Abstract
Purpose
SARS-CoV-2 infection can be associated with destructive thyroiditis and triggers thyroid autoimmunity. More recent evidence suggests that SARS-CoV-2 vaccines may also be associated with permanent or transient thyroid dysfunction in susceptible individuals.
Methods
We observed three patients who developed/exacerbated autoimmune thyroid diseases (AITDs) shortly after receiving mRNA-based vaccines against SARS-CoV2. Clinical histories are reported, and relevant literature in the field is summarized.
Results
Our case series gives a description of the full spectrum of autoimmune disorders that may occur after SARS-CoV-2 vaccines administration, ranging from a case of new-onset Graves’ disease to autoimmune hypothyroidism in two patients with pre-existing AITDs. Our three patients had a personal and/or family history of autoimmune disorders, suggesting that genetic predisposition is an important risk factor for the development of AITDs following vaccination. Moreover, our real-life experience demonstrates that persistent hypothyroidism may occur in the long run and should be overlooked; subjects with a previous AITDs are at risk of developing it. Reviewing the pertinent literature up to date Graves’ disease is the most common vaccine-related AITDs with up to 51 cases reported in the literature, occurring mainly in female patients with no personal history of AIDTs, while only a case of autoimmune hypothyroidism has been reported so far.
Conclusions
SARS-CoV-2 vaccines can trigger autoimmune reactions and the present case series contributes to make clinicians aware of full spectrum of AITDs that may occur following vaccination. Thyroid function monitoring is recommended, mainly in subjects with a personal/family history of AITDs.
Keywords: COVID-19, SARS-COV-2 vaccine, Autoimmune thyroiditis, Graves’ disease, Hypothyroidism, ASIA syndrome
Introduction
Coronavirus disease 2019 (COVID-19) pandemic, caused by the novel coronavirus SARS-CoV-2, emerged in Wuhan in December 2019, rapidly covered the whole world and it is still ongoing [1]. During the COVID-19 pandemic thyroid gland alteration/dysfunction represented a quite common endocrine complication, as a consequence of either direct or indirect effects of SARS-CoV-2 infection [2–4]. Indeed, follicular thyroid cells may represent a direct target for SARS-CoV-2 infection since they express the angiotensin converting enzyme 2 (ACE2) that is the receptor for cellular entry of the virus [5]. Also, antibodies against the SARS-CoV-2 spike protein have been shown to cross-react with thyroid peroxidase, suggesting that mechanisms of molecular mimicry could be responsible for thyroid auto-inflammatory damage [6, 7]. Moreover, COVID-19 is associated with a systemic inflammatory response, involving innate immune cells, T helper (h)1/17 and Th2 lymphocytes, which may involve the thyroid gland [8]. As a consequence, a wide spectrum of SARS-CoV-2-related thyroid disorders has been described, ranging from subacute thyroiditis to autoimmune thyroid diseases and associated features of thyrotoxicosis and/or hypothyroidism [2–4, 8].
In the effort to face the pandemic, a number of vaccines have been developed with various technologies, including mRNA-based vaccine (Pfizer/BioNTech BNT162b2 or Moderna mRNA-1273), viral vector vaccine (Vaxzevria ChAdOx1, AstraZeneca or Janssen or Cansino Biotech), and inactivated virus (Sinovac Biotech CoronaVac or Bharat Biotech BBV152) [9]. In the last year, following massive diffusion of SARS-CoV-2 vaccines administration, an increasing number of case reports have suggested a possible association between SARS-CoV-2 vaccines and thyroid disorders, replicating the spectrum of thyroid dysfunction that have already been described to occur during SARS-CoV-2 infection [10–13].
We describe three patients with autoimmune thyroid diseases (AITDs) developed/exacerbated shortly after receiving mRNA-based vaccines against SARS-CoV-2. In addition, we also reviewed the pertinent literature.
Case series
Case 1
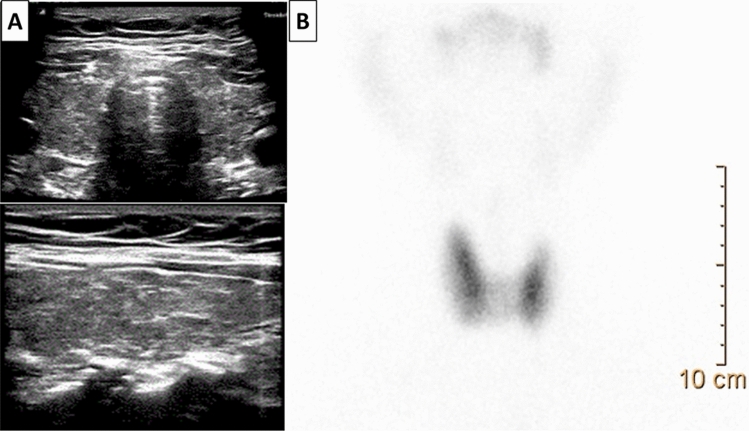
A 50-year-old man without personal history of thyroid disease and/or autoimmune diseases was referred to our endocrinology clinic because of the sudden onset of fatigue, palpitations, distal tremor, insomnia, anxiety, nervousness and irritability. He has received a first dose of anti-SARS-CoV-2 vaccine (Pfizer BioNTech) two weeks before the onset of symptoms. There was a history of autoimmune hypothyroidism and vitiligo in the mother. At clinical examination, the patient was clinically hyperthyroid, with a diffusely enlarged thyroid gland. Thyroid function tests (TFTs) demonstrated suppressed TSH, elevated free triiodothyronine (fT3) and free thyroxine (fT4), and positivity of anti-thyroperoxidase (Ab-TPO) and anti-TSH receptor (TRAb) antibodies (Table 1). Ultrasound (US) examination showed a diffuse enlargement of the thyroid, associated with hypoechogenicity and increased vascularity, and 99m-technezium scintigraphy revealed an enlarged gland with diffuse hypercaptation of the radiotracer (Fig. 1). There was no thyroid eye disease (TED), dermopathy, and/or acropachy. Graves’ disease (GD) was diagnosed, and therapy with methimazole (MMI, 30 mg/day) was started, with progressive attenuation of symptoms and signs of thyrotoxicosis and normalization of FT3 and FT4 values. As thyroid function improved, the daily dosage of MMI was gradually decreased and the patient is still under low-dose MMI treatment (5 mg/day) with normal thyroid function at last evaluation.
Table 1.
Clinical and biochemical features of our patients
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Age (years) | 50 | 55 | 61 |
| Sex | M | F | M |
| Clinical presentation | Fatigue, palpitations, distal tremor, insomnia, anxiety, nervousness and irritability | Fatigue, tremors and palpitations | Fatigue and muscle weakness, decreased concentration, loss of memory, depression, increased sensitivity to cold, constipation |
| Time to onset of symptoms after vaccination (days) | 14 | 10 | 15 |
| Personal history of autoimmune disease | No |
Yes HT |
Yes GD |
| Family history of autoimmune disease | Yes | Yes | Yes |
| TFTs | |||
| At onset of symptoms | |||
| TSH (IUml) | 0.001 | 0.006 | 37.97 |
| FT4 (ng/dl) | 2.0 | 4.56 | 0.56 |
| FT3 (pg/ml) | 10.47 | 6.82 | 2.0 |
| Tg-Ab (IU/l) | 385.49 | 11.0 | 356 |
| TPO-Ab (IU/l) | 529.50 | 344 | 1590 |
| TRAB (IU/l) | 5.0 | < 1.0 | – |
| At follow-up six months later | |||
| TSH (IU/l) | 1.06* | 10.8 | 2.45# |
| FT4 (ng/dl) | 0.92 | 0.80 | 1.1 |
| FT3 (pg/ml) | 3.58 | 2.4 | 2.78 |
HT Hashimoto’s thyroiditis, GD Graves’ disease, TFTs Thyroid Function Tests, TSH Thyroid stimulating hormone (normal values: 0.25–4.5), FT3 free Triiodothyronine (n. 2.0–4.0), FT4 free Thyroxine (n.v. 0.7–1.48), TRAb Thyrotropin receptor antibody (n.v. < 1.0), Anti-TPO Anti-Thyroid peroxidase antibodies (n.v. 0–10), Tg-Ab anti-thyroglobulin antibodies (n.v. 0–40)
*Under methimazole therapy, #under L-Thyroxine therapy
Fig. 1.
Case 1. Thyroid US demonstrates an enlarged, hypoechoic gland, without nodules (panel A), while.99mTc-pertechnetate thyroid scintigraphy reveals an increased and diffuse uptake in both lobes (panel B)
Case 2
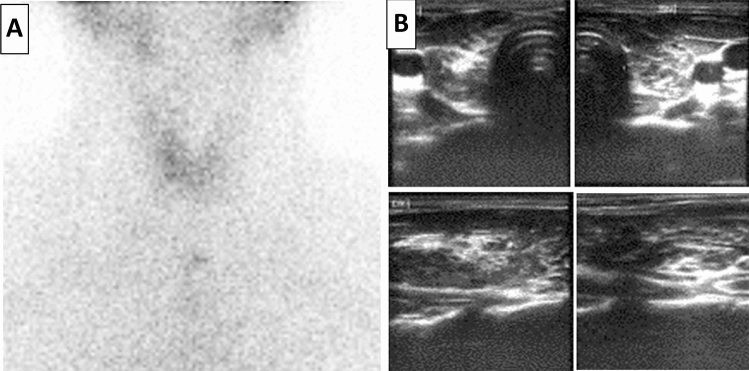
A 55-year-old woman with Hashimoto’s thyroiditis (HT) in spontaneous euthyroidism. She had been diagnosed with HT at the age of 40; her mother and a sister were suffering from the same thyroid disease. At time of diagnosis TTFs were within normal range, and she has been followed up for the following years without therapy. In April 2021, she received the first dose of Moderna mRNA-1273 vaccine. After ten days she suddenly developed fatigue, tremors and palpitations. Thyroid function tests demonstrated suppressed TSH, elevated FT4 and FT3 levels (Table 1). On scintigraphy, tracer uptake was markedly reduced, compatibly with destructive thyroiditis (Fig. 2, panel A). No treatment was prescribed. Four weeks later, FT4 and FT3 normalized, while TSH increased to 6.5 uIU/mL (Table 1). Six months after, serum TSH was still over the upper limit of normal range and slight increased (10.8 uIU/mL), consistently with persistent subclinical hypothyroidism. Levo thyroxine (L-T4) treatment was started with normalization of TFTs at the subsequent check 3 months later.
Fig. 2.
Panel A: 99mTc-pertechnetate thyroid scintigraphy demonstrated very low uptake of the tracer due to inflammation-driven cytolysis (Case 2). Panel B: Thyroid US demonstrates a heterogeneous echostructure with diffuse hypoechogenicity (Case 3)
Case 3
A 61-year-old man with pre-existing GD, under control without therapy. There was a history of autoimmune hypothyroidism in the mother and a daughter. At the age of 38 he had been diagnosed with GD without TED and had been treated with MMI for the following 24 months, gradually tapering the dosages until discontinuation. In the subsequent years, he experienced a stable remission of hyperthyroidism. At the beginning of 2021, he received two doses of the Pfizer/BioNTech vaccine, completing his vaccination program (third dose) in October 2021. Fifteen days after he referred to our outpatient clinic complaining of fatigue and muscle weakness, decreased concentration, loss of memory, depression, increased sensitivity to cold, constipation. Symptoms had appeared some months earlier but were ignored, and had worsened over the last weeks. Physical examination revealed normal body weight (body mass index 24.5 kg/m2), normal body temperature (36.4 °C), bradycardia (pulse rate: 52/min), reduced differential blood pressure (110/80 mmHg), dry skin, mild periorbital oedema and a diffuse painless goitre. TFTs revealed overt hypothyroidism with TPOAb positivity (Table 1), and neck US showed a heterogeneous echo-structure with diffuse hypoechogenicity and increased vascularity of the gland (Fig. 2, panel B). All findings were consistent with autoimmune hypothyroidism and L-T4 therapy was started at an initial dosage of 50 µg/day, gradually increased up to the current dosage of 100 µg/daily, obtaining restoration of euthyroidism.
Discussion
SARS-CoV-2 infection has been demonstrated to cause a wide range of autoimmune/inflammatory thyroid disorders via direct entry of the virus in the follicular cells, molecular mimicry, or dysregulated immune responses [8]. Similarly, an association between SARS-CoV-2 vaccination and inflammatory and autoimmune disorders has been emerging in the last year. Typical subacute thyroiditis (SAT) represents to be the most common thyroid disease secondary to vaccine administration, with more than 60 cases reported in the literature, and affects mostly women [11–13]. Most patients (about 70%) were vaccinated with a mRNA vaccine, and developed SAT after a median of 10 days after the first or second dose. Vaccine-associated SAT does not differ from the classic form of virus-related SAT in terms of clinical and epidemiological features and outcomes. These were usually self-limiting cases of mild/moderate severity that could be easily treated with symptomatic therapy or a short course of anti-inflammatory drugs, with overall favourable outcomes [11–13].
More recently, an increasing number of cases of AITDs potentially triggered by SARS-CoV-2 vaccines administration have been reported [13, 14]. Among these, GD is the most common one, accounting for about one-third of the thyroid abnormalities observed after vaccination [13]. Reviewing the pertinent literature, up to date 51 cases of Graves’ disease following vaccination against COVID-19 have been reported in the literature [13–19]. As briefly summarized in Table 2, most of patients were females (39 F, 76.57% and 12 M, 23.5%), with no previous history of thyroid disease; the median age was 42 years (range 28–73 yr). The onset of hyperthyroidism occurred 1–63 days after the first (26 pts, median: 10 days) or the second dose (25 pts, median: 13 days) of vaccine. According to vaccine type, 39 subjects (76.5%) had received an mRNA-based vaccine and nine (17.6%) the vector-based one, while the remaining three subjects (5.9%) had received an inactivated vaccine (Table 2). Clinical presentation was dominated by the typical signs and symptoms of hyperthyroidism (palpitation, anxiety and insomnia, diarrhoea, weight loss), and both biochemical features (low TSH, elevated FT3 and FT4, TRAb positive) and thyroid ultrasonography or scintigraphy imaging were consistent with the diagnosis of GD. The patients were managed with anti-thyroid drugs and beta-blockers, as appropriate, with favourable responses in all cases. Among these GD patients, three developed a new onset TED, while one male patient experienced a relapse [12–18]. However, some other cases of TED new-onset or recrudescence after vaccination have been reported, not associated with the occurrence of hyperthyroidism (Table 2). Two middle-age females with a silent personal history developed signs and symptoms of TED after receiving their first or second dose of mRNA-based vaccine [20, 21]. Also, three middle-age female patients and a 43-year-old male with a history of inactive TED with stable thyroid function tests experienced a flare of TED few days after the administration of mRNA-based SARS-CoV-2 vaccine [21, 22].
Table 2.
Summary of the cases of autoimmune thyroid disorders (AITDs) occurred after COVID-19 vaccination and reported in the literature
| Autoimmune thyroid disorder | Number of patients | Sex (%) median age (range) | Known pre-existing thyroid disease | Time from vaccine to AITDs onset | Vaccine type |
|---|---|---|---|---|---|
| Graves’ disease (GD) |
N = 51 26 after first dose 25 after second dose |
12 M (23.5%), 44.7 yr (30–70) 39 F (76.5%), 42 yr (28–73) |
None (new onset), n = 34 GD (recurrence,) n = 12 Previous HT, n = 3 Previous SAT, n = 2 |
After first dose 10 days (1–50) After second dose 13 days (2–63) |
mRNA (n = 39, 76.5%) Vector-based (n = 9, 17.6%) Inactivated (n = 3, 5.9%) |
| Thyroid eye disease (TED) |
N = 10 4 with Hyper 6 without Hyper |
With hyper 3 F, 37 yr (34–59) 1 M, 59 yr Without hyper 5 F, 53 yr (45–66) 1 M, 43 yr |
With hyper 3 new onset 1 relapse Without hyper* 2 new onset 4 relapse |
After first dose (n = 5) 14 days (1–21) After second dose (n = 5) 21 days (3–25) |
mRNA (n = 10, 100%) |
| Painless thyroiditis |
N = 8 7 after first dose 1 after second dose |
5 F, 38 yr (29–59) 3 M, 33 yr (32–34) |
None |
After first dose 12 days (7–21) After second dose 10 days |
mRNA (n = 6, 75%) Vector-based (n = 2; 25%) |
| Autoimmune hypothyroidism | N = 1 | F, 63 yr | None |
After second dose 6 weeks |
mRNA |
GD Graves’ disease, Hyper hyperthyroidism, HT Hashimoto’s thyroiditis, SAT subacute thyroiditis, TED thyroid eye disease
*The four patients who experienced a relapse of TED not associated with hyperthyroidism were three GD patient who had been previously treatment with radioiodine and were euthyroid under L-Thyroxine therapy at the time of vaccination, and one GD patient already under medical therapy with methimazole. The two new-onset case were a female with no previous history of thyroid disease and/or TED and another female patient with a previous diagnosis of HT under control without any treatment and no previous TED
A few cases of painless thyroiditis were also reported [13, 23], and only one case of overt hypothyroidism associated with acute thyroid swelling may be due to silent thyroiditis precipitating overt autoimmune hypothyroidism [24]. A clear increase in HT occurrence and hypothyroidism following vaccination has not been reported yet, but this is not surprising may be due to its chronic and slow course with hypothyroidism usually occurring years after the appearance of thyroid autoantibodies [25]. Moreover, it might be underestimated more frequently than hyperthyroidism or SAT, which are characterized by a rapid onset of clinical symptoms that facilitate diagnosis [25]. Long-term prospective studies could clarify the impact of SARS-CoV-2 infection and vaccines on TH epidemiology.
Our case series gives a description of the full spectrum of autoimmune disorders that may occur after SARS-CoV-2 vaccines administration, ranging from new-onset Graves’ disease to autoimmune hypothyroidism in patients with pre-existing autoimmune disorders. All three patients had a personal and/or family history of autoimmune disorders, confirming that genetic predisposition is an important risk factor for the development of AITDs following vaccination. Even if two out of three of our patients were males, female gender is another well-recognized risk factor, as it emerges from the revision of the current literature. Two patients, namely case 1 and 2, presented with typical signs and symptoms of thyrotoxicosis, due to autoimmune hyperthyroidism (GD) in the male patient and to destructive painless thyroiditis in the female. Since the clinical presentation of GD and thyroiditis is similar, early differentiation of these two entities is important for timely initiation of treatment. Indeed, GD requires medical treatment with anti-thyroid drugs to block the excessive thyroid hormone synthesis, while painless thyroiditis does not and rather should be followed without any treatment. In case 2, the occurrence of painless thyroiditis precipitated persistent hypothyroidism as a consequence of inflammatory damage of the gland superimposed on a pre-existing autoimmune disease, whereas in patient 3, vaccine administration might have triggered reactivation of AITD with a shift from pre-existing GD to over-hypothyroidism with the typical features of HT. All patients responded well to medical treatment and became euthyroid within few weeks, with no need to discontinue their vaccination program when scheduled. All cases showed a clear temporal relationship between the administration of the vaccine and the onset of symptoms, suggesting that AITDS may represent an adverse event after SARS- CoV-2 vaccines in susceptible individuals. A history of AITDs and/or positivity of thyroid autoantibodies may represent risk factors for the development of vaccine-related AITDs, as already described for other treatments [26], but further prospective studies and larger cohorts are needed to verify this hypothesis.
Like infections, vaccines can trigger the development of autoimmunity by several mechanisms, including molecular mimicry, epitope spreading, polyclonal activation, bystander activation, and presentation of cryptic antigenic determinants [27]. In the case of SARS-CoV-2 vaccines, a cross-reactivity between the virus spike protein targeted by the vaccines and antigens expressed on the surface of the thyroid follicular cell has been postulated [6, 28]. Vojdani et al. reported cross-reactivity to human antigens, including thyroid peroxidase, of antibodies against spike protein S, nucleoprotein and membrane protein, and demonstrated that numerous thyroid peroxidase peptide sequences shared homology or similarity with sequences in various SARS-CoV-2 proteins [28]. Based on these findings, they suggested that the cross-reactivity between thyroid cell antigens and the spike protein of the coronavirus might represent a possible link between COVID-19 infection and an increase in autoimmune diseases. By the same mechanism, due to the molecular mimicry, SARS-CoV-2 vaccination might trigger autoimmune thyroid disorders in genetically predisposed individuals [6, 7]. These phenomena could be enhanced by adjuvants in the context of the post-vaccination so-called ASIA syndrome. First described in 2011, the autoimmune/inflammatory syndrome induced by adjuvants (ASIA) refers to a number of autoimmune disorders induced by exposure to adjuvants added to vaccines to enhance their immunogenicity [29]. Indeed, vaccine adjuvants can trigger adverse autoimmune reactions in predisposed individuals, as a consequence of dysregulation of both innate and adaptive immune systems [29, 30]. Since the ASIA syndrome was described, a wide range of autoimmune conditions have been reported to occur after the administration of HBV, HPV and influenza vaccines [31] and more recently SARS-CoV-2 vaccine. Among endocrine manifestations, cases of HT, ovarian insufficiency, type1 diabetes mellitus, and autoimmune adrenal insufficiency have already been described with the use of various vaccines, while GD has been reported only after SARS-CoV-2 vaccine administration [13–19, and the present paper]. Peculiarly, ASIA syndrome is often associated with a personal or familial history of autoimmune diseases [31].
In conclusion, it might be hypothesized that SARS-CoV-2 vaccines (mainly mRNA-based) can trigger AITDs, either as a new-onset disease or a recurrence of previously diagnosed ones. To date, mostly cases of Graves’ disease have been reported in the literature but growing evidence and our real-life experience suggest that SARS-CoV-2 vaccines, as other vaccines, can cause autoimmune hypothyroidism in susceptible individuals. The purpose of the present case series is to make clinicians aware of full spectrum of AITDs that may occur following vaccination, so that onset of symptoms with a temporal relationship with the administration of the vaccine are not overlooked and treated timely. However, it should be pointed that up to date, about 70 cases of AITDs following SARS-CoV-2 vaccination have been recorded in the face of several billion vaccine doses that have been administered globally so far [32]. Thus, at a population level, the benefits of mass vaccination largely outweigh any issues concerning thyroid side effects, and the possible and rare occurrence of thyroid disorders should certainly not discourage vaccination.
Author contributions
All authors made substantial contributions to the study conception and design. Data collection and analysis were performed by RMR and AC. RMR drafted the work. LG critically revised the work. All authors approved the final version for submission for publication. All authors agree to be accountable for the accuracy and integrity of the work.
Funding
The research was not supported by any funding.
Declarations
Conflict of interest
The authors have no financial or non-financial competing interests to disclose.
Ethics approval
The authors have no ethical conflict to disclose. The study was performed in accordance with the principles of the Declaration of Helsinki. Local Ethics Research Committee approval was obtained.
Informed consent
Written informed consent was obtained from the patients for publication of this case series.
Research involving human participants and/or animals
No animals were used for this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO Coronavirus (COVID-19) Dashboard. February, 2022
- 2.Giovanella L, Ruggeri RM, Petranović Ovčariček P, Campenni A, Treglia G, Deandreis D. SARS-CoV-2-related thyroid disorders: a synopsis for nuclear medicine thyroidologists. Eur J Nucl Med Mol Imaging. 2021;48:1719–1723. doi: 10.1007/s00259-021-05316-0. [DOI] [PubMed] [Google Scholar]
- 3.Piticchio T, Le Moli R, Tumino D, Frasca F. Relationship between betacoronaviruses and the endocrine system: a new key to understand the COVID-19 pandemic—a comprehensive review. J Endocrinol Invest. 2021;44:1553–1570. doi: 10.1007/s40618-020-01486-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giovanella L, Ruggeri RM, Petranović Ovčariček P, Campenni A, Treglia G, Deandreis D. Prevalence of thyroid dysfunction in patients with COVID-19: a systematic review. Clin Transl Imaging. 2021;9:233–240. doi: 10.1007/s40336-021-00419-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rotondi M, Coperchini F, Ricci G, Denegri M, Croce L, Ngnitejeu ST, Villani L, Magri F, Latrofa F, Chiovato L. Detection of SARS-COV-2 receptor ACE-2 mRNA in thyroid cells: a clue for COVID-19-related subacute thyroiditis. J Endocrinol Invest. 2021;44:1085–1090. doi: 10.1007/s40618-020-01436-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vojdani A, Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020;217:108480. doi: 10.1016/j.clim.2020.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanduc D, Shoenfeld Y. On the molecular determinants of the SARS-CoV-2 279 attack. Clin Immunol. 2020;215:108426. doi: 10.1016/j.clim.2020.108426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruggeri RM, Campennì A, Deandreis D, Siracusa M, Tozzoli R, Petranović Ovčariček P, Giovanella L. SARS-COV-2-related immune-inflammatory thyroid disorders: facts and perspectives. Expert Rev Clin Immunol. 2021;17:737–759. doi: 10.1080/1744666X.2021.1932467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung YH, Beiss V, Fiering SN, Steinmetz NF. COVID-19 vaccine frontrunners and their nanotechnology design. ACS Nano. 2020;14:12522–21253. doi: 10.1021/acsnano.0c07197. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Xu Z, Wang P, Li XM, Shuai ZW, Ye DQ, Pan HF. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology. 2022;165:386–440. doi: 10.1111/imm.13443. [DOI] [PubMed] [Google Scholar]
- 11.Ippolito S, Gallo D, Rossini A, Patera B, Lanzo N, Fazzino GFM, Piantanida E, Tanda ML. SARS-CoV-2 vaccine-associated subacute thyroiditis: insights from a systematic review. J Endocrinol Invest. 2022;29:1–12. doi: 10.1007/s40618-022-01747-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yorulmaz G, Sahin Tekin M. SARS-CoV-2 vaccine-associated subacute thyroiditis. J Endocrinol Invest. 2022;45:1341–1347. doi: 10.1007/s40618-022-01767-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jafarzadeh A, Nemati M, Jafarzadeh S, Nozari P, Mortazavi SMJ. Thyroid dysfunction following vaccination with COVID-19 vaccines: a basic review of the preliminary evidence. J Endocrinol Invest. 2022;26:1–29. doi: 10.1007/s40618-022-01786-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pujol A, Gómez LA, Gallegos C, Nicolau J, Sanchís P, González-Freire M, López-González ÁA, Dotre K, Masmiquel L. Thyroid as a target of adjuvant autoimmunity/inflammatory syndrome due to mRNA-based SARS-CoV2 vaccination: from Graves' disease to silent thyroiditis. J Endocrinol Invest. 2022;45:875–882. doi: 10.1007/s40618-021-01707-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vera-Lastra O, Ordinola Navarro A, Cruz Domiguez MP, Medina G, Sanchez Valadez TI, Jara LJ. Two cases of Graves’ disease following SARS-CoV-2 vaccination: an autoimmune/infammatory syndrome induced by adjuvants. Thyroid. 2021;31:1436–1439. doi: 10.1089/thy.2021.0142. [DOI] [PubMed] [Google Scholar]
- 16.Zettining G, Krebs M. Two further cases of Graves' disease following SARS-CoV-2 vaccination. J Endocrinol Invest. 2021;45:227–228. doi: 10.1007/s40618-021-01650-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taieb A, Sawsen N, Asma BA, Ghada S, Hamza E, Yosra H, Amel M, Molka C, Maha K, Koussay A. A rare case of Grave's disease after SARS-CoV-2 vaccine: is it an adjuvant effect? Eur Rev Med Pharmacol Sci. 2022;26:2627–2630. doi: 10.26355/eurrev_202204_28500. [DOI] [PubMed] [Google Scholar]
- 18.Bostan H, Ucan B, Kizilgul M, Calapkulu M, Hepsen S, Gul U, Ozturk Unsal I, Cakal E. Relapsed and newly diagnosed Graves' disease due to immunization against COVID-19: A case series and review of the literature. J Autoimmun. 2022;128:102809. doi: 10.1016/j.jaut.2022.102809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chee YJ, Liew H, Hoi WH, Lee Y, Lim B, Chin HX, Lai RTR, Koh Y, Tham M, Seow CJ, Quek ZH, Chen AW, Quek TPL, Tan AWK, Dalan R. SARS-CoV-2 mRNA vaccination and Graves' disease: a report of 12 cases and review of the literature. J Clin Endocrinol Metab. 2022;107:e2324–e2330. doi: 10.1210/clinem/dgac119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubinstein TJ. Thyroid eye disease following COVID-19 vaccine in a patient with a history Graves’ disease: a case report. Ophthalmic Plast Reconstr Surg. 2021;37:e221–e223. doi: 10.1097/IOP.0000000000002059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park KS, Fung SE, Ting M, Ozzello DJ, Yoon JS, Liu CY, Korn BS, Kikkawa DO. Thyroid eye disease reactivation associated with COVID-19 vaccination. Taiwan J Ophthalmol. 2022;12:93–96. doi: 10.4103/tjo.tjo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patrizio A, Ferrari SM, Antonelli A, Fallahi P. Worsening of Graves' ophthalmopathy after SARS-CoV-2 mRNA vaccination. Autoimmun Rev. 2022;9:103096. doi: 10.1016/j.autrev.2022.103096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siolos A, Gartzonika K, Tigas S. Thyroiditis following vaccination against COVID-19: Report of two cases and review of the literature. Metabol Open. 2021;12:100136. doi: 10.1016/j.metop.2021.100136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giusti M, Maio A. Acute thyroid swelling with severe hypothyroid myxoedema after COVID-19 vaccination. Clin Case Reports. 2021;9:e05217. doi: 10.1002/ccr3.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruggeri RM, Giuffrida G, Campennì A. Autoimmune endocrine diseases. Minerva Endocrinol. 2018;43:305–322. doi: 10.23736/S0391-1977.17.02757-2. [DOI] [PubMed] [Google Scholar]
- 26.Burch EB. Drug effects on the thyroid. N Engl J Med. 2019;381:749–756. doi: 10.1056/NEJMra1901214. [DOI] [PubMed] [Google Scholar]
- 27.Olivieri B, Betterle C, Zanoni G. Vaccinations and autoimmune diseases. Vaccines (Basel) 2021;9:815. doi: 10.3390/vaccines9080815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vojdani A, Vojdani E, Kharrazian D. Reaction of human monoclonal antibodies to SARS-CoV-2 proteins with tissue antigens: implications for autoimmune diseases. Front Immunol. 2020;11:617089. doi: 10.3389/fimmu.2020.617089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shoenfeld Y, Agmon-Levin N. “ASIA”—autoimmune/infammatory syndrome induced by adjuvants. J Autoimmun. 2021;36:4–8. doi: 10.1016/j.jaut.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Watad A, David P, Brown S, Shoenfeld Y. Autoimmune/inflammatory syndrome induced by adjuvants and thyroid autoimmunity. Front Endocrinol (Lausanne) 2017;7:150. doi: 10.3389/fendo.2016.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bragazzi N, Hejly A, Watad A, Adawi M, Amital H, Shoenfeld Y. ASIA syndrome and endocrine autoimmune disorders. Best Pract Res Clin Endocrinol Metab. 2020;34:101412. doi: 10.1016/j.beem.2020.101412. [DOI] [PubMed] [Google Scholar]
- 32.Mathieu E, Ritchie H, Ortiz-Ospina E, Roser M, Hasell J, Appel C, Giattino C, Rodés-Guirao LA. A global database of COVID-19 vaccinations. Nat Hum Behav. 2021;5:947–953. doi: 10.1038/s41562-021-01122-8. [DOI] [PubMed] [Google Scholar]