Abstract
Background
Immunoscore from tumor tissues was initially established to evaluate the prognosis of solid tumor patients. However, the feasibility of circulating immune score (cIS) for the prognosis of advanced gastrointestinal cancers (AGC) has not been reported.
Material and methods
Peripheral venous blood was collected from 64 untreated AGC patients. We utilized flow cytometry to determine several immune cell subpopulations, including CD8+ and CD4+ T cells, NK cells, and CD4 + CD25 + CD127low Tregs. The circulating immune score 1 (cIS1) was assessed according to the proportions of CD4+, CD8+ T cells, and NK cell, whereas circulating immune score 2 (cIS2) was derived from the proportions of CD4+, CD8+ T cell, and CD4 + CD25 + CD127low Tregs. The prognostic role of cIS for progression-free survival (PFS) and overall survival (OS) was analyzed using Kaplan–Meier curves and Cox multivariate models. Receiver operating characteristic (ROC) curves were depicted to compare the prognostic values of cIS1 and cIS2.
Results
AGC patients with high cIS1(≥ 2) and cIS2(≥ 2) had significantly longer PFS (cIS1: median PFS, 11 vs. 6.7 months, P = 0.001; cIS2: 12 vs. 5.8 months, P < 0.0001) and OS (cIS1: median OS, 12 vs. 7.9 months, P = 0.0004; cIS2: 12.8 vs. 7.4 months, P < 0.0001) than those with low cIS1 and low cIS2. The areas under ROC curves (AUROCs) of cIS1 and cIS2 for OS were 0.526 (95% confidence interval; 95% CI 0.326–0.726) and 0.603 (95% CI 0.427–0.779, P = 0.332), whereas AUROC of cIS2 for PFS was larger than that of cIS1 0.735 (95% CI 0.609–0.837) vs 0.625 (95% CI 0.495–0.743) (P = 0.04)).
Conclusion
The cIS can be applied to predict the prognosis of untreated AGC patients. Compared with cIS1, cIS2 displayed superior prognostic value for PFS prediction.
Keywords: Gastrointestinal cancer, Circulating immune score, Circulating immune cells, Prognosis
Introduction
Gastrointestinal cancer (GC) is one of the most prevalent digestive tract tumors, and the leading cause of cancer-related morbidity and mortality worldwide [1]. Approximately 30–35% of gastric cancer patients presented with distant site metastases at the time of diagnosis [2], while 40–60% of colorectal cancer (CRC) patients have distant metastasis, of which 15–20% are simultaneous metastasis [3, 4]. Less than 25% of patients with advanced CRC are candidates for radical resection of liver and lung metastatic lesions [5]. However, those with peritoneal dissemination are less likely to undergo R0 resection or have a low survival rate [6, 7]. Patients with metastatic gastrointestinal tumors experience a high recurrence rate and low survival rate even if metastatic resection is feasible [8, 9]. Numerous traditional clinicopathological factors, including differentiated subtypes, lymph node condition of the primary tumor, distant metastatic sites, tumor burden, and feasibility of surgical resection, have been closely associated with patient survival in AGC. Various clinical scoring systems employ these indicators to predict patient prognosis [10, 11]. However, prognosis still varies even when patients receive the same treatment, demonstrating that these clinical indicators are not the only element affecting survival.
In recent years, tumor immune microenvironment has been critical in the occurrence and development of gastrointestinal tumors, tumor infiltration by T lymphocytes, including helper T cells (CD4+), cytotoxic T cells (CD8+), and T regulatory cells (Tregs, FOXp3+), is known to predict prognosis in multiple cancers including breast cancer, colorectal cancer, head and neck carcinoma, lung cancer, and esophageal cancer [12–20]. Immunoscore constructed by researchers, which considered the infiltration level of T cell subtype, B cell subtype or macrophage in tumor microenvironment (TME) of primary lesions or metastases, is widely applied to predict the prognosis in patients with gastrointestinal tumors [21–25]. In addition, more and more peripheral-related indicators are used to predict the prognosis of solid tumor patients [26–28]. The simplicity, reproducibility and accuracy of these scoring systems make them the most popular prediction models in clinical practice.
Considering that some patients with advanced tumors cannot acquire sufficient amounts of high-quality tissue specimens for quantitative analysis of tumor-associated immune cells and previous findings, we wondered whether the immune score could be extended to circulatory blood systems and identified it as a predictor of prognosis for unresectable AGC patients. Our research aimed to (1) construct a circulating immune score by determining the main subtypes of circulating immune cells and evaluating its prognostic effect on patients with untreated advanced gastrointestinal tumors and (2) compare the sensitivity and specificity of different circulating immune scoring systems for prognosis.
Methods and materials
Patient selection
This study included consecutive AGC patients who did not receive palliative treatment between July 2018 and April 2021 at the First Affiliated Hospital of University of Science and Technology in China. The inclusion criteria in this study are as follows: (1) pathologically diagnosed patients with gastric or colorectal carcinomas, and the existence of at least a distant metastatic site, (2) untreated AGC patients prior to diagnosis, (3) follow-up period greater than 6 months, and (4) existence of adequate pathological information and blood specimens for analysis. Exclusion criteria comprised those who (1) had autoimmune diseases or (2) were treated with long-term oral immunosuppressive therapy. The protocol for this research was approved by Institutional Review Board (IRB). All subjects provided written consent to participate in this investigation.
Data collection
The patients were followed-up until November 2021 by medical records (inpatient and outpatient) or by telephone contact with patients or relatives aware of their illness. Clinical and pathological data were collected from electronic inpatient records. The data included pathological and histological type, distant metastatic site, expression of CEA and CA199 before the first treatment, ascites existence, physical status, etc. The expression proportion of circulating immune cell subtypes, including CD3+ T cells, CD4+ T cells, CD8+ T cells, NK cells, CD4+ CD25+ T cells, as well as the ratio of CD4+ CD25+ CD127low regulatory T cells (Tregs), were specifically recorded.
Flow cytometry
Peripheral blood samples were obtained before initiating therapy. Peripheral blood mononuclear cells (PBMCs) were splintered off from heparinized peripheral blood. Freshly isolated PBMCs or sorted cells (1 × 106 cells) were stained with UCHT1-anti-CD3 (BioLegend, USA), RPA-T4-anti-CD4 (BioLegend, USA), HIT8a-anti-CD8 (Bio Legend, USA), MEM-188-anti-CD56 (BioLegend, USA), and APC-conjugated anti-CD127 (BioLegend, USA). The cells were then analyzed using a Navios flow cytometer (Beckman Coulter, USA) according to manufacturer’s instructions. The initial singlet gate was set on the lymphocytes on forward scatter and side scatter dot plots to depict immune cells to be analyzed in this study.
Immune cell quantification
The best cutoff value of the expression proportion for each circulating immune cell subtype was selected by X-tile software V. 3.6.1. This software was used to measure the cutoff value of the expression proportions of CD3+ T cells, CD4+ T cells, CD8+ T cells, NK cells, CD4+ CD25+ T cells and CD4+ CD25+ CD127low Tregs in patients, and for PFS, the best cutoff values were 58.1%, 29.5%, 19.3%, 17.7%, 14.6%, and 3.7%, respectively. For OS, the best cutoff points were 54.3%, 24.3%, 19.3%, 17.7%, 12.0%, and 4.0%, respectively. Each circulating immune cell subtype was given a dichotomous score (high expression levels of CD3+, CD4+, CD8+, NK cells scored 1, low expression level scored 0; low expression levels of CD4+ CD25+ CD127low Tregs scored 1, high expression level scored 0) based on the set cutoff point. The circulating immune score 1 (cIS1) was based on expression levels of CD4+ T cells, CD8+ T cells, and NK cells. Patients with a cIS1 ≥ 2 points were classified as a high-score group (cIS1-high), whereas those with a cIS1 < 2 were classified as a low-score group (cIS1-low). Circulating immune score 2 (cIS2) was established according to expression levels of CD4+, CD8+, and CD4+ CD25+ CD127low immune cells. Patients with cIS2 ≥ 2 points were classified as the high-score group (cIS2-high), whereas those with cIS2 < 2 were classified as the low-score group (cIS2-low).
Statistical analysis and endpoint
The statistical analyses were executed using IBM SPSS software (version 22), GraphPad Prism (version 8.0.1), X-tile software (version 3.6.1), and MedCalc (version 19.0.4). The univariate and multivariate analyses were conducted using Cox proportional hazards model to select independent prognostic variables of prognosis. The optimal cutoff value for prognosis was determined based on X-tile software, and all patients were classified into high-level and low-level subgroups. The prognostic value of circulating immune score and various clinicopathological factors was analyzed using Kaplan-Meier curve and log-rank test. Cox multivariate models for PFS and OS were conducted for multivariate analysis to clear independent prognostic factors affecting survival of AGC patients. Receiver operating characteristic (ROC) curves were conducted to compare the predictive ability of cIS1 system with cIS2 system for PFS and OS. The primary endpoints of this study were OS and PFS. OS was defined as the time from the date of diagnosis to the date of death or last follow-up. PFS was defined as the time from the beginning of diagnosis to the date of diagnosis of treatment failure, death, or the last follow-up. P values < 0.05 using two-sided tests were considered statistically significant.
Results
Basic characteristics of AGC patients
This study retrospectively analyzed 64 untreated patients with an initial diagnosis of advanced gastrointestinal cancer. The average age of all patients was 62 years, including 18 (28.1%) females and 46 (71.9%) males. There were 36 cases (56.3%) of colorectal cancer and 28 cases (43.7%) of gastric cancer. A total of 14.1% of AGC patients were histologically diagnosed with mucinous adenocarcinoma or signet ring cell carcinoma. A total of 27 (42.2%) cases manifested with G1-2 histological grade, 31 (48.4%) cases with G3, whereas histological grade of 6 (9.4%) cases was unknown. At baseline, the imaging identified 48 (75%) patients with visceral metastasis and 33 (51.6%) patients with distant lymph node metastasis. The median expressing level of CD3+ T cells in peripheral blood was 68.5% (interquartile range, IQR, 60.65–76.28%), whereas that of CD8+ T cells was 28.85% (IQR, 20.28–34.23%). The median expressing level of CD4+ T cells was 37.15% (IQR, 28.65–41.18%), that of NK cells was 17.1% (IQR, 11.2–22.3%), while that of the ratio of CD4+CD25+CD127low Tregs was 4.25% (IQR, 3.23–5.08%). These clinical characteristics are presented in Table 1.
Table 1.
Clinical characteristics of patients with advanced gastrointestinal cancers
| Variables | N (%) |
|---|---|
| Age, (mean) | 62 (28–82) |
| Sex | |
| Female | 18 (28.1) |
| Male | 46 (71.9) |
| ECOG | |
| 0–1 | 52 (81.3) |
| 2 | 12 (18.7) |
| Primary cancer | |
| Colorectal cancer | 36 (56.3) |
| Gastric cancer | 28 (43.7) |
| Pathological type | |
| Non-adenocarcinoma/signet-ring cell cancer | 55 (85.9) |
| Adenocarcinoma/Signet-ring cell cancer | 9 (14.1) |
| Grade | |
| G1–2 | 27 (42.2) |
| G3 | 31 (48.4) |
| Unknown | 6 (9.4) |
| CEA | |
| Abnormal | 31 (48.4) |
| Normal | 33 (51.6) |
| CA199 | |
| Abnormal | 24 (37.5) |
| Normal | 40 (62.5) |
| Visceral metastasis | |
| Yes | 48 (75.0) |
| No | 16 (25.0) |
| Lymph node metastasis | |
| Yes | 33 (51.6) |
| No | 31 (48.4) |
| Disease progression | |
| Yes | 31 (48.4) |
| No | 33 (51.6) |
| Survival status | |
| Yes | 10 (15.6) |
| No | 54 (84.4) |
Prognostic role of different cIS in AGC patients
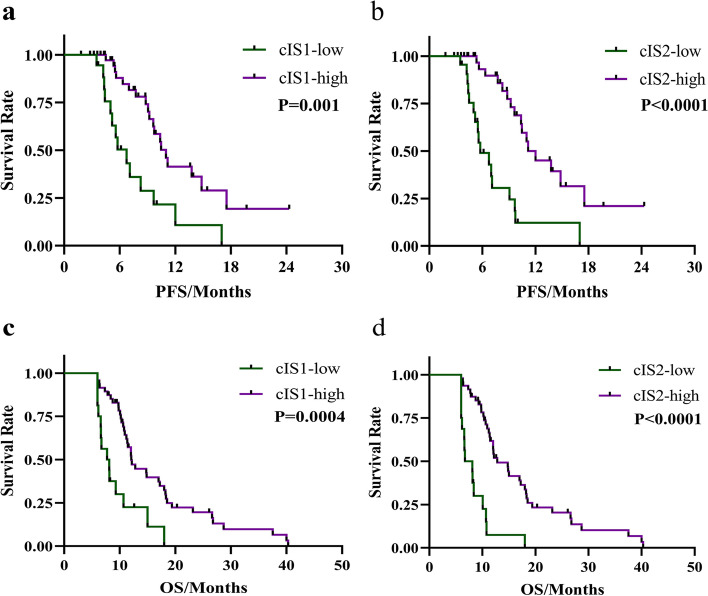
Of 64 AGC patients who were followed up to November 2021, 48.4% had disease progression, while 15.6% died. The lost to follow-up rate was 3% (Two patients were lost to follow up). The median follow-up time was 11 months (IQR, 8.03–17.17 months). A total of 44 (68.7%) and 20 (31.3%) patients were categorized into high and low cIS1 subgroups, whereas 41(64.1%) and 23 (35.9%) were categorized into high and low cIS2 subgroups. The median PFS of cIS1-high and cIS1-low subgroups was 11 and 6.7 months, respectively (P = 0.001, Fig. 1a). The median PFS of cIS2-high and cIS2-low subgroups was 12 and 5.8 months, respectively (P < 0.0001, Fig. 1b). The difference in OS between high-cIS and low-cIS subgroups reach statistical significance (cIS1: median OS, 12 vs. 7.9 months, P = 0.0004 (Fig. 1c); cIS2: 12.8 vs. 7.4 months, P < 0.0001 (Fig. 1d)).
Fig. 1.
Kaplan-Meier estimates and log-rank test PFS according to cIS1 (a) and cIS2 score (b). Kaplan-Meier estimates and log-rank test OS according to cIS1 (c) and cIS2 score (d). a, c Patients with cIS1 ≥ 2 and cIS1 < 2 shown in purple and green, respectively. b, d Patients with cIS2 ≥ 2 and cIS2 < 2 displayed in purple and green, respectively. cIS = circulating immune score
Prognostic factors in univariate and multivariate analyses
For PFS, the results of univariate analysis of subtypes of circulating immune cells displayed that the expression level of CD4+ T cell (high vs. low, HR, 0.41, 95% CI (0.17–0.97), P = 0.01), CD8+ T cell (high vs. low, HR, 0.47, 95% CI (0.20–1.11), P = 0.03), CD4 + CD25 + CD127low Treg (high vs. low, HR, 2.75, 95% CI (1.33–5.69), P= 0.027), cIS1 score (cIS1-high vs. cIS1-low, HR, 0.34, 95% CI (0.14–0.80) P = 0.001), and cIS2 score (cIS2-high vs. cIS2-low, HR, 0.26, 95% CI (0.11–0.59), P < 0.0001) had prognostic value. Other immune cell subtypes and clinicopathological factors were not significantly linked to PFS. In univariate analysis, it was found that the factors related to OS included the expression levels of CD8+ T cell (high vs. low, HR, 0.44, 95% CI (0.19–0.99), P = 0.006), CD4 + CD25+CD127low Treg (high vs. low, HR, 1.87, 95% CI (1.10–3.20), P = 0.02), cIS1 score (cIS1-high vs. cIS1-low, HR, 0.36, 95% CI (0.16–0.84) P = 0.0004) and cIS2 score (cIS2-high vs. cIS2-low, HR, 0.26, 95% CI (0.10–0.66), P < 0.0001) (Table 2). After adjusting for clinicopathologic variables, multivariate Cox regression analyses revealed that both circulating immune scoring systems were independent predictors of PFS and OS (PFS: cIS1 score (cIS1-high vs. cIS1-low, HR, 0.41, 95% CI (0.19–0.86) P = 0.019), cIS2 score (cIS2-high vs. cIS2-low, HR, 0.16, 95% CI (0.06–0.4), P < 0.001). OS: cIS1 score (cIS1-high vs. cIS1-low, HR, 0.34, 95% CI (0.17–0.67) P = 0.002), cIS2 score (cIS2-high vs. cIS2-low, HR, 0.14, 95% CI (0.06–0.32), P<0.001) (Table 3).
Table 2.
Univariate analysis for the prognosis of patients with advanced gastrointestinal cancers
| Variables | PFS | OS | ||
|---|---|---|---|---|
| Univariate analysis | Univariate analysis | |||
| HR (95% CI) | P value | HR (95% CI) | P values | |
| Age, years | ||||
| < 69 vs ≥ 69 | 0.53 (0.25–1.16) | P = 0.11 | 0.61 (0.31–1.20) | P = 0.08 |
| Sex | ||||
| Female vs male | 0.58 (0.28–1.21) | P = 0.15 | 0.76 (0.43–1.35) | P = 0.36 |
| CEA | ||||
| Normal vs abnormal | 0.98 (0.48–1.97) | P = 0.95 | 0.98 (0.57–1.67) | P = 0.93 |
| CA199 | ||||
| Normal vs abnormal | 0.51 (0.25–1.01) | P = 0.06 | 0.63 (0.37–1.09) | P = 0.41 |
| Grade | ||||
| G1–2 vs G3 | 0.76 (0.37–1.57) | P = 0.47 | 0.77 (0.43–1.36) | P = 0.37 |
| Lymph node metastasis | ||||
| No vs yes | 0.54 (0.27–1.10) | P = 0.07 | 0.81 (0.47–1.37) | P = 0.40 |
| Visceral metastasis | ||||
| No vs yes | 0.84 (0.38–1.86) | P = 0.64 | 0.96 (0.51–1.80) | P = 0.90 |
| CD3 + cell | ||||
| High vs low | 0.40 (0.13–1.27) | P = 0.023 | 0.36 (0.09–1.52) | P = 0.02 |
| CD4 + cell | ||||
| High vs low | 0.41 (0.17–0.97) | P = 0.01 | 0.40 (0.14–0.10) | P = 0.07 |
| CD8 + cell | ||||
| High vs low | 0.47 (0.20–1.11) | P = 0.03 | 0.44 (0.19–0.99) | P = 0.006 |
| NK cell | ||||
| High vs low | 1.92 (0.93–3.96) | P = 0.053 | 1.53 (0.86–2.70) | P = 0.11 |
| CD4 + CD25+CD127low | ||||
| High vs low | 2.75 (1.33–5.69) | P = 0.027 | 1.87 (1.10–3.20) | P = 0.02 |
| cIS1 | ||||
| High vs low | 0.34 (0.14–0.80) | P = 0.001 | 0.36 (0.16–0.84) | P = 0.0004 |
| cIS2 | ||||
| High vs low | 0.26 (0.11–0.59) | P < 0.0001 | 0.26 (0.10–0.66) | P < 0.0001 |
Table 3.
Multivariate analyses of prognostic factors of patients with advanced gastrointestinal cancers
| OS | Multivariate analysis 1 | Multivariate analysis 2 | ||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Sex | ||||
| Female vs male | 0.87 (0.46–1.63) | P = 0.66 | 0.75 (0.39–1.42) | P = 0.37 |
| Age | ||||
| < 69 vs ≥ 69 | 0.78 (0.40–1.50) | P = 0.45 | 1.41 (0.65–3.06) | P = 0.39 |
| Lymph node metastasis | ||||
| Yes vs no | 1.42 (0.82–2.46) | P = 0.22 | 1.66 (0.93–2.95) | P = 0.084 |
| cIS1 | ||||
| High vs low | 0.34 (0.17–0.67) | P = 0.002 | ||
| cIS2 | ||||
| High vs low | 0.14 (0.06–0.32) | P < 0.001 | ||
| PFS | Multivariate analysis 1 | Multivariate analysis 2 | ||
| Sex | ||||
| Female vs male | 0.68 (0.28–1.65) | P = 0.40 | 0.51 (0.20–1.28) | P = 0.15 |
| Age | ||||
| < 69 vs ≥ 69 | 0.68 (0.30–1.46) | P = 0.31 | 1.38 (0.52–3.66) | P = 0.52 |
| Lymph node metastasis | ||||
| Yes vs no | 2.03 (0.98–4.22) | P = 0.056 | 2.67 (1.26–5.68) | P = 0.10 |
| cIS1 | ||||
| High vs low | 0.41 (0.19–0.86) | P = 0.019 | ||
| cIS2 | ||||
| High vs low | 0.16 (0.06–0.4) | P < 0.001 | ||
The bold value indicates P < 0.05, HR Hazard ratio, CI Confidence interval, sex, age, lymph node metastasis, and cIS1 were included in multivariate analysis 1; sex, age, lymph node metastasis and cIS2 were included in multivariate analysis 2
Comparison of cIS1 and cIS2
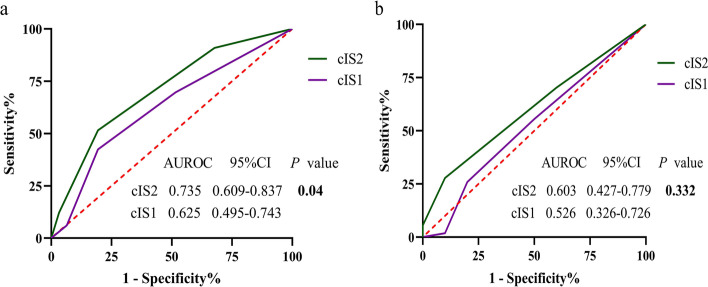
ROC curves were performed to compare sensitivity and specificity of the predictive ability between cIS1 and cIS2 systems. The cIS2 corresponded to a relatively larger AUROC for PFS prediction than cIS1 system, with statistically significant difference (0.735 (95% CI 0.609–0.837) vs. 0.625 (95% CI 0.495–0.743) (P = 0.04)) (Fig. 2a). However, when OS prediction was compared, no statistically significant difference was observed between the two circulating immune scoring systems (P = 0.332, Fig. 2b).
Fig. 2.
Comparison of the sensitivity and specificity for predicting PFS and OS of AGC patients with cIS1 and cIS2 systems. a ROC curves displayed the significant predictive values of both systems for PFS prediction, and cIS2 system was superior to cIS1 system in PFS prediction. b ROC curves indicated the difference between the two systems was insignificant for OS prediction. cIS = circulating immune score
Discussion
In the current study, the circulating immune score system was first applied to assess the prognostic value in AGC patients. The results suggested that cIS system had the potential to predict the outcome of untreated AGC patients. This study successfully created a novel cIS1 scoring model based on an estimated total percentage of CD4+ T cells, CD8+ T cells, and NK cells in the peripheral blood, as well as a cIS2 scoring system based on the proportions of peripheral CD4+ T cells, CD8+ T cells, and CD4 + CD25 + CD127low Tregs. Both scoring systems could serve as an effective index in predicting the outcome of AGC patients in either PFS or OS; high cIS was significantly associated with extended OS and PFS in the survival analysis.
For patients with resectable gastrointestinal cancer, various studies have demonstrated that individual tumor-infiltrating lymphocyte subtypes and the ratio of subsets in tumor microenvironment (TME) are critical predictors of survival and recurrence [13, 18, 21, 29–31]. In recent years, accumulating studies have employed TIL to create an immune scoring system to predict the prognosis of patients with non-advanced gastric cancer or colorectal cancer. Meanwhile, some researchers have investigated the infiltration of tumor-associated immune cells in the metastatic foci of gastrointestinal tumors to establish a novel immune scoring system to analyze its prognostic role in metastatic gastrointestinal tumors. Pagès et al. stated that patients with stage I–III colon cancer with a high immunoscore were associated with the lowest risk of 5-year recurrence [23]. Similarly, Jiang et al. revealed that for patients with stage II-III gastric cancer, compared with the low immunoscore group, the high immunoscore predicted higher 5-year disease-free survival (45.0% vs. 4.4%, respectively; P < 0.001) and 5-year overall survival (48.8% vs. 6.7%, respectively; P < 0.001) [32]. IS and TB scoring systems of metastatic focus constructed by Wang et al. and Mlecnik et al. using immune cell subtypes after resection of common metastatic sites of colorectal cancer (liver, lung, peritoneum, etc.) also revealed good independent predictive values [22, 24]. At present, increasing studies on the exploratory analysis of immune cell subtypes in peripheral blood, regarding its prognostic value, indicated that expression levels of circulating regulatory T cells, NK cells, CD3+ T cells, CD4+ T cells, and CD8+ T cells were also significantly linked to survival [33–36]. Although the above immune score concerning TME demonstrates good predictive function, its evaluation process remains very complex, limiting its extensive application in clinic.
This study performed immune scoring according to peripheral blood immune cell subtypes of AGC patients, which was applied to patients with initially resectable or initially unresectable advanced tumors. Although previous studies involving the immunoscore of TME have confirmed that it could be used as a supplement to TNM staging, and even the prediction efficiency was better than TNM staging, the evaluation criteria for immune cells of each study were different. These researches required sufficient tissue samples and professional pathologists to distinguish types, density, functional localization, and location of adaptive immune cells in different tumor regions [23, 32]. For AGC patients under heavy tumor burden with the inability to obtain enough tumor tissue samples due to loss of operation opportunity, the immune score for TME could not be evaluated. Our current study expanded the immunoscore from TME to peripheral blood and preliminarily explored the prognostic prediction of initial untreated AGC using the circulating immune score constructed by peripheral blood immune cells subtypes. The preliminary results indicated that cIS had a certain predictive value and was straightforward and feasible; it was not limited to whether patients with tumors could obtain sufficient tumor tissue specimens.
CD8+ T and CD3+ T cells were significant subtypes of tumor-related immune cells; the former played an anti-tumor role directly or indirectly through cytotoxicity, and the latter activated and promoted proliferation and acted as effector molecules of CD8+ T and NK cells. In solid tumors, the high-density infiltration of these cells was strongly linked to a favorable prognosis [13, 18, 37, 38]. Tumor tissue-derived and peripheral blood-derived NK cells are damaged in vivo in cancer patients. On the one hand, it might be due to depletion and functional destruction of functional NK cells. On the other hand, it might be due to the existence of immunosuppressive targets (such as PD-L1) in malignant tumor patients, resulting in the damage of NK cell-induced ADCC cytotoxicity. In previous studies, such damage indicated tumor progression and poor prognosis [39–41]. Pernot et al. revealed that a high count of circulating NK correlates with a better prognosis in gastric cancer [36]. CD4+ CD25+ CD127low/-T cell population had the most typical Treg characteristics. CD127 with low or no expression on CD4+ CD25+ markers could recognize Treg more accurately than the combination of other markers [42]. Treg cells promote immunosuppression by inhibiting immune response to cancer cells, and the expression of CD4+ CD25+ CD127low Tregs had a positive correlation with TGF-β1 and IL-10 expressions and was closely linked to tumor occurrence and development and immunotherapy reactivity [33, 43]. Our study indicated that individual circulating tumor-related immune cells also demonstrate prognostic value similar to previous studies. In addition, the scoring system constructed by combining multiple circulating immune cells for the first time displayed good performance in predicting clinical outcomes. Moreover, this study revealed that the prediction performance of cIS2 based on CD4+ T cells, CD8+ T cells, and Treg cells outperforms that of cIS1 based on CD4+ T cells, CD8+ T cells, and NK cells, which also provides a certain direction for our future research on peripheral blood immune score.
This study displayed the first use of circulating immune score based on multiple immune cell subtypes in peripheral blood to predict prognosis in AGC patients. This study included patients with gastrointestinal tumors and simultaneous metastasis and was untreated initially, avoiding the impact of systematic treatment on the patient's immune system before collecting blood samples. However, this research was designed as a preliminary exploratory study and lacked independent validation set for external or internal validation of the immune score, which crippled a certain degree of credibility. In addition, current results were limited by small sample size, single-center, and retrospective design. Inconsistencies in the follow-up treatment of selected patients, including palliative surgery or palliative chemoradiotherapy or immunotherapy, or participation in clinical trials organized by our center, would also cause inevitable confounding factors. As a result, additional investigations involving expanding the sample size and the number of research centers are necessary.
Conclusion
In conclusion, this study demonstrated that a circulating immune score system could be deployed to predict the prognosis of patients with initial untreated AGC. We confirmed that cIS1 system constructed by combining the expression proportions of CD4+, CD8+ T cells, and NK cells, as well as cIS2 system constructed by combining CD3+ T cells, CD8+ T cells, and CD4+ CD25+ CD127low Tregs, could successfully predict PFS and OS of AGC patients. Furthermore, cIS2 displayed a superior prognostic value to cIS1 in PFS prediction.
Acknowledgements
Our special acknowledgments to Mr. Xiang Zhou for helping us with editing
Abbreviations
- cIS
Circulating immune score
- AGC
Advanced gastrointestinal cancers
- OS
Overall survival
- PFS
Progression-free survival
- GC
Gastrointestinal cancer
- TME
Tumor microenvironment
- PBMCs
Peripheral blood mononuclear cells
Authors’ contributions
Study concepts: Yamei Zhao and Changlu Hu. Data acquisition: Yamei Zhao. Manuscript preparation: Yamei Zhao, Yan Tang, Hanlin Qin, and Kehai Feng. Manuscript editing: Yan Tang, Hanlin Qin, and Kehai Feng. Manuscript review: Changlu Hu. The authors read and approved the final manuscript.
Funding
None
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
This study was approved by the local institutional ethical committee of Anhui Cancer Hospital, the First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yamei Zhao and Yan Tang contributed equally to this work.
References
- 1.Torre LA, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87-108. [DOI] [PubMed]
- 2.Ebinger SM, et al. Modest overall survival improvements from 1998 to 2009 in metastatic gastric cancer patients: a population-based SEER analysis. Gastric Cancer. 2016;19(3):723–734. doi: 10.1007/s10120-015-0541-9. [DOI] [PubMed] [Google Scholar]
- 3.Dromain C, et al. Liver, lung and peritoneal metastases in colorectal cancers: is the patient still curable? What should the radiologist know. Diagn Interv Imaging. 2014;95(5):513–523. doi: 10.1016/j.diii.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Vatandoust S, Price TJ, Karapetis CS. Colorectal cancer: metastases to a single organ. World J Gastroenterol. 2015;21(41):11767–11776. doi: 10.3748/wjg.v21.i41.11767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsoulfas G, Pramateftakis MG, Kanellos I. Surgical treatment of hepatic metastases from colorectal cancer. World J Gastroenterol Oncol. 2011;3(1):1–9. doi: 10.4251/wjgo.v3.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakedis J, Schmidt CR. Surgical treatment of metastatic colorectal cancer. Surg Oncol Clin N Am. 2018;27(2):377–399. doi: 10.1016/j.soc.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Veld JV, et al. Synchronous and metachronous peritoneal metastases in patients with left-sided obstructive colon cancer. Ann Surg Oncol. 2020;27(8):2762–2773. doi: 10.1245/s10434-020-08327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shida D, et al. Long-Term Outcomes After R0 resection of synchronous peritoneal metastasis from colorectal cancer without cytoreductive surgery or hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2018;25(1):173–178. doi: 10.1245/s10434-017-6133-7. [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi K, et al. The long-term survival of stage IV gastric cancer patients with conversion therapy. Gastric Cancer. 2018;21(2):315–323. doi: 10.1007/s10120-017-0738-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dias AR, et al. Prediction scores for complication and recurrence after multivisceral resection in gastric cancer. Eur J Surg Oncol. 2020;46(6):1097–1102. doi: 10.1016/j.ejso.2020.01.014. [DOI] [PubMed] [Google Scholar]
- 11.Enblad M, Ghanipour L, Cashin PH. Prognostic scores for colorectal cancer with peritoneal metastases treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Int J Hyperthermia. 2018;34(8):1390–1395. doi: 10.1080/02656736.2018.1464668. [DOI] [PubMed] [Google Scholar]
- 12.Ahmadvand S, et al. Importance of CD45RO+ tumor-infiltrating lymphocytes in post-operative survival of breast cancer patients. Cell Oncol (Dordr) 2019;42(3):343–356. doi: 10.1007/s13402-019-00430-6. [DOI] [PubMed] [Google Scholar]
- 13.Kuwahara T, et al. Intratumoural-infiltrating CD4 + and FOXP3 + T cells as strong positive predictive markers for the prognosis of resectable colorectal cancer. Br J Cancer. 2019;121(8):659–665. doi: 10.1038/s41416-019-0559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spector ME, et al. Prognostic Value of Tumor-Infiltrating Lymphocytes in Head and Neck Squamous Cell Carcinoma. JAMA Otolaryngol Head Neck Surg. 2019;145(11):1012–1019. [DOI] [PMC free article] [PubMed]
- 15.Bremnes RM, et al. The Role of tumor-infiltrating lymphocytes in development, progression, and prognosis of non-small cell lung cancer. J Thorac Oncol. 2016;11(6):789–800. doi: 10.1016/j.jtho.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Okadome K, et al. Prognostic nutritional index, tumor-infiltrating lymphocytes, and prognosis in patients with esophageal cancer. Ann Surg. 2020;271(4):693–700. doi: 10.1097/SLA.0000000000002985. [DOI] [PubMed] [Google Scholar]
- 17.Vihervuori H, et al. Tumor-infiltrating lymphocytes and CD8 T cells predict survival of triple-negative breast cancer. J Cancer Res Clin Oncol. 2019;145(12):3105–3114. doi: 10.1007/s00432-019-03036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galon J, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 19.Wang B, et al. Loss of survival advantage for deficient mismatch repair in patients with advanced colorectal cancer may be caused by changes in prognostic value of CD8+T cell. World J Surg Oncol. 2020;18(1):196. doi: 10.1186/s12957-020-01970-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Yang J. Association of tumor deposits with tumor-infiltrating lymphocytes and prognosis in gastric cancer. World J Surg Oncol. 2022;20(1):58. doi: 10.1186/s12957-022-02507-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang D, et al. Scoring System for Tumor-Infiltrating Lymphocytes and Its Prognostic Value for Gastric Cancer. Front Immunol. 2019;10:71. doi: 10.3389/fimmu.2019.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mlecnik B, et al. Comprehensive Intrametastatic Immune Quantification and Major Impact of Immunoscore on Survival. J Natl Cancer Inst. 2018;110(1):97-108. [DOI] [PubMed]
- 23.Pagès F, et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391(10135):2128–2139. doi: 10.1016/S0140-6736(18)30789-X. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, et al. The Immunoscore system predicts prognosis after liver metastasectomy in colorectal cancer liver metastases. Cancer Immunol Immunother. 2018;67(3):435–444. doi: 10.1007/s00262-017-2094-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao Y, et al. An efficient prognostic immune scoring system for colorectal cancer patients with peritoneal metastasis. Oncoimmunology. 2021;10(1):1901464. doi: 10.1080/2162402X.2021.1901464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schlanger D, et al. The role of systemic immuno-inflammatory factors in resectable pancreatic adenocarcinoma: a cohort retrospective study. World J Surg Oncol. 2022;20(1):144. doi: 10.1186/s12957-022-02606-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan J, et al. Analysis of the expression and prognosis for leukocyte immunoglobulin-like receptor subfamily B in human liver cancer. World J Surg Oncol. 2022;20(1):92. doi: 10.1186/s12957-022-02562-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai X, et al. A novel inflammation-related prognostic biomarker for predicting the disease-free survival of patients with colorectal cancer. World J Surg Oncol. 2022;20(1):79. doi: 10.1186/s12957-022-02550-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eriksen AC, et al. The prognostic value of tumor-infiltrating lymphocytes in stage II colon cancer. A Nationwide Population-Based Study. Transl Oncol. 2018;11(4):979–987. doi: 10.1016/j.tranon.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sideras K, et al. Prognostic value of intra-tumoral CD8(+) /FoxP3(+) lymphocyte ratio in patients with resected colorectal cancer liver metastasis. J Surg Oncol. 2018;118(1):68–76. doi: 10.1002/jso.25091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saito T, et al. Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med. 2016;22(6):679–684. doi: 10.1038/nm.4086. [DOI] [PubMed] [Google Scholar]
- 32.Jiang Y, et al. ImmunoScore Signature: A Prognostic and Predictive Tool in Gastric Cancer. Ann Surg. 2018;267(3):504–513. doi: 10.1097/SLA.0000000000002116. [DOI] [PubMed] [Google Scholar]
- 33.Kang DH, et al. Circulating regulatory T cells predict efficacy and atypical responses in lung cancer patients treated with PD-1/PD-L1 inhibitors. Cancer immunology, immunotherapy: CII. 2021;71(3):579-588. [DOI] [PMC free article] [PubMed]
- 34.Tang Y-P, et al. Prognostic value of peripheral blood natural killer cells in colorectal cancer. BMC Gastroenterol. 2020;20(1):31. doi: 10.1186/s12876-020-1177-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Y-Y, et al. Diagnostic and prognostic value of the peripheral natural killer cell levels in gastric cancer. Exp Ther Med. 2020;20(4):3816–3822. doi: 10.3892/etm.2020.9101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pernot S, et al. Infiltrating and peripheral immune cell analysis in advanced gastric cancer according to the Lauren classification and its prognostic significance. Gastric Cancer. 2020;23(1):73–81. doi: 10.1007/s10120-019-00983-3. [DOI] [PubMed] [Google Scholar]
- 37.Knutson KL, Disis ML. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol Immunother. 2005;54(8):721–728. doi: 10.1007/s00262-004-0653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nazemalhosseini-Mojarad E, et al. Intratumoral infiltrating lymphocytes correlate with improved survival in colorectal cancer patients: Independent of oncogenetic features. J Cell Physiol. 2019;234(4):4768–4777. doi: 10.1002/jcp.27273. [DOI] [PubMed] [Google Scholar]
- 39.Zheng Y, et al. Immune suppressive landscape in the human esophageal squamous cell carcinoma microenvironment. Nat Commun. 2020;11(1):6268. doi: 10.1038/s41467-020-20019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park J-E, et al. Anti-tumor effects of NK cells and anti-PD-L1 antibody with antibody-dependent cellular cytotoxicity in PD-L1-positive cancer cell lines. J Immunother Cancer. 2020;8(2):e000873. [DOI] [PMC free article] [PubMed]
- 41.Schantz SP, et al. Natural killer cell activity and head and neck cancer: a clinical assessment. J Natl Cancer Inst. 1986;77(4):869–875. [PubMed] [Google Scholar]
- 42.Yu N, et al. CD4(+)CD25 (+)CD127 (low/-) T cells: a more specific Treg population in human peripheral blood. Inflammation. 2012;35(6):1773–1780. doi: 10.1007/s10753-012-9496-8. [DOI] [PubMed] [Google Scholar]
- 43.Zhou W, et al. Expression of CD4+CD25+CD127 regulatory T cells and cytokines in peripheral blood of patients with primary liver carcinoma. Int J Med Sci. 2020;17(6):712–719. doi: 10.7150/ijms.44088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.