Abstract
Introduction
There is limited literature on cardiovascular manifestations of post-acute sequelae of SARS-CoV-2 infection (PASC).
Methods
This observational study aimed to describe the characteristics, diagnostic evaluations, and new cardiac diagnoses in patients referred to a cardiovascular disease clinic designed for patients with PASC, and to identify factors associated with cardiovascular symptoms with no identifiable cardiac pathology.
Results
Of 126 patients, average age was 46 years, and 34 % were male. Patients presented on average five months after COVID-19 diagnosis. The most common symptoms were dyspnea (52 %), chest pain/pressure (48 %), palpitations (44 %), and fatigue (42 %), commonly associated with exertion or exercise intolerance. New cardiovascular diseases were present in 23 % of cases. The remainder exhibited common symptoms which we termed “cardiovascular PASC syndrome.”
Discussion
We found that only one in four patients had a new cardiovascular diagnosis, but most displayed a pattern of symptoms associated with exercise intolerance.
Keywords: Coronavirus disease 2019, Long COVID, Post-acute sequelae of COVID-19, Cardiovascular diseases
1. Introduction
Post-acute sequelae of SARS-CoV-2 infection (PASC), also known as Long COVID, is a long-term manifestation of acute coronavirus disease 2019 (COVID-19), affecting up to ten to 30 % of patients, and defined as emergent or persistent symptoms extending beyond four weeks from onset of acute illness [1]. Despite growing reports of cardiopulmonary symptoms associated with PASC, the literature on cardiovascular manifestations in this post-acute period remains limited.
2. Methods
This was an observational study of all consecutive patients referred to the Yale cardiovascular clinic for PASC (part of the CompREhensive Post-COVID CentER at Yale [2], RECOVERY) between May 2020 and September 2021. Clinical data were manually extracted from the electronic medical record. Diagnostic evaluation included baseline vital signs, cardiopulmonary physical examination, ECG, laboratory studies, and echocardiogram. Initially, all patients had a NT-proBNP, troponin, d-dimer, and CRP – however, these tests were overwhelmingly normal and subsequently only checked based on clinical suspicion. An active stand test for postural orthostatic tachycardia syndrome (POTS) was performed in patients with orthostatic intolerance (blood pressure and heart rate measured after five minutes in the supine position and with ten minutes of standing). Further evaluation was guided by clinical judgement to: a) identify cardiovascular disease pathology, and b) evaluate cardiovascular symptoms not readily attributable to a cardiovascular diagnosis, but which may have a cardiopulmonary etiology. We describe the clinical characteristics and diagnostic yield of testing, along with predictors of having cardiovascular symptoms without an identified diagnosis. The study was approved by the Human Investigation Committee of Yale University.
3. Results
Of 126 patients, average age was 44 years (range 19–74 years), 9 % were ≥ 65 years old, and 83 (66 %) were female. Thirty patients (24 %) had been hospitalized for acute COVID-19 (Table 1). Severity of acute COVID-19 was mild in 37 %, moderate in 41 %, severe in 11 %, and critical in 9 % [3]. Patients presented on average (SD) 5.1 months (3.8 months) after acute infection. All but six patients had confirmed antigen studies for COVID-19. The most common referrals were from primary care (54 %), pulmonology (26 %), and cardiology (12 %). Patients were also followed for PASC by other specialties: pulmonology (53 %), neurology (33 %), otolaryngology (11 %), and rheumatology (7 %). Comorbidities included hypertension (23 %), hyperlipidemia (25 %), diabetes (17 %), and obesity (37 %). Twelve patients (10 %) had underlying cardiovascular disease, and 40 patients (32 %) had underlying pulmonary or rheumatologic disease.
Table 1.
Characteristics of patients at time of presentation to a cardiovascular disease clinic for post-acute sequelae of SARS CoV-2 Infection.
| Sociodemographic characteristics | |
|---|---|
| Number | 126 |
| Age (SD) | 44 (15) |
| Male sex (%) | 43 (34) |
| Race (%) | |
| White | 92 (73) |
| Black | 19 (15) |
| Other/unknown | 15 (12) |
| Ethnicity | |
| Hispanic (%) | 20 (16) |
| Non-Hispanic | 99 (79) |
| Other/unknown | 7 (6) |
| Consultants (%) | |
| Pulmonology | 67 (53) |
| Neurology | 42 (33) |
| Otolaryngology | 14 (11) |
| Rheumatology | 9 (7) |
| Hematology | 3 (2) |
| Nephrology | 2 (2) |
| Psychiatry | 2 (2) |
| Hospitalization (%) | 30 (24) |
|---|---|
| Acute COVID-19 severity (%) | |
| Mild | 46 (37) |
| Moderate | 51 (41) |
| Severe | 14 (11) |
| Critical | 11 (9) |
| Unknown | 3 (2) |
| Months since diagnosis (SD) | 5.1 (3.8) |
| Vital signs | |
|---|---|
| Systolic blood pressure (SD) | 128 (17) |
| Diastolic blood pressure (SD) | 77 (11) |
| Heart rate (SD) | 78 (13) |
| Body mass index (SD) | 31 (8) |
| Comorbidities | |
|---|---|
| Hypertension (%) | 29 (23) |
| Diabetes (%) | 21 (17) |
| Hyperlipidemia (%) | 32 (25) |
| Obesity (%) | 47 (37) |
| Cardiovascular disease (%) | 12 (10) |
| Pulmonary/rheumatologic disease (%) | 40 (32) |
| Diagnostic testing | |
|---|---|
| Transthoracic echocardiogram (%) | 106 (84)a |
| LVEF, mean % (SD) | 60 (9) |
| LV systolic dysfunction (%) | 11 (10) |
| LV diastolic dysfunction (%) | 10 (9) |
| RV systolic dysfunction (%) | 9 (7) |
| TAPSE, mean cm (SD) | 2.2 (0.3) |
| RVSP, mean mm Hg (SD) | 22 (6) |
| Global longitudinal strain, mean % (SD) | −19 (3)b |
| Holter monitor (%) | 48 (38) |
| Stress test (%) | 52 (41) |
| Cardiac MRI (%) | 25 (20) |
| Late gadolinium enhancement (%) | 15 (60) |
| T2 enhancement (%) | 12 (48) |
| LVEF, mean % (SD) | 57 (6) |
| RVEF, mean % (SD) | 56 (7) |
| CPET (%) | 19 (15) |
| Reached anaerobic threshold (%) | 18 (95 %) |
| VO2 max, mean ml/kg/min (SD) | 18 (5) |
| Predicted VO2 max, mean % (SD) | 64 (21) |
| Pulmonary and circulatory limitation (%) | 2 (11) |
| Pulmonary limitation (%) | 12 (63) |
| Circulatory limitation (%) | 3 (16) |
| Ve/VCO2 in patients with CL, mean (SD) | 30 (4) |
| RER in patients with CL, mean (SD) | 1.1 (0.8) |
| PET/CT (%) | 6 (5) |
CL, circulatory limitation; CPET, cardiopulmonary exercise testing; LV, left ventricular; LVEF, left ventricular ejection fraction; MRI, magnetic resonance imaging; PET/CT, positron emission tomography/computed tomography; RV, right ventricular; RVEF, right ventricular ejection fraction; RER, respiratory exchange ratio; RVSP, right ventricular systolic pressure; SD, standard deviation; TAPSE, tricuspid annular plane systolic excursion; Ve/VCO2, minute ventilation/carbon dioxide production; VO2, oxygen consumption.
Twenty additional patients had an echocardiogram performed by an outside institution.
Only measured in seven patients.
The most common cardiovascular symptoms on presentation included dyspnea (52 %, almost always exertional), chest pain/pressure (48 %) and palpitations (44 %); 84 % of patients demonstrated at least one of these cardiopulmonary symptoms (Fig. 1).
Fig. 1.
Baseline symptoms of patients referred for cardiovascular symptoms associated with post-acute sequelae of SARS-CoV-2 (PASC).
3.1. Diagnostic evaluation for cardiovascular pathology
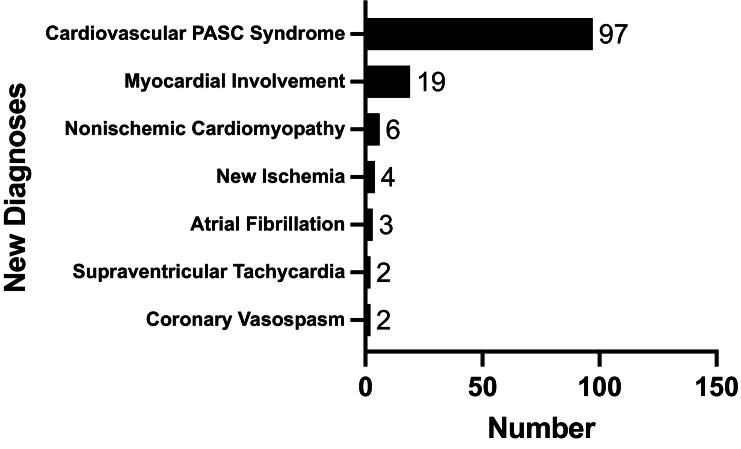
Echocardiography was performed by the Yale Heart and Vascular Center (n = 106); 20 were performed at an outside institution and reported as “normal” (Table 1). Of the remaining 106 patients, the mean left ventricular ejection fraction was 60 %. Eleven patients (9 %) had evidence of left ventricular systolic dysfunction (no baseline TTEs for comparison), ten patients (8 %) had left ventricular diastolic dysfunction, and nine patients (7 %) had right ventricular systolic dysfunction. Mean right ventricular systolic pressure was 22 mm Hg, mean TAPSE was 2.2 cm, and mean global longitudinal strain (measured in seven patients) was −19.4 %. Forty-eight patients (38 %) had a Holter monitor, of whom 43 had non-exertional sinus tachycardia (with symptoms), three had new atrial fibrillation, and two had another supraventricular tachycardia. Four out of 53 patients who underwent stress testing (with SPECT or echocardiogram) had evidence of new ischemia (Fig. 2). Twenty-five patients (20 %) had a cardiac MRI, of whom 76 % had late gadolinium enhancement and/or evidence of T2 inflammation, which we diagnosed as myocardial involvement but not acute myocarditis since the presentation was not acute, troponin was negative, and there were no electrocardiographic changes. Other new diagnoses included: nonischemic cardiomyopathy (n = 6; 5 %) and coronary vasospasm (n = 2; 2 %).
Fig. 2.
New diagnoses identified at evaluation for cardiovascular symptoms associated with post-acute sequelae of SARS-CoV-2 (PASC).a
aNumbers do not add up to 126 since some diagnoses overlap in the same patients.
3.2. Other diagnoses and symptom patterns
The remaining 97 patients (77 %) without new cardiovascular pathology had a “cardiovascular PASC syndrome” of palpitations, chest pain/pressure, fatigue or dyspnea on exertion that limited regular activities. Three patients met criteria for POTS; one of whom had a confirmatory tilt table test. Nineteen (15 %) underwent cardiopulmonary exercise testing (CPET) to further evaluate symptoms (Table 1). 95 % reached anaerobic threshold with a mean max VO2 of 18 ml/kg/min. Of those with cardiac limitation, the mean Ve/VCO2 was 30, and the mean peak respiratory exchange ratio was 1.11. Ten patients did not demonstrate any pulmonary limitation, but had varying degrees of cardiac limitation.
Severe (OR = 0.24, 95 % CI 0.06–0.90) and critical (OR = 0.22, 95 % CI 0.05–0.90) severity of acute COVID-19 was associated with a significantly lower odds of cardiovascular PASC syndrome compared with mild severity. Age, sex, and comorbidities were not significantly associated with cardiovascular PASC syndrome.
4. Discussion
In this large, consecutive series of patients referred to the cardiovascular disease clinic for PASC, we identified a range of cardiovascular disease pathology. Most patients, however, exhibited a “cardiovascular PASC syndrome” without an underlying diagnosis.
Our study continues to show that cardiovascular manifestations of PASC are common [4], affecting primarily non-elderly individuals [5]. While patients were referred for cardiopulmonary symptoms suspected to have a cardiac etiology, only 23 % of evaluations led to a cardiovascular diagnosis, including new cardiomyopathy, ischemia, arrhythmias, and myocardial involvement. Many patients, including those with cardiovascular diagnoses, suffered from exercise intolerance, describing fatigue with activities of daily living and severe post-exertional dyspnea and malaise impairing their ability to work; however, in the majority of cases (77 %), a cardiovascular disease was not found. Many of these patients also suffered from “brain fog,” characterized by memory problems, difficulty concentrating, and sleep disturbance. Many also described body aches. These symptoms overlap with myalgic encephalomyelitis-chronic fatigue syndrome (ME-CFS) [6].
COVID-19 can affect the cardiovascular system through several mechanisms, including viral injury, inflammation, dysregulation of ACE-2, along with metabolic and autonomic impairment; however, in a standard clinical evaluation, these mechanisms are challenging to discern [7]. We did observe downstream physiological dysregulation - including endothelial dysfunction along with cardiometabolic dysfunction – in a subset of patients who underwent invasive coronary vasomotion testing and cardiopulmonary exercise testing. Endothelial dysfunction was directly observed in two patients with ischemia and no obstructive coronary artery disease who developed vasospasm to acetylcholine challenge. For these patients, we prescribed calcium channel blockers, nitrates, statins, and L-arginine. Interestingly, of six patients who underwent stress PET/CT, we detected only one case of microvascular dysfunction. Cardiometabolic impairment was observed on CPET; specifically, more than half of patients had low peak VO2 with normal cardiac and pulmonary function, which may be secondary to bedrest deconditioning and/or impaired oxygen extraction. In bedrest deconditioning, low plasma volume leads to decreased left ventricular filling, cardiac atrophy, and resultant decreased stroke volume which potentiates a compensatory tachycardia to maintain cardiac output [8]. Fatigue and tachycardia result in less physical activity, which further exacerbates the cycle of deconditioning. Impaired oxygen extraction in the peripheral microcirculation was previously demonstrated on invasive CPET in patients with ME-CFS and more recently with PASC [9], [10]. For all patients with exercise intolerance, we employed principles from the Levine protocol, developed for patients with POTS, which includes structured recumbent exercise with gradual increases in duration, along with hydration, salt loading, compression socks, and elevating the head of the bed [11].
This study should be interpreted in the context of the following limitations. First, referrals and diagnostic evaluation were not systematic, but may be generalizable to other cardiology practices seeing high volumes of patients with PASC. We used a probabilistic approach that follows evidence-based diagnostic pathways for evaluating ischemia due to obstructive and non-obstructive disease, structural heart disease, and arrhythmias, along with cardiovascular causes of orthostatic and exertional intolerance. Consistent with this, and as our understanding of PASC evolved with time, we reduced the number of diagnostic tests performed in patients with mild acute COVID-19 infection and who had a low pretest probability of disease, since these tests were either normal or did not advance our understanding of their disease pathology. Still, all patients had a thorough cardiovascular physical examination, ECG, and echocardiogram. Second, the clinical relevance of myocardial involvement based on cardiac MRI is uncertain. Third, new diagnoses that better define the PASC cardiovascular syndrome may emerge, although diagnostic algorithms have yet to be developed. Finally, we do not report on patient recovery since patients in our cohort did not have uniform follow-up and many were treated by multiple specialties, making it difficult to discern treatment effects. Their cardiovascular treatment, when indicated, followed evidence-based standards.
In conclusion, this study provides insight into patients with cardiovascular manifestations of PASC, emphasizing the need for multidisciplinary teams and accelerated research to better phenotype and treat a heterogeneous group of patients.
Declaration of competing interest
Dr Spatz reports that she receives research funding from the Centers for Disease Control and Prevention (ClinicalTrials.gov Identifier: NCT04610515) to study the long-term health outcomes of COVID in adults. She served on an expert panel on Long Covid, hosted by Regeneron. Dr Spatz receives funding from the National Institute on Minority Health and Health Disparities, the U.S. Food and Drug Administration to support projects within the Yale-Mayo Clinic Center of Excellence in Regulatory Science and Innovation (CERSI), and from the National Heart, Lung, and Blood Institute.
Other authors: no conflicts of interest.
Acknowledgments
Acknowledgment
Disclosures
Dr Spatz is a co-PI on a multi-center, Centers for Disease Control and Prevention (CDC)-funded study, “Innovative Support for Patients With SARS-COV2 Infections (COVID-19) Registry (INSPIRE).” She receives funding from the Food and Drug Administration (FDA) as part of the Yale University-Mayo Clinic Center of Excellence in Regulatory Science and Innovation (CERSI). She also receives support from the National Institute on Minority Health and Health Disparities (U54MD010711-01) to study precision-based approaches to diagnosing and preventing hypertension, from the National Institute of Biomedical Imaging and Bioengineering (R01 EB028106-01) to study a cuff-less blood pressure device. The other authors have no relevant disclosures.
References
- 1.Nalbandian A., Sehgal K., Gupta A., et al. Post-acute COVID-19 syndrome. Nat. Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lutchmansingh D.D., Knauert M.P., Antin-Ozerkis D.E., et al. A clinic blueprint for post-coronavirus disease 2019 RECOVERY: learning from the past, looking to the future. Chest. 2021;159(3):949–958. doi: 10.1016/j.chest.2020.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clinical Spectrum of SARS-CoV-2 Infection. 2021. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
- 4.Carfì A., Bernabei R., Landi F. Group ftGAC-P-ACS. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Estiri H., Strasser Z.H., Brat G.A., Semenov Y.R., Patel C.J., Murphy S.N. Evolving phenotypes of non-hospitalized patients that indicate long COVID. BMC Med. 2021;19(1):249. doi: 10.1186/s12916-021-02115-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paul B.D., Lemle M.D., Komaroff A.L., Snyder S.H. Redox imbalance links COVID-19 and myalgic encephalomyelitis/chronic fatigue syndrome. Proc. Natl. Acad. Sci. U. S. A. 2021;118(34) doi: 10.1073/pnas.2024358118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans P.C., Rainger G.E., Mason J.C., et al. Endothelial dysfunction in COVID-19: a position paper of the ESC working Group for Atherosclerosis and Vascular Biology, and the ESC Council of basic cardiovascular science. Cardiovasc. Res. 2020;116(14):2177–2184. doi: 10.1093/cvr/cvaa230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine B.D., Zuckerman J.H., Pawelczyk J.A. Cardiac atrophy after bed-rest deconditioning: a nonneural mechanism for orthostatic intolerance. Circulation. 1997;96(2):517–525. doi: 10.1161/01.cir.96.2.517. [DOI] [PubMed] [Google Scholar]
- 9.Singh I., Joseph P., Heerdt P.M., et al. Persistent exertional intolerance after COVID-19: insights from invasive cardiopulmonary exercise testing. Chest. 2021;161(1):54–63. doi: 10.1016/j.chest.2021.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joseph P., Arevalo C., Oliveira R.K.F., et al. Insights from invasive cardiopulmonary exercise testing of patients with myalgic Encephalomyelitis/Chronic fatigue syndrome. Chest. 2021;160(2):642–651. doi: 10.1016/j.chest.2021.01.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bryarly M., Phillips L.T., Fu Q., Vernino S., Levine B.D. Postural orthostatic tachycardia syndrome: JACC focus seminar. J. Am. Coll. Cardiol. 2019;73(10):1207–1228. doi: 10.1016/j.jacc.2018.11.059. [DOI] [PubMed] [Google Scholar]