Abstract
Haemophagocytic lymphohistiocytosis is a life-threatening systemic inflammatory syndrome defined by persistent fever, cytopenia and multi-organ dysfunction. Primary haemophagocytic lymphohistiocytosis classically presents in childhood as a result of genetically abnormal perforin or inflammasome function, leading to the aberrant release of pro-inflammatory cytokines causing a hyperinflammatory state. Secondary haemophagocytic lymphohistiocytosis is an acquired phenomenon occurring at any age as a result of immune dysregulation to a specific trigger such as infection, haematological malignancy or autoimmune disease. Secondary haemophagocytic lymphohistiocytosis occurring in the pregnant woman represents a diagnostic challenge and carries a significant mortality. This has led to its first inclusion in the fourth Mothers and Babies: Reducing Risk through Audits and Confidential Enquiries across the United Kingdom annual maternal report in 2017. This article presents an overview of haemophagocytic lymphohistiocytosis, reviews the literature on haemophagocytic lymphohistiocytosis in pregnancy, suggests diagnostic pathways and explores the safety and efficacy of existing and potential treatment strategies for haemophagocytic lymphohistiocytosis occurring during pregnancy.
Keywords: Maternal death, haemophagocytosis, inflammation
Introduction
Haemophagocytic lymphohistiocytosis (HLH) is a life-threatening systemic inflammatory syndrome defined by persistent fever, cytopaenia and multi-organ dysfunction. The terms ‘haemophagocytic syndrome’ and ‘macrophage activation syndrome’ may also be used – the latter particularly in the context of autoimmune disease. 1
HLH is classically divided into two categories; familial or primary HLH (pHLH), which most commonly presents in childhood and is caused by abnormal function of perforin and the inflammasome, leading to the aberrant release of pro-inflammatory cytokines. 2 The second and more common form of HLH is known as secondary HLH (sHLH). This is an acquired phenomenon occurring at any age as a result of immune dysregulation to a specific trigger in the context of reduced, rather than absent, cytolytic protein function. 3 Although pHLH may present in adolescents and young adults, sHLH is by far the most prevalent subtype in women of child-bearing age. However, the distinction between genetic mutations resulting in pHLH and the possible aetiologies of sHLH is likely to be an over-simplification; heterozygous perforin pathway mutations have been described in those with sHLH, particularly in Epstein-Barr virus-(EBV) driven adult-onset sHLH, as well as other mutations in pathways leading to hyperinflammation such as NLRC4 mutations impairing inflammasome activity and SH2P1A mutations leading to impaired viral response. 2
Typically, there is a delay in the diagnosis of HLH; in part due to the similarity in presentation to sepsis and other causes of multiorgan failure. 4 Common triggers of sHLH include infection, autoimmune conditions and malignancy (Table 1). 5 Secondary HLH is challenging to diagnose and it is also challenging to treat. Multidisciplinary discussion is best sought early given the associated high mortality. The Histiocytosis UK Haemophagocytosis Across Specialty Collaboration (HASC) is a national initiative to create local and regional multidisciplinary teams to aid in the management of these women to improve outcomes.6–8 However, alerting the local and regional specialist teams relies on the treating clinicians being vigilant to the possibility of the diagnosis and this is a challenge due to its rarity.
Table 1.
| Causes of HLH |
|---|
| Primary mutations |
| Defects in cytolytic function: |
| PRF1 (10q21-22), UNC13D (17q2), STX11 (6q24), STXBP2 (19p13), RAB27A (15q21), LYST (1q42-43), SH2D1A (Xq24-25) |
| Defects in inflammasome regulation: |
| BIRC4 (Xq25), NLRC4 (2p22.3) |
| Secondary aetiologies |
| Infections: |
| Viruses – EBV, CMV, HSV, HHV-6 and -8, VZV, hepatitis, HIV, parvovirus B19, influenza A and B, adenovirus, enterovirus, dengue, Ebola and others |
| Bacteria – Mycobacteria spp., Rickettsia spp., Staphylococcus spp., Escherichia coli, Legionella spp. and others |
| Parasites – Leishmania spp., Plasmodium spp., Toxoplasma spp. and others |
| Fungi – Aspergillus spp., Candida spp., Histoplasma spp., Pneumocystis jiroveci and others |
| Autoimmune conditions: |
| SLE, sJIA, AOSD, RA, systemic vasculitides, periodic fever syndromes, IBD and others |
| Malignancy: |
| Haematological – T-cell, NK-cell, B-cell or Hodgkin’s lymphoma, leukaemia and others |
| Solid organ tumours |
| Miscellaneous: |
| Transplantation – Solid organ or HSCT |
| Iatrogenic – Immunosuppression, vaccination, surgery, haemodialysis |
| Pregnancy |
AOSD: adult-onset Still’s disease; CMV: cytomegalovirus; EBV: Epstein-Barr virus; HHV: human herpesvirus; HIV: human immunodeficiency virus; HSCT: haematopoietic stem cell transplantation; HSV: herpes simplex virus; IBD: inflammatory bowel disease; NK: natural killer; RA: rheumatoid arthritis: sJIA: systemic juvenile inflammatory arthritis; SLE: systemic lupus erythematosus; spp.: species; VZV: varicella zoster virus.
In the UK between 2013 and 2015, 556 women died during pregnancy or up to one year postpartum. Of these, four (0.72%) died as a consequence of HLH. 9 Thus, HLH occurring in pregnancy is thankfully rare but is associated with a poor prognosis. It is generally accepted that early treatment of evolving HLH is associated with better outcomes and as such raising awareness of this condition is a crucial start to improve outcomes. 5
Pathophysiology
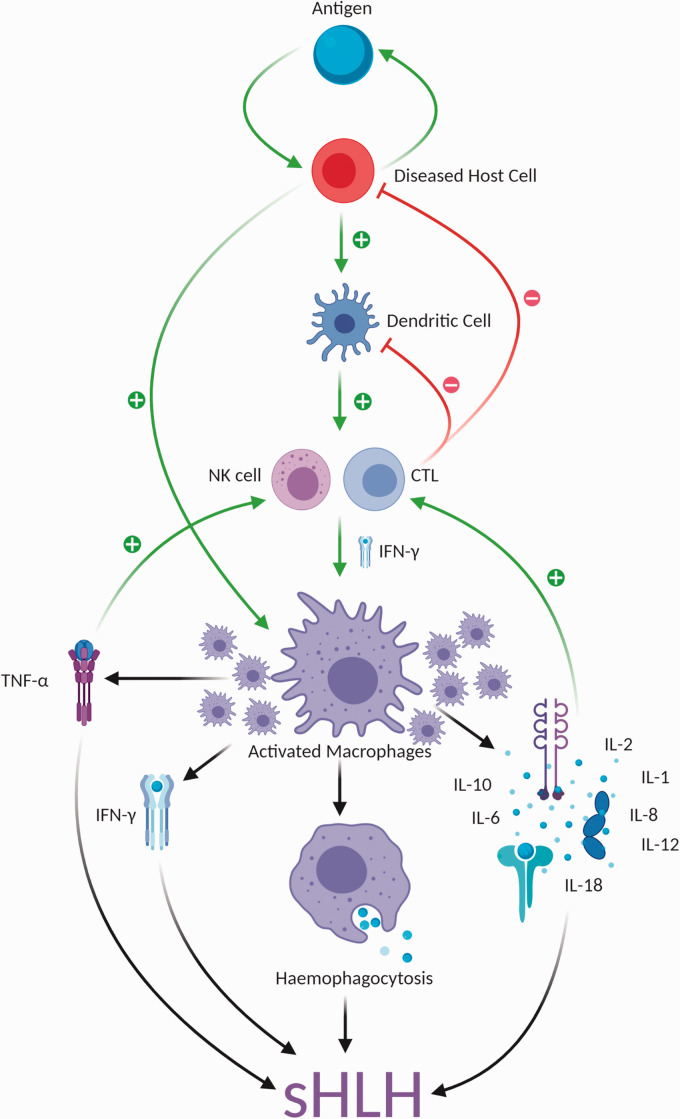
In immunocompetent individuals, natural killer (NK) cells and cytotoxic T-lymphocytes (CTLs) eliminate infected cells by a perforin-dependent pathway. 10 Cytotoxic cells contain secretory lysosomes that themselves contain perforin and granzymes. When these cells are activated their lysosomes are transported along microtubules toward the synapse between effector and target cell before they can release their contents into the synapse. The perforin and granzymes subsequently mediate apoptotic target cell death resulting in a down-regulation of the immune response. 10 This process is interrupted in pHLH due to genetic mutations leading to abnormal perforin function and thus an inability to destroy the diseased antigen-presenting cell (APC). Genetic variants, such as low NK cell numbers, may also contribute to individual susceptibility in those who develop sHLH 11 . Furthermore, some pathogens that persist in histiocytes such as Mycobacterium tuberculosis or Leishmania can directly activate toll-like receptors while others, like certain viruses, have developed complex immune evasion strategies that can interfere with NK and CTL cytotoxicity. 10 Failure to clear such potential antigen triggers from infected, tumour or autoimmune cells leads to uncontrolled activation and proliferation of T-lymphocytes, macrophages and pro-inflammatory cytokines, particularly interferon-gamma (IFN-γ), tumour necrosis factor-alpha (TNF-α), interleukin-10 (IL-10), IL-12, IL-18. This leads to a combination of hyperinflammation, end-organ damage and haemophagocytosis resulting in the clinical manifestations of HLH. The pathophysiology of sHLH will depend on the aetiology but shares the final common pathway of hyperinflammation and organ dysfunction (Figure 1).
Figure 1.
Pathophysiology of sHLH. CTL: cytotoxic T-lymphocyte; IFN-γ: interferon gamma; IL: interleukin; NK: natural killer; sHLH: secondary haemophagocytic lymphohistiocytosis; TNF-α: tumour necrosis factor alpha.
HLH in pregnancy
Primary HLH typically presents in childhood and the diagnosis is often established prior to any pregnancies; however, there is increasing awareness that late onset disease can occur, and genetic testing is, therefore, considered in all cases of HLH in pregnancy. During pregnancy, maternal immune regulation is under additional stress. The role of the placenta is key to acting as an immunological barrier between mother and the semi-allogeneic fetus. 12 There is evidence that a degree of inflammatory stimulus exists in all pregnancies stemming from the apoptotic debris shed from the syncytial surface of the placenta. 13 In specific contexts, the mechanisms that keep this stimulus in check can fail leading to immune dysregulation and hyperinflammation as is observed in pregnancy pathologies such as pre-eclampsia. This context provides a favourable environment for potential secondary HLH triggers such as infection or pre-existing autoimmune diseases such as systemic lupus erythematosus. 14
Clinical presentation
sHLH is characterised by persistent fever, organ dysfunction, lymphadenopathy and hepato- and/or splenomegaly. Fever is almost universally described, but otherwise the presenting clinical signs and symptoms in adult women with HLH are less typical than in children.5,15 Importantly, sHLH can arise in a subacute manner, with a gradual amalgamation of features rather than the florid acute presentation more frequently recognised at the point of multiorgan failure. 5
To date, 36 cases of sHLH in pregnancy have been reported (Table 2). Clinical descriptors are available for 31 cases showing mostly non-specific presentations that could have been due to sepsis alone and other symptoms and signs that could be due to physiological changes in pregnancy or pregnancy-specific complications. In 26 cases (72% of the tabulated reports), enlargement of the liver, spleen or both is described. Splenomegaly is rarely observed in sepsis alone and should alert the clinician to an alternative underlying disease process such as lymphoma or EBV infection, but this is problematic in pregnancy as mild splenomegaly is a normal finding; 4 48% reported either jaundice or liver function test derangement, the latter common in pregnancy. Furthermore, sHLH has been known to lead to other sequelae of liver pathology, namely encephalopathy and ascites. 50 It is also important to note the prevalence of central nervous system (CNS) involvement. Although poorly documented in this cohort, neurological symptoms are an established phenomenon within HLH with a wide spectrum of severity witnessed at presentation. 5 This can range from mild cognitive impairment and delirium to more severe CNS manifestations such as seizures, meningoencephalitis, ataxia, hemiplegia and cranial nerve palsies. 51 CNS involvement is an independent prognostic marker in paediatric HLH. 52
Table 2.
Reported cases of sHLH in pregnancy.
|
Reported cases of pregnancy-associated sHLH | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Case number | Authors | Age (years) | Obstetric history | Gestational age (weeks) | Aetiology | Clinical presentation and laboratory findings | Treatment (except antibiotics) | Maternal outcome | Mode of delivery and fetal outcome |
| 1 | Gill et al. 16 | 30 | – | 18 | – | Fever, hepatomegaly, pancytopenia | IVIg | Remission | 40/40 VD – survived |
| 2 | Tsuda et al. 17 | – | – | – | Parvovirus B19 | – | – | – | – |
| 3 | Yamanaka et al. 18 | – | – | 36 | – | – | Steroids, IVIg, AT III | Remission | Survived |
| 4 | Ishida et al. 19 | 31 | – | – | Hyperemesis | – | Steroids, IVIg | Death | 22/40 – IU death |
| 5 | Mihara et al. 20 | 32 | – | 16 | EBV | Fever, pancytopenia, elevated ferritin, LDH, sIL-2R | Steroids, aciclovir IVIg, gabexate mesilate | Failure Remission | 35/40 VD – survived |
| 6 | Nakabayashi et al. 21 | 30 | – | 21 | Pre-eclampsia | Fever, pancytopenia, elevated ferritin, LDH, LFTs | IVIg AT III | Failure Remission | 29/40 CS – survived |
| 7 | Chmait et al. 22 | 24 | G2 P1 | 29 | EBV | Fever, pancytopenia, elevated LFTs | IVIg, aciclovir, CS | Death | 30/40 CS – survived |
| 8 | Yamaguchi et al. 23 | – | – | – | HSV-2 | Fever, pancytopenia, elevated ferritin, TG, sIL-2R | Steroids CsA | Failure Remission | 40/40 VD – survived |
| 9 | Hanaoka et al. 24 | 33 | G1 P0 | 21 | B-cell lymphoma | Fever, hepatosplenomegaly, pancytopenia, elevated ferritin, TG, LDH, sIL-2R | R-CHOP, HSCT | Remission | 28/40 CS – survived |
| 10 | Perard et al. 25 | 28 | G4 | 22 | SLE | Fever, pancytopenia, elevated ferritin, TG | Steroids IVIg | Failure Remission | 30/40 VD – survived |
| 11 | Gonzalez et al. 26 | 27 | – | 22 | Parvovirus B19 | – | Steroids, AT III | Remission | 36/40 – survived |
| 12 | Chien et al. 27 | 28 | G1 P0 | 23 | – | Fever, bicytopenia, elevated TG | CS delivery | Remission | 28/40 CS – survived |
| 13 | Teng et al. 28 | 28 | G1 P0 | 23 | AIHA | Fever, bicytopenia, elevated ferritin, TG, LDH, sIL-2R | Steroids TOP | Failure Remission | 29/40 CS – death |
| 14 | Yoshida et al 29 | 33 | – | PP | SLE | – | Steroids | Remission | Survived |
| 15 | Arewa and Ajadi 30 | 31 | G1 P0 | 21 | HIV and malaria | Fever, bicytopenia, jaundice | Antimalarials, HAART, VD | Remission | 40/40 VD – survived |
| 16 | Dunn et al. 31 | 41 | – | 19 | AOSD | Fever, rash, headache, anaemia, elevated ferritin, TG, LDH, LFTs | Steroids, CS | Remission | 30/40 CS – survived (twins) |
| 17 | Hannebicque-Montaigne et al. 32 | 21 | – | 29 | SLE | Fever, pancytopenia, elevated ferritin, TG | Steroids, IVIg | Remission | 38/40 VD – survived |
| 18 | Kim et al. 33 | 29 | – | 12 | SLE | Fever, pancytopenia, elevated ferritin, TG, LDH | CsA, Splenectomy | Remission | 14/40 TOP – death |
| 19 | Komaru et al. 34 | 36 | G1 P0 | 40 + 38d | Sjogren’s | Splenomegaly, bicytopenia, jaundice, elevated ferritin, bilirubin, LDH, sIL-2R | Steroids | Remission | 40/40 VD – survived |
| 20 | Shukla et al. 35 | 23 | G1 P0 | 10 | – | Fever, hepatosplenomegaly, pancytopenia, elevated ferritin, TG, bilirubin | Steroids EPL | Failure Remission | 10/40 EPL – death |
| 21 | Goulding et al. 36 | 27 | G1 P0 | 23 | HSV-2 | Fever, pancytopenia, elevated ferritin | Steroids, aciclovir, Valaciclovir | Remission | 24/40 CS – death |
| 22 | Klein et al. 37 | 39 | – | 30 | EBV | Pancytopaenia, elevated ferritin, sIL-2R | Steroids, CsA, etoposide, RTX | Death | 31/40 CS – death (twins) |
| 23 | Mayama et al. 38 | 28 | G2 P2 | 19 | Parvovirus B19 | Fever, pancytopenia, elevated ferritin, LDH | Steroids | Remission | 37/40 VD – survived |
| 24 | Samra et al. 39 | 36 | – | 16 | – | Fever, pancytopenia, elevated ferritin, TG | Steroids | Remission | 40/40 VD – survived |
| 25 | Tumian and Wong. 40 | 35 | G2 P1 | 38 | CMV | Fever, anaemia, jaundice, elevated ferritin, TG, LDH, LFTs, sIL-2R | CS delivery, steroids, CsA | Death | 38/40 CS |
| 26 | Giard et al. 41 | 35 | G2 P1 | 13 | – | Fever, pancytopenia, jaundice, elevated ferritin, TG, LFTs, sCD25 | Steroids, etoposide | Death | 22/40 TOP – death |
| 27 | Ota et al. 42 | 26 | G1 P0 | 23 | Liver abscess | Fever, thrombocytopaenia, elevated ferritin, TG, LDH, LFTs, sIL-2R | Nil | Death | 23/40 – IU death |
| 28 | He et al. 43 | 27 | G2 P0 | 30 | NK/T-cell lymphoma | Fever, splenomegaly, pancytopenia, elevated ferritin, LDH, LFTs | Steroids, etoposide, RTX, CS delivery, splenectomy | Death | 30/40 CS – survived |
| 29 | Ikeda et al. 44 | 32 | – | 11 | EBV | Fever, bicytopenia, elevated ferritin | Steroids, etoposide, CsA | Remission | 11/40 EPL – death |
| 30 | Kerley et al. 45 | 33 | G1 P0 | 22 | – | Fever, bicytopenia, elevated ferritin, LDH, LFTs | Steroids Delivery, etoposide, BMT | Failure Remission | 22/40 VD – death |
| 31 | Rousselin et al. 46 | 44 | G1 P0 | 30 | – | Fever, hepatomegaly, pancytopenia, elevated ferritin, TG, LDH, LFTs | Steroids | Remission | 38/40 VD – survived |
| 32 | Nasser et al. 47 | 36 | G7 P2 | 31 | HSV-2 | Fever, splenomegaly, thrombocytopaenia, elevated ferritin, LDH, LFTs | Steroids, aciclovir, Delivery | Remission | CS – death |
| 33 | Cheng et al. 48 | – | – | 26 | – | Fever, bicytopenia, elevated ferritin, TG | Steroids, etoposide | Remission | 27/40 TOP – death |
| 34 | Parrott et al. 14 | 28 | G2 P1 | 18 | SLE | Fever, splenomegaly, pancytopenia, elevated ferritin, LFTs, sIL-2R, stroke | Steroids, etoposide, IVIg, CsA | Failure | 21/40 VD – IU death |
| 35 | Parrott et al. 14 | 37 | G4 P3 | 24 | CMV | Fever, thrombocytopaenia, jaundice, elevated ferritin, TG, LFTs, bilirubin, LDH, sIL-2R | Steroids, etoposide | Remission | 37/40 VD – survived |
| 36 | Yip et al. 49 | 23 | G2 P1 | 22 | – | Fever, pancytopenia, elevated ferritin, TG | Steroids, IVIg Anakinra | Failure Remission | 31/40 CS – survived |
AOSD: adult-onset Still’s disease; AT III: antithrombin III; BMT: bone marrow transplantation; CMV: cytomegalovirus; CS: caesarean section; CsA: ciclosporin A; d: days; EBV: Epstein-Barr virus; EPL: early pregnancy loss; G: gravida; HAART: highly active anti-retroviral therapy; HIV: human immunodeficiency virus; HSCT: haematopoietic stem cell transplantation; HSV: herpes simplex virus; Ig: immunoglobulin; IU: intrauterine; IVIg: intravenous immunoglobulin; LFTs: liver function tests; LDH: lactate dehydrogenase; NK: natural killer; P: para; PP: postpartum; R-CHOP: rituximab: cyclophosphamide: doxorubicin: vincristine: prednisolone; RTX: rituximab; sCD25: soluble CD25; sIL-2R: soluble interleukin-2 receptor; SLE: systemic lupus erythematosus; TOP: termination of pregnancy; TG: triglycerides; VD: vaginal delivery.
Pulmonary manifestations of sHLH are common but may contribute to the diagnostic delay given the risk of attributing these symptoms to other, more common, diagnoses. A national retrospective cohort study identified lung involvement in 54% of cases with dyspnoea and cough occurring most frequently. 53 These symptoms were not frequently represented in published cases of sHLH in pregnancy (Table 2). However, the authors recommend high clinical suspicion for those with these symptoms and supportive radiological changes such as interstitial infiltrates with centrilobular nodules, ill-defined consolidation and localised ground-glass opacities on chest imaging. 53
A wide spectrum of rashes has been reported in HLH ranging from non-specific erythematous macules to petechiae or purpura. In large non-pregnant cohorts, 25% of adults with HLH have cutaneous involvement. 5 Only one case of pregnancy-associated sHLH reported a diffuse erythematous macular rash at presentation. 31 Nodular lesions should raise suspicion of associated T-cell lymphoma. 5
Diagnosis
The diagnosis of HLH can be difficult and a number of diagnostic criteria exist. The most widely used diagnostic criteria, HLH-2004, were established for paediatric women under 18 years of age (Table 3). 54 To date, there are no validated criteria for diagnosis of HLH in adults, although there is a composite probability score for sHLH, the HScore, which is commonly used but has not been validated in pregnancy. 2 The HScore uses clinical parameters, common laboratory values and bone marrow findings dependent on their HLH specificity and calculates a probability score (Table 4). 55 The higher the HScore out of a total of 337, the higher the likelihood of HLH. It should be further highlighted, however, that there is currently no well-validated means of diagnosing HLH in adult women, and that all available paradigms (including HScore) are limited by their lack of specificity for HLH. Many critically ill women will score highly using these paradigms whether they have HLH or not. 56
Table 3.
HLH-2004 diagnostic criteria (for use in non-pregnant individuals). 54
| HLH-2004 diagnostic criteria (≥5 of 8 criteria below) |
|---|
| 1. Fever |
| 2. Splenomegaly |
| 3. Cytopenia affecting ≥2 of 3 peripheral blood
lineages – Haemoglobin < 90 g/L – Platelets < 100 ×109/L – Neutrophils <1.0 × 109/L |
| 4. Hypertriglyceridaemia and/or hypofibrinogenaemia – Fasting triglycerides ≥3.0 mmol/L – Fibrinogen ≤1.5 g/L |
| 5. Haemophagocytosis in bone marrow, spleen or lymph nodes with no evidence of malignancy |
| 6. Low or no NK cell activity |
| 7. Ferritin ≥500 µg/L |
| 8. Soluble CD25 (i.e. soluble interleukin-2 receptor, sIL-2R) ≥2400 U/ml |
Table 4.
HScore parameters and points. 55
| Parameter | Number of points |
|---|---|
| Known underlying immunosuppression | No 0
Yes +18 |
| Temperature (°C) | <38.4 0 38.4–39.4 +33 >39.4 +49 |
| Organomegaly | No 0 Hepatomegaly or splenomegaly +23 Hepatomegaly and splenomegaly +38 |
| Cytopenia lineage(s) | 1 0
2 +24 3 +34 |
| Ferritin (µg/L) | <2000 0
2000–6000 +35 >6000 +50 |
| Triglyceride (mmol/L) | <1.5 0 1.5 − 4 +44 >4 +64 |
| Fibrinogen (g/L) | >2.5 0
≤2.5 +30 |
| Aspartate aminotransferase (U/L) | <30 0
≥30 +19 |
| Haemophagocytosis on bone marrow aspirate | No 0
Yes +35 |
Importantly, not all markers used in these scoring systems will be routinely checked and they may be initially normal. Trends of biochemical values over time can also be revealing. We suggest the measurement of serum ferritin in any individual who is unwell and has a fever. Subsequently, the other markers of HLH, such as serum lipids and more specific tests such as the soluble interleukin-2 receptor can be requested (Figure 2). Ferritin is a glycoprotein that stores iron which it releases in a controlled manner in the absence of inflammation. 57 It is also an acute phase reactant, and as such is non-specifically elevated in a wide range of inflammatory states, including acute infection, malignancy, chronic kidney disease and a variety of autoimmune conditions. 58 During pregnancy, the serum ferritin initially rises in women with adequate iron stores at conception reaching a peak of approximately 230 µg/L during the second trimester.57,59 This is followed by a progressive fall by 32 weeks due to haemodilution and mobilisation of iron to approximately half of the pre-pregnancy levels, before a further mild elevation in the third trimester.57,60 Marked hyperferritinaemia (>10,000 µg/L) is 96% specific and 90% sensitive for HLH in the paediatric population. 61 It is less specific for HLH in adults, but significantly elevated levels should still raise suspicion. 62 However, in some adults, even with severe life-threatening HLH, milder elevations are observed. 2 Values between 7000 and 10,000 µg/L are often reported in this context, while a separate analysis of 50 adult women with HLH revealed a median ferritin level on presentation of 5823 µg/L. 62
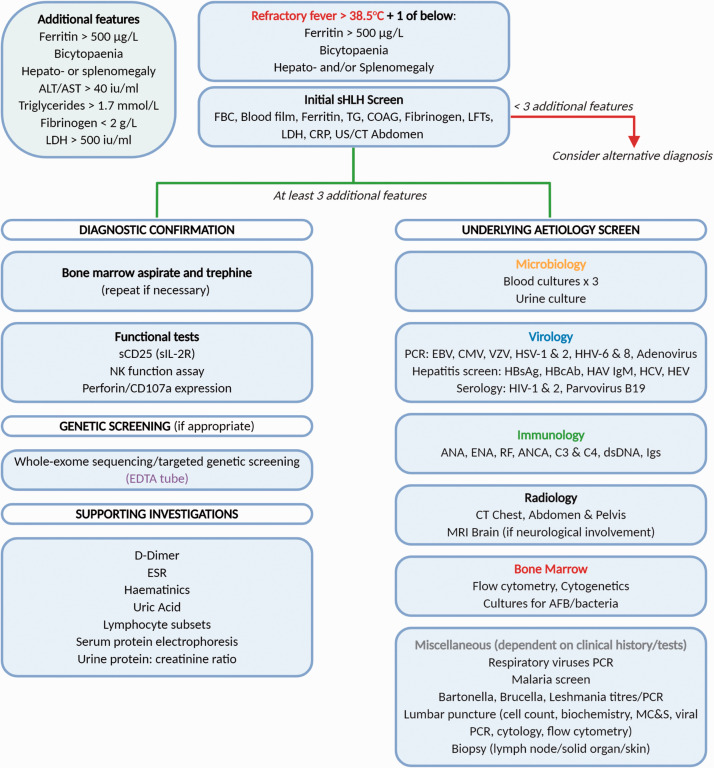
Figure 2.
Proposed algorithm for investigation of suspected HLH in pregnancy. AFB: acid-fast bacillus; ALT: alanine aminotransferase; ANA: antinuclear antibody; ANCA: anti-neutrophil cytoplasmic antibody; AST: aspartate aminotransferase; C: complement; CMV: cytomegalovirus; COAG: coagulation studies; CRP: c-reactive protein; CT: computerised tomography; dsDNA: double-stranded DNA; EBV: Epstein-Barr virus: ENA: extractable nuclear antigen; ESR: erythrocyte sedimentation rate; FBC: full blood count; HAV IgM: hepatitis A immunoglobulin M; HBcAb: hepatitis B core antibody; HBsAg: hepatitis B surface antigen; HCV: hepatitis C virus; HEV: hepatitis E virus; HHV: human herpesvirus; HIV: human immunodeficiency virus; HSV: herpes simplex virus; Igs: immunoglobulins; LDH: lactate dehydrogenase; LFTs: liver function tests; MC&S: microscopy culture and sensitivity; MRI: magnetic resonance imaging; NK: natural killer; PCR: polymerase chain reaction; RF: rheumatoid factor; sCD25: soluble CD25; sIL-2R: soluble interleukin-2 receptor; TG: triglyceride; US: ultrasound.
Soluble CD25 (sCD25), the soluble interleukin-2 receptor (sIL-2R) is a useful diagnostic marker for HLH especially in paediatric populations but is not yet readily available in all laboratories and its use in pregnancy has not been validated. sCD25 is a heterotrimeric transmembrane protein that is upregulated on activated T-cells, thus high levels are found in HLH, lymphoma and other conditions where T-cell activation is prevalent. 63 The test itself is based on a low-cost commercially available assay. A recent study has shown that the specificity of sCD25 in adults fails to match that seen in the paediatric population. 64 Thus, further work is required in adult cohorts with and without HLH to determine more accurate sensitivity and specificity values for this assay. It is also notable that the extent of elevation of sCD25 above 10,000 U/ml has not been shown to be of prognostic significance. 63
Pregnancy will alter the interpretation of some of the biochemical markers typically used in the diagnosis of HLH. In the HScore, it is the ferritin and triglycerides that confer the most diagnostic weight. A pregnant woman with persistent fever and cytopaenia affecting at least 2 peripheral blood lineages presents a particular diagnostic challenge because, as well as infection, a number of pregnancy-specific conditions may present similarly. These include acute fatty liver of pregnancy and haemolysis, elevated liver enzymes and low platelet count (HELLP) syndrome. Indeed, sHLH and HELLP syndrome may coexist, thus a high degree of suspicion is warranted. 45 Lipids including cholesterol and triglycerides tend to increase in pregnancy and are infrequently measured. The normal ranges have not been established and so while it is appropriate to measure triglycerides if HLH is suspected in pregnancy, the results should be interpreted with caution.
In all cohorts of sHLH, including those occurring in pregnancy, many cases are initially treated for sepsis. Indeed, there is a known subset of septic women whose infection will cause an aberrant cytokine cascade due to immune dysregulation, resulting in the clinical syndrome of sHLH. Furthermore, sHLH can further predispose to sepsis, creating a diagnostic and therapeutic challenge. The presentation of the two syndromes may be identical, with multi-organ failure and disseminated intravascular coagulopathy (DIC) occurring in severe phenotypes, making it necessary to consider sHLH in any individual with sepsis not responding to conventional antimicrobials. Very elevated ferritin levels and progressive cytopenia (particularly reduction in platelet count) can be signs of evolving HLH and are useful markers in this context. 54
Initial investigations should be tailored to the diagnostic criteria listed in Table 3 and include full blood count, blood film, coagulation screen (including fibrinogen) and erythrocyte sedimentation rate (ESR). ESR is affected by pregnancy but, like many investigations in HLH, single measurements are unhelpful but trends of measurements over days can be enlightening. A high ESR due to infection or pregnancy will fall due to fibrinogen consumption as HLH evolves. 65 Anaemia is common in pregnancy due to fetal demand for iron and haemodilution. 57 However, the downward haemoglobin trend is much more marked in evolving sHLH in pregnancy, and the blood film often reveals concomitant thrombocytopenia. Microcytic hypochromic red cells and characteristic pencil cells of iron deficiency may also be observed, but these are not accompanied by low ferritin levels in sHLH. Fibrinogen is classically low due to consumption in HLH and raised in infection. Thus, daily haematology tests are required to create trends to guide the suspecting clinician.
Biochemistry to assess for end-organ damage (renal profile and hepatic function), ferritin, lipids (including triglycerides), lactate dehydrogenase (LDH), C-reactive protein (CRP) and haematinics should also be performed and repeated regularly. sCD25 should be sent in all cases, but the results typically take many weeks and do not currently assist in the immediate diagnostic phase unless in a specialist centre. Genetic testing for pHLH genotypes should be considered in all young adults, women with a family history, those with EBV-driven sHLH and also requested in those in whom no cause for their HLH can be identified.
Further investigations should be geared towards elucidating the underlying aetiology. Bacterial blood cultures should be taken alongside serum for EBV and CMV serology and PCR, viral hepatitis screen for Hepatitis A, B and C, human immunodeficiency viruses (HIV) and Parvovirus B19. Given the higher prevalence of autoimmunity in women of childbearing age, we advocate the testing of antinuclear antibodies (ANA), extractable nuclear antigens (ENA), antineutrophil cytoplasmic antibodies (ANCA), complement (C3 and C4) and double-stranded DNA (dsDNA) levels in all cases.
Bone marrow examination should be performed in all cases, where possible, as it will not only confirm the presence of haemophagocytic activity but aid in the diagnosis of underlying haematological malignancies as the driver for the disease. The presence of haemophagocytosis in the bone marrow is not, however, diagnostic for HLH; it can be seen in other settings such as after blood transfusion and chemotherapy, in fulminant sepsis, and after surgery. 66 The absence of haemophagocytosis on the marrow examination does also not exclude the diagnosis, if clinical suspicion and other markers are supportive. 5 A few small studies have investigated the quantification of haemophagocytosis in the marrow and its correlation with the eventual diagnosis of HLH. A case-control study, using aspirates only, showed the extent of haemophagocytosis to be higher in those with HLH with a sensitivity of 83% and a specificity of 60%. 67 A larger blinded retrospective study evaluating 64 bone marrow aspirates and core biopsy specimens from adult women with a clinical phenotype suspicious for sHLH revealed no correlation between the amount of haemophagocytosis observed and clinical disease. 66
Often imaging may be required in the diagnostic work-up. Magnetic resonance imaging (MRI) of the brain and culture of cerebrospinal fluid may be appropriate in the context of neurological involvement, while cross-sectional imaging using computerised and/or positron-emission tomography followed by targeted lymph node or solid organ biopsy can identify malignancy. The use of imaging techniques involving ionising radiation may be appropriate in pregnancy but should be discussed in a multidisciplinary team (MDT), and alternatives considered.
A suspected diagnosis of sHLH in pregnancy should be urgently discussed with a regional expert or in a specialist multidisciplinary team (MDT) HLH meeting, as the complexities of treatment may require the need for early specialist transfer if it safe to do so given the associated high mortality.
Treatment
The treatment of sHLH typically involves management of the underlying cause, which may not be easily identified. In the interim, empirical immunosuppressive treatment based is generally advocated in order to rapidly suppress the hyperinflammation and buy time to identify the underlying trigger, allowing for more targeted definitive treatment of the underlying aetiological driver. Delivery must be considered in each case of HLH in pregnancy and has been shown to lead to rapid clinical and biochemical resolution in some cases. 28
Typically, glucocorticoids and intravenous immunoglobulin are used to rapidly suppress the acute phase response and to allow time for diagnostic investigations to both confirm the clinical suspicion of HLH and look for the underlying aetiology. The widely used paediatric HLH-94 protocol includes the use of high-dose dexamethasone, the topoisomerase II inhibiting alkylating agent etoposide, the calcineurin inhibitor ciclosporin A (CsA) and employs intrathecal methotrexate in cases with central nervous system involvement. 68 However, this is not necessary in many cases of sHLH where immunosuppression and treatment of the underlying driver of the HLH can lead to resolution. In the last decade, the use of targeted cytokine inhibition has been introduced to the management of HLH, and can lead to a rapid clinical response. 69
Non-selective immunosuppressive agents
Glucocorticoids
Glucocorticoids mediate anti-inflammatory effects on both genomic and non-genomic levels when bound to the intracellular glucocorticoid receptor (GR). Classically, high-dose glucocorticoids such as intravenous methylprednisolone or dexamethasone are used; the latter has greater anti-macrophage activity and is the glucocorticoid of choice in this condition. Furthermore, dexamethasone has particular use in the context of neurological involvement as it has the capacity to permeate across the blood–brain barrier, but dose-related caution should be implemented to avoid CNS toxicity. 70 Methylprednisolone is often preferred in pregnancy due to reduced placental transfer compared to dexamethasone, and is therefore the glucocorticoid if no clear CNS involvement.
In cases where the underlying sHLH trigger has been elusive, high-dose steroid use can blur the diagnostic picture. As aforementioned, a common driver of sHLH is lymphoproliferative disease, which can be exquisitely sensitive to glucocorticoids. Therefore, their early use in sHLH cases may obviate the swift diagnosis of lymphoma resulting in additional investigations and the need for repeat sampling once disease-masking doses have been weaned.
Intravenous immunoglobulin
The use of intravenous immunoglobulin (IVIg) is safe and in combination with glucocorticoids forms the backbone of some HLH treatment regimens. Like many interventions in sHLH, however, evidence is lacking.71,72 It achieves its anti-inflammatory effects by competitively binding to the Fc-receptor on macrophages, causing downstream modulation of B- and T-cell activity, and inhibiting complement activation. 73 Clinical experience over many years of its use for the treatment of autoimmune conditions in pregnancy does not suggest any harmful effect to the developing fetus, nor the infant during breastfeeding. 74 IVIg is expensive, however, and supplies can be restricted.
Corticosteroids and IVIg are both used in the management of pregnancy-associated sHLH, but their combined use is not always enough to induce remission.14,75
Selective immunosuppressive agents
Calcineurin inhibitors
Ciclosporin A (CsA) is a calcineurin inhibitor that binds to the receptor cyclophilin-1 leading to downstream effects that inhibit the transcription of key inflammatory cytokines, such as IL-2. This leads to the downregulation of activated T-cells. 76 CsA has been used in severe cases of pregnancy-associated sHLH refractory to corticosteroids with varying results.23,40 It has been seen to be most effective in sHLH secondary to autoimmune disease. 2 Often in these cases there is a suboptimal response to corticosteroids alone, and CsA is used as a potent adjunct to first-line therapy. Its early introduction in those treated with HLH-94 etoposide-dexamethasone based regimens did not improve outcomes, however. 71
CsA has a favourable safety profile in pregnancy derived from long-term solid organ transplant data. 77 Prematurity and intrauterine growth restriction (IUGR) leading to low infant birthweights have been reported but CsA does not appear to be a human teratogen. 77 Long-term data are still required to consolidate its safety during breastfeeding, but there are no reports of adverse effects on growth, development or renal function despite varying recorded levels of CsA in breast milk. 78
Cytokine inhibitors
Anakinra
Anakinra, a recombinant IL-1 receptor antagonist (IL-1Ra), has become favoured in recent years due to its excellent safety profile, rapid onset of action and its short half-life. 79 It provides a physiological steroid-sparing path to IL-1 downregulation resulting in additional time for further investigation of the underlying aetiology. 80 Anakinra requires daily dosing and can be administered either via subcutaneous or intravenous routes. Fewer than 50 cases of anakinra use in pregnancy have been reported.81–84 The majority completed successful pregnancies to term with normal neonatal checks. One case of fetal unilateral hearing loss and two cases of renal agenesis have been reported. A causal link between anakinra and renal agenesis is uncertain as in both cases there was maternal illness (uncontrolled inflammation in one case and the other a twin pregnancy in a woman with autoimmune disease and glucocorticoid-induced diabetes mellitus). 85 Anakinra is considered appropriate to use in pregnancy, and no concerns have been identified with its ongoing use in breastfeeding in a large international multicentre study. 86
Tocilizumab
Tocilizumab (TCZ) is a monoclonal antibody that targets the receptor of IL-6. It is widely used in systemic-onset juvenile arthritis and adult-onset Still’s disease which can lead to sHLH. 69 By inhibiting IL-6, TCZ effectively abrogates the acute phase response leading to normalisation of measurable parameters of inflammation and infection such as fever, CRP and ferritin but is not necessarily synonymous with disease control. 87
There are currently no controlled studies of TCZ use in human pregnancy; the current literature of 360 exposed pregnancies demonstrates cases of congenital anomaly, miscarriage, preterm delivery and low birth weight, seen at a similar incidence when compared to rates in the unexposed pregnant population. 88
Etoposide
Etoposide is a chemotherapeutic drug primarily used in cancer treatment. It selectively inhibits topoisomerase II preventing re-ligation of DNA strands leading to acquired errors in DNA synthesis and apoptosis of cancer cells. Etoposide selectively induces apoptosis of activated T-cells leading to a marked reduction in inflammatory cytokine production while sparing memory T-cells. 89 The HLH-94 paediatric treatment protocol contains etoposide as a key component, and its adapted use in adults, extrapolated from this, is often effective at reversing the life-threatening sequelae of sHLH. 71 Dose reduction is advised if renal impairment is present, as etoposide is primarily renally excreted. 90 Etoposide’s potential toxicity to the fetus is an obvious concern in the treatment of sHLH in pregnancy. Although there is no statistically significant increase in the number of congenital malformations seen in pregnancies exposed to chemotherapy after the first trimester, 91 etoposide use after this time has been seen to result in adverse fetal ovarian development in mice studies. 92 However, the clinical scenario may warrant the use of the drug in pregnant women despite potential risks although delivery would have to be strongly considered. 93
Emapalumab
Emapalumab is a fully human immunoglobulin G1 monoclonal antibody directed against IFN-γ developed for use in HLH. Favourable outcomes have been observed in paediatric cases of refractory pHLH. 94 A phase 2/3 interventional study (NCT03985423) is currently recruiting adult women to assess the efficacy, safety and pharmacokinetics of emapalumab in sHLH, but severe infections have been noted in the existing data. 95 No data exist relating to the use of emapalumab in the context of pregnancy and breastfeeding in humans. 96
Ruxolitinib
Ruxolitinib is a selective inhibitor of the Janus-associated tyrosine kinases JAK1 and JAK2, whose role in the JAK/STAT signalling pathway contributes to the cytokine-mediated hyperinflammatory state seen in sHLH. 97 It is used in the treatment of myeloproliferative neoplasms such as myelofibrosis and polycythaemia vera, but promising results in the context of adult HLH have been observed in pre-clinical trials and case series.97,98 A phase 2 interventional study (NCT02400463) is currently recruiting adult women to assess efficacy and safety of ruxolitinib in sHLH and the preliminary data are encouraging in both regards. 99 There are no current data for its use in the context of pregnancy. Tofacitinib, another JAK inhibitor used in the treatment of rheumatoid arthritis, psoriatic arthritis, and ulcerative colitis, has been shown to be teratogenic in rats, thus its use in pregnancy is currently largely contraindicated despite a human study revealing adverse rates comparable to the unexposed background risk.100,101
B-cell depletion
Rituximab
There are known triggers for sHLH in pregnancy where the use of rituximab, a genetically engineered chimeric murine/human monoclonal antibody that targets CD20, may be indicated. Typically, it is used in conjunction with glucocorticoids and IVIg (and cytokine inhibitors) in the treatment of sHLH due to B-cell lymphoma, severe systemic lupus erythematosus and in EBV-driven HLH where rituximab is used repeatedly to deplete the B-cells in which the virus is actively replicating. 37
Rituximab use in pregnancy can lead to B-cell depletion in the fetus if given beyond 16 weeks of gestation, increasing the potential risks of neonatal infection. 102 Congenital abnormalities have also been reported; however, its use is generally considered acceptable if indicated in a pregnant woman with sHLH, and other conditions where there are no suitable alternative options. 103
Transplantation
Haematopoietic stem cell transplantation (HSCT) is established for pHLH and refractory sHLH including those occurring in pregnancy but only performed postpartum. Successful outcomes have been reported after delivery and focused chemotherapy. 24 Despite the commonly reported occurrence of HSCT-related infertility, women are able to retain or recover fertility and conceive. 104
Delivery
Maternal and fetal outcomes following delivery in reported cases of HLH in pregnancy are mixed (Table 2). Emergency caesarean sections were performed in cases where fetal complications were observed, but they were more frequently undertaken to aid maternal treatment and complete remission was occasionally achieved after delivery.28,45,47 This raises a key question – does early delivery lead to better outcomes? This has yet to be proven and so delivery is not mandated when the diagnosis is made, but close monitoring of mother and fetus is required, and delivery performed if the mother’s condition is deteriorating, or if there is evidence of fetal compromise. 9
Future pregnancies
Future pregnancies for any woman following HLH in pregnancy should be carefully considered and planned. If there was a clear and transient trigger for the sHLH in pregnancy, such as infection, there is no suggestion that subsequent pregnancies will lead to recurrence; however, caution is advised. A caveat to this is if the infection is triggered by EBV, genetic testing for underlying primary HLH is strongly advised. For those with autoimmune disease such as SLE, which may have presented as sHLH in pregnancy, future pregnancies should be carefully planned, taking place following adequate disease control and on non-teratogenic treatment regimens. If the cause for sHLH in pregnancy was not identified, we suggest genetic testing and referral to a specialist HLH centre for investigation prior to the planning of subsequent pregnancies.
Conclusion
HLH in pregnancy is a rare, but likely underdiagnosed, subtype of a complex life-threatening multisystem inflammatory syndrome. It provides significant diagnostic challenges due to its spectrum of underlying aetiologies and the subsequent diagnostic delay contributes to the high mortality seen. Serum ferritin measurement can hint at the diagnosis of sHLH in pregnancy and trigger further investigation and involvement of specialists early in the disease course. The use of immunosuppression and more targeted therapies presents more efficacious and less toxic treatment options. The interleukin-1 receptor antagonist, anakinra, is particularly attractive in this setting due to its established efficacy and physiological nature, but more importantly its short half-life lends itself well to use in hyperinflammation triggered by infection. Refractory cases may require difficult decisions to be made regarding delivery and the use of more toxic treatment regimens. Future pregnancies need to be carefully considered in the context of the underlying aetiology of the HLH. Particularly, caution should be taken in those in whom an aetiological trigger has not been identified.
Declaration of conflicting interests: HW and TY declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. CF is co-Editor-in-Chief of Obstetric Medicine.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Guarantor: TY.
Contributorship: Dr Wilson-Morkeh drafted the initial manuscript. Dr Youngstein (corresponding and guaranteeing author) drafted parts of the manuscript, edited the manuscript with Dr Frise and approved the final draft.
ORCID iD: Taryn Youngstein https://orcid.org/0000-0002-8297-556X
Ethical approval/ informed consent
Obtaining patient consent or ethical approval is not applicable for this manuscript.
References
- 1.Grom AA. Macrophage activation syndrome and reactive hemophagocytic lymphohistiocytosis: the same entities?. Curr Opin Rheumatol 2003; 15: 587–590. [DOI] [PubMed] [Google Scholar]
- 2.La Rosee P, Horne A, Hines M, et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood 2019; 133: 2465–2477. [DOI] [PubMed] [Google Scholar]
- 3.Grom AA. Natural killer cell dysfunction: a common pathway in systemic-onset juvenile rheumatoid arthritis, macrophage activation syndrome, and hemophagocytic lymphohistiocytosis?. Arthritis Rheum 2004; 50: 689–698. [DOI] [PubMed] [Google Scholar]
- 4.Machowicz R, Janka G, Wiktor-Jedrzejczak W. Similar but not the same: differential diagnosis of HLH and sepsis. Crit Rev Oncol Hematol 2017; 114: 1–12. [DOI] [PubMed] [Google Scholar]
- 5.Ramos-Casals M, Brito-Zerón P, López-Guillermo A, et al. Adult haemophagocytic syndrome. Lancet 2014; 383: 1503–1516. [DOI] [PubMed] [Google Scholar]
- 6.Arca M, Fardet L, Galicier L, et al. Prognostic factors of early death in a cohort of 162 adult haemophagocytic syndrome: impact of triggering disease and early treatment with etoposide. Br J Haematol 2015; 168: 63–68. [DOI] [PubMed] [Google Scholar]
- 7.Park HS, Kim DY, Lee JH, et al. Clinical features of adult patients with secondary hemophagocytic lymphohistiocytosis from causes other than lymphoma: an analysis of treatment outcome and prognostic factors. Ann Hematol 2012; 91: 897–904. [DOI] [PubMed] [Google Scholar]
- 8.HistioUK. Histio UK Haemophagocytosis across specialty collaboration (HASC) initiative, www.histiocytosisuk.org/histio-uk-haemophagocytosis-across-specialty-collaboration-hasc-initiative/ (2020, accessed 12 April 2021).
- 9.Knight M, Nelson-Piercy C, Group Obotmasc W. Lessons for the care of women with medical and general surgical disorders. In: Knight M, Nair M, Tuffnell D, et al. (eds) Saving lives, improving mothers’ care – lessons learned to inform maternity care from the UK and Ireland confidential enquiries into maternal deaths and morbidity 2013–15. Oxford: National Perinatal Epidemiology Unit: University of Oxford, 2017, p. 55. [Google Scholar]
- 10.Janka GE, Lehmberg K. Hemophagocytic lymphohistiocytosis: pathogenesis and treatment. Hematology Am Soc Hematol Educ Program 2013; 2013: 605–611. [DOI] [PubMed] [Google Scholar]
- 11.Zhang K, Jordan MB, Marsh RA, et al. Hypomorphic mutations in PRF1, MUNC13-4, and STXBP2 are associated with adult-onset familial HLH. Blood 2011; 118: 5794–5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koch CA, Platt JL. Natural mechanisms for evading graft rejection: the fetus as an allograft. Springer Semin Immunopathol 2003; 25: 95–117. [DOI] [PubMed] [Google Scholar]
- 13.Redman CW, Sargent IL. Pre-eclampsia, the placenta and the maternal systemic inflammatory response – a review. Placenta 2003; 24(Suppl A): S21–27. [DOI] [PubMed] [Google Scholar]
- 14.Parrott J, Shilling A, Male HJ, et al. Hemophagocytic lymphohistiocytosis in pregnancy: a case series and review of the current literature. Case Rep Obstet Gynecol 2019; 2019: 9695367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Z, Wang J, Ji B, et al. Clinical presentation of hemophagocytic lymphohistiocytosis in adults is less typical than in children. Clinics 2016; 71: 205–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gill DS, Spencer A, Cobcroft RG. High-dose gamma-globulin therapy in the reactive haemophagocytic syndrome. Br J Haematol 1994; 88: 204–206. [DOI] [PubMed] [Google Scholar]
- 17.Tsuda H, Shirono K, Shimizu K, et al. Post-partum parvovirus B19-associated acute pure red cell aplasia and haemophagocytic syndrome. Rinsho Ketsueki 1995; 36: 672–676. [PubMed] [Google Scholar]
- 18.Yamanaka S, Katsube Y, Honda H, et al. A case of pregnancy complicated with virus-associated hemophagocytic syndrome. Nihon Sanka Fujinka Gakkai Zasshi 1995; 47: 503–506. [PubMed] [Google Scholar]
- 19.Ishida A, Matsumoto J, Kobayashi S, et al. [A case of reactive hemophagocytic syndrome which occurred during treatment of hyoeremesis]. Kanto J Obstet Gynaecol 1996; 33: 51–54. [Google Scholar]
- 20.Mihara H, Kato Y, Tokura Y, et al. Epstein-Barr virus-associated hemophagocytic syndrome during mid-term pregnancy successfully treated with combined methylprednisolone and intravenous immunoglobulin. Rinsho Ketsueki 1999; 40: 1258–1264. [PubMed] [Google Scholar]
- 21.Nakabayashi M, Adachi T, Izuchi S, et al. Association of hypercytokinemia in the development of severe preeclampsia in a case of hemophagocytic syndrome. Semin Thromb Hemost 1999; 25: 467–471. [DOI] [PubMed] [Google Scholar]
- 22.Chmait R, Meimin D, Koo C, et al. Hemophagocytic syndrome in pregnancy. Obstet Gynecol 2000; 95: 1022–1024. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi K, Yamamoto A, Hisano M, et al. Herpes simplex virus 2-associated hemophagocytic lymphohistiocytosis in a pregnant patient. Obstet Gynecol 2005; 105: 1241–1244. [DOI] [PubMed] [Google Scholar]
- 24.Hanaoka M, Tsukimori K, Hojo S, et al. B-cell lymphoma during pregnancy associated with hemophagocytic syndrome and placental involvement. Clin Lymphoma Myeloma 2007; 7: 486–490. [DOI] [PubMed] [Google Scholar]
- 25.Perard L, Costedoat-Chalumeau N, Limal N, et al. Hemophagocytic syndrome in a pregnant patient with systemic lupus erythematosus, complicated with preeclampsia and cerebral hemorrhage. Ann Hematol 2007; 86: 541–544. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez E, Olvera H, Gonzalez V, et al. Hemophogocytic syndrome secondary to an infection with parvovirus B19. Rev Chil Obstet Ginecol 2008; 73: 406–410. [Google Scholar]
- 27.Chien CT, Lee FJ, Luk HN, et al. Anesthetic management for cesarean delivery in a parturient with exacerbated hemophagocytic syndrome. Int J Obstet Anesth 2009; 18: 413–416. [DOI] [PubMed] [Google Scholar]
- 28.Teng C-L, Hwang G-Y, Lee B-J, et al. Pregnancy-induced hemophagocytic lymphohistiocytosis combined with autoimmune hemolytic anemia. J Chin Med Assoc 2009; 72: 156–159. [DOI] [PubMed] [Google Scholar]
- 29.Yoshida S, Takeuchi T, Itami Y, et al. Hemophagocytic syndrome as primary manifestation in a patient with systemic lupus erythematosus after parturition. Nihon Rinsho Meneki Gakkai Kaishi 2009; 32: 66–70. [DOI] [PubMed] [Google Scholar]
- 30.Arewa OP, Ajadi AA. Human immunodeficiency virus associated with haemophagocytic syndrome in pregnancy: a case report. West Afr J Med 2011; 30: 66–68. [DOI] [PubMed] [Google Scholar]
- 31.Dunn T, Cho M, Medeiros B, et al. Hemophagocytic lymphohistiocytosis in pregnancy: a case report and review of treatment options. Hematology 2012; 17: 325–328. [DOI] [PubMed] [Google Scholar]
- 32.Hannebicque-Montaigne K, Le Roc'h A, Launay D, et al. [Haemophagocytic syndrome in pregnancy: a case report]. Ann Fr Anesth Reanim 2012; 31: 239–242. [DOI] [PubMed] [Google Scholar]
- 33.Kim JM, Kwok SK, Ju JH, et al. Macrophage activation syndrome resistant to medical therapy in a patient with systemic lupus erythematosus and its remission with splenectomy. Rheumatol Int 2013; 33: 767–771. [DOI] [PubMed] [Google Scholar]
- 34.Komaru Y, Higuchi T, Koyamada R, et al. Primary Sjogren syndrome presenting with hemolytic anemia and pure red cell aplasia following delivery due to Coombs-negative autoimmune hemolytic anemia and hemophagocytosis. Intern Med 2013; 52: 2343–2346. [DOI] [PubMed] [Google Scholar]
- 35.Shukla A, Kaur A, Hira HS. Pregnancy induced haemophagocytic syndrome. J Obstet Gynaecol India 2013; 63: 203–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goulding EA, Barnden KR. Disseminated herpes simplex virus manifesting as pyrexia and cervicitis and leading to reactive hemophagocytic syndrome in pregnancy. Eur J Obstet Gynecol Reprod Biol 2014; 180: 198–199. [DOI] [PubMed] [Google Scholar]
- 37.Klein S, Schmidt C, La Rosee P, et al. Fulminant gastrointestinal bleeding caused by EBV-triggered hemophagocytic lymphohistiocytosis: report of a case. Z Gastroenterol 2014; 52: 354–359. [DOI] [PubMed] [Google Scholar]
- 38.Mayama M, Yoshihara M, Kokabu T, et al. Hemophagocytic lymphohistiocytosis associated with a parvovirus B19 infection during pregnancy. Obstet Gynecol 2014; 124: 438–441. [DOI] [PubMed] [Google Scholar]
- 39.Samra B, Yasmin M, Arnaout S, et al. Idiopathic hemophagocytic lymphohistiocytosis during pregnancy treated with steroids. Hematol Rep 2015; 7: 6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tumian NR, Wong CL. Pregnancy-related hemophagocytic lymphohistiocytosis associated with cytomegalovirus infection: a diagnostic and therapeutic challenge. Taiwan J Obstet Gynecol 2015; 54: 432–437. [DOI] [PubMed] [Google Scholar]
- 41.Giard JM, Decker KA, Lai JC, et al. Liver failure secondary to hemophagocytic lymphohistiocytosis during pregnancy. ACG Case Rep J 2016; 3: e162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ota K, Kawahara K, Banno H, et al. Hemophagocytic lymphohistiocytosis caused by pyogenic liver abscess during pregnancy: a case report and literature review. Open J Obstet Gynecol 2016; 06: 287–292. [Google Scholar]
- 43.He M, Jia J, Zhang J, et al. Pregnancy-associated hemophagocytic lymphohistiocytosis secondary to NK/T cells lymphoma: a case report and literature review. Medicine 2017; 96: e8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ikeda M, Oba R, Yoshiki Y, et al. Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis during pregnancy. Rinsho Ketsueki 2017; 58: 216–221. [DOI] [PubMed] [Google Scholar]
- 45.Kerley RN, Kelly RM, Cahill MR, et al. Haemophagocytic lymphohistiocytosis presenting as HELLP syndrome: a diagnostic and therapeutic challenge. BMJ Case Rep 2017; 2017: doi:10.1136/bcr-2017-219516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rousselin A, Alavi Z, Le Moigne E, et al. Hemophagocytic syndrome in pregnancy: case report, diagnosis, treatment, and prognosis. Clin Case Rep 2017; 5: 1756–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nasser MF, Sharma S, Albers E, et al. Pregnancy-related hemophagocytic lymphohistiocytosis associated with herpes simplex virus-2 infection: a diagnostic dilemma. Cureus 2018; 10: e2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng J, Niu J, Wang Y, et al. Hemophagocytic lymphohistiocytosis in pregnancy: a case report and review of the literature. J Obstet Gynaecol 2020; 40: 153–159. [DOI] [PubMed] [Google Scholar]
- 49.Yip KP, Ali M, Avann F, et al. Pregnancy-induced haemophagocytic lymphohistiocytosis. J Intensive Care Soc 2020; 21: 87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Kerguenec C, Hillaire S, Molinie V, et al. Hepatic manifestations of hemophagocytic syndrome: a study of 30 cases. Am J Gastroenterol 2001; 96: 852–857. [DOI] [PubMed] [Google Scholar]
- 51.Rosado FG, Kim AS. Hemophagocytic lymphohistiocytosis: an update on diagnosis and pathogenesis. Am J Clin Pathol 2013; 139: 713–727. [DOI] [PubMed] [Google Scholar]
- 52.Horne A, Trottestam H, Arico M, et al. Frequency and spectrum of central nervous system involvement in 193 children with haemophagocytic lymphohistiocytosis. Br J Haematol 2008; 140: 327–335. [DOI] [PubMed] [Google Scholar]
- 53.Seguin A, Galicier L, Boutboul D, et al. Pulmonary involvement in patients with hemophagocytic lymphohistiocytosis. Chest 2016; 149: 1294–1301. [DOI] [PubMed] [Google Scholar]
- 54.Henter JI, Horne A, Arico M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 2007; 48: 124–131. [DOI] [PubMed] [Google Scholar]
- 55.Fardet L, Galicier L, Lambotte O, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol 2014; 66: 2613–2620. [DOI] [PubMed] [Google Scholar]
- 56.Naymagon L. Can we truly diagnose adult secondary hemophagocytic lymphohistiocytosis (HLH)? A critical review of current paradigms. Pathol Res Pract 2021; 218: 153321. [DOI] [PubMed] [Google Scholar]
- 57.Pavord S, Myers B, Robinson S, et al. UK guidelines on the management of iron deficiency in pregnancy. Br J Haematol 2012; 156: 588–600. [DOI] [PubMed] [Google Scholar]
- 58.Wang W, Knovich MA, Coffman LG, et al. Serum ferritin: past, present and future. Biochim Biophys Acta 2010; 1800: 760–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abbassi-Ghanavati M, Greer LG, Cunningham FG. Pregnancy and laboratory studies: a reference table for clinicians. Obstet Gynecol 2009; 114: 1326–1331. [DOI] [PubMed] [Google Scholar]
- 60.Asif N, Hassan K, Mahmud S, et al. Comparison of serum ferritin levels in three trimesters of pregnancy and their correlation with increasing gravidity. Int J Pathol 2007; 5: 26–30. [Google Scholar]
- 61.Allen CE, Yu X, Kozinetz CA, et al. Highly elevated ferritin levels and the diagnosis of hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 2008; 50: 1227–1235. [DOI] [PubMed] [Google Scholar]
- 62.Schram AM, Campigotto F, Mullally A, et al. Marked hyperferritinemia does not predict for HLH in the adult population. Blood 2015; 125: 1548–1552. [DOI] [PubMed] [Google Scholar]
- 63.Hayden A, Lin M, Park S, et al. Soluble interleukin-2 receptor is a sensitive diagnostic test in adult HLH. Blood Adv 2017; 1: 2529–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Naymagon L, Tremblay D, Troy K, et al. Soluble interleukin-2 receptor (sIL-2r) level is a limited test for the diagnosis of adult secondary hemophagocytic lymphohistiocytosis. Eur J Haematol 2020; 105: 255–261. [DOI] [PubMed] [Google Scholar]
- 65.Schram AM, Berliner N. How I treat hemophagocytic lymphohistiocytosis in the adult patient. Blood 2015; 125: 2908–2914. [DOI] [PubMed] [Google Scholar]
- 66.Ho C, Yao X, Tian L, et al. Marrow assessment for hemophagocytic lymphohistiocytosis demonstrates poor correlation with disease probability. Am J Clin Pathol 2014; 141: 62–71. [DOI] [PubMed] [Google Scholar]
- 67.Goel S, Polski JM, Imran H. Sensitivity and specificity of bone marrow haemophagocytosis in haemophagocytic lymphohistiocytosis. Ann Clin Lab Sci 2012; 42: 21–25. [PubMed] [Google Scholar]
- 68.Trottestam H, Horne A, Arico M, et al. Chemoimmunotherapy for hemophagocytic lymphohistiocytosis: long-term results of the HLH-94 treatment protocol. Blood 2011; 118: 4577–4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carter SJ, Tattersall RS, Ramanan AV. Macrophage activation syndrome in adults: recent advances in pathophysiology, diagnosis and treatment. Rheumatology 2019; 58: 5–17. [DOI] [PubMed] [Google Scholar]
- 70.Ciriaco M, Ventrice P, Russo G, et al. Corticosteroid-related central nervous system side effects. J Pharmacol Pharmacother 2013; 4: S94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bergsten E, Horne A, Arico M, et al. Confirmed efficacy of etoposide and dexamethasone in HLH treatment: long-term results of the cooperative HLH-2004 study. Blood 2017; 130: 2728–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dunogue B, Gerin M, Larroche C, et al. Intravenous immunoglobulin therapy for secondary hemophagocytic lymphohistiocytosis: a retrospective study of 46 patients. ACR/ARHP Ann Meet 2014; 2199, https://acrabstracts.org/abstract/intravenous-immunoglobulin-therapy-for-secondary-hemophagocytic-lymphohistiocytosis-a-retrospective-study-of-46-patients/. [Google Scholar]
- 73.Bayry J, Lacroix-Desmazes S, Kazatchkine MD, et al. Monoclonal antibody and intravenous immunoglobulin therapy for rheumatic diseases: rationale and mechanisms of action. Nat Clin Pract Rheumatol 2007; 3: 262–272. [DOI] [PubMed] [Google Scholar]
- 74.Ferrero S, Esposito F, Pretta S, et al. Fetal risks related to the treatment of multiple sclerosis during pregnancy and breastfeeding. Expert Rev Neurother 2006; 6: 1823–1831. [DOI] [PubMed] [Google Scholar]
- 75.Song Y, Wang Z, Hao Z, et al. Requirement for etoposide in the treatment of pregnancy related hemophagocytic lymphohistiocytosis: a multicenter retrospective study. Orphanet J Rare Dis 2019; 14: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Russell G, Graveley R, Seid J, et al. Mechanisms of action of cyclosporine and effects on connective tissues. Semin Arthritis Rheum 1992; 21: 16–22. [DOI] [PubMed] [Google Scholar]
- 77.Paziana K, Del Monaco M, Cardonick E, et al. Ciclosporin use during pregnancy. Drug Saf 2013; 36: 279–294. [DOI] [PubMed] [Google Scholar]
- 78.Constantinescu S, Pai A, Coscia LA, et al. Breast-feeding after transplantation. Best Pract Res Clin Obstet Gynaecol 2014; 28: 1163–1173. [DOI] [PubMed] [Google Scholar]
- 79.Opal SM, Fisher CJ, Jr., Dhainaut JF, et al. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. The Interleukin-1 Receptor Antagonist Sepsis Investigator Group. Crit Care Med 1997; 25: 1115–1124. [DOI] [PubMed] [Google Scholar]
- 80.Shabbir S, Al-Abdulla A, Kinderlerer A, et al. THU0051 off-licence use of anakinra in critically ill adults with suspected haemophagocytosis – a single centre experience. Ann Rheum Dis 2019; 78: 292–293. [Google Scholar]
- 81.Lachmann HJ, Ozdogan H, Simon A, et al. OR14-002 – ANTI IL-1 therapies and pregnancy outcome. Pediatr Rheumatol 2013; 11: A269. [Google Scholar]
- 82.Ilgen U, Kucuksahin O. Anakinra use during pregnancy: report of a case with familial Mediterranean fever and infertility. Eur J Rheumatol 2017; 4: 66–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Venhoff N, Voll RE, Glaser C, et al. IL-1-blockade with Anakinra during pregnancy: retrospective analysis of efficacy and safety in female patients with familial Mediterranean fever. Z Rheumatol 2018; 77: 127–134. [DOI] [PubMed] [Google Scholar]
- 84.Chang Z, Spong CY, Jesus AA, et al. Anakinra use during pregnancy in patients with cryopyrin-associated periodic syndromes (CAPS). Arthritis Rheumatol 2014; 66: 3227–3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rider RA, Stevenson DA, Rinsky JE, et al. Association of twinning and maternal age with major structural birth defects in Utah, 1999 to 2008. Birth Defects Res A Clin Mol Teratol 2013; 97: 554–563. [DOI] [PubMed] [Google Scholar]
- 86.Youngstein T, Hoffmann P, Gul A, et al. International multi-centre study of pregnancy outcomes with interleukin-1 inhibitors. Rheumatology 2017; 56: 2102–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shimizu M, Nakagishi Y, Kasai K, et al. Tocilizumab masks the clinical symptoms of systemic juvenile idiopathic arthritis-associated macrophage activation syndrome: the diagnostic significance of interleukin-18 and interleukin-6. Cytokine 2012; 58: 287–294. [DOI] [PubMed] [Google Scholar]
- 88.UKTIS. Use of tocilizumab in pregnancy, www.medicinesinpregnancy.org/bumps/monographs/USE-OF-TOCILIZUMAB-IN-PREGNANCY/ (2020, accessed 12 April 2021).
- 89.Johnson TS, Terrell CE, Millen SH, et al. Etoposide selectively ablates activated T cells to control the immunoregulatory disorder hemophagocytic lymphohistiocytosis. J Immunol 2014; 192: 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ehl S, Astigarraga I, von Bahr Greenwood T, et al. Recommendations for the use of etoposide-based therapy and bone marrow transplantation for the treatment of HLH: consensus statements by the HLH steering committee of the histiocyte society. J Allergy Clin Immunol Pract 2018; 6: 1508–1517. [DOI] [PubMed] [Google Scholar]
- 91.Cardonick E, Usmani A, Ghaffar S. Perinatal outcomes of a pregnancy complicated by cancer, including neonatal follow-up after in utero exposure to chemotherapy: results of an international registry. Am J Clin Oncol 2010; 33: 221–228. [DOI] [PubMed] [Google Scholar]
- 92.Stefansdottir A, Johnston ZC, Powles-Glover N, et al. Etoposide damages female germ cells in the developing ovary. BMC Cancer 2016; 16: 482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Inc CM. UK summary of product characteristics, www.drugs.com/pregnancy/etoposide.html#ref_pregnancy (2019, accessed 12 April 2021).
- 94.Locatelli F, Jordan MB, Allen C, et al. Emapalumab in children with primary hemophagocytic lymphohistiocytosis. N Engl J Med 2020; 382: 1811–1822. [DOI] [PubMed] [Google Scholar]
- 95.Vallurupalli M, Berliner N. Emapalumab for the treatment of relapsed/refractory hemophagocytic lymphohistiocytosis. Blood 2019; 134: 1783–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sobi. Gamifant (emapalumab) prescribing information, www.accessdata.fda.gov/drugsatfda_docs/label/2018/761107lbl.pdf (2018, accessed 12 April 2021).
- 97.Goldsmith SR, Saif Ur Rehman S, Shirai CL, et al. Dramatic resolution of HLH after treatment with the JAK 1/2 inhibitor, ruxolitinib Blood 2019; 134: 2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Albeituni S, Verbist KC, Tedrick PE, et al. Mechanisms of action of ruxolitinib in murine models of hemophagocytic lymphohistiocytosis. Blood 2019; 134: 147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ahmed A, Merrill SA, Alsawah F, et al. Ruxolitinib in adult patients with secondary haemophagocytic lymphohistiocytosis: an open-label, single-centre, pilot trial. Lancet Haematol 2019; 6: e630e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pfizer. Xeljanz (tofacitinib) prescribing information. 2018, https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/203214s018lbl.pdf.
- 101.Clowse ME, Feldman SR, Isaacs JD, et al. Pregnancy outcomes in the tofacitinib safety databases for rheumatoid arthritis and psoriasis. Drug Saf 2016; 39: 755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Flint J, Panchal S, Hurrell A, et al. BSR and BHPR guideline on prescribing drugs in pregnancy and breastfeeding-Part I: standard and biologic disease modifying anti-rheumatic drugs and corticosteroids. Rheumatology 2016; 55: 1693–1697. [DOI] [PubMed] [Google Scholar]
- 103.Chakravarty EF, Murray ER, Kelman A, et al. Pregnancy outcomes after maternal exposure to rituximab. Blood 2011; 117: 1499–1506. [DOI] [PubMed] [Google Scholar]
- 104.Salooja N, Michonneau D, Tichelli A, et al. Conception and pregnancy outcomes after haematopoietic stem cell transplant: a retrospective study from the transplant complications working party of the European Society for Blood and Marrow Transplantation. Blood 2018; 132: 2139. [Google Scholar]