Abstract
Background
The rapid development of safe and effective vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been a singular scientific achievement. Confounding due to health-seeking behaviors, circulating variants, and differential testing by vaccination status may bias analyses toward an apparent increase in infection severity following vaccination.
Methods
We used data from the Ontario, Canada, Case and Contact Management Database and a provincial vaccination dataset (COVaxON) to create a time-matched cohort of individuals who were hospitalized with SARS-CoV-2 infection. Vaccinated individuals were matched to up to 5 unvaccinated individuals based on test date. Risk of intensive care unit (ICU) admission and death were evaluated using conditional logistic regression.
Results
In 20 064 individuals (3353 vaccinated and 16 711 unvaccinated) hospitalized with infection due to SARS-CoV-2 between 1 January 2021 and 5 January 2022, vaccination with 1, 2, or 3 doses significantly reduced the risk of ICU admission and death. An inverse dose–response relationship was observed between vaccine doses received and both outcomes (adjusted odds ratio [aOR] per additional dose for ICU admission, 0.66; 95% confidence interval [CI], .62 to .71; aOR for death, 0.78; 95% CI, .72 to .84).
Conclusions
We identified decreased virulence of SARS-CoV-2 infections in vaccinated individuals, even when vaccines failed to prevent infection sufficiently severe to cause hospitalization. Even with diminished efficacy of vaccines against infection with novel variants of concern, vaccines remain an important tool for reduction of ICU admission and mortality.
Keywords: epidemiology, SARS-CoV-2, vaccination, pandemic, outcomes
Reduction in severity of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, even when vaccines fail to prevent infection, would be desirable. Using a time-matched cohort design, we demonstrate that vaccinated individuals hospitalized with SARS-CoV-2 have lower risk of intensive care unit admission and death than unvaccinated individuals.
The global pandemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has sickened hundreds of millions of people and killed millions [1]. The rapid development of safe and effective vaccines against the virus has been a singular scientific achievement and has likely prevented many more illnesses and deaths [2–4]. However, the ongoing emergence of novel viral variants remains a challenge, with reduced protection against infection seen with the B.1.529 (Omicron) variant that emerged in fall 2021 [5]; though vaccines seem to continue to prevent severe illness, resulting in reduced hospitalizations, intensive care unit (ICU) admissions, and deaths. Indeed, the effectiveness of vaccination for prevention of severe outcomes reflects 2 effects that may be difficult to disentangle: prevention of infection and prevention of severe outcomes even when vaccination does not prevent infection. Studies may be further complicated by factors such as decreased propensity to test vaccinated individuals with mild symptoms of respiratory illness, which could produce biases that result in increased apparent severity of infection following vaccination.
Previously, we evaluated the effectiveness of SARS-CoV-2 vaccines to prevent infection in the Canadian province of Ontario [6]. However, vaccines that result in attenuation of severity, even among individuals in whom they failed to protect against infection, would be of considerable value to vaccinated individuals and to the wider community by limiting consumption of scarce critical care resources. We sought to study the impact of prior vaccination on severity of illness among individuals admitted to the hospital with SARS-CoV-2 in Ontario, Canada, because a study limited to hospitalized individuals should limit biases introduced by differential testing according to disease severity and vaccination status. As both the propensity to receive vaccination and the dominant viral variant of concern (VOC) has varied over the course of the pandemic in Ontario, we used a time-matched cohort to evaluate the adjusted risk of ICU admission and death in vaccinated and unvaccinated individuals with identically timed infection admitted to Ontario hospitals. Our primary objective was to determine whether the risks of ICU admission and death were diminished significantly by vaccination among individuals whose vaccination failed to prevent hospitalization. We also performed exploratory analyses to determine whether protective effects were modified by an individual’s characteristics or by infecting viral variant.
METHODS
Data Sources
We created a time-matched cohort of individuals who were hospitalized due to SARS-CoV-2 infection between 1 January 2021 and 5 January 2022. Individuals who had received 1, 2, or 3 doses of a SARS-CoV-2 vaccine were included as exposed and were matched to unvaccinated individuals based on the test date for SARS-CoV-2. Each vaccinated individual was matched with up to 5 unvaccinated individuals who acted as controls [7]. We identified vaccinated and unvaccinated SARS-CoV-2 cases in the province’s Case and Contact Management (CCM) System, as described elsewhere [8, 9].
We included only cases with a unique “pseudo-health card number,” permitting linkage with the provincial vaccination COVaxON database [9], which provided information on dose administration, dates, and vaccines used. We considered individuals to be vaccinated 14 days or more after vaccine dose administration [10]. We used the date of positive testing as a surrogate for onset of infection; when individuals had a test date <14 days from a dose of vaccine, they were considered to be not protected by that dose. For example, an individual tested for infection 2 days after their second dose of vaccine would be considered single-dose vaccinated. While information on third vaccine doses was available in COVaxON, third doses were not widely available at the time of the analysis, and a small number of hospitalized individuals had received a third dose of vaccine. Consequently, we evaluated individuals based on the number of vaccine doses received and by classifying them as unvaccinated, single-dose vaccinated, double-dose vaccinated, or triple-dose vaccinated.
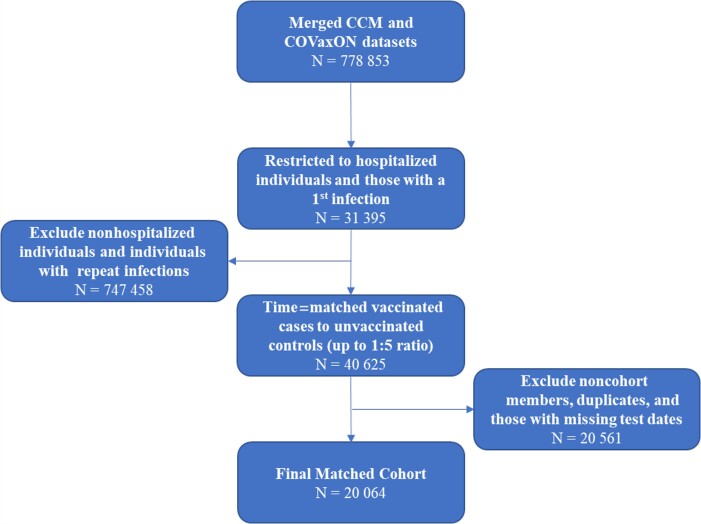
There have been several waves of the SARS-CoV-2 pandemic in Ontario, with different infecting variants prevalent for each. Initial waves were driven by the Wuhan variant, then waves were driven by Alpha in spring 2021, Delta in summer and fall 2021 [9], and finally Omicron in December 2021 [11]. As such, infecting viral variants in our analysis were classified as non-VOC, N501Y+ variant (including the Alpha, Beta, and Gamma variants) or Delta variant, as described elsewhere [9]. Individuals were considered infected with the Omicron variant (B.1.1.529) if they had been identified as such through whole-genome sequencing or if they were infected on or after 10 November 2021 with a strain with S-gene target failure or the N501Y mutation. Individuals were also considered infected with the Omicron variant if they had been classed as infected with an unknown VOC after 13 December 2022. A flow diagram outlining creation of the cohort is presented in Figure 1.
Figure 1.
Flow diagram for the creation of the matched cohort. Abbreviations: CCM, Case and Contact Management System (Ontario’s line list database); COVaxON, Ontario provincial vaccination database.
Analysis
We used our matched cohort to calculate the risk of ICU admission and death among those hospitalized due to SARS-CoV-2 using conditional logistic regression models. The models were specified a priori to adjust for age category (treated as a 9-level ordinal variable), sex, healthcare worker status, long-term care residence, comorbidity, and infecting variant. Vaccine status was treated as a 4-level nominal variable (0, 1, 2, or 3 doses) where 0 doses was used as the referent. Due to the rarity of deaths in healthcare workers, healthcare worker status was not included in models used to evaluate risk of death.
We also performed exploratory restriction analyses in which vaccinated and unvaccinated cases were limited to a single infecting variant (non-VOC, N501Y+ VOC, Delta VOC, or Omicron VOC) and additional exploratory analyses were conducted in which unvaccinated individuals were compared with individuals vaccinated exclusively with viral vector vaccines or to individuals vaccinated exclusively with messenger RNA (mRNA) vaccines. As conditional logistic regression models failed to converge for some of these models, we modeled the effect of vaccination using unmatched logistic regression models, with time trend modeled as a cubic trend function. We investigated heterogeneity in the adjusted odds ratios (aORs) for ICU admission and death according to infecting variant or vaccine product used using meta-analytic techniques (ie, graphically using forest plots, statistically using the meta-analytic Q statistic, and through construction of meta-regression models). The study was conducted in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology Guidelines for observational research [12] and received ethics approval from the University of Toronto Research Ethics Board.
RESULTS
The final matched cohort consisted of 3353 vaccinated individuals and 16 711 unvaccinated individuals. Most cohort members were aged ≥50 years (69.47%), and most were infected with the N501Y+ VOC (33.41%). The majority of the cohort were male (53.69%), and 21.58% of individuals had a recorded major medical comorbidity. In univariable analyses, vaccinated and unvaccinated individuals differed significantly according to age group, residence in long-term care, comorbidity status, and infecting variant (Table 1). Among vaccinated individuals, 51.12% had received 1 dose, 44.23% had received 2 doses, and only 4.65% had received 3 doses.
Table 1.
Baseline Characteristics of the Matched Cohort
| Characteristic | Vaccinated | (%) | Unvaccinated Controls | (%) | Total | (%) | P Value |
|---|---|---|---|---|---|---|---|
| N | 3353 | 16 711 | 20 064 | ||||
| Outcome | |||||||
| ȃIntensive care unit admission | 467 | 13.93 | 3819 | 22.85 | 4286 | 21.36 | <.001 |
| ȃDeath | 517 | 15.42 | 1849 | 11.06 | 2366 | 11.79 | <.001 |
| Age group, y | |||||||
| ȃ0–9 or 10–19a | 32 | 0.95 | 1038 | 6.21 | 1070 | 5.33 | <.001 |
| ȃ20–29 | 52 | 1.55 | 905 | 5.42 | 957 | 4.77 | |
| ȃ30–39 | 96 | 2.86 | 1665 | 9.96 | 1761 | 8.78 | |
| ȃ40–49 | 142 | 4.24 | 2195 | 13.14 | 2337 | 11.65 | |
| ȃ50–59 | 281 | 8.38 | 3108 | 18.60 | 3389 | 16.89 | |
| ȃ60–69 | 576 | 17.18 | 3376 | 20.20 | 3952 | 19.70 | |
| ȃ70–79 | 798 | 23.80 | 2669 | 15.97 | 3467 | 17.28 | |
| ȃ80+ | 1376 | 41.04 | 1754 | 10.50 | 3130 | 15.60 | |
| Male | 1838 | 54.82 | 8935 | 53.47 | 10 773 | 53.69 | .135 |
| Healthcare worker | 16 | 0.48 | 88 | 0.53 | 104 | 0.52 | .716 |
| Long-term care | 102 | 3.04 | 93 | 0.56 | 195 | 0.97 | <.001 |
| Comorbidity | |||||||
| ȃAny significant comorbidity | 1088 | 32.45 | 3242 | 19.40 | 4330 | 21.58 | <.001 |
| ȃAsthma | 79 | 2.36 | 344 | 2.06 | 423 | 2.11 | .274 |
| ȃHematological disease | 47 | 1.40 | 136 | 0.81 | 183 | 0.91 | .001 |
| ȃCardiac disease | 578 | 17.24 | 1662 | 9.95 | 2240 | 11.16 | <.001 |
| ȃ Chronic obstructive pulmonary disease | 142 | 4.24 | 276 | 1.65 | 418 | 2.08 | .628 |
| ȃDiabetes | 344 | 10.26 | 1110 | 6.64 | 1454 | 7.25 | <.001 |
| ȃRenal disease | 138 | 4.12 | 226 | 1.35 | 364 | 1.81 | <.001 |
| ȃNeurological disease | 90 | 2.68 | 186 | 1.11 | 276 | 1.38 | <.001 |
| ȃObesity | 49 | 1.46 | 244 | 1.46 | 293 | 1.46 | .996 |
| ȃLiver disease | 35 | 1.04 | 73 | 0.44 | 108 | 0.54 | <.001 |
| ȃImmune compromise | 215 | 6.41 | 448 | 2.68 | 663 | 3.30 | <.001 |
| Infecting variant of concern | |||||||
| ȃNot detected | 69 | 2.06 | 432 | 2.59 | 501 | 2.50 | <.001 |
| ȃN501Y+ | 1056 | 31.49 | 5648 | 33.80 | 6704 | 33.41 | |
| ȃDelta | 954 | 28.45 | 5362 | 32.09 | 6316 | 31.48 | |
| ȃPresumptive Omicron | 645 | 19.24 | 2917 | 17.46 | 404 | 2.01 | |
| ȃOther | 49 | 1.46 | 207 | 1.24 | 256 | 1.28 | |
| ȃUnknown | 580 | 17.30 | 2145 | 12.84 | 5883 | 29.32 | |
Age groups combined due to small cells (≤5).
We fit 2 conditional logistic regression models to assess the risk of being admitted to the ICU and the risk of death in hospitalized individuals (Table 2). Compared with no vaccination, vaccination with a single dose of vaccine (aOR, 0.57; 95% confidence interval [CI], .49 to .66), with 2 doses (aOR, 0.51; 95% CI, .44 to .60), or with 3 doses (aOR, 0.56; 95% CI, .34 to .93) significantly reduced ICU admission risk. When vaccination status was treated as a 4-level ordinal variable, we identified a significant inverse dose–response relationship between vaccine doses received and ICU admission risk (aOR per additional dose, 0.66; 95% CI, .62 to .71).
Table 2.
Odds Ratios and 95% Confidence Intervals from Conditional Logistic Regression on Intensive Care Unit Admission and Death Due to Severe Acute Respiratory Syndrome Coronavirus 2
| Covariate | Crude OR | Lower CI | Upper CI | P Value | Adjusted ORa | Lower CI | Upper CI | P Value |
|---|---|---|---|---|---|---|---|---|
| Intensive care unit admission | ||||||||
| Vaccinations received | ||||||||
| ȃUnvaccinated (referent) | 1.00 | 1.00 | ||||||
| ȃ1 dose | 0.57 | .49 | .66 | <.001 | 0.51 | .44 | .59 | <.001 |
| ȃ2 doses | 0.51 | .44 | .60 | <.001 | 0.48 | .40 | .56 | <.001 |
| ȃ3 doses | 0.56 | .34 | .93 | .024 | 0.52 | .31 | .86 | .011 |
| Age group (per 10-year increase) | 1.03 | 1.01 | 1.05 | .001 | 1.07 | 1.05 | 1.09 | <.001 |
| Male | 1.42 | 1.32 | 1.53 | <.001 | 1.43 | 1.33 | 1.55 | <.001 |
| Healthcare worker | 0.67 | .38 | 1.18 | .164 | 0.76 | .43 | 1.35 | .348 |
| Long-term care | 0.39 | .23 | .69 | .001 | 0.43 | .25 | .75 | .003 |
| Any significant comorbidity | 1.08 | .98 | 1.18 | .104 | 1.12 | 1.02 | 1.22 | .019 |
| Infecting VOC | ||||||||
| ȃNon-VOC, unknown Other (referent) | 1.00 | 1.00 | ||||||
| ȃN501Y+ | 1.01 | .89 | 1.15 | .831 | 1.16 | 1.01 | 1.33 | .040 |
| ȃDelta | 1.80 | 1.61 | 2.01 | <.001 | 1.78 | 1.56 | 2.04 | <.001 |
| ȃPresumptive Omicron | 0.50 | .39 | .65 | <.001 | 1.04 | .77 | 1.39 | .816 |
| Death | ||||||||
| Vaccinations received | ||||||||
| ȃUnvaccinated (Referent) | 1.00 | 1.00 | ||||||
| ȃ1 dose | 1.52 | 1.32 | 1.75 | <.001 | 0.68 | .58 | .80 | <.001 |
| ȃ2 doses | 1.38 | 1.17 | 1.63 | <.001 | 0.62 | .51 | .74 | <.001 |
| ȃ3 doses | 1.98 | 1.05 | 3.73 | .034 | 0.83 | .42 | 1.61 | .575 |
| Age group (per 10-year increase) | 1.69 | 1.63 | 1.75 | <.001 | 1.77 | 1.70 | 1.84 | <.001 |
| Male | 1.55 | 1.41 | 1.71 | <.001 | 1.75 | 1.58 | 1.95 | <.001 |
| Long-term care | 3.39 | 2.22 | 5.17 | <.001 | 1.67 | 1.05 | 2.66 | .029 |
| Any significant comorbidity | 1.66 | 1.50 | 1.85 | <.001 | 1.40 | 1.25 | 1.57 | <.001 |
| Infecting VOC | ||||||||
| ȃNon-VOC, unknown Other (referent) | 1.00 | 1.00 | ||||||
| ȃN501Y+ | 1.09 | .93 | 1.27 | .301 | 1.06 | .89 | 1.27 | .517 |
| ȃDelta | 1.33 | 1.15 | 1.54 | <.001 | 1.47 | 1.22 | 1.77 | <.001 |
| ȃPresumptive Omicron | 1.31 | .89 | 1.93 | .172 | 2.71 | 1.69 | 4.33 | <.001 |
Abbreviations: CI, confidence interval; OR, odds ratio; VOC, variant of concern.
Intensive care unit admission adjusted for age group, sex, healthcare worker, long-term care, comorbidity, and infecting variant. Death adjusted for age group, sex, long-term care, comorbidity, and infecting variant.
Vaccination also significantly decreased the risk of death conditional on hospitalization for SARS-CoV-2 (aOR for a single vaccine dose, 0.5; 95% CI, .44 to .59; aOR for 2 doses, 0.48; 95% CI, .40 to .56; and aOR for 3 doses, 0.52; 95% CI, .31 to .86). We identified a significant inverse dose–response relationship between the number of vaccine doses received and risk of death (aOR per additional dose, 0.78; 95% CI, .72 to .84).
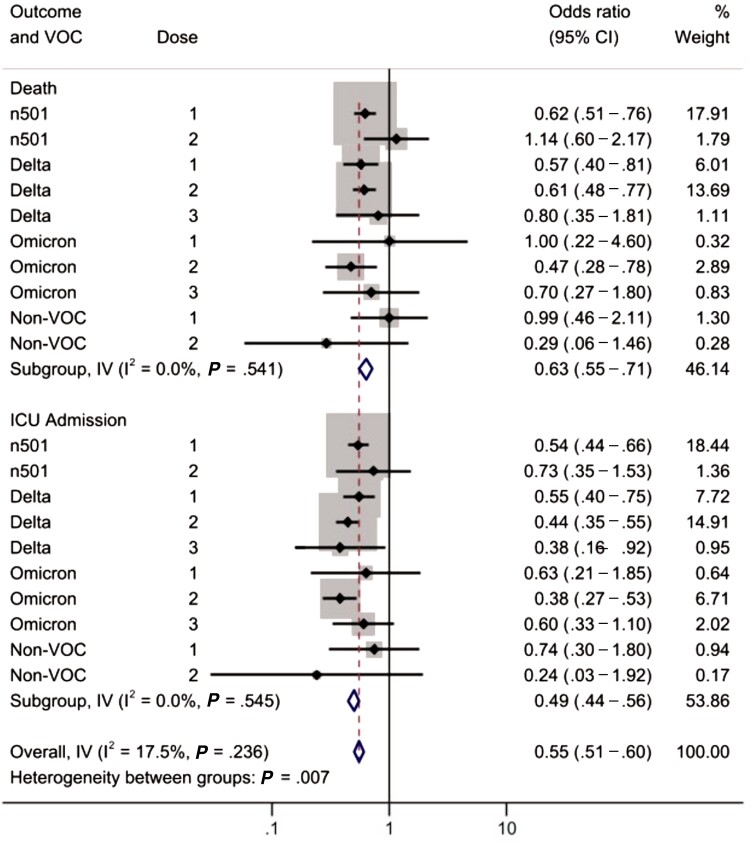
We also performed an exploratory subgroup analysis on the odds of ICU admission and death stratified by the infecting variant. Heterogeneity in the aORs for ICU admission and death is shown in Figure 2. There was no significant heterogeneity between variants within each outcome. However, there was significant heterogeneity in vaccine effects based on the outcome used, with greater protection against ICU admission than death (P < .05). When we created a meta-regression model that included vaccine type (mRNA vs viral vector vaccine), dose number, and outcome (death vs ICU), we found that vaccination was more protective against ICU admission than against death (27% relative reduction in risk; 95% CI, 12% to 39%), and mRNA vaccines were more protective against ICU admission and death than viral vector vaccines (36% relative reduction in risk; 95% CI, 10% to 55%). Results of stratified models and meta-regression are presented in detail in Supplementary Tables 1–3.
Figure 2.
Forest plot to evaluate heterogeneity between estimates by infecting variant and outcome. The analysis is stratified by outcome, with results for death in the upper rows and ICU admission below. Abbreviations: CI, confidence interval; ICU, intensive care unit; IV, contribution of within-stratum variance to overall variance; n501, N501Y-positive variant; VOC, variant of concern.
DISCUSSION
In a cohort of vaccinated and unvaccinated individuals matched on infection timing and hospitalization with SARS-CoV-2 infection in Ontario, Canada, vaccination was associated with a decreased risk of ICU admission and death after adjustment for confounding factors such as age, sex, healthcare worker status, long-term care residence, comorbidity status, and infecting variant. A reduced risk of severe outcomes with increased number of vaccine doses received was also seen. The time-matched nature of our design suggests that our findings are unlikely to be due to changes in vaccine prioritization or dominant circulating variants over time. Restriction to hospitalized individuals makes it less likely that our findings are biased by differential testing in vaccinated and unvaccinated individuals. We postulate that this might result in confounding and selection bias, inasmuch as vaccinated individuals may be less likely to be tested for mild symptoms of infection but may be more likely to be tested overall.
The significant protective effects of vaccination against ICU admission and death were seen for both the N501Y+ and Delta variants in exploratory restriction analyses. While we did not identify significant protection against the Omicron variant in restriction analyses, this likely reflects low statistical power due to recent Omicron emergence. We found no evidence for heterogeneity in effect according to infecting variant. We did, however, identify greater protection against ICU admission than against death with vaccines. It is possible that this relates to residual confounding by long term care residence status, which independently reduced the likelihood of ICU admission while increasing the risk of death.
Protection against infection and symptomatic infection has declined with the emergence of novel VOCs, most notably the Omicron variant [5, 13–16]. While recent data suggest that booster doses of mRNA vaccines substantially restore vaccine efficacy [15, 16], our analysis shows that prior partial vaccination can provide benefits to individuals and health systems even when vaccines fail to prevent infection, or even hospitalization, and remains an important pillar of the public health response to the SARS-CoV-2 pandemic.
Our approach to this analysis emphasized adjustment for confounding both in the design (via matching) and analysis (via multivariable regression). The importance of adjustment for confounding when evaluating vaccine efficacy against SARS-CoV-2 relates to the differential risk profiles of vaccinated and unvaccinated individuals. For example, in Ontario, older individuals and those with medical comorbidities who are expected to have worse outcomes if infected with SARS-CoV-2 have been prioritized for vaccination. This so-called confounding by indication is likely to diminish the apparent crude efficacy of vaccines, as is seen in our unadjusted analyses, simply because those who are more likely to be vaccinated are also more likely to experience adverse outcomes of infection, independent of vaccination status. While confounding by age and comorbidity might be expected, we also adjusted by other factors, including biological sex. As in this analysis, we previously identified male sex as an independent risk factor for poor outcomes in individuals with SARS-CoV-2 infection [17, 18].
The effects we demonstrate here are not unique to SARS-CoV-2, and we have previously identified similar effects with prior pneumococcal and influenza vaccination [19, 20]. As with that earlier work, an important limitation here is the inability to ensure that the effects we observe are not at least in part due to residual confounding. This is a potential limitation of any cohort study and is one that will be the focus of future work. The relatively recent emergence of the Omicron variant and the lags associated with critical illness and death result in the lack of statistical power to estimate Omicron-specific protections, as noted above. Unfortunately, ongoing high rates of SARS-CoV-2 hospitalizations, critical illnesses, and deaths in Ontario mean that we will be able to address this potential limitation in the months ahead.
In summary, we identified a decrease in the risk of ICU admission and death in hospitalized, vaccinated individuals compared with hospitalized, unvaccinated individuals, matched for infection timing, in Ontario, Canada. Our analysis further emphasizes the critical importance of high rates of vaccination for protection of community health and reduction of the impacts of SARS-CoV-2 on ICU capacity during the pandemic.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the staff at Public Health Ontario and Ontario’s public health units for collecting, sequencing, analyzing, and providing access to the data used for this analysis.
Financial support. This work was supported by a grant to D. N. F. from the Canadian Institutes for Health Research (2019 COVID-19 rapid researching funding, OV4-170360). D. N. F. reports support for this work from the Canadian Institutes for Health Research.
Supplementary Material
Contributor Information
Alicia A Grima, Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Canada.
Kiera R Murison, Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Canada.
Alison E Simmons, Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Canada.
Ashleigh R Tuite, Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Canada.
David N Fisman, Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Canada.
References
- 1. Johns Hopkins University Coronavirus Resource Center. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Available at: https://coronavirus.jhu.edu/map.html. Last accessed 27 December 2021.
- 2. Kreier F. “Unprecedented achievement”: who received the first billion COVID vaccinations? Nature 2021. doi: 10.1038/d41586-021-01136-2. [DOI] [PubMed] [Google Scholar]
- 3. Mesle MM, Brown J, Mook P, et al. . Estimated number of deaths directly averted in people 60 years and older as a result of COVID-19 vaccination in the WHO European Region, December 2020 to November 2021. Euro Surveill 2021; 26:2101021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gupta S, Cantor J, Simon KI, Bento AI, Wing C, Whaley CM. Vaccinations against COVID-19 may have averted up to 140,000 deaths in the United States. Health Aff (Millwood) 2021; 40:1465–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hogan AB, Wu SL, Doohan P, et al. . Report 48—The value of vaccine booster doses to mitigate the global impact of the Omicron SARS-CoV-2 variant. 2021. Available at: http://www.imperial.ac.uk/mrc-global-infectious-disease-analysis/covid-19/report-48-global-omicron/. Last accessed 17 December 2021.
- 6. Fisman DN, Lee N, Tuite AR. Timing of breakthrough infection risk after vaccination against SARS-CoV-2. medRxiv. 2022.
- 7. Hennessy S, Bilker WB, Berlin JA, Strom BL. Factors influencing the optimal control-to-case ratio in matched case-control studies. Am J Epidemiol 1999; 149:195–7. [DOI] [PubMed] [Google Scholar]
- 8. Fisman DN, Greer AL, Hillmer M, O’Brien SF, Drews SJ, Tuite AR. COVID-19 case age distribution: correction for differential testing by age. medRxiv 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fisman DN, Tuite AR. Evaluation of the relative virulence of novel SARS-CoV-2 variants: a retrospective cohort study in Ontario, Canada. CMAJ 2021; 193:E1619–E25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ontario Ministry of Health. COVID-19 Fully Vaccinated Status in Ontario. 2021. Available at: https://www.health.gov.on.ca/en/pro/programs/publichealth/coronavirus/docs/vaccine/COVID-19_fully_vaccinated_status_ontario.pdf. Last accessed 2 December 2021.
- 11. Garcia-Beltran WF, St Denis KJ, Hoelzemer, A, et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. medRxiv 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. von Elm E, Altman DG, Egger M, et al. . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Bull World Health Organ 2007; 85:867–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khoury DS, Steain M, Triccas JA, Sigal A, Davenport MP, Cromer D. A meta-analysis of early results to predict vaccine efficacy against Omicron. medRxiv 2021. [Google Scholar]
- 14. Rufino J, Baquero C, Frey D, et al. . Using survey data to estimate the impact of the omicron variant on vaccine efficacy against COVID-19 infection. medRxiv 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buchan SA, Chung H, Brown KA, et al. . Effectiveness of COVID-19 vaccines against Omicron or Delta symptomatic infection and severe outcomes. medRxiv 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kislaya I, Peralta Santos A, Borges V, et al. . Comparative complete scheme and booster effectiveness of COVID-19 vaccines in preventing SARS-CoV-2 infections with SARS-CoV-2 Omicron (BA.1) and Delta (B.1.617.2) variants. medRxiv 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stall NM, Wu W, Lapointe-Shaw L, et al. . Sex- and age-specific differences in COVID-19 testing, cases, and outcomes: a population-wide study in Ontario, Canada. J Am Geriatr Soc 2020; 68:2188–91. [DOI] [PubMed] [Google Scholar]
- 18. Fisman DN, Greer AL, Hillmer M, Tuite R. Derivation and validation of clinical prediction rules for COVID-19 mortality in Ontario, Canada. Open Forum Infect Dis 2020; 7:ofaa463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Spaude KA, Abrutyn E, Kirchner C, Kim A, Daley J, Fisman DN. Influenza vaccination and risk of mortality among adults hospitalized with community-acquired pneumonia. Arch Intern Med 2007; 167:53–9. [DOI] [PubMed] [Google Scholar]
- 20. Fisman DN, Abrutyn E, Spaude KA, Kim A, Kirchner C, Daley J. Prior pneumococcal vaccination is associated with reduced death, complications, and length of stay among hospitalized adults with community-acquired pneumonia. Clin Infect Dis 2006; 42:1093–101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.