Abstract
Background
We studied whether comorbid conditions affect strength and duration of immune responses after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) messenger RNA vaccination in a US-based, adult population.
Methods
Sera (before and after BNT162b2 vaccination) were tested serially up to 12 months after 2 doses of vaccine for SARS-CoV-2-anti-Spike neutralizing capacity by pseudotyping assay in 124 individuals; neutralizing titers were correlated to clinical variables with multivariate regression. Postbooster (third dose) effect was measured at 1 and 3 months in 72 and 88 subjects, respectively.
Results
After completion of primary vaccine series, neutralizing antibody half maximal inhibitory concentration (IC50) values were high at 1 month (14-fold increase from prevaccination), declined at 6 months (3.3-fold increase), and increased at 1 month postbooster (41.5-fold increase). Three months postbooster, IC50 decreased in coronavirus disease (COVID)-naïve individuals (18-fold increase) and increased in prior COVID 2019 (COVID-19+) individuals (132-fold increase). Age >65 years (β = −0.94, P = .001) and malignancy (β = −0.88, P = .002) reduced strength of response at 1 month. Both neutralization strength and durability at 6 months, respectively, were negatively affected by end-stage renal disease ([β = −1.10, P = .004]; [β = −0.66, P = .014]), diabetes mellitus ([β = −0.57, P = .032]; [β = −0.44, P = .028]), and systemic steroid use ([β = −0.066, P = .032]; [β = −0.55, P = .037]). Postbooster IC50 was robust against WA-1 and B.1.617.2. Postbooster neutralization increased with prior COVID-19 (β = 2.9, P < .0001), and malignancy reduced neutralization response (β = −0.68, P = .03), regardless of infection status.
Conclusions
Multiple clinical factors affect the strength and duration of neutralization response after primary series vaccination, but not the postbooster dose strength. Malignancy was associated with lower booster-dose response regardless of prior COVID infection, suggesting a need for clinically guided vaccine regimens.
Keywords: SARS-CoV-2, mRNA vaccine, immune response, neutralization assay, impact on outcome, clinical variables, COVID-19
Multiple clinical factors affect the half maximal inhibitory concentration strength and duration after primary series vaccination. All subjects, regardless of prior coronavirus disease infection, had enhanced neutralization following the third dose. Malignancy decreased response after the third dose, suggesting the importance of clinically guided vaccine-dosing regimens.
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) virus has infected more than 508 million people worldwide as of April 2022, and has caused more than 6 million deaths [1]. Three vaccines have been approved by the US Food and Drug Administration, based on safety and efficacy [2]. The messenger RNA (mRNA) vaccines encode a codon-optimized version of Spike glycoprotein (S) of SARS-CoV-2, which is the viral protein that elicits neutralizing antibodies that block viral entry into cells and subsequent replication [3–5]. Neutralizing antibodies to the Spike protein of SARS-CoV-2 correlate with immunity against the virus after vaccination, reducing rates of coronavirus disease (COVID)-related infection, hospitalization, and death [6, 7]. Neutralizing antibody titers have been shown to decline over a period of 6 months after completion of the initial vaccine series. However, neither the actual duration of humoral protection nor the clinical factors that impact the strength and duration of this protection are well described [8–13]. Unlike anti-SARS-CoV-2 antibodies, Spike-specific T-cell responses may be sustained at least up to 6 months after both infection and vaccination [14]. Poorer concordance between neutralizing antibodies and T-cell responses of the adaptive immune system have been associated with severity of disease in individuals ≥65 years old [15]. It remains to be seen whether other clinical factors and comorbidities affect the strength and duration of the humoral and cellular immune responses after vaccination.
In light of the emerging data on the waning protection of SARS-CoV-2 mRNA vaccines and the clinical benefits of a third dose, the Centers of Disease Control and Prevention recommend an additional dose after completion of the initial mRNA vaccine series for all adults, with further dosing by age and immunocompromised status [16, 17]. Although advancing age and a personal history of malignancy are associated with decreased immunogenicity to vaccination, those with end-stage renal disease or diabetes mellitus had promising neutralizing antibody responses in other studies [18–21]. A better understanding of which clinical factors impact postvaccine immune responses can help guide additional dose requirements over time. We present our results from a longitudinal study on the evaluation of the clinical variables that impact strength and duration of neutralizing antibodies in the sera of individuals vaccinated with Pfizer-BioNTech SARS-CoV-2 mRNA vaccine.
METHODS
Study Design
Starting in December 2020, we conducted a prospective longitudinal study to collect both clinical information and peripheral blood samples from recipients of BNT162b2 (Pfizer-BioNTech) mRNA vaccine, including veterans and healthcare workers at the Veterans Affairs Connecticut Healthcare System located in West Haven, CT, USA. Venous blood was obtained within 48 hours before the first and second doses of vaccine, and at 1 month (up to 1.5 months), 3 months (up to 3.5 months), 6 months (± 2 weeks), and 12 months (± 3 weeks) after the second dose. Sera were additionally drawn 1 month after the third (booster) dose (up to 1.5 months), which was on average 10 months after the second dose (standard deviation, 0.8). By the 12-month blood draw, only 2 subjects had chosen not to receive their third dose. Venous blood was processed to obtain serum and plasma. which were cryopreserved at −80°C. Clinical and demographic variables were collected via retrospective review of medical records, and included age, race, ethnicity, sex, body mass index, medical comorbidities (>80 variables including cardiovascular, pulmonary, oncological, renal, hepatic disease, and others), laboratory values (hemoglobin, serum creatinine, hemoglobin A1c, reverse transcriptase-polymerase chain reaction test results for COVID 2019 [COVID-19] before or during study participation), and concomitant medications. Current steroid use was defined as systemic steroid use for >2 weeks within 1 month before primary vaccine series or during the study period. Renal function was calculated by estimating glomerular filtration rate (GFR) by using the Chronic Kidney Disease Epidemiology Collaboration equation (Supplementary Table 1) [22]. Subjects whose medical charts were not complete within the Veterans Affairs system signed release of information for us to obtain their medical records. Per protocol, subjects reported COVID-19 infection, exposure, or diagnosis. Three subjects, who had clinically consistent symptoms and exposures but did not get tested, were presumed COVID-positive.
Ethics
The study was approved by the institutional review board at the Veterans Affairs Connecticut Healthcare System. Written informed consent was obtained from each subject.
Evaluation of Anti-SARS-CoV-2 Neutralizing Antibodies
Sera were tested for neutralization activity against SARS-CoV-2 using a single-cycle infectivity assay with Spike-pseudotyped virus particles as described previously [23]. SARS-CoV-2 Spike- (codon-optimized WA1 or Wuhan-1) pseudotyped lentiviral cores expressing firefly luciferase were produced. Spike for codon-optimized B1.617.2 (Delta variant) were similarly prepared for testing of a small subset of sera (N = 36) to compare with WA1-pseudotyped particles at the 1-month after the third-dose timepoint. These pseudotyped particles were titered on 293T-hACE2 cells seeded in 96-well plates. Each serum sample was tested in duplicate by premixing 75 µL of 4-fold serial dilutions (1:9.21–1:603587) with 30 µL of pseudotyped virus, then adding 95 µL of this mixture to the 293T-hACE2 cells. After an overnight incubation, 165 µL of fresh medium was added. After another 48 hours, cells were lysed and luciferase luminometry performed. Curve-fitting using GraphPad PRISM was used to calculate the neutralization titer half maximal inhibitory concentration (IC50 value) for each sample at each timepoint.
Statistical Analysis
Variable Selection
Subject morbidity data were grouped based on organ system involved and clinical characteristics (Supplementary Table 1). Undetectable IC50 values (<9.21 μg/mL) were changed to 9.21 for statistical analyses. The IC50 values were then log-normalized. Paired t test analysis was used to compare log-normalized IC50 values at various timepoints: 1, 3, 6, 10, and 12 months with baseline log2IC50 values before vaccination. To compare IC50 values in subjects with prior COVID-19 at 12 months, an unpaired t test was performed after log normalization.
To assess the neutralization assay over time and as a comparison with baseline values, the fold-change (Figure 1B) was calculated by dividing the IC50 at each timepoint by the baseline IC50. As shown in the y-axis of Figure 1B, the fold changes (FC) were found to follow a logarithmic distribution and were thus log-normalized. The steps to calculate log2FC (LFC) are shown in the equation and were used in all univariate and multivariate analyses (Figures 2–4).
Log2ΔIC50 was also considered as an alternate outcome variable in which the prevaccination IC50 was subtracted from the IC50 at each timepoint. Ultimately, LFC was chosen to adequately normalize the outcome for parametric statistical tests and consistent reporting with prior studies. However, using LFC results in a measurement artifact in which higher prevaccination IC50 values are associated with lower increases in LFC [24].
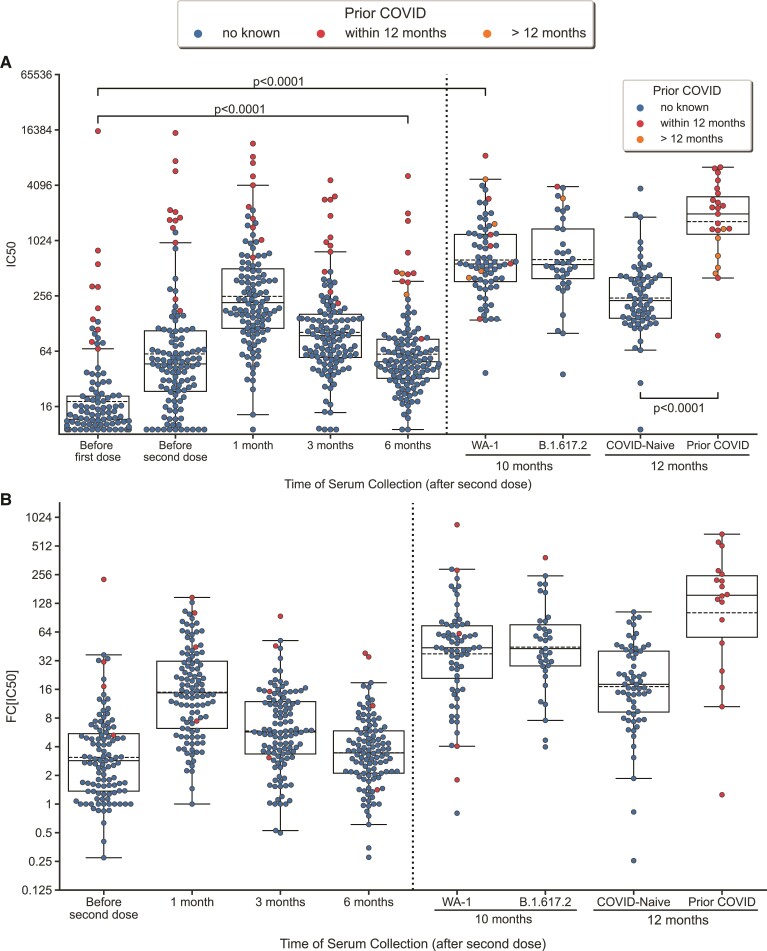
Figure 1.
Neutralization antibody titers following vaccination. A, Swarmplot/boxplot of IC50 plotted over time before and after vaccination, second dose, and third dose. Boxplot shows median, lower/upper quartile, and extremes. B, Swarmplot/boxplot of fold-change (FC[IC50]) over pre-vaccination IC50 plotted over time after the first dose of vaccine. A values are derived from paired A test. Outliers, values further than 1.5 times the interquartile range (Q1–1.5*IQR and Q3+1.5*IQR), are shown beyond the boxplot. Subjects with COVID prior to first dose of vaccination were excluded in figure 1A because it artifactually lowers the fold-change. The 1- month time point was 1 month post third dose. The 12-month timepoint was 3 months post third dose.
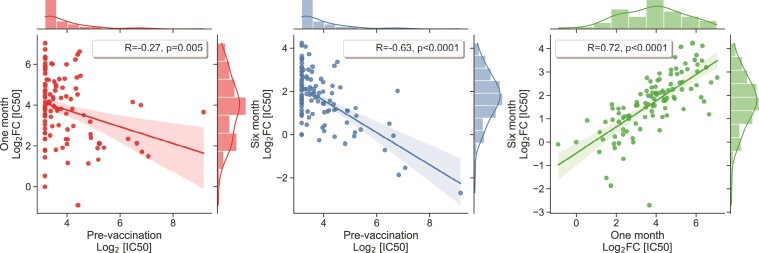
Figure 2.
Association between prevaccination IC50 and fold-changes in neutralizing antibody response. Scatterplot and Pearson correlation coefficients between prevaccination IC50 and fold-change response at 1 and 6 months. Not shown: similar correlation with prevaccination IC50 found at 10 and 12 months (R = −0.40, P = .0015; and R = −0.54, P < .0001 respectively). IC50, half maximal inhibitory concentration.
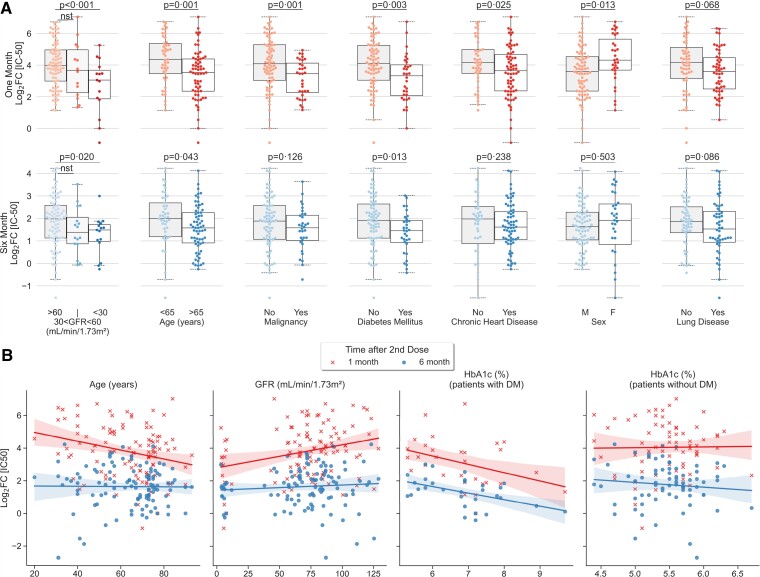
Figure 3.
Univariate analysis showing clinical factors that correlated significantly with neutralizing antibody titer peak and duration at 6 months. A, Categorical analysis between significant (p < 0.05) and trending variables (p < 0.10) of vaccination response at one month (left) and six months following second dose of the vaccine. B, Scatterplot illustrating continuous variables: age, glomerular filtration rate, and hemoglobin A1c plotted against vaccination response at one month (crosses) and six months (circles). Correlation analyses of hemoglobin A1c was conducted separately for patients with and without diabetes. Colored lines represent lines of best fit, with shading showing 95% confidence intervals. Subjects with prior COVID-19 diagnosis were excluded from univariate analysis. NS represents non-significant or trending association.
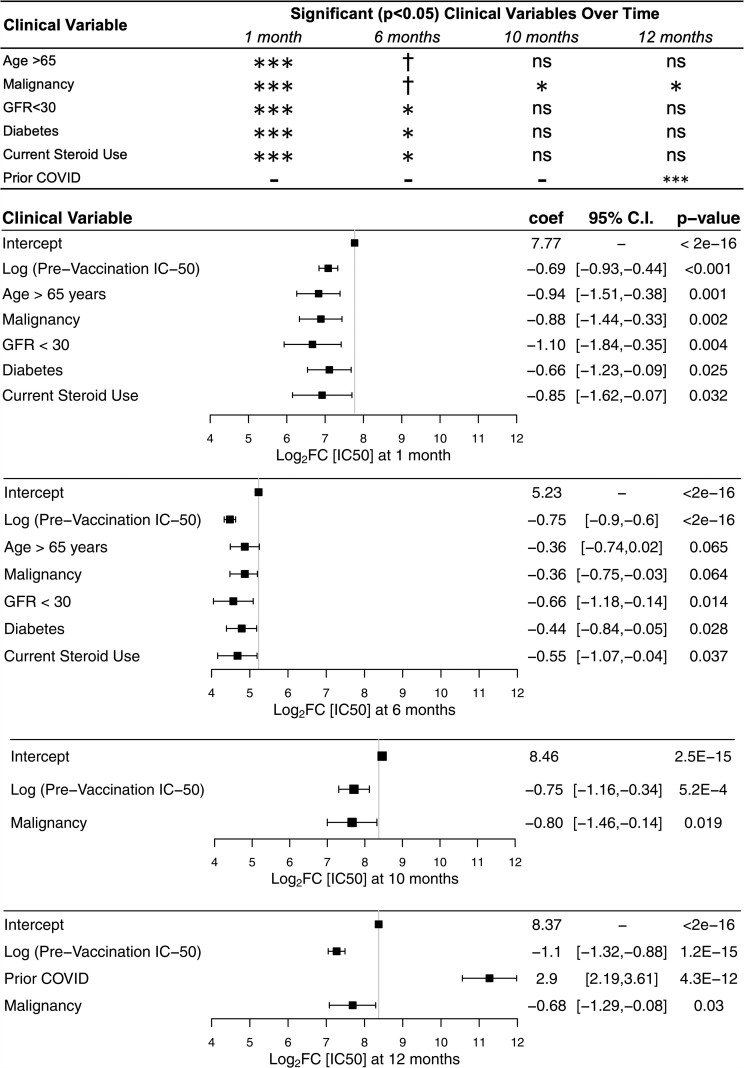
Figure 4.
Multivariable linear regression of clinical variables associated with neutralizing antibody response. Summary plot (top) showing variables retained in the stepwise regression with corresponding significant P values. Nonsignificance denoted by “ns”, P value between .10 and .05 denoted by †. P value between .05 and .01 denoted by ∗. P value between .01 and .001 denoted by ∗∗. P value less than .001 denoted by ∗∗∗. Log2FC = log normalized fold-change over prevaccination IC50. Forest plots showing multivariable linear regression variables of response at 1, 6, 10, and 12 months following administration of second dose. Variables not reaching significance not shown in the figure are sex, race, chronic heart disease, lung disease, liver disease, current steroid use, cerebrovascular disease, HIV-Transplant-Rheum, and dementia. Intercept represents baseline comparison values: age less than 65 years, GFR greater than 60 mL/min/1.73 m2, no steroid use, and no comorbidities. The 10-month time point was 1 month after the third dose. The 12-month timepoint was 3 months after the third dose. GFR, glomerular filtration rate; IC50, half maximal inhibitory concentration.
We used the highest LFC as a measure of “strength” of the humoral response. Additionally, we calculated the LFC of titers at 6 and 12 months compared with titers at prevaccination to represent the “duration” of the response after the primary series and third dose, respectively [24]. Positive LFC values represent increase in IC50 compared with prevaccination IC50 and negative values represent a decrease in IC50.
Univariate Analysis
Continuous variables (Xi) were used in analyses with outcomes log2(FC) (neutralization titers). Pearson or Spearman correlation tests were used depending on the distribution of the response variable:
In this equation, Y represents log2FC. R values reported represent the strength and direction of correlation.
For dichotomous categorical variables, if both subgroups maintained normal distributions (P > .10 using Shapiro-Wilk test) of the outcome variable, then we reported the results of 2-sided Student (independent) or t test (1-sided). If, however, at least 1 subgroup did not maintain a normal distribution (P < .10), then we used the nonparametric test, Wilcoxon rank-sum test (1-sided). All univariate analyses were conducted using the Python (v3.8.5) statsmodels package [25].
Multiple Regression Analysis
Variables (Xi) were added and subtracted in a stepwise manner to optimize the Akaike information criterion, an established metric quantifying the trade-off between underfitting and overfitting the data [26, 27].
To account for the artifactual negative correlation between prevaccination IC50 and log2FC values, log2prevaccination IC50 values were added as a variable in our analysis. Reported are the coefficient estimates representing the slope of each variable, βi. Nonsignificant variables (P > .10) were excluded from the figure. Positive and negative values (full range between negative infinity and positive infinity) represent positive and negative associations between clinical variable and response. The intercept represents y-intercept of the model (ie, younger subjects without comorbidities and higher GFR response variables). Goodness of fit was analyzed using Q-Q plots. Multiple regression analysis was conducted using R (v4.1.1) with the MASS package.
Visualizations
All univariate association figures were created using the Python package seaborn. Forest plots, illustrating multivariable linear regression models, were generated using R package forest plot.
RESULTS
We included 124 subjects (91 veterans, 33 healthcare workers), of whom 74% were male, 66% were White, 93% non-Hispanic, and 60% were older than age 65 years (Table 1). Ten subjects had COVID-19 diagnosed by reverse transcriptase-polymerase chain reaction before the first dose of vaccine and were thus excluded from the univariate and multivariate analyses. Two subjects developed COVID-19 before month 6 after the second dose of vaccine, and their 6-month IC50 values were excluded from analyses. All 124 subjects had sera serially collected until 6 months after their second dose. Of these, 72 subjects had sera drawn 1 month after their third dose (10 months after the second dose), and 88 subjects had sera drawn 3 months after the third dose (12 months after the second dose) by the time of this publication. Two individuals did not receive their third dose by 12 months, 1 of whom contracted COVID. By 12 months, 23 subjects had contracted COVID-19 and COVID-19 was added as a variable into the multivariate regression.
Table 1.
Cohort Demographics and Morbidities (n = 124)
| n (%) | |
|---|---|
| Male | 91 (73.4) |
| Age, y | |
| 20–64 | 50 (40.3) |
| 65–95 | 74 (59.7) |
| Race | |
| White | 82 (66.1) |
| Black | 22 (17.7) |
| Other | 20 (16.1) |
| Ethnicity | |
| Non-Hispanic | 108 (87.1) |
| Hispanic | 8 (6.5) |
| COVID-positive | |
| No | 110 (88.7) |
| Yes, before first dose | 10/121 (8.3) |
| Yes, at 1-m serum collection | 10/121 (8.3) |
| Yes, at 3-m serum collection | 12/121 (9.9) |
| Yes, at 6-m serum collection | 12/121 (9.9) |
| Yes, at 10-m serum collection | 10/72 (5.8) |
| Yes, at 12-m serum collection | 23/88 (26.1) |
| Chronic heart disease | 77 (62.1) |
| Lung disease | 67 (54.0) |
| Current steroid use | 19 (15.3) |
| Liver disease | 8 (6.5) |
| GFR in mL/min/1.73 m2, mean (SD)a | 69.6 (31.2) |
| >60 | 87 (70.2) |
| 30–60 | 18 (14.5) |
| <30 | 17 (13.7) |
| Malignancy | |
| Solid | 31 (25) |
| Hematological | 5 (4) |
| Both | 2 (1.6) |
| Diabetes mellitus | 39 (31.5) |
| TIA-CVA | 13 (10.5) |
| Dementia | 5 (4.0) |
| HIV-Transplant-Rheum | 18 (14.6) |
| Hemoglobin A1c, median [Q1,Q3]b,c | 5.6 [5.2,6.1] |
| BMI, median [Q1,Q3]b,c | 28.4 [24.8,31.5] |
Abbreviations: BMI, body mass index; COVID, coronavirus disease; GFR, glomerular filtration rate; SD, standard deviation; TIA-CVA, transient ischemic attack-cerebrovascular accident.
Multimodal distributions (Hartigan dip test).
Non-normal distributions (Shapiro-Wilk test).
Far outliers in Tukey test.
Time Since Vaccination and Anti-SARS-CoV-2 Neutralizing Antibody Titers
Among the timepoints tested, IC50 values were highest at 1 month after the second dose of vaccine, with a median fold change (FC) of 14.1 compared to pre-vaccination titers, thereafter declining to a median FC of 5.6 and 3.3 at 3 and 6 months, respectively. Consistent with previous reports [24], we found that higher prevaccination IC50 values were associated with a lower FC response at 1 month (R = −0.27, P = .005) and 6-month timepoints (R = −0.63, P < .0001) (Figure 2). Furthermore, we found that individuals with stronger response strength (higher FC at 1 month) also had better duration of vaccination response (higher FC at 6 months) (R = 0.72, P < .0001).
At 1 month after the third-dose (10 months after the second dose), the median FC increased to 41.5 (Figure 1). This peak was significantly higher than the peak response measured 1 month after the second dose (P < .0001). Although the median FC for WA-1 and B1.617.2 at 1 month after the third dose was 41.5 and 41.2, respectively, the FC for WA-1 was consistently higher for each subject compared with B1.617.2 in a paired analysis of 36 subjects (P ≤.0001). Prevaccination IC50 maintained a negative correlation with fold-change at 1 month (R = −0.40, P = .0015) and 3 months (R = −0.54, P < .0001) after the third dose. FC response at 6 months positively correlated with 1-month FC (R = 0.35, P = .0061) and 3-month FC (R = 0.50, P < .0001) after the third-dose response.
Hybrid Immune Response
By the 12-month timepoint (3 months after the third dose), 23 subjects had contracted COVID-19. The IC50 was significantly higher in these subjects (median FC, 132) compared with those who were COVID-19 naïve (median FC, 18.1; P < .0001). Those with COVID-19 >12 months prior, had significantly lower IC50 values than those with COVID-19 within 12 months (P = .004), but still significantly higher than those who were COVID naïve (P = .001).
Correlation of Neutralizing Antibody Response to Clinical Variables
Univariate Analysis
To understand the role of demographic and clinical factors in the strength and duration of the humoral response, univariate analysis was conducted after excluding subjects with prior COVID-19 diagnoses. Results are shown in Figure 3 and Supplementary Figure 1.
Multivariable Linear Regression
To account for the multimorbidity of our subjects, we conducted a stepwise multivariable linear regression both for the response strength and response duration (Figure 4). Body mass index was intentionally excluded because of collinearity with chronic heart disease. Because of the strong negative correlation between prevaccination IC50 values and response measured as log2FC, the baseline log2IC50 value was added to the stepwise regression. Unsurprisingly, higher prevaccination IC50 maintained a significant association with lower fold-change values at 1, 6, 10, and 12 months, consistent with our univariate analyses as well as previously reported neutralization assays [24].
Significant clinical factors associated with decreased strength of response at 1 month after primary vaccine series completion, included age >65 years (β = −0.94, P = .001), malignancy (β = −0.88, P = .002), GFR < 30 mL/min/1.73 m2 (β = −1.10, P = .004), current steroid use (β = −0.066, P = .032), and diabetes mellitus (β = −0.57, P = .032) (Figure 4). GFR < 30 mL/min/1.73 m2 (β = −0.66, P = .014), current steroid use (β = −0.55, P = .037), and diabetes mellitus (β = −0.44, P = .028) were significantly associated with decreased duration of response at 6 months. Meanwhile, age >65 years (β = −0.36, P = 0.065) and malignancy (β = −0.36, P = .064) showed a trend toward reduced duration without meeting significance (Figure 4). Malignancy significantly decreased the after third-dose response at 1 month (β = −0.80, P = .019) and 3 months (β = −0.68, P = .03).
DISCUSSION
SARS-CoV-2 vaccines have played a key role in curbing the spread, morbidity, and mortality of COVID-19. With rising concerns regarding the duration of protection after vaccination, efforts to understand the optimal timing and number of additional doses of vaccine are under way. It is imperative to understand clinical factors that affect the strength and duration of the immune responses to these vaccines and the role of adaptive immune responses on duration of protection. Previous studies have examined 1 or 2 clinical factors to assess the effect of vaccination on immune response [18–21]. In this study, with full access to medical records, we evaluated multiple clinical factors with multivariable regression to identify which clinical variables had the most significant negative (or positive) impact on the strength and duration of immune response over the course of 1 year.
This study was conducted on a US-based population of veterans and healthcare workers, which offers a unique study demographic. About one-half of our sample population for this cohort was >65 years old and the majority was male with multiple comorbidities. The predominantly older male population typical of veterans is also a predisposed demographic group to suffer severe/fatal COVID-19, especially if there are coexistent comorbid conditions [28, 29]. It is critical to determine how persistent the vaccine response will be in individuals at high risk for contracting severe COVID-19. We included healthcare workers in this analysis to enhance the diversity of the study population in terms of demographic and clinical factors.
In this cohort, we found that IC50 values peaked at 1 month after completion of the primary series of BNT162b2 mRNA vaccine, with the 6-month median level at one-fourth of the peak level. Our data suggest that age and malignancy play a role in reducing the initial peak response, but do not significantly impact the duration of response at 6 months, although they trend toward significance.
The third dose of mRNA vaccine has been shown to boost the waning immune response. Our results confirm that the immediate after-third-dose response was robust against variants WA-1 and B1.617.2, and other studies have demonstrated protection against the Omicron variant as well [30, 31]. At 1 and 3 months after the third dose, a history of malignancy was the only clinical variable that significantly reduced the IC50. Given that the level of neutralizing antibody titers has been shown to be highly correlated with immune protection [7], these findings support the use of booster doses, with prioritization for vulnerable populations, in particular those with a history of malignancy, as other studies suggest [32, 33].
We found that SARS-CoV-2 infection enhanced the neutralization antibody response to third dose. This effect was most prominent if the subject had COVID-19 within 12 months, but both the COVID-19 naïve and those with prior COVID-19 had significantly higher IC50 from the additional dose. This finding is supported by prior literature highlighting the synergistic response between the immune response from vaccination and infection with various variants, facilitated by B- and T-cell activation [34, 35].
Antibody titers after a third dose of mRNA vaccine have been shown to correlate with preexisting B-cell frequency and may benefit from T- and B-cell memory that allows for efficient, rapid, and high-level production of neutralizing antibodies [36]. Although a fourth dose seems to provide only short-term protection against contracting COVID-19, the longer term protective effect against severe disease suggests that certain populations may benefit from additional immune memory priming [37]. Further study will be needed to understand how many doses are beneficial over time, whether immune memory will offer sufficient protection, and whether different clinical factors will impact long-term immune memory.
This study has some important limitations. Our sample size is relatively small and limited in ethnicity distribution. More generalizable results could be generated from larger distributions of ethnicity. Subjects who had 12-month IC50 analysis had received third dose 3 months before. Although this provided an insight into clinical variables affecting duration of third dose response, evaluation of 12-month IC50 without third dose would enrich the information further. Similarly, we defined strength of response as the highest IC50 obtained by each individual. Because the timepoints of collection were predetermined, it is possible that the true peak response occurs before or after the 1-month timepoint. Although this may affect FC values, it will likely not alter the correlation of strength of response with clinical factors.
In conclusion, although age >65 years negatively affects the initial strength of the humoral immune response as quantified by levels of neutralizing antibodies, clinical comorbidities of end-stage renal disease, diabetes mellitus, and steroid use negatively impact the initial strength and duration of the humoral immune response against SARS-CoV-2 at 6 months following the primary series. The response to the third dose was universally robust suggesting a sustained immune memory response. Prior COVID-19 enhanced vaccine-generated response after the third dose, but the effect waned with time. Those with malignancy had a significantly lower neutralization response in both COVID-infected and naïve groups, suggesting that this population may benefit from further doses of vaccine. Further exploration is needed into the durability of immunologic memory of SARS-CoV-2-specific T and B cells for vulnerable populations.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. M. Z.: methodology, investigation, visualization, writing–original draft; R. S.: data curation, formal analysis, investigation, visualization, validation, writing–original draft, writing–review & editing; A. H. S.: formal analysis, visualization, writing–original draft; L. P.: formal analysis, visualization; T. C. K.: formal analysis, validation, visualization, writing–review and editing; S. S.: methodology, investigation, visualization; B. E.: methodology, investigation, resources, validation, writing–review & editing; C. M.: data curation, project administration; A. M. F.: data curation; J. X.: data curation; E. M.: data curation; J. H.: data curation; J. D.: data curation; Q. S.: methodology, investigation; R. E. S.: conceptualization, investigation, methodology, funding acquisition, resources, supervision, validation, visualization, writing–review & editing; S. G.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, resources, project administration, supervision, visualization, validation, writing–original draft, writing–review & editing.
Acknowledgments. The authors thank the Veterans and healthcare workers who have donated their samples to make this study possible.
Financial support. Funding for this study was provided by the National Institutes of Health (awards R01 AI150334 to RES and 5T32AI007517-20 to LP). The analysis was supported by Fogarty International Center (FIC D43TW010540 to RS), and Yale School of Medicine (AHS). The funders of the study had no role in the study design, data collection, data analysis, writing of the report, or in the decision to submit results for publication.
Potential conflicts of interest. None of the authors declare any conflict of interests: Q. S. reports support for the present manuscript from VA Connecticut Research and Education Foundation. E. M. and S. G. report being members of IRB committee at West Haven VA, recused themselves during discussion of study. R. S. reports support for the present manuscript from WHVA Research Service and serving on DSMB for Moderna for several clinical mRNA vaccine studies. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Supplementary Material
Contributor Information
Min Zhao, Department of Medicine, Division of Infectious Diseases, Yale School of Medicine, New Haven, Connecticut, USA.
Rebecca Slotkin, Yale School of Public Health, New Haven, Connecticut, USA.
Amar H Sheth, Yale School of Medicine, New Haven, Connecticut, USA.
Lauren Pischel, Department of Medicine, Division of Infectious Diseases, Yale School of Medicine, New Haven, Connecticut, USA; Yale School of Public Health, New Haven, Connecticut, USA.
Tassos C Kyriakides, Department of Veterans Affairs Office of Research and Development, Cooperative Studies Program Coordinating Center, West Haven, Connecticut, USA; Yale Center for Analytical Sciences, Yale School of Public Health, New Haven, Connecticut, USA.
Brinda Emu, Department of Medicine, Division of Infectious Diseases, Yale School of Medicine, New Haven, Connecticut, USA; Department of Medicine, Division of Infectious Diseases, Veterans Affairs Healthcare Systems of Connecticut, West Haven, Connecticut, USA.
Cynthia McNamara, Department of Medicine, Veterans Affairs Healthcare Systems of Connecticut, West Haven, Connecticut, USA; Department of Medicine, Yale School of Medicine, New Haven, Connecticut, USA.
Qiaosu Shi, Department of Medicine, Division of Infectious Diseases, Yale School of Medicine, New Haven, Connecticut, USA.
Jaden Delgobbo, Department of Medicine, Veterans Affairs Healthcare Systems of Connecticut, West Haven, Connecticut, USA; University of Connecticut, Storrs, Connecticut, USA.
Jin Xu, Department of Medicine, Veterans Affairs Healthcare Systems of Connecticut, West Haven, Connecticut, USA; Department of Medicine, Yale School of Medicine, New Haven, Connecticut, USA.
Elizabeth Marhoffer, Department of Medicine, Veterans Affairs Healthcare Systems of Connecticut, West Haven, Connecticut, USA; Department of Medicine, Yale School of Medicine, New Haven, Connecticut, USA.
Aleagia Mercer-Falkoff, Department of Medicine, Veterans Affairs Healthcare Systems of Connecticut, West Haven, Connecticut, USA; Department of Medicine, Yale School of Medicine, New Haven, Connecticut, USA.
Jürgen Holleck, Department of Medicine, Veterans Affairs Healthcare Systems of Connecticut, West Haven, Connecticut, USA; Department of Medicine, Yale School of Medicine, New Haven, Connecticut, USA.
David Ardito, Department of Medicine, Veterans Affairs Healthcare Systems of Connecticut, West Haven, Connecticut, USA.
Richard E Sutton, Department of Medicine, Division of Infectious Diseases, Yale School of Medicine, New Haven, Connecticut, USA; Department of Medicine, Division of Infectious Diseases, Veterans Affairs Healthcare Systems of Connecticut, West Haven, Connecticut, USA.
Shaili Gupta, Department of Medicine, Division of Infectious Diseases, Veterans Affairs Healthcare Systems of Connecticut, West Haven, Connecticut, USA; Department of Medicine, Veterans Affairs Healthcare Systems of Connecticut, West Haven, Connecticut, USA; Department of Medicine, Yale School of Medicine, New Haven, Connecticut, USA.
References
- 1. Johns Hopkins Coronavirus resource center. Available at: https://coronavirus.jhu.edu. Accessed 30 November 2021.
- 2. CDC . Different COVID-19 vaccines. 2021. Available at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines.html. Accessed 17 November 2021.
- 3. Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020; 586(7830):594–9. [DOI] [PubMed] [Google Scholar]
- 4. Ou X, Liu Y, Lei X, et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun 2020; 11:1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dos Santos WG. Impact of virus genetic variability and host immunity for the success of COVID-19 vaccines. Biomed Pharmacother 2021; 136:111272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Earle KA, Ambrosino DM, Fiore-Gartland A, et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 2021; 39:4423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021; 27:1205–11. [DOI] [PubMed] [Google Scholar]
- 8. Chia WN, Zhu F, Ong SWX, et al. Dynamics of SARS-CoV-2 neutralising antibody responses and duration of immunity: a longitudinal study. Lancet Microbe 2021; 2:e240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fergie J, Srivastava A. Immunity to SARS-CoV-2: lessons learned. Front Immunol 2021; 12:654165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dispinseri S, Secchi M, Pirillo MF, et al. Neutralizing antibody responses to SARS-CoV-2 in symptomatic COVID-19 is persistent and critical for survival. Nat Commun 2021; 12:2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Breton G, Mendoza P, Hägglöf T, et al. Persistent cellular immunity to SARS-CoV-2 infection. J Exp Med. 2021; 218:e20202515. doi: 10.1084/jem.20202515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cromer D, Juno JA, Khoury D, et al. Prospects for durable immune control of SARS-CoV-2 and prevention of reinfection. Nat Rev Immunol 2021; 21:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goldberg Y, Mandel M, Bar-On YM, et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med 2021; 385:e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guerrera G, Picozza M, D’Orso S, et al. BNT162b2 vaccination induces durable SARS-CoV-2-specific T cells with a stem cell memory phenotype. Sci Immunol. 2021; 6:eabl5344. doi: 10.1126/sciimmunol.abl5344. [DOI] [PubMed] [Google Scholar]
- 15. Rydyznski Moderbacher C, Ramirez SI, Dan JM, et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell 2020; 183:996–1012.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med 2021; 385:1393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. CDC . COVID-19 vaccine booster shots. Available at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html. Accessed 30 November 2021.
- 18. Herishanu Y, Avivi I, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood 2021; 137:3165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dispinseri S, Lampasona V, Secchi M, et al. Robust neutralizing antibodies to SARS-CoV-2 develop and persist in subjects with diabetes and COVID-19 pneumonia. J Clin Endocrinol Metab 2021; 106:1472–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carr EJ, Wu M, Harvey R, et al. Neutralising antibodies after COVID-19 vaccination in UK haemodialysis patients. Lancet 2021; 398:1038–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Collier DA, Ferreira IATM, Kotagiri P, et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature 2021; 596:417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. National Institute of Diabetes and Digestive and Kidney Diseases . CKD-EPI adults (conventional units). National Institute of Diabetes and Digestive and Kidney Diseases. U.S. Department of Health and Human Services. Available at: https://www.niddk.nih.gov/health-information/professionals/clinical-tools-patient-management/kidney-disease/laboratory-evaluation/glomerular-filtration-rate-calculators/ckd-epi-adults-conventional-units. Accessed 30 November 2021. [Google Scholar]
- 23. Zhao M, Su P-Y, Castro DA, et al. Rapid, reliable, and reproducible cell fusion assay to quantify SARS-Cov-2 spike interaction with hACE2. PLoS Pathog 2021; 17:e1009683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zaccaro DJ, Wagener DK, Whisnant CC, Staats HF. Evaluation of vaccine-induced antibody responses: impact of new technologies. Vaccine 2013; 31:2756–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Seabold S, Perktold J. Statsmodels: econometric and statistical modeling with python. In: Proceeding of the 9th Python in Science Conference (SCIPY 2010). Available at: http://conference.scipy.org/proceedings/scipy2010/pdfs/seabold.pdf. Accessed 30 November 2021. [Google Scholar]
- 26. Zhang Z. Variable selection with stepwise and best subset approaches. Ann Transl Med 2016; 4:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Venables B, Ripley B. Modern applied statistics with S. Fourth edition. New York: Springer; 2002. [Google Scholar]
- 28. Bienvenu LA, Noonan J, Wang X, Peter K. Higher mortality of COVID-19 in males: sex differences in immune response and cardiovascular comorbidities. Cardiovasc Res 2020; 116:2197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. King JT Jr, Yoon JS, Rentsch CT, et al. Development and validation of a 30-day mortality index based on pre-existing medical administrative data from 13,323 COVID-19 patients: the Veterans Health Administration COVID-19 (VACO) Index. PLoS One 2020; 15:e0241825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shen X. Boosting immunity to Omicron. Nat Med 2022; 28:445–6. [DOI] [PubMed] [Google Scholar]
- 31. Garcia-Beltran WF, St Denis KJ, Hoelzemer A, et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell 2022; 185:457–466.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guven DC, Sahin TK, Kilickap S, Uckun FM. Antibody responses to COVID-19 vaccination in cancer: a systematic review. Front Oncol 2021; 11:759108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tran S, Truong TH, Narendran A. Evaluation of COVID-19 vaccine response in patients with cancer: an interim analysis. Eur J Cancer 2021; 159:259–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rössler A, Riepler L, Bante D, von Laer D, Kimpel J. SARS-CoV-2 Omicron variant neutralization in serum from vaccinated and convalescent persons. N Engl J Med 2022; 386:698–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Crotty S. Hybrid immunity. Science 2021; 372:1392–3. [Google Scholar]
- 36. Goel RR, Painter MM, Apostolidis SA, et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science 2021; 374:abm0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bar-On YM, Goldberg Y, Mandel M, et al. Protection by a fourth dose of BNT162b2 against Omicron in Israel. N Engl J Med 2022; 386:1712–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.