiomyomata. Epidemiology. 2007;18(6):747-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vines AI, Ta M, Esserman DA. The association between self-reported major life events and the presence of uterine fibroids. Womens Health Issues. 2010;20(4):294-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Thompson VL. Perceived experiences of racism as stressful life events. Community Ment Health J. 1996;32(3):223-233. [DOI] [PubMed] [Google Scholar]
- 57. Paradies Y, Ben J, Denson N, et al. Racism as a determinant of health: a systematic review and meta-analysis. Plos One. 2015;10(9):e0138511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dhama K, Latheef SK, Dadar M, et al. Biomarkers in stress related diseases/disorders: diagnostic, prognostic, and therapeutic values. Front Mol Biosci. 2019;6:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Matyakhina L, Freedman RJ, Bourdeau I, et al. Hereditary leiomyomatosis associated with bilateral, massive, macronodular adrenocortical disease and atypical Cushing syndrome: a clinical and molecular genetic investigation. J Clin Endocrinol Metab. 2005;90(6):3773-3779. [DOI] [PubMed] [Google Scholar]
- 60. Herrera AY, Nielsen SE, Mather M. Stress-induced increases in progesterone and cortisol in naturally cycling women. Neurobiol Stress. 2016;3:96-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Puder JJ, Freda PU, Goland RS, Ferin M, Wardlaw SL. Stimulatory effects of stress on gonadotropin secretion in estrogen-treated women. J Clin Endocrinol Metab. 2000;85(6):2184-2188. [DOI] [PubMed] [Google Scholar]
- 62. Simons RL, Lei MK, Beach SRH, et al. Discrimination, segregation, and chronic inflammation: Testing the weathering explanation for the poor health of Black Americans. Dev Psychol. 2018;54(10):1993-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Romieu I, Dossus L, Barquera S, et al. ; IARC working group on Energy Balance and Obesity . Energy balance and obesity: what are the main drivers? Cancer Causes Control. 2017;28(3):247-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Afzal S, Brøndum-Jacobsen P, Bojesen SE, Nordestgaard BG. Vitamin D concentration, obesity, and risk of diabetes: a Mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2(4):298-306. [DOI] [PubMed] [Google Scholar]
- 65. Sun K, Xie Y, Zhao N, Li Z. A case-control study of the relationship between visceral fat and development of uterine fibroids. Exp Ther Med. 2019;18(1):404-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci. 2017;13(4):851-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Shozu M, Murakami K, Inoue M. Aromatase and leiomyoma of the uterus. Semin Reprod Med. 2004;22(1):51-60. [DOI] [PubMed] [Google Scholar]
- 68. Bulun SE, Imir G, Utsunomiya H, et al. Aromatase in endometriosis and uterine leiomyomata. J Steroid Biochem Mol Biol. 2005;95(1-5):57-62. [DOI] [PubMed] [Google Scholar]
- 69. Bulun SE, Lin Z, Imir G, et al. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol Rev. 2005;57(3):359-383. [DOI] [PubMed] [Google Scholar]
- 70. Hautanen A. Synthesis and regulation of sex hormone-binding globulin in obesity. Int J Obes Relat Metab Disord. 2000;24 Suppl 2:S64-S70. [DOI] [PubMed] [Google Scholar]
- 71. Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. Association of physical activity with development of uterine leiomyoma. Am J Epidemiol. 2007;165(2):157-163. [DOI] [PubMed] [Google Scholar]
- 72. Shikora SA, Niloff JM, Bistrian BR, Forse RA, Blackburn GL. Relationship between obesity and uterine leiomyomata. Nutrition. 1991;7(4):251-255. [PubMed] [Google Scholar]
- 73. Yang Y, He Y, Zeng Q, Li S. Association of body size and body fat distribution with uterine fibroids among Chinese women. J Womens Health (Larchmt). 2014;23(7):619-626. [DOI] [PubMed] [Google Scholar]
- 74. Parazzini F, Negri E, La Vecchia C, Chatenoud L, Ricci E, Guarnerio P. Reproductive factors and risk of uterine fibroids. Epidemiology. 1996;7(4):440-442. [DOI] [PubMed] [Google Scholar]
- 75. Sommer EM, Balkwill A, Reeves G, Green J, Beral DV, Coffey K; Million Women Study Collaborators . Effects of obesity and hormone therapy on surgically-confirmed fibroids in postmenopausal women. Eur J Epidemiol. 2015;30(6):493-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Okolo S. Incidence, aetiology and epidemiology of uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2008;22(4):571-588. [DOI] [PubMed] [Google Scholar]
- 77. Baird DD, Dunson DB. Why is parity protective for uterine fibroids? Epidemiology. 2003;14(2):247-250. [DOI] [PubMed] [Google Scholar]
- 78. Laughlin SK, Herring AH, Savitz DA, et al. Pregnancy-related fibroid reduction. Fertil Steril. 2010;94(6):2421-2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Boynton-Jarrett R, Rich-Edwards J, Malspeis S, Missmer SA, Wright R. A prospective study of hypertension and risk of uterine leiomyomata. Am J Epidemiol. 2005;161(7):628-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Radin RG, Rosenberg L, Palmer JR, Cozier YC, Kumanyika SK, Wise LA. Hypertension and risk of uterine leiomyomata in US black women. Hum Reprod. 2012;27(5):1504-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Takeda T, Sakata M, Isobe A, et al. Relationship between metabolic syndrome and uterine leiomyomas: a case-control study. Gynecol Obstet Invest. 2008;66(1):14-17. [DOI] [PubMed] [Google Scholar]
- 82. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281. [DOI] [PubMed] [Google Scholar]
- 83. Bikle D. Vitamin D: production, metabolism, and mechanisms of action. In: Feingold KR, Anawalt B, Boyce A, et al. , eds. Endotext. MDText.com, Inc; 2000. [PubMed] [Google Scholar]
- 84. Nair R, Maseeh A. Vitamin D: The “sunshine” vitamin. J Pharmacol Pharmacother. 2012;3(2):118-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Norman AW. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr. 2008;88(2):491S-499S. [DOI] [PubMed] [Google Scholar]
- 86. Wacker M, Holick MF. Sunlight and Vitamin D: A global perspective for health. Dermatoendocrinol. 2013;5(1):51-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Prentice A, Schoenmakers I, Jones KS, Jarjou LM, Goldberg GR. Vitamin D deficiency and its health consequences in Africa. Clin Rev Bone Miner Metab. 2009;7:94-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zhao G, Ford ES, Tsai J, Li C, Croft JB. Factors associated with vitamin D deficiency and inadequacy among women of childbearing age in the United States. ISRN Obstet Gynecol. 2012;2012:691486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1(8263):74-76. [DOI] [PubMed] [Google Scholar]
- 90. Pilz S, Zittermann A, Trummer C, et al. Vitamin D testing and treatment: a narrative review of current evidence. Endocr Connect. 2019;8(2):R27-R43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19(2):73-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. ; Endocrine Society . Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-1930. [DOI] [PubMed] [Google Scholar]
- 93. Rosen CJ, Abrams SA, Aloia JF, et al. IOM committee members respond to Endocrine Society vitamin D guideline. J Clin Endocrinol Metab. 2012;97(4):1146-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kennel KA, Drake MT, Hurley DL. Vitamin D deficiency in adults: when to test and how to treat. Mayo Clin Proc. 2010;85(8):752-7; quiz 757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Jukic AMZ, Upson K, Harmon QE, Baird DD. Increasing serum 25-hydroxyvitamin D is associated with reduced odds of long menstrual cycles in a cross-sectional study of African American women. Fertil Steril. 2016;106(1):172-179.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Nandi A, Sinha N, Ong E, Sonmez H, Poretsky L. Is there a role for vitamin D in human reproduction? Horm Mol Biol Clin Investig. 2016;25(1):15-28. [DOI] [PubMed] [Google Scholar]
- 97. Triunfo S, Lanzone A. Potential impact of maternal vitamin D status on obstetric well-being. J Endocrinol Invest. 2016;39(1):37-44. [DOI] [PubMed] [Google Scholar]
- 98. Bläuer M, Rovio PH, Ylikomi T, Heinonen PK. Vitamin D inhibits myometrial and leiomyoma cell proliferation in vitro. Fertil Steril. 2009;91(5):1919-1925. [DOI] [PubMed] [Google Scholar]
- 99. Halder SK, Osteen KG, Al-Hendy A. Vitamin D3 inhibits expression and activities of matrix metalloproteinase-2 and -9 in human uterine fibroid cells. Hum Reprod. 2013;28(9):2407-2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Halder SK, Osteen KG, Al-Hendy A. 1,25-dihydroxyvitamin d3 reduces extracellular matrix-associated protein expression in human uterine fibroid cells. Biol Reprod. 2013;89(6):150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Paffoni A, Somigliana E, Vigano’ P, et al. Vitamin D status in women with uterine leiomyomas. J Clin Endocrinol Metab. 2013;98(8):E1374-E1378. [DOI] [PubMed] [Google Scholar]
- 102. Chiaffarino F, Parazzini F, La Vecchia C, Chatenoud L, Di Cintio E, Marsico S. Diet and uterine myomas. Obstet Gynecol. 1999;94(3):395-398. [DOI] [PubMed] [Google Scholar]
- 103. Parker WH. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril. 2007;87(4):725-736. [DOI] [PubMed] [Google Scholar]
- 104. Wise LA, Radin RG, Palmer JR, Kumanyika SK, Boggs DA, Rosenberg L. Intake of fruit, vegetables, and carotenoids in relation to risk of uterine leiomyomata. Am J Clin Nutr. 2011;94(6):1620-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wise LA, Radin RG, Kumanyika SK, Ruiz-Narváez EA, Palmer JR, Rosenberg L. Prospective study of dietary fat and risk of uterine leiomyomata. Am J Clin Nutr. 2014;99(5):1105-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Harris SS. Vitamin D and African Americans. J Nutr. 2006;136(4):1126-1129. [DOI] [PubMed] [Google Scholar]
- 107. Chwalisz K, Taylor H. Current and emerging medical treatments for uterine fibroids. Semin Reprod Med. 2017;35(6):510-522. [DOI] [PubMed] [Google Scholar]
- 108. Marshall LM, Spiegelman D, Goldman MB, et al. A prospective study of reproductive factors and oral contraceptive use in relation to the risk of uterine leiomyomata. Fertil Steril. 1998;70(3):432-439. [DOI] [PubMed] [Google Scholar]
- 109. Marshall LM, Spiegelman D, Manson JE, et al. Risk of uterine leiomyomata among premenopausal women in relation to body size and cigarette smoking. Epidemiology. 1998;9(5):511-517. [PubMed] [Google Scholar]
- 110. Chiaffarino F, Parazzini F, La Vecchia C, Marsico S, Surace M, Ricci E. Use of oral contraceptives and uterine fibroids: results from a case-control study. Br J Obstet Gynaecol. 1999;106(8):857-860. [DOI] [PubMed] [Google Scholar]
- 111. Islam MS, Segars JH, Castellucci M, Ciarmela P. Dietary phytochemicals for possible preventive and therapeutic option of uterine fibroids: Signaling pathways as target. Pharmacol Rep. 2017;69(1):57-70. [DOI] [PubMed] [Google Scholar]
- 112. Islam MS, Akhtar MM, Segars JH, Castellucci M, Ciarmela P. Molecular targets of dietary phytochemicals for possible prevention and therapy of uterine fibroids: Focus on fibrosis. Crit Rev Food Sci Nutr. 2017;57(17):3583-3600. [DOI] [PubMed] [Google Scholar]
- 113. Ali M, Al-Hendy A. Uterine fibroid therapy: the pharmacokinetic considerations. Expert Opin Drug Metab Toxicol. 2018;14(9):887-889. [DOI] [PubMed] [Google Scholar]
- 114. Gao M, Wang H. Frequent milk and soybean consumption are high risks for uterine leiomyoma: A prospective cohort study. Medicine (Baltimore). 2018;97(41):e12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Orta OR, Terry KL, Missmer SA, Harris HR. Dairy and related nutrient intake and risk of uterine leiomyoma: a prospective cohort study. Hum Reprod. 2020;35(2):453-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Croce S, Ribeiro A, Brulard C, et al. Uterine smooth muscle tumor analysis by comparative genomic hybridization: a useful diagnostic tool in challenging lesions. Mod Pathol. 2015;28(7):1001-1010. [DOI] [PubMed] [Google Scholar]
- 117. Mehine M, Mäkinen N, Heinonen HR, Aaltonen LA, Vahteristo P. Genomics of uterine leiomyomas: insights from high-throughput sequencing. Fertil Steril. 2014;102(3):621-629. [DOI] [PubMed] [Google Scholar]
- 118. Mäkinen N, Heinonen HR, Moore S, Tomlinson IP, van der Spuy ZM, Aaltonen LA. MED12 exon 2 mutations are common in uterine leiomyomas from South African patients. Oncotarget. 2011;2(12):966-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Kämpjärvi K, Mäkinen N, Kilpivaara O, et al. Somatic MED12 mutations in uterine leiomyosarcoma and colorectal cancer. Br J Cancer. 2012;107(10):1761-1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Je EM, Kim MR, Min KO, Yoo NJ, Lee SH. Mutational analysis of MED12 exon 2 in uterine leiomyoma and other common tumors. Int J Cancer. 2012;131(6):E1044-E1047. [DOI] [PubMed] [Google Scholar]
- 121. Markowski DN, Bartnitzke S, Löning T, Drieschner N, Helmke BM, Bullerdiek J. MED12 mutations in uterine fibroids–their relationship to cytogenetic subgroups. Int J Cancer. 2012;131(7):1528-1536. [DOI] [PubMed] [Google Scholar]
- 122. Markowski DN, Huhle S, Nimzyk R, Stenman G, Löning T, Bullerdiek J. MED12 mutations occurring in benign and malignant mammalian smooth muscle tumors. Genes Chromosomes Cancer. 2013;52(3):297-304. [DOI] [PubMed] [Google Scholar]
- 123. Sadeghi S, Khorrami M, Amin-Beidokhti M, et al. The study of MED12 gene mutations in uterine leiomyomas from Iranian patients. Tumour Biol. 2016;37(2):1567-1571. [DOI] [PubMed] [Google Scholar]
- 124. Shahbazi S, Fatahi N, Amini-Moghaddam S. Somatic mutational analysis of MED12 exon 2 in uterine leiomyomas of Iranian women. Am J Cancer Res. 2015;5(8):2441-2446. [PMC free article] [PubMed] [Google Scholar]
- 125. Halder SK, Laknaur A, Miller J, Layman LC, Diamond M, Al-Hendy A. Novel MED12 gene somatic mutations in women from the Southern United States with symptomatic uterine fibroids. Mol Genet Genomics. 2015;290(2):505-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Wang H, Ye J, Qian H, Zhou R, Jiang J, Ye L. High-resolution melting analysis of MED12 mutations in uterine leiomyomas in Chinese patients. Genet Test Mol Biomarkers. 2015;19(3):162-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Wu B, Słabicki M, Sellner L, et al. MED12 mutations and NOTCH signalling in chronic lymphocytic leukaemia. Br J Haematol. 2017;179(3):421-429. [DOI] [PubMed] [Google Scholar]
- 128. Park MJ, Shen H, Kim NH, et al. Mediator kinase disruption in MED12-mutant uterine fibroids from Hispanic women of south Texas. J Clin Endocrinol Metab. 2018;103(11):4283-4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Kämpjärvi K, Park MJ, Mehine M, et al. Mutations in Exon 1 highlight the role of MED12 in uterine leiomyomas. Hum Mutat. 2014;35(9):1136-1141. [DOI] [PubMed] [Google Scholar]
- 130. Hodge JC, Pearce KE, Clayton AC, Taran FA, Stewart EA. Uterine cellular leiomyomata with chromosome 1p deletions represent a distinct entity. Am J Obstet Gynecol. 2014;210(6):572.e1-572.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Nezhad MH, Drieschner N, Helms S, et al. 6p21 rearrangements in uterine leiomyomas targeting HMGA1. Cancer Genet Cytogenet. 2010;203(2):247-252. [DOI] [PubMed] [Google Scholar]
- 132. Vanharanta S, Pollard PJ, Lehtonen HJ, et al. Distinct expression profile in fumarate-hydratase-deficient uterine fibroids. Hum Mol Genet. 2006;15(1):97-103. [DOI] [PubMed] [Google Scholar]
- 133. Vanharanta S, Wortham NC, Laiho P, et al. 7q deletion mapping and expression profiling in uterine fibroids. Oncogene. 2005;24(43):6545-6554. [DOI] [PubMed] [Google Scholar]
- 134. Mehine M, Kaasinen E, Heinonen HR, et al. Integrated data analysis reveals uterine leiomyoma subtypes with distinct driver pathways and biomarkers. Proc Natl Acad Sci U S A. 2016;113(5):1315-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. George JW, Fan H, Johnson B, et al. Integrated epigenome, exome, and transcriptome analyses reveal molecular subtypes and homeotic transformation in uterine fibroids. Cell Rep. 2019;29(12):4069-4085.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. He C, Nelson W, Li H, et al. Frequency of MED12 mutation in relation to tumor and patient’s clinical characteristics: a meta-analysis. Reprod Sci. Published online February 10, 2021. doi: 10.1007/s43032-021-00473-x [DOI] [PubMed] [Google Scholar]
- 137. Clark AD, Oldenbroek M, Boyer TG. Mediator kinase module and human tumorigenesis. Crit Rev Biochem Mol Biol. 2015;50(5):393-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Lim WK, Ong CK, Tan J, et al. Exome sequencing identifies highly recurrent MED12 somatic mutations in breast fibroadenoma. Nat Genet. 2014;46(8):877-880. [DOI] [PubMed] [Google Scholar]
- 139. Yoshida M, Sekine S, Ogawa R, et al. Frequent MED12 mutations in phyllodes tumours of the breast. Br J Cancer. 2015;112(10):1703-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Piscuoglio S, Murray M, Fusco N, et al. MED12 somatic mutations in fibroadenomas and phyllodes tumours of the breast. Histopathology. 2015;67(5):719-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Nagasawa S, Maeda I, Fukuda T, et al. MED12 exon 2 mutations in phyllodes tumors of the breast. Cancer Med. 2015;4(7):1117-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Mishima C, Kagara N, Tanei T, et al. Mutational analysis of MED12 in fibroadenomas and phyllodes tumors of the breast by means of targeted next-generation sequencing. Breast Cancer Res Treat. 2015;152(2):305-312. [DOI] [PubMed] [Google Scholar]
- 143. Tan J, Ong CK, Lim WK, et al. Genomic landscapes of breast fibroepithelial tumors. Nat Genet. 2015;47(11):1341-1345. [DOI] [PubMed] [Google Scholar]
- 144. Kämpjärvi K, Järvinen TM, Heikkinen T, et al. Somatic MED12 mutations are associated with poor prognosis markers in chronic lymphocytic leukemia. Oncotarget. 2015;6(3):1884-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Mittal P, Shin YH, Yatsenko SA, Castro CA, Surti U, Rajkovic A. Med12 gain-of-function mutation causes leiomyomas and genomic instability. J Clin Invest. 2015;125(8):3280-3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Croce S, Chibon F. MED12 and uterine smooth muscle oncogenesis: state of the art and perspectives. Eur J Cancer. 2015;51(12):1603-1610. [DOI] [PubMed] [Google Scholar]
- 147. Heinonen HR, Sarvilinna NS, Sjöberg J, et al. MED12 mutation frequency in unselected sporadic uterine leiomyomas. Fertil Steril. 2014;102(4):1137-1142. [DOI] [PubMed] [Google Scholar]
- 148. Markowski DN, Helmke BM, Bartnitzke S, Löning T, Bullerdiek J. Uterine fibroids: do we deal with more than one disease? Int J Gynecol Pathol. 2014;33(6):568-572. [DOI] [PubMed] [Google Scholar]
- 149. Matsubara A, Sekine S, Yoshida M, et al. Prevalence of MED12 mutations in uterine and extrauterine smooth muscle tumours. Histopathology. 2013;62(4):657-661. [DOI] [PubMed] [Google Scholar]
- 150. Mäkinen N, Vahteristo P, Kämpjärvi K, Arola J, Bützow R, Aaltonen LA. MED12 exon 2 mutations in histopathological uterine leiomyoma variants. Eur J Hum Genet. 2013;21(11):1300-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Pérot G, Croce S, Ribeiro A, et al. MED12 alterations in both human benign and malignant uterine soft tissue tumors. Plos One. 2012;7(6):e40015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Zhang Q, Ubago J, Li L, et al. Molecular analyses of 6 different types of uterine smooth muscle tumors: emphasis in atypical leiomyoma. Cancer. 2014;120(20):3165-3177. [DOI] [PubMed] [Google Scholar]
- 153. Äyräväinen A, Pasanen A, Ahvenainen T, et al. Systematic molecular and clinical analysis of uterine leiomyomas from fertile-aged women undergoing myomectomy. Hum Reprod. 2020;35(10):2237-2244. [DOI] [PubMed] [Google Scholar]
- 154. Nadine Markowski D, Tadayyon M, Bartnitzke S, Belge G, Maria Helmke B, Bullerdiek J. Cell cultures in uterine leiomyomas: rapid disappearance of cells carrying MED12 mutations. Genes Chromosomes Cancer. 2014;53(4):317-323. [DOI] [PubMed] [Google Scholar]
- 155. Bloch J, Holzmann C, Koczan D, Helmke BM, Bullerdiek J. Factors affecting the loss of MED12-mutated leiomyoma cells during in vitro growth. Oncotarget. 2017;8(21):34762-34772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Holdsworth-Carson SJ, Zaitseva M, Vollenhoven BJ, Rogers PA. Clonality of smooth muscle and fibroblast cell populations isolated from human fibroid and myometrial tissues. Mol Hum Reprod. 2014;20(3):250-259. [DOI] [PubMed] [Google Scholar]
- 157. Salo T, Sutinen M, Hoque Apu E, et al. A novel human leiomyoma tissue derived matrix for cell culture studies. BMC Cancer. 2015;15:981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Wu X, Serna VA, Thomas J, Qiang W, Blumenfeld ML, Kurita T. Subtype-specific tumor-associated fibroblasts contribute to the pathogenesis of uterine leiomyoma. Cancer Res. 2017;77(24):6891-6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Serna VA, Wu X, Qiang W, Thomas J, Blumenfeld ML, Kurita T. Cellular kinetics of MED12-mutant uterine leiomyoma growth and regression in vivo. Endocr Relat Cancer. 2018;25(7):747-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Barbieri CE, Baca SC, Lawrence MS, et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat Genet. 2012;44(6):685-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Arai E, Sakamoto H, Ichikawa H, et al. Multilayer-omics analysis of renal cell carcinoma, including the whole exome, methylome and transcriptome. Int J Cancer. 2014;135(6):1330-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Kämpjärvi K, Kim NH, Keskitalo S, et al. Somatic MED12 mutations in prostate cancer and uterine leiomyomas promote tumorigenesis through distinct mechanisms. Prostate. 2016;76(1):22-31. [DOI] [PubMed] [Google Scholar]
- 163. Knuesel MT, Meyer KD, Donner AJ, Espinosa JM, Taatjes DJ. The human CDK8 subcomplex is a histone kinase that requires Med12 for activity and can function independently of mediator. Mol Cell Biol. 2009;29(3):650-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Knuesel MT, Meyer KD, Bernecky C, Taatjes DJ. The human CDK8 subcomplex is a molecular switch that controls Mediator coactivator function. Genes Dev. 2009;23(4):439-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Turunen M, Spaeth JM, Keskitalo S, et al. Uterine leiomyoma-linked MED12 mutations disrupt mediator-associated CDK activity. Cell Rep. 2014;7(3):654-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Park MJ, Shen H, Spaeth JM, et al. Oncogenic exon 2 mutations in Mediator subunit MED12 disrupt allosteric activation of cyclin C-CDK8/19. J Biol Chem. 2018;293(13):4870-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Li YC, Chao TC, Kim HJ, et al. Structure and noncanonical Cdk8 activation mechanism within an argonaute-containing mediator kinase module. Sci Adv. 2021;7(3). doi: 10.1126/sciadv.abd4484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Spaeth JM, Kim NH, Boyer TG. Mediator and human disease. Semin Cell Dev Biol. 2011;22(7):776-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Fant CB, Taatjes DJ. Regulatory functions of the Mediator kinases CDK8 and CDK19. Transcription. 2019;10(2):76-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Aranda-Orgilles B, Saldaña-Meyer R, Wang E, et al. MED12 regulates HSC-specific enhancers independently of mediator kinase activity to control hematopoiesis. Cell Stem Cell. 2016;19(6):784-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Papadopoulou T, Kaymak A, Sayols S, Richly H. Dual role of Med12 in PRC1-dependent gene repression and ncRNA-mediated transcriptional activation. Cell Cycle. 2016;15(11):1479-1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Huang S, Hölzel M, Knijnenburg T, et al. MED12 controls the response to multiple cancer drugs through regulation of TGF-β receptor signaling. Cell. 2012;151(5):937-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Ding N, Zhou H, Esteve PO, et al. Mediator links epigenetic silencing of neuronal gene expression with x-linked mental retardation. Mol Cell. 2008;31(3):347-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. El Andaloussi A, Al-Hendy A, Ismail N, Boyer TG, Halder SK. Introduction of somatic mutation in MED12 induces Wnt4/β-catenin and disrupts autophagy in human uterine myometrial cell. Reprod Sci. 2020;27(3):823-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Liu S, Yin P, Kujawa SA, Coon JS 5th, Okeigwe I, Bulun SE. Progesterone receptor integrates the effects of mutated MED12 and altered DNA methylation to stimulate RANKL expression and stem cell proliferation in uterine leiomyoma. Oncogene. 2019;38(15):2722-2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Asano R, Asai-Sato M, Matsukuma S, et al. Expression of erythropoietin messenger ribonucleic acid in wild-type MED12 uterine leiomyomas under estrogenic influence: new insights into related growth disparities. Fertil Steril. 2019;111(1):178-185. [DOI] [PubMed] [Google Scholar]
- 177. Lynch CJ, Bernad R, Martínez-Val A, et al. Global hyperactivation of enhancers stabilizes human and mouse naive pluripotency through inhibition of CDK8/19 Mediator kinases. Nat Cell Biol. 2020;22(10):1223-1238. [DOI] [PubMed] [Google Scholar]
- 178. Martinez-Val A, Lynch CJ, Calvo I, et al. Dissection of two routes to naïve pluripotency using different kinase inhibitors. Nat Commun. 2021;12(1):1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Adler AS, McCleland ML, Truong T, et al. CDK8 maintains tumor dedifferentiation and embryonic stem cell pluripotency. Cancer Res. 2012;72(8):2129-2139. [DOI] [PubMed] [Google Scholar]
- 180. Fukasawa K, Kadota T, Horie T, et al. CDK8 maintains stemness and tumorigenicity of glioma stem cells by regulating the c-MYC pathway. Oncogene. 2021;40(15):2803-2815. [DOI] [PubMed] [Google Scholar]
- 181. Técher H, Koundrioukoff S, Nicolas A, Debatisse M. The impact of replication stress on replication dynamics and DNA damage in vertebrate cells. Nat Rev Genet. 2017;18(9):535-550. [DOI] [PubMed] [Google Scholar]
- 182. Bullerdiek J, Rommel B. Factors targeting MED12 to drive tumorigenesis? F1000Res. 2018;7:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Helmink BA, Khan MAW, Hermann A, Gopalakrishnan V, Wargo JA. The microbiome, cancer, and cancer therapy. Nat Med. 2019;25(3):377-388. [DOI] [PubMed] [Google Scholar]
- 184. Łaniewski P, Ilhan ZE, Herbst-Kralovetz MM. The microbiome and gynaecological cancer development, prevention and therapy. Nat Rev Urol. 2020;17(4):232-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Gallagher CS, Morton CC. Genetic association studies in uterine fibroids: risk alleles presage the path to personalized therapies. Semin Reprod Med. 2016;34(4):235-241. [DOI] [PubMed] [Google Scholar]
- 186. Quade BJ, Weremowicz S, Neskey DM, et al. Fusion transcripts involving HMGA2 are not a common molecular mechanism in uterine leiomyomata with rearrangements in 12q15. Cancer Res. 2003;63(6):1351-1358. [PubMed] [Google Scholar]
- 187. Gross KL, Morton CC. Genetics and the development of fibroids. Clin Obstet Gynecol. 2001;44(2):335-349. [DOI] [PubMed] [Google Scholar]
- 188. Gross KL, Neskey DM, Manchanda N, et al. HMGA2 expression in uterine leiomyomata and myometrium: quantitative analysis and tissue culture studies. Genes Chromosomes Cancer. 2003;38(1):68-79. [DOI] [PubMed] [Google Scholar]
- 189. Mehine M, Kaasinen E, Heinonen HR, et al. Integrated data analysis reveals uterine leiomyoma subtypes with distinct driver pathways and biomarkers. Proc Natl Acad Sci U S A. 2016;113(5):1315-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Stewart L, Glenn GM, Stratton P, et al. Association of germline mutations in the fumarate hydratase gene and uterine fibroids in women with hereditary leiomyomatosis and renal cell cancer. Arch Dermatol. 2008;144(12):1584-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Badeloe S, Bladergroen RS, Jonkman MF, et al. Hereditary multiple cutaneous leiomyoma resulting from novel mutations in the fumarate hydratase gene. J Dermatol Sci. 2008;51(2):139-143. [DOI] [PubMed] [Google Scholar]
- 192. Wheeler KC, Warr DJ, Warsetsky SI, Barmat LI. Novel fumarate hydratase mutation in a family with atypical uterine leiomyomas and hereditary leiomyomatosis and renal cell cancer. Fertil Steril. 2016;105(1):144-148. [DOI] [PubMed] [Google Scholar]
- 193. Popp B, Erber R, Kraus C, et al. Targeted sequencing of FH-deficient uterine leiomyomas reveals biallelic inactivating somatic fumarase variants and allows characterization of missense variants. Mod Pathol. 2020;33(11):2341-2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Berta DG, Kuisma H, Välimäki N, et al. Deficient H2A.Z deposition is associated with genesis of uterine leiomyoma. Nature. 2021;596(7872):398-403. [DOI] [PubMed] [Google Scholar]
- 195. Leistico JR, Saini P, Futtner CR, et al. Epigenomic tensor predicts disease subtypes and reveals constrained tumor evolution. Cell Rep. 2021;34(13):108927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Linder D, Gartler SM. Glucose-6-phosphate dehydrogenase mosaicism: utilization as a cell marker in the study of leiomyomas. Science. 1965;150(3692):67-69. [DOI] [PubMed] [Google Scholar]
- 197. Bulun SE. Uterine fibroids. N Engl J Med. 2013;369(14):1344-1355. [DOI] [PubMed] [Google Scholar]
- 198. Ono M, Maruyama T, Masuda H, et al. Side population in human uterine myometrium displays phenotypic and functional characteristics of myometrial stem cells. Proc Natl Acad Sci U S A. 2007;104(47):18700-18705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Chang HL, Senaratne TN, Zhang L, et al. Uterine leiomyomas exhibit fewer stem/progenitor cell characteristics when compared with corresponding normal myometrium. Reprod Sci. 2010;17(2):158-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Mas A, Cervelló I, Gil-Sanchis C, et al. Identification and characterization of the human leiomyoma side population as putative tumor-initiating cells. Fertil Steril. 2012;98(3):741-751.e6. [DOI] [PubMed] [Google Scholar]
- 201. Mas A, Nair S, Laknaur A, Simón C, Diamond MP, Al-Hendy A. Stro-1/CD44 as putative human myometrial and fibroid stem cell markers. Fertil Steril. 2015;104(1):225-34.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Patterson AL, George JW, Chatterjee A, et al. Putative human myometrial and fibroid stem-like cells have mesenchymal stem cell and endometrial stromal cell properties. Hum Reprod. 2020;35(1):44-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Ono M, Qiang W, Serna VA, et al. Role of stem cells in human uterine leiomyoma growth. Plos One. 2012;7(5):e36935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Elkafas H, Qiwei Y, Al-Hendy A. Origin of uterine fibroids: conversion of myometrial stem cells to tumor-initiating cells. Semin Reprod Med. 2017;35(6):481-486. [DOI] [PubMed] [Google Scholar]
- 205. Afify SM, Seno M. Conversion of stem cells to cancer stem cells: undercurrent of cancer initiation. Cancers (Basel). 2019;11(3). doi: 10.3390/cancers11030345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Mas A, Stone L, O’Connor PM, et al. Developmental exposure to endocrine disruptors expands murine myometrial stem cell compartment as a prerequisite to leiomyoma tumorigenesis. Stem Cells. 2017;35(3):666-678. [DOI] [PubMed] [Google Scholar]
- 207. Hu WY, Shi GB, Hu DP, Nelles JL, Prins GS. Actions of estrogens and endocrine disrupting chemicals on human prostate stem/progenitor cells and prostate cancer risk. Mol Cell Endocrinol. 2012;354(1-2):63-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Cook JD, Davis BJ, Cai SL, Barrett JC, Conti CJ, Walker CL. Interaction between genetic susceptibility and early-life environmental exposure determines tumor-suppressor-gene penetrance. Proc Natl Acad Sci U S A. 2005;102(24):8644-8649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Treviño LS, Dong J, Kaushal A, et al. Epigenome environment interactions accelerate epigenomic aging and unlock metabolically restricted epigenetic reprogramming in adulthood. Nat Commun. 2020;11(1):2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Zhang J, Liu J, Ren L, et al. PM2.5 induces male reproductive toxicity via mitochondrial dysfunction, DNA damage and RIPK1 mediated apoptotic signaling pathway. Sci Total Environ. 2018;634:1435-1444. [DOI] [PubMed] [Google Scholar]
- 211. Hendryx M, Luo J, Chojenta C, Byles JE. Air pollution exposures from multiple point sources and risk of incident chronic obstructive pulmonary disease (COPD) and asthma. Environ Res. 2019;179(Pt A):108783. [DOI] [PubMed] [Google Scholar]
- 212. Herbert C, Kumar RK. Ambient air pollution and asthma. Eur Respir J. 2017;49(5). doi: 10.1183/13993003.00230-2017 [DOI] [PubMed] [Google Scholar]
- 213. Shukla A, Bunkar N, Kumar R, et al. Air pollution associated epigenetic modifications: Transgenerational inheritance and underlying molecular mechanisms. Sci Total Environ. 2019;656:760-777. [DOI] [PubMed] [Google Scholar]
- 214. Fuchs LFP, Veras MM, Saldiva PHN, et al. Ambient levels of concentrated PM2.5 affects cell kinetics in adrenal glands: an experimental study in mice. Gynecol Endocrinol. 2017;33(6):490-495. [DOI] [PubMed] [Google Scholar]
- 215. VoPham T, Bertrand KA, Tamimi RM, Laden F, Hart JE. Ambient PM2.5 air pollution exposure and hepatocellular carcinoma incidence in the United States. Cancer Causes Control. 2018;29(6):563-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Mahalingaiah S, Hart JE, Laden F, et al. Air pollution and risk of uterine leiomyomata. Epidemiology. 2014;25(5):682-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Lin CY, Wang CM, Chen ML, Hwang BF. The effects of exposure to air pollution on the development of uterine fibroids. Int J Hyg Environ Health. 2019;222(3):549-555. [DOI] [PubMed] [Google Scholar]
- 218. Chiaffarino F, Cipriani S, Ricci E, et al. Alcohol consumption and risk of uterine myoma: a systematic review and meta analysis. Plos One. 2017;12(11):e0188355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Wise LA, Palmer JR, Harlow BL, et al. Risk of uterine leiomyomata in relation to tobacco, alcohol and caffeine consumption in the Black Women’s Health Study. Hum Reprod. 2004;19(8):1746-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Templeman C, Marshall SF, Clarke CA, et al. Risk factors for surgically removed fibroids in a large cohort of teachers. Fertil Steril. 2009;92(4):1436-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Nagata C, Nakamura K, Oba S, Hayashi M, Takeda N, Yasuda K. Association of intakes of fat, dietary fibre, soya isoflavones and alcohol with uterine fibroids in Japanese women. Br J Nutr. 2009;101(10):1427-1431. [DOI] [PubMed] [Google Scholar]
- 222. Reichman ME, Judd JT, Longcope C, et al. Effects of alcohol consumption on plasma and urinary hormone concentrations in premenopausal women. J Natl Cancer Inst. 1993;85(9):722-727. [DOI] [PubMed] [Google Scholar]
- 223. Hankinson SE, Willett WC, Manson JE, et al. Alcohol, height, and adiposity in relation to estrogen and prolactin levels in postmenopausal women. J Natl Cancer Inst. 1995;87(17):1297-1302. [DOI] [PubMed] [Google Scholar]
- 224. Katsouyanni K, Boyle P, Trichopoulos D. Diet and urine estrogens among postmenopausal women. Oncology. 1991;48(6):490-494. [DOI] [PubMed] [Google Scholar]
- 225. Garaycoechea JI, Crossan GP, Langevin F, et al. Alcohol and endogenous aldehydes damage chromosomes and mutate stem cells. Nature. 2018;553(7687):171-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Sadikot RT, Bedi B, Li J, Yeligar SM. Alcohol-induced mitochondrial DNA damage promotes injurious crosstalk between alveolar epithelial cells and alveolar macrophages. Alcohol. 2019;80:65-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Kruman II, Henderson GI, Bergeson SE. DNA damage and neurotoxicity of chronic alcohol abuse. Exp Biol Med (Maywood). 2012;237(7):740-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Zhao M, Howard EW, Guo Z, Parris AB, Yang X. p53 pathway determines the cellular response to alcohol-induced DNA damage in MCF-7 breast cancer cells. Plos One. 2017;12(4):e0175121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Chiaffarino F, Ricci E, Cipriani S, Chiantera V, Parazzini F. Cigarette smoking and risk of uterine myoma: systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2016;197:63-71. [DOI] [PubMed] [Google Scholar]
- 230. Ross RK, Pike MC, Vessey MP, Bull D, Yeates D, Casagrande JT. Risk factors for uterine fibroids: reduced risk associated with oral contraceptives. Br Med J (Clin Res Ed). 1986;293(6543):359-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Lumbiganon P, Rugpao S, Phandhu-fung S, Laopaiboon M, Vudhikamraksa N, Werawatakul Y. Protective effect of depot-medroxyprogesterone acetate on surgically treated uterine leiomyomas: a multicentre case–control study. Br J Obstet Gynaecol. 1996;103(9):909-914. [DOI] [PubMed] [Google Scholar]
- 232. D’Aloisio AA, Baird DD, DeRoo LA, Sandler DP. Association of intrauterine and early-life exposures with diagnosis of uterine leiomyomata by 35 years of age in the Sister Study. Environ Health Perspect. 2010;118(3):375-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Faerstein E, Szklo M, Rosenshein NB. Risk factors for uterine leiomyoma: a practice-based case-control study. II. Atherogenic risk factors and potential sources of uterine irritation. Am J Epidemiol. 2001;153(1):11-19. [DOI] [PubMed] [Google Scholar]
- 234. Wang Q, Trevino LS, Wong RL, et al. Reprogramming of the epigenome by MLL1 links early-life environmental exposures to prostate cancer risk. Mol Endocrinol. 2016;30(8):856-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Walker CL, Ho SM. Developmental reprogramming of cancer susceptibility. Nat Rev Cancer. 2012;12(7):479-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. McCampbell AS, Walker CL, Broaddus RR, Cook JD, Davies PJ. Developmental reprogramming of IGF signaling and susceptibility to endometrial hyperplasia in the rat. Lab Invest. 2008;88(6):615-626. [DOI] [PubMed] [Google Scholar]
- 237. Gore AC, Walker DM, Zama AM, Armenti AE, Uzumcu M. Early life exposure to endocrine-disrupting chemicals causes lifelong molecular reprogramming of the hypothalamus and premature reproductive aging. Mol Endocrinol. 2011;25(12):2157-2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Sirohi D, Al Ramadhani R, Knibbs LD. Environmental exposures to endocrine disrupting chemicals (EDCs) and their role in endometriosis: a systematic literature review. Rev Environ Health. 2021;36(1):101-115. [DOI] [PubMed] [Google Scholar]
- 239. Liu Q. Effects of environmental endocrine-disrupting chemicals on female reproductive health. Adv Exp Med Biol. 2021;1300:205-229. [DOI] [PubMed] [Google Scholar]
- 240. Prusinski L, Al-Hendy A, Yang Q. Developmental exposure to endocrine disrupting chemicals alters the epigenome: identification of reprogrammed targets. Gynecol Obstet Res. 2016;3(1):1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Ono M, Yin P, Navarro A, et al. Paracrine activation of WNT/β-catenin pathway in uterine leiomyoma stem cells promotes tumor growth. Proc Natl Acad Sci U S A. 2013;110(42):17053-17058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Hall JM, Greco CW. Perturbation of nuclear hormone receptors by endocrine disrupting chemicals: mechanisms and pathological consequences of exposure. Cells. 2019;9(1). doi: 10.3390/cells9010013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Toporova L, Balaguer P. Nuclear receptors are the major targets of endocrine disrupting chemicals. Mol Cell Endocrinol. 2020;502:110665. [DOI] [PubMed] [Google Scholar]
- 244. Moore AB, Castro L, Yu L, et al. Stimulatory and inhibitory effects of genistein on human uterine leiomyoma cell proliferation are influenced by the concentration. Hum Reprod. 2007;22(10):2623-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Baird DD, Newbold R. Prenatal diethylstilbestrol (DES) exposure is associated with uterine leiomyoma development. Reprod Toxicol. 2005;20(1):81-84. [DOI] [PubMed] [Google Scholar]
- 246. Mahalingaiah S, Hart JE, Wise LA, Terry KL, Boynton-Jarrett R, Missmer SA. Prenatal diethylstilbestrol exposure and risk of uterine leiomyomata in the Nurses’ Health Study II. Am J Epidemiol. 2014;179(2):186-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Zota AR, Geller RJ, Calafat AM, Marfori CQ, Baccarelli AA, Moawad GN. Phthalates exposure and uterine fibroid burden among women undergoing surgical treatment for fibroids: a preliminary study. Fertil Steril. 2019;111(1):112-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Zota AR, Geller RJ, VanNoy BN, et al. Phthalate exposures and MicroRNA expression in uterine fibroids: The FORGE Study. Epigenet Insights. 2020;13:2516865720904057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Lee J, Jeong Y, Mok S, et al. Associations of exposure to phthalates and environmental phenols with gynecological disorders. Reprod Toxicol. 2020;95:19-28. [DOI] [PubMed] [Google Scholar]
- 250. Hassan MH, Eyzaguirre E, Arafa HM, Hamada FM, Salama SA, Al-Hendy A. Memy I: a novel murine model for uterine leiomyoma using adenovirus-enhanced human fibroid explants in severe combined immune deficiency mice. Am J Obstet Gynecol. 2008;199(2):156.e1-156.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Ishikawa H, Ishi K, Serna VA, Kakazu R, Bulun SE, Kurita T. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology. 2010;151(6):2433-2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Croy A, Fritsch M, Schmidt N, et al. Application of a patient derived xenograft model for predicative study of uterine fibroid disease. Plos One. 2015;10(11). doi: 10.1371/journal.pone.0142429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Suo G, Sadarangani A, Lamarca B, Cowan B, Wang JY. Murine xenograft model for human uterine fibroids: an in vivo imaging approach. Reprod Sci. 2009;16(9):827-842. [DOI] [PubMed] [Google Scholar]
- 254. Mas A, Nair S, Laknaur A, Simón C, Diamond MP, Al-Hendy A. Stro-1/CD44 as putative human myometrial and fibroid stem cell markers. Fertil Steril. 2015;104(1):225-34.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Prizant H, Sen A, Light A, et al. Uterine-specific loss of Tsc2 leads to myometrial tumors in both the uterus and lungs. Mol Endocrinol. 2013;27(9):1403-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Varghese BV, Koohestani F, McWilliams M, et al. Loss of the repressor REST in uterine fibroids promotes aberrant G protein-coupled receptor 10 expression and activates mammalian target of rapamycin pathway. Proc Natl Acad Sci U S A. 2013;110(6):2187-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Tanwar PS, Lee HJ, Zhang L, et al. Constitutive activation of Beta-catenin in uterine stroma and smooth muscle leads to the development of mesenchymal tumors in mice. Biol Reprod. 2009;81(3):545-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Field KJ, Griffith JW, Lang CM. Spontaneous reproductive tract leiomyomas in aged guinea-pigs. J Comp Pathol. 1989;101(3):287-294. [DOI] [PubMed] [Google Scholar]
- 259. Walker CL, Hunter D, Everitt JI. Uterine leiomyoma in the Eker rat: a unique model for important diseases of women. Genes Chromosomes Cancer. 2003;38(4):349-356. [DOI] [PubMed] [Google Scholar]
- 260. Everitt JI, Wolf DC, Howe SR, Goldsworthy TL, Walker C. Rodent model of reproductive tract leiomyomata. Clinical and pathological features. Am J Pathol. 1995;146(6):1556-1567. [PMC free article] [PubMed] [Google Scholar]
- 261. Leppert PC, Baginski T, Prupas C, Catherino WH, Pletcher S, Segars JH. Comparative ultrastructure of collagen fibrils in uterine leiomyomas and normal myometrium. Fertil Steril. 2004;82 Suppl 3:1182-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Brody JR, Cunha GR. Histologic, morphometric, and immunocytochemical analysis of myometrial development in rats and mice: II. Effects of DES on development. Am J Anat. 1989;186(1):21-42. [DOI] [PubMed] [Google Scholar]
- 263. Newbold RR, Jefferson WN, Grissom SF, Padilla-Banks E, Snyder RJ, Lobenhofer EK. Developmental exposure to diethylstilbestrol alters uterine gene expression that may be associated with uterine neoplasia later in life. Mol Carcinog. 2007;46(9):783-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Cook JD, Davis BJ, Goewey JA, Berry TD, Walker CL. Identification of a sensitive period for developmental programming that increases risk for uterine leiomyoma in Eker rats. Reprod Sci. 2007;14(2):121-136. [DOI] [PubMed] [Google Scholar]
- 265. Branham WS, Sheehan DM. Ovarian and adrenal contributions to postnatal growth and differentiation of the rat uterus. Biol Reprod. 1995;53(4):863-872. [DOI] [PubMed] [Google Scholar]
- 266. Yang Q, Diamond MP, Al-Hendy A. Early life adverse environmental exposures increase the risk of uterine fibroid development: role of epigenetic regulation. Front Pharmacol. 2016;7:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Greathouse KL, Bredfeldt T, Everitt JI, et al. Environmental estrogens differentially engage the histone methyltransferase EZH2 to increase risk of uterine tumorigenesis. Mol Cancer Res. 2012;10(4):546-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Treviño LS, Wang Q, Walker CL. Phosphorylation of epigenetic “readers, writers and erasers”: Implications for developmental reprogramming and the epigenetic basis for health and disease. Prog Biophys Mol Biol. 2015;118(1-2):8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Wong RL, Wang Q, Treviño LS, et al. Identification of secretaglobin Scgb2a1 as a target for developmental reprogramming by BPA in the rat prostate. Epigenetics. 2015;10(2):127-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Li S, Washburn KA, Moore R, et al. Developmental exposure to diethylstilbestrol elicits demethylation of estrogen-responsive lactoferrin gene in mouse uterus. Cancer Res. 1997;57(19):4356-4359. [PubMed] [Google Scholar]
- 271. Yin Y, Lin C, Veith GM, Chen H, Dhandha M, Ma L. Neonatal diethylstilbestrol exposure alters the metabolic profile of uterine epithelial cells. Dis Model Mech. 2012;5(6):870-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Yang Q, Trevino LS, Mas A, et al. Early life developmental exposure to endocrine disrupting chemicals increases the risk of adult onset of uterine fibroids by permanently reprograming the epigenome of myometrial stem cells towards a pro-fibroid landscape. Fertil Steril. 2016; 106(3):suppl e2. [Google Scholar]
- 273. Elkafas H, Badary OA, Elmorsy E, Kamel R, Al-Hendy A, Yang Q. Targeting activated pro-inflammatory pathway in primed myometrial stem cells with vitamin D3 and Paricalcitol. Fertil Steril. 2019;112(3):e100-e101. [Google Scholar]
- 274. Yang Q, Treviño L, Mas A, Diamond M, Walker C, Al-Hendy A. Identification of novel epigenetic reprogrammed genes in myometrial stem cells developmentally exposed to endocrine disrupting chemicals. Reprod Sci. 2017;24 suppl 1:103A. [Google Scholar]
- 275. Mohamed Ali HE, Ismail N, Al-Hendy A, Yang Q.. Inhibition of MLL1 and HDAC activity reverses reprogrammed inflammatory components induced by developmental exposure to an endocrine disruptor (diethylstibesterol) in myometrial stem cells. Reprod Sci. 2020;27 suppl 1:229A. doi:10.1007/s43032-020-00176-9 [Google Scholar]
- 276. Yang Q, , Al-HendyA. Integrated multi-omics analyses of high-risk myometrial progenitor/stem cells: implication for uterine fibroid pathogenesis. Reprod Sci. 2021;28 suppl 1:37A. [Google Scholar]
- 277. Levy G, Malik M, Britten J, Gilden M, Segars J, Catherino WH. Liarozole inhibits transforming growth factor-β3–mediated extracellular matrix formation in human three-dimensional leiomyoma cultures. Fertil Steril. 2014;102(1):272-281.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Malik M, Britten J, Segars J, Catherino WH. Leiomyoma cells in 3-dimensional cultures demonstrate an attenuated response to fasudil, a rho-kinase inhibitor, when compared to 2-dimensional cultures. Reprod Sci. 2014;21(9):1126-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Xie J, Xu X, Yin P, et al. Application of ex-vivo spheroid model system for the analysis of senescence and senolytic phenotypes in uterine leiomyoma. Lab Invest. 2018;98(12):1575-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Thompson WE, Al-Hendy A, Yang Q, et al. OR20-04 modeling uterine disorders utilizing adult uterine stem cells. J Endocr Soc. 2020;4(Supplement_1). doi: 10.1210/jendso/bvaa046.668 [DOI] [Google Scholar]
- 281. Ishikawa H, Reierstad S, Demura M, et al. High aromatase expression in uterine leiomyoma tissues of African-American women. J Clin Endocrinol Metab. 2009;94(5):1752-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Nierth-Simpson EN, Martin MM, Chiang TC, et al. Human uterine smooth muscle and leiomyoma cells differ in their rapid 17beta-estradiol signaling: implications for proliferation. Endocrinology. 2009;150(5):2436-2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Borahay MA, Asoglu MR, Mas A, Adam S, Kilic GS, Al-Hendy A. Estrogen receptors and signaling in fibroids: role in pathobiology and therapeutic implications. Reprod Sci. 2017;24(9):1235-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Bulun SE, Moravek MB, Yin P, et al. Uterine leiomyoma stem cells: linking progesterone to growth. Semin Reprod Med. 2015;33(5):357-365. [DOI] [PubMed] [Google Scholar]
- 285. Hodges LC, Houston KD, Hunter DS, et al. Transdominant suppression of estrogen receptor signaling by progesterone receptor ligands in uterine leiomyoma cells. Mol Cell Endocrinol. 2002;196(1-2):11-20. [DOI] [PubMed] [Google Scholar]
- 286. Cermik D, Arici A, Taylor HS. Coordinated regulation of HOX gene expression in myometrium and uterine leiomyoma. Fertil Steril. 2002;78(5):979-984. [DOI] [PubMed] [Google Scholar]
- 287. Kawaguchi K, Fujii S, Konishi I, et al. Immunohistochemical analysis of oestrogen receptors, progesterone receptors and Ki-67 in leiomyoma and myometrium during the menstrual cycle and pregnancy. Virchows Arch A Pathol Anat Histopathol. 1991;419(4):309-315. [DOI] [PubMed] [Google Scholar]
- 288. Matsuo H, Kurachi O, Shimomura Y, Samoto T, Maruo T. Molecular bases for the actions of ovarian sex steroids in the regulation of proliferation and apoptosis of human uterine leiomyoma. Oncology. 1999;57 Suppl 2:49-58. [DOI] [PubMed] [Google Scholar]
- 289. Ishikawa H, Ishi K, Serna VA, Kakazu R, Bulun SE, Kurita T. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology. 2010;151(6):2433-2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Ali M, Al-Hendy A. Selective progesterone receptor modulators for fertility preservation in women with symptomatic uterine fibroids. Biol Reprod. 2017;97(3):337-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Leppert PC, Baginski T, Prupas C, Catherino WH, Pletcher S, Segars JH. Comparative ultrastructure of collagen fibrils in uterine leiomyomas and normal myometrium. Fertil Steril. 2004;82 Suppl 3:1182-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Rogers R, Norian J, Malik M, et al. Mechanical homeostasis is altered in uterine leiomyoma. Am J Obstet Gynecol. 2008;198(4):474.e1-474.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Martino F, Perestrelo AR, Vinarský V, Pagliari S, Forte G. Cellular mechanotransduction: from tension to function. Front Physiol. 2018;9:824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Ko YA, Jamaluddin MFB, Adebayo M, et al. Extracellular matrix (ECM) activates β-catenin signaling in uterine fibroids. Reproduction. 2018;155(1):61-71. [DOI] [PubMed] [Google Scholar]
- 295. Flake GP, Moore AB, Sutton D, et al. The natural history of uterine leiomyomas: light and electron microscopic studies of fibroid phases, interstitial ischemia, inanosis, and reclamation. Obstet Gynecol Int. 2013;2013:528376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Flake GP, Moore AB, Sutton D, et al. The natural history of uterine leiomyomas: light and electron microscopic studies of fibroid phases, interstitial ischemia, inanosis, and reclamation. Obstet Gynecol Int. 2013;2013:528376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Flake GP, Moore AB, Sutton D, et al. The life cycle of the uterine fibroid myocyte. Curr Obstet Gynecol Rep. 2018;7(2):97-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Cho S, Vashisth M, Abbas A, et al. Mechanosensing by the lamina protects against nuclear rupture, DNA damage, and cell-cycle arrest. Dev Cell. 2019;49(6):920-935.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Gilbert HTJ, Mallikarjun V, Dobre O, et al. Nuclear decoupling is part of a rapid protein-level cellular response to high-intensity mechanical loading. Nat Commun. 2019;10(1):4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Leavy M, Trottmann M, Liedl B, et al. Effects of elevated β-estradiol levels on the functional morphology of the testis—new insights. Sci Rep. 2017;7:39931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Malik M, Segars J, Catherino WH. Integrin β1 regulates leiomyoma cytoskeletal integrity and growth. Matrix Biol. 2012;31(7-8):389-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Stewart EA, Friedman AJ, Peck K, Nowak RA. Relative overexpression of collagen type I and collagen type III messenger ribonucleic acids by uterine leiomyomas during the proliferative phase of the menstrual cycle. J Clin Endocrinol Metab. 1994;79(3):900-906. [DOI] [PubMed] [Google Scholar]
- 303. Islam MS, Ciavattini A, Petraglia F, Castellucci M, Ciarmela P. Extracellular matrix in uterine leiomyoma pathogenesis: a potential target for future therapeutics. Hum Reprod Update. 2018;24(1):59-85. [DOI] [PubMed] [Google Scholar]
- 304. Ciebiera M, Ali M, Zgliczynska M, Skrzypczak M, Al-Hendy A. Vitamins and uterine fibroids: current data on pathophysiology and possible clinical relevance. Int J Mol Sci. 2020;21(15). doi: 10.3390/ijms21155528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Jayes FL, Liu B, Moutos FT, Kuchibhatla M, Guilak F, Leppert PC. Loss of stiffness in collagen-rich uterine fibroids after digestion with purified collagenase Clostridium histolyticum. Am J Obstet Gynecol. 2016;215(5):596.e1-596.e8. [DOI] [PubMed] [Google Scholar]
- 306. Suzuki A, Kariya M, Matsumura N, et al. Expression of p53 and p21(WAF-1), apoptosis, and proliferation of smooth muscle cells in normal myometrium during the menstrual cycle: implication of DNA damage and repair for leiomyoma development. Med Mol Morphol. 2012;45(4):214-221. [DOI] [PubMed] [Google Scholar]
- 307. Karowicz-Bilinska A, Plodzidym M, Krol J, Lewinska A, Bartosz G. Changes of markers of oxidative stress during menstrual cycle. Redox Rep. 2008;13(5):237-240. [DOI] [PubMed] [Google Scholar]
- 308. Atashgaran V, Wrin J, Barry SC, Dasari P, Ingman WV. Dissecting the biology of menstrual cycle-associated breast cancer risk. Front Oncol. 2016;6:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Yang Q, Laknaur A, Elam L, et al. Identification of polycomb group protein EZH2-mediated DNA mismatch repair gene MSH2 in human uterine fibroids. Reprod Sci. 2016;23(10):1314-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Yang Q, Nair S, Laknaur A, Ismail N, Diamond MP, Al-Hendy A. The polycomb group protein EZH2 impairs DNA damage repair gene expression in human uterine fibroids. Biol Reprod. 2016;94(3):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Prusinski Fernung LE, Al-Hendy A, Yang Q. A preliminary study: human fibroid Stro-1+/CD44+ stem cells isolated from uterine fibroids demonstrate decreased DNA repair and genomic integrity compared to adjacent myometrial Stro-1+/CD44+ Cells. Reprod Sci. 2019;26(5):619-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Prusinski Fernung LE, Yang Q, Sakamuro D, Kumari A, Mas A, Al-Hendy A. Endocrine disruptor exposure during development increases incidence of uterine fibroids by altering DNA repair in myometrial stem cells. Biol Reprod. 2018;99(4):735-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Chatterjee N, Walker GC. Mechanisms of DNA damage, repair, and mutagenesis. Environ Mol Mutagen. 2017;58(5):235-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Thakur R, Mishra DP. Pharmacological modulation of beta-catenin and its applications in cancer therapy. J Cell Mol Med. 2013;17(4):449-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene. 2017;36(11):1461-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Al-Hendy A, Laknaur A, Diamond MP, Ismail N, Boyer TG, Halder SK. Silencing Med12 gene reduces proliferation of human leiomyoma cells mediated via Wnt/β-catenin signaling pathway. Endocrinology. 2017;158(3):592-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Ko YA, Jamaluddin MFB, Adebayo M, et al. Extracellular matrix (ECM) activates β-catenin signaling in uterine fibroids. Reproduction. 2018;155(1):61-71. [DOI] [PubMed] [Google Scholar]
- 318. Zaitseva M, Holdsworth-Carson SJ, Waldrip L, et al. Aberrant expression and regulation of NR2F2 and CTNNB1 in uterine fibroids. Reproduction. 2013;146(2):91-102. [DOI] [PubMed] [Google Scholar]
- 319. Tai CT, Lin WC, Chang WC, Chiu TH, Chen GT. Classical cadherin and catenin expression in normal myometrial tissues and uterine leiomyomas. Mol Reprod Dev. 2003;64(2):172-178. [DOI] [PubMed] [Google Scholar]
- 320. Ulin M, Ali M, Chaudhry ZT, Al-Hendy A, Yang Q. Uterine fibroids in menopause and perimenopause. Menopause. 2020;27(2):238-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Al-Hendy A, Diamond MP, Boyer TG, Halder SK. Vitamin D3 inhibits Wnt/β-catenin and mTOR signaling pathways in human uterine fibroid cells. J Clin Endocrinol Metab. 2016;101(4):1542-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Corachán A, Ferrero H, Aguilar A, et al. Inhibition of tumor cell proliferation in human uterine leiomyomas by vitamin D via Wnt/β-catenin pathway. Fertil Steril. 2019;111(2):397-407. [DOI] [PubMed] [Google Scholar]
- 323. Dieffenbach PB, Haeger CM, Coronata AMF, et al. Arterial stiffness induces remodeling phenotypes in pulmonary artery smooth muscle cells via YAP/TAZ-mediated repression of cyclooxygenase-2. Am J Physiol Lung Cell Mol Physiol. 2017;313(3):L628-L647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Mulder CL, Eijkenboom LL, Beerendonk CCM, Braat DDM, Peek R. Enhancing the safety of ovarian cortex autotransplantation: cancer cells are purged completely from human ovarian tissue fragments by pharmacological inhibition of YAP/TAZ oncoproteins. Hum Reprod. 2019;34(3):506-518. [DOI] [PubMed] [Google Scholar]
- 325. Park JH, Shin JE, Park HW. The role of Hippo pathway in cancer stem cell biology. Mol Cells. 2018;41(2):83-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Purdy MP, Ducharme M, Haak AJ, et al. YAP/TAZ are activated by mechanical and hormonal stimuli in myometrium and exhibit increased baseline activation in uterine fibroids. Reprod Sci. 2020;27(4):1074-1085. [DOI] [PubMed] [Google Scholar]
- 327. Islam MS, Afrin S, Singh B, et al. Extracellular matrix and Hippo signaling as therapeutic targets of antifibrotic compounds for uterine fibroids. Clin Transl Med. 2021;11(7):e475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Norian JM, Owen CM, Taboas J, et al. Characterization of tissue biomechanics and mechanical signaling in uterine leiomyoma. Matrix Biol. 2012;31(1):57-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Berdasco M, Esteller M. Clinical epigenetics: seizing opportunities for translation. Nat Rev Genet. 2019;20(2):109-127. [DOI] [PubMed] [Google Scholar]
- 330. Eckschlager T, Plch J, Stiborova M, Hrabeta J. Histone deacetylase inhibitors as anticancer drugs. Int J Mol Sci. 2017;18(7). doi: 10.3390/ijms18071414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Yang Q, Mas A, Diamond MP, Al-Hendy A. The mechanism and function of epigenetics in uterine leiomyoma development. Reprod Sci. 2016;23(2):163-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Yang Q, Al-Hendy A. Non-coding RNAs: an important regulatory mechanism in pathogenesis of uterine fibroids. Fertil Steril. 2018;109(5):802-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Navarro A, Yin P, Ono M, et al. 5-Hydroxymethylcytosine promotes proliferation of human uterine leiomyoma: a biological link to a new epigenetic modification in benign tumors. J Clin Endocrinol Metab. 2014;99(11):E2437-E2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Ciebiera M, Wlodarczyk M, Zgliczynski S, Lozinski T, Walczak K, Czekierdowski A. The role of miRNA and related pathways in pathophysiology of uterine fibroids-from bench to bedside. Int J Mol Sci. 2020;21(8). doi: 10.3390/ijms21083016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Wang T, Zhang X, Obijuru L, et al. A micro-RNA signature associated with race, tumor size, and target gene activity in human uterine leiomyomas. Genes Chromosomes Cancer. 2007;46(4):336-347. [DOI] [PubMed] [Google Scholar]
- 336. Falahati Z, Mohseni-Dargah M, Mirfakhraie R. Emerging roles of long non-coding RNAs in uterine leiomyoma pathogenesis: a review. Reprod Sci. 2021. doi: 10.1007/s43032-021-00571-w [DOI] [PubMed] [Google Scholar]
- 337. Zhou W, Wang G, Li B, Qu J, Zhang Y. LncRNA APTR promotes uterine leiomyoma cell proliferation by targeting erα to activate the Wnt/β-catenin pathway. Front Oncol. 2021;11:536346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Chuang TD, Rehan A, Khorram O. Functional role of the long noncoding RNA X-inactive specific transcript in leiomyoma pathogenesis. Fertil Steril. 2021;115(1):238-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Borahay MA, Al-Hendy A, Kilic GS, Boehning D. Signaling pathways in leiomyoma: understanding pathobiology and implications for therapy. Mol Med. 2015;21:242-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Weiss G, Goldsmith LT, Taylor RN, Bellet D, Taylor HS. Inflammation in reproductive disorders. Reprod Sci. 2009;16(2):216-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. AlAshqar A, Reschke L, Kirschen GW, Borahay MA. Role of inflammation in benign gynecologic disorders: from pathogenesis to novel therapies†. Biol Reprod. 2021;105(1):7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Sassi F, Tamone C, D’Amelio P. Vitamin D: nutrient, hormone, and immunomodulator. Nutrients. 2018;10(11). doi: 10.3390/nu10111656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Singh N, Baby D, Rajguru JP, Patil PB, Thakkannavar SS, Pujari VB. Inflammation and cancer. Ann Afr Med. 2019;18(3):121-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Laughlin SK, Schroeder JC, Baird DD. New directions in the epidemiology of uterine fibroids. Semin Reprod Med. 2010;28(3):204-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Moore KR, Cole SR, Dittmer DP, Schoenbach VJ, Smith JS, Baird DD. Self-reported reproductive tract infections and ultrasound diagnosed uterine fibroids in African-American Women. J Womens Health (Larchmt). 2015;24(6):489-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Pietrowski D, Thewes R, Sator M, Denschlag D, Keck C, Tempfer C. Uterine leiomyoma is associated with a polymorphism in the interleukin 1-beta gene. Am J Reprod Immunol. 2009;62(2):112-117. [DOI] [PubMed] [Google Scholar]
- 348. Prokofiev V, Konenkov V, Koroleva E, Shevchenko A, Dergacheva T, Novikov A. The structure of the cytokine gene network in uterine fibroids. Presented at: 2020 Cognitive Sciences, Genomics and Bioinformatics (CSGB); 2020. doi: 10.1109/CSGB51356.2020.9214588 [DOI] [Google Scholar]
- 349. Sosna O, Kolesár L, Slavčev A, et al. Th1/Th2 cytokine gene polymorphisms in patients with uterine fibroid. Folia Biol (Praha). 2010;56(5):206-210. [PubMed] [Google Scholar]
- 350. Hsieh YY, Chang CC, Tsai FJ, Lin CC, Yeh LS, Tsai CH. Tumor necrosis factor-alpha-308 promoter and p53 codon 72 gene polymorphisms in women with leiomyomas. Fertil Steril. 2004;82 Suppl 3:1177-1181. [DOI] [PubMed] [Google Scholar]
- 351. Litovkin KV, Domenyuk VP, Bubnov VV, Zaporozhan VN. Interleukin-6 -174G/C polymorphism in breast cancer and uterine leiomyoma patients: a population-based case control study. Exp Oncol. 2007;29(4):295-298. [PubMed] [Google Scholar]
- 352. Hsieh YY, Chang CC, Tsai CH, Lin CC, Tsai FJ. Interleukin (IL)-12 receptor beta1 codon 378 G homozygote and allele, but not IL-1 (beta-511 promoter, 3953 exon 5, receptor antagonist), IL-2 114, IL-4-590 intron 3, IL-8 3’-UTR 2767, and IL-18 105, are associated with higher susceptibility to leiomyoma. Fertil Steril. 2007;87(4):886-895. [DOI] [PubMed] [Google Scholar]
- 353. Chegini N. Proinflammatory and profibrotic mediators: principal effectors of leiomyoma development as a fibrotic disorder. Semin Reprod Med. 2010;28(3):180-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Wegienka G. Are uterine leiomyoma a consequence of a chronically inflammatory immune system? Med Hypotheses. 2012;79(2):226-231. [DOI] [PubMed] [Google Scholar]
- 355. Protic O, Toti P, Islam MS, et al. Possible involvement of inflammatory/reparative processes in the development of uterine fibroids. Cell Tissue Res. 2016;364(2):415-427. [DOI] [PubMed] [Google Scholar]
- 356. Liu ZQ, Lu MY, Sun RL, Yin ZN, Liu B, Wu YZ. Characteristics of peripheral immune function in reproductive females with uterine leiomyoma. J Oncol. 2019;2019:5935640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Elkafas H, Bariani MV, Ali M, et al. Pro-inflammatory and immunosuppressive environment contributes to the development and progression of uterine fibroids. Fertil Steril. 2020;114(3). doi: 10.1016/j.fertnstert.2020.08.264 [DOI] [Google Scholar]
- 358. Shanes ED, Friedman LA, Mills AM. PD-L1 expression and tumor-infiltrating lymphocytes in uterine smooth muscle tumors: implications for immunotherapy. Am J Surg Pathol. 2019;43(6):792-801. [DOI] [PubMed] [Google Scholar]
- 359. Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum Reprod Update. 2005;11(4):411-423. [DOI] [PubMed] [Google Scholar]
- 360. Yang Q, Trevino LS, El Andaloussi A, Ismail N, Walker C, Al-Hendy A.. Developmental Reprogramming of Pro-Inflammatory Pathway Mediates Adult Onset of Uterine Fibroids. Fertil Steril. 2018; 110: 4 suppl e377-378. [Google Scholar]
- 361. Pike JW, Christakos S. Biology and mechanisms of action of the vitamin D hormone. Endocrinol Metab Clin North Am. 2017;46(4):815-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Young MRI, Xiong Y. Influence of vitamin D on cancer risk and treatment: Why the variability? Trends Cancer Res. 2018;13:43-53. [PMC free article] [PubMed] [Google Scholar]
- 363. Halder SK, Goodwin JS, Al-Hendy A. 1,25-Dihydroxyvitamin D3 reduces TGF-beta3-induced fibrosis-related gene expression in human uterine leiomyoma cells. J Clin Endocrinol Metab. 2011;96(4):E754-E762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Sharan C, Halder SK, Thota C, Jaleel T, Nair S, Al-Hendy A. Vitamin D inhibits proliferation of human uterine leiomyoma cells via catechol-O-methyltransferase. Fertil Steril. 2011;95(1):247-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Ciebiera M, Wlodarczyk M, Wrzosek M, et al. Role of transforming growth factor beta in uterine fibroid biology. Int J Mol Sci. 2017;18(11). doi: 10.3390/ijms18112435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366. Halder SK, Sharan C, Al-Hendy A. 1,25-dihydroxyvitamin D3 treatment shrinks uterine leiomyoma tumors in the Eker rat model. Biol Reprod. 2012;86(4):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367. Al-Hendy A, Diamond MP, El-Sohemy A, Halder SK. 1,25-dihydroxyvitamin D3 regulates expression of sex steroid receptors in human uterine fibroid cells. J Clin Endocrinol Metab. 2015;100(4):E572-E582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368. Elhusseini H, Elkafas H, Abdelaziz M, et al. Diet-induced vitamin D deficiency triggers inflammation and DNA damage profile in murine myometrium. Int J Womens Health. 2018;10:503-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369. Ciebiera M, Wlodarczyk M, Zgliczynska M, et al. The role of tumor necrosis factor alpha in the biology of uterine fibroids and the related symptoms. Int J Mol Sci. 2018;19(12). doi: 10.3390/ijms19123869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370. Ali M, Shahin SM, Sabri NA, Al-Hendy A, Yang Q. Hypovitaminosis D exacerbates the DNA damage load in human uterine fibroids, which is ameliorated by vitamin D3 treatment. Acta Pharmacol Sin. 2019;40(7):957-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Corachán A, Ferrero H, Escrig J, et al. Long-term vitamin D treatment decreases human uterine leiomyoma size in a xenograft animal model. Fertil Steril. 2020;113(1):205-216.e4. [DOI] [PubMed] [Google Scholar]
- 372. Ciavattini A, Delli Carpini G, Serri M, et al. Hypovitaminosis D and “small burden” uterine fibroids: Opportunity for a vitamin D supplementation. Medicine (Baltimore). 2016;95(52):e5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373. Arjeh S, Darsareh F, Asl ZA, Azizi Kutenaei M. Effect of oral consumption of vitamin D on uterine fibroids: a randomized clinical trial. Complement Ther Clin Pract. 2020;39:101159. [DOI] [PubMed] [Google Scholar]
- 374. Ciebiera M, Ali M, Prince L, Zgliczyński S, Jakiel G, Al-Hendy A. The significance of measuring vitamin D serum levels in women with uterine fibroids. Reprod Sci. 2021;28(8):2098-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375. Marchesi JR, Ravel J. The vocabulary of microbiome research: a proposal. Microbiome. 2015;3:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376. Lv LX, Fang DQ, Shi D, et al. Alterations and correlations of the gut microbiome, metabolism and immunity in patients with primary biliary cirrhosis. Environ Microbiol. 2016;18(7):2272-2286. [DOI] [PubMed] [Google Scholar]
- 377. Wang W, Li Y, Wu Q, Pan X, He X, Ma X. High-throughput sequencing study of the effect of transabdominal hysterectomy on intestinal flora in patients with uterine fibroids. BMC Microbiol. 2020;20(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378. Qin J, Li R, Raes J, et al. ; MetaHIT Consortium . A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379. Claesson MJ, Cusack S, O’Sullivan O, et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci U S A. 2011;108 Suppl 1:4586-4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Plottel CS, Blaser MJ. Microbiome and malignancy. Cell Host Microbe. 2011;10(4):324-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381. Rajagopala SV, Vashee S, Oldfield LM, et al. The human microbiome and cancer. Cancer Prev Res (Phila). 2017;10(4):226-234. [DOI] [PubMed] [Google Scholar]
- 382. Flores R, Shi J, Fuhrman B, et al. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: a cross-sectional study. J Transl Med. 2012;10:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383. Breban M. Gut microbiota and inflammatory joint diseases. Joint Bone Spine. 2016;83(6):645-649. [DOI] [PubMed] [Google Scholar]
- 384. Fuhrman BJ, Feigelson HS, Flores R, et al. Associations of the fecal microbiome with urinary estrogens and estrogen metabolites in postmenopausal women. J Clin Endocrinol Metab. 2014;99(12):4632-4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385. Verstraelen H, Vilchez-Vargas R, Desimpel F, et al. Characterisation of the human uterine microbiome in nonpregnant women through deep sequencing of the V1-2 region of the 16S rRNA gene. Peerj. 2016;4:e1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386. Moreno I, Franasiak JM. Endometrial microbiota-new player in town. Fertil Steril. 2017;108(1):32-39. [DOI] [PubMed] [Google Scholar]
- 387. Morgan XC, Segata N, Huttenhower C. Biodiversity and functional genomics in the human microbiome. Trends Genet. 2013;29(1):51-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388. Green KA, Zarek SM, Catherino WH. Gynecologic health and disease in relation to the microbiome of the female reproductive tract. Fertil Steril. 2015;104(6):1351-1357. [DOI] [PubMed] [Google Scholar]
- 389. Pelzer E, Gomez-Arango LF, Barrett HL, Nitert MD. Review: maternal health and the placental microbiome. Placenta. 2017;54:30-37. [DOI] [PubMed] [Google Scholar]
- 390. Abdeljabar El Andaloussi JG, Anukrit S, Al-Hendy A, Ismail N. Impact of uterine microbiota on the prevalence of uterine fibroids in women of colors. Reprod Sci. 2019;26, Supplement 1:202A. [Google Scholar]
- 391. Nikitovic D, Jayes FL, Liu B, et al. Evidence of biomechanical and collagen heterogeneity in uterine fibroids. Plos One. 2019;14(4). doi: 10.1371/journal.pone.0215646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392. Berta DG, Kuisma H, Välimäki N, et al. Deficient H2A.Z deposition is associated with genesis of uterine leiomyoma. Nature. 2021;596(7872):398-403. [DOI] [PubMed] [Google Scholar]
- 393. Omar M, Laknaur A, Al-Hendy A, Yang Q. Myometrial progesterone hyper-responsiveness associated with increased risk of human uterine fibroids. BMC Womens Health. 2019;19(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394. Paul EN, Burns GW, Carpenter TJ, Grey JA, Fazleabas AT, Teixeira JM. Transcriptome analyses of myometrium from fibroid patients reveals phenotypic differences compared to non-diseased myometrium. Int J Mol Sci. 2021;22(7). doi: 10.3390/ijms22073618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395. Ali M, Shahin SM, Sabri NA, Al-Hendy A, Yang Q. 1,25 Dihydroxyvitamin D3 enhances the antifibroid effects of ulipristal acetate in human uterine fibroids. Reprod Sci. 2019;26(6):812-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396. Elkafas H, Ali M, Elmorsy E, et al. Vitamin D3 ameliorates DNA damage caused by developmental exposure to endocrine disruptors in the uterine myometrial stem cells of eker rats. Cells. 2020;9(6). doi: 10.3390/cells9061459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397. Pilgrim J, Arismendi J, DeAngelis A, et al. Characterization of the role of activator protein 1 signaling pathway on extracellular matrix deposition in uterine leiomyoma. F&S Sci. 2020;1(1):78-89. [DOI] [PubMed] [Google Scholar]
- 398. Shalaby S, Khater M, Laknaur A, Arbab A, Al-Hendy A. molecular bio-imaging probe for noninvasive differentiation between human leiomyoma versus leiomyosarcoma. Reprod Sci. 2020;27(2):644-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399. Bharambe BM, Deshpande KA, Surase SG, Ajmera AP. Malignant transformation of leiomyoma of uterus to leiomyosarcoma with metastasis to ovary. J Obstet Gynaecol India. 2014;64(1):68-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400. Giuntoli RL 2nd, Metzinger DS, DiMarco CS, et al. Retrospective review of 208 patients with leiomyosarcoma of the uterus: prognostic indicators, surgical management, and adjuvant therapy. Gynecol Oncol. 2003;89(3):460-469. [DOI] [PubMed] [Google Scholar]
- 401. Dinh TA, Oliva EA, Fuller AF Jr, Lee H, Goodman A. The treatment of uterine leiomyosarcoma. Results from a 10-year experience (1990-1999) at the Massachusetts General Hospital. Gynecol Oncol. 2004;92(2):648-652. [DOI] [PubMed] [Google Scholar]
- 402. Tsikouras P, Liberis V, Galazios G, et al. Uterine sarcoma: a report of 57 cases over a 16-year period analysis. Eur J Gynaecol Oncol. 2008;29(2):129-134. [PubMed] [Google Scholar]
- 403. Kho KA, Lin K, Hechanova M, Richardson DL. Risk of occult uterine sarcoma in women undergoing hysterectomy for benign indications. Obstet Gynecol. 2016;127(3):468-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404. Hosh M, Antar S, Nazzal A, Warda M, Gibreel A, Refky B. Uterine sarcoma: analysis of 13 089 cases based on surveillance, epidemiology, and end results database. Int J Gynecol Cancer. 2016;26(6):1098-1104. [DOI] [PubMed] [Google Scholar]
- 405. Brooks SE, Zhan M, Cote T, Baquet CR. Surveillance, epidemiology, and end results analysis of 2677 cases of uterine sarcoma 1989-1999. Gynecol Oncol. 2004;93(1):204-208. [DOI] [PubMed] [Google Scholar]
- 406. Sun S, Bonaffini PA, Nougaret S, et al. How to differentiate uterine leiomyosarcoma from leiomyoma with imaging. Diagn Interv Imaging. 2019;100(10):619-634. [DOI] [PubMed] [Google Scholar]
- 407. Al Ansari AA, Al Hail FA, Abboud E. Malignant transformation of uterine leiomyoma. Qatar Med J. 2012;2012(2):71-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408. Bertsch E, Qiang W, Zhang Q, et al. MED12 and HMGA2 mutations: two independent genetic events in uterine leiomyoma and leiomyosarcoma. Mod Pathol. 2014;27(8):1144-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409. Ravegnini G, Mariño-Enriquez A, Slater J, et al. MED12 mutations in leiomyosarcoma and extrauterine leiomyoma. Mod Pathol. 2013;26(5):743-749. [DOI] [PubMed] [Google Scholar]
- 410. Christacos NC, Quade BJ, Dal Cin P, Morton CC. Uterine leiomyomata with deletions of Ip represent a distinct cytogenetic subgroup associated with unusual histologic features. Genes Chromosomes Cancer. 2006;45(3):304-312. [DOI] [PubMed] [Google Scholar]
- 411. Hodge JC, Morton CC. Genetic heterogeneity among uterine leiomyomata: insights into malignant progression. Hum Mol Genet. 2007;16 Spec No 1:R7-13. [DOI] [PubMed] [Google Scholar]
- 412. Moyo MB, Parker JB, Chakravarti D. Altered chromatin landscape and enhancer engagement underlie transcriptional dysregulation in MED12 mutant uterine leiomyomas. Nat Commun. 2020;11(1):1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413. Omar MK, Yang Q, Laknaur A, Al-Hendy A. Myometrial progesterone hyper-responsivness is associated with increased risk for the development of human uterine fibroids. Fertil Steril. 2016;106(3). doi: 10.1016/j.fertnstert.2016.07.280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414. Huang H, Weng H, Chen J. The Biogenesis and Precise Control of RNA m6A Methylation. Trends Genet. 2020;36(1):44-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415. Nachtergaele S, He C. Chemical modifications in the life of an mRNA transcript. Annu Rev Genet. 2018;52:349-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416. Yang Q, Bariani M, He C, Boyer T, Al-Hendy A. Aberrant expression of N6-Methyladenosine regulators in uterine fibroids from the Eker rat model (Abstract P-314). American Society for Reproductive Medicine, Baltimore, MD; 2021. [Google Scholar]
- 417. Yang Q, Shanmugasundaram K, He C, Al-Hendy A, Boyer T. Pathological reprogramming of epitranscriptomics via METTL3 in uterine fibroids (Abstract W-046). Society for Reproductive Investigation’s 68th Annual Scientific Meeting; 2021, Boston, MA. [Google Scholar]
- 418. Zhang X, Yang Q. A Pyroptosis-Related Gene Panel in Prognosis Prediction and Immune Microenvironment of Human Endometrial Cancer. Front Cell Dev Biol. 2021;9:705828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419. Rackow BW, Taylor HS. Submucosal uterine leiomyomas have a global effect on molecular determinants of endometrial receptivity. Fertil Steril. 2010;93(6):2027-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420. Yang Q, Diamond MP, Al-Hendy A. The emerging role of extracellular vesicle-derived miRNAs: implication in cancer progression and stem cell related diseases. J Clin Epigenet. 2016;2(1):13. [PMC free article] [PubMed] [Google Scholar]
- 421. Li Z, Gao X, Peng X, et al. Multi-omics characterization of molecular features of gastric cancer correlated with response to neoadjuvant chemotherapy. Sci Adv. 2020;6(9):eaay4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422. Mars RAT, Yang Y, Ward T, et al. Longitudinal multi-omics reveals subset-specific mechanisms underlying irritable bowel syndrome. Cell. 2020;183(4):1137-1140. [DOI] [PubMed] [Google Scholar]
- 423. Mas A, Alonso R, Garrido-Gómez T, et al. The differential diagnoses of uterine leiomyomas and leiomyosarcomas using DNA and RNA sequencing. Am J Obstet Gynecol. 2019;221(4):320.e1-320.e23. [DOI] [PubMed] [Google Scholar]
- 424. Croce S, Ducoulombier A, Ribeiro A, et al. Genome profiling is an efficient tool to avoid the STUMP classification of uterine smooth muscle lesions: a comprehensive array-genomic hybridization analysis of 77 tumors. Mod Pathol. 2018;31(5):816-828. [DOI] [PubMed] [Google Scholar]
- 425. Croce S, Chibon F. Molecular prognostication of uterine smooth muscle neoplasms: from CGH array to CINSARC signature and beyond. Genes Chromosomes Cancer. 2021;60(3):129-137. [DOI] [PubMed] [Google Scholar]
- 426. Mas A, Simón C. Molecular differential diagnosis of uterine leiomyomas and leiomyosarcomas. Biol Reprod. 2019;101(6):1115-1123. [DOI] [PubMed] [Google Scholar]
- 427. Garcia N, Ulin M, Al-Hendy A, Yang Q. The role of hedgehog pathway in female cancers. J Cancer Sci Clin Ther. 2020;4(4):487-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428. Garcia N, Bozzini N, Baiocchi G, et al. May Sonic Hedgehog proteins be markers for malignancy in uterine smooth muscle tumors? Hum Pathol. 2016;50:43-50. [DOI] [PubMed] [Google Scholar]
- 429. Garcia N, Al-Hendy A, Baracat EC, Carvalho KC, Yang Q. Targeting hedgehog pathway and DNA methyltransferases in uterine leiomyosarcoma cells. Cells. 2020;10(1). doi: 10.3390/cells10010053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430. Brunengraber LN, Jayes FL, Leppert PC. Injectable Clostridium histolyticum collagenase as a potential treatment for uterine fibroids. Reprod Sci. 2014;21(12):1452-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431. Jayes FL, Liu B, Moutos FT, Kuchibhatla M, Guilak F, Leppert PC. Loss of stiffness in collagen-rich uterine fibroids after digestion with purified collagenase Clostridium histolyticum. Am J Obstet Gynecol. 2016;215(5):596.e1-596.e8. [DOI] [PubMed] [Google Scholar]
- 432. Nair S, Curiel DT, Rajaratnam V, Thota C, Al-Hendy A. Targeting adenoviral vectors for enhanced gene therapy of uterine leiomyomas. Hum Reprod. 2013;28(9):2398-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433. Abdelaziz M, Sherif L, ElKhiary M, et al. Targeted adenoviral vector demonstrates enhanced efficacy for in vivo gene therapy of uterine leiomyoma. Reprod Sci. 2016;23(4):464-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434. Al-Hendy A, Lee EJ, Wang HQ, Copland JA. Gene therapy of uterine leiomyomas: adenovirus-mediated expression of dominant negative estrogen receptor inhibits tumor growth in nude mice. Am J Obstet Gynecol. 2004;191(5):1621-1631. [DOI] [PubMed] [Google Scholar]
- 435. Shalaby SM, Khater MK, Perucho AM, et al. Magnetic nanoparticles as a new approach to improve the efficacy of gene therapy against differentiated human uterine fibroid cells and tumor-initiating stem cells. Fertil Steril. 2016;105(6):1638-1648.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436. Senturk LM, Sozen I, Gutierrez L, Arici A. Interleukin 8 production and interleukin 8 receptor expression in human myometrium and leiomyoma. Am J Obstet Gynecol. 2001;184(4):559-566. [DOI] [PubMed] [Google Scholar]
- 437. Orciani M, Caffarini M, Biagini A, et al. Chronic inflammation may enhance leiomyoma development by the involvement of progenitor cells. Stem Cells Int. 2018;2018:1716246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438. Santulli P, Even M, Chouzenoux S, et al. Profibrotic interleukin-33 is correlated with uterine leiomyoma tumour burden. Hum Reprod. 2013;28(8):2126-2133. [DOI] [PubMed] [Google Scholar]
- 439. Chegini N, Tang XM, Ma C. Regulation of transforming growth factor-beta1 expression by granulocyte macrophage-colony-stimulating factor in leiomyoma and myometrial smooth muscle cells. J Clin Endocrinol Metab. 1999;84(11):4138-4143. [DOI] [PubMed] [Google Scholar]
- 440. Kurachi O, Matsuo H, Samoto T, Maruo T. Tumor necrosis factor-alpha expression in human uterine leiomyoma and its down-regulation by progesterone. J Clin Endocrinol Metab. 2001;86(5):2275-2280. [DOI] [PubMed] [Google Scholar]
- 441. Ciebiera M, Włodarczyk M, Wrzosek M, et al. TNF-α serum levels are elevated in women with clinically symptomatic uterine fibroids. Int J Immunopathol Pharmacol. 2018;32:2058738418779461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442. Moridi I, Mamillapalli R, Kodaman PH, Habata S, Dang T, Taylor HS. CXCL12 attracts bone marrow-derived cells to uterine leiomyomas. Reprod Sci. 2020;27(9):1724-1730. [DOI] [PubMed] [Google Scholar]
- 443. Sozen I, Olive DL, Arici A. Expression and hormonal regulation of monocyte chemotactic protein-1 in myometrium and leiomyomata. Fertil Steril. 1998;69(6):1095-1102. [DOI] [PubMed] [Google Scholar]
- 444. Syssoev KA, Kulagina NV, Chukhlovin AB, Morozova EB, Totolian AA. Expression of mRNA for chemokines and chemokine receptors in tissues of the myometrium and uterine leiomyoma. Bull Exp Biol Med. 2008;145(1):84-89. [DOI] [PubMed] [Google Scholar]
- 445. Ali M, Chaudhry ZT, Al-Hendy A. Successes and failures of uterine leiomyoma drug discovery. Expert Opin Drug Discov. 2018;13(2):169-177. [DOI] [PubMed] [Google Scholar]
- 446. Mohammed NH, Al-Taie A, Albasry Z. Evaluation of goserelin effectiveness based on assessment of inflammatory cytokines and symptoms in uterine leiomyoma. Int J Clin Pharm. 2020;42(3):931-937. [DOI] [PubMed] [Google Scholar]
- 447. Alfini P, Bianco V, Felice R, Magro B. [Treatment of uterine fibroma with goserelin]. Ann Ostet Ginecol Med Perinat. 1991;112(6):359-367. [PubMed] [Google Scholar]
- 448. Eizenberg DH. Goserelin reduction of uterine fibroids prior to vaginal hysterectomy. Aust N Z J Obstet Gynaecol. 1995;35(1):109-110. [DOI] [PubMed] [Google Scholar]
- 449. Ahmed RS, Liu G, Renzetti A, et al. Biological and mechanistic characterization of novel prodrugs of green tea polyphenol epigallocatechin gallate analogs in human leiomyoma cell lines. J Cell Biochem. 2016;117(10):2357-2369. [DOI] [PubMed] [Google Scholar]
- 450. Roshdy E, Rajaratnam V, Maitra S, Sabry M, Allah AS, Al-Hendy A. Treatment of symptomatic uterine fibroids with green tea extract: a pilot randomized controlled clinical study. Int J Womens Health. 2013;5:477-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451. Shime H, Kariya M, Orii A, et al. Tranilast inhibits the proliferation of uterine leiomyoma cells in vitro through G1 arrest associated with the induction of p21(waf1) and p53. J Clin Endocrinol Metab. 2002;87(12):5610-5617. [DOI] [PubMed] [Google Scholar]
- 452. Chuang TD, Rehan A, Khorram O. Tranilast induces MiR-200c expression through blockade of RelA/p65 activity in leiomyoma smooth muscle cells. Fertil Steril. 2020;113(6):1308-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453. Chuang TD, Khorram O. Tranilast inhibits genes functionally involved in cell proliferation, fibrosis, and epigenetic regulation and epigenetically induces miR-29c expression in leiomyoma cells. Reprod Sci. 2017;24(9):1253-1263. [DOI] [PubMed] [Google Scholar]
- 454. Tsuiji K, Takeda T, Li B, et al. Inhibitory effect of curcumin on uterine leiomyoma cell proliferation. Gynecol Endocrinol. 2011;27(7):512-517. [DOI] [PubMed] [Google Scholar]
- 455. Malik M, Mendoza M, Payson M, Catherino WH. Curcumin, a nutritional supplement with antineoplastic activity, enhances leiomyoma cell apoptosis and decreases fibronectin expression. Fertil Steril. 2009;91(5 Suppl):2177-2184. [DOI] [PubMed] [Google Scholar]
- 456. Salama SA, Diaz-Arrastia CR, Kilic GS, Kamel MW. 2-Methoxyestradiol causes functional repression of transforming growth factor β3 signaling by ameliorating Smad and non-Smad signaling pathways in immortalized uterine fibroid cells. Fertil Steril. 2012;98(1):178-184. [DOI] [PubMed] [Google Scholar]
- 457. Han M, Kim JY, Park JE, Kim JM, Lee KS. Effects of letrozole on proliferation and apoptosis in cultured leiomyoma cells treated with prostaglandin E(2). Eur J Obstet Gynecol Reprod Biol. 2008;138(1):83-88. [DOI] [PubMed] [Google Scholar]
- 458. Malik M, Webb J, Catherino WH. Retinoic acid treatment of human leiomyoma cells transformed the cell phenotype to one strongly resembling myometrial cells. Clin Endocrinol (Oxf). 2008;69(3):462-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459. Ciebiera M, Ali M, Prince L, et al. The evolving role of natural compounds in the medical treatment of uterine fibroids. J Clin Med. 2020;9(5):1479. doi: 10.3390/jcm9051479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460. Chen HY, Lin PH, Shih YH, et al. Natural antioxidant resveratrol suppresses uterine fibroid cell growth and extracellular matrix formation in vitro and in vivo. Antioxidants (Basel). 2019;8(4). doi: 10.3390/antiox8040099 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.