Abstract
The highly pathogenic, novel coronavirus disease (COVID-19) outbreak has emerged as a once-in-a-century pandemic with poor consequences, urgently calling for new therapeutics, cures, and supportive interventions. It has already affected over 250 million people worldwide; thereby, there is a need for novel therapies to alleviate the related complications. There is a paradigm shift in developing drugs and clinical practices to combat COVID-19. Several clinical trials have been performed or are testing diverse pharmacological interventions to alleviate viral load and complications such as cytokine release storm (CRS). Kinase-inhibitors have appeared as potential antiviral agents for COVID-19 patients due to their efficacy against CRS. Combination of kinase inhibitors with other therapies can achieve more efficacy against COVID-19. Based on the pre-clinical trials, kinase inhibitors such as Janus kinase-signal transducer and activator of transcription (JAK/STAT) inhibitors, Brutton's tyrosin kinase (BTK) inhibitors, p38 mitogen-activated protein kinases (p38 MAPK) inhibitors, Glycogen synthase kinase 3 (GSK-3) inhibitors can be a promising strategy against COVID-19. Kinase inhibitors possess crucial pharmacological properties for a successful re-purposing in terms of dual anti-inflammatory and anti-viral effects. This review will address the current clinical evidence and the newest discovery regarding the application of kinase inhibitors in COVID-19. An outlook on ongoing clinical trials (clinicaltrials.gov) and unpublished data is also presented here. Besides, Kinase inhibitors' function on COVID-19-mediated CRS is discussed.
Keywords: Kinase inhibitor, COVID-19, Signaling, JAK/STAT, BTK, p38 MAPK, GSK-3, CRS
Graphical abstract
Nomenclature
- SARS-CoV-2
-
severe acute respiratory syndrome coronavirus-2
COVID-19
coronavirus disease 2019
ARDS
acute respiratory distress syndrome
CRS
cytokine release syndrome
IL
interleukin
TNF
tumor necrosis factor
ICU
intensive care unit
INF
interferon
MCP
monocyte chemoattractant protein
ACE2
angiotensin-converting enzyme 2
NF-kB
nuclear factor-kappa
IFN-γ
interferon-gamma
G-CSF
granulocyte colony-stimulating factor
IP
inducible protein
MIP-1α
macrophage inflammatory protein
JAK/STAT
Janus kinase/signal transducer and activator of transcription
VEGF
vascular endothelial growth factor
ADAM17
A disintegrin and metalloprotease 17
TGF-β
transforming growth factor-beta
RA
rheumatoid arthritis
RCTs
randomized controlled trials
CRP
C-reactive protein
MCD
multicentric castleman disease
MAPKs
mitogen-associated protein kinases
ERKs
extracellular signal-regulated kinases
APCs
antigen-presenting cells
DCs
dendritic cells
CTLs
cytotoxic T-lymphocytes
MMPs
matrix metalloproteinases
IL-17RA
anti-IL-17 receptor A
1. Introduction
The outbreak and rapid spread of the novel coronavirus disease (COVID-19) has posed a severe problem for public health organizations. COVID-19 patients experience a broad spectrum of clinical manifestations, such as acute respiratory distress syndrome (ARDS) and multi-organ failure [114]. The leading cause of clinical complications is an exuberant immune response called cytokine release syndrome (CRS) [60]. CRS is defined by systemic immune activation with elevated levels of pro-inflammatory cytokines and chemokines. Indeed, viral entry can stimulate immune responses to release the excess pro-inflammatory cytokines such as interleukin (IL)-1β, IL2, IL-6, IL10, IL-17, tumor necrosis factor-α (TNFα), interferon-γ (IFN-γ), granulocyte-colony stimulating factor (G-CSF), macrophage inflammatory protein 1α (MIP-1α) [59]. In addition, chemokines such as CCL2, CXCL8, and CXCL10 are involved in different stage of SARS-CoV-2 infection [42]. Excessive release of chemokine leads to massive infiltration of inflammatory-related cells to the lung and eventually develops ARDS [36]. Growing evidence has shown the presence of higher levels of pro-inflammatory cytokines in plasma of severe patients as compared to pateints with mild condition, implying that the CRS is directly associated with the severity of COVID-19 infection [70]. As a result, there is a need to develop novel interventions targeting viral infection and the CRS-elicited deteriorations. Several attempts have been made to counteract the disease progression and its mortality. Growing evidence showed that inhibiting pro-inflammatory signaling could dampen the inflammatory process, improving clinical outcomes [102]. Re-purposing pre-existing FDA-approved drugs to treat SARS-CoV-2 and other coronaviruses offer an alternative opportunity for rapid development of therapeutics against the current pandemic.
Several kinases have been reported to be essential mediators for viral entry, metabolism, or reproduction, such as SARS-CoV-2. Given the contribution of multiple kinases in viral infections, kinase inhibitors may be a beneficial therapeutic option in alleviating the severe symptoms of COVID-19 [68]. In this regard, kinase inhibitors can be re-purposed as a therapeutic agent against COVID-19 infection to reduce viral-mediated infection. Along with this strategy, it is worth noting that combining kinase inhibitors with antiviral agents or other targeted therapies has shown promise in clinical trials for COVID-19, resulting in more remarkable benefits than any individual agent alone. Kinases can regulate many inflammatory reactions in response to pro-inflammatory cytokines (e.g., IL-6 and TNF-α) and protein molecules associated with inflammatory responses and pulmonary fibrosis (e.g., transforming growth factor-β) [104]. The direct effects of kinase inhibitors are mediated by their immunomodulatory effects (e.g., anti-inflammation, cytokine-suppression, and antifibrotic effects), exhibiting beneficial efficacy against viral infections like COVID-19. Accordingly, various kinase inhibitors are being examined in clinical trials to treat COVID-19 disease [72]. With this respect, we briefly review the mechanism action of kinase and their inhibitors, its original application, and the re-purposing for COVID-19 treatment. We also provide an outlook on the recent clinical evidence associated with COVID-19 infection. The potential of antiviral, anti-inflammatory, cytokine-suppressive, and antifibrotic activity of kinase inhibitors is discussed, too.
2. Kinase-mediated signaling pathways
Kinases are responsible for catalyzing the transfer of phosphate from adenosine triphosphate (ATP) to the substrate with a hydroxyl group. Kinases regulate many cellular processes, including differentiation, transcriptional activation, apoptosis, metabolism, etc., which are essential in signal transduction [108]. Kinase inhibitors are a prospective treatment for many disorders with the uncontrolled activity of kinase as a sign of inflammation and tumor [4], [113]. Chemokines have an essential role in the migration and binding of cells to cognate receptors at the site of inflammation/infection. Chemokine receptors that belong to G protein-coupled receptors (GPCRs) mediate downstream intracellular signaling events. A variety of kinases, such as phosphoinositide 3-kinase (PI3K), mitogen-activated protein kinases/extracellular signal-regulated kinases (MAPK/ERK), G protein-coupled receptor kinases (GRKs), Janus kinases (JAKs), etc., regulate the activation, expression, and administration of the GPCR-based signaling [95]. The chemokine binding to the cognate receptor induces the GDP/GTP exchange and activation of several signaling molecules, secondary messengers, and phosphorylation processes catalyzed by intracellular kinases to control chemokine-mediated signaling [63]. Collectively, the kinase system induced by the chemokines and their receptor plays a pivotal role in the regulation of the immune system and initiation of diverse pathophysiological conditions.
3. Method
Different databases, including Pubmed, EMBASE, Scopus, and Web of Science, and numerous journals, including New England Journal of Medicine (NEJM), the Lancet, and Nature, were searched to collect articles about the effects of four main kinase pathways inhibitors which described in this article (JAK/STAT pathway inhibitors, BTK pathway inhibitors, MAPK pathway inhibitors, and GSK-3 pathway inhibitors). Although we reviewed case reports, cohort studies, in vitro and animal studies, our focus was on reviewing the papers that include clinical trials (Table 1, Table 2, Table 3 ).
Table 1.
JAK/STAT inhibitors COVID-19 randomized clinical trials information.
| Clinical identifier/Status | Sponsor | Population | Intervention | Control group | Primary outcome |
|---|---|---|---|---|---|
| NCT04421027 (phase III, completed) | Eli Lilly and Company | N = 1585 | Baricitinib (orally) | Placebo | Mortality, needing mechanical ventilation or ECMO |
| NCT04970719 (phase III, recruiting) | Bangladesh Institute of Research and Rehabitation in Diabetes, Endocrine, and Metabolic Disorders | N = 382 | Baricitinib (orally) | Remdesivir (IV) plus dexamethasone | requiring “rescue treatment” |
| NCT05056558 (phase III, not yet recruiting) | Incepta Pharmaceuticals Ltd | N = 480 | Baricitinib (orally) | Placebo | Clinical status assessed by a 7-point ordinal scale on Day 14: (mortality, hospitalization’ needing mechanical ventilation or ECMO) |
| NCT05082714 (phase: not applicable, recruiting) | University Hospital of Patras | N = 164 | Baricitinib (orally) plus usual care versus tocilizumab (IV) plus usual care | – | Mortality and mechanical ventilation |
| NCT04693026 (phase III, recruiting) | M Abdur Rahim Medical College and Hospital | N = 150 | Remdesivir (IV) plus baricitinib (orally) | Remdesivir (IV) plus tocilizumab (IV) | Time for clinical improvement |
| NCT04832880 (phase III, not yet recruiting) | ASST Fatebenefratelli Sacco | N = 4000 | Remdesivir (IV) plus dexamethasone (IV)/baricitinib (orally) plus dexamethasine (IV) | Control arm: dexamethasone arm (IV)/remdesivir (IV) plus baricitinib (IV) plus dexamethasone (IV) | Mortality or respiratory failure |
| NCT04393051 (phase II, not yet recruiting) | Azienda Ospedaliero, Universitaria Pisana | N = 126 | Baricitinib (orally) | Standard of care | Mechanical ventilation |
| NCT04346147 (phase II, active, not recruiting) | Hospital Universitario de Fuenlabrada | N = 168 | Imatinib (orally)/baricitinib (orally) | Supportive treatment | Time for clinical improvement |
| NCT04401579 (phase III, completed) | National Institute of Allergy and Infectious Diseases (NIAID) | N = 1033 | Remdesivir (IV) plus baricitinib (orally) | Remdesivir (IV) plus placebo | Time to recovery, time to hospitalization, and needing supplemental oxygen |
| NCT04640168 (phase III, completed) | National Institute of Allergy and Infectious Diseases (NIAID) | N = 1010 | Baricitinib (orally) plus remdesivir (IV) plus dexamethasone (IV) | Dexamethasone (IV) plus placebo plus remdesivir (IV) | Death, hospitalization, needing mechanical ventilation or ECMO |
| NCT04390464 (phase IV, recruiting) | Cambridge University Hospitals NHS Foundation Trust | N = 1167 | Baricitinib (orally) plus standard care/ravulizumab (IV) plus standard care | Standard care | Mortality, needing mechanical ventilation, ECMO or cardiovascular organ support, renal failure |
| NCT04891133 (phase II and III, recruiting) | Oslo University Hospital | N = 1900 | Baricitinib (orally) | Placebo | Mortality, disease progression, SpO2/FiO2-ratio |
| NCT04890626 (phase III, recruiting) | Instituto de Investigación Hospital Universitario La Paz | N = 2193 | Main randomization.Arm: Emtricitabine/Tenofovir disoproxil fumarate Emtricitabine/Tenofovir disoproxil fumarate/Rescue randomization: Arm: Dexamethasone (IV) + Baricitinib (orally)/Arm: Dexamethasone (IV) |
No Intervention: Main randomization.Arm: No treatment | Mortality rate |
| NCT04381936 (phase II and III, recruiting) | University of Oxford | N = 50,000 | Baricitinib (orally) but not limited to it (Lopinavir-Ritonavir, Corticosteroid, and 13 other medications) | Standard care | Mortality rate |
| NCT05187793 (phase III, recruiting) | R-Pharm | N = 204 | Olokizumab (IV) plus standard therapy (baricitinib (orally), favipiravir (orally), dexamethasone (IV) | Standard therapy (baricitinib (orally), favipiravir (orally), dexamethasone (IV) | Clinical recovery |
| NCT04469114 (phase III, completed) | Hospital Israelita Albert Einstein (Pfizer) | N = 289 | Tofacitinib (orally) | placebo | Death, mechanical ventilation, or ECMO |
| NCT04469114 (phase III, completed) | Hospital Israelita Albert Einstein (collaborator: Pfizer) | N = 289 | Tofacitinib (orally) | placebo | Death, mechanical ventilation, or ECMO |
| NCT04390061 (phase II, not yet recruiting) | Università Politecnica delle Marche | N = 116 | Tofacitinib (orally) | Standard care (Hydroxychloroquine (orally)) | Prevention of severe respiratory failure and requiring mechanical ventilation |
| NCT05080218 (phase IV, not yet recruiting) | Jeffrey Curtis | N = 1000 | Tofacitinib (orally)/Upadacitinib/Abatacept and 3 other types of medications | No, Intervention: treatment continuation treatment continuation of all immunomodulatory therapy at the time of COVID vaccine booster (mRNA vaccine dose 3) | Quantitative ratio post booster vs. pre-booster of IgG against SARS-CoV-2 using electrochemiluminescent (ECL) technology against the receptor-binding domain (RBD) of spike protein, stratified by treatment arm |
| NCT04362137 (phase III, completed) | Novartis Pharmaceuticals | N = 432 | Ruxolitinib (orally) | placebo | Mortality, respiratory failure, ICU admission |
| NCT04424056 (phase III, not yet recruiting) | Assistance Publique Hopitaux De Marseille | N = 216 | Administration of Anakinra ± ruxolitinib (orally) in the severe stage/Tocilizumab ± ruxolitinib (orally) in the severe stage | Standard care in sever stage | Ventilation free days |
| NCT04403243 (phase II, recruiting) | Lomonosov Moscow State University Medical Research and Educational Center | N = 70 | Arm1: Colchicine (orally)/Arm2:ruxolitinib (orally)/Arm 3: Secukinumab (subcutaneously) | Standard therapy | Changes in clinical status: respiratory rate, body temperature, Sp02 without support oxygen, ventilation, CRP, D-dimer, exposure area on lung CT |
| NCT04581954 (phase I and II, recruiting) | Imperial College London | N = 456 | Fostamatinib (orally)/ruxolitinib (orally) | Standard care | Mortality, needing mechanical ventilation |
| NCT04348695 (phase II, recruiting) | Fundación de investigación HM | N = 94 | Ruxolitinib (orally) | Standard care | Respiratory failure |
| NCT04348695 (phase II, recruiting) | Fundación de investigación HM | N = 94 | Ruxolitinib (orally) plus simvastatin (orally) | Standard care | Respiratory failure |
Table 2.
BTK inhibitors COVID-19 randomized clinical trials information.
| Trial Identifier/Status | Sponsor | Population | Intervention | Control Group | Primary Outcome |
|---|---|---|---|---|---|
| NCT04380688 (phase II, completed) | AstraZeneca | N = 62 | Acalabrutinib (orally) | Best supportive care | Serious adverse events, death, and respiratory failure |
| NCT04346199 (phase II, completed) | AstraZeneca | N = 177 | Acalabrutinib (orally) | Best supportive care | Death and respiratory failure |
| NCT04647669 (phase III, not yet recruiting) | The University of The West Indies | N = 100 | Acalabrutinib (orally)/remdesivir (IV)/Interferon beta-1a (IV) | Local standard of care | Mortality rate |
| NCT05024006 (phase: Not Applicable, completed) | University of the Philippines | N = 1314 | Acalabrutinib (orally) but not limited to it | Local standard of care | Mortality rate |
| NCT04375397 (phase II, completed) | AbbVie | N = 46 | Ibrutinib (orally) | Placebo | Death and respiratory failure |
| NCT04439006 (phase II, active, not recruiting) | Jennifer Woyach | N = 10 | Ibrutinib (orally) | Usual care | Death, respiratory failure |
| NCT04382586 (phase II, completed) | BeiGene | N = 63 | Zanubrutinib (orally) + supportive care | Supportive care + placebo | Respiratory failure and time to breathe room air |
Table 3.
An overview of the newest kinase inhibitors on COVID-19-mediated CRS.
| Medication (brand name) | Mechanism of action | Approval | Approved or recommended indications | Cytokines decreased by intervention in Covid-19 model | References |
|---|---|---|---|---|---|
| Baricitinib (Olumiant) | JAK1/JAK2 inhibitor | FDA approved | Moderate to severe active rheumatoid arthritis in adult patients | IFN-γ, IL-1β, IL-6, TNF-α, IL-17, IL-4, IL-13, IL-10, IL-1ra, IP-10, MCP-1, IP-10, MCP-1, FGF, GM-CSF, IL-2, IL-5, CXCL8, CCL5, CCL3, CCL20, IL-18, type I IFN, IFN-β | [67], [31], [8] |
| Ruxolitinib (Jakafi and Jakavi) | JAK1/JAK2 Inhibitor | FDA approved | Intermediate or high-risk myelofibrosis and resistant forms of polycythemia vera and graft-vs-host disease | IL-6, nerve growth factor β, IL-12, macrophage migration inhibitory factor, MIP-1α, macrophage inflammatory protein 1β (MIP-1β), and vascular endothelial growth factor (VEGF), IFN-γ producing T cells and TNF-α producing NK cells, IL-1β, IL-6, IL-12, IP-10, MCP1, | [13], [98], [62] |
| Tofacitinib (Xeljanz) | JAK1/JAK3 inhibitor | Rheumatoid arthritis | |||
| Itolizumab (Alzumab) | anti-CD6 monoclonal antibody | Experimental drug (phase III) | Acute psoriasis | IL-6 | [99] |
| Acalabrutinib (Calquence) | BTK inhibitor | FDA approved | B cell malignancies including refractory mantle cell lymphoma and chronic lymphocytic leukemia | IL-6 | [79] |
| Ibrutinib (Imbruvica) | BTK inhibitor | FDA approved | Refractory chronic Lymphocytic leukemia (CLL) and mantle cell lymphoma | TNF-α, IFN-γ, IL-6 | [22] |
| Zanubrutinib (Brukinsa) | BTK inhibitor | FDA approved | Refractory mantle cell lymphoma (MCL) | ||
| SB 203580 | P48 MAPK inhibitor (p38α and p38β) | Experimental drug | Non-steroidal anti-inflammatory agent | IL-6, TNF-α, CXCL8, CCL20, and CCL2, | [7] |
| Gilteritinib (Xospata) | AXL and FLT3 inhibitor | FDA approved | Acute myeloid leukemia (ALM) | ||
| Bemcentinib | AXL inhibitor | Experimental drug | Acute myeloid leukemia (ALM) | ||
| Ralimetinib | P38 MAPK inhibitor (p38α and p38β) | Experimental drug | Ovarian cancer | ||
| MAPK-13-IN-1 | P38 MAPK inhibitor (p38 δ) | Experimental drug | |||
| ARRY371797 | P38 MAPK inhibitor (p38α) | Experimental drug | LMNA-related dilated cardiomyopathy | ||
| Lithium chloride (Licl) | GSK-3 inhibitor | FDA approved | The main drug used for bipolar disorder, psychiatric disorders |
4. Pharmacological aspects of Janus-associated kinase (JAK) inhibitors
As noted above, there is a strong correlation between severe COVID-19 and hyperactivity of the immune system. COVID-19-induced hyper inflammation contributes to the pathophysiology of ARDS and organ damage in affected patients. For this reason, anti-inflammatory agents are utilized against COVID-19 by interfering with pro-inflammatory cascades [46]. Several cytokines are involved in distinct signaling pathways mediated by the Janus kinase signal transducer and activator of transcription (JAK/STAT) pathway. JAK/STAT pathway mediates the signaling of extracellular stimuli such as cytokines into the nuclei of cells; thereby, interrupting this pathway could alleviate the immunopathology of SARS-CoV-2 infection [83] (Fig. 1 ).
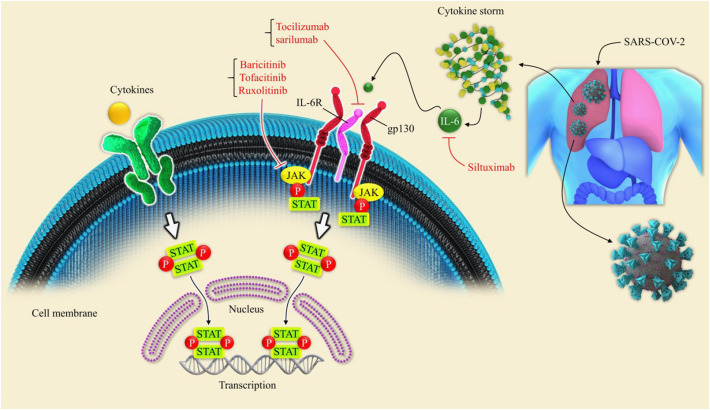
Fig. 1.
The role of the JAK/STAT pathway in COVID-19 hyperinflammatory response. The SARS-CoV-2 induces a cytokine storm. IL-6 binds to the soluble or membrane-bound receptor, forming a complex with ubiquitously expressed gp130 protein. The intracellular domain of gp130 activates JAK/STAT and downstream signaling pathways that promote the expression of pro-inflammatory genes involved in inflammation and tissue damage. Antagonists of IL-6 (tocilizumab, sarilumab, and siltuximab) antagonize ligand-receptor engagement and prevent IL-6 mediated signaling. sgp130Fc is an exclusive inhibitor of IL-6 trans-signaling. Baricitinib, Tofacitinib, and Ruxolitinib inhibit activation of JAKs and prevent activation of immune cells and inflammatory reactions.
4.1. Baricitinib
Baricitinib (a drug approved for rheumatoid arthritis) is a potent molecule that selectively inhibits the JAK/STAT pathway and reduces cell inflammation. JAK proteins transduce signals through the activation of STAT proteins. Activated STAT proteins can mediate nuclear signaling and promote inflammatory gene transcription such as interferon-1 and IL-6. Baricitinib exerts its effects by reversibly inhibiting JAK1/JAK2 and preventing inflammatory cytokine production [38], [76]. Beyond anti-inflammatory effects, baricitinib exerts direct antiviral effects by blocking viral entry into cells. Angiotensin-converting enzyme 2 (ACE2) receptors are the main binding site for entry of SARS-CoV-2 and subsequent viral replication. Upon binding, two host-derived kinases are activated, including AP2-associated protein kinase 1 (AAK1) and cyclin G-associated kinase (GAK). AAK1 and GAK belong to the numb-associated kinase (NAK) family and play an essential role in life cycles and virus infection. AAK1 and GAK activate adaptor protein AP that binds to the cell-surface receptors to recruit clathrins for endocytosis. AAK1 promotes clathrin-mediated endocytosis, and GAK mediates viral endocytosis [39].
In this context, baricitinib suppresses AAK1 and GAK and inhibits the Clarithrin-associated entry by reducing viral assembly [69]. Baricitinib can mitigate IFN-elicited auto-inflammatory events via blocking the type-I IFN signaling pathway and downregulating CD80/CD86 expression. Also, it could suppress the production of type I IFN from plasmacytoid dendritic cells, which are the main source of type-I IFN production. Based on these properties, baricinitib is a promising option in every phase of the viral disease [45]. Several studies proposed that baricitinib is a possible choice for disease management in SARS-CoV-2 infection due to its dual actions of reducing cytokine release and inhibiting viral endocytosis [12].
An in vitro study on 39 blood samples of patients with COVID-19 demonstrated that administering 10 nM and 1000 nM baricitinib remarkabely decreased interferon-gamma (IFN-γ) level in response to spike-antigen. In addition to this, bericitinib at a dose of 1000 nM could significantly reduce spike-specific response mediated by pro-inflammatory cytokines, including IL-1β, IL-6, TNF-α, IL-17, IL-4, IL-13, Fibroblast growth factors, and other inflammatory cytokines [67]. In another study on the rhesus macaque model of COVID-19 infection, Hoang et al. showed beneficial effects of baricitinib drug in alleviating inflammation and decreasing lung pathology. NETosis activity was also reduced in animals treated with baricitinib [31]. The investigations on beneficial antiviral effects of baricitinib against COVID-19 are not limited to experimental assays; indeed, many clinical trials and observational studies confirm this drug's anti-inflammatory and antiviral effects. In a non-controlled study on 15 patients with COVID-19 positive test, administration of baricitinib and hydroxychloroquine decreased CRP level and attenuated clinical symptoms and oxygen saturation. Although, three patients died, and some thrombotic events were observed [94]. An observational study on 20 patients with severe COVID-19 showed that daily administration of baricitinib markedly reduced inflammatory markers in serum samples, including IL-6, IL-1β, and TNF-α. Besides, the number of T and B cells was rapidly recovered, and antibody production against S protein of SARS-CoV-2 was elevated following baricitinib administration [9]. Another retrospective observational study of 60 COVID-19 patients showed that treatment with baricitinib or tocilizumab did not cause serious side effects [78]. In addition, two other studies investigated the effects of bericitinib in combination with other antiviral and anti-inflammatory drugs, including remdesivir and corticosteroids. A randomized controlled trials (RCT) study in 1033 hospitalized patients with COVID-19 compared the combination of baricitinib with remdesivir versus remdesivir alone. They showed that combination therapy could reduce recovery time and improve clinical outcomes, with lower adverse events and even mortality rates than patients who received remdesivir alone [40]. In another similar research, combination therapy of baricitinib and corticosteroids improved SpO2/FiO2 ratio in hospitalized patients until discharged compared to corticosteroid treatment alone [77].
Nevertheless, the immunosuppressive effects of baricitinib may enhance the vulnerability to secondary opportunistic infections. Besides, baricitinib is associated with an increased risk of thromboembolic events and hypercoagulable state in patients with COVID-19. Evidence indicated that baricitinib could not be used in patients with a neutrophil count <1 × 109 cells/L or lymphocyte count <0.5 × 109 cells/L. Since lymphopenia is one of the most prominent findings in COVID-19 patients, associated with more disease severity [69].
On the other hand, interferon is one of the most robust innate immune responses to inhibit viral replication at the early stage of infection. JAK/STAT signaling pathway (mainly JAK1 and JAK2) is activated by interferons. This leads to the upregulation of interferon-controlled genes for killing viruses. As a result, blocking JAK/STAT signal by baricitinib produces an impairment of interferon-mediated antiviral response, facilitating the evolution of SARS-CoV-2 infection [20].
4.2. Ruxolitinib
Ruxolitinib is an FDA-approved JAK1/2 inhibitor used to treat polycythemia vera and myelofibrosis. It inhibits JAK kinase activity and stops STAT activation and subsequent nuclear translocation [1]. Ruxolitinib could block the IL6/JAK/STAT3 pathway, thereby down-regulate the circulating IL-6 levels. One small sample size RCT project assessed the effectiveness and safety of ruxolitinib for COVID-19 pneumonia treatment. The patients who received ruxolitinib significantly improved pulmonary CT findings and lymphopenia. The severe adverse effects have not been reported [111]. Ruxolitinib has great potency in overcoming complications developed by immune hyperactivation due to the JAK/STAT signaling pathway. As the JAK/STAT pathway induces multiple molecular immune pathways, inhibition of it may impede various cellular processes [34], [35], [57].
Many studies (RCT, observational, case report) investigated ruxolitinib's clinical benefits and toxicity in COVID-19 patients, and many trials registered on Clinicaltrails.gov [23]. A multicenter, single-blind randomized controlled trial investigated the beneficial effects of ruxolitinib on 43 patients with severe COVID-19. Their results demonstrated that patients on ruxolitinib exhibited faster clinical improvement, reduced levels of CRP, IL-6, IL-12, MIP-1α, and vascular endothelial growth factor (VEGF), but no significant reduction was observed in IFN-γ level [13]. In an observational study in 34 patients suffering from COVID-19, the ruxolitinib treatment led to clinical improvement (82.4 % cumulative incidence) and reduced levels of IL-6, monocyte chemoattractant protein-1 (MCPI), Interferon-γ inducible protein 10 (IP-10), TNF-α, and IL-1β. Some adverse events such as anemia and thrombocytopenia, were observed, too [98]. In a non-randomized multicenter study on patients with severe COVID-19 with ARDS, the efficacy of ruxolitinib was evaluated. Sixteen hospitalized COVID-19 patients with ARDS requiring mechanical ventilation received ruxolitinib and dexamethazon (for the standard care of severe cases based on oxford trials). The results showed that 13 patients stayed alive at day 28 (81 % overall survival), and the level of CRP and IL-6 was markedly reduced. Although, some adverse events have been reported, including increased hepatic enzymes, requiring dialysis, and anemia [62]. Moreover, in a controlled study, the combination therapy of ruxolitinib and eculizumab (approved monoclonal antibodies for paroxysmal nocturnal hemoglobinuria) was assessed to treat severe SARS-CoV-2-associated ARDS. The results demonstrated fewer radiographic pulmonary lesions and improved respiratory function following administration [24]. Generally, ruxolitinib is a promising option in treating COVID-19 infection, but the adverse effects like opportunistic infections must be considered [3].
4.3. Tofacitinib
Tofacitinib is an effective and potent JAK inhibitor that prevents JAK 1/3 signaling pathways. This drug indirectly modulates the production of pro-inflammatory cytokines through autocrine and paracrine feedback cascades. Tofacitinib can be used to treat ADRS by suppressing pro-inflammatory mediators. In randomly assigned 289 patients hospitalized with COVID-19 pneumonia, administration of tofacitinib at a dose of 10 mg twice daily (for up to 14 days or until hospital discharge) could reduce death or respiratory dysfunction [27].
In an observational study between March and September 2020 on 269 hospitalized patients with COVID-19, tofacitinib was administered alone or combined with dexamethasone. Tofacitinib and dexamethasone co-treatment resulted in lower mortality rates than dexamethasone alone. Based on their results, it can be inferred that adding anti-inflammatory therapy such as tofacitinib to standard treatments like corticosteroids has a beneficial impact on the survival rate in COVID-19 cases with inflammation [29]. In another retrospective study, the anti-inflammatory effects of tofacitinib were evaluated on 32 patients treated with tofacitinib versus 30 patients who did not receive any anti-cytokine agent. The mortality and ICU admission was lower in the tofacitinib-treated group than in the control group. Also, tofacitinib showed beneficial effects in diminishing CRS in COVID-19 subjects [56]. Despite its medical safety, a high dose of tofacitinib is sometimes associated with thrombotic events [90]. Aside from its effects on the JAK pathway, tofacitinib can suppress inflammation in the airways by targeting tissue memory CD8+ cells. With this consideration, tofacitinib could be an effective agent in ameliorating chronic lung disease caused by SARS-CoV-2 [97].
4.4. Itolizumab
Itolizumab is a humanized anti-CD6 monoclonal antibody first developed to treat various cancers and psoriasis. It was then re-purposed for COVID-19 treatment to mitigate CRS. This receptor is expressed on a variety of immune cells, including mature T cells, immature B cells, and the B1a subset of B lymphocytes. Stimulation of CD6 as a co-stimulatory molecule leads to Th1 activation and T cells differentiation, promoting pro-inflammatory responses. The binding of itolizumab to CD6 domain 1 can block the co-stimulation pathway and inhibits naïve T-cells proliferation. This leads to the downregulation of pro-inflammatory cytokines and adhesion molecules such as interleukin IL-17A, TNF-α, IL-6, IFN-γ, IL-6, and IL-2, along with a decreased level of T-cell infiltration at the site of inflammation [52], [81].
Itolizumab does not deplete T-cells but prevents T-cell proliferation caused by a higher level of IL-2 and ALCAM (activated leukocyte cell adhesion molecule). In fact, itolizumab downregulates the phosphorylation of some intracellular proteins involved in the CD6-mediated activation pathways. As a result, reducing pro-inflammatory cytokine can inhibit downstream inflammatory events [81].
Moreover, itolizumab inhibits the differentiation of T cells and reduces IL-17 levels, downregulating pSTAT3 [10]. A multi-centric RCT evaluated the efficacy and safety of itolizumab in patients with moderate to severe COVID-19-related ARDS. It was shown that itolizumab administration had a remarkable clinical improvement, especially SpO2 and PaO2 ratios. Although the CRP level decreased, it was not significant [47]. In a case report of 3 severe COVID-19 patients, itolizumab treatment could reduce inflammation and improved respiratory and radiological alteration in 2 patients [21]. Moreover, a small size, single-center study showed that a single dose of itolizumab could decrease plasma level of IL-6 as an important factor in CRS in affected patients [99].
5. Brutton's tyrosin kinase (BTK) inhibitor
Bruton's tyrosine kinase (BTK) is highly expressed in B-cells and all hematopoietic cell lineages except T cells. BTKs participate in innate immune responses and regulate the production of pro-inflammatory cytokines. BTK has a crucial role in multiple signaling pathways, including multiple Toll-like receptors (TLRs), macrophages, and dendritic cells, releasing pro-inflammatory cytokines [103]. BTK also regulates some transcription factors, such as NF-ƙB and IFN-regulatory factors, which are imperative for macrophage M1 polarization and subsequent production of several inflammatory cytokines, chemokines, and phagocytosis [15], [43], [73]. The BTK pathway is up-regulated in pro-inflammatory macrophages and activates downstream signaling pathways.
There was a higher level of BTK phosphorylation in macrophages from patients with a severe form of COVID-19. Indeed, an increased phosphorylated BTK is linked with lung injury and ARDS. BTKs play a critical regulatory role in lung macrophages and release cytokines and chemokines such as IL-6, IL-10, TNFa, and MCP-1. Evidence suggests that inhibition of BTK-mediated activities could reduce cytokine and chemokine levels. On the other hand, blocking the B-cell receptor signaling may impede B-cell activation and BTK-mediated downstream signaling. Noteworthy, BTK inhibitors are commonly used to treat B-cell malignancy and chronic graft-versus-host disease (GvHD). With this notion, BTK inhibitors can be therapeutic interventions in patients with severe COVID-19 to ameliorate lung damage [71].
5.1. Acalabrutinib
Acalabrutinib is a highly selective and potent inhibitor of Bruton tyrosine kinase used as a therapeutic approach for chronic lymphocytic leukemia [101]. It is hypothesized that acalabrutinib may benefit individuals with COVID-19. A prospective off-label study was conducted on 19 hospitalized patients with severe COVID-19 and ARDS. They treated with acalabrutinib for 10 to 14 days. Evidence of inflammation (CRP > 10 mg/dL, ferritin >500 ng/mL) and/or lymphopenia was present in COVID-19 affected patients. Within 1 to 3 days of acalabrutinib administration, most patients experienced an improvement in oxygenation. With increasing oxygen saturation, inflammation-related markers such as CRP and IL-6 quickly returned to normal levels in most patients, as did lymphopenia. At the end of treatment, 72.7 % of patients did not need oxygen supplementation and were discharged on room air. These results highlight the potential benefit of BTK inhibitor in the severe form of COVID-19. Besides, no toxicities were observed via using BTK inhibitors [79]. Although it is unclear how lymphopenia affects the severity of COVID-19, numerous studies show that lymphopenia is strongly associated with CRS in COVID-19 [43].
Other BTK inhibitors are ibrutinib and zanubrutinib could be re-purposed for the treatment of COVID-19 patients. In an ex-vivo study, blood samples of CLL patients with COVID-19 were examined to determine the efficacy of iburtinib treatment on the cytokine release mediated by T-cells and monocytes. The results showed that ibrutinib remarkably normalized the expression of TNF-α and IFN-γ released by monocytes in SARS-CoV-2 infection. Moreover, ibrutinib could mitigate the expression level of IL-6 caused by SARS-CoV-2 infection [22] (Fig. 2 ).
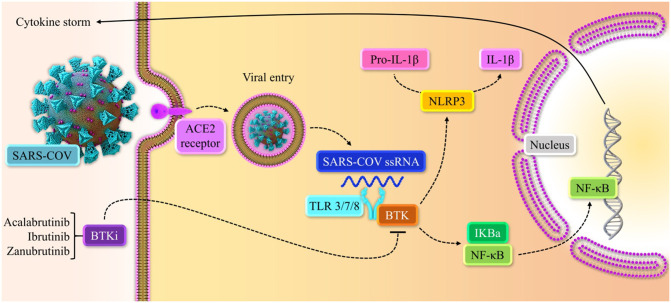
Fig. 2.
Model of BTK signaling pathway in COVID-19 hyper inflammation. SARS-CoV-2 entry is mediated by binding to ACE2 receptors in epithelial cells, stimulating the BTK pathway in COVID-19 inflammation. In macrophages, binding toll-like receptors (TLR3/7/8) to SARS-CoV-2 viral ssRNA stimulates the nuclear factor kappa B (NF-kβ) signaling pathway through BTK activation and leads to the production of multiple inflammatory cytokines [64], [79], [91]. BTK-depend NF-kβ stimulation could increase cytokine release and recruit monocytes, macrophages, and neutrophils in severe COVID-19 infection [79]. The binding of BTK to NLRP3 could phosphorylate and converts it into NLRP3 inflammasome, which turns pro-IL-1β to mature IL-1β [6], [50], [79].
6. Pharmacological aspects of p38 MAPK inhibitors
The p38 mitogen-activated protein kinases (p38 MAPK) signaling pathway is activated by various pathological factors. p38 MAPK plays a crucial role in cell apoptosis, autophagy, and cell differentiation [16], [89]. Several viruses such as SARS-CoV, DENV, MERS-COV, IBA, and SARS-CoV-2 could activate p38 MAPK pathway and release some pro-inflammatory cytokines, including IL-6, TNF-α, and IL-1β, which enhance the regulation of NF-kβ pathway [5], [28]. Previous studies in animal models have shown the role of the p38 MAPK pathway in the hyperinflammatory response in acute lung injury and inflammatory cardiomyopathy [19], [54]. Non-canonically upregulation of the p38 MAPK pathway in COVID-19 patients could be explained by losing ACE2. SARS-CoV-2 spike (S) protein attaches ACE2 receptor and leads to loss of ACE2 upon viral entry. ACE2 is expressed in multi organs, but its abundant is in lungs and heart tissue. ACE2 converts Angiotensin II (Ang II) into Angiotensin (Ang 1–7). The binding of Ang II and its receptor angiotensin II type 1 receptor (AT1R) could activate the ERK1/JNK/p38 MAPK pathway, which disproportionately releases pro-inflammatory cytokines such as IL-1 and IL-10, IL-12, and TNF-α [26], [55]. Also, Ang II stimulates the NF-kβ pathway to up-regulate pro-inflammatory mediators by phosphorylation of the P65 subunit of NF-kβ [55], [86]. Ang II-induced p38 MAPK phosphorylation promotes pro-inflammatory, pro-vasoconstrictive, and pro-thrombotic cascades. These events could be suppressed by Ang 1–7 activity [26], [112]. Ang 1–7 regulates Mas receptor to attenuate p38 MAPK pathway and counterbalance the detrimental effects of Ang II. The Mas receptor involves in angiotensin Ang1–7 vasodilatory activity. Downregulation of Ang 1–7 and ACE2 and upregulation of Ang II can induce positive feedback through p38 MAPK pathway, which is mediated by ADAM17 (an enzyme that cleaves the ectodomain of ACE2), reducing ACE2 expression [84]. In addition to downregulating ACE2 by attaching to that receptor, COVID-19 might also increase p38 MAPK signaling by a particular virus protein similar to SARS-CoV [26], [44], [53]. Considering the pro-inflammatory, vasoconstriction, and thrombotic effects of Ang II and p38 MAPK pathway, it can be explained the reasons behind the additional symptoms in patients hospitalized with SARS-CoV-2 [53], [85], [110]. Several variants of SARS-CoV-2 have been classified by the World Health Organization (WHO) as a variant of concern (VOC). The classified variants included B.1.1.7 (Alpha variant, first found in the UK, September 2020), B.1.351 (Beta variant, first found in South Africa, August 2020), B.1.617.2 (Delta variant, first found in India, October 2020), and B.1.1.529 (omicron variant, first found in Botswana and South Africa, November 2021). An ex-vivo study that compared the replication of these mutant and wild types. They showed that the replication of Delta and Omicron variants is significantly more than the wild type of SARS-CoV-2 in the human bronchus. However, no significant difference was found between Delta and Omicron variants in terms of replication [32]. Another in vitro study revealed high viral replication of omicron variant in the nasal epithelium of humans compared to Delta and wild type of SARS-CoV-2 [66]. It should be noted that Omicron infection still depends on spike protein to bind ACE2 receptors similar to other variants; however, higher mutations were found in the Omicron spike protein compared to wild-type of SARS-CoV-2 [11], [32].
Interestingly, Omicron replication is faster than all other variants of SARS-CoV-2 on human bronchus but less remarkably on the lung parenchyma [14]. Regarding the impact of the p38 MAPK pathway in COVID-19, in this section, we peruse the efficacy of p38 MAPK inhibitors in SARS-Cov-2 trials.
6.1. SB203580
SB203580 is a selective inhibitor of MAPK14 (p38 β) and MAPK11 (p38 α), which reduces the phosphorylation of the downstream signaling molecules of p-38 MAPK, including MAPK AP kinase-2 and heat shock protein 27 (HSP-27) [88]. Previous studies confirmed the beneficial effects of SB203580 on the inflammatory response, specifically in the pulmonary tissue. In an in vitro assay on chronic obstructive pulmonary disease (COPD), SB203580 could suppress CCL5 and TNF-α and enhance IL-10 expression [58]. Moreover, another experiment in lung edema and inflammatory response in ALI (acute lung injury) demonstrated that SB203580 inhibits the expression of TNF-α and IL-1β as pro-inflammatory cytokines [48]. Interestingly, SB203580 can moderate vital entry and inflammatory response during viral infection caused by p38 MAPK activation; hence, pharmacological blocking of this pathway is an effective strategy for combating viral infection. In an animal model of non-typeable haemophilus influenza (NTHI) infection, SB203580 treatment could reduce the airway epithelial hyper-responsiveness and mucus-hyper-secretion. However, SB203580 failed to suppress neutrophilic inflammation in steroid-resistant allergic airway disease (SRAAD). It was shown that co-treatment of dexamethasone with SB203580 significantly suppressed SRAAD [100]. More recently, in vitro study investigated p38 MAPK blockage by SB203580 in cells infected with the SARS-CoV-2 virus. Phospho-proteomic analysis showed that SB203580 suppressed viral replication and inflammatory cytokines expression in Vero E6 and ACE2-A549 human lung carcinoma cells infected with the SARS-CoV-2 virus. The investigation also revealed that the transcription of IL-6, TNF-α was suppressed in a dose-depend manner. Furthermore, SB203580 significantly reduced the protein levels of IL-6, CXCL8, CCL8, CCL20, and CCL2 which were elevated after viral entry. Regarding another data from this study, other MAPK pharmacological inhibitors including gilteritinib, ralimetinib, MAPK-13_IN-1, ARRY-797, and bencentinib displayed potent antiviral activities in A549-ACE2 cells [7].
6.2. Gilteritinib
Gilteritinib is a potent, selective inhibitor of AXL receptor tyrosine kinase and its downstream targets, including p-38 MAPK pathway. It is sold under Xospata brand name. Gilteritinib inhibits FLT3 tyrosine kinase receptor and its downstream pathway STAT5 and ERK. Both AXL and FLT3 are implicated in cancer cells growth [17]. Gilteritinib got approval in the USA, the EV, Japan, and Canada to treat relapsed or refractory acute myeloid leukemia (AML) in adults. The integrated safety analysis of gilteritinib found some treatment-related adverse events (AES) in treated patients, including anemia, febrile neutropenia, and thrombocytopenia. Because of the risk of embryo-fetal toxicity, patients with reproductive potential must use contraceptives while receiving gilteritinib [41]. In a case report study, a 27 years old patient with AML and SARS-CoV-2, giltritinib administration ameliorated COVID-19 symptoms. It can be inferred that giltiritinib is safe drug and may induce remission in AML patients, but further studies are needed to elucidate its efficacy [105].
6.3. MAPK-13-IN-1
MAPK-13-IN-1 is a MAPK (p38 δ) inhibitor designed to reduce IL-13- activated mucous overproduction caused by MAPK-13 activation in airway epithelial cells. It was approved for application in some respiratory diseases, including asthma and COPD [2], [115]. It was observed that MAPK-13-IN-1 could significantly decrease the replication of SARS-CoV-2 in A549-ACE2 cells [7].
6.4. ARRY371797
Arry 371,797 is an oral Arry Pharmed developed drug used for lamin A/C gene (LMNA) related to dilated cardiomyopathy treatment in the clinical stage [25]. Previous studies elucidated that the kinase ½ (ERK1/2) and JNK downstream branches of MAPK pathway non-conically hyperactivation could contribute to cardiac impairment [61]. It should be noted that ARRY371797 could reduce SARS-CoV-2 replication significantly [7].
6.5. Bencentinib
Bencentinib, also known as GBG324, is a selective AXL receptor inhibitor developed for non-small cell lung cancer treatment. The correlation between AXL receptor and its downstream pathways, including MAPK plays an essential role in cancer progression [96]. In an in silico experiment on FDA-approved drugs, including bencentinib, two proteases that control duplication and life-cycle of COVID-19 (PLPRO and CLPRO) inhibition was assayed. Bencentinib attached to active sites of both proteases and inhibited their activity. CLPRO up-regulates the proteolysis of two important replication polyprotein of SARS-CoV-2 virus (ppla and pplab). Therefore, it seems that PLPRO and CLPRO play a crucial role in viral replication, implicating in polyproteins translating from the viral RNA [33].
According to the effects of p38 MAPK pathway in SARS-CoV-2 entry and replication and the remarkable role of p38 MAPK pathway and its downstream targets in inflammation, the authors suggest more clinical studies about the inhibitors of this pathway. Although a clinical trial study was performed recently about the effects of bemcentinib as a AXL inhibitor (upstream kinase of p38 MAPK), the results are not published yet (clinical identifier: NCT04890509) (Fig. 3 ).
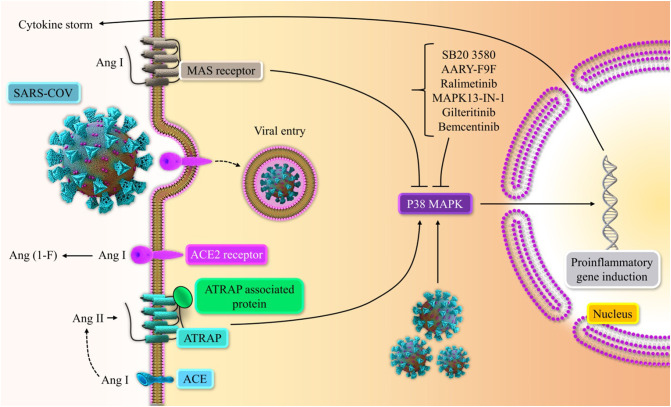
Fig. 3.
The role of p38 MAPK pathway inhibitors in alleviating COVID-19 inflammatory response. Ang II and AT1R binding activate p38 MAPK pathway, which could remarkably increase pro-inflammatory cytokines release. On the other hand, Ang (1–7) and Mas receptor binding could diminish p38 MAPK pathway and counterbalance the effect of Ang II. ACE2 receptor transfers Ang II to Ang (1–7), and its binding to S protein of SARS-CoV-2 leads to the loss of ACE2 upon viral entry [26], [55], [84].
7. The signaling pathway of glycogen synthase kinase 3 (GSK-3)
Glycogen synthase kinase 3 (GSK-3) is a type of serine/threonine protein kinase that exists in GSK-3α and GSK-3β isoforms in mammals. These isoforms are remarkably similar but encoded by two specific separated genes [30], [65]. GSK-3 is involved in numerous biological activities, including cell signaling, inflammatory response, and modulating of innate immunity. This pathway implicates many clinical disorders such as cancer, hyper-inflammation, type2 diabetes, Alzheimer's disease, and other neurological diseases [18], [30], [65]. According to the previous studies, GSK-3 pathway is lesser-known in comparison to other biological pathways like MAPK, STAT, and protein kinase C (PKC) in virology studies. Based on new research, GSK-3 plays an essential role in viral replication, especially in COVID-19 [68]. From four structural proteins of SARS-CoV-1, N protein is necessary for viral replication and holds RNA genome, while the other structural proteins in this virus create the envelope. N protein consists 3 domains, including the N terminal domain (NTD, domain 1), G terminal domain (CTD, domain3), and linker region (LKR, domain 2). Both domain 1 and domain 3 of N protein connect through the linker region, which contains the serine-arginine motif as a target of GSK-3 pathway [74], [80]. Inhibiting GSK-3 with small molecule inhibitors (SMIs) has been involved in reducing SARS-CoV-1 N protein phosphorylation and induced viral replication limitation [49], [106], [107]. Investigations showed a remarkable similarity between the key phosphorylation site of N protein in SARS-CoV-1 and SARS-CoV-2 viruses. Given the identical N protein sequences, GSK-3 phosphorylation will occur in related together [37], [80]. Likewise, GSK-3 inhibitors prevented coronavirus protease (Mpro) activation. Mpro cleaves the encoded polyproteins of SARS-CoV-2 (pp1 a and ppl ab) necessary for the virus replication and its transcription [80], [109].
The most noticeable symptom in SARS-CoV-2 patients is reduced T cells population, especially in severe hospitalized cases. Compared to the mild cases, the helper T cells (D4+), cytotoxic T cells (CD8+), and regulatory T cells levels were reduced significantly in severe cases [37]. The recent investigations suggested that the N protein in SARS-CoV-1 could interact with NF-kβ as an essential factor in pro-inflammatory responses. Accordingly, it enhances IL-6 and other pro-inflammatory cytokines involved in a cytokine storm [116]. According to clinical reports, the plasma IL-6 level is higher than in non-severe cases [37]. These evidences confirm that GSK-3 inhibitors prevent SARS-CoV-2 replication as same as SARS-CoV-1. Recent research elucidated that GSK-3 pathway interferes negatively with T-cells proliferation and function [92]. GSK-3 inhibiting can also enhance adaptive CD8+ cytolytic cells with upregulation of T-beb (Tbx21) transcription factor, which promotes the expression of cytolytic genes, including granzyme B, perforin, and interferon-gamma [93]. The contribution of GSK-3 pathway in CD8 CTLs function was obviously observed as well as GSK-3 negatively regulates CD4+ T cells function and proliferation as an effective cell in CRS, especially in severe hospitalized cases [80]. Briefly, accumulating evidence indicated that GSK-3 inhibition with small molecules could induce high expression of IL-10 as a limiting factor of the immune response, specifically in autoimmune diseases [82]. Thereby, according to the roles of GSK-3 in SARS-CoV-2 viral replication and CD8+ T cell responses, this article reviews the GSK-3 pathway inhibitors briefly. But it needs to be noted that further experiments and trials specially in combination with other effective and new drugs are necessary.
7.1. Lithium
Lithium is a widely used drug for treating bipolar disorders (BPT) and a selective GSK-3 inhibitor that does not remarkably implicate other protein kinase pathways. The antiviral effects of lithium have been interrogated for a long time. Briefly, investigations approved that lithium has antiviral effects against multi types of DNA and RNA viruses such as hepatitis C, adenoviruses, Dengue viruses, parvoviruses, and human papillomavirus (HPV) [87]. It has been reported that lithium chloride (Licl) can help to treat gastroenteritis coronavirus in pigs [75]. Also, it could help to overcome the infectious bronchitis coronavirus infection by inhibiting the genomic RNA and subgenomic mRNA synthesis in avians [74]. But the remarkable point is that recent research on the SARS-CoV-2 virus indicated that lithium could have antiviral activity against this new virus. An in vitro experiment showed Licl inhibited the SARS-CoV-2 N protein phosphorylation in human embryonic kidney 293 T cells with IC ~ 10 mM. Likewise, the collected data from a cohort study in 3 major health center in U.S.A in described study demonstrated that patients on lithium who were tasted for COVID-19 showed a significant reduced risk of infection of COVID-19 [odds-ratio = 0.51 [0.35–0.74], P = 0.05] [51].
8. Conclusion and perspective remarks
The novel SARS-CoV-2 is defined as a causative agent for COVID-19. To combat COVID-19, numerous therapeutic agents are being examined in clinical trials to be used as a single therapy or combination with other medicines. Based on the evidence provided in this review, the combination of kinase inhibitors with other immunomodulatory agents could achieve more efficacy than monotherapy against COVID-19- induced inflammation. Various kinas inhibitors are under clinical investigation to target critical proteins responsible for COVID-19 symptoms, including CRS, ARDS, and systemic inflammation. Multiple kinase inhibitors were approved, and some are being clinically tested in Phase I–III trials. However, some points should be considered for re-purposing them for the treatment of COVID-19, including adverse effects, pharmacokinetic properties, and long-term application. Balancing the potential benefits of kinase inhibitors and the risks of off-target effects of these agents must be kept in mind for COVID-19 therapy. Some drugs should be prescribed at long-term dosing to achieve the optimal concentrations and exert anti-inflammatory effects; thus, these can not be used for COVID-19 patients because of the immediacy of treatment. Overall, kinase inhibitors can be one of the beneficial therapeutic strategies in controlling the COVID-19 pandemic by targeting JAK/STAT, BTK, p38 MAPK, GSK-3 signaling pathways. These signaling pathways mediate a hyperimmune activity that leads to ARD, organ failure, and death in patients afflicted with COVID-19. Accordingly, re-purposing already FDA-approved targeted therapies for the abovementioned signaling pathways may benefit affected patients.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
Not applicable
CRediT authorship contribution statement
Z.M, S.M, and R.F contributed to manuscript writing and critical review of the manuscript; A.N and B·B cooperated in the editing; S.J,Y·B and F.R contributed to the first draft design, A.B collaborated in drawing the figures.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgements
Not applicable.
Data availability
No data was used for the research described in the article.
References
- 1.Ajayi S., Becker H., Reinhardt H., Engelhardt M., Zeiser R., von Bubnoff N. Small Molecules in Hematology. Springer; 2018. Ruxolitinib; pp. 119–132. [DOI] [PubMed] [Google Scholar]
- 2.Alevy Y.G., Patel A.C., Romero A.G., Patel D.A., Tucker J., Roswit W.T., et al. IL-13–induced airway mucus production is attenuated by MAPK13 inhibition. J. Clin. Invest. 2012;122:4555–4568. doi: 10.1172/JCI64896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagca B.G., Avci C.B. The potential of JAK/STAT pathway inhibition by ruxolitinib in the treatment of COVID-19. Cytokine Growth Factor Rev. 2020;54:51–61. doi: 10.1016/j.cytogfr.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes P.J. Kinases as novel therapeutic targets in asthma and chronic obstructive pulmonary disease. Pharmacol. Rev. 2016;68:788–815. doi: 10.1124/pr.116.012518. [DOI] [PubMed] [Google Scholar]
- 5.Battagello D.S., Dragunas G., Klein M.O., Ayub A.L., Velloso F.J., Correa R.G. Unpuzzling COVID-19: tissue-related signaling pathways associated with SARS-CoV-2 infection and transmission. Clin. Sci. 2020;134:2137–2160. doi: 10.1042/CS20200904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bittner Z.A., Liu X., Mateo Tortola M., Tapia-Abellán A., Shankar S., Andreeva L., et al. BTK operates a phospho-tyrosine switch to regulate NLRP3 inflammasome activity. J. Exp. Med. 2021;218 doi: 10.1084/jem.20201656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouhaddou M., Memon D., Meyer B., White K.M., Rezelj V.V., Marrero M.C., et al. The global phosphorylation landscape of SARS-CoV-2 infection. Cell. 2020;182(685–712) doi: 10.1016/j.cell.2020.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bronte V., Ugel S., Tinazzi E., Vella A., De Sanctis F., Canè S., et al. Baricitinib restrains the immune dysregulation in patients with severe COVID-19. J. Clin. Invest. 2020;130:6409–6416. doi: 10.1172/JCI141772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bronte V., Ugel S., Tinazzi E., Vella A., De Sanctis F., Canè S., et al. Baricitinib restrains the immune dysregulation in patients with severe COVID-19. J. Clin. Invest. 2020;130 doi: 10.1172/JCI141772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bughani U., Saha A., Kuriakose A., Nair R., Sadashivarao R.B., Venkataraman R., et al. T cell activation and differentiation is modulated by a CD6 domain 1 antibody itolizumab. PloS one. 2017;12 doi: 10.1371/journal.pone.0180088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cameroni E., Bowen J.E., Rosen L.E., Saliba C., Zepeda S.K., Culap K., et al. Broadly neutralizing antibodies overcome SARS-CoV-2 omicron antigenic shift. Nature. 2022;602:664–670. doi: 10.1038/s41586-021-04386-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cantini F., Niccoli L., Nannini C., Matarrese D., Di Natale M.E., Lotti P. Beneficial Impact of Baricitinib in COVID-19 Moderate Pneumonia; Multicentre Study. Vol. 81. 2020. pp. 647–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao Y., Wei J., Zou L., Jiang T., Wang G., Chen L., et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): a multicenter, single-blind, randomized controlled trial. J. Allergy Clin. Immunol. 2020;146(137–46) doi: 10.1016/j.jaci.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan M.C., Hui K.P., Ho J., Cheung M.-C., Ng K.-C., Ching R. 2021. SARS-CoV-2 Omicron Variant Replication in Human Respiratory Tract Ex Vivo. [Google Scholar]
- 15.Chong E.A., Roeker L.E., Shadman M., Davids M.S., Schuster S.J., Mato A.R. BTK inhibitors in cancer patients with COVID-19:“The winner will be the one who controls that chaos”(Napoleon Bonaparte) Clin. Cancer Res. 2020;26:3514–3516. doi: 10.1158/1078-0432.CCR-20-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuadrado A., Nebreda A.R. Mechanisms and functions of p38 MAPK signalling. Biochem. J. 2010;429:403–417. doi: 10.1042/BJ20100323. [DOI] [PubMed] [Google Scholar]
- 17.Dhillon S. Gilteritinib: first global approval. Drugs. 2019;79:331–339. doi: 10.1007/s40265-019-1062-3. [DOI] [PubMed] [Google Scholar]
- 18.Eldar-Finkelman H. Glycogen synthase kinase 3: an emerging therapeutic target. Trends Mol. Med. 2002;8:126–132. doi: 10.1016/s1471-4914(01)02266-3. [DOI] [PubMed] [Google Scholar]
- 19.Fang W., Cai S.X., Wang C.L., Sun X.X., Li K., Yan X.W., et al. Modulation of mitogen-activated protein kinase attenuates sepsis-induced acute lung injury in acute respiratory distress syndrome rats. Mol. Med. Rep. 2017;16:9652–9658. doi: 10.3892/mmr.2017.7811. [DOI] [PubMed] [Google Scholar]
- 20.Favalli E.G., Biggioggero M., Maioli G., Caporali R. Baricitinib for COVID-19: a suitable treatment? Lancet Infect. Dis. 2020;20:1012–1013. doi: 10.1016/S1473-3099(20)30262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Filgueira L.M., Cervantes J.B., Lovelle O.A., Herrera C., Figueredo C., Caballero J.A., et al. An anti-CD6 antibody for the treatment of COVID-19 patients with cytokine-release syndrome: report of three cases. Immunotherapy. 2021;13:289–295. doi: 10.2217/imt-2020-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiorcari S., Atene C.G., Maffei R., Debbia G., Potenza L., Luppi M., et al. Ibrutinib interferes with innate immunity in chronic lymphocytic leukemia patients during COVID-19 infection. Haematologica. 2021;106:2265. doi: 10.3324/haematol.2020.277392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gatti M., Turrini E., Raschi E., Sestili P., Fimognari C. Janus kinase inhibitors and coronavirus disease (COVID)-19: rationale, clinical evidence and safety issues. Pharmaceuticals. 2021;14:738. doi: 10.3390/ph14080738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giudice V., Pagliano P., Vatrella A., Masullo A., Poto S., Polverino B.M., et al. Combination of ruxolitinib and eculizumab for treatment of severe SARS-CoV-2-related acute respiratory distress syndrome: a controlled study. Front. Pharmacol. 2020;11:857. doi: 10.3389/fphar.2020.00857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldstein D.M., Kuglstatter A., Lou Y., Soth M.J. Selective p38α inhibitors clinically evaluated for the treatment of chronic inflammatory disorders. J. Med. Chem. 2010;53:2345–2353. doi: 10.1021/jm9012906. [DOI] [PubMed] [Google Scholar]
- 26.Grimes J.M., Grimes K.V. p38 MAPK inhibition: a promising therapeutic approach for COVID-19. J. Mol. Cell. Cardiol. 2020;144:63–65. doi: 10.1016/j.yjmcc.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guimarães P.O., Quirk D., Furtado R.H., Maia L.N., Saraiva J.F., Antunes M.O., et al. Tofacitinib in patients hospitalized with Covid-19 pneumonia. N. Engl. J. Med. 2021;385(5):406–415. doi: 10.1056/NEJMoa2101643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hariharan A., Hakeem A.R., Radhakrishnan S., Reddy M.S., Rela M. The role and therapeutic potential of NF-kappa-B pathway in severe COVID-19 patients. Inflammopharmacology. 2021;29:91–100. doi: 10.1007/s10787-020-00773-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayek M.E., Mansour M., Ndetan H., Burkes Q., Corkern R., Dulli A., et al. Anti-inflammatory treatment of COVID-19 pneumonia with tofacitinib alone or in combination with dexamethasone is safe and possibly superior to dexamethasone as a single agent in a predominantly african american cohort. Mayo Clin. Proc. 2021;5:605–613. doi: 10.1016/j.mayocpiqo.2021.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hermida M.A., Kumar J.D., Leslie N.R. GSK3 and its interactions with the PI3K/AKT/mTOR signalling network. Adv. Biol. Regul. 2017;65:5–15. doi: 10.1016/j.jbior.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Hoang T.N., Pino M., Boddapati A.K., Viox E.G., Starke C.E., Upadhyay A.A., et al. Baricitinib treatment resolves lower-airway macrophage inflammation and neutrophil recruitment in SARS-CoV-2-infected rhesus macaques. Cell. 2021;184(460–75) doi: 10.1016/j.cell.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hui K.P., Ho J.C., Cheung M.-c., Ng K.-c., Ching R.H., Lai K.-l., et al. SARS-CoV-2 omicron variant replication in human bronchus and lung ex vivo. Nature. 2022:1–5. doi: 10.1038/s41586-022-04479-6. [DOI] [PubMed] [Google Scholar]
- 33.Jade D., Ayyamperumal S., Tallapaneni V., Nanjan C.M.J., Barge S., Mohan S., et al. Virtual high throughput screening: potential inhibitors for SARS-CoV-2 PLPRO and 3CLPRO proteases. Eur. J. Pharmacol. 2021;901 doi: 10.1016/j.ejphar.2021.174082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jafari S., Heydarian S., Lai R., Aghdam E.M., Molavi O. Silibinin induces immunogenic cell death in cancer cells and enhances the induced immunogenicity by chemotherapy. BIOIMPACTS. 2022;12 doi: 10.34172/bi.2022.23698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jafari S., Lavasanifar A., Hejazi M.S., Maleki-Dizaji N., Mesgari M., Molavi O. STAT3 inhibitory stattic enhances immunogenic cell death induced by chemotherapy in cancer cells. DARU J. Pharm. Sci. 2020;28:159–169. doi: 10.1007/s40199-020-00326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang Y., Rubin L., Peng T., Liu L., Xing X., Lazarovici P., et al. Cytokine storm in COVID-19: from viral infection to immune responses, diagnosis and therapy. Int. J. Biol. Sci. 2022;18:459. doi: 10.7150/ijbs.59272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin Z., Du X., Xu Y., Deng Y., Liu M., Zhao Y., et al. Structure of M pro from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582:289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 38.Jorgensen S.C., Tse C.L., Burry L., Dresser L.D. Baricitinib: a review of pharmacology, safety, and emerging clinical experience in COVID-19. Pharmacotherapy. 2020;40:843–856. doi: 10.1002/phar.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaksonen M., Roux A. Mechanisms of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2018;19:313–326. doi: 10.1038/nrm.2017.132. [DOI] [PubMed] [Google Scholar]
- 40.Kalil A.C., Patterson T.F., Mehta A.K., Tomashek K.M., Wolfe C.R., Ghazaryan V., et al. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N. Engl. J. Med. 2021;384:795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang C., Blair H.A. Gilteritinib: a review in relapsed or refractory FLT3-mutated acute myeloid leukaemia. Target. Oncol. 2020;1–9 doi: 10.1007/s11523-020-00749-3. [DOI] [PubMed] [Google Scholar]
- 42.Khalil B.A., Elemam N.M., Maghazachi A.A. Chemokines and chemokine receptors during COVID-19 infection. Comput. Struct. Biotechnol. J. 2021;19:976–988. doi: 10.1016/j.csbj.2021.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kifle Z.D. Bruton tyrosine kinase inhibitors as potential therapeutic agents for COVID-19: a review. Metab. Open. 2021;11 doi: 10.1016/j.metop.2021.100116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kopecky-Bromberg S.A., Martinez-Sobrido L., Palese P. 7a protein of severe acute respiratory syndrome coronavirus inhibits cellular protein synthesis and activates p38 mitogen-activated protein kinase. J. Virol. 2006;80:785–793. doi: 10.1128/JVI.80.2.785-793.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kubo S., Nakayamada S., Tanaka Y. Baricitinib for the treatment of rheumatoid arthritis and systemic lupus erythematosus: a 2019 update. Expert. Rev. Clin. Immunol. 2019;15:693–700. doi: 10.1080/1744666X.2019.1608821. [DOI] [PubMed] [Google Scholar]
- 46.Kumar M., Al Khodor S. Pathophysiology and treatment strategies for COVID-19. J. Transl. Med. 2020;18:1–9. doi: 10.1186/s12967-020-02520-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar S., De Souza R., Nadkar M., Guleria R., Trikha A., Joshi S.R., et al. A two-arm, randomized, controlled, multi-centric, open-label phase-2 study to evaluate the efficacy and safety of itolizumab in moderate to severe ARDS patients due to COVID-19. Expert. Opin. Biol. Ther. 2021;21:675–686. doi: 10.1080/14712598.2021.1905794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li G., Dai Y., Tan J., Zou J., Nie X., Yang Z., et al. SB203580 protects against inflammatory response and lung injury in a mouse model of lipopolysaccharide-induced acute lung injury. Mol. Med. Rep. 2020;22:1656–1662. doi: 10.3892/mmr.2020.11214. [DOI] [PubMed] [Google Scholar]
- 49.Lin L., Shao J., Sun M., Liu J., Xu G., Zhang X., et al. Identification of phosphorylation sites in the nucleocapsid protein (N protein) of SARS-coronavirus. Int. J. Mass Spectrom. 2007;268:296–303. doi: 10.1016/j.ijms.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu X., Pichulik T., Wolz O.-O., Dang T.-M., Stutz A., Dillen C., et al. Human NACHT, LRR, and PYD domain–containing protein 3 (NLRP3) inflammasome activity is regulated by and potentially targetable through Bruton tyrosine kinase. J. Allergy Clin. Immunol. 2017;140(1054–67) doi: 10.1016/j.jaci.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 51.Liu X., Verma A., Ramage H., Garcia G., Myers R.L., Lucas A. medRxiv; 2021. Targeting the Coronavirus Nucleocapsid Protein Through GSK-3 Inhibition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Loganathan S., Athalye S.N., Joshi S.R. Itolizumab, an anti-CD6 monoclonal antibody, as a potential treatment for COVID-19 complications. Expert. Opin. Biol. Ther. 2020;20:1025–1031. doi: 10.1080/14712598.2020.1798399. [DOI] [PubMed] [Google Scholar]
- 53.Ma Q., Pan W., Li R., Liu B., Li C., Xie Y., et al. Liu Shen capsule shows antiviral and anti-inflammatory abilities against novel coronavirus SARS-CoV-2 via suppression of NF-κB signaling pathway. Pharmacol. Res. 2020;158 doi: 10.1016/j.phrs.2020.104850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma X.L., Kumar S., Gao F., Louden C.S., Lopez B.L., Christopher T.A., et al. Inhibition of p38 mitogen-activated protein kinase decreases cardiomyocyte apoptosis and improves cardiac function after myocardial ischemia and reperfusion. Circulation. 1999;99:1685–1691. doi: 10.1161/01.cir.99.13.1685. [DOI] [PubMed] [Google Scholar]
- 55.Mahmudpour M., Roozbeh J., Keshavarz M., Farrokhi S., Nabipour I. COVID-19 cytokine storm: the anger of inflammation. Cytokine. 2020;133 doi: 10.1016/j.cyto.2020.155151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maslennikov R., Ivashkin V., Vasilieva E., Chipurik M., Semikova P., Semenets V., et al. Tofacitinib reduces mortality in coronavirus disease 2019 tofacitinib in COVID-19. Pulm. Pharmacol. Ther. 2021;69 doi: 10.1016/j.pupt.2021.102039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Masoumzadeh H., Hoseinzad N., Jafari S., Shayanfar A., Molavi O., Vaez H. Development of polymeric micelles loaded with STAT3 inhibitory, stattic, for cancer treatment. Med. J. Tabriz Univ. Med. Sci. Health Serv. 2021;43 [Google Scholar]
- 58.Meng A., Zhang X., Wu S., Wu M., Li J., Yan X., et al. In vitro modeling of COPD inflammation and limitation of p38 inhibitor–SB203580. Int. J. Chron. Obstruct. Pulmon. Dis. 2016;11:909. doi: 10.2147/COPD.S99810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Montazersaheb S., Hosseiniyan Khatibi S.M., Hejazi M.S., Tarhriz V., Farjami A., Ghasemian Sorbeni F., et al. COVID-19 infection: an overview on cytokine storm and related interventions. Virol. J. 2022;19:1–15. doi: 10.1186/s12985-022-01814-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 61.Muchir A., Wu W., Choi J.C., Iwata S., Morrow J., Homma S., et al. Abnormal p38α mitogen-activated protein kinase signaling in dilated cardiomyopathy caused by Lamin A/C gene mutation. Hum. Mol. Genet. 2012;21:4325–4333. doi: 10.1093/hmg/dds265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Neubauer A., Johow J., Mack E., Burchert A., Meyn D., Kadlubiec A., et al. The janus-kinase inhibitor ruxolitinib in SARS-CoV-2 induced acute respiratory distress syndrome (ARDS) Leukemia. 2021;35:2917–2923. doi: 10.1038/s41375-021-01374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O'Hayre M., Salanga C.L., Handel T.M., Allen S.J. Chemokines and cancer: migration, intracellular signalling and intercellular communication in the microenvironment. Biochem. J. 2008;409:635–649. doi: 10.1042/BJ20071493. [DOI] [PubMed] [Google Scholar]
- 64.Page T.H., Urbaniak A.M., Santo A.I.E., Danks L., Smallie T., Williams L.M., et al. Bruton's tyrosine kinase regulates TLR7/8-induced TNF transcription via nuclear factor-κB recruitment. Biochem. Biophys. Res. Commun. 2018;499:260–266. doi: 10.1016/j.bbrc.2018.03.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pandey M.K., DeGrado T.R. Glycogen synthase kinase-3 (GSK-3)-targeted therapy and imaging. Theranostics. 2016;6:571. doi: 10.7150/thno.14334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peacock T.P., Brown J.C., Zhou J., Thakur N., Newman J., Kugathasan R. BioRxiv; 2022. The SARS-CoV-2 Variant, Omicron, Shows Rapid Replication in Human Primary Nasal Epithelial Cultures and Efficiently Uses the Endosomal Route of Entry. 2021.12. 31.474653. [Google Scholar]
- 67.Petrone L., Petruccioli E., Alonzi T., Vanini V., Cuzzi G., Fard S.N., et al. In-vitro evaluation of the immunomodulatory effects of baricitinib: implication for COVID-19 therapy. J. Infect. 2021;82:58–66. doi: 10.1016/j.jinf.2021.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pillaiyar T., Laufer S. Kinases as potential therapeutic targets for anti-coronaviral therapy. J. Med. Chem. 2021;65(2):955–982. doi: 10.1021/acs.jmedchem.1c00335. [DOI] [PubMed] [Google Scholar]
- 69.Praveen D., Chowdary P., Aanandhi V. Int J Antimicrob Agents; 2020. Baricitinib-a Januase Kinase Inhibitor-not an Ideal Option for Management of COVID 19. 105967- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Que Y., Hu C., Wan K., Hu P., Wang R., Luo J., et al. Cytokine release syndrome in COVID-19: a major mechanism of morbidity and mortality. Int. Rev. Immunol. 2022;41:217–230. doi: 10.1080/08830185.2021.1884248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rada M., Qusairy Z., Massip-Salcedo M., Macip S. Relevance of the Bruton tyrosine kinase as a target for COVID-19 therapy. Mol. Cancer Res. 2021;19:549–554. doi: 10.1158/1541-7786.MCR-20-0814. [DOI] [PubMed] [Google Scholar]
- 72.Raghuvanshi R., Bharate SBJJoMC. 2021. Recent Developments in the Use of Kinase Inhibitors for Management of Viral Infections. [DOI] [PubMed] [Google Scholar]
- 73.Rahimi M., Bagheri A., Bagheri Y., Fathi E., Bagheri S., Nia A., et al. Renoprotective effects of prazosin on ischemia-reperfusion injury in rats. Hum. Exp. Toxicol. 2021;40:1263–1273. doi: 10.1177/0960327121993224. [DOI] [PubMed] [Google Scholar]
- 74.Rana A.K., Rahmatkar S.N., Kumar A., Singh D. Glycogen synthase kinase-3: a putative target to combat severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic. Cytokine Growth Factor Rev. 2021;58:92–101. doi: 10.1016/j.cytogfr.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ren X., Meng F., Yin J., Li G., Li X., Wang C., et al. Action mechanisms of lithium chloride on cell infection by transmissible gastroenteritis coronavirus. PloS one. 2011;6 doi: 10.1371/journal.pone.0018669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Richardson P., Griffin I., Tucker C., Smith D., Oechsle O., Phelan A. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease (vol 395, pg e30, 2020) Lancet. 2020;395:1906. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rodriguez-Garcia J.L., Sanchez-Nievas G., Arevalo-Serrano J., Garcia-Gomez C., Jimenez-Vizuete J.M., Martinez-Alfaro E. Baricitinib improves respiratory function in patients treated with corticosteroids for SARS-CoV-2 pneumonia: an observational cohort study. Rheumatology. 2021;60:399–407. doi: 10.1093/rheumatology/keaa587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rosas J., Liaño F.P., Cantó M.L., Barea J.M.C., Beser A.R., Rabasa J.T.A., et al. Experience with the use of baricitinib and tocilizumab monotherapy or combined, in patients with interstitial pneumonia secondary to coronavirus COVID19: a real-world study. Reumatol. Clin. 2020;18(3):150–156. doi: 10.1016/j.reuma.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roschewski M., Lionakis M.S., Sharman J.P., Roswarski J., Goy A., Monticelli M.A., et al. Inhibition of Bruton tyrosine kinase in patients with severe COVID-19. Sci. Immunol. 2020;5 doi: 10.1126/sciimmunol.abd0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rudd C.E. GSK-3 inhibition as a therapeutic approach against SARs CoV2: dual benefit of inhibiting viral replication while potentiating the immune response. Front. Immunol. 2020;11:1638. doi: 10.3389/fimmu.2020.01638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saavedra D., Añé-Kourí A.L., Sánchez N., Filgueira L.M., Betancourt J., Herrera C., et al. An anti-CD6 monoclonal antibody (itolizumab) reduces circulating IL-6 in severe COVID-19 elderly patients. Immun. Ageing. 2020;17:1–8. doi: 10.1186/s12979-020-00207-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saraiva M., O'garra A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 83.Satarker S., Tom A.A., Shaji R.A., Alosious A., Luvis M., Nampoothiri M. JAK-STAT pathway inhibition and their implications in COVID-19 therapy. Postgrad. Med. 2021;133:489–507. doi: 10.1080/00325481.2020.1855921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Scott A.J., O'Dea K.P., O'Callaghan D., Williams L., Dokpesi J.O., Tatton L., et al. Reactive oxygen species and p38 mitogen-activated protein kinase mediate tumor necrosis factor α-converting enzyme (TACE/ADAM-17) activation in primary human monocytes. J. Biol. Chem. 2011;286:35466–35476. doi: 10.1074/jbc.M111.277434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Simões e Silva A., Silveira K., Ferreira A., Teixeira M. ACE2, angiotensin-(1-7) and M as receptor axis in inflammation and fibrosis. Br. J. Pharmacol. 2013;169:477–492. doi: 10.1111/bph.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Skurk T., van Harmelen V., Hauner H. Angiotensin II stimulates the release of interleukin-6 and interleukin-8 from cultured human adipocytes by activation of NF-κB. Arterioscler. Thromb. Vasc. Biol. 2004;24:1199–1203. doi: 10.1161/01.ATV.0000131266.38312.2e. [DOI] [PubMed] [Google Scholar]
- 87.Snitow M.E., Bhansali R.S., Klein P.S. Lithium and therapeutic targeting of GSK-3. Cells. 2021;10:255. doi: 10.3390/cells10020255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sreekanth G.P., Chuncharunee A., Sirimontaporn A., Panaampon J., Noisakran S., Yenchitsomanus P.-t., et al. SB203580 modulates p38 MAPK signaling and dengue virus-induced liver injury by reducing MAPKAPK2, HSP27, and ATF2 phosphorylation. PloS one. 2016;11 doi: 10.1371/journal.pone.0149486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stramucci L., Pranteda A., Bossi G. Insights of crosstalk between p53 protein and the MKK3/MKK6/p38 MAPK signaling pathway in cancer. Cancers. 2018;10:131. doi: 10.3390/cancers10050131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Suria C., Bosca-Watts M.M., Navarro P., Tosca J., Anton R., Sanahuja A., et al. Management of patients with intestinal bowel disease and COVID-19: a review of current evidence and future perspectives. Gastroenterol. Hepatol. 2021;45(5):383–389. doi: 10.1016/j.gastrohep.2021.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tatematsu M., Nishikawa F., Seya T., Matsumoto M. Toll-like receptor 3 recognizes incomplete stem structures in single-stranded viral RNA. Nat. Commun. 2013;4:1–13. doi: 10.1038/ncomms2857. [DOI] [PubMed] [Google Scholar]
- 92.Taylor A., Harker J.A., Chanthong K., Stevenson P.G., Zuniga E.I., Rudd C.E. Glycogen synthase kinase 3 inactivation drives T-bet-mediated downregulation of co-receptor PD-1 to enhance CD8+ cytolytic T cell responses. Immunity. 2016;44:274–286. doi: 10.1016/j.immuni.2016.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Taylor A., Rudd C.E. Glycogen synthase kinase 3 inactivation compensates for the lack of CD28 in the priming of CD8+ cytotoxic T-cells: implications for anti-PD-1 immunotherapy. Front. Immunol. 2017;8:1653. doi: 10.3389/fimmu.2017.01653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Titanji B.K., Farley M.M., Mehta A., Connor-Schuler R., Moanna A., Cribbs S.K., et al. Use of baricitinib in patients with moderate to severe coronavirus disease 2019. Clin. Infect. Dis. 2021;72:1247–1250. doi: 10.1093/cid/ciaa879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tripathi D.K., Poluri K.M. Molecular insights into kinase mediated signaling pathways of chemokines and their cognate G protein coupled receptors. Front. Biosci. 2020;25:1361–1385. doi: 10.2741/4860. [DOI] [PubMed] [Google Scholar]
- 96.Tsukita Y., Fujino N., Miyauchi E., Saito R., Fujishima F., Itakura K., et al. Axl kinase drives immune checkpoint and chemokine signalling pathways in lung adenocarcinomas. Mol. Cancer. 2019;18:1–6. doi: 10.1186/s12943-019-0953-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vahed S.Z., Khatibi S.M.H., Ahmadian E., Ardalan M. Targeting chronic COVID-19 lung injury; tofacitinib can be used against tissue-resident memory T cells. Biomed. Pharmacother. 2022;147 doi: 10.1016/j.biopha.2022.112614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vannucchi A.M., Sordi B., Morettini A., Nozzoli C., Poggesi L., Pieralli F., et al. Compassionate use of JAK1/2 inhibitor ruxolitinib for severe COVID-19: a prospective observational study. Leukemia. 2021;35:1121–1133. doi: 10.1038/s41375-020-01018-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vityala Y., Tagaev T., Mamatov S., Aidarov Z., Harinath P. Potential effects of itolizumab treatment on plasma interleukin-6 levels in patients with severe COVID-19. Indian J. Pharmacol. 2021;53:246. doi: 10.4103/ijp.IJP_33_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang G., Pang Z., Hsu A.C.-Y., Guan X., Ran N., Yuan Y., et al. Combined treatment with SB203580 and dexamethasone suppresses non-typeable Haemophilus influenzae-induced Th17 inflammation response in murine allergic asthma. Eur. J. Pharmacol. 2019;862 doi: 10.1016/j.ejphar.2019.172623. [DOI] [PubMed] [Google Scholar]
- 101.Wang M., Rule S., Zinzani P.L., Goy A., Casasnovas O., Smith S.D., et al. Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): a single-arm, multicentre, phase 2 trial. Lancet. 2018;391:659–667. doi: 10.1016/S0140-6736(17)33108-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang Y., Perlman S. COVID-19: inflammatory profile. Annu. Rev. Med. 2022;73:65–80. doi: 10.1146/annurev-med-042220-012417. [DOI] [PubMed] [Google Scholar]
- 103.Weber A.N., Bittner Z., Liu X., Dang T.-M., Radsak M.P., Brunner C. Bruton’s tyrosine kinase: an emerging key player in innate immunity. Front. Immunol. 2017;8:1454. doi: 10.3389/fimmu.2017.01454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Weisberg E., Parent A., Yang P.L., Sattler M., Liu Q., Liu Q. Re-purposing of Kinase Inhibitors for Treatment of COVID-19. Vol. 37. 2020. pp. 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wilson A.J., Troy-Barnes E., Subhan M., Clark F., Gupta R., Fielding A.K., et al. Successful remission induction therapy with gilteritinib in a patient with de novo FLT3-mutated acute myeloid leukaemia and severe COVID-19. Br. J. Haematol. 2020;190:e189–e191. doi: 10.1111/bjh.16962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu C.-H., Chen P.-J., Yeh S.-H. Nucleocapsid phosphorylation and RNA helicase DDX1 recruitment enables coronavirus transition from discontinuous to continuous transcription. Cell Host Microbe. 2014;16:462–472. doi: 10.1016/j.chom.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wu C.-H., Yeh S.-H., Tsay Y.-G., Shieh Y.-H., Kao C.-L., Chen Y.-S., et al. Glycogen synthase kinase-3 regulates the phosphorylation of severe acute respiratory syndrome coronavirus nucleocapsid protein and viral replication. J. Biol. Chem. 2009;284:5229–5239. doi: 10.1074/jbc.M805747200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wu P., Nielsen T.E., Clausen M.H. FDA-approved small-molecule kinase inhibitors. Trends Pharmacol. Sci. 2015;36:422–439. doi: 10.1016/j.tips.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 109.Yang H., Yang M., Ding Y., Liu Y., Lou Z., Zhou Z., et al. The crystal structures of severe acute respiratory syndrome virus main protease and its complex with an inhibitor. Proc. Natl. Acad. Sci. 2003;100:13190–13195. doi: 10.1073/pnas.1835675100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang P., Gu H., Zhao Z., Wang W., Cao B., Lai C., et al. Angiotensin-converting enzyme 2 (ACE2) mediates influenza H7N9 virus-induced acute lung injury. Sci. Rep. 2014;4:1–6. doi: 10.1038/srep07027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yeleswaram S., Smith P., Burn T., Covington M., Juvekar A., Li Y., et al. Inhibition of cytokine signaling by ruxolitinib and implications for COVID-19 treatment. Clin. Immunol. 2020;218 doi: 10.1016/j.clim.2020.108517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yu X., Cui L., Hou F., Liu X., Wang Y., Wen Y., et al. Angiotensin-converting enzyme 2-angiotensin (1–7)-mas axis prevents pancreatic acinar cell inflammatory response via inhibition of the p38 mitogen-activated protein kinase/nuclear factor-κB pathway. Int. J. Mol. Med. 2018;41:409–420. doi: 10.3892/ijmm.2017.3252. [DOI] [PubMed] [Google Scholar]
- 113.Yuan X., Wu H., Bu H., Zhou J., Zhang H. Targeting the immunity protein kinases for immuno-oncology. Eur. J. Med. Chem. 2019;163:413–427. doi: 10.1016/j.ejmech.2018.11.072. [DOI] [PubMed] [Google Scholar]
- 114.Yuki K., Fujiogi M., Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin. Immunol. 2020;215 doi: 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yurtsever Z., Patel D.A., Kober D.L., Su A., Miller C.A., Romero A.G. First comprehensive structural and biophysical analysis of MAPK13 inhibitors targeting DFG-in and DFG-out binding modes. Biochim. Biophys. Acta Gen. Subj. 2016;1860:2335–2344. doi: 10.1016/j.bbagen.2016.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang X., Wu K., Wang D., Yue X., Song D., Zhu Y., et al. Nucleocapsid protein of SARS-CoV activates interleukin-6 expression through cellular transcription factor NF-κB. Virology. 2007;365:324–335. doi: 10.1016/j.virol.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
No data was used for the research described in the article.