Abstract
Overexpression of human epidermal growth factor receptor 2 (HER2) occurs in approximately 15–20% of breast cancer cases. HER2 is a member of the epidermal growth factor receptor (EGFR) family with tyrosinase kinase activity, and its overexpression is linked to poor prognosis and shorter progression-free survival (PFS) and overall survival (OS). Among various treatment options, HER2-targeting monoclonal antibodies and tyrosine kinase inhibitors (TKIs) have mostly been applied in recent decades to treat HER2-positive (HER2+) breast cancer patients. Although positive clinical outcomes were documented in both advanced disease and neoadjuvant settings, the development of resistance mechanisms to such approaches has been one of the major challenges with the continuous usage of these drugs. In addition, patients who experience disease progression after treatment with multiple HER2-targeted therapies often have limited treatment options. The Food and Drug Administration (FDA) has recently approved a new TKI (i.e., tucatinib) for use in combination with immunotherapy and/or chemotherapeutic agents for the treatment of advanced-stage/metastatic HER2+ breast cancer. This review highlights recent updates on the efficacy of tucatinib-based therapeutic approaches in experimental models as well as in the clinical settings of HER2+ breast cancer.
Keywords: HER2-positive (HER2+), Breast cancer, Targeted therapy, Tucatinib, Immunotherapy
Background
Breast cancer is one of the most frequently diagnosed human malignancies worldwide and the second most common cause of cancer-related mortality among females in the United States [1–3]. Breast cancer is categorized into four primary clinically relevant molecular subtypes based on the prognosis and levels of gene expression: luminal A or hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2−), luminal B or HR+ /HER2+, HER2+, and triple-negative breast cancer (TNBC) or HR−/HER2− [4–7]. In addition, a fifth subtype that closely resembles luminal A is known as normal-like breast cancer [8]. This review is focused on HER2+ breast cancer. Importantly, the most common modifiable and non-modifiable risk factors associated with breast cancer include lifestyle factors (such as alcohol use and tobacco smoking) and other factors [such as age, body weight, breast density, benign breast conditions, hormonal intake (e.g., oral contraceptives), menopausal status, and family history of malignancy] [9–15].
HER2 has been implicated in mammary carcinogenesis induction in early studies using in vitro and in vivo model systems [16, 17]. HER2 and other members (e.g., HER3/4) of the epidermal growth factor receptor (EGFR) family are receptor tyrosine kinases, which are located on the cellular membrane and respond to a wide variety of ligands [18, 19]. Except for HER2, all other isoforms have a ligand. Activation (i.e., phosphorylation) of HER2 is dependent on homodimerization (i.e., HER2-HER2 homodimer) or heterodimerization with other HER receptors (i.e., dimerization partners), such as HER2-EGFR, HER2-HER3, and HER2-HER4 [20–22]. This activation induces downstream signaling pathways, such as the phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) and Ras/Raf/mitogen-activated protein kinase (Ras/MAPK) pathways, which are related to increased cell proliferation and survival as well as augmentation of primary tumor growth and tumor metastasis [19, 23, 24].
Importantly, HER2 gene is overexpressed or amplified in approximately 15–20% of breast cancer patients, which is linked with poor prognosis, tumor relapse, and worse outcomes, such as shorter progression-free survival (PFS) and overall survival (OS) [20, 25, 26]. Several agents possessing anticancer properties, including taxanes and capecitabine, have been evaluated for the treatment of HER2+ breast cancer [27–30]. However, the efficacy of different therapeutic regimens has been shown to have low-to-moderate response rates and is associated with adverse side effects [20, 24, 31]. One of the possible explanations for these adverse events is that most therapeutic agents target multiple cellular pathways in addition to HER2 signaling, contributing to the development of tumor resistance mechanisms and resulting in reduced effectiveness of such approaches [20, 24, 31]. This has led to the development of targeted therapy with specific targetability against HER2+ breast cancer cells.
Although monoclonal antibodies (e.g., trastuzumab) and tyrosine kinase inhibitors (TKIs) (e.g., lapatinib) have mostly been used in recent decades to treat HER2+ breast cancer patients [32–36], current regimens typically use a combination of taxanes, trastuzumab, and pertuzumab as first-line agents, with ado-trastuzumab emtansine (T-DMI) used as a second-line agent. TKIs (e.g., tucatinib and lapatinib) have also been reserved for use in combination with chemotherapy or HER2-targeted therapy as third-line agents [37–40]. Previous review articles have covered the mechanisms of other HER2-targeting immunotherapies and TKIs [41–45]. Therefore, to avoid repetition, we discuss the mechanisms of one of the most commonly used immunotherapies (e.g., trastuzumab) and recently FDA-approved TKI (i.e., tucatinib) along with their resistance mechanisms, as this combination has been explored in most clinical studies [33, 46, 47]. We also highlight the therapeutic efficacy of tucatinib combination approaches in experimental models and clinical settings of HER2+ breast cancer.
Mechanisms of action and resistance to trastuzumab and tucatinib
Mechanisms of action and resistance to trastuzumab
Trastuzumab was one of the first drugs used to target HER2 receptors, which led to a significant improvement in therapeutic efficacy, including increased PFS and OS for HER2+ breast cancer patients. Trastuzumab exerts its effect by binding to the extracellular and juxtamembrane domains of the HER2 receptor. Binding to the extracellular domain blocks HER2 proteolytic cleavage and triggers an immune-mediated response against HER2-overexpressing tumor cells, whereas binding to the juxtamembrane domain selectively blocks HER2-HER3 dimerization independently of a ligand [32, 48–50]. Trastuzumab’s mechanism of action involves antibody-dependent cell cytotoxicity (ADCC), which is dependent on the frequency of CD16 and CD56 co-expressing lymphocytes. Overall, it has been shown that the amount and lytic effectiveness of CD16+ lymphocytes are important determinants in trastuzumab-induced ADCC, indicating a potential mechanism for the short-term clinical benefit of trastuzumab as a monotherapy [51]. Another critical factor contributing to the therapeutic efficacy of trastuzumab is the cyclin-dependent kinase inhibitor p27kip1 [52]. In this regard, a study performed by Nahta et al. [52] exploited two cellular models known as trastuzumab-resistant (TR) pools generated from SKBR3 HER2-overexpressing breast cancer cells to determine trastuzumab-mediated cytotoxicity escape mechanisms. According to the data, TR cells exhibited an extended S-phase of the cell cycle, resulting in an increased growth rate measured by doubling time compared to the parental cells, which was accompanied by decreased p27kip1 expression and increased cyclin-dependent kinase 2 (CDK2) activity. Notably, induced expression of p27kip1 was found to enhance the sensitivity of TR cells to trastuzumab, indicating that downregulation of p27kip1 might be associated with the development of resistance to trastuzumab therapy.
Despite promising clinical benefits in both advanced disease and neoadjuvant settings [32–36], a substantial proportion of HER2+ breast cancers show a proclivity toward resistance or become refractory to HER2-targeted therapies, including trastuzumab, which has been a major challenge with the use of these drugs [53, 54]. Along similar lines, patients have experienced disease progression after treatment with multiple HER2-targeted therapies, with often limited treatment options [25, 55, 56]. Several mechanisms have been proposed to explain the development of resistance to trastuzumab, such as HER2 signal transduction via activation of the PI3K-AKT-mTOR pathway [57], leading to the accumulation of p95-HER2, which modifies and adapts the signaling from alternative receptors. Another mechanism of resistance involves a mutation in the HER2 structural protein or elevation of other receptor tyrosine kinases, such as insulin-like growth factor receptor [21, 54, 58–61]. Therefore, resistance to HER2 immunotherapy may involve genetic or epigenetic changes that modulate the receptor to be degraded.
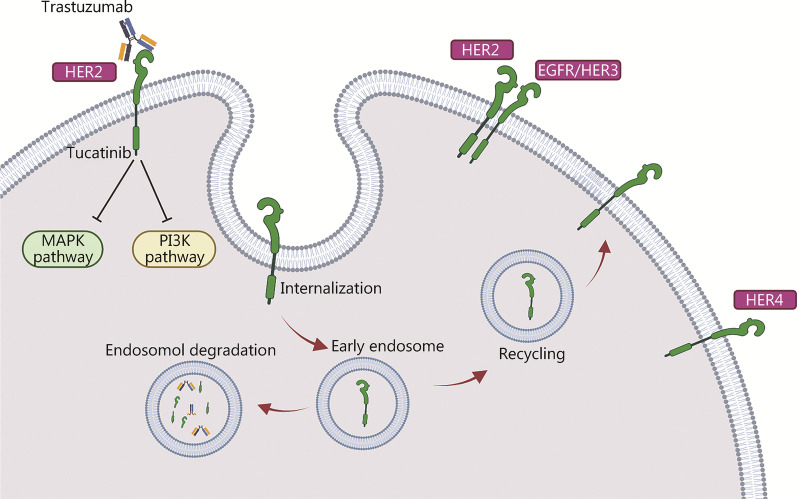
Similarly, HER2 heterodimerization with EGFR/HER3 has been shown to mediate antibody-induced internalization, resulting in subsequent ubiquitination and proteolysis (Fig. 1). Notably, this internalization is the mechanism by which HER2-specific antibodies disable the transforming activity of the receptor [24]. In addition, clathrin-mediated endocytosis of cell surface proteins from the endosomal compartment to the lysosome is responsible for HER2 internalization and degradation [59]. Other trafficking mechanisms also play critical roles in HER2 endocytosis. For example, as the dynamic surface pool of the HER2 receptor is created by varying rates of endocytosis and recycling, trastuzumab efficacy is disrupted when the HER2 surface pool is diminished [62]. It was found that the caveolin-1 (CAV1) protein is important for HER2 cell membrane dynamics during receptor endocytosis. Using in vivo biological models and fresh human tumor cells, Pereira et al. [62] demonstrated that temporal CAV1 reduction with lovastatin enhances the HER2 half-life and its availability at the cell membrane, resulting in better trastuzumab binding to the extracellular HER2 receptor domain.
Fig. 1.
Mechanisms of HER2 internalization and recycling by trastuzumab mediated via different processes resulting in endocytosis and subsequent degradation/recycling. The mechanism of action of tucatinib is mediated via inhibition of HER2 and HER3 phosphorylation, resulting in blockade of the downstream MAPK and AKT signaling pathways and leading to decreased cell proliferation. HER2/3/4 human epidermal growth factor receptor 2/3/4, EGFR epidermal growth factor receptor, MAPK mitogen-activated protein kinase, PI3K phosphatidylinositol 3-kinase
It has also been demonstrated that sortilin-related receptor 1 (SORLA) interacts with and regulates HER2 subcellular localization by enhancing dynamic recycling of the endosomal receptor back to the cell surface, and levels of SORLA protein and HER2 correlate positively in malignant cells. Importantly, SORLA protein silencing results in decreased HER2 signaling and localization to late endosomes or lysosomes due to impaired normal lysosome function. This mechanism impacts tumor development as well as the resistance/sensitivity of HER2 targeting antibody approaches [63].
Overall, HER2 endocytosis impacts the efficacy of anti-HER2 therapies such as trastuzumab. Study utilizing NSCLC cell lines and patient-derived xenograft models has found ubiquitination and internalization of the HER2 receptor to be the critical mechanisms of endocytosis, augmenting the therapeutic efficacy of anti-HER2 antibody–drug conjugates (ADC), including T-DMI, as well as its synergy with irreversible pan-HER inhibitors [64].
Mechanisms of action and resistance to tucatinib
Tucatinib is an oral, potent, and selective HER2-specific targeting agent that was approved by the FDA in April 2020 for the treatment of advanced unresectable or metastatic breast cancer. In a preclinical study and phase I clinical trials, tucatinib showed promising activity as a single agent, with improved effectiveness when combined with chemotherapy or trastuzumab [65–69]. Insight into the signaling mechanism revealed that unlike other TKIs, such as lapatinib and neratinib, which act as dual inhibitors for both EGFR and HER2, tucatinib is a specific and reversible inhibitor of the protein tyrosine kinase (PTK) activity of HER2 and exerts minimal inhibition of EGFR [69–71]. Kaur et al. [72] performed an in silico study to determine the molecular basis of synergism between tucatinib and trastuzumab. Given that the mechanism of trastuzumab action occurs via HER2 extracellular domain IV binding, the authors explored the basis of tucatinib action, particularly addressing whether tucatinib binds to the intracellular HER2 tyrosine kinase domain, which occurs intracellularly [73, 74]. Of note, the synergism of combination therapy is achieved when one drug binds to the extracellular domain and the other to the intracellular domain of the HER2 receptor [72]. By using a web-based docking tool, the authors docked tucatinib at the ATP binding site of the HER2 tyrosine kinase domain, and the data showed that tucatinib binds to the tyrosine kinase domain with substantial stability and binding energy. These studies indicate one of the possible reasons for the synergism between trastuzumab and tucatinib resulting in improved clinical responses in HER2+ breast cancer patients [72].
However, another study explored the resistance mechanisms of TKIs, including tucatinib, through gene profiling of different HER2- and non-HER2-overexpressing breast cancer cell lines [75], and identified three novel markers relevant to TKI sensitivities: V-set domain-containing T-cell activation inhibitor 1 (VTCN1), cyclin-dependent kinase 12 (CDK12), and Ras-related C3 botulinum toxin substrate 1 (RAC1). In addition, adenomatous polyposis coli (APC) mutations have been suggested as markers of tucatinib resistance [75]. The efficacy of tucatinib as a single agent vs. in combination with other therapies, such as trastuzumab and capecitabine, of HER2+ breast cancer is summarized below.
Cellular, preclinical and clinical studies of tucatinib and tucatinib combination approaches
Evidence from in vitro and in vivo studies
Several studies have evaluated the effects of tucatinib and its combination with other anticancer agents in various experimental models of HER2+ breast cancer. For example, Kulukian et al. [69] demonstrated that tucatinib treatment specifically decreases phosphorylation (i.e., activation) and viability in HER2-expressing BT-474 cells in a dose-dependent manner compared to EGFR-expressing A431 cells. Importantly, tucatinib was found to inhibit not only activation of the HER2 receptor but also binding with its heterodimer partner HER3 receptor, as well as downstream signaling cascades such as MAPK (i.e., MEK and ERK) and PI3K/AKT. Such decreased phosphorylation of AKT, as well as induction of apoptosis, were found to have additive effects in combination with trastuzumab, the HER2-targeted immunotherapy [69]. Preclinical data showed that tucatinib alone suppresses the growth of subcutaneously implanted BT-474 tumor xenografts in a dose-dependent manner compared to vehicle control-treated mice, and enhanced tumor growth suppression was observed with the combination of trastuzumab or docetaxel chemotherapy [69]. Relatively similar in vivo tumor growth-suppressive effects of tucatinib, trastuzumab, and the combination of tucatinib and trastuzumab have also been observed in other HER2+ tumor models, such as gastric, colorectal, and esophageal cancers [69]. Overall, these findings indicate the promising applicability of tucatinib in diverse HER2+ tumor models and that enhanced antitumor efficacy can be achieved via tucatinib in combination with immunotherapy or chemotherapy.
Along similar lines, Conlon et al. [75] compared the antiproliferative efficacy of three TKIs with regard to drug response and gene profiling using different breast cancer cell lines. The authors compared the efficacies and IC50 values of neratinib, lapatinib, and tucatinib with other anticancer agents. The tucatinib profile was found to be similar to that of trastuzumab for HER2 selectivity. Additionally, neratinib, lapatinib, and tucatinib were effective against HER2-overexpressing breast cancer models. Neratinib showed the greatest potency; tucatinib exhibited high selectivity for HER2-amplified cell lines but minimal effect on HER2 mutant cell lines [75].
Evidence from clinical studies
Evaluation of tucatinib efficacy with or without other agents in HER2+ breast cancer patients previously treated with HER2-targeted therapies
Multiple studies have explored the efficacy of tucatinib in combination with chemotherapeutic agents or immunotherapy in HER2+ breast cancer patients who were previously treated with HER2-targeted therapies. A summary of these studies is presented in Table 1.
Table 1.
Summary of clinical studies evaluating tucatinib or its combination with other agents in HER2+ metastatic breast cancer patients
| Treatment(s) | Other factor(s) | Study design and finding(s) |
|---|---|---|
| Tucatinib [65] | – |
To determine the MTD, antitumor activity, and pharmacokinetic properties of tucatinib The tucatinib MTD was determined to be 600 mg twice daily; 22% of patients treated at the MTD had a partial response + SD |
| Tucatinib + trastuzumab + capecitabine [33] | ALT/AST |
To evaluate the therapeutic responses of tucatinib vs. placebo combination with trastuzumab and capecitabine Tucatinib-combination resulted in improved PFS and increased OS with common adverse events compared to the placebo-combination group |
HER2+ HER2-positive, MTD maximum tolerable dose, SD stable disease, ALT alanine aminotransferase, AST aspartate aminotransferase, PFS progression-free survival, OS overall survival
In a phase I dose-escalation study, Moulder et al. [65] evaluated the maximum tolerable dose (MTD), antitumor activity, and pharmacokinetic properties of tucatinib. The MTD was found to be 600 mg twice daily, with dose-limiting toxicity at 800 mg twice daily. In addition, tucatinib had a favorable pharmacokinetic profile. When given at the MTD, tucatinib maintained the mean steady-state concentration at or above the predicted IC90 value for HER2 inhibition [65]. The overall median Tmax for tucatinib was approximately 2 h, and the overall median half-life was more than 5 h. No significant food effect was observed for tucatinib or metabolite exposure; however, variability among patients was high [65]. Of all patients treated with MTD doses, 22% had a partial response with stable disease (SD) when evaluated at ≥ 24 weeks, but 28% experienced disease progression [65].
In the HER2CLIMB study, Murthy et al. [33] determined the therapeutic responses of the tucatinib combination in patients who received prior treatments involving trastuzumab, pertuzumab, and trastuzumab emtansine and experienced disease progression. The patients were assigned randomly into two groups: tucatinib-combination group (administered tucatinib in combination with trastuzumab and capecitabine) and placebo-combination group (treated with a placebo in combination with trastuzumab and capecitabine). The primary endpoint was PFS at the one-year time point, which was found to be 33.1% in the tucatinib-combination group and 12.3% in the placebo-combination group, with a median PFS of 7.8 months and 5.6 months, respectively [33]. OS after two years was 44.9%, with a median OS of 21.9 months in the tucatinib-combination therapy group compared to 26.6% and 17.4 months in the placebo-combination group. Notably, patients in the tucatinib-combination group experienced common adverse effects of grade 3 or higher, including diarrhea, fatigue, and increased levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) compared to the placebo-combination group. Overall, the findings indicate that tucatinib combined with trastuzumab and capecitabine can be used to decrease the risk of disease progression or to increase survival benefits in HER2+ metastatic breast cancer patients with or without brain metastasis [33].
Evaluation of tucatinib combination in HER2+ breast cancer patients with brain metastasis
The incidence rate of breast cancer with brain metastasis ranges from 10 to 30% and is associated with significant morbidity and mortality [70, 76]. Therefore, several studies have evaluated the efficacy of tucatinib combination therapy in these patients. A summary of these studies is presented in Table 2.
Table 2.
Summary of clinical studies evaluating tucatinib combination approaches in HER2+ metastatic breast cancer patients with brain metastasis
| Treatment(s) | Parameter(s) | Study design and finding(s) |
|---|---|---|
| Tucatinib + trastuzumab + capecitabine [47] | OS | To evaluate safety. The OS was 20.7 months with the tucatinib-combination |
| Tucatinib + T-DMI [77] | mPFS | Phase I dose-escalation study to evaluate effect. The mPFS was 8.2 months |
| Tucatinib + trastuzumab [78] | MTD | Phase I dose-escalation study to evaluate response. The combination therapy was tolerable and effective in HER2+ breast cancer patients having brain metastases |
| Tucatinib + capecitabine, tucatinib + trastuzumab, tucatinib + capecitabine + trastuzumab [46] | mPFS | Phase I dose-escalation study to evaluate response. ORR of tucatinib + capecitabine was 83%, tucatinib + trastuzumab was 40%, and tucatinib + capecitabine + trastuzumab was 61%. The mPFS of the triple combination in patients with no brain metastasis was 7.8 months; however, it was 6.7 months in patients with brain metastasis |
| Tucatinib + trastuzumab + capecitabine [79] | OS | Phase II study to evaluate safety and efficacy. The median OS was 11.9 months and the median time to CNS progression 6.9 months |
HER2+ HER2-positive, OS overall survival, T-DMI ado-trastuzumab emtansine, mPFS median progression-free survival, MTD maximum tolerable dose, ORR objective response rate, SD stable disease, CNS central nervous system
In a subset of the HER2CLIMB study, Lin et al. [47] evaluated the effects of tucatinib in combination with trastuzumab and capecitabine in breast cancer patients who were previously treated or remained untreated with progressing brain metastasis. In patients not previously treated, OS in the tucatinib combination group was 20.7 months compared with 11.6 months in the placebo combination group. It was also found that the tucatinib-combination regimen delayed the time between the initial and second brain progression by 7.6 months compared with 3.1 months in the placebo combination group [47].
Borges et al. [77] conducted an open-label phase Ib clinical trial with the primary objective of evaluating the MTD of tucatinib in combination with T-DMI in T-DMI-naive advanced-stage metastatic HER2+ breast cancer patients, including those who had brain metastasis. The secondary objectives of this combination were determination of the safety, tolerability, pharmacokinetics, and preliminary antitumor activity, as assessed by the objective response rate (ORR) and PFS. The MTD of tucatinib was 300 mg twice daily, with dose-related toxic effects observed at 350 mg when given twice a day. Overall, the ORR of tucatinib combined with T-DMI at the MTD was 48%, and the median PFS (mPFS) was 8.2 months; the mPFS of patients previously treated with trastuzumab and pertuzumab was 6.5 months [77]. The clinical benefit rate of tucatinib combination therapy in patients, which was defined by the complete response, partial response, or stable response for over 6 months, was 58%, with a median response duration of 6.9 months. Among patients with brain metastasis, the mPFS of tucatinib combination therapy was 6.7 months, with a median overall response duration of 6.9 months, as per the response evaluation criteria in solid tumors (RECIST) version 1.1. Notably, the three most common adverse events observed were nausea, diarrhea, and fatigue [77].
Along similar lines, in a phase I study, Metzger Filho et al. [78] evaluated response to tucatinib when combined with trastuzumab. The primary objective of the study was to determine the MTD; the secondary objective included responses such as intracranial and extracranial parameters using modified RECIST and the clinical benefit rate. The study evaluated tucatinib MTD of 750 mg given once daily in cohort B, and a dose-limiting grade 3 adverse effect (i.e., transaminitis) was observed in one patient. Importantly, the combination regimen was found to be tolerable and effective in HER2+ breast cancer patients having brain metastases. The most common dose-limiting toxicities included thrombocytopenia and elevation of ALT/AST [78].
The MTD, safety, tolerability, and antitumor activity of tucatinib in combination with capecitabine and trastuzumab were evaluated in another phase I open-label nonrandomized dose-escalation study [46]. For this study, the authors recruited HER2+ breast cancer patients with or without brain metastasis who were previously treated with trastuzumab, pertuzumab, or trastuzumab emtansine. The tucatinib MTD was determined to be 300 mg twice daily. The ORR of the tucatinib and capecitabine combination was 83%, that of the tucatinib and trastuzumab combination was 40%, and that of the tucatinib, capecitabine, and trastuzumab combination was 61% [46]. Importantly, the mPFS for patients treated with the triple combination was 7.8 months compared to 7.1 months with tucatinib and capecitabine and 5.5 months with tucatinib and trastuzumab. Moreover, the median response duration was 5.2 months with tucatinib and capecitabine compared to 8.9 months with tucatinib and trastuzumab and 11.0 months with tucatinib, capecitabine, and trastuzumab [46]. Notably, 56% of patients enrolled at the time of the study had brain metastasis. Of these, 59% were either untreated for brain metastasis or had previously been treated, with progressive brain metastasis. The mPFS of the patients with brain metastasis treated with the combination of tucatinib, capecitabine, and trastuzumab was 6.7 months. Adverse effects reported irrespective of dose, causality, and grade were fatigue, diarrhea, and palmar-plantar erythrodysesthesia syndrome [46].
The same combination regimen was also evaluated for its safety and efficacy in HER2+ breast cancer patients with leptomeningeal metastasis (LM) who had poor prognosis and limited therapeutic options. In this nonrandomized phase II clinical trial, Murthy et al. [79] enrolled 17 adult HER2+ breast cancer patients based on Karnofsky performance status (KPS) of > 50 who were newly diagnosed with untreated LM, as confirmed by positive cerebrospinal fluid (CSF) cytology and/or radiographic evidence of LM plus other signs and symptoms. The primary endpoint of this study was OS. The median age of the patients was 53 years old. All patients had radiographic evidence of LM in the brain and were treated for the brain metastases; 8 patients had abnormal CSF cytology. The results showed a median OS of 11.9 months and a median time to central nervous system (CNS) progression of 6.9 months. These findings for the first time indicated the clinical benefits of tucatinib, trastuzumab, and capecitabine for the treatment of HER2+ metastatic breast cancer patients with LM. Importantly, a recent highlight published by the American Society of Clinical Oncology (ASCO) suggested that the combination of tucatinib with trastuzumab and capecitabine constitutes a novel standard therapy for pretreated HER2+ breast cancer patients with active brain metastasis [80].
In addition to HER2+ metastatic breast cancer, this combination regimen has been evaluated in other human HER2+ malignancies, including metastatic colorectal cancer (mCRC). For example, the MOUNTAINEER open-label phase II study determined the safety and efficacy of tucatinib, trastuzumab, and capecitabine combination in HER2+ RAS wild-type mCRC, and interim analysis of 26 patients showed an ORR of 52.2%, mPFS of 8.1 months, and mOS of 18.7 months [81]. Of significance, the MOUNTAINEER-02 phase II/III study is ongoing (NCT04499924) and aims to evaluate the safety and efficacy of tucatinib and trastuzumab combined with ramucirumab and paclitaxel in patients with gastric or gastroesophageal junction adenocarcinoma (GEC) [82].
Conclusions
Several studies have suggested that the combination of tucatinib with trastuzumab and capecitabine exhibits increased effectiveness over trastuzumab and capecitabine alone in treating HER2+ breast cancer patients. However, despite an increased therapeutic efficacy of combination therapies, one of the ongoing challenges is therapy-induced adverse effects, indicating the mechanisms involved in mediating such effects, which should be investigated. As tucatinib is a relatively new FDA-approved targeted therapy, its efficacy with other combinations should also be explored to assess their overall effectiveness and compare it with published data. Importantly, some clinical trials are ongoing and recruiting patients to evaluate tucatinib in combination with other anticancer agents.
Acknowledgements
The authors would like to acknowledge the Biorender.com software that was used to create Fig. 1.
Abbreviations
- ADCC
Antibody-dependent cell cytotoxicity
- ADC
Antibody–drug conjugates
- ALT
Alanine aminotransferase
- APC
Adenomatous polyposis coli
- ASCO
American Society of Clinical Oncology
- AST
Aspartate aminotransferase
- CAV1
Caveolin-1
- CDK2
Cyclin-dependent kinase 2
- CDK12
Cyclin-dependent kinase 12
- CNS
Central nervous system
- CSF
Cerebrospinal fluid
- EGFR
Epidermal growth factor receptor
- FDA
Food and Drug Administration
- GEC
Gastroesophageal junction adenocarcinoma
- HER
Human epidermal growth factor
- HER2+
HER2-positive
- KPS
Karnofsky performance status
- LM
Leptomeningeal metastasis
- mCRC
Metastatic colorectal cancer
- mPFS
Median progression-free survival
- MTD
Maximum tolerable dose
- ORR
Objective response rate
- OS
Overall survival
- PFS
Progression-free survival
- PI3k/AKT
Phosphatidylinositol 3-kinase/protein kinase B
- PTK
Protein tyrosine kinase
- RAC1
Ras-related C3 botulinum toxin substrate 1
- Ras/MAPK
Ras/Raf/mitogen-activated protein kinase
- RECIST
Response evaluation criteria in solid tumors
- SD
Stable disease
- SORLA
Sortilin-related receptor 1
- T-DMI
Ado-trastuzumab emtansine
- TKI
Tyrosine kinase inhibitor
- TNBC
Triple-negative breast cancer
- TR
Trastuzumab-resistant
- VTCN1
V-set domain-containing T-cell activation inhibitor 1
Author contributions
All the authors were involved in the discussion, writing, and editing of the manuscript. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Zaid Sirhan, Email: sirhan.2@wright.edu.
Anita Thyagarajan, Email: anita.thyagarajan@wright.edu.
Ravi P. Sahu, Email: ravi.sahu@wright.edu
References
- 1.DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69(6):438–451. doi: 10.3322/caac.21583. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 3.DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69(3):211–233. doi: 10.3322/caac.21555. [DOI] [PubMed] [Google Scholar]
- 4.Feng Y, Spezia M, Huang S, Yuan C, Zeng Z, Zhang L, et al. Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018;5(2):77–106. doi: 10.1016/j.gendis.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeo SK, Guan JL. Breast cancer: Multiple subtypes within a tumor? Trends Cancer. 2017;3(11):753–760. doi: 10.1016/j.trecan.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harbeck N, Gnant M. Breast cancer. Lancet. 2017;389(10074):1134–1150. doi: 10.1016/S0140-6736(16)31891-8. [DOI] [PubMed] [Google Scholar]
- 7.Howlader N, Cronin KA, Kurian AW, Andridge R. Differences in breast cancer survival by molecular subtypes in the United States. Cancer Epidemiol Biomark Prev. 2018;27(6):619–626. doi: 10.1158/1055-9965.EPI-17-0627. [DOI] [PubMed] [Google Scholar]
- 8.Dai X, Li T, Bai Z, Yang Y, Liu X, Zhan J, et al. Breast cancer intrinsic subtype classification, clinical use and future trends. Am J Cancer Res. 2015;5(10):2929–2943. [PMC free article] [PubMed] [Google Scholar]
- 9.Huszno J, Nowara E. Risk factors for disease progression in HER2-positive breast cancer patients based on the location of metastases. Prz Menopauzalny. 2015;14(3):173–177. doi: 10.5114/pm.2015.54341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnard ME, Boeke CE, Tamimi RM. Established breast cancer risk factors and risk of intrinsic tumor subtypes. Biochim Biophys Acta. 2015;1856(1):73–85. doi: 10.1016/j.bbcan.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Hamajima N, Hirose K, Tajima K, Rohan T, Calle EE, Heath CWJ, et al. Alcohol, tobacco and breast cancer–collaborative reanalysis of individual data from 53 epidemiological studies, including 58,515 women with breast cancer and 95,067 women without the disease. Br J Cancer. 2002;87(11):1234–1245. doi: 10.1038/sj.bjc.6600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung S, Wang M, Anderson K, Baglietto L, Bergkvist L, Bernstein L, et al. Alcohol consumption and breast cancer risk by estrogen receptor status: in a pooled analysis of 20 studies. Int J Epidemiol. 2016;45(3):916–928. doi: 10.1093/ije/dyv156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makarem N, Chandran U, Bandera EV, Parekh N. Dietary fat in breast cancer survival. Annu Rev Nutr. 2013;33:319–348. doi: 10.1146/annurev-nutr-112912-095300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Catsburg C, Miller AB, Rohan TE. Active cigarette smoking and risk of breast cancer. Int J Cancer. 2015;136(9):2204–2209. doi: 10.1002/ijc.29266. [DOI] [PubMed] [Google Scholar]
- 15.Gaudet MM, Carter BD, Brinton LA, Falk RT, Gram IT, Luo J, et al. Pooled analysis of active cigarette smoking and invasive breast cancer risk in 14 cohort studies. Int J Epidemiol. 2017;46(3):881–893. doi: 10.1093/ije/dyw288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Fiore PP, Pierce JH, Kraus MH, Segatto O, King CR, Aaronson SA. erbB-2 is a potent oncogene when overexpressed in NIH/3T3 cells. Science. 1987;237(4811):178–182. doi: 10.1126/science.2885917. [DOI] [PubMed] [Google Scholar]
- 17.Muller WJ, Sinn E, Pattengale PK, Wallace R, Leder P. Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell. 1988;54(1):105–115. doi: 10.1016/0092-8674(88)90184-5. [DOI] [PubMed] [Google Scholar]
- 18.Du Z, Lovly CM. Mechanisms of receptor tyrosine kinase activation in cancer. Mol Cancer. 2018;17(1):58. doi: 10.1186/s12943-018-0782-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dittrich A, Gautrey H, Browell D, Tyson-Capper A. The HER2 signaling network in breast cancer-like a spider in its web. J Mammary Gland Biol Neoplasia. 2014;19(3–4):253–270. doi: 10.1007/s10911-014-9329-5. [DOI] [PubMed] [Google Scholar]
- 20.Bredin P, Walshe JM, Denduluri N. Systemic therapy for metastatic HER2-positive breast cancer. Semin Oncol. 2020;47(5):259–269. doi: 10.1053/j.seminoncol.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Gajria D, Chandarlapaty S. HER2-amplified breast cancer: mechanisms of trastuzumab resistance and novel targeted therapies. Expert Rev Anticancer Ther. 2011;11(2):263–275. doi: 10.1586/era.10.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nami B, Maadi H, Wang Z. Mechanisms underlying the action and synergism of trastuzumab and pertuzumab in targeting HER2-positive breast cancer. Cancers (Basel) 2018;10(10):342. doi: 10.3390/cancers10100342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hubbard SR, Till JH. Protein tyrosine kinase structure and function. Annu Rev Biochem. 2000;69:373–398. doi: 10.1146/annurev.biochem.69.1.373. [DOI] [PubMed] [Google Scholar]
- 24.Freudenberg JA, Wang Q, Katsumata M, Drebin J, Nagatomo I, Greene MI. The role of HER2 in early breast cancer metastasis and the origins of resistance to HER2-targeted therapies. Exp Mol Pathol. 2009;87(1):1–11. doi: 10.1016/j.yexmp.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cronin KA, Harlan LC, Dodd KW, Abrams JS, Ballard-Barbash R. Population-based estimate of the prevalence of HER-2 positive breast cancer tumors for early stage patients in the US. Cancer Invest. 2010;28(9):963–968. doi: 10.3109/07357907.2010.496759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu JL, Hung MC. The role of HER2, EGFR, and other receptor tyrosine kinases in breast cancer. Cancer Metastasis Rev. 2016;35(4):575–588. doi: 10.1007/s10555-016-9649-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masuda N, Lee SJ, Ohtani S, Im YH, Lee ES, Yokota I, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376(22):2147–2159. doi: 10.1056/NEJMoa1612645. [DOI] [PubMed] [Google Scholar]
- 28.Nakatsukasa K, Koyama H, Oouchi Y, Imanishi S, Mizuta N, Sakaguchi K, et al. Docetaxel and cyclophosphamide as neoadjuvant chemotherapy in HER2-negative primary breast cancer. Breast Cancer. 2017;24(1):63–68. doi: 10.1007/s12282-016-0666-7. [DOI] [PubMed] [Google Scholar]
- 29.Ferguson T, Wilcken N, Vagg R, Ghersi D, Nowak AK. Taxanes for adjuvant treatment of early breast cancer. Cochrane database Syst Rev. 2007;4:CD004421. [DOI] [PubMed]
- 30.Nabholtz JM, Gligorov J. Docetaxel in the treatment of breast cancer: current experience and future prospects. Expert Rev Anticancer Ther. 2005;5(4):613–633. doi: 10.1586/14737140.5.4.613. [DOI] [PubMed] [Google Scholar]
- 31.File D, Curigliano G, Carey LA. Escalating and de-escalating therapy for early-stage HER2-positive breast Cancer. Am Soc Clin Oncol Educ Book. 2020;40:1–11. doi: 10.1200/EDBK_100023. [DOI] [PubMed] [Google Scholar]
- 32.Kreutzfeldt J, Rozeboom B, Dey N, De P. The trastuzumab era: current and upcoming targeted HER2+ breast cancer therapies. Am J Cancer Res. 2020;10(4):1045–1067. [PMC free article] [PubMed] [Google Scholar]
- 33.Murthy RK, Loi S, Okines A, Paplomata E, Hamilton E, Hurvitz SA, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020;382(7):597–609. doi: 10.1056/NEJMoa1914609. [DOI] [PubMed] [Google Scholar]
- 34.Duchnowska R, Loibl S, Jassem J. Tyrosine kinase inhibitors for brain metastases in HER2-positive breast cancer. Cancer Treat Rev. 2018;67:71–77. doi: 10.1016/j.ctrv.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 36.Figueroa-Magalhães MC, Jelovac D, Connolly R, Wolff AC. Treatment of HER2-positive breast cancer. Breast. 2014;23(2):128–136. doi: 10.1016/j.breast.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swain SM, Miles D, Kim SB, Im YH, Im SA, Semiglazov V, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(4):519–530. doi: 10.1016/S1470-2045(19)30863-0. [DOI] [PubMed] [Google Scholar]
- 38.Cesca MG, Vian L, Cristóvão-Ferreira S, Pondé N, de Azambuja E. HER2-positive advanced breast cancer treatment in 2020. Cancer Treat Rev. 2020;88:102033. doi: 10.1016/j.ctrv.2020.102033. [DOI] [PubMed] [Google Scholar]
- 39.Tesch ME, Gelmon KA. Targeting HER2 in Breast Cancer: Latest developments on treatment sequencing and the introduction of biosimilars. Drugs. 2020;80(17):1811–1830. doi: 10.1007/s40265-020-01411-y. [DOI] [PubMed] [Google Scholar]
- 40.Santa-Maria CA, Nye L, Mutonga MB, Jain S, Gradishar WJ. Management of metastatic HER2-positive breast cancer: Where are we and where do we go from here? Oncology. 2016;30(2):148–155. [PubMed] [Google Scholar]
- 41.Xuhong JC, Qi XW, Zhang Y, Jiang J. Mechanism, safety and efficacy of three tyrosine kinase inhibitors lapatinib, neratinib and pyrotinib in HER2-positive breast cancer. Am J Cancer Res. 2019;9(10):2103–2119. [PMC free article] [PubMed] [Google Scholar]
- 42.Schlam I, Swain SM. HER2-positive breast cancer and tyrosine kinase inhibitors: the time is now. NPJ Breast Cancer. 2021;7(1):56. doi: 10.1038/s41523-021-00265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang X, Wu D, Yuan S. Tyrosine kinase inhibitors in the combination therapy of HER2 positive breast cancer. Technol Cancer Res Treat. 2020;19:1533033820962140. doi: 10.1177/1533033820962140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barok M, Joensuu H, Isola J. Trastuzumab emtansine: mechanisms of action and drug resistance. Breast Cancer Res. 2014;16(2):209. doi: 10.1186/bcr3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krasniqi E, Barchiesi G, Pizzuti L, Mazzotta M, Venuti A, Maugeri-Saccà M, et al. Immunotherapy in HER2-positive breast cancer: state of the art and future perspectives. J Hematol Oncol. 2019;12(1):111. doi: 10.1186/s13045-019-0798-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murthy R, Borges VF, Conlin A, Chaves J, Chamberlain M, Gray T, et al. Tucatinib with capecitabine and trastuzumab in advanced HER2-positive metastatic breast cancer with and without brain metastases: a non-randomised, open-label, phase 1b study. Lancet Oncol. 2018;19(7):880–888. doi: 10.1016/S1470-2045(18)30256-0. [DOI] [PubMed] [Google Scholar]
- 47.Lin NU, Borges V, Anders C, Murthy RK, Paplomata E, Hamilton E, et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. J Clin Oncol. 2020;38(23):2610–2619. doi: 10.1200/JCO.20.00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cuello M, Ettenberg SA, Clark AS, Keane MM, Posner RH, Nau MM, et al. Down-regulation of the erbB-2 receptor by trastuzumab (herceptin) enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in breast and ovarian cancer cell lines that overexpress erbB-2. Cancer Res. 2001;61(12):4892–4900. [PubMed] [Google Scholar]
- 49.Junttila TT, Akita RW, Parsons K, Fields C, Lewis Phillips GD, Friedman LS, et al. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009;15(5):429–440. doi: 10.1016/j.ccr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 50.Molina MA, Codony-Servat J, Albanell J, Rojo F, Arribas J, Baselga J. Trastuzumab (herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells. Cancer Res. 2001;61(12):4744–4749. [PubMed] [Google Scholar]
- 51.Varchetta S, Gibelli N, Oliviero B, Nardini E, Gennari R, Gatti G, et al. Elements related to heterogeneity of antibody-dependent cell cytotoxicity in patients under trastuzumab therapy for primary operable breast cancer overexpressing Her2. Cancer Res. 2007;67(24):11991–11999. doi: 10.1158/0008-5472.CAN-07-2068. [DOI] [PubMed] [Google Scholar]
- 52.Nahta R, Takahashi T, Ueno NT, Hung MC, Esteva FJ. P27(kip1) down-regulation is associated with trastuzumab resistance in breast cancer cells. Cancer Res. 2004;64(11):3981–3986. doi: 10.1158/0008-5472.CAN-03-3900. [DOI] [PubMed] [Google Scholar]
- 53.Zhang H, Berezov A, Wang Q, Zhang G, Drebin J, Murali R, et al. ErbB receptors: from oncogenes to targeted cancer therapies. J Clin Invest. 2007;117(8):2051–2058. doi: 10.1172/JCI32278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4(12):988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 55.Derakhshani A, Rezaei Z, Safarpour H, Sabri M, Mir A, Sanati MA, et al. Overcoming trastuzumab resistance in HER2-positive breast cancer using combination therapy. J Cell Physiol. 2020;235(4):3142–3156. doi: 10.1002/jcp.29216. [DOI] [PubMed] [Google Scholar]
- 56.Brandão M, Pondé NF, Poggio F, Kotecki N, Salis M, Lambertini M, et al. Combination therapies for the treatment of HER2-positive breast cancer: current and future prospects. Expert Rev Anticancer Ther. 2018;18(7):629–649. doi: 10.1080/14737140.2018.1477596. [DOI] [PubMed] [Google Scholar]
- 57.Paplomata E, O’Regan R. The PI3K/AKT/mTOR pathway in breast cancer: targets, trials and biomarkers. Ther Adv Med Oncol. 2014;6(4):154–166. doi: 10.1177/1758834014530023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luque-Cabal M, García-Teijido P, Fernández-Pérez Y, Sánchez-Lorenzo L, Palacio-Vázquez I. Mechanisms behind the resistance to trastuzumab in HER2-amplified breast cancer and strategies to overcome it. Clin Med Insights Oncol. 2016;10(Suppl 1):21–30. doi: 10.4137/CMO.S34537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maier LA, Xu FJ, Hester S, Boyer CM, McKenzie S, Bruskin AM, et al. Requirements for the internalization of a murine monoclonal antibody directed against the HER-2/neu gene product c-erbB-2. Cancer Res. 1991;51(19):5361–5369. [PubMed] [Google Scholar]
- 60.Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6(2):117–127. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 61.Scaltriti M, Rojo F, Ocaña A, Anido J, Guzman M, Cortes J, et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst. 2007;99(8):628–638. doi: 10.1093/jnci/djk134. [DOI] [PubMed] [Google Scholar]
- 62.Pereira PMR, Sharma SK, Carter LM, Edwards KJ, Pourat J, Ragupathi A, et al. Caveolin-1 mediates cellular distribution of HER2 and affects trastuzumab binding and therapeutic efficacy. Nat Commun. 2018;9(1):5137. doi: 10.1038/s41467-018-07608-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pietilä M, Sahgal P, Peuhu E, Jäntti NZ, Paatero I, Närvä E, et al. SORLA regulates endosomal trafficking and oncogenic fitness of HER2. Nat Commun. 2019;10(1):2340. doi: 10.1038/s41467-019-10275-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li BT, Michelini F, Misale S, Cocco E, Baldino L, Cai Y, et al. HER2-mediated internalization of cytotoxic agents in ERBB2 amplified or mutant lung cancers. Cancer Discov. 2020;10(5):674–687. doi: 10.1158/2159-8290.CD-20-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moulder SL, Borges VF, Baetz T, Mcspadden T, Fernetich G, Murthy RK, et al. Phase I study of ONT-380, a HER2 inhibitor, in patients with HER2+-advanced solid tumors, with an expansion cohort in HER2+ metastatic breast cancer (MBC) Clin Cancer Res. 2017;23(14):3529–3536. doi: 10.1158/1078-0432.CCR-16-1496. [DOI] [PubMed] [Google Scholar]
- 66.Borges VF, Ferrario C, Aucoin N, Falkson CI, Khan QJ, Krop IE, et al. Efficacy results of a phase 1b study of ONT-380, a CNS-penetrant TKI, in combination with T-DM1 in HER2+ metastatic breast cancer (MBC), including patients (pts) with brain metastases. J Clin Oncol. 2016;34(Suppl 15):513. doi: 10.1200/JCO.2016.34.15_suppl.513. [DOI] [Google Scholar]
- 67.Dinkel V, Anderson D, Winski S, Winkler J, Koch K, Lee PA. ARRY-380, a potent, small molecule inhibitor of ErbB2, increases survival in intracranial ErbB2+ xenograft models in mice. Cancer Res. 2012;72(Suppl 8):852. doi: 10.1158/1538-7445.AM2012-852. [DOI] [Google Scholar]
- 68.Vo AC, Walker L, Hausman D, Peterson S. Abstract B152: Clinical pharmacokinetics of an improved tablet formulation of ONT-380 in HER2+ metastatic breast cancer patients. Mol Cancer Ther. 2015;14(12 Supplement 2):B152. doi: 10.1158/1535-7163.TARG-15-B152. [DOI] [Google Scholar]
- 69.Kulukian A, Lee P, Taylor J, Rosler R, de Vries P, Watson D, et al. Preclinical activity of her2-selective tyrosine kinase inhibitor tucatinib as a single agent or in combination with trastuzumab or docetaxel in solid tumor models. Mol Cancer Ther. 2020;19(4):976–987. doi: 10.1158/1535-7163.MCT-19-0873. [DOI] [PubMed] [Google Scholar]
- 70.O’Sullivan CC, Davarpanah NN, Abraham J, Bates SE. Current challenges in the management of breast cancer brain metastases. Semin Oncol. 2017;44(2):85–100. doi: 10.1053/j.seminoncol.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 71.Kunte S, Abraham J, Montero AJ. Novel HER2-targeted therapies for HER2-positive metastatic breast cancer. Cancer. 2020;126(19):4278–4288. doi: 10.1002/cncr.33102. [DOI] [PubMed] [Google Scholar]
- 72.Kaur S, Bhattacharyya R, Banerjee D, Shukla J. Application of tucatinib and trastuzumab: dual anti HER2 therapy against HER2 positive breast cancer. Indian J Clin Biochem. 2021;36(1):124–125. doi: 10.1007/s12291-019-00863-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hudis CA. Trastuzumab–mechanism of action and use in clinical practice. N Engl J Med. 2007;357(1):39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 74.Zhao J, Mohan N, Nussinov R, Ma B, Wu WJ. Trastuzumab blocks the receiver function of HER2 leading to the population shifts of HER2-containing homodimers and heterodimers. Antibodies. 2021;10(1):7. doi: 10.3390/antib10010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Conlon NT, Kooijman JJ, van Gerwen SJC, Mulder WR, Zaman GJR, Diala I, et al. Comparative analysis of drug response and gene profiling of HER2-targeted tyrosine kinase inhibitors. Br J Cancer. 2021;124(7):1249–1259. doi: 10.1038/s41416-020-01257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zimmer AS, Van Swearingen AED, Anders CK. HER2-positive breast cancer brain metastasis: a new and exciting landscape. Cancer Rep. 2022;5(4):e1274. doi: 10.1002/cnr2.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Borges VF, Ferrario C, Aucoin N, Falkson C, Khan Q, Krop I, et al. Tucatinib combined with ado-trastuzumab emtansine in advanced ERBB2/HER2-positive metastatic breast cancer: a phase 1b clinical trial. JAMA Oncol. 2018;4(9):1214–1220. doi: 10.1001/jamaoncol.2018.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Metzger Filho O, Leone JP, Li T, Tan-Wasielewski Z, Trippa L, Barry WT, et al. Phase I dose-escalation trial of tucatinib in combination with trastuzumab in patients with HER2-positive breast cancer brain metastases. Ann Oncol. 2020;31(9):1231–1239. doi: 10.1016/j.annonc.2020.05.014. [DOI] [PubMed] [Google Scholar]
- 79.Murthy RK, O’Brien B, Berry DA, Singareeka-Raghavendra A, Monroe MG, Johnson J, et al. Abstract PD4-02: Safety and efficacy of a tucatinib-trastuzumab-capecitabine regimen for treatment of leptomeningeal metastasis (LM) in HER2-positive breast cancer: Results from TBCRC049, a phase 2 non-randomized study. Cancer Res. 2022;82(4_Supplement):PD4-02.
- 80.Bartsch R. ASCO 2020: highlights in breast cancer. Memo. 2021;14(1):58–61. doi: 10.1007/s12254-021-00674-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Strickler JH, Ng K, Cercek A, Fountzilas C, Sanchez FA, Hubbard JM, et al. MOUNTAINEER: open-label, Phase II study of tucatinib combined with trastuzumab for HER2-positive metastatic colorectal cancer (SGNTUC-017, trial in progress). J Clin Oncol. 2021;39(Sppl 3):TPS153.
- 82.Strickler JH, Nakamura Y, Yoshino T, Catenacci DVT, Janjigian YY, Barzi A, et al. MOUNTAINEER-02: Phase II/III study of tucatinib, trastuzumab, ramucirumab, and paclitaxel in previously treated HER2+ gastric or gastroesophageal junction adenocarcinoma—trial in progress. J Clin Oncol. 2021;39(Suppl 3):TPS252.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.