Abstract
Background
Renal schwannomas are very rare and are usually benign. Its clinical symptoms and imaging features are nonspecific, and the diagnosis is usually confirmed by pathology after surgical resection.
Case presentation
A 46-year-old Chinese female was admitted to the hospital with right flank pain that had persisted for the six months prior to admission. This pain had worsened for 10 days before admission, and dyspnea occurred when she was supine and agitated. A right abdominal mass could be palpated on physical examination. Computed tomography and magnetic resonance imaging examinations revealed a large, nonenhanced, cystic and solid mass in the right kidney. The patient received radical nephrectomy for the right kidney. The diagnosis of schwannoma was confirmed by pathological examination.
Conclusions
We report a case of a large renal schwannoma with obvious hemorrhage and cystic degeneration, which can be used as a reference for further study.
Keywords: Renal tumor, Renal schwannoma, Computed tomography, Magnetic resonance imaging
Background
Schwannomas are predominantly benign peripheral nerve sheath tumors. These tumors rarely undergo malignant transformation. Schwannomas are most commonly seen in the extremities, head, neck, retroperitoneum and mediastinum. Rarer locations include the kidney, duodenum [1], bronchus [2], and other internal regions. Renal schwannomas more frequently arise from the hilum and less frequently arise from the parenchyma because nerve tissues congregate at the hilum [3]. We report a case of large schwannoma originating from the renal parenchyma.
Case presentation
A 46-year-old Chinese female had right flank of unclear origin pain that lasted more than six months. It began with slight and persistent dull pain and no other symptoms. Ten days before admission, however, the pain worsened, and dyspnea occurred when she was supine and agitated. A right abdominal mass with poor mobility and a clear boundary between the surrounding structures could be palpated on physical examination. There was no tenderness. The patient had no genetic history of neurofibromatosis. No abnormal findings were found on blood, biochemistry, routine urine and antibody laboratory examination. Nonenhanced CT showed a large cystic and solid mass in the right kidney with septation and a few areas of calcification that increased the volume of the right kidney. The renal cortex had become thinner, and the renal pelvis and calices were obviously hydrous and dilated. The adjacent organs were compressed and displaced (Fig. 1). MRI revealed that the mass was slightly hyperintense on T1-weighted imaging, and had high-low mixed signal intensity on T2-weighted imaging. The edge of the lesion showed hyperintensity on diffusion-weighted imaging, and ring-like and septal enhancement was observed on enhanced T1-weighted imaging (Fig. 2). Because the mass was so large, the patient underwent radical nephrectomy of the right kidney, which revealed that the mass had adhered tightly to the inferior vena cava and duodenum. Postoperative pathology showed that the mass from the renal parenchyma measured 20.5 × 17.5 × 10.0 cm and was encapsulated. On cut sections, it was soft and reddish-brown with a massive amount of hemorrhage and necrosis. The boundary between the mass and renal parenchyma was clear. Immunostaining with S-100 protein and Ki-67 (positivity in approximately five percent of neoplastic cells) was positive and supported a diagnosis of a benign schwannoma (Fig. 3). Postoperatively, the patient recovered well, and no complications were observed.
Fig. 1.
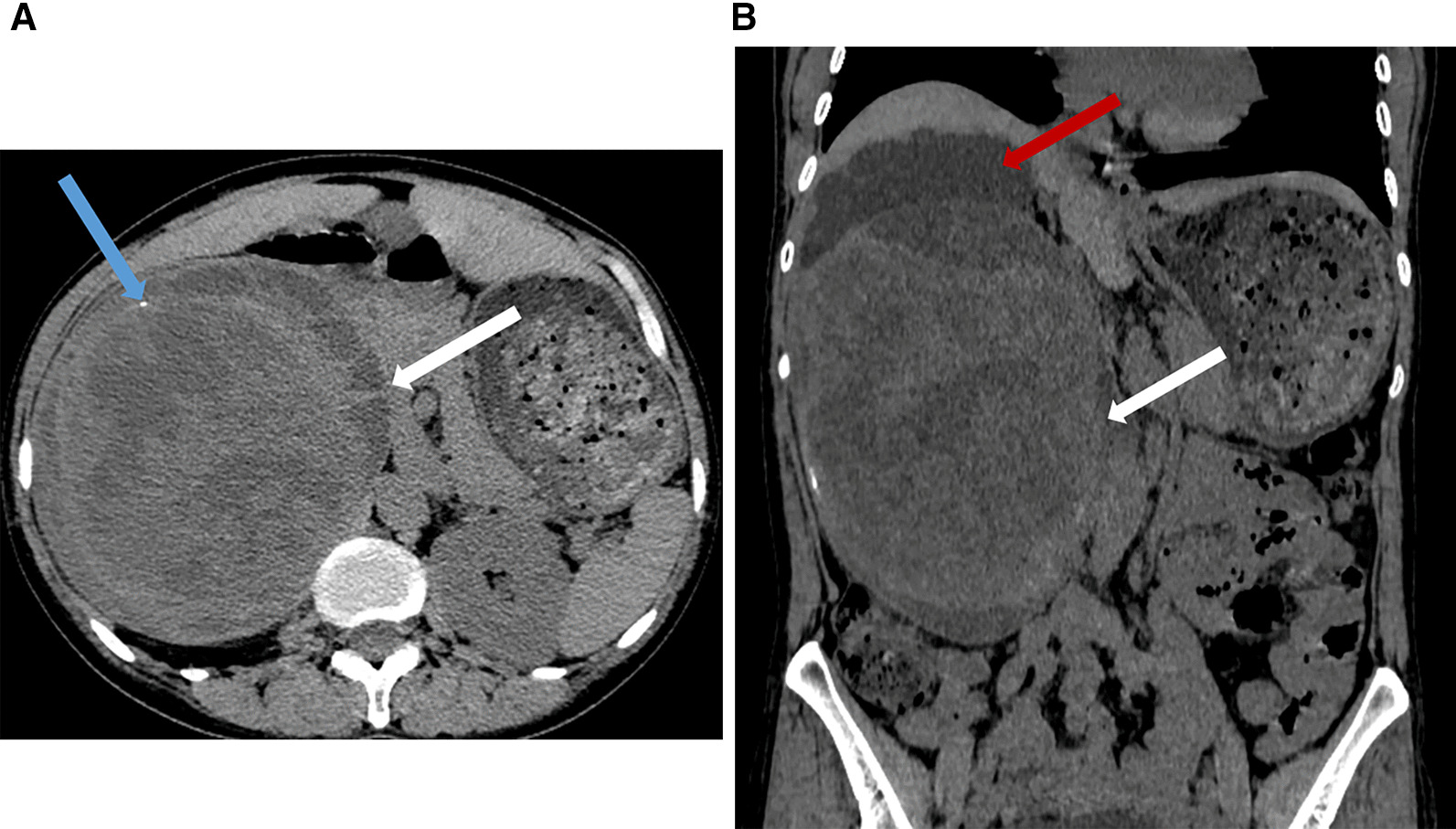
CT findings of renal schwannoma. Axial (a) and coronal (b) CT scan shows a giant cystic and solid mass (white arrow) is located at right kidney with a few calcification (blue arrow) and hydronephrosis (red arrow)
Fig. 2.
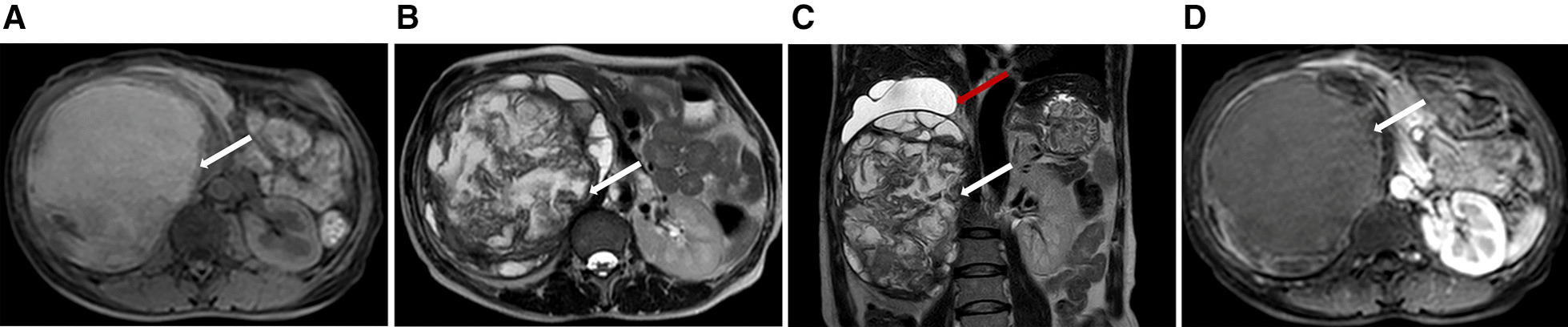
MR findings of renal schwannoma. a Precontrast T1-weighted MR shows a slightly hyperintense mass (white arrow) arises from the kidney. T2-weighted axial (b) and cronal (c) plane MR shows the high-low mixed signal intensity mass (white arrow) with hydronephrosis (red arrow). No obvious enhancement (white arrow) on enhanced T1-weighted imaging (d)
Fig. 3.

Final pathology slide of nephrectomy specimen. a H&E staining showed the tumor was fairly cellular showing spindle cells (× 100). b Immunostaining with S-100 protein was positive (× 100)
Discussion and conclusions
Peripheral schwannoma is an uncommon tumor that originates from Schwann cells of nerve sheaths. Approximately three percent of schwannomas occur retroperitoneally, but renal involvement is uncommon [4]. Most of them are benign, and malignancy is rare. There are different types of schwannomas: plexiform, ancient, cellular, melanotic, epithelioid, and microcystic [5]. In the 2020 WHO Classification of Tumors of Soft Tissue, melanotic schwannoma was reclassified as a malignant tumor because of its aggressive clinical behavior [6].
There are only 37 cases of renal schwannoma reported in the English literature. Table 1 summarizes the data for these cases, including the present case. Among the 37 cases, the mean age of the patients was 52.0 ± 14.0 years (range 18–74 years), and the male to female ratio was 1:1.5. The mean size of the lesions was 8.0 ± 4.3 cm (range 2.6–20.5 cm). The renal schwannomas were all solitary. There were 16 lesions in the left kidney and 21 lesions in the right kidney, and the ratio of left to right was approximately 1:1.3. These lesions were located at the hilum and pelvis (51.4%), parenchyma (43.2%) and capsule (5.4%). Among all cases, 33 were benign, and 4 were malignant. Malignant renal schwannoma can metastasize to the lung, bone, diaphragm, liver, colon, mesentery, peritoneum, and subcutaneous tissues, of which lung metastasis is the most common.
Table 1.
Cases of renal schwannoma
| Author | Year | Sex | Age (years) | Side | Location | Size (cm) | Malignancy | Symptom | Imaging features |
|---|---|---|---|---|---|---|---|---|---|
| Phillips [7] | 1955 | M | 56 | L | Hilum | 12 | No | Fever, chills, weight loss | A large, diffuse, smooth shadow on excretory and retrograde pyelography |
| Fein [8] | 1965 | F | 51 | R | Hilum | 6 | No | Fever, chills, right flank pain, dysuria | Renal hypertrophy, pyelectasis and caliectasis on retrograde pyelography |
| Bair [9] | 1978 | M | 56 | R | Hilum | 7 | No | Microscopic hematuria | Neovascularity within a solid mass on selective right renal arteriography |
| Steers [10] | 1985 | F | 50 | R | Hilum | 9 | No | Microscopic hematuria | A noncalcified, cystic renal mass with hemorrhage and nerosis on CT; hypovascular exophytic mass on renal arteriography |
| Somers [11] | 1988 | F | 55 | L | Parenchyma | 5 | No | Incidental finding | Solid mass on arteriography |
| Kitagawa [12] | 1990 | M | 51 | L | Hilum | 2.8 | No | Epigastric pain, high fever | Hypoechoic mass on US; an extrinsic compression of the left renal pelvis and mild hydronephrosis on excretory pyelography; homogeneous tumor without enhanced on CT; isointense on T1WI, homogeneous hyperintense on T2WI |
| Ma [13] | 1990 | M | 67 | R | Parenchyma | 8 | No | Epigastric pain | Renal hypertrophy on US; hypovascular tumor on arteriography |
| Naslund [14] | 1991 | F | 50 | L | Parenchyma | 14 | Yes | Mild abdominal discomfort,decreased appetite, weight loss | – |
| Romics [15] | 1992 | M | 52 | R | Capsule | A large invasve mass | Yes | Back and the right flank pain, recurrent fever | Extensive, cystic-necrotic space occupation in the right kidney on imaging techniques |
| Singer [16] | 1996 | F | 70 | L | Hilum | 6 | No | Asymptomatic | Extrinsic compression of the upper and middle infundibula on excretory pyelography; soft tissue mass with moderate enhancement on CT; slightly hypointense on T1WI, slightly hyperintense on T2WI |
| Alvarado-Cabrero [17] | 2000 | F | 18 | R | Parenchyma | 6.2 | No | Flank pain | – |
| Alvarado-Cabrero [17] | 2000 | F | 40 | L | Parenchyma | 12.5 | No | Flank pain | – |
| Alvarado-Cabrero [17] | 2000 | M | 45 | L | Parenchyma | 16 | No | Flank and abdominal pain | – |
| Tsurusaki [18] | 2001 | F | 69 | L | Capsule | – | No | Incidental finding | Severe extrinstic compression of the left ureter on excretory pyelography; heterogeneous hypoechoic mass on US; low-attenuation area with moderately enhanced rim on CT; hypointense on T1WI, slightly hyperintense on T2WI |
| Cachay [19] | 2003 | F | 74 | R | Parenchyma | 9 | Yes | – | An unique, well-demarcated, round hypodense mass on CT |
| Singh [20] | 2005 | M | 40 | L | Pelvis | 3 | No | Left renal colicky pain | A soft tissue mass on US; enhancing mass compresses the pelvis of the left kidney on CT |
| Singh [20] | 2005 | M | 35 | R | Hilum | – | No | Fank pain, hematuria | A heterogenous mass on US; enhancing mass compresses the renal parenchyma and pelvis on CT |
| Umphrey [21] | 2007 | F | 63 | R | Parenchyma | 7 | No | Hypertension and hot flashes | A markedly hypoechoic mass with lobulation and septations on US; a lower attenuation large lobulated mass with a few faint calcifications and slight enhancement on CT |
| Hung [22] | 2007 | F | 36 | L | Parenchyma | 7.3 | No | Low-grade fever | Homogeneous isointense on T1WI, homogeneous hyperintense on T2WI, enhancement of outer rim of tumor on gadolinium-enhanced T1WI |
| Gobbo [23] | 2008 | F | 59 | L | Hilum | 4.8 | No | Asymptomatic | – |
| Gobbo [23] | 2008 | F | 27 | R | Parenchyma | 8.5 | No | Incidental finding | – |
| Gobbo [23] | 2008 | F | 35 | L | Hilum | 7 | No | Abdominal pain, nausea | – |
| Chen [24] | 2010 | M | 34 | R | Hilum | 2.6 | No | Hematuria | Solid mass on US; the edge of right renal calices was irregular on excretory pyelography; solid mass with slight enhancement on CT |
| Nayyar [25] | 2011 | F | 44 | R | Hilum | 10 | No | Flank pain, nausea, vomiting | A large cystic area with large extrarenal pelvis and gross hydronephrosis on US, CT and excretory pyelography |
| Yang [26] | 2012 | F | 40 | L | Pelvic | 6.8 | No | Flank pain | A low-attenuated, lobulated, and minimally enhanced on CT; on retrograde pyelography, the left ureteropelvic junction was kinked, the upper calyces were obliterated, and the calyx was filled with an irregular collection of contrast |
| Wang [27] | 2013 | M | 66 | L | Parenchyma | 2.7 | No | Intermittent painless gross hematuria | A solid mass on CT |
| Mikkilineni [28] | 2013 | F | 36 | R | Parenchyma | 4.6 | No | Fever, malaise, right flank discomfort, nignt sweat, hematuria | A complex cystic leision on US; a complex cystic leision with thick, irregular, nodular rim of enhancement on CT |
| Verze [3] | 2014 | M | 59 | R | Parenchyma | 15 | Yes | Incidental finding | A mass with a large central necrosis on CT |
| Yong [29] | 2015 | F | 55 | R | Pelvic | 5.1 | No | Colicky pain, microscopic haematuria | A soft tissue density lesion with mildly enhancement on CT; |
| Hall [30] | 2015 | F | 73 | L | Hilum | 4.9 | No | Vague abdominal pain | An echo poor mass on US; smooth filling defect affecting the renal pelvic on retrograde pyelography |
| Kumano [31] | 2015 | M | 73 | R | Hilum | 3.5 | No | – | The tumor was poorly enhanced on CT; MRI showed that the inside was uniform on T1WI and heterogeneous contrast on T2WI |
| Herden [32] | 2015 | M | 60 | R | Parenchyma | 11 | No | Incidental finding, asymptomatic | A polycystic, centrally hypodense space-occupying mass with rim of enhancement on CT |
| Herden [32] | 2015 | F | 69 | R | Hilum | 6.5 | No | Microscopic hematuria | A tumorous space-occupying process with partially central colliquations, compressing the vena cava on CT |
| Vidal [33] | 2020 | M | 66 | R | Parenchyma | 3.5 | No | Incidental finding | A focal solid mass on US and CT |
| Wang [34] | 2020 | F | 56 | L | Hilum | 11.5 | No | Left lower back pain | A massive tumor with soft tissue density and inhomogeneous enhancement on CT |
| Dahmen [35] | 2021 | M | 47 | R | Hilum | 12 | No | Flank pain | Enhancing large right renal mass with no filling defect of the renal pelvis on CTU |
| Present | 2021 | F | 46 | R | Parenchyma | 20.5 | No | Flank pain | A cystic and solid mass with septation and local calcification on CT; slightly hyperintense on T1WI, high-low mixed signal intensity on T2WI, ringlike and septal enhancement |
We further analyzed the radiological images of these previously reported renal schwannomas and summarized some of its radiological features. On ultrasound (US), renal schwannomas are hypoechoic, well-defined masses and may contain cystic areas, which are more commonly seen in renal schwannomas, as they are larger than 5 cm. The larger the tumor is, the more cystic degeneration and necrosis are present. Further investigation is usually performed with CT or MR imaging. On nonenhanced CT, they are typically well-defined and round or fusiform hypoattenuating masses. Large tumors also show cystic degeneration, calcifications and hemorrhage. On contrast-enhanced CT, renal schwannomas show mild to moderate homogeneous or heterogeneous enhancement. On MR imaging, most renal schwannomas often appear isointense or hypointense relative to muscle on T1-weighted imaging and hyperintense on T2-weighted imaging with variable enhancement; however, cystic degeneration and hemorrhage can complicate the signal intensity. In addition, pyelectasis and caliectasis on excretory and retrograde pyelography and hypovascular tumors on renal arteriography can be seen. There are no clear imaging features that can differentiate between benign and malignant lesions unless metastasis in other regions is found.
There are two important differences between the present case and the previous cases. First, the patient in question here had a large renal schwannoma, which is quite rare, making it the largest tumor reported thus far. Second, to the best of our knowledge, this case presents with more extensive bleeding and cystic degeneration than any of the previous reported cases. In our case, due to the large range of cystic lesions and hemorrhage, the mass was slightly hyperintense on T1-weighted imaging and had high-low mixed signal intensity on T2-weighted imaging with ring-like and septal enhancement.
The clinical symptoms of renal schwannoma are nonspecific; a small number of patients do not have any symptoms, and the mass is only found incidentally during physical examination for any number of reasons. Among the 37 previous cases, the most common symptoms were abdominal pain (51.4%, mostly flank pain), hematuria (21.6%) and fever (16.2%). Other symptoms included nausea, vomiting, loss of appetite, and weight loss. In our report, the patient exhibited persistent flank pain. As the tumor grew, it pressed on the surrounding organs and tissues, resulting in pain that worsened and caused dyspnea during emotional agitation and when lying in the supine position. In addition, renal schwannomas grow slowly. When the tumor grows to a certain volume, the abdominal mass can be palpated.
Radical nephrectomy or partial nephrectomy are recommended as first-line treatments for renal schwannomas. Histologically, a typical schwannoma consists of Antoni A and Antoni B tissue. Antoni A tissue is composed of spindle cells arranged in a palisade with Verocay bodies, while Antoni B tissue is composed of loose and scattered cells with many myxoid changes [33]. S-100 protein immunostaining was positive in all cases.
In conclusion, renal schwannomas are rare and grow slowly. Cystic degeneration in the tumor is a common imaging feature. When a middle aged-elderly patient has a well-defined renal tumor with obvious cystic degeneration and shows mild to moderate homogeneous or heterogeneous enhancement, renal schwannoma should be considered. However, pathological examination is the gold standard for diagnosis. We report a large renal schwannoma with obvious hemorrhage and cystic degeneration, which could be used as a reference for further study.
Acknowledgements
Not applicable.
Abbreviations
- CT
Computed tomography
- MRI
Magnetic resonance imaging
- US
Ultrasound
Author contributions
CFY composed the manuscript preparation. HZ provided figures. CFY, JHY, and YL had the acquisition, analysis or interpretation of data. SKD and YL revised manuscript. All authors have reviewed the final version of the manuscript and approve it for publication. All authors read and approved the final manuscript.
Funding
This research is supported by the People's Hospital of Yuechi County (Grant No. YY21-01,02), the affiliated hospital of north Sichuan medical college (Grant No. 2021LC004) and the opening project of medical imaging key laboratory of Sichuan province (Grant No. MIKLSP2021010). The funding bodies did not have anything to do with the study design, data collection, analysis, interpretation or writing.
Availability of data and materials
The datasets used and analysed during the current study available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report. A copy of the consent form is available for review and can be provided on request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chao Feng Yang, Email: 1164842730@qq.com.
Hui Zuo, Email: 574048990@qq.com.
Jin Hong Yu, Email: 3255417708@qq.com.
Sushant Kumar Das, Email: sus_mak4u@yahoo.co.in.
Yang Li, Email: 552410618@qq.com.
References
- 1.Zhang Z, Gong T, Rennke HG, Hayashi R. Duodenal schwannoma as a rare association with membranous nephropathy: a case report. Am J Kidney Dis. 2019;73(2):278–280. doi: 10.1053/j.ajkd.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Guerreiro C, Dionisio J, Duro DCJ. Endobronchial schwannoma involving the carina. Arch Bronconeumol. 2017;53(8):452. doi: 10.1016/j.arbres.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 3.Verze P, Somma A, Imbimbo C, Mansueto G, Mirone V, Insabato L. Melanotic schwannoma: a case of renal origin. Clin Genitourin Cancer. 2014;12(1):e37–e41. doi: 10.1016/j.clgc.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Gubbay AD, Moschilla G, Gray BN, Thompson I. Retroperitoneal schwannoma: a case series and review. Aust N Z J Surg. 1995;65(3):197–200. doi: 10.1111/j.1445-2197.1995.tb00607.x. [DOI] [PubMed] [Google Scholar]
- 5.Iannaci G, Crispino M, Cifarelli P, Montella M, Panarese I, Ronchi A, Russo R, Tremiterra G, Luise R, Sapere P. Epithelioid angiosarcoma arising in schwannoma of the kidney: report of the first case and review of the literature. World J Surg Oncol. 2016;14(1):29. doi: 10.1186/s12957-016-0789-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi JH, Ro JY. The 2020 WHO Classification of tumors of soft tissue: selected changes and new entities. Adv Anat Pathol. 2021;28(1):44–58. doi: 10.1097/PAP.0000000000000284. [DOI] [PubMed] [Google Scholar]
- 7.Phillips CA, Baumrucker G. Neurilemmoma (arising in the hilus of left kidney) J Urol. 1955;73(4):671–673. doi: 10.1016/S0022-5347(17)67452-4. [DOI] [PubMed] [Google Scholar]
- 8.Fein RL, Hamm FC. Malignant schwannoma of the renal pelvis: a review of the literature and a case report. J Urol. 1965;94(4):356–361. doi: 10.1016/S0022-5347(17)63631-0. [DOI] [PubMed] [Google Scholar]
- 9.Bair ED, Woodside JR, Williams WL, Borden TA. Perirenal malignant schwannoma presenting as renal cell carcinoma. Urology. 1978;11(5):510–512. doi: 10.1016/0090-4295(78)90173-5. [DOI] [PubMed] [Google Scholar]
- 10.Steers WD, Hodge GB, Johnson DE, Chaitin BA, Charnsangavej C. Benign retroperitoneal neurilemoma without von Recklinghausen's disease: a rare occurrence. J Urol. 1985;133(5):846–848. doi: 10.1016/S0022-5347(17)49251-2. [DOI] [PubMed] [Google Scholar]
- 11.Somers WJ, Terpenning B, Lowe FC, Romas NA. Renal parenchymal neurilemoma: a rare and unusual kidney tumor. J Urol. 1988;139(1):109–110. doi: 10.1016/S0022-5347(17)42309-3. [DOI] [PubMed] [Google Scholar]
- 12.Kitagawa K, Yamahana T, Hirano S, Kawaguchi S, Mikawa I, Masuda S, Kadoya M. MR imaging of neurilemoma arising from the renal hilus. J Comput Assist Tomogr. 1990;14(5):830–832. doi: 10.1097/00004728-199009000-00034. [DOI] [PubMed] [Google Scholar]
- 13.Ma KF, Tse CH, Tsui MS. Neurilemmoma of kidney—a rare occurrence. Histopathology. 1990;17(4):378–380. doi: 10.1111/j.1365-2559.1990.tb00746.x. [DOI] [PubMed] [Google Scholar]
- 14.Naslund MJ, Dement S, Marshall FF. Malignant renal schwannoma. Urology. 1991;38(5):477–479. doi: 10.1016/0090-4295(91)80243-Z. [DOI] [PubMed] [Google Scholar]
- 15.Romics I, Bach D, Beutler W. Malignant schwannoma of kidney capsule. Urology. 1992;40(5):453–455. doi: 10.1016/0090-4295(92)90463-7. [DOI] [PubMed] [Google Scholar]
- 16.Singer AJ, Anders KH. Neurilemoma of the kidney. Urology. 1996;47(4):575–581. doi: 10.1016/S0090-4295(99)80500-7. [DOI] [PubMed] [Google Scholar]
- 17.Alvarado-Cabrero I, Folpe AL, Srigley JR, Gaudin P, Philip AT, Reuter VE, Amin MB. Intrarenal schwannoma: a report of four cases including three cellular variants. Mod Pathol. 2000;13(8):851–856. doi: 10.1038/modpathol.3880150. [DOI] [PubMed] [Google Scholar]
- 18.Tsurusaki M, Mimura F, Yasui N, Minayoshi K, Sugimura K. Neurilemoma of the renal capsule: MR imaging and pathologic correlation. Eur Radiol. 2001;11(9):1834–1837. doi: 10.1007/s003300000767. [DOI] [PubMed] [Google Scholar]
- 19.Cachay M, Sousa-Escandon A, Gibernau R, Benet JM, Valcacel JP. Malignant metastatic perirenal schwannoma. Scand J Urol Nephrol. 2003;37(5):443–445. doi: 10.1080/00365590310019026. [DOI] [PubMed] [Google Scholar]
- 20.Singh V, Kapoor R. Atypical presentations of benign retroperitoneal schwannoma: report of three cases with review of literature. Int Urol Nephrol. 2005;37(3):547–549. doi: 10.1007/s11255-004-4705-5. [DOI] [PubMed] [Google Scholar]
- 21.Umphrey HR, Lockhart ME, Kenney PJ. Benign renal schwannoma: a case report and literature review. Radiol Case Rep. 2007;2(2):52–55. doi: 10.2484/rcr.v2i2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hung SF, Chung SD, Lai MK, Chueh SC, Yu HJ. Renal Schwannoma: case report and literature review. Urology. 2008;72(3):713–716. doi: 10.1016/j.urology.2007.12.056. [DOI] [PubMed] [Google Scholar]
- 23.Gobbo S, Eble JN, Huang J, Grignon DJ, Wang M, Martignoni G, Brunelli M, Cheng L. Schwannoma of the kidney. Mod Pathol. 2008;21(6):779–783. doi: 10.1038/modpathol.2008.52. [DOI] [PubMed] [Google Scholar]
- 24.Qiguang C, Zhe Z, Chuize K. Neurilemoma of the renal hilum. Am Surg. 2010;76(11):E197–E198. doi: 10.1177/000313481007601103. [DOI] [PubMed] [Google Scholar]
- 25.Nayyar R, Khattar N, Sood R, Bhardwaj M. Cystic retroperitoneal renal hilar ancient schwannoma: report of a rare case with atypical presentation masquerading as simple cyst. Indian J Urol. 2011;27(3):404–406. doi: 10.4103/0970-1591.85450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang HJ, Lee MH, Kim DS, Lee HJ, Lee JH, Jeon YS. A case of renal schwannoma. Korean J Urol. 2012;53(12):875–878. doi: 10.4111/kju.2012.53.12.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Zhu B. A case of schwannoma in kidney. Quant Imaging Med Surg. 2013;3(3):180–181. doi: 10.3978/j.issn.2223-4292.2013.06.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mikkilineni H, Thupili CR. Benign renal schwannoma. J Urol. 2013;189(1):317–318. doi: 10.1016/j.juro.2012.10.037. [DOI] [PubMed] [Google Scholar]
- 29.Yong A, Kanodia AK, Alizadeh Y, Flinn J. Benign renal schwannoma: a rare entity. BMJ Case Rep. 2015;20:bcr2015211642. doi: 10.1136/bcr-2015-211642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall SJ, Williams ST, Jackson TA, Lee AT, McCulloch TA. Retroperitoneal gastrointestinal type schwannoma presenting as a renal mass. Urol Case Rep. 2015;3(6):206–208. doi: 10.1016/j.eucr.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumano Y, Kawahara T, Chiba S, Maeda Y, Ohtaka M, Kondo T, Mochizuki T, Hattori Y, Teranishi J, Miyoshi Y, et al. Retroperitoneal schwannoma in the renal hilum: a case report. Case Rep Oncol. 2015;8(3):394–398. doi: 10.1159/000440612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herden J, Drebber U, Ural Y, Zimmer S, Wille S, Engelmann UH. Retroperitoneal schwannomas of renal and pararenal origin: presentation of two case reports. Rare Tumors. 2015;7(1):5616. doi: 10.4081/rt.2015.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vidal CN, Lopez CP, Ferri NB, Aznar ML, Gomez GG. Benign renal schwannoma: case report and literature review. Urol Case Rep. 2020;28:101018. doi: 10.1016/j.eucr.2019.101018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang C, Gao W, Wei S, Ligao W, Beibei L, Jianmin L, Xiaohuai Y, Yuanyuan G. Laparoscopic nephrectomy for giant benign renal schwannoma: a case report and review of literature. Aging Male. 2020;23(5):1504–1508. doi: 10.1080/13685538.2020.1812566. [DOI] [PubMed] [Google Scholar]
- 35.Dahmen A, Juwono T, Griffith J, Patel T. Renal schwannoma: a case report and literature review of a rare and benign entity mimicking an invasive renal neoplasm. Urol Case Rep. 2021;37:101637. doi: 10.1016/j.eucr.2021.101637. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analysed during the current study available from the corresponding author on reasonable request.


