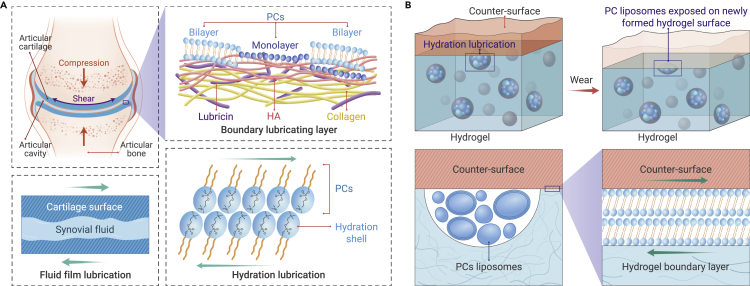
In nature, when two solid interfaces contact and slide with each other, adverse friction and wear ensue. Lubrication is an important means to reduce them. Fluid film lubrication and boundary lubrication are the most typical lubrication paradigms that are different in the principle of formation.1 More generally, in fluid film lubrication mode, the two sliding surfaces are completely separated by a fluid film with a thickness much larger than the surface asperities. At this time, the energy dissipation is mainly caused by the fluid being sheared. Boundary lubrication occurs when two surfaces are in molecular contact, and the energy dissipation mainly comes from van der Waals force, Coulomb force, and dis-entanglement of surface polymers. Therefore, boundary lubrication is directly related to the physical and chemical properties of the surface.
So far, no artificial lubrication system has been as successful as human synovial joints such as hips and knees. Despite the high local pressure (nearly 20 MPa) and a wide range of shear rates (from rest to 105_106 s−1) on the articular surface, this surface maintains enduring sliding friction coefficients as low as μ ≈ 0.001.1,2 This extraordinary phenomenon has led to a decade-long quest to date. Physiological studies have shown that the articular cartilage covers the surface of the end of the articular bone and plays a lubricating role.1,2 Articular cartilage is a highly specific and structurally connective tissue with a dense extracellular matrix structure and relatively sparse chondrocytes. It mainly contains of up to 70% water, as well as collagen, proteoglycans, and lipids dominated by phospholipids, and can thus be regarded as a hydrogel. Within the joint cavity, viscous synovial fluid exists between the cartilage surfaces (Figure 1A).
Figure 1.
Lubrication models of cartilage, and hydrogel inspired by it
(A) The physiological structure of the joint and the distribution of various molecules in the boundary lubricating layer. The fluid film lubrication and hydration lubrication models are derived from these structures.
(B) A cartilage-inspired self-lubrication hydrogel model. The surface and the bulk of the hydrogel are distributed with microreservoirs containing PC liposomes. PCs induce hydration lubrication on the surface. When frictional wear occurs, the hydrogel surface is exposed, and boundary layers of lipids are self-supplemented.
According to the structure, many cartilage lubrication models have been derived (Figure 1A).1 In earlier models, the viscous synovial fluid was thought to act as a fluid film for hydrodynamic lubrication. Later, considering the complex properties of cartilage, such as elasticity and permeability, researchers have gradually proposed models of elastic surfaces and mechanisms such as squeeze film. In this mechanism, the interstitial fluid forms a surface liquid film during cartilage extrusion to reduce friction. To explain what properties of synovial fluid and cartilage surfaces allow interstitial fluid to be retained on the loading surface, weeping lubrication, boosted lubrication, and ultrafiltration theory have been proposed.
However, more in-depth experimental trials reveal an important role for boundary lubrication of cartilage surfaces. The pressure on the cartilage surface is extremely uneven, and the high-pressure regions usually have boundary lubrication. In the synovial fluid and the superficial zone of cartilage, hyaluronic acid (HA), aggrecan, lubricin, and phospholipids have been proved to be the major boundary lubrication molecules (Figure 1A).1,2 HA is linear polysaccharides with long and soft molecular chains with abundant charges. Aggrecan is embedded in a collagen network and consists of a protein core and several glycosaminoglycan chains with a bottlebrush-like structure. It is non-covalently linked to HA through link proteins. Separate HA is not considered to have excellent boundary lubrication capability. HA with aggrecan linked is more lubricated but only at low pressures. Lubricin is an elongated glycoprotein with a highly conserved structure, but the specific role in lubrication has not been fully explained. Surface-active phospholipids involved in boundary lubrication are primarily phosphatidylcholines (PCs). They have two fatty acids tails and a zwitterionic phosphocholine headgroup, wherein the choline group is positively charged, and the phosphate group is negatively charged.3
Based on boundary lubrication, hydration lubrication is proposed to allow for the influence of water at the interface.1 In simple terms, the hydration shell surrounding a charge is very tenaciously held due to the charge-dipole interactions and very rapidly relaxing due to the ultrafast exchange rates of surrounding water molecules.3 This dual property makes it difficult for water molecules to be squeezed out during compression between cartilage surfaces. But when shear occurs, the water molecules of the hydration shell exchange quickly, sliding easily against each other like a fluid. Such hydration lubrication can occur in charged polymers, polyzwitterionic brushes, and especially zwitterionic phosphocholine groups exposed on vesicles and bilayers of PCs. At 200 atm, the friction coefficient μ can be reduced to 10−4 or even lower by the hydration lubrication of PCs (Figure 1A).1,3 The continuity of lubrication can be maintained by cellular replenishment and self-assembly after frictional wear. Note that as a newly emerged model, the hydration lubrication model of cartilage may contradict some existing models and hence needs more direct evidence. Nevertheless, it provides a cutting-edge direction for the study of cartilage lubrication.
The exploration of cartilage lubrication not only contributes to solve health problems such as arthritis but also inspires novel biolubricant materials. Although cartilage-inspired homopolymers, polymer brushes, etc., have been designed, the long-term lubrication under extreme conditions remains a major challenge. Recently, Lin, Weifeng, et al. reported their ground-breaking study, cartilage-inspired hydrogels by lipid-based hydration lubrication, in Science.3 In this study, low concentrations of PCs were mixed with pregel to form multilamellar vesicles (MLVs) under ultrasonic emulsification. After cross-linking, the hydrogels with microreservoirs spread across the surface, and the bulk was prepared. Thus, MLVs were in the microreservoirs. Due to PCs, the boundary layer of the hydrogel is similar to the boundary lubrication layers of articular cartilage surface. Analogously, the hydrated headgroup of PCs, which was attached to the hydrogel surface, provided efficient hydration lubrication (Figure 1B), which possessed an extremely low coefficient of friction even under extremely high pressure. After frictional wear on the original surface of the hydrogel and the exposure of the new surface, the liposomes originally embedded in the interior were also released. It allowed the hydration lubrication to continuously renew and reconfigure itself, endowing the hydrogel with self-lubricating properties (Figure 1B).
The team has applied this lubrication strategy to a series of hydrogels that contain a wide range of modulus and water content successfully. The universality is related to the multiple interaction modes of PCs liposomes on the hydrogel surface. For negatively charged hydrogels, the attachment of liposomes is due to the dipole interaction of the negative charges of the hydrogel with PC molecules. For neutral hydrogels, the source of such attachment is zwitterionic-dipole/induced-dipole attraction. Next, the team focuses on the widely exploited poly(hydroxyethylmethacrylate) hydrogel. The PCs used were dimyristoylphosphatidylcholine or hydrogenated soy phosphatidylcholine. Characterization by laser scanning confocal microscope and scanning electron microscopy confirmed that these MLVs exist in the form of being isolated into clusters or forming spherical microreservoirs and are widely distributed both on the surface and inside the hydrogel. The rheology showed that the PCs had a lesser effect on the original storage and loss modulus of the hydrogel.
Furthermore, in the friction experiment with the stainless-steel surface, the distribution and reconstruction of the PCs’ lubricating boundary in the interface are described by fluorescence means and formula calculations, thereby proving its self-renewal ability. Under a wide range of pressure and speed, compared with the lipid-free group, the lipid-incorporating hydrogel showed an 80%–99.3% reduction in friction and wear. In another experiment, compared with the lipid-free hydrogels washed after absorbing PCs and the lipid-free gels immersed in lipid dispersions, lipid-incorporating hydrogels immersed in water provided significantly lower coefficients of friction. Moreover, properties such as the ability of hydrogels to maintain lubrication for a long time and to restore lubrication after rehydration were also investigated. Friction and wear are also at a low level in these conditions. While there are huge advantages over lipid-free equivalent hydrogels, it is a limitation compared with other lubrication strategies that do not rely on wears, as this hydrogel cannot perform cellular replenishment and self-assembly of their components like cartilage tissue.
To summarize, this strategy can construct long-term, wide-ranging, self-renewing, highly efficient hydrogel lubrication that can be rehydrated after drying, greatly reducing friction and wear. Prior to this study, this cartilage-inspired lipid-based mechanism had rarely been explored and applied in the field of hydrogels. In terms of research methods, its simplicity, universality, and excellent performance are amazing. In the future, this strategy might show unlimited potentials in many fields such as biomedicine. For instance, this cartilage-inspired lubrication strategy can be combined with existing functionalized and structured hydrogels.4 Or, combining it with other biomimetic articular cartilage materials with high mechanical properties and low friction strategies might ultimately enable all-around capabilities far beyond nature.5 Furthermore, vesicles also have functions such as drug carriers rather than lubrication, but how to effectively synergize these functions for biomedical demands is still an issue to be explored. At the theoretical level, this type of hydrogel model is helping to continuously explore the debatable cartilage lubrication mechanism; a more precise mechanism will inspire more materials with excellent characteristics. Far from being the end point of cartilage lubrication and the materials it inspires, the research is a new cornerstone of the intersection of biotribology, materials science, and more.
Acknowledgments
The work was supported by the National Natural Science Foundation of China (NSFC) (81930048, 81627805, and 32101057); the Hong Kong Research Grant Council (15217721, R5029-19, and C7074-21GF); the Hong Kong Innovation and Technology Commission (GHP/043/19SZ and GHP/044/19GD); the Guangdong Science and Technology Commission (2019A1515011374 and 2019BT02X105); and WIU-CASQD2022009 of the Wenzhou Institute at University of Chinese Academy of Science.
Declaration of interests
The authors declare no competing interests.
Published Online: June 23, 2022
Contributor Information
Yan Zu, Email: zuyan@foxmail.com.
Puxiang Lai, Email: puxiang.lai@polyu.edu.hk.
References
- 1.Lin W., Klein J. Recent progress in cartilage lubrication. Adv. Mater. 2021;33:2005513. doi: 10.1002/adma.202005513. [DOI] [PubMed] [Google Scholar]
- 2.Zhou Y., Ni J., Wen C., et al. Light on osteoarthritic joint: from bench to bed. Theranostics. 2022;12:542–557. doi: 10.7150/thno.64340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin W., Kluzek M., Iuster N., et al. Cartilage-inspired, lipid-based boundary-lubricated hydrogels. Science. 2020;370:335–338. doi: 10.1126/science.aay8276. [DOI] [PubMed] [Google Scholar]
- 4.Lei Y., Wang Y., Shen J., et al. Injectable hydrogel microspheres with self-renewable hydration layers alleviate osteoarthritis. Sci. Adv. 2022;8:eabl6449. doi: 10.1126/sciadv.abl6449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rong M., Liu H., Scaraggi M., et al. High lubricity meets load capacity: cartilage mimicking bilayer structure by brushing up stiff hydrogels from subsurface. Adv. Funct. Mater. 2020;30:2004062. [Google Scholar]