Abstract
Aims
Concern about the cardiovascular safety of coronavirus disease 2019 (COVID-19) vaccines among individuals with cardiovascular disease (CVD) may lead to vaccine hesitancy. We sought to assess the association between two COVID-19 vaccines, BNT162b2 and CoronaVac, and the risk of major adverse cardiovascular events (MACE) in individuals with established CVD.
Methods and results
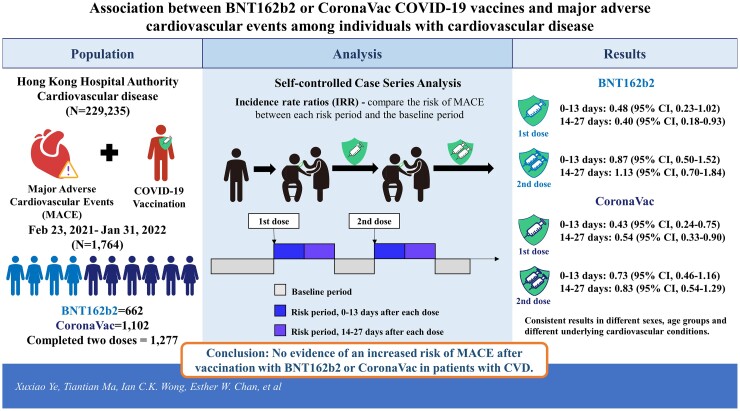
We identified individuals with a history of CVD before 23 February 2021 and a diagnosis of MACE between 23 February 2021 and 31 January 2022 in Hong Kong. MACE was defined as a composite of myocardial infarction, stroke, revascularization, and cardiovascular death. Electronic health records from the Hong Kong Hospital Authority were linked to vaccination records from the Department of Health. A self-controlled case-series method was used to evaluate the risk of MACE for 0–13 and 14–27 days after two doses of COVID-19 vaccine. We estimated incidence rate ratios (IRRs) to compare the risk of MACE between each risk period and the baseline period. A total of 229 235 individuals with CVD were identified, of which 1764 were vaccinated and had a diagnosis of MACE during the observation period (BNT162b2 = 662; CoronaVac = 1102). For BNT162b2, IRRs were 0.48 [95% confidence interval (CI) 0.23–1.02] for the first dose and 0.87 (95% CI 0.50–1.52) for the second dose during the 0–13 days risk period, 0.40 (95% CI 0.18–0.93) for the first dose and 1.13 (95% CI 0.70–1.84) for the second dose during the 14–27 days risk period. For CoronaVac, the IRRs were 0.43 (95% CI 0.24–0.75) for the first dose and, 0.73 (95% CI 0.46–1.16) for the second dose during the 0–13 days risk period, 0.54 (95% CI 0.33–0.90) for the first dose and 0.83 (95% CI 0.54–1.29) for the second dose during the 14–27 days risk period. Consistent results were found in subgroup analyses for different sexes, age groups and different underlying cardiovascular conditions.
Conclusion
Our findings showed no evidence of an increased risk of MACE after vaccination with BNT162b2 or CoronaVac in patients with CVD. Future research is required to monitor the risk after the third dose of each vaccine.
Keywords: COVID-19 vaccine, BNT162b2, CoronaVac, Major adverse cardiovascular events, Self-controlled case series
Graphical Abstract
Graphical Abstract.
There was no evidence of an increased risk of major adverse cardiovascular events after vaccination with BNT162b2 or CoronaVac in patients with cardiovascular disease.
Time of primary review: 27 days
1. Introduction
Since the global rollout of coronavirus disease 2019 (COVID-19) vaccines, reports of possible vaccine-related cardiovascular adverse events have raised concerns about their safety issues. Many case reports and a study in England reported an increased risk of thrombocytopenia and venous thromboembolism after ChAdOx1 nCoV-19 (Oxford-AstraZeneca, Oxford, UK) vaccination in the general population.1–4 Cases of myocardial infarction (MI) and stroke were also reported after BNT162b2 (Comirnaty, BioNTech/Pfizer/Fosun, Mainz, Germany) and CoronaVac (Sinovac Life Sciences, Beijing, China), the only two COVID-19 vaccines authorized for emergency use in Hong Kong.5,6 Although these vaccines have demonstrated efficacy against COVID-19 with good safety and tolerability profiles in clinical trials,7–9 the situation is likely to be complicated among individuals with established cardiovascular disease (CVD), as they have a higher risk of a major adverse cardiovascular event (MACE) and recurrent events.10,11
The American College of Cardiology considers patients with CVD to be a priority group for COVID-19 vaccination as CVD is associated with severer outcomes and a higher risk of death after COVID-19 infection.12 The COVID-19 infection is also reported to increase the risk of MI, stroke, arrhythmia, acute coronary syndrome, and venous thromboembolism.13,14 Therefore, vaccination in this population is important to prevent potential severe outcomes after COVID-19 infection. However, current evidence is limited in examining the association between the risk of MACE and BNT162b2 or CoronaVac vaccine in individuals with CVD. Limitations of case reports and previous observational studies include the small number of cases, potential or recording biases, confounding issues, lack of post-marketing safety data on CoronaVac, and only focusing on the general population.1–4,15 To gain insight into the cardiovascular safety profile of COVID-19 vaccines in individuals with CVD, this study utilized the self-controlled case-series (SCCS) study design to assess the risk of MACE in the periods after COVID-19 vaccination.
2. Methods
2.1. Study design
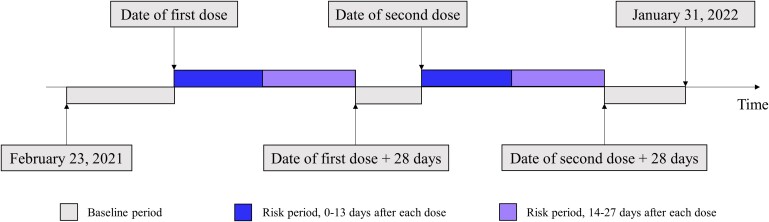
We undertook an SCCS study to examine the association between BNT162b2 or CoronaVac and the risk of MACE during the ongoing COVID-19 vaccination programme in Hong Kong. The SCCS is a within-individual study design that was developed to assess the risk of adverse events after vaccination16 and has been widely used for vaccine safety monitoring.13,17 The SCCS determines the relative incidence of the outcome for specific risk periods compared with the non-risk baseline periods in individuals with the outcome of interest (Figure 1). The inference is within each individual so all time-invariant covariates are inherently controlled during the study period and time-varying covariates can be manually adjusted.16
Figure 1.
Visualization of the self-controlled case-series observation period (23 February 2021 to 31 January 2022), baseline and risk periods following coronavirus disease 2019 vaccination. Unvaccinated individuals who developed major adverse cardiovascular event during the observation period were also included for adjustment of seasonal effects.
2.2. Data sources
We linked data of electronic health records managed by the Hong Kong Hospital Authority (HA) and vaccination records provided by the Department of Health (DH). The HA serves as a major publicly funded healthcare provider and gives services to over 7.4 million Hong Kong residents covering around 80% of all hospital admissions.18 Individual patient-specific data include diagnoses, prescriptions and dispensing information, demographic information, hospital admissions and discharges, inpatient, outpatient, and emergency department admission records. The DH provided COVID-19 vaccination records of BNT162b2 and CoronaVac vaccines on 23 February 2021, when the mass COVID-19 vaccination programme in Hong Kong was launched, until 31 January 2022. Individuals are not permitted to switch between vaccine types for the first two doses but can switch vaccine types for the third dose. All the data are anonymized to protect patient confidentiality. These data have been used for a prior COVID-19 vaccine safety study.19
2.3. Ethics statement
This study was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (reference number: UW21-149) and by the Department of Health Ethics Committee (LM21/2021). All procedures performed in these studies were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed written consent has been waived by the ethics committees, as this is an observational study using de-identified electronic health records. This study does not contain any studies with animals performed by any of the authors.
2.4. Inclusion criteria
We identified all individuals aged 16 years and above who had a record of CVD from 1 January 2018 to 22 February 2021. The CVD was defined as coronary heart disease, cerebrovascular disease, peripheral vascular disease, or a prior intervention including angioplasty, stenting, atherectomy, peripheral arterial bypass grafting, or amputation.10 The records were identified using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostics and procedure codes from inpatient, outpatient, and emergency department diagnosis records and International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) codes were used to identify the cause of death in the demographic data (see Supplementary material online, Table S1).
2.5. Exposure
Our main exposures were the vaccination of BNT162b2 or CoronaVac. The currently recommended dosing intervals in Hong Kong are 28 days for CoronaVac and 21 days for BNT162b2. The vaccination date was considered as day 0 (Figure 1). The risk periods were defined as 0–13 and 14–27 days after the first and second doses, respectively. As the interval between the two doses might be less than 27 days, the risk period was defined as day 14 to the day before the second dose in this case. The baseline non-risk period was defined as all periods before vaccination of the first dose, after the first dose plus 28 days and before the second dose, and after the second dose plus 28 days until 31 January 2022 or the date of death. As the number of patients in this study who received a third dose and developed outcomes within 28 days are limited, only the risk periods after the first two doses were measured.
2.6. Outcome
The MACE was defined as a composite of the first admission or procedure date for MI, stroke, revascularization, or cardiovascular death from 23 February 2021 to 31 January 2022.20 The ICD-9-CM and ICD-10-CM codes were used to identify outcome events (see Supplementary material online, Table S1). We considered only the first event within the observation period to ensure the outcome events were independent.
2.7. Statistical analysis
Conditional Poisson regression was used to estimate the incidence rate ratio (IRR) and its corresponding 95% confidence interval by comparing the incidence rate of each risk period vs. the baseline period. All the analyses were stratified by the type of vaccines, BNT162b2 and CoronaVac. Since the outcome event occurring after the first dose could affect the subsequent second dose exposure, we applied the modified SCCS extension, event-dependent exposure, using the function ‘eventdepenexp’ in the R-package ‘SCCS’ which is designed for the situation where the assumption that occurrence of an event does not influence subsequent exposures might be violated.16 As the priority group for vaccination is defined by the government and altered throughout the process of the vaccination programme, the population characteristics of individuals receiving the vaccines are dynamic during the observation period. To account for the dynamic populational characteristics and the higher incidence of MACE in certain seasons or months, we also adjusted the seasonal effect including the unvaccinated cases by modelling a piecewise constant with each month set as cut points.16 It is important to note that unvaccinated individuals did not act as controls and the inclusion of the unvaccinated group in the modified SCCS is essential because the lack of vaccination records may indicate cancellation of vaccination appointments, and may tend to occur more often for earlier events (before they had the opportunity to be vaccinated). Thus, absence of vaccination may be informative regarding the timing of the event and excluding unvaccinated individuals may therefore introduce bias. A comprehensive discussion on the use of modified SCCS for COVID-19 vaccine research can be found in a recent publication which highlights the important consideration of addressing event-dependent exposures.21 In the French study that they used as an example,17 the researchers considered the event-dependent exposures and high event-related mortality that can cancel or defer subsequent vaccination or increase short-term mortality and therefore used the modified SCCS and included the unvaccinated individuals. Due to the similar considerations, the use of modified SCCS and the inclusion of unvaccinated individuals were more appropriate in addressing our study objectives. We further conducted subgroup studies and stratified individuals by sex groups (male and female), age groups (65 years old or older and under 65 years old), and history of different cardiovascular conditions.
2.8. Sensitivity analyses
A series of sensitivity analyses were conducted to assess the robustness of this study: (i) We excluded individuals who died during the observation period to assess whether the occurrence of death might influence the probability of subsequent observation16; (ii) We removed revascularization from the definition of MACE so that it consisted of MI, stroke, or cardiovascular death; (iii) We applied standard SCCS for the main analysis instead of the event-dependent exposure extension; (iv) We included only vaccinated individuals and did not adjust for seasonal effect as the different characteristics of vaccinated and unvaccinated groups might generate potential bias; (v) We added thrombotic events to the definition of MACE; (vi) We added myocarditis to the definition of MACE; (vii) We excluded those who were diagnosed with COVID-19 before or during the observation period; (viii) We excluded those who received the third dose during the observation period; (ix) We also conducted a negative control outcome analysis using fracture since no association between fracture and COVID-19 vaccination has been reported. A 5% significance level was considered statistically significant in all analyses. All analyses were performed using the SCCS package in R, version 4.0.3 (http://www.R-project.org).
3. Results
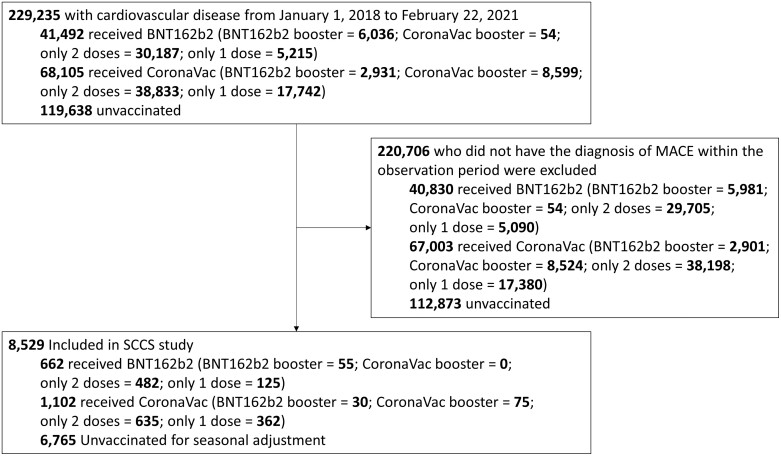
A total of 229 235 individuals with CVD before 23 February 2021 were identified. About 8529 individuals who developed MACE during the observation period were included, of which 1764 received COVID-19 vaccination (Figure 2). Among vaccinated individuals, 662 received BNT162b2 and 1102 received CoronaVac and in total 1277 individuals completed two doses by 31 January 2022 and 160 received a third dose. Individuals who received BNT162b2 were slightly younger than those who received CoronaVac and both vaccinated groups were younger than the unvaccinated group. Among the 1764 individuals who developed MACE and received COVID-19 vaccination during the observation period, 116 individuals died with 42 deaths (31 cardiovascular deaths, 6 not recorded, and 5 other causes) in the BNT162b2 group and 74 (48 cardiovascular deaths, 15 not recorded, and 11 other causes) in the CoronaVac group. Other causes of death include sepsis, bacteraemia, thrombotic microangiopathy, pneumonitis, pneumonia, pulmonary oedema, respiratory failure, and lung cancer. The majority of included individuals had a history of coronary heart disease, or cerebrovascular disease, and only 38 individuals had peripheral vascular disease. No vaccinated individual with history of cardiovascular intervention had an event of MACE during the observation period (Table 1). The characteristics of all individuals with history of CVD stratified by each brand of vaccination and the unvaccinated group and whether they developed MACE are presented in Supplementary material online, Table S2. Most of vaccinated individuals had prescription records of antiplatelet drugs, beta-blockers, calcium channel blockers, diuretics, lipid-lowering agents, renin-angiotensin-system agents, or oral anticoagulants within 3 months before receiving the first dose of vaccination and the majority had a prescription of lipid-lowering agents. A total of five individuals had a positive test for severe acute respiratory syndrome–related coronavirus 2 (SARS-CoV-2) before or during the observation period, among which two received BNT162b2 before testing positive, two received BNT162b2 after a positive test, and one received CoronaVac before testing positive.
Figure 2.
Flow chart illustrating patient inclusion and reasons for exclusion in the self-controlled case-series study.
Table 1.
Characteristics of vaccinated and unvaccinated individuals with established cardiovascular disease who experienced a major adverse cardiovascular event during the observation period (23 February 2021 to 31 January 2022)
| Characteristics | BNT162b2 | CoronaVac | Unvaccinated | P-value |
|---|---|---|---|---|
| Number | 662 | 1102 | 6765 | |
| Died during observation period | 42 (6.3) | 74 (6.7) | 3121 (46.1) | <0.001 |
| Age, mean (SD) | 67.90 (11.94) | 71.23 (11.76) | 78.66 (12.34) | <0.001 |
| Male | 506 (76.4) | 778 (70.6) | 3918 (57.9) | <0.001 |
| Completed two vaccine doses by 31 January 2022 | 537 (81.1) | 740 (67.2) | 0 (0.0) | |
| Received a booster by 31 January 2022 | 55 (8.3) | 105 (9.5) | 0 (0.0) | |
| Baseline conditions | ||||
| Myocardial infarction | 87 (13.1) | 120 (10.9) | 1013 (15.0) | 0.001 |
| Ischaemic stroke | 130 (19.6) | 214 (19.4) | 1260 (18.6) | 0.699 |
| Coronary heart disease | 384 (58.0) | 553 (50.2) | 3396 (50.2) | 0.001 |
| Cerebrovascular disease | 267 (40.3) | 522 (47.4) | 3058 (45.2) | 0.015 |
| Peripheral vascular disease | 11 (1.7) | 27 (2.5) | 304 (4.5) | <0.001 |
| Cardiovascular surgery | 0 (0.0) | 0 (0.0) | 7 (0.1) | 0.401 |
| Diabetes | 231 (34.9) | 394 (35.8) | 2963 (43.8) | <0.001 |
| Liver disease | 1 (0.2) | 2 (0.2) | 68 (1.0) | 0.003 |
| Renal disease | 45 (6.8) | 71 (6.4) | 1231 (18.2) | <0.001 |
| Hypertension | 417 (63.0) | 739 (67.1) | 4870 (72.0) | <0.001 |
| Atrial fibrillation | 77 (11.6) | 133 (12.1) | 1751 (25.9) | <0.001 |
| Cardiovascular drug dispensed within 90 days before vaccination | ||||
| Lipid lowering agents | 602 (90.9) | 1007 (91.4) | 4852 (71.7) | <0.001 |
| Antiplatelet drugs | 571 (86.3) | 955 (86.7) | 4568 (67.5) | <0.001 |
| Renin-angiotensin-system agents | 377 (56.9) | 621 (56.4) | 3418 (50.5) | <0.001 |
| Beta-blockers | 343 (51.8) | 541 (49.1) | 3164 (46.8) | 0.024 |
| Calcium channel blockers | 308 (46.5) | 526 (47.7) | 2966 (43.8) | 0.031 |
| Diuretics | 103 (15.6) | 172 (15.6) | 2026 (29.9) | <0.001 |
| Oral anticoagulants | 67 (10.1) | 106 (9.6) | 958 (14.2) | <0.001 |
Figures are number (%) unless stated otherwise. To account for the dynamic populational characteristics and the higher incidence of MACE in certain seasons or months, we also adjusted the seasonal effect including the unvaccinated cases by modelling a piecewise constant with each month set as cut points.16 Therefore, the unvaccinated cases did not serve as controls.
SD, standard deviation.
3.1. Main analyses
In total, there were 82 MACE events within 28 days after the first two doses for BNT162b2 and 115 events for CoronaVac. Only four and three MACE events were observed within 28 days following the third dose of BNT162b2 and CoronaVac, respectively. Due to the scant number of events for the third dose, safety evaluations were performed only on the first two doses. The event-dependent SCCS model detected no evidence of an increased risk of MACE during the 28 days after both doses of BNT162b2 or CoronaVac (Table 2). For BNT162b2, IRRs were 0.48 [95% confidence interval (CI) 0.23–1.02] for the first dose and 0.87 (95% CI 0.50–1.52) for the second dose during the 0–13 days risk period, 0.40 (95% CI 0.18–0.93) for the first dose, and 1.13 (95% CI 0.70–1.84) for the second dose during the 14–27 days risk period. For CoronaVac, the IRRs were 0.43 (95% CI 0.24–0.75) for the first dose and 0.73 (95% CI 0.46–1.16) for the second dose during the 0–13 days risk period, 0.54 (95% CI 0.33–0.90) for the first dose and 0.83 (95% CI 0.54–1.29) for the second dose during the 14–27 days risk period.
Table 2.
Results of the main self-controlled case-series analysis
| Number of events | Patient-days | Crude incidence (per 1000 patient-days) |
Incidence rate ratio (95% CI) |
P-value | |
|---|---|---|---|---|---|
| BNT162b2 (n = 662) | |||||
| Baseline | 580 | 195 945 | 3 | ||
| First dose | |||||
| 0–13 days after | 18 | 9015 | 2 | 0.48 (0.23–1.02) | 0.06 |
| 14–27 days after | 12 | 5112 | 2.3 | 0.40 (0.18–0.93) | 0.03 |
| Second dose | |||||
| 0–13 days after | 25 | 7228 | 3.5 | 0.87 (0.50–1.52) | 0.63 |
| 14–27 days after | 27 | 6687 | 4 | 1.13 (0.70–1.84) | 0.61 |
| CoronaVac (n = 1102) | |||||
| Baseline | 987 | 324 557 | 3 | ||
| First dose | |||||
| 0–13 days after | 27 | 14 724 | 1.8 | 0.43 (0.24–0.75) | <0.01 |
| 14–27 days after | 28 | 12 414 | 2.3 | 0.54 (0.33–0.90) | 0.02 |
| Second dose | |||||
| 0–13 days after | 30 | 10 062 | 3 | 0.73 (0.46–1.16) | 0.19 |
| 14–27 days after | 30 | 9571 | 3.1 | 0.83 (0.54–1.29) | 0.41 |
CI = confidence interval.
3.2. Subgroup and sensitivity analyses
Consistent with the results of the main analysis, subgroup analyses did not show an association between MACE and vaccination with BNT162b2 or CoronaVac vaccine within the first 28 days after vaccination (Table 3) stratified by sex groups, age groups, and history of different cardiovascular conditions. The individuals with a history of peripheral vascular disease or prior cardiovascular intervention were not analysed individually because of the small sample size (38 and 0, respectively). Results of the sensitivity analyses were generally consistent with the main analysis. They were robust when excluding individuals who died during the observation period and when we defined MACE as the composite of MI, stroke, or cardiovascular death (see Supplementary material online, Tables S3 and 4). Similarly, no association between the two COVID-19 vaccines and MACE was observed using the standard SCCS study design (see Supplementary material online, Table S5); only including vaccinated individuals in the analysis without adjustment of the seasonal effect (see Supplementary material online, Table S6); including thrombotic events or myocarditis into the definition of MACE (see Supplementary material online, Tables S7 and 8); excluding those who were diagnosed with COVID-19 (see Supplementary material online, Table S9); or excluding those who received the third dose during the observation period (see Supplementary material online, Tables S10). The result of negative control outcome analysis showed no association between COVID-19 vaccination and the risk of fracture (see Supplementary material online, Table S11).
Table 3.
Results of subgroup analyses
| Subgroups and time period | Number of events |
Incidence rate ratio (95% CI) |
Number of events |
Incidence rate ratio (95% CI) |
|---|---|---|---|---|
| BNT162b2 vaccine | ||||
| Sex | Men | Women | ||
| Baseline | 450 | 130 | ||
| First dose | ||||
| 0–13 days after | 13 | 0.45 (0.19–1.08) | 5 | 0.65 (0.17–2.48) |
| 14–27 days after | 6 | 0.26 (0.08–0.81) | 6 | 0.93 (0.28–3.12) |
| Second dose | ||||
| 0–13 days after | 19 | 0.77 (0.40–1.50) | 6 | 1.18 (0.41–3.38) |
| 14–27 days after | 18 | 1.00 (0.56–1.79) | 9 | 1.52 (0.62–3.74) |
| Age groups | 65 years old or older | Under 65 years old | ||
| Baseline | 353 | 227 | ||
| First dose | ||||
| 0–13 days after | 11 | 0.40 (0.16–1.02) | 7 | 0.77 (0.33–1.80) |
| 14–27 days after | 11 | 0.54 (0.21–1.35) | 1 | 0.14 (0.02–1.20) |
| Second dose | ||||
| 0–13 days after | 14 | 0.71 (0.34–1.50) | 11 | 1.12 (0.53–2.39) |
| 14–27 days after | 21 | 1.22 (0.67–2.22) | 6 | 0.88 (0.38–2.03) |
| History of different cardiovascular conditions | Coronary heart disease | Cerebrovascular disease | ||
| Baseline | 342 | 242 | ||
| First dose | ||||
| 0–13 days after | 8 | 0.74 (0.35–1.58) | 9 | 0.97 (0.48–1.93) |
| 14–27 days after | 5 | 0.71 (0.28–1.83) | 4 | 0.69 (0.25–1.91) |
| Second dose | ||||
| 0–13 days after | 9 | 0.98 (0.48–1.99) | 12 | 1.42 (0.76–2.67) |
| 14–27 days after | 13 | 1.38 (0.76–2.49) | 11 | 1.32 (0.67–2.57) |
| CoronaVac vaccine | ||||
| Sex | Men | Women | ||
| Baseline | 704 | 283 | ||
| First dose | ||||
| 0–13 days after | 19 | 0.37 (0.18–0.76) | 8 | 0.57 (0.22–1.48) |
| 14–27 days after | 17 | 0.42 (0.21–0.82) | 11 | 0.89 (0.41–1.92) |
| Second dose | ||||
| 0–13 days after | 20 | 0.60 (0.33–1.11) | 10 | 1.08 (0.50–2.33) |
| 14–27 days after | 18 | 0.63 (0.37–1.08) | 12 | 1.53 (0.70–3.38) |
| Age groups | 65 years old or older | Under 65 years old | ||
| Baseline | 697 | 290 | ||
| First dose | ||||
| 0–13 days after | 23 | 0.50 (0.26–0.97) | 4 | 0.15 (0.03–0.67) |
| 14–27 days after | 23 | 0.61 (0.32–1.18) | 5 | 0.24 (0.08–0.72) |
| Second dose | ||||
| 0–13 days after | 18 | 0.54 (0.28–1.02) | 12 | 1.08 (0.59–1.98) |
| 14–27 days after | 22 | 0.86 (0.50–1.46) | 8 | 0.74 (0.34–1.60) |
| History of different cardiovascular conditions | Coronary heart disease | Cerebrovascular disease | ||
| Baseline | 522 | 472 | ||
| First dose | ||||
| 0–13 days after | 8 | 0.49 (0.23–1.04) | 13 | 0.80 (0.45–1.42) |
| 14–27 days after | 12 | 0.73 (0.39–1.34) | 11 | 0.72 (0.39–1.33) |
| Second dose | ||||
| 0–13 days after | 7 | 0.54 (0.25–1.15) | 19 | 1.36 (0.83–2.22) |
| 14–27 days after | 13 | 1.05 (0.59–1.87) | 14 | 1.11 (0.63–1.96) |
4. Discussion
To our knowledge, this is the first study to examine the association between COVID-19 vaccines and the risk of MACE in individuals with CVD. Our analysis did not demonstrate an association during the first 28 days after vaccination with either BNT162b2 or CoronaVac. The subgroup analyses were consistent among men and women, individuals aged under and above 65 years old, and individuals with a history of coronary heart disease or cerebrovascular disease.
Since the appearance of reports on possible COVID-19 vaccine-related MACE, the potential cardiovascular safety issues of vaccination have been raised.5,6,22 The possible mechanism of the relationship between MACE and COVID-19 vaccine is still unknown, and it is hypothesized that there may be a correlation between vaccine-induced immune syndrome and CVD.23 Cases of apparent secondary immune thrombocytopenia have been reported after both BNT162b2 and Moderna vaccination.24 Although the pathogenesis is unclear, the autoimmune reaction following vaccination is of great concern, particularly for individuals with a complex disease history.25 The immune system makes an important contribution to cardiac composition and function, as well as an ischaemic injury such as MI and ischaemic stroke, which have a diverse impact on innate and adaptive immune cells.23 Among the reports of adverse cardiovascular events, most were related to the ChAdOx1 nCoV-19 vaccine.1–4 In an SCCS study in England1 and a case–control study in Scotland,15 ChAdOx1 nCoV-19 vaccine was significantly related to an increased risk of thrombocytopenia, venous thromboembolism, and cerebral venous sinus thrombosis in the general population. Neither of the two studies found any association between these outcomes and the BNT162b2 mRNA vaccine. Our study was consistent as there was no increased risk with the combined outcome of thrombotic events and MACE following vaccination with BNT162b2 or CoronaVac. A surveillance study in the USA reported no increased risk of MI or stroke after the BNT162b2 vaccine within 1–21 days post-vaccination compared with 22–42 days post-vaccination in the general population26 and a French study found no increase in cardiovascular events after the BNT162b2 vaccine among older individuals.17 The findings on the BNT162b2 vaccine in our study are consistent with these studies, suggesting no increased risk of MACE after vaccination in individuals with CVD. In addition, myocarditis is a major concern of the cardiovascular safety of the COVID-19 vaccines, especially in young adults and adolescent males.27–29 In our studied population with history of CVD, the average age is much older and the results also showed no increased risk after including myocarditis into the definition of MACE.
On the other hand, BNT162b2 raised concerns of long-term adverse effects because of the relatively new mRNA vaccine technology.30 CoronaVac, an inactivated COVID-19 vaccine, is an alternative for individuals who are concerned about the mRNA vaccine technology; however, aside from the initial clinical trials, published post-marketing safety data are limited.31,32 To date, the association between the CoronaVac and MACE has not been explored. Our study found no increased risk of MACE after receiving CoronaVac in individuals with previous CVD, providing additional evidence of cardiovascular safety for CoronaVac in this group of individuals.
4.1. Clinical implications
In clinical practice, fear and anxiety about the possible adverse effects of COVID-19 vaccines may induce vaccine hesitancy, low vaccination rates, and subsequent severe illness after infection.33 Despite being given priority for COVID-19 vaccination, only 47.8% (109 597/229 235) of individuals with a history of CVD were vaccinated by 31 January 2022 in Hong Kong, which demonstrates a significant challenge to achieving optimal vaccine coverage in this high-risk population. Our findings support the short-term safety of both BNT162b2 and CoronaVac vaccines among individuals with CVD. To improve the vaccination rate in individuals with established CVD in Hong Kong, strategies are needed to eliminate concerns and strengthen awareness of the vaccines’ role in preventing COVID-19 infection, and more importantly, in reducing the likelihood of mortality and comorbidities. One strategy could be to provide more real-world evidence from population-based observational studies to assist this population in weighing the benefits and risks of COVID-19 vaccines. Another strategy requires cardiologists and primary-care clinicians to provide easy-to-access and easy-to-understand information as well as vaccination and, in the meantime, address the common misconceptions about adverse effects.
4.2. Strengths and limitations
The evaluation of COVID-19 vaccine safety requires rapid response and ongoing assessment. In the context of vaccine surveillance, cohort, and case–control studies are limited by confounding and residual healthy vaccine effects.34 Therefore, we used the SCCS study design, specifically, the event-dependent SCCS extension, which is designed for situations where individuals may not want the second dose if a MACE event occurred after the first dose. Various sensitivity analyses further supported the robustness of our conclusion. Furthermore, unlike previous studies that focused on the general population,1,15,26 we specifically focused on individuals with CVD and conducted subgroup analyses in individuals with a history of coronary heart disease or cerebrovascular disease. The rationale for choosing this population is that individuals with CVD are at a higher risk of developing MACE, and this risk varies in individuals with different disease histories.10,11 In a previous study with a median follow-up duration of 27.4 months, the results reported that 9.6% of MI individuals would have a recurrent MI,35 and 26% of stroke individuals were reported to have a recurrent stroke within 5 years.36 Our results can be applied to general CVD individuals as well as individuals with specific CVDs for the evaluation of vaccine safety.
Our study has some limitations. First, some of the subgroup analyses present a small sample size and further studies on a larger population are required to confirm our findings. Second, as the majority of Hong Kong residents are Chinese, the generalizability of our results to different ethnic groups or outside Hong Kong may be limited. Third, a previous study reported an increased risk of MI and stroke after diagnosis of COVID-19, which suggests a potential bias of MACE-related studies during the COVID-19 pandemic.37 However, our sensitivity analysis which excluded all individuals who had a positive test for SARS-CoV-2 before or during the observation period showed consistent results as the main analysis, and the potential bias was minimized. Fourth, we were unable to include data from private clinics and hospitals, and potential bias could exist to include all the outcome cases. As the public data already cover around 80% of all hospital admissions, the missing captured outcome events would be rare. Also, as the unvaccinated individuals had a different risk profile to the vaccinated individuals, adjusting the seasonal effect using the unvaccinated individuals might introduce potential bias. Therefore, we conducted a sensitivity analysis to only include vaccinated individuals without adjustment of the seasonal effect with consistent results. Last, the available data only allowed for an evaluation between MACE and the two doses of vaccines. Future studies with longer observation periods are required for risks following the third ‘booster’ dose.
5. Conclusion
In this SCCS study among individuals with CVD in Hong Kong, there was no evidence of an increased risk of MACE during the 28 days after BNT162b2 or CoronaVac vaccination when compared with the baseline period. Clinicians and public health professionals should emphasize the cardiovascular safety of these two vaccines, which could alleviate the potential concerns among individuals with CVD.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Authors’ contributions
X.Y., T.M., I.C.K.W., and E.W.C. designed the research; X.Y. and T.M. performed the research; X.Y., T.M., J.E.B., V.K.C.Y., and W.K. analysed the data; C.S.L.C, F.T.T.L., X.L., E.Y.F.W., C.K.H.W., H.F.T., and C.W.S. provided discussion and revision; I.C.K.W. and E.W.C. provided funding and supervision; X.Y. and T.M. prepared the manuscript with input from all co-authors.
Supplementary Material
Acknowledgements
The authors thank colleagues from the Drug Office of the Department of Health and the Hospital Authority for the generous provision of vaccination and clinical data. They also thank Lisa Y. Lam for proofreading the manuscript. F.T.T.L. and I.C.K.W.'s post were partly funded by D24H; hence this work was partly supported by AIR@InnoHK administered by Innovation and Technology Commission.
Conflict of interest: C.S.L.C. has received grants from the Food and Health Bureau of the Hong Kong Government, Hong Kong Research Grant Council, Hong Kong Innovation and Technology Commission, Pfizer, IQVIA, and Amgen; personal fees from Primevigilance Ltd.; outside the submitted work. E.Y.F.W. has received research grants from the Food and Health Bureau of the Government of the Hong Kong SAR, and the Hong Kong Research Grants Council, outside the submitted work. F.T.T.L. has been supported by the RGC Postdoctoral Fellowship under the Hong Kong Research Grants Council and has received research grants from Food and Health Bureau of the Government of the Hong Kong SAR, outside the submitted work. X.L. received research grants from Research Fund Secretariat of the Food and Health Bureau (HMRF, HKSAR), Research Grants Council Early Career Scheme (RGC/ECS, HKSAR), Janssen and Pfizer; internal funding from the University of Hong Kong; consultancy fee from Merck Sharp & Dohme, unrelated to this work. I.C.K.W. reports research funding outside the submitted work from Amgen, Bristol-Myers Squibb, Pfizer, Janssen, Bayer, GSK, Novartis, the Hong Kong RGC, and the Hong Kong Health and Medical Research Fund, National Institute for Health Research in England, European Commission, National Health and Medical Research Council in Australia, and also received speaker fees from Janssen and MEDICE in the previous 3 years. He is also an independent non-executive director of Jacobson Medical in Hong Kong. E.W.C. has received research grants from Research Grants Council (RGC, HKSAR), Research Fund Secretariat of the Food and Health Bureau (HMRF, HKSAR), National Natural Science Fund of China, National Health and Medical Research Council (NHMRC, Australia), Welcome Trust, Bayer, Bristol-Myers Squibb, Pfizer, Janssen, Amgen, Takeda, Novartis, and Narcotics Division of the Security Bureau of HKSAR; and honorarium from the hospital authority, outside the submitted work. All other authors declare no competing interests.
Contributor Information
Xuxiao Ye, Centre for Safe Medication Practice and Research, Department of Pharmacology and Pharmacy, General Office, L02-56 2/F, Laboratory Block, LKS Faculty of Medicine, The University of Hong Kong, 21 Sassoon Road, Pokfulam, Hong Kong SAR, China.
Tiantian Ma, Centre for Safe Medication Practice and Research, Department of Pharmacology and Pharmacy, General Office, L02-56 2/F, Laboratory Block, LKS Faculty of Medicine, The University of Hong Kong, 21 Sassoon Road, Pokfulam, Hong Kong SAR, China; Laboratory of Data Discovery for Health (D24H), Hong Kong SAR, China.
Joseph E Blais, Centre for Safe Medication Practice and Research, Department of Pharmacology and Pharmacy, General Office, L02-56 2/F, Laboratory Block, LKS Faculty of Medicine, The University of Hong Kong, 21 Sassoon Road, Pokfulam, Hong Kong SAR, China.
Vincent K C Yan, Centre for Safe Medication Practice and Research, Department of Pharmacology and Pharmacy, General Office, L02-56 2/F, Laboratory Block, LKS Faculty of Medicine, The University of Hong Kong, 21 Sassoon Road, Pokfulam, Hong Kong SAR, China.
Wei Kang, Centre for Safe Medication Practice and Research, Department of Pharmacology and Pharmacy, General Office, L02-56 2/F, Laboratory Block, LKS Faculty of Medicine, The University of Hong Kong, 21 Sassoon Road, Pokfulam, Hong Kong SAR, China.
Celine S L Chui, Laboratory of Data Discovery for Health (D24H), Hong Kong SAR, China; School of Nursing, LKS Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China; School of Public Health, LKS Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China.
Francisco T T Lai, Centre for Safe Medication Practice and Research, Department of Pharmacology and Pharmacy, General Office, L02-56 2/F, Laboratory Block, LKS Faculty of Medicine, The University of Hong Kong, 21 Sassoon Road, Pokfulam, Hong Kong SAR, China; Laboratory of Data Discovery for Health (D24H), Hong Kong SAR, China.
Xue Li, Centre for Safe Medication Practice and Research, Department of Pharmacology and Pharmacy, General Office, L02-56 2/F, Laboratory Block, LKS Faculty of Medicine, The University of Hong Kong, 21 Sassoon Road, Pokfulam, Hong Kong SAR, China; Laboratory of Data Discovery for Health (D24H), Hong Kong SAR, China; Department of Medicine, School of Clinical Medicine, LKS Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China.
Eric Y F Wan, Centre for Safe Medication Practice and Research, Department of Pharmacology and Pharmacy, General Office, L02-56 2/F, Laboratory Block, LKS Faculty of Medicine, The University of Hong Kong, 21 Sassoon Road, Pokfulam, Hong Kong SAR, China; Laboratory of Data Discovery for Health (D24H), Hong Kong SAR, China; Department of Family Medicine and Primary Care, School of Clinical Medicine, LKS Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China.
Carlos K H Wong, Centre for Safe Medication Practice and Research, Department of Pharmacology and Pharmacy, General Office, L02-56 2/F, Laboratory Block, LKS Faculty of Medicine, The University of Hong Kong, 21 Sassoon Road, Pokfulam, Hong Kong SAR, China; Laboratory of Data Discovery for Health (D24H), Hong Kong SAR, China; Department of Family Medicine and Primary Care, School of Clinical Medicine, LKS Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China.
Hung Fat Tse, Cardiology Division, Department of Medicine, School of Clinical Medicine, LKS Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China; Hong Kong-Guangdong Stem Cell and Regenerative Medicine Research Centre, The University of Hong Kong and Guangzhou Institutes of Biomedicine and Health, Hong Kong SAR, China; Cardiac and Vascular Center, The University of Hong Kong-Shenzhen Hospital, Shenzhen, China.
Chung Wah Siu, Cardiology Division, Department of Medicine, School of Clinical Medicine, LKS Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China.
Ian C K Wong, Centre for Safe Medication Practice and Research, Department of Pharmacology and Pharmacy, General Office, L02-56 2/F, Laboratory Block, LKS Faculty of Medicine, The University of Hong Kong, 21 Sassoon Road, Pokfulam, Hong Kong SAR, China; Laboratory of Data Discovery for Health (D24H), Hong Kong SAR, China; Research Department of Practice and Policy, School of Pharmacy, University College London, London, UK; Expert Committee on Clinical Events Assessment Following COVID-19 Immunization, Department of Health, The Government of the Hong Kong SAR, Hong Kong SAR, China.
Esther W Chan, Centre for Safe Medication Practice and Research, Department of Pharmacology and Pharmacy, General Office, L02-56 2/F, Laboratory Block, LKS Faculty of Medicine, The University of Hong Kong, 21 Sassoon Road, Pokfulam, Hong Kong SAR, China; Laboratory of Data Discovery for Health (D24H), Hong Kong SAR, China; Department of Pharmacy, The University of Hong Kong-Shenzhen Hospital, Shenzhen, China; The University of Hong Kong Shenzhen Institute of Research and Innovation, Shenzhen, China.
Funding
This was a regulatory pharmacovigilance study initiated by the Department of Health and funded via the Food and Health Bureau of the Government of the Hong Kong Special Administrative Region (reference COVID19F01). The sponsor of the study was involved in study design, data collection, data analysis, data interpretation, and writing of the report via the Department of Health.
Data availability
Data will not be available for others as the data custodians have not given permission.
Code availability
All the analysis codes support the findings are available from the corresponding author upon reasonable requests.
References
- 1. Hippisley-Cox J, Patone M, Mei XW, Saatci D, Dixon S, Khunti K, Zaccardi F, Watkinson P, Shankar-Hari M, Doidge J, Harrison DA, Griffin SJ, Sheikh A, Coupland CAC. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. BMJ 2021;374:n1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lund LC, Hallas J, Nielsen H, Koch A, Mogensen SH, Brun NC, Christiansen CF, Thomsen RW, Pottegård A. Post-acute effects of SARS-CoV-2 infection in individuals not requiring hospital admission: a Danish population-based cohort study. Lancet Infect Dis 2021;21:1373–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schultz NH, Sørvoll IH, Michelsen AE, Munthe LA, Lund-Johansen F, Ahlen MT, Wiedmann M, Aamodt AH, Skattør TH, Tjønnfjord GE, Holme PA. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med 2021;384:2124–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med 2021;384:2092–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. The Government of the Hong Kong Special Administrative Region . Expert Committee on Clinical Events Assessment Following COVID-19 Immunisation assessed a suspected serious adverse event following COVID-19 vaccination. 2021. https://www.info.gov.hk/gia/general/202103/03/P2021030300753.htm? fontSize=1 (27 August 2021, date last accessed).
- 6. South China Morning Post . Hong Kong authorities looking into death of chronically ill man two days after receiving Sinovac Covid-19 vaccine. 2021. https://www.scmp.com/news/hong-kong/health-environment/article/3123818/hong-kong-authorities-looking-death-chronically (27 August 2021, date last accessed).
- 7. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Al Kaabi N, Zhang Y, Xia S, Yang Y, Al Qahtani MM, Abdulrazzaq N, Al Nusair M, Hassany M, Jawad JS, Abdalla J, Hussein SE, Al Mazrouei SK, Al Karam M, Li X, Yang X, Wang W, Lai B, Chen W, Huang S, Wang Q, Yang T, Liu Y, Ma R, Hussain ZM, Khan T, Saifuddin Fasihuddin M, You W, Xie Z, Zhao Y, Jiang Z, Zhao G, Zhang Y, Mahmoud S, ElTantawy I, Xiao P, Koshy A, Zaher WA, Wang H, Duan K, Pan A, Yang X. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA 2021;326:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jara A, Undurraga EA, González C, Paredes F, Fontecilla T, Jara G, Pizarro A, Acevedo J, Leo K, Leon F, Sans C, Leighton P, Suárez P, García-Escorza H, Araos R. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med 2021;385:875–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miao B, Hernandez AV, Alberts MJ, Mangiafico N, Roman YM, Coleman CI. Incidence and predictors of major adverse cardiovascular events in patients with established atherosclerotic disease or multiple risk factors. J Am Heart Assoc 2020;9:e014402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tessitore E, Carballo D, Poncet A, Perrin N, Follonier C, Assouline B, Carballo S, Girardin F, Mach F. Mortality and high risk of major adverse events in patients with COVID-19 and history of cardiovascular disease. Open Heart 2021;8:e001526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Driggin E, Maddox TM, Ferdinand KC, Kirkpatrick JN, Ky B, Morris AA, Mullen JB, Parikh SA, Philbin DM, Vaduganathan M. ACC health policy statement on cardiovascular disease considerations for COVID-19 vaccine prioritization. J Am Coll Cardiol 2021;77:1938–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Katsoularis I, Fonseca-Rodríguez O, Farrington P, Lindmark K, Fors Connolly AM. Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: a self-controlled case series and matched cohort study. Lancet 2021;398:599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol 2020;17:543–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Simpson CR, Shi T, Vasileiou E, Katikireddi SV, Kerr S, Moore E, McCowan C, Agrawal U, Shah SA, Ritchie LD, Murray J, Pan J, Bradley DT, Stock SJ, Wood R, Chuter A, Beggs J, Stagg HR, Joy M, Tsang RSM, de Lusignan S, Hobbs R, Lyons RA, Torabi F, Bedston S, O’Leary M, Akbari A, McMenamin J, Robertson C, Sheikh A. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat Med 2021;27:1290–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Petersen I, Douglas I, Whitaker H. Self controlled case series methods: an alternative to standard epidemiological study designs. BMJ 2016;354:i4515. [DOI] [PubMed] [Google Scholar]
- 17. Jabagi MJ, Botton J, Bertrand M, Weill A, Farrington P, Zureik M, Dray-Spira R. Myocardial infarction, stroke, and pulmonary embolism after BNT162b2 mRNA COVID-19 vaccine in people aged 75 years or older. JAMA 2022;327:80–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leung GM, Wong I OL, Chan W-S, Choi S, Lo SV. The ecology of health care in Hong Kong. Soc Sci Med 2005;61:577–590. [DOI] [PubMed] [Google Scholar]
- 19. Wan EYF, Chui CSL, Lai FTT, Chan EWY, Li X, Yan VKC, Gao L, Yu Q, Lam ICH, Chun RKC, Cowling BJ, Fong WC, Lau AYL, Mok VCT, Chan FLF, Lee CK, Chan LST, Lo D, Lau KK, Hung IFN, Leung GM, Wong ICK. Bell's palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect Dis 2022;22:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lopes RD, de Barros e Silva PGM, Damiani LP, Santos RHN, Alexander JH, Granger CB, Berwanger O. Major adverse cardiovascular events after 12 months among patients with acute coronary syndrome receiving loading doses of atorvastatin prior to planned PCI. JAMA 2020;323:787–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ghebremichael-Weldeselassie Y, Jabagi MJ, Botton J, Bertrand M, Baricault B, Drouin J, Weill A, Zureik M, Dray-Spira R, Farrington P. A modified self-controlled case series method for event-dependent exposures and high event-related mortality, with application to COVID-19 vaccine safety. Stat Med 2022;41:1735–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sung JG, Sobieszczyk PS, Bhatt DL. Acute myocardial infarction within 24 hours after COVID-19 vaccination. Am J Cardiol 2021;156:129–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Swirski FK, Nahrendorf M. Cardioimmunology: the immune system in cardiac homeostasis and disease. Nat Rev Immunol 2018;18:733–744. [DOI] [PubMed] [Google Scholar]
- 24. Lee E-J, Cines DB, Gernsheimer T, Kessler C, Michel M, Tarantino MD, Semple JW, Arnold DM, Godeau B, Lambert MP, Bussel JB. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am J Hematol 2021;96:534–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cines DB, Bussel JB. SARS-CoV-2 Vaccine–induced immune thrombotic thrombocytopenia. N Engl J Med 2021;384:2254–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Klein NP, Lewis N, Goddard K, Fireman B, Zerbo O, Hanson KE, Donahue JG, Kharbanda EO, Naleway A, Nelson JC, Xu S, Yih WK, Glanz JM, Williams JTB, Hambidge SJ, Lewin BJ, Shimabukuro TT, DeStefano F, Weintraub ES. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA 2021;326:1390–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA vaccines. Circulation 2021;144:471–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chua GT, Kwan MYW, Chui CSL, Smith RD, Cheung EC, Tian T, Leung MTY, Tsao SSL, Kan E, Ng WKC, Man Chan VC, Tai SM, Yu TC, Lee KP, Wong JSC, Lin YK, Shek CC, Leung ASY, Chow CK, Li KW, Ma J, Fung WY, Lee D, Ng MY, Wong WHS, Tsang HW, Kwok J, Leung D, Chung KL, Chow CB, Chan GCF, Leung WH, To KKW, Yuen KY, Lau YL, Wong ICK, Ip P. Epidemiology of acute myocarditis/pericarditis in Hong Kong adolescents following Comirnaty vaccination. Clin Infect Dis 2021:ciab989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lai FTT, Li X, Peng K, Huang L, Ip P, Tong X, Chui CSL, Wan EYF, Wong CKH, Chan EWY, Siu DCW, Wong ICK. Carditis after COVID-19 vaccination with a messenger RNA vaccine and an inactivated virus vaccine: a case-control study. Ann Intern Med 2022;175:362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hitti FL, Weissman D. Debunking mRNA vaccine misconceptions-an overview for medical professionals. Am J Med 2021;134:703–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, Han W, Chen Z, Tang R, Yin W, Chen X, Hu Y, Liu X, Jiang C, Li J, Yang M, Song Y, Wang X, Gao Q, Zhu F. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis 2021;21:181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tanriover MD, Doğanay HL, Akova M, Güner HR, Azap A, Akhan S, Köse Ş, Erdinç F, Akalın EH, Tabak Ö F, Pullukçu H, Batum Ö, Şimşek Yavuz S, Turhan Ö, Yıldırmak MT, Köksal İ, Taşova Y, Korten V, Yılmaz G, Çelen MK, Altın S, Çelik İ, Bayındır Y, Karaoğlan İ, Yılmaz A, Özkul A, Gür H, Unal S, CoronaVac Study Group . Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet 2021;398:213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rief W. Fear of adverse effects and COVID-19 vaccine hesitancy: recommendations of the treatment expectation expert group. JAMA Health Forum 2021;2:e210804. [DOI] [PubMed] [Google Scholar]
- 34. Lipsitch M, Jha A, Simonsen L. Observational studies and the difficult quest for causality: lessons from vaccine effectiveness and impact studies. Int J Epidemiol 2016;45:2060–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thune JJ, Signorovitch JE, Kober L, McMurray JJV, Swedberg K, Rouleau J, Maggioni A, Velazquez E, Califf R, Pfeffer MA, Solomon SD. Predictors and prognostic impact of recurrent myocardial infarction in patients with left ventricular dysfunction, heart failure, or both following a first myocardial infarction. Eur J Heart Fail 2011;13:148–153. [DOI] [PubMed] [Google Scholar]
- 36. Flach C, Muruet W, Wolfe CDA, Bhalla A, Douiri A. Risk and secondary prevention of stroke recurrence. Stroke 2020;51:2435–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Modin D, Claggett B, Sindet-Pedersen C, Lassen MCH, Skaarup KG, Jensen JUS, Fralick M, Schou M, Lamberts M, Gerds T, Fosbøl EL, Phelps M, Kragholm KH, Andersen MP, Køber L, Torp-Pedersen C, Solomon SD, Gislason G, Biering-Sørensen T. Acute COVID-19 and the incidence of ischemic stroke and acute myocardial infarction. Circulation 2020;142:2080–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will not be available for others as the data custodians have not given permission.