Abstract
The highly transmissible severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron variant has caused high rates of breakthrough infections in those previously vaccinated with ancestral strain coronavirus disease 2019 (COVID-19) vaccines. Here, we demonstrate that a booster dose of UB-612 vaccine candidate delivered 7–9 months after primary vaccination increased neutralizing antibody levels by 131-, 61-, and 49-fold against ancestral SARS-CoV-2 and the Omicron BA.1 and BA.2 variants, respectively. Based on the receptor-binding domain protein binding antibody responses, the UB-612 third-dose booster may lead to an estimated approximately 95% efficacy against symptomatic COVID-19 caused by the ancestral strain. Our results support UB-612 as a potential potent booster against current and emerging SARS-CoV-2 variants.
Keywords: SARS-CoV-2, COVID-19, Omicron, variant, subunit, vaccine, booster, receptor-binding domain, neutralizing antibody, clinical trial
A booster dose of UB-612, a next-generation subunit protein-peptide vaccine, induced high cross-reactive antibodies against SARS-CoV-2 variants and is predicted to confer approximately 95% efficacy against symptomatic COVID-19 caused by the ancestral SARS-CoV-2 strain.
In November 2021, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron (B.1.1.529) variant of concern was first reported in South Africa and quickly spread, eventually displacing Delta to become the global predominant circulating variant [1]. Currently, the Omicron variant has 5 major circulating sublineages: BA.1, BA.2, BA.4, BA.5, and BA.2.12.1. BA.1 was the dominant variant, circulating throughout November 2021–February 2022 until it was displaced by BA.2 during the spring of 2022. Three new sublineages—BA4, BA5 (emerged in South Africa), and BA.2.12.1 (emerged in the United States)—are poised to become dominant variants and likely to result in new waves of global infection and disease [2].
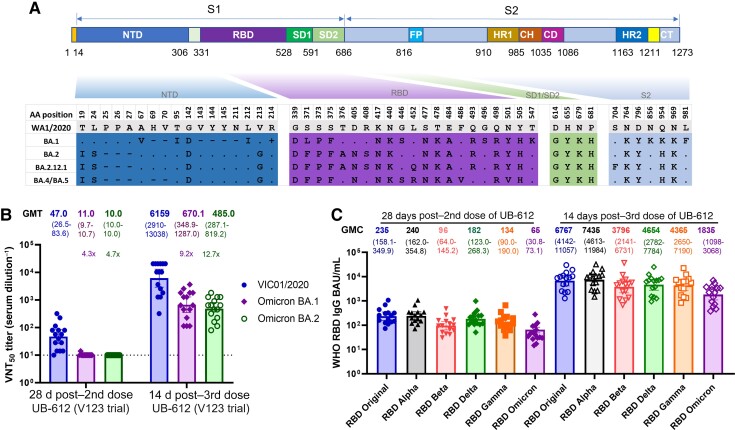
The Omicron variants have >50 new amino acid substitutions, 15–20 of which are in the receptor-binding domain (RBD) of the spike (S) protein [1]. The newly emerged Omicron variants carry additional RBD substitutions: L452R, F486V (BA4 and BA5), and L452Q (BA.12.1) (Figure 1A) [2].
Figure 1.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron BA.1 and BA.2 amino acid substitutions and UB-612 vaccine–induced antibody responses. A, Amino acid substitutions in the spike protein of Omicron’s BA.1, BA.2, BA.2.12.1, BA.4 and BA.5 sublineages. The upper part is the S protein diagram, and the lower part shows the substitutions. The “.”, “-”, and “+” represent sequence identical, deletion, and insertion, respectively, in Omicron variants compared with the US-WA1/2020 virus. B, 50% virus-neutralization antibody titers (VNT50, geometric mean titer [GMT]) against SARS-CoV-2 ancestral strain Victoria/1/2020 (VIC01/2020) and Omicron (B.1.1.529) variant sublineages BA.1 and BA.2 in sera from phase clinical 1 trial (V123) participants (n = 15). The sera were collected at 28 days after 2 doses and at 14 days after the booster dose with UB-612 (100 µg). Data expressed in the reciprocal dilutions for each serum sample and GMT (95% confidence interval) are plotted. C, Receptor-binding domain (RBD)–specific immunoglobulin G (IgG) binding titers (geometric concentration) against SARS-CoV-2 major variants of concern in sera collected 28 days after 2 doses and 14 days after 3 doses with UB-612 (100 µg) from phase 1 clinical trial participants (n = 15). The loss of antibody binding to the RBD of variants compared with the original RBD (ancestral strain) remains stable between 2 and 3 doses of UB-612 vaccine, despite an increase in levels of binding antibodies to RBD. Abbreviations: CD, connector domain; CH, central helix; CT, cytoplasmic tail; FP, fusion peptide; GMC, geometric mean concentration; GMT, geometric mean titer; HR, heptad-repeat; NTD, N-terminal domain; RBD, receptor-binding domain; VNT50, 50% virus neutralization antibody titer.
More than 90% of neutralizing antibodies in plasma of convalescent individuals and up to 99% of neutralizing antibodies elicited by the messenger RNA [mRNA]–1273 vaccine are directed to RBD [3]. The RBD mutations could largely be responsible for Omicron’s ability to evade neutralizing antibodies induced by the authorized coronavirus disease 2019 (COVID-19) vaccines [4]. In multiple studies, sera from primary series vaccineees had 20- to 30-fold reduced neutralization activity against Omicron, compared to the ancestral SARS-CoV-2 or D614G viruses [4–6]. The emergence of new variants, in addition to rapidly waning immunity over time, has raised concerns about breakthrough infections and highlights the need for boosters. Homologous booster vaccines, all based on the full-length S protein, were capable of restoring protective neutralizing antibodies to levels achieved after primary immunization [4].
In contrast to most of the authorized COVID-19 vaccines, which encode the full-length S protein, the UB-612 vaccine candidate is composed of a Wuhan-Hu S1-RBD-sFc fusion protein, and is enriched with an additional 5 peptides representing pan-sarbecovirus-conserved T helper (Th) and Cytotoxic T Lymphocyte (CTL) epitopes on the S2 subunit, membrane (M), and nucleocapsid (N) proteins [7]. A favorable safety and tolerability profile for UB-612 was demonstrated in approximately 4000 participants in phase 1 and phase 2 trials [7]. Two immunizations with UB-612 elicited long-lasting neutralizing antibody titers similar to levels detected in convalescent patients [7]. These antibodies were shown to be cross-reactive against Delta and Omicron variants [7]. A third dose, delivered at 7–9 months after the second dose, boosted the level of neutralizing antibodies by 37-fold compared to its peak titer after the 2-dose primary immunization [7].
The objectives of this study were to evaluate the magnitude of neutralizing antibodies elicited by a third dose (booster) of UB-612 against Omicron and their associated activity to recombinant S and RBD protein antigens across various SARS-CoV-2 variants.
METHODS
After receiving a 2-dose primary vaccine series or a booster given at 7–9 months after the second dose, sera from 15 participants in the phase 1 clinical trial (UB-612, 100-µg dose) (Supplementary Table 1) were tested in a SARS-CoV-2 live virus cytopathic effect (CPE) microneutralization test at Vismederi, Siena, Italy (a Coalition for Epidemic Preparedness Innovations testing laboratory for COVID-19 vaccines). Previously, live virus CPE-based neutralization assays were identified to be the most stringent compared to other plaque reduction–, foci reduction–, or pseudotyped virus–based neutralization assays [9]. The immunoglobulin G (IgG) antibodies binding to SARS-CoV-2 RBD and trimeric spike antigen were tested in a multiplex solid-phase chemiluminescence assay (Meso Scale Discovery) as described previously [10]. The detailed Materials and Methods for clinical trial and sample information, neutralization assays, immunological assays, and statistical analyses are described in the Supplementary Materials.
RESULTS
A UB-612 Booster Dose Significantly Increased the Levels of Neutralizing Antibodies Against the Ancestral Strain and Omicron Variants
Two doses of UB-612 stimulated neutralizing antibody activity against the wild-type SARS-CoV-2 ancestral strain (Victoria/1/2020) (geometric mean titer [GMT], 50% virus neutralization antibody titer [VNT50] of 47.0) and Omicron BA.1 and BA.2 sublineages (GMT VNT50 of 11 and 10, respectively) (Figure 1B) (n = 15). Comparable levels of Omicron neutralizing antibodies were also reported after the primary immunization with mRNA vaccines: mRNA-1273 (GMT pVNT50 of 14) and BNT162b2 (GMT pVNT50 of 7) [5, 6].
A booster dose of UB-612 delivered at 7–9 months after the primary series increased neutralizing antibody titers against the ancestral Victoria strain and the BA.1 and BA.2 variants by 131-, 61-, and 49-fold, respectively, compared to the peak titers achieved after 2 doses (Figure 1B). When compared to the ancestral Victoria strain, the neutralization of Omicron BA.1 and BA.2 was reduced by 9.2- and 12.7-fold, respectively. However, there was only a 1.38-fold reduction in the neutralization of BA.2 compared to BA.1. Previously, in pseudovirus-based neutralization assay, we reported a 5.5-fold decrease in neutralizing activity against BA.1 compared to the Wuhan strain [7].
Expansion of Antibody Breadth Following UB-612 Booster
We evaluated the reactivity of UB-612–elicited antibodies to S and RBD proteins in multiplex enzyme-linked immunosorbent–based assays (ELISAs), using sera from 15 subjects who participated in a phase 1 trial (V123) and 92 randomly selected subjects, including 8 placebos, from a phase 2 clinical trial (V205) (Supplementary Tables 1 and 2). A third dose of UB-612 booster stimulated broadly reactive IgG antibodies, effectively binding to RBDs of 14 divergent SARS-CoV-2 variants, including Alpha, Beta, Gamma, Delta, and Omicron (Figure 1C and Supplementary Figure 1). Compared to the peak titers after the second dose, the UB-612 booster increased IgG-binding titers against the Omicron RBD by >40-fold, and RBDs of other SARS-CoV-2 variants by 30- to 50-fold. The loss of IgG-binding activity to RBD (binding antibody units [BAU]/mL) was variant dependent and did not depend on the number of UB-612 immunizations: Alpha (0.98-fold), Beta (2.44-fold), Delta (1.33-fold), Gamma (1.77-fold), and Omicron (3.3-fold) after 2 UB-612 immunizations; and Alpha (0.91-fold), Beta (1.8-fold), Delta (1.4-fold), Gamma (1.55-fold), and Omicron (3.7-fold) after a third-dose booster.
Similar to RBD binding, the results of S protein–binding antibody responses (S:ACE2- and RBD:ACE2-blocking antibody titers) confirmed the extent of cross-reactivity of UB-612 immune sera after 2 immunizations or a third-dose booster (Supplementary Figures 2 and 3). These data confirm the breadth of UB-612–elicited humoral responses, measured by functional antibody (eg neutralization and ACE2 inhibition) and IgG-binding assays (against S and RBD), a differentiating property of UB-612 primarily attributed to its antigenic component based on the RBD subunit protein [7].
Vaccine Efficacy Prediction
We compared the level of UB-612–elicited IgG antibodies (expressed as geometric mean concentration [GMC]) with data previously reported for several authorized vaccines determined in equivalent S- and RBD-binding assays [8]. After a 2-dose primary immunization series, the GMCs of UB-612–elicited IgG antibodies were 69 (phase 1, n = 15) and 127 (phase 2, n = 84) BAU/mL against the Wuhan S protein, and 235 (phase 1, n = 15) and 494 (phase 2, n = 84) BAU/mL against the RBD antigen (Supplementary Figure 4). These IgG responses were comparable to those observed in individuals after the primary immunization with adenovirus-vectored vaccines (1-dose Ad26.COV2.S or 2-dose ChAdOx1-S) but were lower than the responses observed after 2 immunizations with mRNA vaccines. The additional third dose with UB-612 boosted the levels of both S- and RBD protein–binding IgG antibodies by more than 16- and 13-fold, reaching the GMCs of 2138 and 6767 (BAU/mL), respectively, and matching the levels achieved by 2 immunizations with the mRNA vaccines.
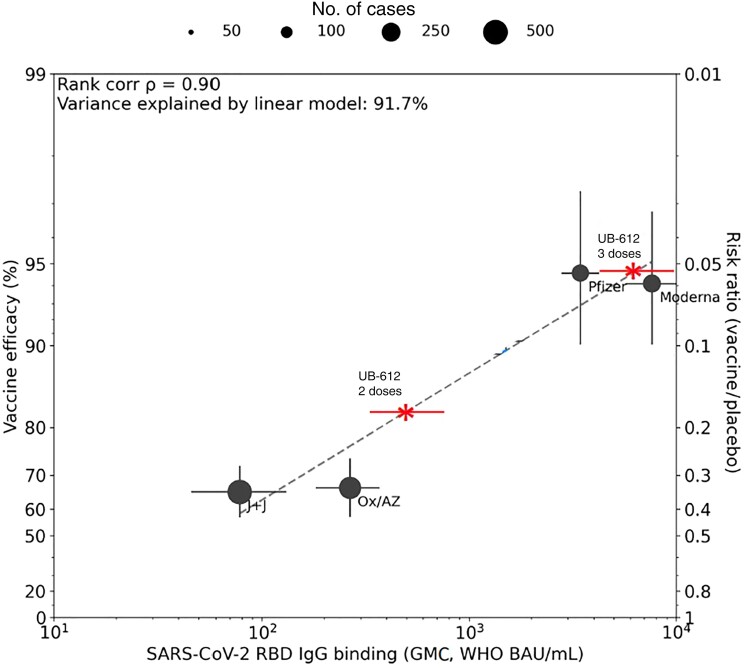
We further utilized a vaccine efficacy prediction model based on the RBD activity of IgG antibodies to the ancestral Wuhan strain, extending on previous reports based on neutralizing antibodies [11] or S protein–binding activities [8]. According to this model, the predicted efficacy of a 2-dose primary immunization with UB-612 against symptomatic COVID-19 caused by the prototype strain is approximately 72% (considering the GMC of 235 BAU/mL from 15 phase 1 subjects) or approximately 82% (considering the GMC of 494 BAU/mL from 84 phase 2 subjects), and after the booster dose is approximately 95% (considering the GMC of 6767 BAU/mL from 15 phase 1 subjects) (Figure 2 and Supplementary Figure 4).
Figure 2.
Estimated efficacy of UB-612 vaccine after 2 and 3 doses. A model bridging vaccine-induced receptor-binding domain (RBD) immunoglobulin G (IgG) response to vaccine efficacy against symptomatic coronavirus disease 2019 caused by the ancestral Wuhan strain [5]. Estimated efficacy of UB-612 after 2 doses is ∼72% (95% confidence interval [CI], 70%–80%) based on RBD-binding IgG antibodies from 15 participants (phase 1) (geometric mean concentration [GMC], 235 binding antibody units [BAU]/mL [95% CI, 158–350]), ∼82% (95% CI, 80%–85%) based on RBD-binding IgG antibodies from 84 randomly selected phase 2 participants (GMC, 494 BAU/mL [95% CI, 337–725], shown in this graph), and ∼95% (95% CI, 93%–97%) after a booster vaccination (GMC, 6767 [95% CI, 4142–11 057]). Abbreviations: BAU, binding antibody units; GMC, geometric mean concentration; IgG, immunoglobulin G; J+J, Johnson & Johnson; Ox/AZ, Oxford/AstraZeneca; RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WHO, World Health Organization.
DISCUSSION
We evaluated humoral immune responses stimulated by UB-612 against SARS-CoV-2 variants and compared the results to the data reported or generated internally for several authorized COVID-19 vaccines, recognizing the caveats in doing such comparisons (including lack of international standard reagents and use of different assays across manufacturers). Based on available reports, the level of neutralizing antibodies against SARS-CoV-2 variants after the UB-612 booster dose was comparable to the levels elicited by a booster dose of the mRNA BNT162b2 [6] and mRNA-1273 [5] vaccines. The homologous booster of mRNA-1273 or BNT162b2 vaccines dramatically increased neutralizing antibodies to Omicron (20- to 30-fold) compared with the modest increase reported for the ancestral strain (1- to 4-fold), likely due to a higher baseline titer for the ancestral strain [5, 6]. UB-612 vaccine elicited high levels of neutralizing antibodies to Omicron variants with a 49- to 61-fold increase in VNT50 compared to the preboost baseline.
There is growing clinical evidence linking protection against COVID-19 with humoral and T-cell immune responses. Previously, several groups bridged the level of vaccine-induced antibodies to protection against SARS-CoV-2 infection [8, 12]. It is well established that the RBD-binding antibodies have a strong correlation to virus neutralization [3] and that neutralizing antibody responses directly correlate with the vaccine-mediated protection against COVID-19 [10]. Since the RBD is the principal neutralizing antibody target in UB-612, we evaluated anti-RBD-binding IgG antibodies stimulated by UB-612 and several authorized COVID-19 vaccines concurrently in the same laboratory and test conditions at University College London, United Kingdom. Booster immunization with UB-612 recalled a high level of RBD-binding IgG antibodies matching the levels achieved by the primary immunization with mRNA or exceeded those for the adenovirus-vectored vaccines, across all tested SARS-CoV-2 variants.
By bridging clinical efficacy and RBD-binding IgG responses for the authorized COVID-19 vaccines, we predicted that UB-612 may confer efficacy of 95% against symptomatic COVID-19 resulting from the infection with ancestral SARS-CoV-2 strains. Based on our data showing that a booster dose of UB-612 induced comparable levels of Omicron-specific neutralizing and RBD-binding IgG antibodies to those elicited by mRNA vaccines, it is likely that the UB-612 booster could match the efficacy of mRNA vaccine boosters to combat Omicron variants.
Moreover, in a recent study, a booster of a SARS-CoV-2 mRNA vaccine was shown to develop new memory B-cell clones specific to the conserved epitopes of RBD, enhancing the breadth of cross-variant neutralization [13]. We believe that UB-612, which contains only the RBD immunogen, unlike most S protein–based vaccines, may be more effective in recalling, persisting, or generating new memory B-cell clones against the conserved RBD epitopes. Another potential advantage of the UB-612 vaccine is the inclusion of Th/CTL peptides from S2, M, and N proteins [7], which are highly conserved in Omicron variants (data not shown) and may provide long-lasting antibody responses [12] that could further differentiate UB-612 from many authorized vaccines. There is growing evidence supportive of the importance of vaccine-elicited CD8+ T cells in protection against Omicron [14].
In summary, a booster dose of UB-612 elicited robust S- and RBD-specific binding IgG and virus-neutralizing antibodies against multiple SARS-CoV-2 variants, including Omicron BA.1 and BA.2 sublineages. The magnitude and extent of reactivity of the neutralizing antibody responses after the UB-612 booster match those reported for the authorized vaccines, including BNT162b2 and mRNA-1273.
Our results indicate that UB-612 could offer an alternative strategy for a booster vaccine, with a predicted efficacy of 95% against the original SARS-CoV-2. As SARS-CoV-2 continues to evolve and COVID-19 cases increase due to the newly emerged Omicron variants, concerns have been raised about the effectiveness of current vaccines. Because the UB-612 vaccine is not currently authorized, its efficacy/effectiveness against Omicron is unknown. UB-612 is currently being evaluated in noninferiority clinical trials as a heterologous booster for multiple authorized COVID-19 vaccines including BNT162b2 and ChAdOx nCoV-19, both of which have shown substantially increased protection by a homologous or heterologous booster; however, immunity waned quickly at approximately 10 weeks or more (from 62%–73% to 39%–64%, depending on the type of vaccine used as the primary or the booster dose) [15]. It remains to be seen if UB-612, as shown to elicit long-lasting neutralizing antibody responses (half-life of ∼27 weeks) [7] with neutralization against multiple currently circulating variants, will extend its longevity and breadth of protection as heterologous booster to other authorized vaccines.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Supplementary Material
Contributor Information
Farshad Guirakhoo, Vaxxinity Inc, Dallas, Texas, USA.
Shixia Wang, Vaxxinity Inc, Dallas, Texas, USA.
Chang Yi Wang, United Biomedical Inc Asia, Hsinchu, Taiwan.
Hui Kai Kuo, United Biomedical Inc Asia, Hsinchu, Taiwan.
Wen Jiun Peng, United Biomedical Inc Asia, Hsinchu, Taiwan.
Hope Liu, United Biomedical Inc Asia, Hsinchu, Taiwan.
Lixia Wang, Vaxxinity Inc, Dallas, Texas, USA.
Marina Johnson, Great Ormond Street Institute of Child Health, University College London, London, United Kingdom.
Adam Hunt, Great Ormond Street Institute of Child Health, University College London, London, United Kingdom.
Mei Mei Hu, Vaxxinity Inc, Dallas, Texas, USA.
Thomas P Monath, Vaxxinity Inc, Dallas, Texas, USA.
Alexander Rumyantsev, Vaxxinity Inc, Dallas, Texas, USA.
David Goldblatt, Great Ormond Street Institute of Child Health, University College London, London, United Kingdom.
Notes
Author contributions. F. G. conceived, conceptualized, and wrote the manuscript. S. W. analyzed data, interpreted data, and wrote the manuscript. C. Y. W., W.-J. P., H. L., and H. K. K. received consent from the patients for the samples to be used for this study and organized shipment of their sera for binding and neutralization assays and reviewed the manuscript. M. M. H. and T. P. M. assisted in planning of the study. A. R. wrote and reviewed the manuscript. L. W. analyzed the data. D. G., M. G., and A. H. performed experiments and interpreted the data.
Acknowledgments. We thank many colleagues at UBI Asia, United BioPharma (UBP), and Vaxxinity who designed, developed, and produced the UB-612 vaccine candidate and performed the phase 1 and 2 clinical trials in Taiwan. We also thank the phase 1 (NCT04545749) and phase 2 (NCT04773067) clinical trial participants, from whom the postvaccination sera were obtained. We are also grateful to the Vismederi team for their work on live virus-neutralizing antibody assays.
Financial support. This work was supported by Vaxxinity Inc.
References
- 1. Viana R, Moyo S, Amoako DG, et al. . Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in Southern Africa. Nature 2022; 603:679–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khan K, Karim F, Ganga Y, et al. . Omicron sub-lineages BA.4/BA.5 escape BA.1 infection elicited neutralizing immunity. medRxiv [Preprint]. Posted online 1 May 2022. doi: 10.1101/2022.04.29.22274477. [DOI] [Google Scholar]
- 3. Kleanthous H, Silverman JM, Makar KW, Yoon I-K, Jackson N, Vaughn DW. Scientific rationale for developing potent RBD-based vaccines targeting COVID-19. NPJ Vaccines 2021; 6:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pérez-Then E, Lucas C, Monteiro VS, et al. . Neutralizing antibodies against the SARS-CoV-2 Delta and Omicron variants following heterologous CoronaVac plus BNT162b2 booster vaccination. Nat Med 2022; 28:481–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pajon R, Doria-Rose NA, Shen X, et al. . SARS-CoV-2 Omicron variant neutralization after mRNA-1273 booster vaccination. N Engl J Med 2022; 386:1088–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Muik A, Lui BG, Wallisch A-K, et al. . Neutralization of SARS-CoV-2 Omicron by BNT162b2 mRNA vaccine-elicited human sera. Science 2022; 375:678–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang CY, Hwang K-P, Kuo H-K, et al. . A multitope SARS-COV-2 vaccine provides long-lasting B cell and T cell immunity against Delta and Omicron variants. J Clin Invest 2022; 132:e157707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goldblatt D, Fiore-Gartland A, Johnson M, et al. . Towards a population-based threshold of protection for COVID-19 vaccines. Vaccine 2022; 40:306–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mattiuzzo G, Bentley EM, Hassall M, et al. . Establishment of the WHO International Standard and Reference Panel for anti-SARS-CoV-2 antibody. 2020. https://www.who.int/publications/m/item/WHO-BS-2020.2403. Accessed 14 February 2022.
- 10. Maciola AK, Raja ML, Pacenti M, et al. . Neutralizing antibody responses to SARS-CoV-2 in recovered COVID-19 patients are variable and correlate with disease severity and receptor-binding domain recognition. Front Immunol 2022; 13:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cromer D, Steain M, Reynaldi A, et al. . Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis. Lancet Microbe 2022; 3:e52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khoury DS, Cromer D, Reynaldi A, et al. . Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021; 27:1205–11. [DOI] [PubMed] [Google Scholar]
- 13. Muecksch F, Wang Z, Cho A, et al. . Increased memory B cell potency and breadth after a SARS-CoV-2 mRNA boost [manuscript published online ahead of print 21 April 2022]. Nature 2022. doi: 10.1038/s41586-022-04778-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chandrashekar A, Yu J, McMahan K, et al. . Vaccine protection against the SARS-CoV-2 Omicron variant in macaques. Cell 2022; 185:1549–55.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Andrews A, Stowe J, Kirsebom F, et al. . Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med 2022; 386:1532–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.