Abstract
Background
Observable symptoms of Bell’s palsy following vaccinations arouse concern over the safety profiles of novel coronavirus disease 2019 (COVID-19) vaccines. However, there are only inconclusive findings on Bell’s palsy following messenger (mRNA) COVID-19 vaccination. This study aims to update the previous analyses on the risk of Bell’s palsy following mRNA (BNT162b2) COVID-19 vaccination.
Methods
This study included cases aged ≥16 years with a new diagnosis of Bell’s palsy within 28 days after BNT162b2 vaccinations from the population-based electronic health records in Hong Kong. Nested case-control and self-controlled case series (SCCS) analyses were used, where the association between Bell’s palsy and BNT162b2 was evaluated using conditional logistic and Poisson regression, respectively.
Results
Totally 54 individuals were newly diagnosed with Bell’s palsy after BNT162b2 vaccinations. The incidence of Bell’s palsy was 1.58 (95% confidence interval [CI], 1.19–2.07) per 100 000 doses administered. The nested case-control analysis showed significant association between BNT162b2 vaccinations and Bell’s palsy (adjusted odds ratio [aOR], 1.543; 95% CI, 1.123–2.121), with up to 1.112 excess events per 100 000 people who received 2 doses of BNT162b2. An increased risk of Bell’s palsy was observed during the first 14 days after the second dose of BNT162b2 in both nested case-control (aOR, 2.325; 95% CI, 1.414–3.821) and SCCS analysis (adjusted incidence rate ratio, 2.44; 95% CI, 1.32–4.50).
Conclusions
There was an overall increased risk of Bell’s palsy following BNT162b2 vaccination, particularly within the first 14 days after the second dose, but the absolute risk was very low.
Keywords: Bell’s palsy, COVID-19, vaccination, safety, BNT162b2
There was an overall increased risk of Bell’s palsy following BNT162b2 vaccination, particularly within the first 14 days after the second dose, but absolute risk was low. The benefits from coronavirus disease 2019 vaccination still outweigh the small risk of Bell’s palsy.
Our previous local study revealed an overall increased risk of Bell’s palsy associated with CoronaVac vaccinations (odds ratio [OR], 2.39; 95% confidence interval [CI], 1.42–4.02) but a nonsignificantly increased risk after messenger RNA (mRNA) BNT162b2 vaccination (OR, 1.76; 95% CI, .89–3.48), which may be attributed to the insufficient number of events reported [1]. Despite its rare occurrence, observable symptoms of Bell’s palsy following vaccinations may arouse concern over the safety profiles of novel coronavirus disease 2019 (COVID-19) vaccines in the general public, devaluing their motivation to take up the COVID-19 vaccines. Currently, the clinical trial of BNT162b2 reported imbalanced incidence of Bell’s palsy between vaccinated and control groups (4 cases of Bell’s palsy in the vaccinated group and none in the control group) [2]. There are only a few studies on Bell’s palsy following mRNA COVID-19 vaccination, including observational studies and ecological studies [1,3–9]. The findings from those studies remain inconclusive. Hence, the actual risk of Bell’s palsy after mRNA COVID-19 vaccinations remains unclear. Several international organizations have recommended the monitoring of mRNA COVID-19 vaccine recipients for Bell’s palsy [2,10,11]. More importantly, none of these studies have evaluated the impact of the first and second doses on the risk of Bell’s palsy. A retrospective cohort study showed a nonsignificant increased risk of Bell’s palsy associated with BNT162b2 vaccination (risk ratio, 1.32; 95% CI, .92–1.86), but suggested that the association of Bell’s palsy may be linked to the increased risk of herpes zoster infection after BNT162b2 vaccination (risk ratio, 1.43; 95% CI, 1.20–1.73) [8]. As suggested in a commentary, the controversial findings on the association between Bell’s palsy and various types of COVID-19 vaccines requires more comprehensive evidence on the safety of COVID-19 vaccines [12].
In this study, our aim was to update our previous analysis on the risk of Bell’s palsy following BNT162b2 vaccination [1] by extending the inclusion period from 4 May 2021 to 31 July 2021.
METHODS
Study Design and Data Sources
Two study designs, including nested case-control and self-controlled case series (SCCS), were applied in this study. We analyzed the data in the clinical management system from the Hospital Authority (HA), which was used to conduct the study on the risk of Bell’s palsy after COVID-19 vaccinations and other COVID-19 pharmacovigilance studies [1,13–26]. Currently, the COVID-19 vaccination program led by the Government of Hong Kong Special Administrative Region (HKSAR) authorized BNT162b2 vaccine (from Fosun–BioNTech; equivalent to Pfizer–BioNTech; Mainz, Germany) and CoronaVac vaccine for emergency use. Individuals can freely choose BNT162b2 or CoronaVac as their first dose and are restricted to receive the same vaccine as their second dose except if there is a clinical reason approved by a clinician, for example, anaphylaxis. The rollout schedule of the vaccination program since 6 March 2021 is shown in Supplementary Table 1. Vaccination status was ascertained by linking data from the clinical management system to COVID-19 vaccination records from the Department of Health (DH), the Government of HKSAR. Incident Bell’s palsy was defined as the first diagnosis in the database using the International Classification of Diseases, Ninth Revision, Clinical Modification, codes 351.0, 351.8, and 351.9.
Nested Case-Control Analysis
Cases were defined as patients with a first primary diagnosis of Bell’s palsy in the emergency room or inpatient setting between 6 March 2021 and 31 July 2021. Patients who attended HA emergency rooms or were hospitalized during the same period without being diagnosed with Bell’s palsy were selected as controls. Patients aged <16 years, those with a history of Bell’s palsy, or those vaccinated with CoronaVac were excluded from the analysis. Up to 10 controls were randomly matched with each case according to sex, age, Charlson comorbidity index, and date of emergency room attendance or hospitalization. Vaccine recipients were defined as patients who received the first or second dose of vaccine on or 28 days before the date of first diagnosis of Bell’s palsy for cases and the date of the hospitalization or emergency room visit for controls.
Self-Controlled Case Series Analysis
The SCCS has been applied in several studies of COVID-19 vaccine safety [27–32]. Individuals who were aged ≥16 years and had at least 1 episode of Bell’s palsy in the emergency room or as a primary diagnosis in the hospital admission records between 6 March 2021 and 31 July 2021 were included in the analysis. Patients vaccinated with CoronaVac during the observation period were excluded. To satisfy the assumptions of SCCS, we applied extension of the SCCS model including unvaccinated individuals with Bell’s palsy and considered first incidence of Bell's palsy during the observation period. Details are provided in the Supplementary material.
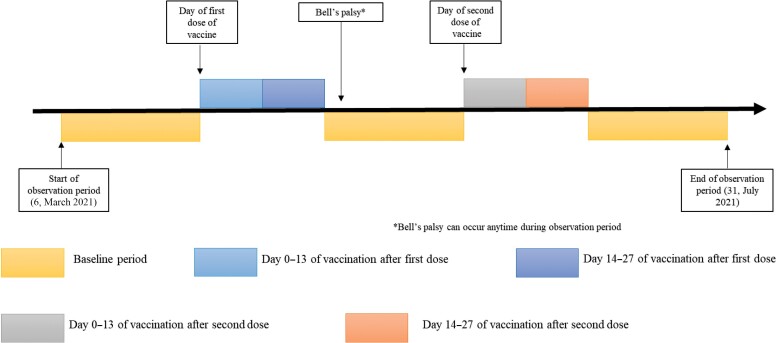
The individual observation period started on 6 March 2021 and ended on 31 July 2021. We divided the observation period into 5 discrete periods: day 0–13 after the first dose, day 14–27 after the first dose (ie, day 14 to day 27 for people with single dose only; day 14 to the day before the second dose but up to 27 days for people with 2 doses of vaccine), day 0–13 after second dose, day 14–27 after second dose, and baseline period (ie, any other periods that do not fall into the above risk windows). A graphic representation of the study design timeline for a single hypothetical participant is provided in Figure 1. Some eligible individuals did not receive the second dose of the vaccine within the observation period so the time after risk window 2 was considered to be the baseline period.
Figure 1.
Illustration of the study design timeline for a hypothetical patient in the self-controlled case series analysis.
A total of 88 matched sets (1 case and 10 controls) and 71 cases was required for the nested case-control and SCCS analyses, respectively. A detailed sample size calculation for nested case-control and SCCS analyses is provided in Supplementary Figures 1 and 2.
Statistical Analyses
Nested Case-Control Analysis
Conditional logistic regression was applied to estimate the association between the vaccine and risk of Bell’s palsy, with adjustment for patient characteristics, including smoking status, medical history (diabetes mellitus, hypertension, asthma, neoplasms, acute respiratory infections, viral infections, rheumatoid arthritis, stroke or systemic embolism, Guillain-Barré syndrome, migraine, and herpes zoster infection), and medication use in the past 90 days (antiviral drugs, systemic corticosteroids, antibacterial drugs, immunosuppressants, lipid-lowering agents). Adjusted ORs (aORs) and 95% CIs were calculated to quantify the risk of Bell’s palsy. Events before and after the second dose were individually analyzed in 2 additional analyses. Subgroup analysis was conducted according to sex (male and female) and age group (<60 years, ≥60 years).
Self-Controlled Case Series Analysis
SCCS extension for event-dependent exposure was applied using the R function eventdepenexp in the R-package SCCS [33]. Season of the year was adjusted in monthly categories. The adjusted incidence rate ratio (IRR) and its corresponding 95% CI were estimated by comparing the rates of Bell’s palsy in different risk windows with that in the baseline period using conditional Poisson regression.
In both nested case-control and SCCS analyses, we conducted a sensitivity analysis by excluding patients with a subsequent diagnosis of stroke or Ramsay Hunt syndrome after incident diagnosis of Bell’s palsy to avoid any potential risk of misdiagnosis of Bell’s palsy. As suggested in a recent study, herpes zoster infection is one of the potential causes of facial-nerve palsy [8]. Hence, another sensitivity analysis was conducted by excluding patients with a history of herpes zoster infection and patients who were prescribed antiviral medications in the 90 days prior to the incident diagnosis of Bell’s palsy. Finally, we also excluded patients with COVID-19 infection during the observation period in order to minimize the effect of COVID-19 infection. Post hoc analysis by extending the risk periods to 42 days in order to investigate the risk of Bell’s palsy in the 28–41 days after vaccination was performed.
All statistical tests were 2-sided and P values < .05 were considered significant. Statistical analysis was conducted using R version 4.0.3. At least 2 investigators (E. Y. F. W., V. W. S. N., Y. W., V. K. C. Y., M. F.) independently did the statistical analyses for each study design for quality assurance.
Ethical Approval
The University of Hong Kong/Hospital Authority Hong Kong West Cluster Institutional Review Board and the DH Ethics Committee provided ethical approval for the study.
RESULTS
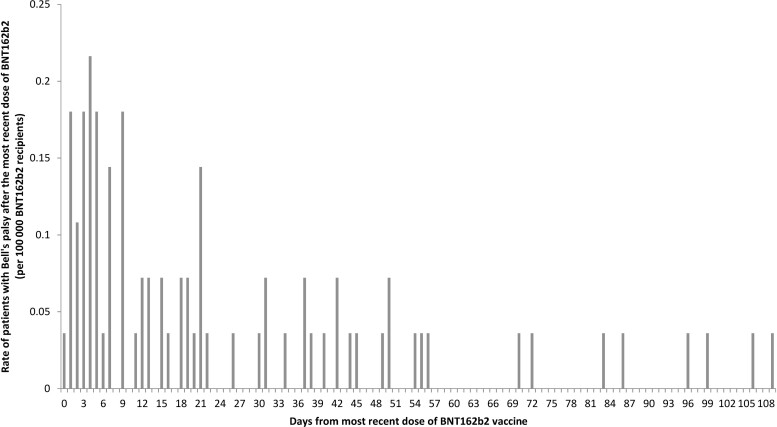
After excluding 45 people with different vaccine types between first and second doses, 1655 people with a second-dose record but without a first-dose record, and 16 people with inconsistent duplicated records, 1 957 612 individuals were recorded to have received their first dose of BNT162b2 between 6 March 2021 and 31 July 2021. Among them, 1 451 858 (74.2%) received both doses of BNT162b2. A total of 3 409 470 doses of BNT162b2 were administered. The number of first diagnosis of Bell’s palsy within 28 days after the first or second dose of BNT162b2 vaccine was 54 of 80 cases (67.5%) after vaccination (Figure 2). Hence, the incidence of Bell’s palsy was 1.58 (95% CI, 1.19–2.07) per 100 000 doses of BNT162b2 vaccine administered.
Figure 2.
Distribution of the time of onset of Bell’s palsy after the most recent dose of BNT162b2 vaccine.
Nested Case-Control Analysis
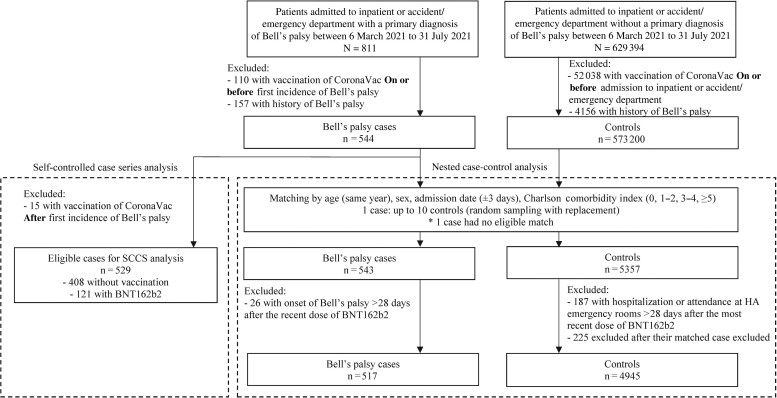
The selection flow for cases and matched controls is summarized in Figure 3. A total of 517 cases and 4945 matched controls were identified. The characteristics of cases and controls by vaccine exposure are listed in Supplementary Tables 2 and 3. There were 54 cases and 348 controls who received BNT162b2. There were significant positive associations between vaccination group and Bell’s palsy (aOR, 1.543; 95% CI, 1.123–2.121; Table 1). Findings from the additional analyses demonstrated a significantly increased risk during the first 14 days following the second dose (aOR, 2.325; 95% CI, 1.414–3.821) of BNT162b2 vaccine. However, the risk of Bell’s palsy was not significantly increased in the 0–13 and 14–27 days after the first dose and in the 14–27 days after the second dose of BNT162b2 vaccine. Results from the subgroup and sensitivity analyses showed similar findings (see Supplementary Tables 4–7).
Figure 3.
Flow chart of inclusion and exclusion criteria in the nested case-control analysis and SCCS analysis. Abbreviations: HA, Hospital Authority; SCCS, self-controlled case series.
Table 1.
Risk of Bell’s Palsy Among Patients in the Nested Case-Control Analysis
| Vaccination | Case (N = 517) | Control (N = 4945) | Crude Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI) |
|---|---|---|---|---|
| Total | ||||
| Not vaccinated | 463 | 4597 | ||
| BNT162b2 | 54 | 348 | 1.559 (1.136–2.141) | 1.543 (1.123–2.121) |
| Additional analysis | ||||
| Events after first dose and before second dose | ||||
| 0–13 days | ||||
| Not vaccinated | 463 | 4292 | ||
| BNT162b2 | 18 | 104 | 1.491 (.889–2.5) | 1.471 (.876–2.468) |
| 14–27 days | ||||
| Not vaccinated | 463 | 4222 | ||
| BNT162b2 | 8 | 49 | 1.382 (.647–2.953) | 1.354 (.634–2.891) |
| Events after second dose | ||||
| 0–13 days | ||||
| Not vaccinated | 463 | 4337 | ||
| BNT162b2 | 22 | 91 | 2.315 (1.411–3.799) | 2.325 (1.414–3.821) |
| 14–27 days | ||||
| Not vaccinated | 463 | 4205 | ||
| BNT162b2 | 6 | 56 | 0.884 (.373–2.097) | 0.871 (.366–2.073) |
Abbreviation: CI, confidence interval.
Cases and controls were matched according to age, sex, admission date, and Charlson comorbidity index. Odds ratios for Bell’s palsy events were estimated using conditional logistic regression adjusted for smoking status, medical history (diabetes mellitus, hypertension, asthma, neoplasms, acute respiratory infections, viral infections, rheumatoid arthritis, stroke or systemic embolism, Guillain-Barré syndrome, migraine, and herpes zoster) and medication use in the past 90 days (antiviral drugs, systemic corticosteroids, antibacterial drugs, immunosuppressants, lipid-lowering agents).
Self-Controlled Case Series Analysis
A total of 529 patients who had their incident primary diagnosis of Bell’s palsy either at the inpatient or emergency setting were included in the analysis (Figure 3). The patients’ demographics and baseline comorbidities are summarized in Supplementary Table 8.
Compared with the baseline period, the risk of Bell’s palsy was approximately 2.4-fold higher during the first 14 days after the second dose of BNT162b2 vaccine (IRR, 2.44; 95% CI, 1.32–4.50), while no association was found in other risk windows. The details of the results are illustrated in Table 2. Results of the sensitivity analyses remained robust and did not change the overall findings (Supplementary Table 9).
Table 2.
Results of Self-Controlled Case Series Analysis
| Risk Periods | Number of Events | Person-Years | Incidence Rate Ratioa (95% Confidence Interval) |
|---|---|---|---|
| Extension for the self-controlled case series model: event-dependent exposure | |||
| BNT162b2 (n = 529) | |||
| 0–13 days after first dose | 18 | 4.51 | 1.21 (.66–2.20) |
| 14–27 days after first dose | 8 | 2.74 | 1.18 (.49–2.83) |
| 0–13 days after second dose | 22 | 3.00 | 2.44 (1.32–4.50) |
| 14–27 days after second dose | 6 | 2.52 | 0.97 (.37–2.58) |
| Baseline | 475 | 201.58 | … |
Incidence rate ratio was determined using conditional Poisson regression and adjusted with seasonal effect.
Post Hoc Analysis
The nested case-control analysis showed an insignificant risk of Bell’s palsy 28–41 days after the first or second dose (Supplementary Table 10). The SCCS analysis showed a significant risk of Bell’s palsy 28–41 days after the first dose (IRR, 13.54; 95% CI, 1.93–94.87) but not after the second dose (Supplementary Table 11). The reason is clear that 95% of vaccine recipients received a second dose within 28 days after the first dose in the current study. Since only 1 event occurred in this period, it was sufficient to produce a significant result due to a very short follow-up time (0.64 person-years in 28–41 days after the first dose and without the second dose).
DISCUSSION
The risk of Bell’s palsy was found to increase following BNT162b2 vaccination using both between- and within-individual comparison study designs. Based on the incidence of Bell’s palsy of 3.16 (2 × 1.58) per 100 000 people vaccinated with 2 doses and the relative risks of 1.543 in nested case-control analysis, there is an excess 1.112 events per 100 000 people receiving 2 doses of BNT162b2. To the best of our knowledge, we are the first to investigate the risk of Bell’s palsy following the first and second doses of BNT162b2 vaccine. We observed a higher risk in the first 14 days after the second dose compared with the other risk period using 2 methods. Although the booster dose of COVID-19 vaccines have already been rolled out to increase the levels of immunity against new variants of COVID-19 in several countries, there are still uncertainties as to whether the booster dose would increase the risk and thus create public health concerns. It is important to conduct additional studies to evaluate the risk of Bell’s palsy before vaccination with a booster dose is fully implemented in the general public.
While the randomized, controlled trials of BNT162b2 showed a higher incidence of Bell’s palsy in the vaccinated group compared with the control group and did not yield statistical significant results, the US Food and Drug Administration (FDA) stated that the frequency of the observed incident was similar to the background rate [2]. However, the result was not adjusted for the follow-up time, and thus the conclusion is debatable [4]. Nevertheless, the FDA did not exclude the possibility of a causal relationship and urged additional study on the risk of Bell’s palsy after vaccination in larger populations [2]. Randomized, controlled trials often recruit relatively healthy individuals due to stringent inclusion criteria, and thus findings may not be fully generalizable to more diverse populations. Furthermore, the limited size of the study population may not provide sufficient power to reveal outcomes of rare occurrence. Although a disproportionality analysis using the World Health Organization Pharmacovigilance Database later revealed that the reporting rate of facial paralysis incidence after mRNA COVID-19 vaccination was not higher than that for other viral vaccines [6], previous studies have shown a positive association between Bell’s palsy and some viral vaccines such as parenteral seasonal influenza vaccines and the influenza A (H1N1) inactivated monovalent pandemic vaccine [34,35]. In addition, this database relied on self-reported cases by healthcare professionals and the public and it lacked the capacity to take other confounding risk factors into account. Hence, this indirect evidence may not conclude the risk of Bell’s palsy between people with and without mRNA COVID-19 vaccination. Another small nested case-control study that was conducted in Israel on 37 patients who developed Bell’s palsy following vaccinations (21 received the BNT162b2 vaccine) with 74 matched controls (44 received the BNT162b2 vaccine) also showed no association between Bell’s palsy and BNT162b2 [36]. An additional cohort study that used an Israeli population reported a nonsignificant increased risk of Bell’s palsy after BNT162b2 vaccination with a risk ratio of 1.32 (95% CI, .92–1.86); however, they acknowledged that the results may be limited by confounding and selection bias [8]. Another cohort study in the United States showed no association (adjusted rate ratio, 1.00; 95% CI, .86–1.17) between the risk of Bell’s palsy and mRNA vaccines (BNT162b2/mRNA-1273) [9], but that study was limited by low statistical power [9]. The SCCS analysis performed in our study can eliminate both measured and unmeasured time-invariant confounding factors, such as family history, genetic factors, and underlying disease severity. Moreover, our study, with larger sample size and number of events, possessed sufficient power to detect the lowest OR of 1.3 in nested case-control analysis and the lowest IRR of 2.0 in SCCS analysis. Indeed, a previous ecological analysis deduced a similar relative risk of 1.5- to 3-times higher for COVID-19 mRNA vaccines associated with the risk of Bell’s palsy compared with our estimation [3,4]. Given the similar relative risk and trend in dose analysis from different study designs, results from our study should provide robust evidence.
The exact pathophysiology of Bell’s palsy remains unclear. It has been speculated that the cause may be due to a combination of factors including autoimmune reaction and viral and innate immune activation [1]. An evaluation of COVID-19 vaccine immunogenicity in Hong Kong showed that the antibody concentrations increased substantially following both the first and second doses of BNT162b2 [37]. Such drastic increase in antibody concentrations caused by triggering of the immune system may potentially lead to the development of Bell’s palsy. A recent cohort study in Israel suggested that BNT162b2 increased the risk of herpes zoster infection, which is a potential cause of facial-nerve palsy [8]. However, in our study, no patient was diagnosed with herpes zoster infection between the date of the first dose and the date of the incident diagnosis of Bell’s palsy. By excluding patients with a history of herpes zoster and those who were previously prescribed antiviral drugs, the results of the sensitivity analysis were similar to those of the primary analyses in both study designs. Hence, herpes zoster cannot explain the association between Bell’s palsy and BNT162b2 vaccines in our study.
There are several limitations of this study that need to be acknowledged. The HA clinical management system only captures patients who have used HA services, for example, public hospitals and emergency rooms. However, HA covers more than 70% of hospitalizations. In addition, HA is the major healthcare service provider of affordable care, and thus the vast majority of patients were likely to be included in our study. In addition to the database limitation, there are some limitations of the study designs. First, the control group in a nested case-control analysis may be misclassified and potentially bias the results. However, the incidence of Bell’s palsy with the estimation of approximately 27 new cases per 100 000 person-years in Hong Kong is relatively low [1]; therefore, the chance of misclassification in the control group should be minimal and would not affect our estimates. Second, like other observational studies that used between-individual comparisons, there might be some important unmeasured confounders (eg, socioeconomic status and educational level) that could bias the results. However, as a within-individual comparison, SCCS can eliminate both measured and unmeasured time-invariant confounding factors; the results from both study designs are similar and support the robustness of the results. Last, the arrangement of a booster dose of COVID-19 vaccine had not been rolled out during the inclusion period in this study. Also, our study included only patients with a new diagnosis of Bell’s palsy in Hong Kong. Moreover, due to the short observation period, a seasonal association could not be evaluated. Hence, additional studies that include a booster dose of vaccine and patients with a history of Bell’s palsy, that have a longer observational period, and that are performed other populations with different ethnicities are warranted to confirm the generalizability of our findings.
CONCLUSIONS
In this study, we found an overall increased risk of Bell’s palsy following BNT162b2 vaccinations, particular in the first 14 days after vaccination. It appears that herpes zoster infection cannot explain the association between Bell’s palsy and COVID-19 vaccinations in our study. As Bell’s palsy is a rare and largely self-limiting adverse event, the benefits from COVID-19 vaccination still outweigh the small risk of Bell’s palsy. However, the adverse events related to COVID-19 vaccines should be continuously monitored.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Eric Yuk Fai Wan, Centre for Safe Medication Practice and Research, Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong Special Administrative Region, China; Department of Family Medicine and Primary Care, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong Special Administrative Region, China; Laboratory of Data Discovery for Health, Hong Kong Science and Technology Park, Sha Tin, Hong Kong Special Administrative Region, China.
Celine Sze Ling Chui, Laboratory of Data Discovery for Health, Hong Kong Science and Technology Park, Sha Tin, Hong Kong Special Administrative Region, China; School of Nursing, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong Special Administrative Region, Hong Kong, China; School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong Special Administrative Region, China.
Vanessa Wai Sei Ng, Centre for Safe Medication Practice and Research, Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong Special Administrative Region, China.
Yuan Wang, Department of Family Medicine and Primary Care, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong Special Administrative Region, China.
Vincent Ka Chun Yan, Centre for Safe Medication Practice and Research, Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong Special Administrative Region, China.
Ivan Chun Hang Lam, Centre for Safe Medication Practice and Research, Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong Special Administrative Region, China.
Min Fan, Centre for Safe Medication Practice and Research, Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong Special Administrative Region, China.
Francisco Tsz Tsun Lai, Centre for Safe Medication Practice and Research, Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong Special Administrative Region, China; Laboratory of Data Discovery for Health, Hong Kong Science and Technology Park, Sha Tin, Hong Kong Special Administrative Region, China.
Esther Wai Yin Chan, Centre for Safe Medication Practice and Research, Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong Special Administrative Region, China; Laboratory of Data Discovery for Health, Hong Kong Science and Technology Park, Sha Tin, Hong Kong Special Administrative Region, China.
Xue Li, Centre for Safe Medication Practice and Research, Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong Special Administrative Region, China; Laboratory of Data Discovery for Health, Hong Kong Science and Technology Park, Sha Tin, Hong Kong Special Administrative Region, China; Department of Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong Special Administrative Region, China.
Carlos King Ho Wong, Centre for Safe Medication Practice and Research, Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong Special Administrative Region, China; Department of Family Medicine and Primary Care, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong Special Administrative Region, China; Laboratory of Data Discovery for Health, Hong Kong Science and Technology Park, Sha Tin, Hong Kong Special Administrative Region, China.
Raccoon Ka Cheong Chung, Department of Health, Government of the Hong Kong Special Administrative Region, Hong Kong Special Administrative Region, China.
Benjamin John Cowling, Laboratory of Data Discovery for Health, Hong Kong Science and Technology Park, Sha Tin, Hong Kong Special Administrative Region, China; School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong Special Administrative Region, China.
Wing Chi Fong, Department of Medicine, Queen Elizabeth Hospital, Hospital Authority, Hong Kong Special Administrative Region, China; Expert Committee on Clinical Events Assessment Following COVID-19 Immunization, Department of Health, Government of the Hong Kong Special Administrative Region, Hong Kong Special Administrative Region, China.
Alexander Yuk Lun Lau, Department of Medicine and Therapeutics, Faculty of Medicine, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China.
Vincent Chung Tong Mok, Expert Committee on Clinical Events Assessment Following COVID-19 Immunization, Department of Health, Government of the Hong Kong Special Administrative Region, Hong Kong Special Administrative Region, China; Department of Medicine and Therapeutics, Faculty of Medicine, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China.
Frank Ling Fung Chan, Department of Health, Government of the Hong Kong Special Administrative Region, Hong Kong Special Administrative Region, China.
Cheuk Kwong Lee, Expert Committee on Clinical Events Assessment Following COVID-19 Immunization, Department of Health, Government of the Hong Kong Special Administrative Region, Hong Kong Special Administrative Region, China; Hong Kong Red Cross Blood Transfusion Service, Hospital Authority, Hong Kong, China.
Lot Sze Tao Chan, Department of Health, Government of the Hong Kong Special Administrative Region, Hong Kong Special Administrative Region, China.
Dawin Lo, Department of Health, Government of the Hong Kong Special Administrative Region, Hong Kong Special Administrative Region, China.
Kui Kai Lau, Department of Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong Special Administrative Region, China; State Key Laboratory of Brain and Cognitive Sciences, The University of Hong Kong, Hong Kong Special Administrative Region, China.
Ivan Fan Ngai Hung, Department of Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong Special Administrative Region, China; Expert Committee on Clinical Events Assessment Following COVID-19 Immunization, Department of Health, Government of the Hong Kong Special Administrative Region, Hong Kong Special Administrative Region, China.
Chak Sing Lau, Division of Rheumatology and Clinical Immunology, Department of Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong Special Administrative Region, China.
Gabriel Matthew Leung, Laboratory of Data Discovery for Health, Hong Kong Science and Technology Park, Sha Tin, Hong Kong Special Administrative Region, China; School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong Special Administrative Region, China.
Ian Chi Kei Wong, Centre for Safe Medication Practice and Research, Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong Special Administrative Region, China; Laboratory of Data Discovery for Health, Hong Kong Science and Technology Park, Sha Tin, Hong Kong Special Administrative Region, China; Expert Committee on Clinical Events Assessment Following COVID-19 Immunization, Department of Health, Government of the Hong Kong Special Administrative Region, Hong Kong Special Administrative Region, China; Research Department of Practice and Policy, School of Pharmacy, University College London, London, United Kingdom.
Notes
Author contributions. E. Y. F. W., C. S. L. C., and I. C. K. W. had the original idea for the study, contributed to the development of the study, extracted data from the source database, constructed the study design and the statistical model, reviewed the literature, and acted as guarantors for the study. E. Y. F. W., V. W. S. N., Y. W., V. K. C. Y., and M. F. conducted statistical analyses. E. Y. F. W., V. W. S. N., and I. C. K. W. wrote the first draft of the manuscript. E. Y. F. W., C. S. L. C., V. W. S. N., Y. W., and V. K. C. Y. extracted data from the source database and validated case reports and the diagnosis codes from the database. I. C. K. W. is the principal investigator and provided oversight for all aspects of the project. V. W. S. N., I. C. H. L., Y. W., V. K. C. Y., M. F., F. T. T. L., E. W. Y. C., X. L., C. K. H. W., R. K. C. C., B. J. C., W. C. F., A. Y. L. L., C. K. L., L. S. T. C., D. L., K. K. L., I. F. N. H., C. S. L., and G. M. L. provided critical input to the analyses, design, and discussion. All authors contributed to the interpretation of the analyses, critically reviewed and revised the manuscript, and approved the final manuscript as submitted.
Acknowledgments. The authors thank the members of the Committee on Clinical Events Assessment Following Covid-19 Immunization for case assessment, colleagues from the Drug Office of the Department of Health, the Hospital Authority for providing vaccination and clinical data, and Dr Yonas Weldeselassie for statistical advice in the self-controlled case series analysis.
Disclaimer. The funder of the study had no role in study design, data collection, data analyses, data interpretation, or writing of the manuscript. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
Financial support. The project was funded by a research grant from the Food and Health Bureau, the Government of the HKSAR (ref. COVID19F01). F. T. T. L.’s and I. C. K. W.’s posts were partly funded by Data Discovery for Health; hence, this work was partly supported by AIR@InnoHK administered by the Innovation and Technology Commission.
Potential conflicts of interest. E. Y. F. W. has received research grants from the Food and Health Bureau of the Government of the HKSAR and the Hong Kong Research Grants Council (RGC), outside the submitted work. C. S. L. C. has received grants from the Food and Health Bureau of the Hong Kong Government, Hong Kong RGC, Hong Kong Innovation and Technology Commission, Pfizer, IQVIA, and Amgen (all paid to institution) and a personal fee from Primevigilance Ltd, outside the submitted work. E. W. Y. C. reports honorarium from the Hospital Authority and grants from the Hong Kong RGC, Research Fund Secretariat of the Food and Health Bureau, National Natural Science Fund of China, Wellcome Trust, Bayer, Bristol-Myers Squibb, Pfizer, Janssen, Amgen, Takeda, and the Narcotics Division of the Security Bureau of HKSAR, outside the submitted work (no payments from Wellcome Trust, all other payments to institution). F. T. T. L. has been supported by the RGC Postdoctoral Fellowship under the Hong Kong RGC (paid to institution Chinese University of Hong Kong) and has received research grants from the Food and Health Bureau of the Government of the HKSAR, outside the submitted work. X. L. has received research grants from the Food and Health Bureau of the Government of the HKSAR, RGC Early Career Scheme, and RGC Research Matching Grant Scheme; research and educational grants from Janssen and Pfizer (all paid to institution); internal funding from the University of Hong Kong; a consultancy fee from Merck Sharp & Dohme, paid to author and unrelated to this work; and payment to author for lectures, presentations, speakers bureaus, manuscript writing, and educational events from Pfizer. K. K. L. received grants from the Research Fund Secretariat of the Food and Health Bureau, Innovation and Technology Bureau, RGC, Amgen, Boehringer Ingelheim, Eisai, and Pfizer and consultation fees from Amgen, Boehringer Ingelheim, Daiichi Sankyo, and Sanofi, all outside the submitted work. B. J. C. received consulting fees paid to author from AstraZeneca, Fosun Pharma, GSK, Moderna, Pfizer, Roche, and Sanofi Pasteur. I. F. N. H. received speaker fees from MSD for the COVID-19 Regional Expert Input Forum 2021 and Herpes Zoster Lecture 2021. I. C. K. W. reports research funding outside the submitted work from Amgen, Bristol-Myers Squibb, Pfizer, Janssen, Bayer, GSK, Novartis, the Hong Kong RGC, the Hong Kong Health and Medical Research Fund, the National Institute for Health Research in England, European Commission, National Health and Medical Research Council in Australia, Narcotics Division of the Security Bureau of HKSAR, and the Wellcome Trust; received speaker fees paid to author from Janssen, Medice, and Amgen; and is an independent nonexecutive director of Jacobson Medical in Hong Kong. C. K. H. W. has received grants or contracts paid to institution from Health and Medical Research Fund, Food and Health Bureau, HKSAR Government, General Research Fund, RGC, HKSAR Government, and Euroqol Research Foundation. All remaining authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Reference
- 1. Wan EYF, Chui CSL, Lai FTT, et al. Bell's palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect Dis 2022; 21:00451–5. doi: 10.1016/S1473-3099(21)00451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vaccines and Related Biological Products Advisory Committee . Vaccines and Related Biological Products Advisory Committee December 10, 2020, meeting: FDA Review of Efficacy and Safety of Pfizer-BioNTech COVID-19 Vaccine Emergency Use Authorization Request. Available at: https://www.fda.gov/media/144337/download. Accessed 1 January 2022.
- 3. Cirillo N, Doan R. Bell's palsy and SARS-CoV-2 vaccines—an unfolding story. Lancet Infect Dis 2021; 21:1210–1. doi: 10.1016/S1473-3099(21)00273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ozonoff A, Nanishi E, Levy O. Bell's palsy and SARS-CoV-2 vaccines. Lancet Infect Dis 2021; 21:450–2. doi: 10.1016/S1473-3099(21)00076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383:2603–15. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Renoud L, Khouri C, Revol B, et al. Association of facial Paralysis with mRNA COVID-19 vaccines: a disproportionality analysis using the World Health Organization pharmacovigilance database. JAMA Intern Med 2021; 181:1243–5. doi: 10.1001/jamainternmed.2021.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shemer A, Pras E, Einan-Lifshitz A, et al. Association of COVID-19 vaccination and facial nerve palsy: a case-control study. JAMA Otolaryngol Head Neck Surg 2021; 147:739–43. doi: 10.1001/jamaoto.2021.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barda N, Dagan N, Ben-Shlomo Y, et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med 2021; 385:1078–90. doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Klein NP, Lewis N, Goddard K, et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA 2021; 326(14):1390–9. doi: 10.1001/jama.2021.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization . Interim recommendations for use of the inactivated COVID-19 vaccine, CoronaVac, developed by Sinovac. Available at: https://apps.who.int/iris/bitstream/handle/10665/341454/WHO-2019-nCoV-vaccines-SAGE-recommendation-Sinovac-CoronaVac-2021.1-eng.pdf. Accessed 12 September 2021.
- 11. Medicines and Healthcare Products Regulatory Agency, UK. Coronavirus vaccine—weekly summary of Yellow Card reporting. Available at: https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting. Accessed 12 September 2021.
- 12. Cirillo N, Doan R. The association between COVID-19 vaccination and Bell's palsy. Lancet Infect Dis 2021; 22:5–6. doi: 10.1016/S1473-3099(21)00467-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li X, Tong X, Yeung WWY, et al. Two-dose COVID-19 vaccination and possible arthritis flare among patients with rheumatoid arthritis in Hong Kong. Ann Rheum Dis 2022; 81:564–8. doi: 10.1136/annrheumdis-2021-221571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lai FTT, Li X, Peng K, et al. Carditis after COVID-19 vaccination with a messenger RNA vaccine and an inactivated virus vaccine: a case-control study. Ann Intern Med 2022; 175:362–70. doi: 10.7326/M21-3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blais JE, Wei Y, Chui CSL, et al. Inconsistent safety outcome reporting in randomized clinical trials of COVID-19 vaccines complicates informed medical decisions. Drug Safety 2021; 44:1121–3. doi: 10.1007/s40264-021-01108-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chan EWW, Leung MTY, Lau LKW, et al. Comparing self-reported reactogenicity between adolescents and adults following the use of BNT162b2 (Pfizer-BioNTech) messenger RNA COVID-19 vaccine: a prospective cohort study. Int J Infect Dis 2022; 116:47–50. doi: 10.1016/j.ijid.2021.12.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chua GT, Kwan MYW, Chui CSL, et al. Epidemiology of acute myocarditis/pericarditis in Hong Kong adolescents following Comirnaty vaccination. Clin Infect Dis 2021. Nov 28:ciab989. doi: 10.1093/cid/ciab989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lai FTT, Huang L, Chui CSL, et al. Multimorbidity and adverse events of special interest associated with Covid-19 vaccines in Hong Kong. Nat Commun 2022; 13:411. doi: 10.1038/s41467-022-28068-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lai FTT, Huang L, Peng K, et al. Post-Covid-19-vaccination adverse events and healthcare utilization among individuals with or without previous SARS-CoV-2 infection. J Intern Med 2022. 291: 864–9 doi: 10.1111/joim.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lai FTT, Leung MTY, Chan EWW, et al. Self-reported reactogenicity of CoronaVac (Sinovac) compared with Comirnaty (Pfizer-BioNTech): a prospective cohort study with intensive monitoring. Vaccine 2022; 40:1390–6. doi: 10.1016/j.vaccine.2022.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wong CKH, Xiong X, Lau KTK, et al. Impact of a delayed second dose of mRNA vaccine (BNT162b2) and inactivated SARS-CoV-2 vaccine (CoronaVac) on risks of all-cause mortality, emergency department visit, and unscheduled hospitalization. BMC Med 2022; 20:119. doi: 10.1186/s12916-022-02321-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li X, Lai FTT, Chua GT, et al. Myocarditis following COVID-19 BNT162b2 vaccination among adolescents in Hong Kong. JAMA Pediatr 2022; 176(6):612–614. doi: 10.1001/jamapediatrics.2022.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xiong X, Wong CKH, Au ICH, et al. Safety of inactivated and mRNA COVID-19 vaccination among patients treated for hypothyroidism: a population-based cohort study. Thyroid 2022; 32:505–4. doi: 10.1056/NEJMoa2200797.. [DOI] [PubMed] [Google Scholar]
- 24. Li X, Tong X, Wong ICK, et al. Lack of inflammatory bowel disease flare-up following two-dose BNT162b2 vaccine: a population-based cohort study. Gut 2022. doi: 10.1136/gutjnl-2021-326860. [DOI] [PubMed] [Google Scholar]
- 25. Sing C-W, Tang CTL, Chui CSL, et al. COVID-19 vaccines and risks of hematological abnormalities: nested case-control and self-controlled case series study. Am J Hematol 2022; 97:470–80. doi: 10.1002/ajh.26478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wan EYF, Chui CSL, Wang Y, et al. Herpes zoster related hospitalization after inactivated (CoronaVac) and mRNA (BNT162b2) SARS-CoV-2 vaccination: a self-controlled case series and nested case-control study. Lancet Reg Health–West Pac 2022; 21:100393. doi: 10.1016/j.lanwpc.2022.100393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ghebremichael-Weldeselassie Y, Jabagi MJ, Botton J, et al. A modified self-controlled case series method for event-dependent exposures and high event-related mortality, with application to COVID-19 vaccine safety. Stat Med 2022; 41:1735–50. doi: 10.1002/sim.9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Simpson CR, Shi T, Vasileiou E, et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat Med 2021; 27:1290–7. doi: 10.1038/s41591-021-01408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hippisley-Cox J, Patone M, Mei XW, et al. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. bmj 2021; 374:n1931. doi: 10.1136/bmj.n1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Patone M, Handunnetthi L, Saatci D, et al. Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. Nat Med 2021; 27:2144–53. doi: 10.1038/s41591-021-01556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jabagi MJ, Botton J, Bertrand M, et al. Myocardial infarction, stroke, and pulmonary embolism after BNT162b2 MRNA COVID-19 vaccine in people aged 75 years or older. JAMA 2022; 327:80–2. doi: 10.1001/jama.2021.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sing CW, Tang CTL, Chui CSL, et al. COVID-19 vaccines and risks of hematological abnormalities: nested case-control and self-controlled case series study. Am J Hematol 2022; 94:470–80. doi: 10.1002/ajh.26478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Farrington P, Whitaker H, Ghebremichael-Weldeselassie Y. Self-controlled case series studies: a modelling guide with R. Chapman and Hall/CRC, 2018. doi: 10.1201/9780429491313. [DOI]
- 34. Zhou W, Pool V, DeStefano F, et al. A potential signal of Bell's palsy after parenteral inactivated influenza vaccines: reports to the Vaccine Adverse Event Reporting System (VAERS)—United States, 1991–2001. Pharmacoepidemiol Drug Saf 2004; 13:505–10. doi: 10.1002/pds.998. [DOI] [PubMed] [Google Scholar]
- 35. Bardage C, Persson I, Ortqvist A, et al. Neurological and autoimmune disorders after vaccination against pandemic influenza A (H1N1) with a monovalent adjuvanted vaccine: population based cohort study in Stockholm, Sweden. BMJ 2011; 343:d5956. doi: 10.1136/bmj.d5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shemer A, Pras E, Einan-Lifshitz A, et al. Association of COVID-19 vaccination and facial nerve palsy: a case-control study. JAMA Otolaryngol Head Neck Surg 2021; 147:739–43. doi: 10.1001/jamaoto.2021.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lim WW, Mak L, Leung GM, et al. Comparative immunogenicity of mRNA and inactivated vaccines against COVID-19. Lancet Microbe 2021; 2:e423. doi: 10.1016/S2666-5247(21)00177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.