Abstract
Background
A more severe course of COVID-19 was associated with low levels of Vitamin D (VitD). Moreover in vitro data showed that VitD up-regulates the mRNA of the Angiotensin Converting Enzyme 2 (ACE-2), the SARS-COV-2 receptor in different type of cells. ACE-2 is expressed in several type of tissues including thyroid cells, on which its mRNA was shown to be up-regulated by interferon-gamma (IFN-γ). The aim of the present study was to investigate if treatment with VitD alone or in combination with IFN-γ would increase ACE-2 both at mRNA and protein levels in primary cultures of human thyrocytes.
Materials and methods
Primary thyroid cell cultures were treated with VitD and IFN-γ alone or in combination for 24 h. ACE-2 mRNA levels were measured by Real-time Polymerase Chain Reaction (RT-PCR). The presence of ACE-2 on thyroid cell membrane was assessed by immunocytochemistry basally and after the previous mentioned treatments.
Results
ACE-2 mRNA levels increased after treatment with VitD and IFN-γ alone. The combination treatment (VitD + IFN-γ) showed an additive increase of ACE-2-mRNA. Immunocytochemistry experiments showed ACE-2 protein on thyroid cells membrane. ACE-2 expression increased after treatment with VitD and IFN-γ alone and further increased by the combination treatment with VitD + IFN-γ.
Conclusions
VitD would defend the body by SARS-COV2 both by regulating the host immune defense and by up-regulating of the expression of the ACE-2 receptor. The existence of a co-operation between VitD and IFN-γ demonstrated in other systems is supported also for ACE-2 up-regulation. These observations lead to an increased interest for the potential therapeutic benefits of VitD supplementation in COVID-19.
Keywords: Thyroid, Thyrocytes, SARS-COV-2, COVID-19, ACE-2
Introduction
The COVID-19 pandemic is not over yet, and the world population is still fighting to defeat SARS-CoV-2. Since its first description, the virus has undergone several mutations, becoming more contagious and, in some cases, is still lethal. However, only a small proportion of infected people experience relevant clinical symptoms and an even lower percentage of them will require intensive medical care [1]. Thus, the clinical course and outcome of COVID-19 remain very variable. Several studies trying to explain these marked differences suggested, among other factors, a potential role of low levels of Vitamin D (VitD), a hormone displaying several immunomodulatory functions, in driving a more severe SARS-CoV-2 infection [2–4].
Indeed, cohort studies reported lower VitD levels in SARS-CoV-2-infected patients than in SARS-CoV-2-negative subjects, suggesting a relationship between insufficient VitD levels and SARS-Cov-2 infection [5]. Interestingly, low VitD levels were also associated with a more severe course of the disease [6, 7].
These clinical observations appear supported by several animals and in vitro studies. Briefly, VitD receptor gene knockout mice showed more severe acute lung injury and increased mortality in an lipopolysaccharides sepsis (LPS-sepsis) model of ARDS with amelioration by treatment with angiotensin II antagonists and/or by suppressing the expression of renin [8, 9]. In vitro data demonstrated that VitD could modulate the mRNA levels and the protein expression of ACE-2 [8, 10], the receptor through which SARS-COV-2 infects the host cells. The above examples suggest a potential role of VitD in driving the clinical course of COVID-19.
ACE-2 is expressed by various tissues, including the thyroid [11, 12]. The possibility that SARS-CoV-2 could also infect thyroid cells was hypothesized by description of patients developing subacute thyroiditis following SARS-CoV-2 infection and subsequently proven by studies demonstrating ACE-2 mRNA expression on thyrocytes [11, 13–19]. Furthermore, interferon-γ (IFN-γ), a pro-inflammatory cytokine involved in the well-characterized cytokine storm of COVID-19, was demonstrated to up-regulate the expression levels of the mRNA encoding for ACE-2 in thyroid cells [20]. However, several issues remain to be elucidated. Indeed, we still lack the demonstration that the protein of ACE-2 receptor is expressed on thyroid cells membrane. Furthermore, while VitD is known to up-regulate ACE-2 mRNA expression in several human cells, no data are available regarding the possible effect of VitD on mRNA and protein of ACE-2 receptor expression in thyroid cells.
The present in vitro study aimed to investigate whether VitD, alone or combined with IFN-γ, could modulate ACE-2 mRNA levels in primary cultures of human thyroid cells. Elucidating this issue might provide further insights into the intriguing clinical association between COVID-19, thyroid diseases, and VitD status.
Materials and methods
Primary cultures of human thyroid cells
Surgical specimens of normal human thyroid (NHT) were obtained from the contralateral disease-free lobe of patients who underwent thyroidectomy for a solitary benign nodule (n=4). Surgical specimens were minced and then incubated with collagenase type II (Sigma, Saint Louis, MO, USA) 5 mg/ml, in 5 ml of Coon’s F12 medium, for 4 hours at 37 °C. Then, 10 ml of Coon’s F12 medium were added, following which, cells were filtered, spun at 1000 × g for 10 min, washed with Coon’s F12 medium, spun again, and finally re-suspended in a complete medium containing 5% newborn calf serum and a mixture of six hormones including insulin (5μg/ml), hydrocortisone (50 μg/ml), transferrin (5 μg/ml), somatostatin (10 ng/ml), gly-his-lysine (10 ng/ml) and bovine TSH (1 mU/ml).
Treatment of thyroid cells with VitD and IFN-γ
NHT cells were grown in a complete medium until an 80% confluence was reached. Cells were then detached and seeded in 6-well flat plates at a 1 × 105 cell/well density. After 24 h, the growth medium was removed, and cells were incubated for 24 h in a serum-free medium containing Vitamin D (1,25-OH2 VitD3 1000 nM) (Sigma Aldrich) alone or in combination with 1000 U/ml IFN-γ (R&D systems, Minneapolis, MN), for 24 h. These concentrations were chosen based on previous experiments [21]. After 24-h incubation, supernatants were removed, and total RNA was extracted and purified from cells.
Real-time PCR
According to the manufacturer’s instructions, total RNA was isolated from thyroid specimens using a Total RNA purification kit (Norgen Biotek, Canada). Genomic DNA was digested using the DNAse enzyme (Norgen Biotek, Canada) at room temperature for 15 minutes and following the manufacturer's protocol. Total RNA from samples was reverse-transcribed into cDNA using a Sensi Fast cDNA synthesis kit (Bioline, London, UK). Real-time PCR was performed using Sensi-Fast SYBR Green Hi-ROX kit (Bioline, London, UK) on a StepOne Plus Applied Biosystems real-time PCR system. Amplification was done under the following conditions: 95 °C for 2 minutes; followed by 40 cycles of 95 °C, 5 seconds, and 60°C, 10 seconds. GAPDH was used as endogenous control. Pre-designed primers targeting human ACE-2 (F: GGGATCAGAGATCGGAAGAAGAAA; R: AGGAGGTCTGAACATCATCAGTG) GAPDH (F: AAATCCCATCACCATCTTCC; R: GGTTCACACCCATGACGAAC) were obtained from Biomers.net GMBH (Soflinger, Germany). Primers of ACE-2 were chosen based on the study by Ma et al. [22]. All samples were run in triplicate. The mean number of Cycles-1 of ACE-2 and GAPDH genes was compared and the expression of ACE-2 in GAPDH was calculated among all the samples.
Immunocytochemistry
Thyrocytes were seeded onto 12 mm glass cover slips in a 24-well plate at a concentration of 10 × 104 cells per well. Cells were incubated for 24 h with complete medium alone (basal condition) or with 1000 ng/ml 1,25-OH2 or VitD, 1000 U/ml IFN-γ, or IFN-γ + 1,25-OH2 VitD. At the end of incubation, samples were washed with PBS, fixed with 4% paraformaldehyde (PFA) for 8 min, and blocked with 10% FBS for 1 h at room temperature. For immunofluorescence evaluation, samples were stained with anti-ACE-2 antibody (1:200) (Atlas antibodies AB, Bromma, Sweden) overnight at 4 °C. The day after, samples were washed three times with PBS for 10 min and then incubated with Alexa Fluor secondary antibody (1:250) (Life Technologies, Monza, Italy) in the dark for 1 h at room temperature. Nuclei were stained with Hoechst 33,258 (1:2000) (Life Technologies, Monza, Italy). Finally, the cover slips were mounted onto glass slides with DAKO (Invitrogen, Milan, Italy). Negative controls were performed by omitting the primary antibody in some untreated samples. Images were acquired by an Olympus XM10 microscope (Olympus, Deutschland GmbH, Hamburg, Germany), using the same settings for all specimens.
Statistical analysis
Statistical analysis was performed using the SPSS software (SPSS, Inc., Evanston, IL). Paired data were compared using the Student’s t test according to a normal distribution of the analyzed variable. Values are given in the text as mean ± SD. A p value < 0.05 was considered statistically significant.
Results
VitD and IFN-ɣ alone and in combination strongly induce ACE-2 mRNA and protein expression in human thyrocytes
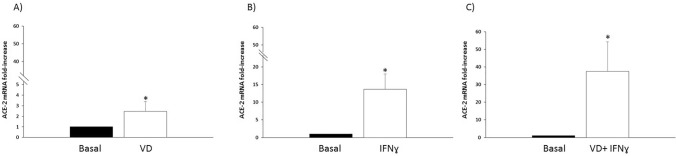
ACE-2 mRNA was basally expressed by thyroid cells in primary cultures, confirming previous data [11, 20]. A significant up-regulation of the basal mRNA levels of ACE-2 in NHT cells in primary cultures was registered following treatment with both VitD (2,4 ± 0,9 ACE-2 mRNA fold increase; Student’s t test p < 0.05 VitD vs. Basal) (Fig. 1A), and IFN-γ (13,6 ± 4,3 ACE-2 mRNA fold increase; Student’s t test p < 0.05IFN-γ vs. Basal) (Fig. 1B). Of note, an additive effect of the co-treatment with VitD + IFN-γ in terms of mRNA levels of expression of ACE-2 was observed (37,5 ± 16,7 ACE-2 mRNA fold increase; Student’s t test p < 0.05 VitD + IFN-γ vs. Basal) (Fig. 1C).
Fig. 1.
Up-regulation of ACE-2 mRNA levels. A A significant up-regulation of the basal mRNA levels of ACE-2 in normal human thyroid cells in primary cultures, was registered following treatment with Vitamin D (2,4±0,9 ACE-2 mRNA fold increase; Student-T test p<0.05 Vitamin D vs. Basal); B a significant up-regulation of the basal mRNA levels of ACE-2 in normal human thyroid cells in primary cultures, was registered following treatment with IFNɣ (13,6±4,3 ACE-2 mRNA fold increase; Student-T test p<0.05 IFNɣ vs. Basal); Panel C A significant increase with the co-treatment with Vitamin D + IFNɣ was observed in terms of increase in ACE-2 mRNA levels (37,5±16,7 ACE-2 mRNA fold increase; Student-T test p<0.05 Vitamin D + IFNɣ vs. Basal)
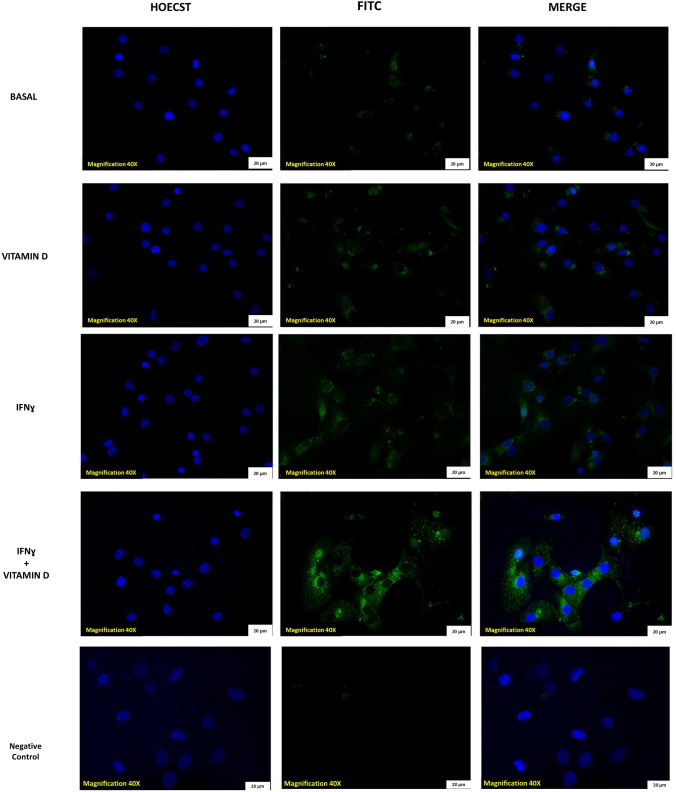
ACE-2 protein expression on thyroid cells was evaluated by immunofluorescence. Representative images in (Fig. 2) show that ACE-2 protein was detected in basal condition in thyroid cells, and its expression increased after treatment with VitD and after treatment with IFN-γ. In line with what was found for mRNA, an additive effect of the combination treatment with VitD + IFN-γ, in terms of a further increase of ACE-2 protein expression, was also observed. The negative control showed no specific binding of the secondary antibody (Fig. 2).
Fig. 2.
ACE-2 expression on human thyroid cells in primary cultures. Representative immunofluorescence staining for ACE-2 receptor in primary cultures of human thyroid cells. Images shows ACE-2 expression in thyroid cells in basal condition. After treatment with VitD alone, the expression increased. After treatment with IFNɣ the expression of ACE-2 also increased. Finally a further increase of ACE-2 expression was observed after co-treatment with VitD + IFNɣ (staining: green = ACE-2, blue = nuclei, scale bar = 100 µm objective 20X). Negative control (secondary antibody) do not show aspecific binding
Discussion
The results of the present study demonstrate, for the first time, the expression of the ACE-2 receptor protein in human thyroid cells and that VitD treatment leads to an up-regulation of the expression of ACE-2 both in terms of mRNA and protein in human thyroid cells in primary cultures. In line with previous findings, ACE-2 mRNA was also increased by treatment with IFN-γ, and, more interestingly, it was further increased, in an additive manner, by co-treatment with the combination VitD + IFN-γ. Consistently, immunofluorescent experiments showed an increase in the expression of ACE-2 protein on the thyroid cell membrane after treatment with either VitD or IFN-γ. Again, an additive effect was observed by the combination treatment with VitD plus IFN-γ. Recent evidence demonstrated that VitD would play a beneficial role in SARS-CoV2 infected patients both by regulating the host immune defense and by displaying a direct action on the renin-angiotensin-system through the up-regulation of the expression of the ACE-2 receptor [8, 23]. Given that ACE-2 was identified as the “access door” for SARS-CoV-2 in host cells [24, 25], it would be reasonable expecting that a higher expression of this receptor would cause a more severe COVID-19 infection. However, accumulating evidences support an opposite scenario. ACE-2 is crucially involved in the regulation of AngII system, reducing AngII presence by increasing its metabolism. The downregulation of the expression and the activity of ACE-2 induced by its binding to Sars-CoV-2 would lead to increased activity of AngII. It is known that higher levels of Ang II promote lung injury and ARDS, as demonstrated by the finding that respiratory distress in COVID-19 was consistently associated with reduced ACE-2 receptor expression [26, 27]. Thus, to prevent AngII accumulation, an adequate expression of ACE-2 is required. The above evidence supported by the results of recently published studies, account for the need to up-regulate ACE-2 expression to mitigate the clinical impact of COVID-19.
On a clinical basis, it should be remembered that individuals with reduced organ ACE-2 expression like diabetic, hypertensive patients, and the elderly, represent setting of subjects proven to be more prone to higher mortality following SARS-CoV-2 infection [28]. On one hand, high levels of ACE-2 favor the entry of the virus into the host cells, on the other hand, low levels of the ACE-2 receptor would be translated into adverse consequences for the host in terms of the progression of the disease. Thus, according to the evidence provided above, it is not surprising the finding that the up-regulation of the ACE-2 receptor by VitD would translate into a less aggressive course of COVID-19.
The above results suggest that the combination of VitD and IFN-γ further increases ACE-2 expression compared with the two compounds alone appears rather attractive.
Previous evidences supports the notion of a co-operation between VitD and IFN-γ. To give a few examples, it was demonstrated that: (1) treatment with VitD + IFN-γ produces a further increase in anti-tuberculosis activity of human monocytes as compared to IFN-γ alone [29]; (2) the IFN-γ-mediated activity of monocytes/macrophages, in terms of autophagy, antimicrobial peptide expression, and phagosome–lysosome fusion, do not occur in the absence of adequate levels of VitD [30]; (3) a synergism between VitD and IFN-γ was found in shaping a unique tolerogenic dendritic cells activation state[31]; (4) moving on in vivo models, the beneficial effect of vit D3 in experimental autoimmune encephalomyelitis (EAE) was demonstrated to be twofold less prominent in IFN-γ gene knockout mice [32]. The above evidence would support that co-operation of VitD + IFN-γ could be also in terms of up-regulation of the ACE-2 expression.
The here reported in vitro data could be relevant from a clinical point of view. Indeed, according to a recently published review, including fifty-four studies for a total of 1.403.715 individuals, severe deficiency, deficiency, and insufficiency of VitD were associated with intensive care unit (ICU) admission, mortality, and COVID-19 hospitalization [23]. More recently, a prospective study reported that severely symptomatic COVID-19 patients displayed lower circulating VitD levels than mildly symptomatic COVID-19 patients and non-SARS-CoV-2-infected controls. These observation increased interest for the potential therapeutic benefits of VitD supplementation in COVID-19 patients [33].
Of note, also bioinformatics and systems biology approaches using transcriptome data provided evidence that VitD could have a role in both suppressing the cytokine storm and inducing an antiviral response in severe COVID-19 patients [34].
Although the design of the present study does not allow drawing conclusions on how the effects of vitamin D and IFN-γ on ACE-2 expression might have a role in thyroidal repercussions of COVID-19, a few notions should be recalled. IFN-γ plays a pivotal role in COVID-19 pathogenesis [33, 34], but it is also crucially involved in autoimmune thyroid diseases [35] which are also associated with a high prevalence of vitamin D deficiency [35]. Thus, patients with AITD could potentially be characterized by increased ACE-2 expression (IFN-γ) or by reduced ACE-2 expression (VitD deficiency). Thus, it could be speculated that patients with AITD might display a more severe course of COVID-19 (lower ACE-2 expression), while they could be more prone to develop COVID-19-related subacute thyroiditis (higher ACE-2 expression). Future specifically designed in vivo studies will be required to further support this speculation.
In conclusion, the results of the present study show that, as reported for other cell types, VitD increases the expression of ACE-2 both at mRNA and protein levels on thyroid cells. Moreover, the further increase of ACE-2 by VitD combined with IFN-γ would suggest a potentially beneficial role of combining these molecules. Future studies will be required to clarify further the relation between VitD status, the course of COVID-19, and the thyroid-related repercussions of SARS-CoV-2 infection.
Funding
This paper was not supported by any grant or funding.
Declarations
Conflict of interest
Flavia Magri, Laura Croce, Luca Chiovato and Mario Rotondi are members of the Editorial Board of Journal of Endocrinological Investigation. On behalf of other authors, the corresponding author states that there is no conflict of interest.
Ethical approval
This research was performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fu L, Wang B, Yuan T, Chen X, Ao Y, Fitzpatrick T, et al. Clinical characteristics of coronavirus disease 2019 (Covid-19) in China: a systematic review and meta-analysis. J Infect. 2020;80(6):656–665. doi: 10.1016/j.jinf.2020.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prietl B, Treiber G. Pieber Tr, Amrein K vitamin D and immune function. Nutrients. 2013;5(7):2502–2521. doi: 10.3390/nu5072502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cangiano B, Lm Fatti, Danesi L, Gazzano G, Croci M, Vitale G, et al. Mortality in an Italian nursing home during Covid-19 pandemic: correlation with gender, age, Adl, vitamin D supplementation, and limitations of the diagnostic tests. Aging (Albany Ny) 2020;12(24):24522–34. doi: 10.18632/aging.202307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peng MY, Liu WC, Zheng JQ, Cl Lu, Hou YC, Zheng CM, et al. Immunological aspects of Sars-Cov-2 infection and the putative beneficial role of vitamin-D. Int J Mol Sci. 2021;22(10):5251. doi: 10.3390/ijms22105251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’avolio A, Avataneo V, Manca A, Cusato J, De Nicolò A, Lucchini R, et al. 25-Hydroxyvitamin D concentrations are lower in patients with positive Pcr For Sars-Cov-2. Nutrients. 2020;12(5):1359. doi: 10.3390/nu12051359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hafez W, Saleh H, Arya A, Alzouhbi M, Fdl Alla O, Lal K, et al. Vitamin D status in relation to the clinical outcome of hospitalized Covid-19 patients. Front Med (Lausanne) 2022;9:843737. doi: 10.3389/fmed.2022.843737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mera S, Barros WMA, Fernandes MSS, Silva ABJ, Souza VON. Letter to the editor: vitamin D deficiency in critically Ill Covid-19 Ards patients. Clin Nutr. 2022 doi: 10.1016/j.clnu.2022.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu J, Yang J, Chen J, Luo Q, Zhang Q, Zhang H. Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system. Mol Med Rep. 2017;16(5):7432–7438. doi: 10.3892/mmr.2017.7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kong J, Zhu X, Shi Y, Liu T, Chen Y, Bhan I, et al. Vdr Attenuates acute lung injury by blocking Ang-2-Tie-2 pathway and renin-angiotensin system. Mol Endocrinol. 2013;27(12):2116–2125. doi: 10.1210/me.2013-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riera M, Anguiano L, Clotet S, Roca-Ho H, Rebull M, Pascual J, et al. Paricalcitol modulates Ace2 shedding and renal Adam17 in nod mice beyond proteinuria. Am J Physiol Renal Physiol. 2016;310(6):F534–F546. doi: 10.1152/ajprenal.00082.2015. [DOI] [PubMed] [Google Scholar]
- 11.Rotondi M, Coperchini F, Ricci G, Denegri M, Croce L, Ngnitejeu ST, et al. Detection of Sars-Cov-2 receptor Ace-2 Mrna in thyroid cells: a clue for Covid-19-related subacute thyroiditis. J Endocrinol Invest. 2020;44:1085–1090. doi: 10.1007/s40618-020-01436-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li MY, Li L, Zhang Y, Wang XS. Expression of the Sars-Cov-2 cell receptor gene Ace2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9(1):45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brancatella A, Viola N, Rutigliano G, Sgrò D, Santini F, Latrofa F. Subacute thyroiditis during the Sars-Cov-2 pandemic. J Endocr Soc. 2021;5(10):Bvab130. doi: 10.1210/jendso/bvab130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trimboli P, Cappelli C, Croce L, Scappaticcio L, Chiovato L, Rotondi M. Covid-19-associated subacute thyroiditis: evidence-based data from a systematic review. Front Endocrinol (Lausanne) 2021;12:707726. doi: 10.3389/fendo.2021.707726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pirola I, Gandossi E, Rotondi M, Marini F, Cristiano A, Chiovato L, et al. Incidence of De Quervain's thyroiditis during the Covid-19 pandemic in an area heavily affected by Sars-Cov-2 infection. Endocrine. 2021;74:215–218. doi: 10.1007/s12020-021-02841-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brancatella A, Ricci D, Cappellani D, Viola N, Sgrò D, Santini F, et al. Is subacute thyroiditis an underestimated manifestation of Sars-Cov-2 infection? Insights from a case series. J Clin Endocrinol Metab. 2020;105(10):e3742–e3746. doi: 10.1210/clinem/dgaa537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanda ML, Ippolito S, Gallo D, Baj A, Novazzi F, Genoni A, et al. Sars-Cov-2 detection in primary thyroid sarcoma: coincidence or interaction? J Endocrinol Invest. 2022;45(5):1059–63. doi: 10.1007/s40618-021-01722-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poma AM, Bonuccelli D, Giannini R, Macerola E, Vignali P, Ugolini C, et al. Covid-19 autopsy cases: detection of virus in endocrine tissues. J Endocrinol Invest. 2022;45(1):209–14. doi: 10.1007/s40618-021-01628-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ippolito S, Dentali F, Tanda ML. Sars-Cov-2: a potential trigger for subacute thyroiditis? Insights from a case report. J Endocrinol Invest. 2020;43(8):1171–2. doi: 10.1007/s40618-020-01312-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coperchini F, Ricci G, Croce L, Denegri M, Ruggiero R, Villani L, Al Et. Modulation of Ace-2 Mrna by inflammatory cytokines in human thyroid cells: a pilot study. Endocrine. 2021;74:638–645. doi: 10.1007/s12020-021-02807-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rotondi M, Falorni A, De Bellis A, Laureti S, Ferruzzi P, Romagnani P, et al. Elevated serum interferon-gamma-inducible chemokine-10/Cxc chemokine ligand-10 in autoimmune primary adrenal insufficiency and in vitro expression in human adrenal cells primary cultures after stimulation with proinflammatory cytokines. J Clin Endocrinol Metab. 2005;90(4):2357–2363. doi: 10.1210/jc.2004-1062. [DOI] [PubMed] [Google Scholar]
- 22.Ma D, Chen CB, Jhanji V, Xu C, Xl Yuan, Liang JJ, et al. Expression of Sars-Cov-2 receptor Ace2 And Tmprss2 in human primary conjunctival and pterygium cell lines and in mouse cornea. Eye (Lond) 2020;34(7):1212–9. doi: 10.1038/s41433-020-0939-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiodini I, Gatti D, Soranna D, Merlotti D, Mingiano C, Fassio A, et al. Vitamin D status and Sars-Cov-2 infection and Covid-19 clinical outcomes. Front Public Health. 2021;9:736665. doi: 10.3389/fpubh.2021.736665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. Sars-Cov-2 cell entry depends on Ace2 and Tmprss2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280 E8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, et al. Angiotensin-converting enzyme 2 is a functional receptor for the sars coronavirus. Nature. 2003;426(6965):450–4. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cs M. The angiotensin-converting enzyme 2 (Ace2) receptor in the prevention and treatment of Covid-19 are distinctly different paradigms. Clin Hypertens. 2020;26:14. doi: 10.1186/s40885-020-00147-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glowacka I, Bertram S, Herzog P, Pfefferle S, Steffen I, Muench MO, et al. Differential downregulation of Ace2 By the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus Nl63. J Virol. 2010;84(2):1198–205. doi: 10.1128/JVI.01248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for Covid-19 infection? Lancet Respir Med. 2020;8(4):E21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rook GA, Taverne J, Leveton C, Steele J. The role of gamma-interferon, vitamin D3 metabolites and tumour necrosis factor in the pathogenesis of tuberculosis. Immunology. 1987;62(2):229–34. [PMC free article] [PubMed] [Google Scholar]
- 30.Fabri M, Stenger S, Shin DM, Yuk JM, Liu PT, Realegeno S, et al. Vitamin D is required for Ifn-gamma-mediated antimicrobial activity of human macrophages. Sci Transl Med. 2011;3(104):104ra2. doi: 10.1126/scitranslmed.3003045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Švajger U, Rožman PJ. Synergistic effects of interferon-Γ and vitamin D. Front Immunol. 2019;10:2627. doi: 10.3389/fimmu.2019.02627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spanier JA, Nashold FE, Olson JK, Hayes CE. The Ifng gene is essential for Vdr gene expression and vitamin D3-mediated reduction of the pathogenic T cell burden in the central nervous system in experimental autoimmune encephalomyelitis. A Multiple Sclerosis Model. J Immunol. 2012;189(6):3188–97. doi: 10.4049/jimmunol.1102925. [DOI] [PubMed] [Google Scholar]
- 33.Campi I, Gennari L, Merlotti D, Mingiano C, Frosali A, Giovanelli L, et al. Vitamin D and Covid-19 severity and related mortality: a prospective study in Italy. Bmc Infect Dis. 2021;21(1):566. doi: 10.1186/s12879-021-06281-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmed F. A network-based analysis reveals the mechanism underlying vitamin D in suppressing cytokine storm and virus in sars-Cov-2 infection. Front Immunol. 2020;11:590459. doi: 10.3389/fimmu.2020.590459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao R, Zhang W, Ma C, Zhao Y, Xiong R, Wang H, et al. Immunomodulatory function of vitamin D and its role in autoimmune thyroid disease. Front Immunol. 2021;12:574967. doi: 10.3389/fimmu.2021.574967. [DOI] [PMC free article] [PubMed] [Google Scholar]