Abstract
Purpose
To report a case of iatrogenic vitrectomy trochar-induced cyclodialysis cleft successfully treated with intraoperative argon endolaser.
Observations
A 68-year-old Caucasian male with a history of high myopia underwent pars plana vitrectomy to clear symptomatic vitreous opacities but developed early postoperative hypotony that was recalcitrant to medical management for the first 6 postoperative months. Intraoperative gonioscopy demonstrated a cyclodialysis cleft and argon endolaser was applied to close the cleft.
Conclusions and Importance
Endolaser is an effective treatment for cyclodialysis clefts and intraoperative gonioscopy allows direct visualization of the cleft in a controlled operating room setting. Placement of vitrectomy ports should be done with care in high myopes to avoid accidental piercing of the ciliary body and inducing a cyclodialysis cleft.
Keywords: Cyclodialysis cleft, Endolaser, Cleft repair, Iatrogenic complication
1. Introduction
Cyclodialysis clefts develop due to disruption of the longitudinal ciliary muscle fibers from their insertion on the scleral spur. This subsequently forms an alternative low-resistance pathway for aqueous humor to flow into the supraciliary space, thus often resulting in hypotony. Historically, the most common cause of cyclodialysis clefts is blunt trauma, but increasingly, clefts have been reported as a complication of intraocular surgery.1, 2, 3, 4
Cases of cyclodialysis cleft that are refractory to medical therapy of cycloplegia require intervention. Laser is commonly used to close clefts, with the most common methods including argon laser applied via a gonioscopy lens, or transscleral cyclophotocoagulation.5,6 Persistent clefts can also be treated with repeat laser, transscleral exocyclopexy, sutured endocyclopexy, cryopexy, exotamponade with a scleral buckle, endotamponade via sutured segments or capsular tension rings, or vitrectomy with gas or oil tamponade.2,7, 8, 9, 10, 11, 12, 13
In this report, we present the first case in the literature of an iatrogenically-induced cyclodialysis cleft caused by the placement of a vitrectomy trochar in a high myope during a vitrectomy for removal of vitreous opacities. We also describe a unique technique of applying intraoperative endolaser to close the cleft, which has only been presented once previously in the literature.
2. Case report
A 68-year-old Caucasian male presented with visually significant vitreous opacities in the left eye (OS) due to a posterior vitreous detachment. Visual acuity (VA) was 20/20 on presentation, and intraocular pressure (IOP) was 10 mm Hg. His past ocular history was significant for high myopia and cataract surgery in the distant past with an axial length of 25.57 mm. He underwent pars plana vitrectomy to clear the vitreous opacities OS. The trochar ports were placed 3.5 mm posterior to the limbus with the trochar inserter blade at approximately a 45-degree angle relative to the sclera to create a short tunnel before being redirected towards the optic nerve to complete biplanar sclerotomies. In the immediate postoperative period, the patient developed vitreous hemorrhage and choroidal detachment associated with hypotony (IOP 5 mmHg).
The vitreous hemorrhage spontaneously cleared, and the choroidal detachment resolved with topical prednisolone acetate 1% four times daily for one month. However, the hypotony persisted, and the macula developed persistent macular folds that did not respond to topical steroid use (Fig. 1A). At post-operative week 10, the intraocular pressure remained 5 mmHg, and sub-Tenon's triamcinolone was administered. One month following the periocular steroid injection, the IOP improved to 10 mmHg, but the macular folds persisted. Five months after surgery, gonioscopy demonstrated a small cyclodialysis cleft nasally. It was thought that the cleft was formed by an anteriorly-placed trochar piercing the ciliary body in a myopic eye with a relatively posterior location of the ciliary body. Despite a month trial of atropine, the choroidal folds persisted, and the IOP remained at 8 mmHg with a VA of 20/30.
Fig. 1.
A. Preoperative macular optical coherence tomography demonstrating hypotonus choroidal folds distorting the fovea despite sub-Tenon's triamcinolone. B. Resolution of choroidal folds three months following endolaser repair of cyclodialysis cleft.
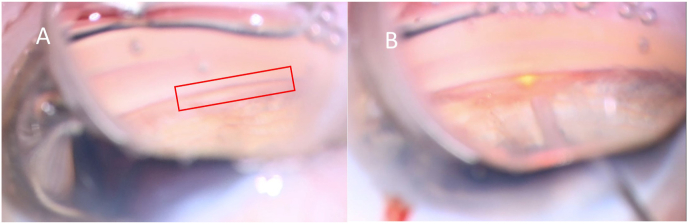
At postoperative month six, the patient was taken back to the operating room. Intraoperative gonioscopy demonstrated a cyclodialysis cleft from 9 to 11 o'clock (Fig. 2A). A paracentesis incision was made inferiorly and temporally into the anterior chamber, and the anterior chamber was filled with viscoelastic. 82 shots of 300 mW argon endolaser were applied to the site of the cleft, with the laser held approximately 1 mm from the cleft (Fig. 2B). Intraoperatively, there was peripheral shrinkage of the iris in response to the laser and the cleft was noted to be closed. Subconjunctival dexamethasone and cefazolin were administered. On postoperative day 1, the cleft was closed on gonioscopy, the IOP had elevated to 15 mmHg and the VA was 20/30. By post-operative month 3, his VA had improved to 20/20, his IOP was 9 mmHg on no topical steroids, and his choroidal folds had resolved (Fig. 1B).
Fig. 2.
A. Intraoperative gonioscopy demonstrating cyclodialysis cleft. B. Argon endolaser applied to cleft using intraoperative gonioscopy.
3. Discussion
Cyclodialysis clefts refractory to medical management with steroids and cycloplegia require intervention to prevent or treat sequelae of intraocular hypotony.1 Laser has often been used to treat cyclodialysis clefts. Argon laser is an in-office procedure that allows direct application of to the site of the cleft with direct visualization using a gonioscopy lens.5,14 However, performing argon laser at the slit lamp is painful to the patient, and often requires an in-office retrobulbar block without the assistance of anesthesiology. It also requires the patient to maintain adequate positioning at the slit lamp. Transcleral photocoagulation can be performed in the operating room with minimal patient positioning requirements and with greater anesthesia control, but does not allow direct visualization of the cleft during treatment.6 Other methods of treatment, including cyclopexy, gas tamponade, and capsular tension ring placement can require extensive surgical manipulation of intraocular tissues and therefore are prone to additional complications.1,2
Intraoperative gonioscopy-assisted endolaser is a relatively novel technique that allows for direct visualization of the cleft without relying on patient positioning and can be performed with minimal or no discomfort to the patient using a retrobulbar block and monitored anesthesia care. Additionally, the power of the laser can be rapidly titrated based on visualization of peripheral iris shrinkage as the laser is applied; the effective laser power can be increased simply by positioning the probe closer to the site of treatment. This method also allows for the instillation of viscoelastic into the anterior chamber, thus deepening the chamber and permitting an excellent view of the cleft. This aids treatment in cases where visualization is limited due to a shallow anterior chamber from hypotony.
Endolaser treatment of a cyclodialysis cleft has only been described once previously in the literature, in a pediatric patient using a 20-gauge 810nm wavelength diode endolaser.4 Our case highlights its wider applicability to the adult population, and we were able to utilize a smaller, 25-gauge argon endolaser probe, which is in the standard inventory at any surgical center where vitreoretinal surgery is performed. Ophthalmic argon lasers produce light with wavelengths of 488 nm and 514 nm, which are selectively absorbed by hemoglobin and melanin.15 This makes argon laser an ideal tool for treating uveal tissue. The previously reported pediatric case described the use of 3W of laser power on a continuous setting, and we were able to achieve efficacy with 300mW applied for a duration of 0.1 seconds and with a spot size of 100 μm, which is in line with reports of direct gonioscopic argon laser cyclopexy.4,14
Another unique aspect of our case was the cause of cyclodialysis cleft. Cataract surgery and glaucoma surgery have been noted to be common causes of iatrogenic cyclodialysis clefts, particularly with the advent of minimally-invasive glaucoma surgery devices which are inserted directly in the angle.1, 2, 3, 4 However, the formation of cyclodiaysis clefts are rare after vitrectomy. Cyclodialysis cleft has been reported following the placement of a scleral-fixated intraocular lens via the Yamane technique.16 There is a single case report of cyclodialysis following successful pars plana vitrectomy for epiretinal membrane in a highly myopic eye. The authors hypothesized that there was traction from residual anteroperipheral vitreous leading to the cycloidalysis cleft and that a second vitrectomy with removal of anterior vitreous and placement of a gas endotamponade was sufficient to close the cleft.17
The cyclodialysis cleft in this case was most likely caused from the insertion of a vitrectomy port and is the first case in the literature of a cleft caused by a vitrectomy for vitreous opacities. Additional vitrectomy as reported by Minamoto and colleagues was not required, suggesting that vitreous traction was not the cause of the cleft. We suspect that the patient's ciliary body was more posteriorly located than usual due to his history of high axial myopia. A prior study found that patients with high myopia had an average position of the ciliary body more than 3 mm posterior to the limbus18; this highlights the need to be particularly cautious in the location of vitrectomy ports in highly myopic eyes, and perhaps obtaining axial lengths in the appropriate population preoperatively.
4. Conclusions
Endolaser is an effective treatment for cyclodialysis clefts and intraoperative gonioscopy allows for direct visualization of the cleft in a controlled operating room setting. The placement of vitrectomy ports should be done with care in high myopes. This report is the first case of cyclodialysis cleft attributed to the iatrogenic piercing of the ciliary body and development of a cyclodialysis cleft associated with vitrectomy trochar insertion.
Patient consent
Consent to publish this case report has been obtained from the patient orally. This case report does not contain any personal identifying information.
Funding
This study was supported by the NIH Center Core Grant P30EY014801 and an unrestricted grant to the University of Miami from the Research to Prevent Blindness.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
The following authors have no financial disclosure: SD, YI, TL, JF, AM.
Acknowledgments
None.
References
- 1.Ioannidis A.S., Barton K. Cyclodialysis cleft: causes and repair. Curr Opin Ophthalmol. 2010;21(2):150–154. doi: 10.1097/ICU.0b013e3283366a4d. [DOI] [PubMed] [Google Scholar]
- 2.Popovic M., Shareef S., Mura J.J., et al. Cyclodialysis cleft repair: a multi‐centred, retrospective case series. Clin Exp Ophthalmol. 2019;47(2):201–211. doi: 10.1111/ceo.13378. [DOI] [PubMed] [Google Scholar]
- 3.Shue A., Levine R.M., Gallousis G.M., Teng C.C. Cyclodialysis cleft associated with Kahook dual blade goniotomy. J Curr Glaucoma Pract. 2019;13(2):74–76. doi: 10.5005/jp-journals-10078-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caronia R.M., Sturm R.T., Marmor M.A., Berke S.J. Treatment of a cyclodialysis cleft by means of ophthalmic laser microendoscope endophotocoagulation. Am J Ophthalmol. 1999;128(6):760–761. doi: 10.1016/S0002-9394(99)00245-7. [DOI] [PubMed] [Google Scholar]
- 5.Han J.C., Kwun Y.K., Cho S.H., Kee C. Long-term outcomes of argon laser photocoagulation in small size cyclodialysis cleft. BMC Ophthalmol. 2015;15(1):123. doi: 10.1186/s12886-015-0113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amini H., Razeghinejad M.R. Transscleral diode laser therapy for cyclodialysis cleft induced hypotony. Clin Exp Ophthalmol. 2005;33(4):348–350. doi: 10.1111/j.1442-9071.2005.01008.x. [DOI] [PubMed] [Google Scholar]
- 7.Selvan H., Gupta V., Gupta S. Cyclodialysis: an updated approach to surgical strategies. Acta Ophthalmol. 2019;97(8):744–751. doi: 10.1111/aos.14210. [DOI] [PubMed] [Google Scholar]
- 8.Yuen N.S.Y., Hui S.P., Woo D.C.F. New method of surgical repair for 360-degree cyclodialysis. J Cataract Refract Surg. 2006;32(1):13–17. doi: 10.1016/j.jcrs.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 9.Rodrigues I.A., Goyal S., Lim K.S., Shah B. Gonioscopically guided nonpenetrating cyclodialysis cleft repair: a novel surgical technique. J Curr Glaucoma Pract. 2017;11(1):31–34. doi: 10.5005/jp-journals-10008-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamiya R., Akimoto M. Repairing cyclodialysis by riveting: a flanged polypropylene suture. Case Rep Ophthalmol. 2021;12(3):784–790. doi: 10.1159/000518431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feiler D.L., Browne A.W., Rachitskaya A.V., et al. Indirect cyclopexy for repair of cyclodialysis clefts. Retina. 2019;39(1):S177–S181. doi: 10.1097/IAE.0000000000002091. [DOI] [PubMed] [Google Scholar]
- 12.Metrikin D.C., Allinson R.W., Snyder R.W. Transscleral repair of recalcitrant, inadvertent, postoperative cyclodialysis cleft. Ophthalmic Surg. 1994;25(6):406–408. [PubMed] [Google Scholar]
- 13.Wiechens B., Rochels R. Circular surgical cyclopexy after extensive traumatic cyclodialysis. Ophthalmic Surg Laser. 1996;27(6):479–483. [PubMed] [Google Scholar]
- 14.Hamidovic L., Khatib T., Dimitriou C., Lin Z. XEN-related hypotonous maculopathy from iatrogenic cyclodialysis cleft treated with argon laser gonio-cyclopexy. BMJ Case Rep. 2022;15(3) doi: 10.1136/bcr-2021-244933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaarawy T., Sherwood M.B., Hitchings R.A., Crowston J.G. second ed. Elsevier, Saunders; 2015. Glaucoma. [Google Scholar]
- 16.Nguyen M.T., Rajanala A., Chen P.P. Cyclodialysis cleft formation following Yamane secondary intraocular lens implantation. Am J Ophthalmol Case Rep. 2022;26 doi: 10.1016/j.ajoc.2022.101457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minamoto A., Nakano K.E., Tanimoto S., Mizote H., Takeda Y. Ultrasound biomicroscopy in the diagnosis of persistent hypotony after vitrectomy. Am J Ophthalmol. 1997;123(5):711–713. doi: 10.1016/S0002-9394(14)71095-5. [DOI] [PubMed] [Google Scholar]
- 18.Agrawal P., Martin K.R. Ciliary body position variability in glaucoma patients assessed by scleral transillumination. Eye. 2008;22(12):1499–1503. doi: 10.1038/eye.2008.79. [DOI] [PubMed] [Google Scholar]