Abstract
Background:
Atrial fibrillation (AF) is the most common heart rhythm disorder in adults and a major cause of stroke. Unfortunately, current treatments of AF are suboptimal as they are not targeted to the molecular mechanisms underlying AF. Using a highly novel gene therapy approach in a canine, rapid atrial pacing model of AF, we demonstrate that NADPH oxidase 2 (NOX2) generated oxidative injury, by causing upregulation of a constitutively active form of acetylcholine-dependent K+ current (IKACh) - called IKH – is an important mechanism underlying not only the genesis but also the perpetuation of electrical remodeling in the intact, fibrillating atrium.
Methods:
To understand the mechanism by which oxidative injury promotes the genesis and/or maintenance of AF, we performed targeted injection of NOX2 shRNA (followed by electroporation to facilitate gene delivery) in atria of normal dogs followed by rapid atrial pacing. We used in-vivo high density electrical mapping, isolation of atrial myocytes, whole-cell patch clamping, in-vitro tachypacing of atrial myocytes, lucigenin chemiluminescence assay, immunoblotting, real-time PCR, immunohistochemistry and Masson’s trichrome staining.
Results:
First, we demonstrate that generation of oxidative injury in atrial myocytes is a frequency-dependent process, with rapid pacing in canine atrial myocytes inducing oxidative injury through induction of NOX2 and generation of mitochondrial reactive oxygen species. We show that oxidative injury likely contributes to electrical remodeling in AF by upregulating IKH by a mechanism involving frequency-dependent activation of protein kinase C epsilon (PKCε). The time to onset of non-sustained AF increased by more than 5-fold in NOX2 shRNA treated dogs. Furthermore, animals treated with NOX2 shRNA did not develop sustained AF for up to 12 weeks. The electrophysiological mechanism underlying AF prevention was prolongation of atrial effective refractory periods, at least in part due to attenuation of IKH. Attenuated membrane translocation of PKCε appeared to be a likely molecular mechanism underlying this beneficial electrophysiological remodeling.
Conclusions:
NOX2 oxidative injury: a) underlies onset as well as maintenance of electrical remodeling in AF, and b) can be successfully prevented with a novel, gene-based approach. Future optimization of this approach may lead to a novel, mechanism-guided therapy for AF.
Keywords: Oxidative injury, NOX2, I KH , Gene Therapy, Atrial Fibrillation
Introduction
Atrial fibrillation (AF) is the most common heart rhythm disorder, affecting 2.7 to 6.1 million adults in 2010 in the US alone. AF is a cause of significant morbidity and mortality, and because the incidence of AF increases with age, it is fast becoming an epidemic worldwide.1, 2 Despite its clinical importance, AF is a difficult condition to treat. Current therapies for AF include anti-arrhythmic drugs and ablation procedures to electrically isolate the pulmonary veins.3 Anti-arrhythmic drugs have limited long-term efficacy and can be associated with significant adverse effects, including pro-arrhythmia. Ablation procedures have suboptimal efficacy in the setting of persistent AF, and can be associated with significant complications.4 A major reason for the low efficacy of the aforementioned therapies – especially in the setting of persistent AF – is that these therapies do not target the molecular mechanisms underlying the electrical and structural remodeling that is characteristic of persistent AF.3 A better understanding of the mechanisms underlying AF is essential for the development of innovative and improved therapeutic approaches for this condition.
Oxidative injury, which is an imbalance between generation and neutralization of reactive oxygen species (ROS), is thought be an important mechanism underlying AF and is regarded as a possible therapeutic target for this condition.5 Oxidative injury has been closely linked with inflammation, with both oxidative injury and inflammasome activation having been described in atrial myocytes from patients with AF.6 Redox potentials of glutathione have been associated with increased prevalence and incidence of AF.7 ROS generated in the cardiovascular system are primarily derived from NADPH oxidases (NOX), mitochondrial electrical transport chain, xanthine oxidase and uncoupled eNOS.8 NOX2 has been suggested as a major source of oxidative injury in atrial appendages of patients with AF.5, 9 More recent studies suggest that with increasing duration of AF, there is an increase in not only expression of NOX2 but also mitochondrial ROS in atrial tissue.10 Although these studies suggest a likely association between oxidative injury (especially NOX2-generated oxidative injury) and AF, they do not demonstrate a causative role for oxidative injury in the genesis and/or maintenance of AF. Specifically, it is not known whether oxidative injury actually leads to electrical remodeling in the intact, fibrillating atrium. Furthermore, the precise mechanisms by which oxidative injury creates a vulnerable substrate are not known. For instance, it is not known which atrial ion channels involved in electrical remodeling in AF are most vulnerable to oxidative injury.
Indeed, although a number of ion channels and transporters including L-type Ca2+ channel, Na+ channel, transient outward K+ channel and type 2 ryanodine receptor (RyR2) have been shown to be redox-sensitive,11 only a few studies have looked at this in the context of AF.12 Among the ion channels that have been suggested to play a key role in action potential duration (APD) and effective refractory period (ERP) shortening in AF, the best studied are the channels responsible for the L-type Ca2+ current (ICaL), which is downregulated in AF, and the inward-rectifier K+ current (IK1), which is elevated in AF. Lately, several studies have described a form of the acetylcholine (ACh)-activated inward rectifier Kir3.1/3.4 potassium channel current (IKACh) that becomes constitutively active in the rapid atrial pacing (RAP) model as well as in patients with paroxysmal and persistent AF13, 14 and has been invoked in ERP shortening in AF. However, the precise mechanisms underlying the emergence of the constitutively active form of IKACh (IKH) in the setting of AF are not known. What is known is that IKH is protein kinase C (PKC) sensitive, with IKH activity in chronic AF appearing to be closely related to abnormal PKC function.13 While the conventional PKC isoform PKCα appears to be involved in inhibition of this current, the novel PKC isoform PKCε, which is upregulated in human persistent AF, has been shown to stimulate the emergence of IKH.15 This PKCε-mediated increase in IKH was demonstrated to be a frequency-dependent phenomenon, with increasing frequency of atrial tachypacing leading to membrane translocation of PKCε and an increase in magnitude of IKH. Because PKC isoforms are well known to be acute phase reactants in the heart, with PKCε having been shown to be highly sensitive to stressors such as oxidative injury,16, 17 we hypothesize that upregulation of IKH in RAP-induced AF is mediated by a frequency-dependent increase in oxidative injury, with resulting activation of PKCε.
In order to determine the precise role of oxidative injury in causing electrical remodeling in the intact atrium, we further hypothesize that oxidative injury leads to not only the initiation but also the maintenance of ERP shortening in the intact, fibrillating atrium. To examine this hypothesis, we performed targeted expression of anti-NOX2 short hairpin RNA (NOX2 shRNA) in the intact atria of dogs, and then subjected these animals to RAP for a period of several weeks to months. Using this novel gene therapy approach in a prevention model, we demonstrate for the first time a clear, causative role for NOX2-generated oxidative injury in the creation as well as the maintenance of electrical remodeling in AF. Furthermore, we demonstrate a likely cellular (ion channel) and molecular mechanism by which oxidative injury creates a vulnerable substrate for AF. The results of this study yield valuable mechanistic insights into the pathogenesis of AF and have important therapeutic implications for the clinical management of this common arrhythmia.
Materials and Methods
Please see the Online Supplemental Material for detailed materials and methods. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study design.
The objective of this study is to determine the precise role of oxidative injury in causing electrical remodeling in the intact, fibrillating atrium. Our pre-specified hypotheses were: 1) Upregulation of IKH – an ion channel thought to be an important contributor to electrical remodeling in AF – is mediated by a frequency-dependent increase in oxidative injury in the setting of AF, with resulting activation of PKCε; and 2) oxidative injury leads to not only the initiation but also the maintenance of ERP shortening in the intact, fibrillating atrium.
This was an experimental study that was performed in large animals, specifically dogs and one pig. Animals used in this study were maintained in accordance to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85–23, revised 1996) as approved by the IACUC of the Northwestern University.
A total of 35 dogs were used for this study. Dogs used were either normal controls (n=10) or were subjected to RAP (n=25). In control animals, the following types of experiments were performed: i) tissue analysis (n=5), ii) atrial myocyte isolation (n=8). Atrial myocytes isolated from normal dogs were subjected to in vitro tachypacing or to single cell electrophysiological analysis. Participation of PKCε in the induction of IKH by oxidative injury was assessed in in vitro tachypaced atrial myocytes.
Dogs were subjected to rapid atrial pacing (RAP) for a period of 3–12 weeks. A subset of dogs undergoing RAP also underwent gene injection. The RAP dogs were divided into three groups: Group 1 - No gene injection (n=15); Group 2 - NOX2 shRNA injection (n=7); and Group 3 - Scrambled shRNA injection (n=3). After pacemaker implantation, all RAP dogs underwent periodic pacemaker interrogations to assess for duration of induced AF. In dogs that underwent gene injection, NOX2 shRNA or scramble shRNA was injected sub-epicardially in canine atria prior to initiation of RAP. NOX2 shRNA was used to inhibit NOX2, a major enzymatic source of oxidative injury in the AF atrium. One week after gene injection, RAP was initiated. At the time of terminal surgery, each group of RAP dogs underwent one or more of the following procedures (the procedures are not mutually exclusive): Group 1: Electrophysiological testing (includes ERP analysis, AF recordings) (n=11); Tissue analysis (n=8); Atrial myocyte isolation (n=8).
Group 2: Electrophysiological testing (includes ERP analysis, AF recordings) (n=5); Tissue analysis (n=7); Atrial myocyte isolation (n=2). Group 3: Electrophysiological testing (includes ERP analysis, AF recordings) (n=2); Tissue analysis (n=3); Atrial myocyte isolation (n=1).
Atrial myocytes isolated from RAP dogs were subjected to single cell electrophysiological analysis. Sensitivity of specific ion channels to ROS inhibition was determined by whole cell patch clamp experiments in control and RAP atrial myocytes, performed in the presence of different ROS inhibitors. Lastly, atrial myocytes were isolated from one pig and two normal dogs and subjected to increasing frequency of in-vitro tachypacing, to assess for ROS generation.
Statistical Analysis
All data is presented as mean ± SEM.
For each ion current, current amplitude was compared at each voltage step by unpaired t-tests.
To assess differences in ERP between different groups of animals, individual ERPs obtained in each animal were combined by region and group, to determine a statistically significant difference in means between treatment groups and between atrial regions by using two-way ANOVA with Holm-Sidak method for multiple testing correction.
Time to sustained AF between the control and active gene groups was compared by log-rank tests on interval censored data. Time to AF was compared for both short duration AF (>30 minutes) and long duration AF (>8 hours). Due to censoring of observations, the median time in AF per occasion per dog was calculated. Difference between groups was determined by a Wilcoxon rank sum test.
AF characteristics were compared between treatment groups and between atrial regions (PLA, LAA) by using two-way ANOVA with Holm-Sidak method for multiple testing correction.
Mean fluorescence in isolated atrial myocytes was compared using one way ANOVA. Other cellular and tissue parameters (O2- levels, density of protein bands on western blot, mRNA levels by PCR, 8-hydroxy-2-deoxyguanosine (8-OHdG) stained nuclei on immunohistochemistry) were compared between treatment groups by unpaired t-tests.
Gene and protein expression were compared by unpaired t-tests or ANOVA with Holm-Sidak method for multiple testing correction when more than two groups were examined.
The values were considered significantly different at p < 0.05.
Results
ROS generation in atrial myocytes is a frequency-dependent phenomenon
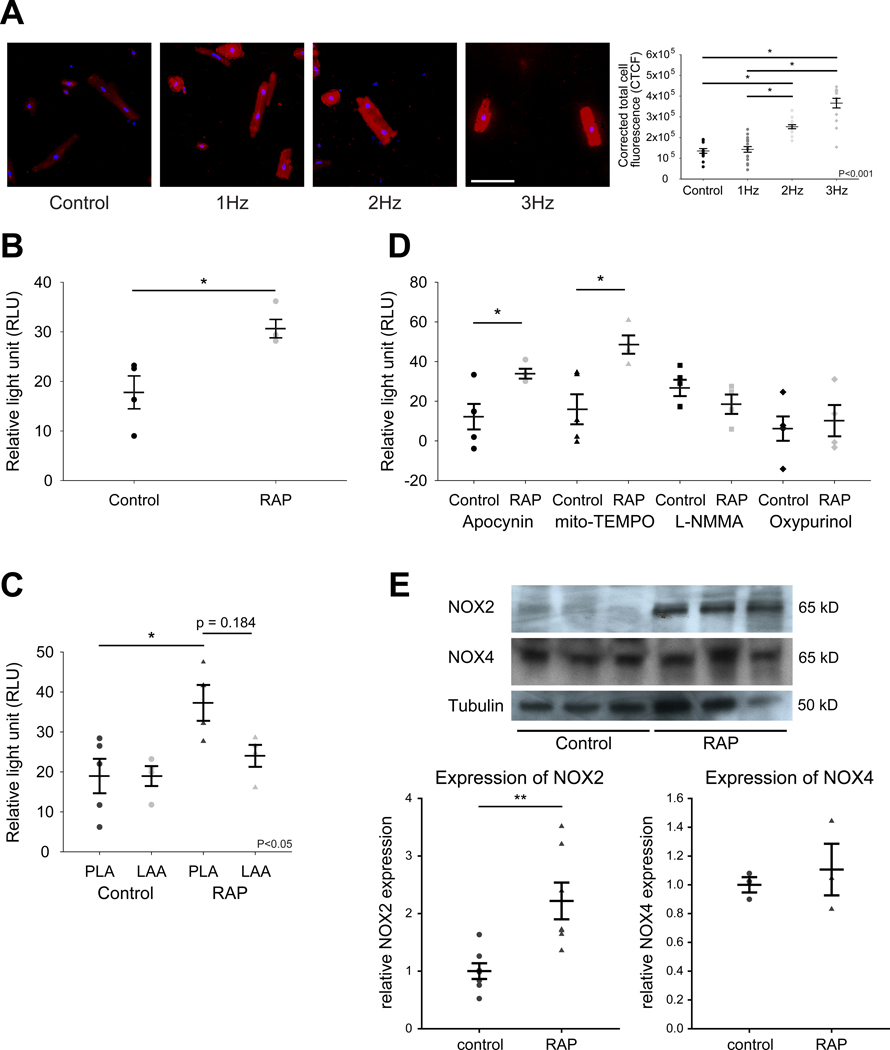
Electrical remodeling in the AF atrium is largely thought to be the result of rapid atrial rates, with rapid stimulation leading to ion channel remodeling. Frequency-dependent IKH upregulation was recently demonstrated in atrial myocytes. The upstream signalling mechanisms underlying this ion channel remodeling are not well understood. We hypothesized that ROS generation in atrial myocytes is a frequency-dependent phenomenon, with increasing stimulation frequency leading to progressively increasing ROS. We therefore investigated ROS generation in isolated, paced canine atrial myocytes in response to increasing pacing frequency. Total cellular fluorescence from a ROS sensitive indicator, CellROX Deep Red, increased in a frequency dependent manner from control (unpaced) to 3 Hz (Figure 1A). In addition, we obtained similar results from isolated, paced swine myocytes (Figure IA in the Supplement). We verified the validity of our experimental system using copper [II] diisopropyl salicylate (CuDIPS) which is a superoxide dismutase 1 (SOD1) mimetic. Incubation of 3 μM CuDIPS for 10 hrs attenuated CellROX Deep Red signal in paced myocytes (Figure IB in the Supplement). Taken together, these results support our postulate that ROS generation in atrial myocytes is a frequency-dependent phenomenon.
Figure 1. Frequency-dependent ROS generation in canine atrial myocytes and RAP left atrium.
(A) ROS imaging in tachypaced atrial myocytes at 0 Hz (n=13), 1 Hz (n=18), 2 Hz (n=17) and 3 Hz (n=14). Red, CellROX red. Blue, DAPI (nucleus). Scale bar, 100 μm. (B) O2- generation in control (n=4) and RAP (n=4) left atrium. (C) Superoxide generation in PLA versus LAA, in RAP (n=4) and control (n=4) atria. (D) Relative contribution of different enzymatic sources of ROS to O2- generation in control (n=4) and RAP (n=4) PLA. (E) Immunoblot and densitometric measurements of NOX2 and NOX4 (normalized to tubulin) from control and RAP atria. N=7 for NOX2, n=3 for NOX4. Uncropped NOX2 and NOX4 immunoblots are shown in Figure XII in the Supplement. Data are presented as mean ± SEM; * p < 0.05 and ** p < 0.01. One or two way ANOVA significance indicated in graphs.
NOX2 and mitochondria-generated superoxide (O2-) significantly increases in rapidly paced left atrium
Next, we looked for evidence of oxidative injury secondary to RAP in the intact, fibrillating atrium. Lucigenin chemiluminescence assay on left atrial tissue homogenates from RAP dogs revealed a significant increase in overall O2- generation as compared to control (Figure 1B). While O2- generation increased in both the posterior left atrium (PLA) and left atrial appendage (LAA) in RAP atria, the increase was significant only in the PLA (Figure 1C). Next, we determined relative contributions to O2- generation by various enzymatic sources of ROS by the application of ROS inhibitors. There was higher activity of mitochondrial ROS and NADPH oxidase (NOX2) in RAP PLA, compared to control PLA (Figure 1D). As the specificity of apocynin for NOX2 has been questioned by some studies,18 we carried out immunoblot analysis to assess the level of NOX2. Consistent with the putative increase in activity of NOX2 in RAP PLA, expression of the gp91 subunit of NOX2 was also significantly greater in RAP as compared to control. Though NOX4 is another major cardiac NOX isoform that was recently found to be upregulated in patients with AF,19 we did not find an increase in NOX4 protein in RAP atria (Figure 1E).
IKH is highly sensitive to inhibition of mitochondrial ROS and NOX2
Since ROS are elevated in the rapidly paced atrium, and since IKH - which is thought to contribute to ERP shortening in AF – is stimulated by the acute phase reactant PKCε, we hypothesized that IKH induction in RAP is modulated by ROS. We therefore investigated the effect of various inhibitors of mitochondrial ROS and NOX2 (the two sources of ROS found to be elevated in the rapidly paced atrium) on the inward rectifying potassium currents IK1, IKACh and IKH. As explained in the Methods, in the absence of agonist (CCh), IKH is defined as tertiapin-Q (TQ) sensitive current; IK1 is the residual current after IKH blockade with TQ. IKACh refers to CCh-activated current.
Mitochondrial ROS inhibition
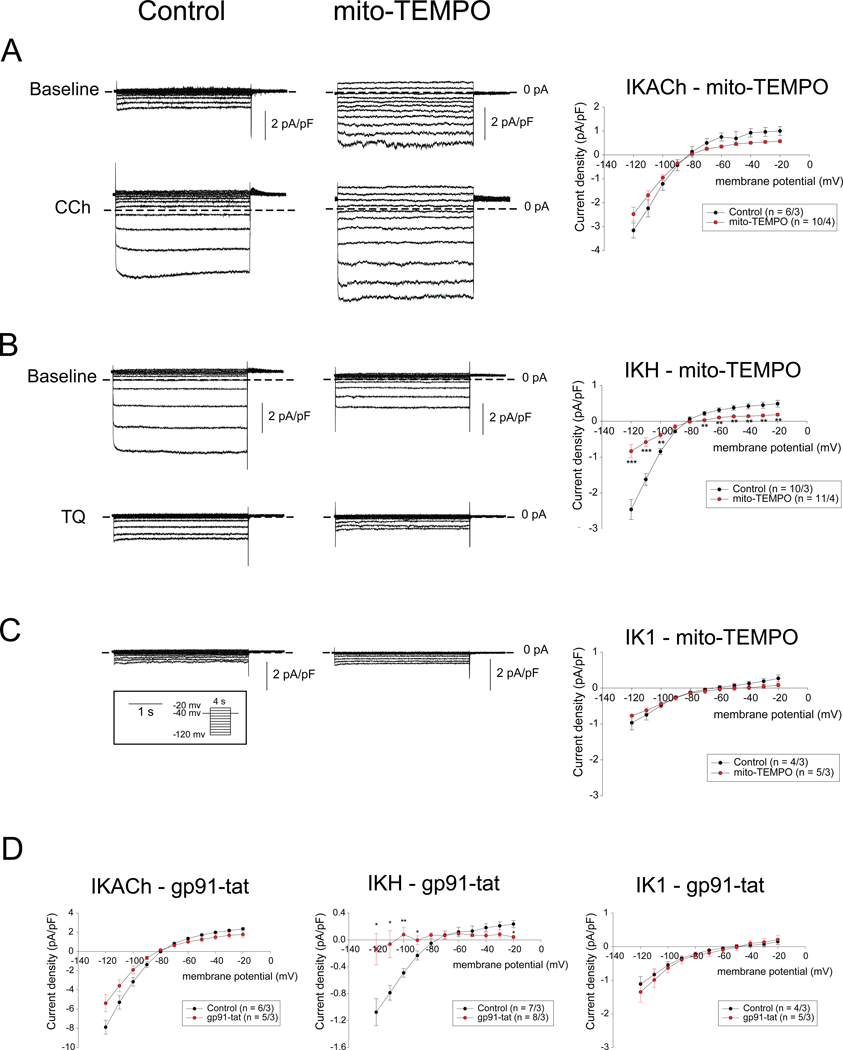
We first examined the effect of mitochondrial ROS inhibition in RAP myocytes using mito-TEMPO on the inward rectifying potassium currents IK1, IKACh and IKH. Figure 2A–C shows currents elicited by 4 second step pulses from a holding potential of −40 mV to test potentials between −120 mV and −20 mV in control and mito-TEMPO treated left atrial myocytes. Representative current traces (left and middle panels) and mean current-voltage relations (I-V curve, right panel) demonstrate that whereas there is no significant difference in IKACh or IK1 between control and mito-TEMPO pre-incubated RAP myocytes, IKH was significantly attenuated by mito-TEMPO.
Figure 2. Effect of mito-TEMPO and gp91-tat on inwardly rectifying currents in RAP atrial myocytes.
(A - C) Raw traces (left and middle panels) of IKACh (A), IKH (B) and IK1 (C) elicited by 4 seconds step pulses from a holding potential of −40 mV to voltage between −120 mV and – 20 mV(pulse protocol shown in inset) and I-V curve (right panels) for control and mito-TEMPO pre-incubated RAP atrial myocytes. (D) I-V curve of IKACh (left), IKH (middle) and IK1 (right) for control and in the presence of gp91-tat elicited by 400 ms ramp pulses from a holding potential of −40 mV to voltage between 20 mV and −120 mV. Number of cells/animals is given in each Figure panel. Data in I-V plots are presented as mean ± SEM at given membrane potentials; * p < 0.05, ** p < 0.01 and *** p < 0.001.
Next, since ICaL is known to be downregulated in AF atria and is thought to play an important role in ERP shortening in AF,1 we also examined the effect of mitochondrial ROS inhibition on ICaL. Consistent with the literature, our own data demonstrated a significant reduction of ICaL in RAP myocytes, compared to normal atrial myocytes (Figure IIA in the Supplement). Pre-incubation with mito-TEMPO did not cause any significant change in ICaL in RAP myocytes.
Taken together, these results indicate that of the major ion channels contributing to ERP shortening in the rapidly paced atrium, only IKH was sensitive to mitochondrial ROS inhibition.
NOX2 inhibition
Next, we examined the effect of NOX2 inhibition on IKACh, IKH and IK1 in isolated RAP myocytes. We examined currents elicited by 400 millisecond (ms) ramp pulses from a holding potential of −40 mV to voltage between 10 mV and −120 mV in control condition and in the presence of 50 μM of gp91-tat, which is a specific NOX2 inhibitory peptide, in the pipette solution in right atrial myocytes. The mean I-V curve (left panel) demonstrates that there is no significant difference in IKACh between control and gp91-tat-treated RAP myocytes (Figure 2D). Of note, the average amplitude of IKACh in right atrial myocytes was noted to be 2-fold larger than in left atrial myocytes (Figure 2A), consistent with prior studies.20
We next examined the effect of gp91-tat on IKH. Figure 2D (middle panel) shows the mean I-V curve for IKH elicited by same ramp pulse protocol as IKACh in right atrial myocytes. These results demonstrate that IKH was significantly attenuated in the presence of gp91-tat in the pipette solution compared to control conditions. There was no significant difference in IK1 in the presence of gp91-tat compared to control condition in right atrial myocytes (see figure 2D, right panel).
Taken together, IKH was the only inwardly rectifying K+ current significantly affected by NOX2 inhibition. In view of NOX2 being a major enzymatic source of ROS in the rapidly paced atria (Figure 1D), the clear attenuation of IKH in the presence of gp91-tat supports a likely role for NOX2 in the emergence of IKH in RAP myocytes.
ROS-induced increase in IKH during RAP is mediated by enhanced PKCε signalling
Previous studies have demonstrated that enhanced PKC signalling may be contributing to the emergence of IKH in RAP myocytes as well as in myocytes from patients with AF.13, 15 Furthermore, Makary et al. showed that membrane translocation of the stimulatory PKC isoform PKCε may be essential to the emergence of IKH in canine RAP myocytes.15 Since PKC signalling and the isoform PKCε have been shown to be acute phase reactants,16, 17 we hypothesized that ROS-mediated emergence of IKH in RAP myocytes is at least partially mediated by an increase in PKC – and specifically, PKCε - signalling.
We therefore first examined the effect of bisindolylmaleimide-1 (BIM1) – a non-specific PKC inhibitor – on IKH in the absence and presence of mito-TEMPO. As anticipated, IKH was attenuated by the application of 2 μM BIM1, as compared to control (Figure IIB in the Supplement, top; control and middle; BIM1). Since BIM-1 is a non-specific PKC inhibitor, we also examined the effect of a specific PKCε inhibitory peptide on IKH; the peptide also attenuated IKH (Figure IIB in the Supplement, bottom; PKCε inhibitory peptide). In mito-TEMPO pre-incubated myocytes, which exhibited around 70% attenuation of IKH (Figure 2B), BIM1 had no additional effect on IKH (Figure IIC in the Supplement). These results indicate that BIM1 effect on IKH may be at least partially mediated by ROS. Similarly, the specific PKCε inhibitory peptide was found to have no synergistic effect on mito-TEMPO-induced attenuation of IKH in RAP myocytes (Figure IID in the Supplement), suggesting that the effect of ROS on IKH is at least partially mediated via PKCε.
We further hypothesized ROS is upstream of PKCε signalling in the rapidly-paced atrium. We therefore assessed membrane translocation of PKCε in isolated atrial myocytes from normal dogs subjected to in-vitro tachypacing, in the absence and presence of the non-specific ROS inhibitor, N-acetylcysteine (NAC) as well as a specific NOX2 inhibitor, gp91-tat. Consistent with the previous report by Makary et al.,15 we first observed that pacing of isolated atrial myocytes at 3 Hz compared to 1 Hz led to increased membrane translocation of PKCε (Figure IIIA and B in the Supplement). However, in the presence of 10 mM NAC, the membrane translocation of PKCε induced by 3Hz tachypacing was significantly attenuated (Figure IIIA and C in the Supplement). Similar to NAC, incubation of gp91 also attenuated the membrane translocation of PKCε (Figure IIID in the Supplement). Taken together, these data indicate that the ROS-induced increase in IKH in the rapidly paced atrium is at least partially mediated by increased PKCε signalling.
AF inducibility and duration is markedly reduced after NOX2 shRNA treatment
Since NOX2 appears to be a major contributor to ROS generation in rapidly-paced atria, and since NOX2 inhibition was noted to attenuate IKH in RAP myocytes, we hypothesized that targeted inhibition of NOX2 in the atria would prevent RAP-induced ERP shortening and consequent AF. We elected to selectively target NOX2 in the atrium by using NOX2 shRNA. We generated a shRNA to canine NOX2 (see Figure IVA in the Supplement for target sequence). Using this shRNA, we were able to achieve significant knockdown of NOX2 in HEK 293 cells (Figure IVB in the Supplement).
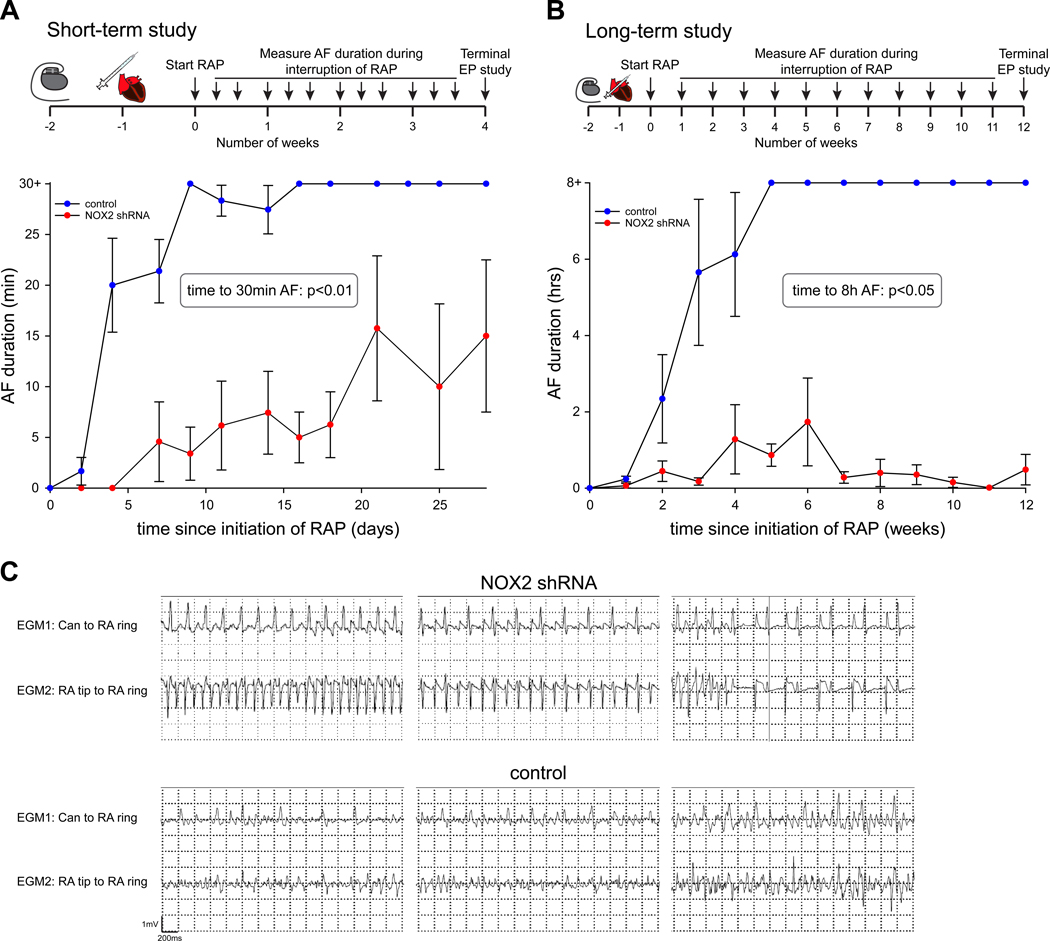
Seven dogs underwent sub-epicardial injection of NOX2 shRNA in the atria, followed by electroporation to facilitate myocardial gene transfer. The gene injection and electroporation procedure was limited to the PLA in the first 3 animals, with subsequent 4 animals receiving gene injection in the left atrial free wall (LAFW), LAA, and right atrium as well. Eighteen animals receiving either injection of scrambled shRNA or no gene injection were used as control. Figure 3A and 3B show detail of the experimental design for assessment of AF both in the short term (i.e. 4 weeks of RAP) and in the long term (i.e. 12 weeks of RAP). After gene injection, animals were subjected to RAP and duration of induced AF was subsequently recorded during periods in which RAP was interrupted. Figure 3A shows the duration of AF after initiation of RAP: whereas control animals developed sustained AF for more than 30 minutes within a median of 4 days of RAP (interquartile range (IQR) 4–9 days), it took a median of 21 days for NOX2 shRNA animals to develop this burden of AF (p<0.01). Three animals in each group were followed for twelve weeks to assess development of persistent AF (defined as AF duration longer than 8 hours; also see Methods). Figure 3B shows that it took a median of 14 days for control animals to develop >8 hours of AF. In contrast, it took animals receiving NOX2 shRNA a median of 28 days to develop AF>8 hours (p<0.05). Over the entire recorded period, control animals spent a median of 60 minutes in AF (IQR 30–60 minutes), whereas NOX2 shRNA animals spent a median of 0 minutes in AF (IQR 0–2 minutes) (p = 0.003). Representative examples of intra-cardiac electrograms are shown in Figure 3C. The dominant rhythm in NOX2 shRNA animals was either sinus rhythm (top right) or atrial flutter (top left and top middle). In distinct contrast, AF was the dominant rhythm in all control animals (bottom panels). Importantly, there was no evidence of loss of capture of tachypacing over the entire studied period (Figure V in the Supplement).
Figure 3. NOX2 shRNA prevents development of sustained AF.
(A) For our short-term study, animals who received NOX2 shRNA developed significantly shorter AF, with a delay in development of sustained AF > 30 minutes. N = 3–12 for controls, n = 3–5 for NOX2 shRNA. (B) For our long-term study, NOX2 shRNA gene injection prevented development of sustained AF > 8 hours. For A and B, n = 3–12 for controls, n = 3–7 for NOX2 shRNA. Data are mean ± SEM. Significance by log-rank test indicated in graph. (C) Representative examples of intracardiac EGMs are shown for NOX2 shRNA animals (top) and control (bottom). Corresponding Can to RA ring and RA tip to RA ring EGMs are shown, with evidence of atrial flutter or sinus rhythm for NOX2 shRNA, and AF for control.
Left atrial ERPs are prolonged after NOX2 shRNA treatment
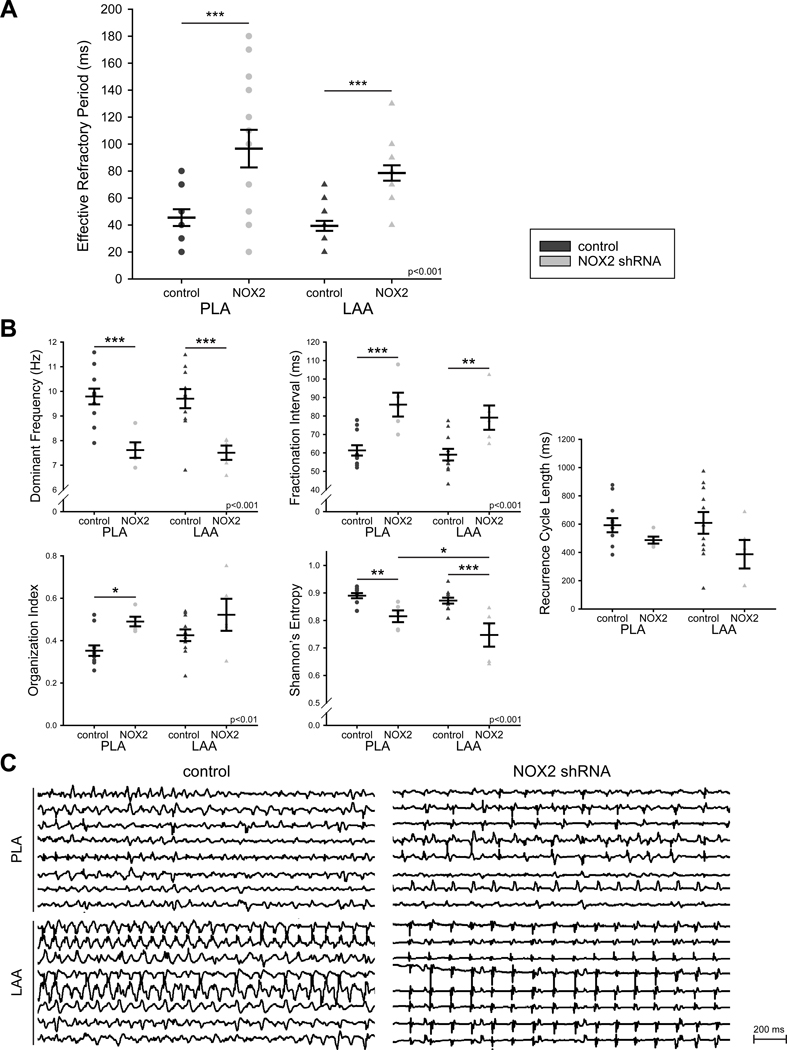
At the terminal study, atrial ERPs were measured in the PLA and LAA in animals that were in sinus rhythm or in which sinus rhythm was restored by burst pacing or electrical cardioversion. As shown in Figure 4A, combined ERPs were significantly longer in NOX2 shRNA-injected dogs as compared to controls. This ERP lengthening was noted in the PLA as well as the LAA.
Figure 4. NOX2 shRNA prevents electrical remodeling induced by RAP.
(A) ERPs were measured in the PLA and LAA of 6 control and 4 NOX2 shRNA animals after RAP. Results are shown as mean ± SEM of combined ERPs. (B). AF electrograms are slower and more organized after NOX2 shRNA gene injection (n=5) compared to control (n=11) with higher dominant frequency, longer fractionation interval, higher organization index and lower Shannon’s Entropy. Recurrence cycle length did not change significantly between groups. Data are mean ± SEM; * p<0.05, ** p<0.01, ***p<0.001. Two way ANOVA significance indicated in graphs. (C) Representative AF electrograms of control (left) and NOX2 shRNA animals (right) in the PLA (top) and LAA (bottom).
Residual AF after NOX2 shRNA treatment is slower, more organized and less complex than in controls
Periods of AF were recorded during the terminal study, either when the animals were spontaneously in AF, or after AF induction with burst pacing. AF in NOX2 shRNA animals showed a decrease in dominant frequency (DF, frequency domain measure of activation rate), an increase in fractionation interval (FI, mean interval between deflections detected in the electrogram segment), an increase in organization index (OI, frequency domain measure of temporal organization or regularity), and a decrease in Shannon’s entropy (ShEn, statistical measure of complexity) when compared to controls (Figure 4B and 4C). Recurrence cycle length (CLR) did not change significantly between groups. Taken together, these data demonstrate the limited AF that could be induced in NOX2 shRNA animals was significantly slower and more organized than the AF noted in control animals.
NOX2 shRNA treatment does not prevent RAP induced changes in ventricular function
A transthoracic echocardiogram was performed at baseline and prior to the terminal study in 5 control animals and 3 NOX2 shRNA animals. Table I in the Supplement details our findings. There was no significant difference in any echocardiographic parameters between groups at baseline. RAP caused a reduction in left ventricular ejection fraction (LVEF) and left ventricular (LV) global longitudinal strain in both groups, without significant difference between animals having received NOX2 shRNA and controls. Right ventricular (RV) systolic function also appeared significantly reduced in control animals as determined by tricuspid annular plane systolic excursion (TAPSE) by M mode and RV s’ velocity, with a similar trend in NOX2 shRNA animals. Importantly, there was no difference in left atrium size and left atrium reservoir strain between baseline and after RAP, and between groups.
NOX2 is attenuated in NOX2 shRNA injected atria
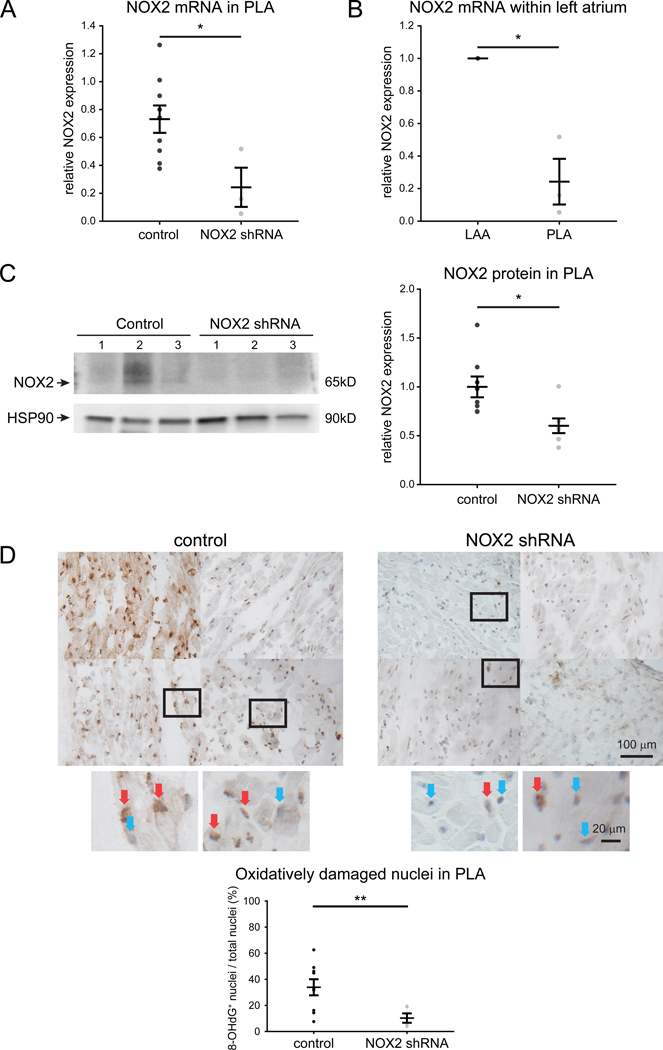
After the terminal electrophysiological study, atrial tissue was removed to measure gene expression, oxidative injury, fibrosis and effect of gene on signaling. As shown in Figure 5A, animals that underwent NOX2 shRNA injection demonstrated >50% decrease in native NOX2 expression in gene-injected PLA when compared to the same region in control animals and also when compared to a non-injected region (i.e. LAA), as shown in Figure 5B. This decrease in NOX2 mRNA was accompanied by a decrease in NOX2 protein levels in the left atrium (Figure 5C). The level of NOX2 was persistently decreased throughout the study period (Figure VI in the Supplement), indicating continued expression of NOX2 shRNA.
Figure 5. NOX2 shRNA attenuates NOX2 levels and attenuates DNA oxidative damage in RAP atrium.
(A) NOX2 qPCR in animals injected in the PLA alone (n=3) shows effective gene suppression when compared to controls PLA (n=9) and (B) when compared to an uninjected LAA (n=3). All samples were normalized to respective LAA. (C) Protein expression of NOX2 is attenuated by injection of NOX2 shRNA (n=8 for control and 7 for NOX2 shRNA). Uncropped NOX2 immunoblots are shown in Figure XII in the Supplement. (D) 8-OHdG stained tissue sections in control RAP PLA (n=9) and NOX2 shRNA transfected PLA (n=5). 8-OHdG positive nuclei were stained in brown and indicate oxidatively damaged nuclei. Blue stained nuclei indicate undamaged nuclei. Inset; undamaged and oxidatively damaged nuclei are designated by blue and red arrows, respectively. Ratio of 8-OHdG positive nuclei against total number of nuclei is shown on the left. Scale bars, 100 μm and 20 μm. Data are presented as mean ± SEM; * p< 0.05 and ** p<0.01.
DNA oxidative damage is attenuated by NOX2 shRNA
To determine whether the decrease in native NOX2 by NOX2 shRNA was accompanied by a decrease in oxidative injury in the atrium, we examined the levels of 8-OHdG - a biomarker of oxidative damage of DNA – in gene-transfected PLA. Figure 5D demonstrates significant attenuation in the percentage of oxidatively damaged nuclei (i.e. 8-OHdG stained nuclei) in NOX2 shRNA compared to control dogs.
To determine whether the effects of NOX2 shRNA were spatially homogeneous in the injected atria, we assessed spatial distribution of oxidatively damaged nuclei. As shown in Figure VII in the Supplement, 4 random low magnification images of 8-OHdG staining from NOX2 shRNA injected tissue sections from 3 different animals showed quite uniform distribution of oxidatively damaged nuclei.
Fibrosis and inflammasome are not affected by NOX2 shRNA
Excessive generation of ROS is likely involved not only in electrical remodeling but also in structural remodeling of the heart, with induction of fibrosis.19 Furthermore, recent studies demonstrate an increase in inflammation in the AF atrium.21 A recent study demonstrated that NLRP3 (NOD-like receptor family, pyrin domain containing 3) inflammasome activation – which can be activated by ROS - may be playing a role in the creation of a vulnerable AF substrate in patients and in animal models.6 We therefore examined whether NOX2 shRNA attenuates the generation of fibrosis and inflammasome activation in the RAP atrium.
Similar to previous studies that showed tachycardia-induced atrial fibrosis,22 we discovered a significant increase in fibrosis after 12 weeks of RAP as compared to 4 weeks of RAP or normal atria (Figure VIII in the Supplement). Interestingly, there was no significant change in fibrosis in NOX2 shRNA injected atria when compared to atria injected with scrambled gene (Figure VIII in the Supplement). These results indicate that whereas fibrosis is induced in persistent AF, it is not affected by NOX2 shRNA injection in the RAP model for AF.
Inflammasomes are oligomeric protein signaling complexes that consist of an upstream sensor protein of the NLR family, an adapter protein ASC (Apoptosis-associated Speck-like Protein Containing a Caspase Recruitment Domain), and caspase I. We examined expression of inflammasome-related genes in NOX2 shRNA-injected atria. Figure IX in the Supplement shows an increase in expression of NLRP3 and caspase I in RAP atria compared to normal atria. However, there was no significant difference between RAP control and NOX2 shRNA-injected atria. Similar to fibrosis, these results indicate that the inflammasome is not affected by NOX2 shRNA injection in RAP.
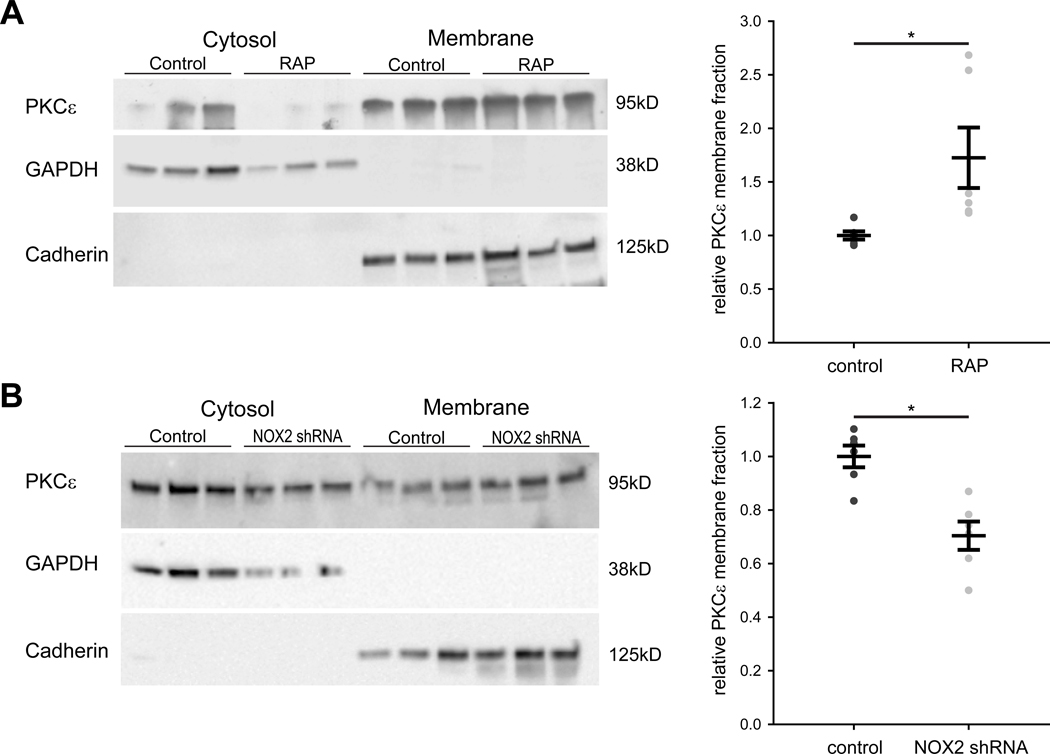
RAP-induced membrane translocation of PKCε is attenuated by NOX2 shRNA treatment
As had been done in isolated atrial myocytes, we also determined PKCε expression in separated cytosolic and membrane protein fractions of left atrial tissue from animals subjected to RAP. Similar to a previous report,15 we observed that RAP increased the relative membrane expression of PKCε as compared to controls (Figure 6A), consistent with the in-vitro tachypacing-induced translocation of PKCε from the cytosol noted earlier (Figure III in the Supplement). We also examined the effect of RAP on the level of PKCα which has been shown to decrease in atria after 1 week of RAP.15 However, we did not discover a significant change in the level of PKCα in our prolonged RAP model (Figure X in the Supplement).
Figure 6. RAP causes membrane translocation of PKCε, which is inhibited after NOX2 shRNA gene injection.
(A) Representative western blots of atrial tissue from control and RAP animals for PKCε, GAPDH as cytosol loading control, and Cadherin as membrane loading control are shown (left panel). Mean ± SEM of relative membrane fraction of PKCε in control and RAP animals (right panel). N=6 for both groups. (B) Representative western blots of atrial tissue from control and NOX2 shRNA animals after RAP for PKCε, GAPDH as cytosol loading control, and Cadherin as membrane loading control are shown (left panel). Mean ± SEM of relative membrane fraction of PKCε in control and NOX2 shRNA animals (right panel). N=6 for both groups. * p<0.05.
Due to the increase in membrane translocation of PKCε noted in RAP atria, we also assessed the effect of NOX2 shRNA on membrane translocation of PKCε in intact atrium. When compared to control animals, relative membrane expression of PKCε was reduced in dogs receiving NOX2 shRNA (Figure 6B). This demonstrates that NOX2 shRNA attenuates RAP-dependent translocation of PKCε from the cytosol to the membrane.
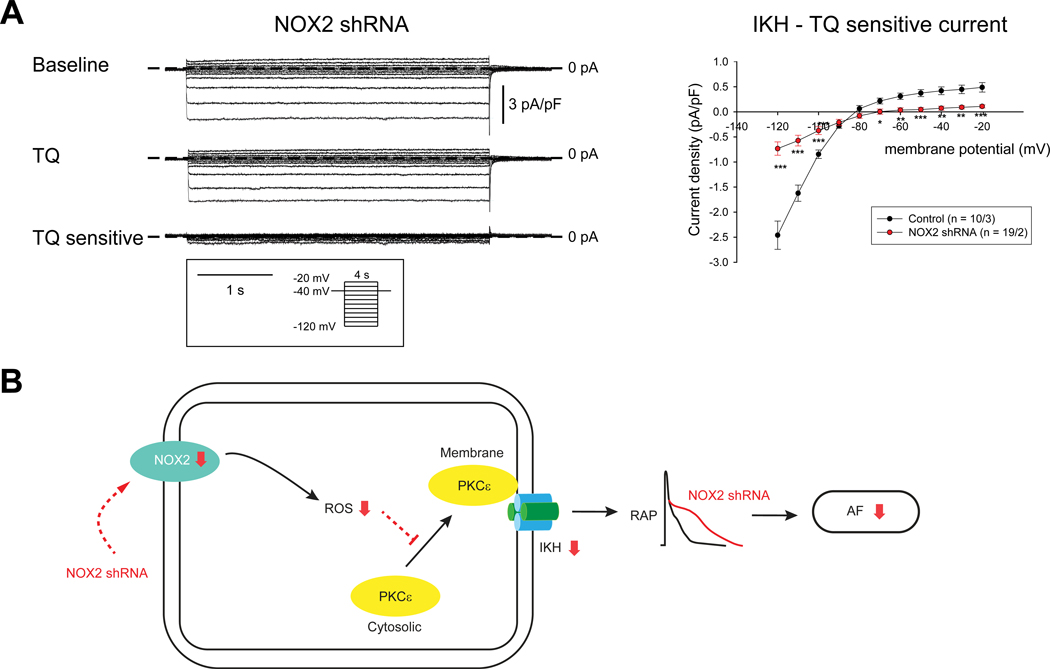
Attenuation of IKH in isolated atrial myocytes from NOX2 shRNA injected dogs
We then measured IKH in isolated atrial myocytes from NOX2 shRNA injected dogs in order to determine whether the decrease in NOX2 expression and attenuation of PKCε translocation is responsible for the emergence of IKH as well as ERP shortening in RAP. Figure 7A shows representative recordings (left panels) and mean I-V curve (right panels) for the currents by step-pulse protocol from isolated myocytes of NOX2 shRNA injected animals. IKH was significantly attenuated in NOX2 shRNA myocytes, compared to control myocytes. These data support our original hypothesis that the emergence of IKH in RAP is mediated by oxidative injury.
Figure 7. NOX2 shRNA attenuates IKH.
(A) Measurements of IKH in NOX2 shRNA injected dogs. Data in I-V plots are presented as mean ± SEM at given membrane potentials; Number of cells/animals is given in the Figure. (B) Schematic illustration of potential mechanisms by which NOX2 shRNA transfection prevents electrical remodeling in AF. * p < 0.05, ** p < 0.01 and *** p < 0.001.
Expression of ion channels involved in ERP shortening during RAP
We evaluated left atrial mRNA expression of ion channels thought to underlie ERP shortening in AF, in animals that received NOX2 shRNA and controls. Figure XI in the Supplement summarizes our qPCR results. Expression of KCNJ2, KCNJ3, and KCNJ5 did not differ significantly in the left atrium of NOX2 shRNA animals when compared to controls. Overall ANOVA was significant when evaluating KCNJ12 expression, but pairwise comparisons between conditions or left atrial regions did not reach significance. CACNA1C was significantly higher in the control PLA when compared to control LAA or LAFW but there was no significant difference between NOX2 shRNA animals and controls in any region. Taken together, NOX2 shRNA injection did not lead to significant changes in mRNA expression of ion channels thought to contribute to atrial ERP shortening in RAP. The lack of change in expression of KCNJ3 and KCNJ5 (which encode Kir3.1 and Kir3.4 respectively) further supports our postulate that attenuation of IKH in NOX2 shRNA atria is likely mediated by a decrease in membrane translocation of PKCε.
Discussion
In this study, we examined the role of oxidative injury in causing electrical remodeling in a RAP model of AF. We demonstrated that ROS generation in the atrium is a frequency-dependent phenomenon, with continued RAP leading to preferential elevation of mitochondrial and NOX2-generated ROS in the fibrillating atrium. Of the major ion channels invoked in causing ERP shortening in the RAP model (and in human AF), we discovered that the PKCε-regulated, constitutively active IKAch channel, IKH, is uniquely sensitive to inhibition of mitochondrial ROS and NOX2. To determine whether oxidative injury is involved in the initiation and/or maintenance of RAP-induced ERP shortening in the intact atrium – and resulting AF - we then performed targeted inhibition of ROS in the atria in an AF prevention model with a novel, gene-based approach. Targeted atrial expression of NOX2 shRNA led to a 113% increase in ERP and inability to induce persistent AF despite up to 12 weeks of continued RAP. Furthermore, NOX2 shRNA attenuated PKCε membrane translocation as well as RAP-induced upregulation of IKH. Importantly, while NOX2 shRNA prevented electrical remodeling in the RAP atrium, it did not affect structural remodeling (fibrosis) or the activation of the NLRP3 inflammasome. Figure 7B shows our proposed model of the mechanism by which NOX2 shRNA is attenuating electrical remodeling and consequent AF in the intact atrium.
Likely sources of ROS that contribute to creation of a vulnerable AF substrate
ROS are unstable, reactive oxygen derivatives playing significant roles in cardiac physiology as crucial second messengers for growth and gene regulation.17 However, excessive ROS can elicit pathologic cellular responses and lead to a number of cardiac diseases.17
Cellular, animal and clinical studies have shown high levels of ROS in fibrillating atria, and suggested a role for oxidative injury in the pathophysiology of AF with stimulation of ROS-sensitive kinases and phosphatases leading to electrical remodeling, as well as inflammation and fibrosis.19 However, the precise mechanisms leading to electrophysiological remodeling in AF and the specific enzymatic source(s) of oxidative injury involved have not been established.10, 19, 23 Whereas prior studies have demonstrated a role for ROS in modulation of several ion channels and EC coupling proteins, the data for ROS modulation of ion channels in the context of AF is limited.12 Finally, prior studies supporting a role for oxidative injury in the development or persistence of AF have been limited to the study of explanted atria or isolated cardiomyocytes. ROS generated in the cardiovascular system are primarily derived from NADPH oxidases (NOX), the mitochondrial electrical transport chain, xanthine oxidases and uncoupled eNOS.8 Among these, NOX have emerged as a major source for ROS in cardiovascular diseases. Mice with cardiac-specific overexpression of Rac-1, a necessary activator for NOX2, develop AF spontaneously.24 In humans, atrial NOX activity was independently associated with risk of developing AF after cardiac surgery.5, 9 In this study, we confirmed a role for NOX2 in the initiation and maintenance of AF in the canine RAP model: attenuating NOX2 with an shRNA-based gene therapy approach not only delayed the onset of AF, but also prevented development of sustained AF. This effect appeared to be largely mediated by NOX2 shRNA preventing RAP-induced ERP shortening. This suggests a role for NOX2 not only in post-operative AF but also as a continuous trigger involved in the maintenance of persistent AF.
A further contribution of other ROS sources (mitochondrial, xanthine oxidases, uncoupled eNOS) to AF has also been hypothesized. Reilly et al. showed that in addition to NOX2, uncoupled NOS and mitochondrial ROS were important sources of oxidative injury in human AF.10 Indeed, our study also demonstrates a significant elevation of mitochondrial ROS in the rapidly paced atrium. Whether inhibition of mitochondrial ROS leads to beneficial effects on electrical remodeling similar to those noted in the current study with targeted inhibition of NOX2 remains to be determined.
Ion channel(s) likely mediating ROS-induced ERP shortening in AF
As in human AF, ERP or action potential duration shortening is a major determinant of AF in the canine RAP model.25 ICaL is downregulated in persistent AF,1 an effect that we also demonstrated in this study (Figure IIA in the Supplement). Downregulation of ICaL α-subunit mRNA appears to be the main contributor to this effect, but posttranscriptional mechanisms such as protein dephosphorylation and breakdown may also be involved.1 Carnes et al. demonstrated higher nitrosylation of the α1c subunit of L-type Ca2+ channels in the LAA of patients with long-standing AF.26 Nitrosylation appeared inversely related to cellular glutathione content, and superfusion of NAC resulted in an increase in ICaL. Notably, ICaL did not appear to be sensitive to ROS inhibition in our model. Potential explanations include species-related differences (canine versus human) or region of origin of atrial myocytes (LAA myocytes were studied by Carnes et al., whereas the current study examined PLA myocytes).
An increase in the inward rectifier current IK1 leading to a more negative resting potential is also described in AF.27 Redox-dependent regulation of IK1 has been reported, with S-nitrosylation of the Cys76 residue of Kir2.1 increasing the channel open probability in isolated atrial myocytes from patients in sinus rhythm.28 In our study, IK1 did not appear to be sensitive to ROS inhibition.
Among voltage gated K+ currents, the transient outward K+ current (Ito) is consistently decreased in RAP due to reduction of Kv4.3 expression.25 Studies on ultra-rapid delayed rectifier K+ current (IKur) point towards attenuation of this current in AF, though with some conflicting results.29 IKur was shown to be ROS-sensitive, with both S-nitrosylation and sulphenylation leading to reduced current density.30 Overall, oxidation dependent regulation of Kv1.5, which forms the ion conducting pore for IKur, is unlikely to lead to ERP shortening; in fact, an opposite effect would be expected. Based on this rationale, we chose to forego evaluation of this channel in this study.
IKACh causes APD shortening and cell membrane hyperpolarization. Increased vagal activity promotes AF by stabilizing atrial reentrant rotors.31 Patients with AF also display IKH current.14, 27, 32 Whereas protein expression of Kir3 subunits is unchanged in RAP models32, 33 and even decreased in AF patients,34 IKH is increased due to enhanced open probability of the single channel secondary to slowed channel closure.33 Frequency-dependent membrane translocation of PKCε leads to abnormal channel phosphorylation that favors enhanced basal IKH.15 In this study, we showed that IKH was uniquely sensitive to ROS inhibition, as opposed to the acetylcholine-regulated fraction of IKACh. Furthermore, ROS inhibition prevented tachypacing-induced membrane translocation of PKCε, providing a mechanistic basis for ROS-dependent IKH activation. Our findings strongly support a mechanistic role for ROS in causing PKCε-induced upregulation of IKH in the fibrillating atrium.
Recent studies have also suggested a role for aberrant Ca2+/Calmodulin dependent protein kinase II (CaMKII) signaling in the creation of a vulnerable substrate for AF.35 Data from our own group demonstrated that oxidative injury, via CaMKII signaling, underlies the development of triggered Ca2+ waves in atrial myocytes of dogs subjected to ventricular tachypacing-induced congestive heart failure.36 However, arrhythmogenic Ca2+ waves have not been convincingly described in the setting of RAP-induced AF.37 Furthermore, we have confirmed this absence of Ca2+ waves in the RAP model in our own laboratory (unpublished data). It is therefore not clear whether oxidative injury-induced CaMKII signaling is critical to the maintenance of atrial arrhythmias in the canine RAP model.
Oxidative injury –a potential therapeutic target in AF
Despite significant evidence that oxidative injury plays an important role in AF, clinical trials with conventional antioxidants have had mixed results. Carnes et al. demonstrated a 50% reduction of post-operative AF with ascorbic acid.38 However, subsequent larger studies with ascorbic acid produced mixed results.23 NAC has also been used in several small clinical trials, but results have also been mixed thus far.23 It has been argued that these studies were likely underpowered to see a significant effect. In addition, there is concern that systemically administered NAC does not reach a sufficient concentration at the myocardium for effective ROS scavenging. Instead of attempting to scavenge existing ROS, strategies aimed at preventing ROS formation might be more successful. For instance, statins reduce ROS formation by inhibiting Rac1 GTPase and hence decreasing NOX2 expression.10 In small studies on patients undergoing cardiac surgery, there was an improvement in redox state after statin therapy. While a meta-analysis showed a significant association between statin use and reduction in post-operative AF and secondary prevention of AF,39 a subsequent blinded, placebo-controlled randomized trial did not reveal any effect of statin therapy on post-operative AF.40 The renin-angiotensin-aldosterone system (RAAS) has also been proposed as an upstream regulator of NOX.19 Several meta-analyses have shown a beneficial effect of RAAS inhibition on incidence or recurrence of AF, though with significant heterogeneity.41 However, it remains difficult to separate the antioxidant effect of RAAS inhibition with its multiple other effects on the cardiovascular system.
This variable efficacy of small molecule approaches is likely to be at least in part secondary to variable drug bioavailability and distribution, affinity for the target, and pleiotropic effects. In contrast, gene therapy has the advantage of a localized, upstream effect allowing for targeted suppression of one or more major enzymatic sources of oxidative injury. The present study suggests efficacy of NOX2 shRNA gene transfer for treatment of sustained AF, with a durable effect lasting at least 12 weeks in the canine RAP model.
A targeted, gene based therapy for AF – one step closer to clinical practice
Gene therapy for AF has been investigated over the last decade, but has remained in pre-clinical testing. ERP prolongation was targeted by gene therapy with an adenovirus vector expressing a dominant-negative mutant of the IKr channel KCNH2. Gene vector was delivered in a pig model of RAP-induced AF either with the gene painting method42 or with direct myocardial injection followed by electroporation.43 Both groups showed prolongation of the APD or ERP. Short-term expression of the vector (<3 weeks) was present in both studies, with a delay in AF induction by 5–10 days. Conduction velocity was also targeted by using an adenovirus vector expressing atrial connexins, either Cx4044 or Cx4344, 45 in pig models of RAP-induced AF. Both studies showed improved conduction velocity with gene therapy, and short-term efficacy with reduced AF induction up to 7 days44 or 14 days.45 Other mechanisms such as cardiomyocyte apoptosis were also targeted with a similar approach. Administration of an adenovirus vector expressing a siRNA for Caspase 3 via myocardial injection and electroporation resulted in improved conduction velocity and short-term reduction in AF inducibility (14 day follow-up) in a pig model of RAP-induced AF. Vagal signaling was also targeted by using a non-viral vector expressing C-terminal peptides of Gα subunits in a canine model.46 This short-term study (3 days) showed effective attenuation of vagal-induced ERP shortening and AF inducibility. Finally, atrial fibrosis was targeted in the canine rapid ventricular pacing model of heart failure by injection and electroporation of a dominant negative TGF-β type II receptor.47 This study showed a decrease in interstitial fibrosis associated with reduction in conduction inhomogeneity and decreased duration of AF after 3–4 weeks of rapid ventricular pacing.
While promising, previously published gene therapy for AF has been mostly focused on short-term efficacy. In this study, we show that NOX2 shRNA has a long-lasting effect of at least 12 weeks after initiation of RAP with a marked and sustained reduction in AF burden. This is consistent with prior studies demonstrating long-lasting plasmid-based gene expression in murine skeletal muscle (at least 78 days).48 Demonstration of longer term gene expression with our non-viral approach is therefore an important step towards translation to humans with AF. While viral vectors – specifically adeno-associated virus and lentivirus – have been shown to have long-lasting gene expression, a non-viral approach may have certain advantages compared to viral approaches e.g. reduced inflammation, less probability of gene spillover to surrounding myocardium, etc. Nonetheless, the need for electroporation to facilitate non-viral gene delivery does add to the complexity of this approach, and requires the development of surgical or percutaneous devices that can facilitate safe and effective electroporation in the human atrium.
Limitations
In our study, we focused on inwardly rectifying potassium currents (i.e. IK1, IKACh, and IKH) that have been most consistently associated with ERP shortening in AF. However, it is certainly possible that other potassium currents, including IKur, may also be contributing to oxidative injury induced electrical remodeling in the RAP atrium. This needs to be investigated in future studies.
Even though we discovered ROS sensitive- and PKCε mediated-activation of IKH in our study, we cannot exclude the possibility of direct oxidation of the Kir3.1/Kir3.4 on emergence of IKH. Assessment of direct oxidation and investigation of sites of oxidation in the channel needs to be performed in future studies. In future studies, single channel recordings may also be performed to assess the effect of ROS on the biophysical properties of IKH.
Since mitochondrial ROS was increased in RAP atrium – and acute inhibition of mitochondrial ROS attenuated IKH – mitochondrial ROS may be playing an important role in electrical remodeling in the intact atrium. Future studies should therefore consider performing targeted inhibition of mitochondrial ROS with a gene-based approach in the RAP model e.g. by overexpression of mitochondrial catalase. Another gene target that deserves further investigation is PKCε. It is also well described in the literature – especially in ischemia reperfusion models - that PKCε affects generation of ROS, including NOX2-generated ROS.49 Whether this is also the case in the AF atrium – with there being a feedback loop between NOX2 and PKCε in the fibrillating atrium – is not known. Studies with PKCε shRNA in the intact canine atrium would help address this question.
The RAP model is primarily a model of electrical remodeling in AF, and as demonstrated in our study, development of fibrosis is limited and occurs late (see Figure VIII in the Supplement). Whereas NOX2 shRNA did not affect fibrosis in our study, it is conceivable that oxidative injury might lead to fibrosis in a different, more pro-fibrotic environment. Future studies need to examine the effect of this gene therapy approach on atrial fibrosis in a more relevant model such as the canine heart failure model of AF. Similarly, whereas the RAP model is well suited to study pathways that affect atrial ERP shortening and AF characteristics, it does not recapitulate all changes observed in human AF. It however remains a valid and effective model to better understand electrical remodeling in AF.
Supplementary Material
Clinical perspective.
What is new?
NADPH oxidase 2 (NOX2)-dependent oxidative injury causes electrical remodeling in a large animal model of atrial fibrillation.
This is dependent on membrane translocation of PKCε, leading to upregulation of a constitutively active form of the acetylcholine-dependent K+ current.
Atrial gene therapy with a short hairpin RNA targeting NOX2 prevents the development of electrical remodeling and sustained atrial fibrillation.
What are the clinical implications?
Current therapies for atrial fibrillation have limited efficacy and do not target the molecular mechanisms underlying this disease.
This study suggests that gene therapy suppressing atrial oxidative injury may be a novel, mechanism-guided approach to treat or prevent atrial fibrillation.
Acknowledgments:
We acknowledge S. Nattel for expert opinion and critical discussion regarding the properties of IKH.
Funding:
AP: This work is supported, in part, by The Kenneth M. Rosen Fellowship in Cardiac Pacing and Electrophysiology from the Heart Rhythm Society and funded via an unrestricted research grant by Medtronic.
RA: R01 HL093490; R01 HL140061; the American Heart Association (AHA) Strategically Focused Research Networks AF Center grant; NIH Center for Accelerated Innovations at Cleveland Clinic (NCAI-CC)
JAW: R01 HL119095
Non-Standard Abbreviations and Acronyms
- 8-OHdG
8-hydroxy-2-deoxyguanosine
- ACh
Acetylcholine
- AF
Atrial fibrillation
- APD
Action potential duration
- BIM-1
Bisindolylmaleimide-1
- CCh
Carbachol
- CLR
Recurrence cycle length
- CuDIPs
Copper [II] diisopropyl salicylate
- DF
Dominant frequency
- ERP
Effective refractive period
- FI
Fractionation interval
- H2O2
Hydrogen peroxide
- I CaL
L-type Ca2+ current
- I K1
Inward-rectifier K+ current
- I KACh
Acetylcholine-dependent K+ current
- I KH
Constitutively active form of IKACh
- I Kur
Ultra-rapid delayed rectifier K+ current
- IQR
Interquartile range
- I to
Transient outward K+ current
- LAA
Left atrial appendage
- LAFW
Left atrial free wall
- LV
Left ventricular
- LVEF
Left ventricular ejection fraction
- ms
millisecond
- NAC
N-acetylcysteine
- NOX
NADPH oxidase
- O2-
Superoxide
- OCl-
hypochlorite ion
- OI
Organization index
- ONOO-
Peroxinitrites
- PKC
Protein Kinase C
- PLA
Posterior left atrium
- RAP
Rapid atrial pacing
- ROS
Reactive oxygen species
- RV
Right ventricular
- ShEn
Shannon’s entropy
- shRNA
Short hairpin RNA
- siRNA
Silencing RNA
- TAPSE
Tricuspid annular plane systolic excursion
- TQ
Tertiapin-Q
Footnotes
Disclosures:
RA: Ownership interest, Rhythm Therapeutics, Inc. Other authors have no conflicts related to this study.
References
- 1.Nattel S, Burstein B and Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol. 2008;1:62–73. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Chen PS, Bild DE, Mascette AM, Albert CM, Alonso A, Calkins H, Connolly SJ, Curtis AB, Darbar D, Ellinor PT, Go AS, Goldschlager NF, Heckbert SR, Jalife J, Kerr CR, Levy D, Lloyd-Jones DM, Massie BM, Nattel S, Olgin JE, Packer DL, Po SS, Tsang TS, Van Wagoner DR, Waldo AL and Wyse DG. Prevention of atrial fibrillation: report from a national heart, lung, and blood institute workshop. Circulation. 2009;119:606–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dobrev D and Nattel S. New antiarrhythmic drugs for treatment of atrial fibrillation. Lancet. 2010;375:1212–23. [DOI] [PubMed] [Google Scholar]
- 4.Piccini JP and Fauchier L. Rhythm control in atrial fibrillation. Lancet. 2016;388:829–840. [DOI] [PubMed] [Google Scholar]
- 5.Chung MK, Shemanski L, Sherman DG, Greene HL, Hogan DB, Kellen JC, Kim SG, Martin LW, Rosenberg Y and Wyse DG. Functional status in rate- versus rhythm-control strategies for atrial fibrillation: results of the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Functional Status Substudy. J Am Coll Cardiol. 2005;46:1891–9. [DOI] [PubMed] [Google Scholar]
- 6.Yao C, Veleva T, Scott L Jr., Cao S, Li L, Chen G, Jeyabal P, Pan X, Alsina KM, Abu-Taha ID, Ghezelbash S, Reynolds CL, Shen YH, LeMaire SA, Schmitz W, Muller FU, El-Armouche A, Tony Eissa N, Beeton C, Nattel S, Wehrens XHT, Dobrev D and Li N. Enhanced Cardiomyocyte NLRP3 Inflammasome Signaling Promotes Atrial Fibrillation. Circulation. 2018;138:2227–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samman TA, Sandesara PB, Hayek SS, Alkhoder A, Chivukula K, Hammadah M, Mohamed-Kelli H, O’Neal WT, Topel M, Ghasemzadeh N, Ko YA, Aida H, Gafeer M, Sperling L, Vaccarino V, Liang Y, Jones DP and Quyyumi AA. Association between oxidative stress and atrial fibrillation. Heart Rhythm. 2017;14:1849–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai H. Hydrogen peroxide regulation of endothelial function: origins, mechanisms, and consequences. Cardiovasc Res. 2005;68:26–36. [DOI] [PubMed] [Google Scholar]
- 9.Kim YM, Kattach H, Ratnatunga C, Pillai R, Channon KM and Casadei B. Association of atrial nicotinamide adenine dinucleotide phosphate oxidase activity with the development of atrial fibrillation after cardiac surgery. J Am Coll Cardiol. 2008;51:68–74. [DOI] [PubMed] [Google Scholar]
- 10.Reilly SN, Jayaram R, Nahar K, Antoniades C, Verheule S, Channon KM, Alp NJ, Schotten U and Casadei B. Atrial sources of reactive oxygen species vary with the duration and substrate of atrial fibrillation: implications for the antiarrhythmic effect of statins. Circulation. 2011;124:1107–1117. [DOI] [PubMed] [Google Scholar]
- 11.Van Wagoner DR. Oxidative stress and inflammation in atrial fibrillation: role in pathogenesis and potential as a therapeutic target. J Cardiovasc Pharmacol. 2008;52:306–313. [DOI] [PubMed] [Google Scholar]
- 12.Wolke C, Bukowska A, Goette A and Lendeckel U. Redox control of cardiac remodeling in atrial fibrillation. Biochim Biophys Acta. 2015;1850:1555–1565. [DOI] [PubMed] [Google Scholar]
- 13.Voigt N, Friedrich A, Bock M, Wettwer E, Christ T, Knaut M, Strasser RH, Ravens U and Dobrev D. Differential phosphorylation-dependent regulation of constitutively active and muscarinic receptor-activated IK,ACh channels in patients with chronic atrial fibrillation. Cardiovasc Res. 2007;74:426–437. [DOI] [PubMed] [Google Scholar]
- 14.Cha TJ, Ehrlich JR, Chartier D, Qi XY, Xiao L and Nattel S. Kir3-based inward rectifier potassium current: potential role in atrial tachycardia remodeling effects on atrial repolarization and arrhythmias. Circulation. 2006;113:1730–1737. [DOI] [PubMed] [Google Scholar]
- 15.Makary S, Voigt N, Maguy A, Wakili R, Nishida K, Harada M, Dobrev D and Nattel S. Differential protein kinase C isoform regulation and increased constitutive activity of acetylcholine-regulated potassium channels in atrial remodeling. Circ Res. 2011;109:1031–1043. [DOI] [PubMed] [Google Scholar]
- 16.Gopalakrishna R and Anderson WB. Ca2+- and phospholipid-independent activation of protein kinase C by selective oxidative modification of the regulatory domain. Proc Natl Acad Sci U S A. 1989;86:6758–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aggarwal NT and Makielski JC. Redox control of cardiac excitability. Antioxid Redox Signal. 2013;18:432–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kleniewska P, Piechota A, Skibska B and Goraca A. The NADPH oxidase family and its inhibitors. Arch Immunol Ther Exp (Warsz ). 2012;60:277–294. [DOI] [PubMed] [Google Scholar]
- 19.Youn JY, Zhang J, Zhang Y, Chen H, Liu D, Ping P, Weiss JN and Cai H. Oxidative stress in atrial fibrillation: an emerging role of NADPH oxidase. J Mol Cell Cardiol. 2013;62:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voigt N, Trausch A, Knaut M, Matschke K, Varro A, Van Wagoner DR, Nattel S, Ravens U and Dobrev D. Left-to-right atrial inward rectifier potassium current gradients in patients with paroxysmal versus chronic atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu YF, Chen YJ, Lin YJ and Chen SA. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol. 2015;12:230–243. [DOI] [PubMed] [Google Scholar]
- 22.Everett THt Wilson EE, Verheule S Guerra JM, Foreman S and Olgin JE. Structural atrial remodeling alters the substrate and spatiotemporal organization of atrial fibrillation: a comparison in canine models of structural and electrical atrial remodeling. Am J Physiol Heart Circ Physiol. 2006;291:H2911–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simon JN, Ziberna K and Casadei B. Compromised redox homeostasis, altered nitroso-redox balance, and therapeutic possibilities in atrial fibrillation. Cardiovasc Res. 2016;109:510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adam O, Frost G, Custodis F, Sussman MA, Schafers HJ, Bohm M and Laufs U. Role of Rac1 GTPase activation in atrial fibrillation. J Am Coll Cardiol. 2007;50:359–367. [DOI] [PubMed] [Google Scholar]
- 25.Nattel S, Maguy A, Le BS and Yeh YH. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev. 2007;87:425–456. [DOI] [PubMed] [Google Scholar]
- 26.Carnes CA, Janssen PM, Ruehr ML, Nakayama H, Nakayama T, Haase H, Bauer JA, Chung MK, Fearon IM, Gillinov AM, Hamlin RL and Van Wagoner DR. Atrial glutathione content, calcium current, and contractility. J Biol Chem. 2007;282:28063–28073. [DOI] [PubMed] [Google Scholar]
- 27.Dobrev D, Friedrich A, Voigt N, Jost N, Wettwer E, Christ T, Knaut M and Ravens U. The G protein-gated potassium current I(K,ACh) is constitutively active in patients with chronic atrial fibrillation. Circulation. 2005;112:3697–706. [DOI] [PubMed] [Google Scholar]
- 28.Gomez R, Caballero R, Barana A, Amoros I, Calvo E, Lopez JA, Klein H, Vaquero M, Osuna L, Atienza F, Almendral J, Pinto A, Tamargo J and Delpon E. Nitric oxide increases cardiac IK1 by nitrosylation of cysteine 76 of Kir2.1 channels. Circ Res. 2009;105:383–392. [DOI] [PubMed] [Google Scholar]
- 29.Christ T, Wettwer E, Voigt N, Hala O, Radicke S, Matschke K, Varro A, Dobrev D and Ravens U. Pathology-specific effects of the IKur/Ito/IK,ACh blocker AVE0118 on ion channels in human chronic atrial fibrillation. Br J Pharmacol. 2008;154:1619–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Svoboda LK, Reddie KG, Zhang L, Vesely ED, Williams ES, Schumacher SM, O’Connell RP, Shaw R, Day SM, Anumonwo JM, Carroll KS and Martens JR. Redox-sensitive sulfenic Acid modification regulates surface expression of the cardiovascular voltage-gated potassium channel kv1.5. Circ Res. 2012;111:842–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kneller J, Zou R, Vigmond EJ, Wang Z, Leon LJ and Nattel S. Cholinergic atrial fibrillation in a computer model of a two-dimensional sheet of canine atrial cells with realistic ionic properties. Circ Res. 2002;90:E73–E87. [DOI] [PubMed] [Google Scholar]
- 32.Ehrlich JR, Cha TJ, Zhang L, Chartier D, Villeneuve L, Hebert TE and Nattel S. Characterization of a hyperpolarization-activated time-dependent potassium current in canine cardiomyocytes from pulmonary vein myocardial sleeves and left atrium. J Physiol. 2004;557:583–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Voigt N, Maguy A, Yeh YH, Qi X, Ravens U, Dobrev D and Nattel S. Changes in I K, ACh single-channel activity with atrial tachycardia remodelling in canine atrial cardiomyocytes. Cardiovasc Res. 2008;77:35–43. [DOI] [PubMed] [Google Scholar]
- 34.Dobrev D, Graf E, Wettwer E, Himmel HM, Hala O, Doerfel C, Christ T, Schuler S and Ravens U. Molecular basis of downregulation of G-protein-coupled inward rectifying K(+) current (I(K,ACh) in chronic human atrial fibrillation: decrease in GIRK4 mRNA correlates with reduced I(K,ACh) and muscarinic receptor-mediated shortening of action potentials. Circulation. 2001;104:2551–2557. [DOI] [PubMed] [Google Scholar]
- 35.Swaminathan PD, Purohit A, Hund TJ and Anderson ME. Calmodulin-dependent protein kinase II: linking heart failure and arrhythmias. Circ Res. 2012;110:1661–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoo S, Aistrup G, Shiferaw Y, Ng J, Mohler PJ, Hund TJ, Waugh T, Browne S, Gussak G, Gilani M, Knight BP, Passman R, Goldberger JJ, Wasserstrom JA and Arora R. Oxidative stress creates a unique, CaMKII-mediated substrate for atrial fibrillation in heart failure. JCI Insight. 2018;3:e120728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greiser M, Kerfant BG, Williams GS, Voigt N, Harks E, Dibb KM, Giese A, Meszaros J, Verheule S, Ravens U, Allessie MA, Gammie JS, van d, V, Lederer WJ, Dobrev D and Schotten U. Tachycardia-induced silencing of subcellular Ca2+ signaling in atrial myocytes. J Clin Invest. 2014;124:4759–4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carnes CA, Chung MK, Nakayama T, Nakayama H, Baliga RS, Piao S, Kanderian A, Pavia S, Hamlin RL, McCarthy PM, Bauer JA and Van Wagoner DR. Ascorbate attenuates atrial pacing-induced peroxynitrite formation and electrical remodeling and decreases the incidence of postoperative atrial fibrillation. Circ Res. 2001;89:E32–E38. [DOI] [PubMed] [Google Scholar]
- 39.Fauchier L, Clementy N and Babuty D. Statin therapy and atrial fibrillation: systematic review and updated meta-analysis of published randomized controlled trials. Curr Opin Cardiol. 2013;28:7–18. [DOI] [PubMed] [Google Scholar]
- 40.Zheng Z, Jayaram R, Jiang L, Emberson J, Zhao Y, Li Q, Du J, Guarguagli S, Hill M, Chen Z, Collins R and Casadei B. Perioperative Rosuvastatin in Cardiac Surgery. N Engl J Med. 2016;374:1744–53. [DOI] [PubMed] [Google Scholar]
- 41.Schneider MP, Hua TA, Bohm M, Wachtell K, Kjeldsen SE and Schmieder RE. Prevention of atrial fibrillation by Renin-Angiotensin system inhibition a meta-analysis. J Am Coll Cardiol. 2010;55:2299–307. [DOI] [PubMed] [Google Scholar]
- 42.Amit G, Kikuchi K, Greener ID, Yang L, Novack V and Donahue JK. Selective molecular potassium channel blockade prevents atrial fibrillation. Circulation. 2010;121:2263–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soucek R, Thomas D, Kelemen K, Bikou O, Seyler C, Voss F, Becker R, Koenen M, Katus HA and Bauer A. Genetic suppression of atrial fibrillation using a dominant-negative ether-a-go-go-related gene mutant. Heart Rhythm. 2012;9:265–72. [DOI] [PubMed] [Google Scholar]
- 44.Igarashi T, Finet JE, Takeuchi A, Fujino Y, Strom M, Greener ID, Rosenbaum DS and Donahue JK. Connexin gene transfer preserves conduction velocity and prevents atrial fibrillation. Circulation. 2012;125:216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bikou O, Thomas D, Trappe K, Lugenbiel P, Kelemen K, Koch M, Soucek R, Voss F, Becker R, Katus HA and Bauer A. Connexin 43 gene therapy prevents persistent atrial fibrillation in a porcine model. Cardiovasc Res. 2011;92:218–25. [DOI] [PubMed] [Google Scholar]
- 46.Aistrup GL, Cokic I, Ng J, Gordon D, Koduri H, Browne S, Arapi D, Segon Y, Goldstein J, Angulo A, Wasserstrom JA, Goldberger JJ, Kadish AH and Arora R. Targeted nonviral gene-based inhibition of Galpha(i/o)-mediated vagal signaling in the posterior left atrium decreases vagal-induced atrial fibrillation. Heart Rhythm. 2011;8:1722–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kunamalla A, Ng J, Parini V, Yoo S, McGee KA, Tomson TT, Gordon D, Thorp EB, Lomasney J, Zhang Q, Shah S, Browne S, Knight BP, Passman R, Goldberger JJ, Aistrup G and Arora R. Constitutive Expression of a Dominant-Negative TGF-beta Type II Receptor in the Posterior Left Atrium Leads to Beneficial Remodeling of Atrial Fibrillation Substrate. Circ Res. 2016;119:69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Escoffre JM, Debin A, Reynes JP, Drocourt D, Tiraby G, Hellaudais L, Teissie J and Golzio M. Long-lasting in vivo gene silencing by electrotransfer of shRNA expressing plasmid. Technol Cancer Res Treat. 2008;7:109–16. [DOI] [PubMed] [Google Scholar]
- 49.Barnett ME, Madgwick DK and Takemoto DJ. Protein kinase C as a stress sensor. Cell Signal. 2007;19:1820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kennedy DJ, Vetteth S, Periyasamy SM, Kanj M, Fedorova L, Khouri S, Kahaleh MB, Xie Z, Malhotra D, Kolodkin NI, Lakatta EG, Fedorova OV, Bagrov AY and Shapiro JI. Central role for the cardiotonic steroid marinobufagenin in the pathogenesis of experimental uremic cardiomyopathy. Hypertension. 2006;47:488–95. [DOI] [PubMed] [Google Scholar]
- 51.Everett THt Moorman JR, Kok LC Akar JG and Haines DE. Assessment of global atrial fibrillation organization to optimize timing of atrial defibrillation. Circulation. 2001;103:2857–61. [DOI] [PubMed] [Google Scholar]
- 52.Ng J, Borodyanskiy AI, Chang ET, Villuendas R, Dibs S, Kadish AH and Goldberger JJ. Measuring the complexity of atrial fibrillation electrograms. J Cardiovasc Electrophysiol. 2010;21:649–55. [DOI] [PubMed] [Google Scholar]
- 53.Ng J, Gordon D, Passman RS, Knight BP, Arora R and Goldberger JJ. Electrogram morphology recurrence patterns during atrial fibrillation. Heart Rhythm. 2014;11:2027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.