Abstract
During malolactic fermentation (MLF) in grape must and wine, heterofermentative lactic acid bacteria may degrade arginine, leading to the formation of ammonia and citrulline, among other substances. This is of concern because ammonia increases the pH and thus the risk of growth by spoilage bacteria, and citrulline is a precursor to the formation of carcinogenic ethyl carbamate (EC). Arginine metabolism and growth of Lactobacillus buchneri CUC-3 and Oenococcus oeni strains MCW and Lo111 in wine were investigated. In contrast to L. buchneri CUC-3, both oenococci required a higher minimum pH for arginine degradation, and arginine utilization was delayed relative to the degradation of malic acid, the main aim of MLF. This allows the control of pH increase and citrulline formation from arginine metabolism by carrying out MLF with pure oenococcal cultures and inhibiting cell metabolism after malic acid depletion. MLF by arginine-degrading lactobacilli should be discouraged because arginine degradation may lead to the enhanced formation of acids from sugar degradation. A linear relationship was found between arginine degradation and citrulline excretion rates. From this data, strain-specific arginine-to-citrulline conversion ratios were calculated that ranged between 2.2 and 3.9% (wt/wt), and these ratios can be used to estimate the contribution of citrulline to the EC precursor pool from a given amount of initial arginine. Increasing arginine concentrations led to higher rates of growth of L. buchneri CUC-3 but did not increase the growth yield of either oenococcus. These results suggest the use of non-arginine-degrading oenococci for inducing MLF.
The term MLF refers to the microbial conversion of l-malic acid to l-lactic acid in grape musts or wine by MLB. MLB are wine LAB belonging to three genera and include homo- and heterofermentative lactobacilli, homofermentative pediococci, and the heterofermentative species Oenococcus oeni (formerly Leuconostoc oenos) (15). MLF may be due to MLB naturally present in wine, but nowadays MLF is often induced with commercial starter cultures. Its main effects are to reduce the acidity of wines by converting dicarboxylic malic acid to monocarboxylic lactic acid and the modification of flavor properties (8). O. oeni is the preferred species for carrying out MLF, whereas most lactobacilli and pediococci are considered undesirable or spoilage bacteria because of flavor depreciation (6).
Besides malic acid, some heterofermentative MLB degrade arginine, which is quantitatively one of the most important amino acids in grape musts and wines (25, 27). Complete degradation of arginine by MLB occurs via the ADI pathway, leading to the production of ammonia, ornithine, ATP, and CO2 (14). During the degradation of arginine, some citrulline is excreted (13). Arginine degradation by MLB has several enological implications (Fig. 1): the production of ammonia increases the pH and the risk of growth of spoilage microorganisms; formation of ATP may give arginine-positive MLB, including spoilage MLB, an ecological advantage; and the excretion of citrulline is toxicologically of concern, since citrulline is a precursor in the formation of carcinogenic EC (urethane) in wine (31).
FIG. 1.
ADI pathway for arginine degradation by LAB and its enological significance.
In a recent study of major commercial MLB, it was shown that all strains tested degraded arginine and excreted citrulline (19). Investigations with resting cells in a wine buffer revealed a linear relationship between arginine degradation and citrulline excretion rates (20). Further study of this relationship and the kinetics of arginine degradation in wines is of paramount importance for the control of citrulline formation by MLB.
Over the last few years, many MLB have been isolated and prepared commercially for the successful induction of MLF in wine. This requires good growth under the harsh conditions found in wine and effective malic acid degradation. While successful MLF may still remain difficult to achieve in some wine types and further optimizations are needed, it is now necessary to concentrate on key metabolic aspects and organoleptic effects of MLB.
This paper reports new findings of arginine metabolism of MLB in wine and its implications. Two commercial strains of O. oeni and one strain of Lactobacillus buchneri were investigated in time course studies of wine at several pH values and arginine concentrations. The effect of the strains on arginine and citrulline concentrations and the relationship to malic acid degradation was investigated, as well as the effect of arginine on the growth of MLB.
MATERIALS AND METHODS
Abbreviations.
MLB, malolactic bacteria; MLF, malolactic fermentation; LAB, lactic acid bacteria; ADI, arginine deiminase; EC, ethyl carbamate; OD750, optical density at 750 nm; OTC, ornithine transcarbamylase; Ymax, maximum growth yield.
Microorganisms.
The MLB used were from the Wine Microbiology Laboratory Culture Collection of the Institute of Molecular BioSciences, Massey University, Palmerston North, New Zealand. L. buchneri CUC-3 was originally isolated from a Californian wine undergoing MLF (22) and has been used previously as an arginine-degrading model organism (13, 19, 20). O. oeni strains MCW and Lo111 are commercially available from Lallemand, Inc., Montreal, Canada; strain Lo111 is part of the two-strain product Bitec D1.
Wine.
A natural, pure-white grape juice without preservatives (Grapetise; Pacific Beverages, Bayswater, Australia) was adjusted with sucrose to a total soluble content of 17 Brix (specific gravity, 1.0696 g ml−1) and used for fermentations without further modification. Alcoholic fermentation was carried out at 18°C after inoculation with 2% (vol/vol) Saccharomyces bayanus strain Première Cuvée, pregrown in grape juice with 5 g of yeast extract liter−1 added (pH 4.5). After completion of alcoholic fermentation, as assessed by a colorimetric test for reducing sugars (Clinitest Ames; Miles, Inc., Elkhart, Ind.), the wines were racked off, cold settled overnight and filtered through sterilization-grade cellulose pads (Ekwip D9; Revesby, New South Wales, Australia). The wine had 9.4% (vol/vol) ethanol; no free SO2 was detected. Glucose, fructose, and malic acid concentrations were 20 mg liter−1, 390 mg liter−1, and 1.2 g liter−1, respectively. Ammonia, urea, and arginine were present in trace amounts. The pH after degassing was 3.2. The wine was adjusted to 3 g of malic acid liter−1 (Sigma M-1000) and 1 g of glucose liter−1 (BDH 101174Y), separated into 1-liter batches, and adjusted to several pH values (pH 3.3, 3.6, and 3.9 with NaOH) and arginine (Sigma A-5131) concentrations (0, 0.5, 1, and 1.5 g liter−1).
Experimental conditions.
After sterile filtration of the wine (0.45-μm-pore-size filters; Sartorius, Göttingen, Germany), 1 liter of wine was poured into glass bottles (each, 1 liter; Schott Duran, Mainz, Germany), and MLF was induced at 20°C by inoculation with 2% (vol/vol) L. buchneri CUC-3 and O. oeni strains MCW and Lo111, pregrown in 50% grape juice with 5 g of yeast extract liter−1 added (pH 3.6). The initial population of bacteria was 1.8 × 106, 9.2 × 105, and 7.8 × 105 CFU per ml for strains CUC-3, MCW, and Lo111, respectively. When samples were taken, the wines were protected from oxidation by flushing bottles with CO2.
Analytical methods.
Growth during MLF was measured by determining the OD750 of samples after mixing the wine in the fermentation bottles. Cell enumeration was carried out by counting CFU after plating out appropriately diluted samples on MRS agar (7) containing 20% pure apple juice (without preservatives; Frucor Beverages, Wiri, Aukland, New Zealand) and incubating at 27°C for 5 days. Arginine concentration was determined colorimetrically by the Staron-Allard method (18). Citrulline concentration was also determined colorimetrically using the method of Archibald as modified by Spector and Jones (26). Concentrations of malic acid, glucose, fructose, and ethanol were determined with enzymatic test kits from Roche (previously Boehringer Mannheim [2]). Free SO2 was determined by the procedure of Ripper as modified by Amerine and Ough (1).
RESULTS
Kinetics of arginine degradation at different pH values.
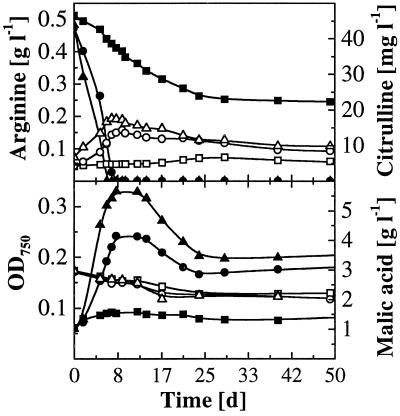
Figure 2 shows the time course of arginine and malic acid degradation and citrulline formation by L. buchneri CUC-3 at several pH values. Whereas degradation of malic acid was only partially achieved at all pH values, arginine was rapidly depleted at pH 3.9 and 3.6 and degraded to 50% at pH 3.3. Degradation of arginine and excretion of citrulline concurred with the increase in biomass, and citrulline was partially reutilized at the end of arginine degradation.
FIG. 2.
Time course of arginine and malic acid degradation and citrulline formation by L. buchneri CUC-3 in wine with 0.5 g of initial arginine liter−1 at several initial pH values. Arginine and OD750, solid symbols; citrulline and malic acid, open symbols. Initial pH values of arginine: ▪, 3.3; ●, 3.6; ▴, 3.9.
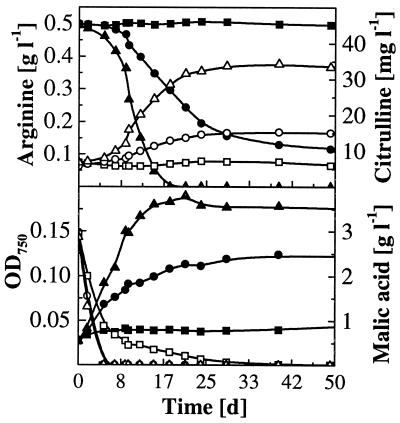
In contrast, O. oeni MCW depleted malic acid at all pH values tested (Fig. 3). Arginine was fully consumed only at pH 3.9 after 19 days and degraded to 80% at pH 3.6. At all pH values, malic acid degradation was completed before significant degradation of arginine and excretion of citrulline occurred. At pH 3.3, where malic acid degradation was delayed, arginine was not degraded at all. Similar results were obtained with O. oeni strain Lo111, which depleted arginine at pH 3.9 within 25 days, but degraded only 20% at pH 3.6 and none at pH 3.3. As for strain MCW, malic acid degradation by Lo111 was finished well ahead of arginine degradation and citrulline excretion. In contrast to L. buchneri strain CUC-3, citrulline was not reutilized by either oenococcus.
FIG. 3.
Time course of arginine and malic acid degradation and citrulline formation by O. oeni MCW in wine with 0.5 g of initial arginine liter−1at several initial pH values. Arginine and OD750, solid symbols; citrulline and malic acid, open symbols. Initial pH values: ▪, 3.3; ●, 3.6; ▴, 3.9.
Arginine degradation at different arginine concentrations.
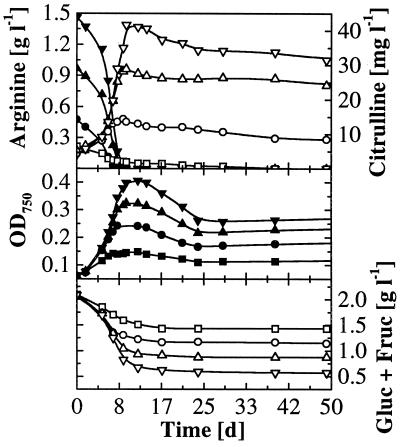
Figure 4 shows arginine and sugar utilization and citrulline formation by L. buchneri CUC-3 at several initial arginine concentrations. Higher initial arginine concentrations led to more rapid growth with the result of faster arginine degradation. This resulted in 1.5 g of arginine liter−1 being degraded in the same time as 0.5 g liter−1. Arginine degradation rates and corresponding citrulline excretion rates from all experiments carried out with strain CUC-3 (data pooled from experiments with several initial pH values and arginine concentrations) correlated well. A linear regression analysis (method of least squares) performed with all the data sets gave the following function: citrulline excretion rate = −0.003 (±0.008) + 0.023 (±0.002) × arginine degradation rate (where the standard error is given in parentheses, the correlation coefficient [r] is 0.91, and the number of samples [n] is 41). The slope of this function constitutes an arginine-to-citrulline conversion ratio (wt/wt) with a value of 2.3% ± 0.2%. Likewise, arginine-to-citrulline conversion ratios were calculated from pooled data for both oenococci and were 3.8% ± 0.1% (r = 0.96; n = 78) for strain MCW and 3.9% ± 0.2% (r = 0.96; n = 45) for strain Lo111.
FIG. 4.
Time course of arginine and sugar utilization and citrulline formation by L. buchneri CUC-3 in wine at initial pH 3.6 and several initial arginine concentrations. Arginine and OD750, solid symbols; citrulline and combined glucose and fructose, open symbols. Initial arginine concentrations: ▪, 0 g liter−1; ●, 0.5 g liter−1; ▴, 1 g liter−1; ▾, 1.5 g liter−1.
Effect of arginine concentrations on wine pH and growth.
Table 1 shows the pH values after malic acid depletion (oenococci only) and at the end of incubations for all MLB at several arginine concentrations. Because of the ability of L. buchneri CUC-3 to degrade arginine effectively, the fermentation of this strain led to higher pH values at higher arginine concentrations. However, with the exception of the fermentations at 1.5 g of arginine liter−1, the final pH values achieved by strain CUC-3 were lower than those attained at the end of the incubation time by strains MCW and Lo111.
TABLE 1.
Wine pH values at the end of incubation of L. buchneri CUC-3 (49 days) and O. oeni strains MCW (49 days) and Lo111 (37 days) at several initial arginine concentrationsa
| Strain | pH valuesb at initial arginine concn (g liter−1) of:
|
|||
|---|---|---|---|---|
| 0 | 0.5 | 1 | 1.5 | |
| CUC-3 | 3.61 | 3.7 | 3.84 | 3.93 |
| MCW | (3.8) 3.88 | (3.8) 3.90 | (3.8) 3.94 | (3.82) 3.87 |
| Lo111 | (3.81) 3.84 | (3.8) 3.85 | (3.8) 3.85 | (3.8) 3.85 |
The wine pH prior to MLF was 3.6.
Values in parentheses show pH values during incubation on completion of malic acid depletion (for strain MCW, after 7.3 days; for Lo111, after 6.9 days).
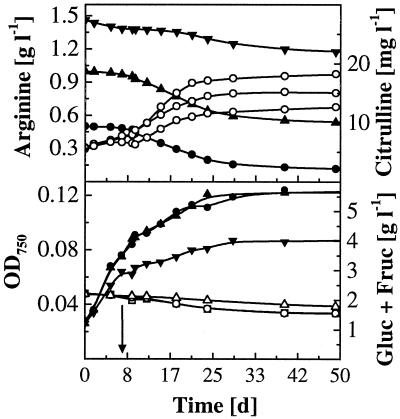
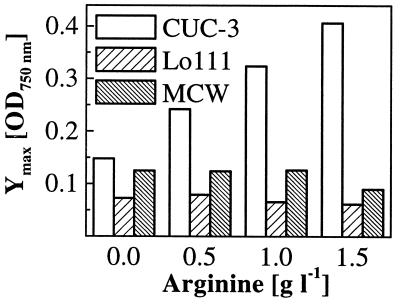
For L. buchneri CUC-3, higher initial arginine concentrations led to increased growth and faster degradation of fructose and glucose (Fig. 4). The extent and duration of growth were determined by the arginine available, since growth ceased after arginine depletion even though fermentable hexoses were still present. In contrast, high initial arginine concentrations did not increase growth and degradation of glucose and fructose by O. oeni MCW. Instead, growth inhibition was observed at the highest arginine concentration, 1.5 g liter−1 (Fig. 5). The same was found for O. oeni Lo111, where sugar degradation was similarly uniform and growth was inhibited at 1 and 1.5 g of initial arginine liter−1. The maximum growth yield data of all MLB are summarized in Fig. 6. A biphasic growth pattern was observed for both O. oeni strains MCW and Lo111, where growth continued for several days at a lower rate after depletion of malic acid. This is shown for strain MCW in Fig. 5.
FIG. 5.
Time course of arginine and sugar utilization and citrulline formation by O. oeni MCW in wine at initial pH 3.6 and several initial arginine concentrations. Arginine and OD750, solid symbols; citrulline and combined glucose and fructose, open symbols. Initial arginine concentrations: ●, 0.5 g liter−1; ▴, 1 g liter−1; ▾, 1.5 g liter−1. Arrow, time of malic acid depletion for all treatments.
FIG. 6.
Ymax values for L. buchneri CUC-3 and O. oeni strains MCW and Lo111 from malolactic fermentations at several initial arginine concentrations.
DISCUSSION
Two major precursors for the formation of carcinogenic EC in wine are urea (9) and citrulline (13, 31). Both are products of microbial arginine degradation. Urea is formed by yeast arginase, and citrulline is an intermediate in the ADI pathway of heterofermentative MLB. Since alcoholic fermentation by yeast traditionally is carried out before MLF, control of EC formation has been mainly by the reduction of arginine levels in musts and the selection of low-urea-producing yeast or yeast that reutilizes most of the produced urea (Ethyl carbamate preventative action manual, Department of Viticulture and Enology, Cooperative Extension, University of California; http://vm.cfsan.fda.gov/∼frf/ecintro.html). This is understandable, since most arginine is degraded during alcoholic fermentation. However, some wines have been reported to have arginine levels as high as 2 to 5 g liter−1 after alcoholic fermentation (4, 10, 27). In a recent long-term study of the formation of EC in table wines, it was found that 20 mg of citrulline liter−1 would react to yield 30 μg of EC liter−1 after 3 years of storage at 15°C, and at this temperature citrulline equaled the EC formation potential of urea (R. Morenzoni, personal communication). Canada has a legal EC limit of 30 μg liter−1 (5), and in the United States there is a voluntary limit of 15 μg liter−1 (3; Urethane in alcoholic beverages under investigation, U.S. Food and Drug Administration [http://vm.cfsan.fda.gov/∼frf/fc0293ur.html]). Statistical data for United States table wines show that a general EC limit of 15 μg liter−1 on wines is feasible (http://vm.cfsan.fda.gov/∼frf/fc0293ur.html), and this would be reasonable from a toxicological point of view, as well (24). Considering these values, addition of citrulline to the EC precursor pool by arginine-degrading MLB may lead to exceeding existing or future voluntary or legal EC limits.
In this study, the arginine metabolism of two commercial oenococcal strains and one lactobacillus strain was investigated under laboratory winemaking conditions. All strains were able to degrade arginine in wine and to excrete considerable amounts of citrulline, underpinning the need to control arginine degradation by wine MLB. However, differences were found in the minimum pH necessary for degradation of arginine and the kinetics of its degradation. Liu et al. showed that arginine was degraded by oenococci in a synthetic wine at pH 4 but not pH 3.2 (12). In this study, oenococci were able to degrade arginine at pH 3.9 and partially at pH 3.6, but no degradation occurred at pH 3.3. In contrast, L. buchneri CUC-3 degraded arginine at all pH values tested. In addition to the higher minimum pH required, arginine degradation by oenococci was delayed in comparison to malic acid degradation. In practice, this allows the winemaker to avoid arginine degradation by carefully monitoring malic acid degradation and removing cells or inhibiting cell activity after malolactic conversion in the wine by oenococci. This might be desirable from a sensory point of view, too, since the concentrations of diacetyl, an important flavor compound produced by MLB, have been reported to be highest at the end of malolactic conversion (21).
As in previous studies with resting cells (20), a linear proportionality was found between arginine degradation and citrulline excretion rates in wine. Arginine-to-citrulline conversion ratios were calculated that ranged between 2.2 and 3.9% (wt/wt). These ratios are important, since they allow estimation of the potential addition to the EC precursor pool by citrulline from a given amount of initial arginine. Additionally, ratios could be used for the comparative assessment of the strain-specific risk of citrulline excretion. The results are interesting from a metabolic viewpoint, too. The excretion of citrulline suggests that the citrulline-degrading OTC (Fig. 1) is a limiting step in the ADI pathway, and it was previously shown that citrulline accumulates intracellularly during arginine degradation (19). The accumulation and excretion of citrulline may be attributable to the fact that the degradation of citrulline by OTC is thermodynamically unfavorable (23) or to the inhibition of OTC through ATP formed by carbamate kinase (29), the last enzyme of the ADI pathway.
Although L. buchneri CUC-3 effectively degraded arginine, it led only to a moderate pH increase because arginine degradation favored acid formation from sugar degradation (Fig. 4). Additionally, strain CUC-3 was able to reutilize some of the excreted citrulline, whereas both oenococci were not. Nonetheless, strain CUC-3 would not be the preferred MLB to induce MLF, since sugar degradation by heterofermentative LAB leads to the formation of variable amounts of acetic acid which can render a wine unacceptable from a sensory viewpoint or can exceed legal limits for acetic acid. Moreover, malic acid was not degraded efficiently by strain CUC-3. This further verifies the preference for the use of oenococci for MLF. Oenococci degraded sugars only marginally, and both the degradation of arginine (Fig. 5) and the resulting pH rise (Table 1) could have been avoided by stopping further microbial activity after complete malolactic conversion.
Liu studied the effect of arginine on growth of MLB in a defined medium at wine pH and reported that arginine degradation increased growth of two lactobacilli but not the oenococcus examined (11). In this study, we confirmed this for the wine environment. Only L. buchneri CUC-3 was able to increase Ymax values at higher initial arginine concentrations, suggesting effective energy coupling from arginine degradation to growth. Both oenococci were not able to increase Ymax under these conditions. Manca de Nadra et al. (16) found growth inhibition of a L. buchneri strain by high concentrations of arginine (>5 g liter−1), and Liu observed prolonged lag phases for two lactobacilli under similar conditions (11). We found growth inhibition of oenococci in wine already existing at lower arginine concentrations, but no inhibition was observed for L. buchneri CUC-3. It has been suggested for Streptococcus lactis (now Lactococcus lactis subsp. lactis) that high arginine concentrations reduce growth by inhibiting uptake of other amino acids by a common amino acid carrier (28), and a similar mechanism could be valid for oenococci. Although growth inhibition by high arginine concentrations is not likely to be important because of the rarity of this occurring in wine, the inability of oenococci to use arginine to increase growth in wine is enologically significant. That is, non-arginine-degrading oenococci could be used for inducing MLF without the risk of being overgrown by arginine-positive strains.
Growth of L. buchneri CUC-3 was clearly driven by the presence of arginine in wine, but growth of both oenococci correlated with malic acid degradation. Significant arginine concentrations were left in oenococcal cultures entering stationary phase (Fig. 5), and therefore low arginine degradation rates were more likely a result of growth cessation than a reason for it. However, arginine degradation by oenococci may be beneficial in maintaining some cell growth for a limited time, since a biphasic growth pattern was observed for both strains. Malolactic conversion has been reported to contribute to the acid tolerance of MLB at the low pH values present in wine (30). Our data suggest that whereas oenococci increase their acid tolerance by the degradation of malic acid, L. buchneri CUC-3 achieves this more efficiently by degrading arginine, as has been shown for several LAB of nonwine origin (17).
From the results presented in this paper, we can conclude that it is possible to reduce the risk of formation of citrulline by MLB in wines with high residual arginine concentrations by carrying out MLF with pure oenococcal cultures and precise determination of complete malolactic conversion, followed by inhibition of bacterial activity. In the long term, non-arginine-degrading O. oeni should be used for induction of MLF. Further work will focus on the influence of different wine constituents on the degradation of arginine by MLB to further understand and control arginine metabolism.
ACKNOWLEDGMENTS
This investigation was supported by a grant from the American Vineyard Foundation (AVF E101).
We wish to thank Patrice Laforce and Sybille Krieger (Lallemand, Inc.) for providing commercial malolactic strains.
REFERENCES
- 1.Amerine M A, Ough C S. Methods for analysis of musts and wine. New York, N.Y: Wiley-Interscience Publications; 1974. [Google Scholar]
- 2.Boehringer GmbH. Methods of biochemical analysis and food analysis. Mannheim, Germany: Boehringer GmbH; 1989. [Google Scholar]
- 3.Canas B J, Joe F L, Diachenko G W, Burns G. Determination of ethyl carbamate in alcoholic beverages and soy sauce by gas chromatography with mass selective detection: collaborative study. J AOAC Int. 1994;77:1530–1536. [PubMed] [Google Scholar]
- 4.Capela A B C, Bakker J. Determination of free amino acids in port wine. In: Rantz J M, editor. Proceedings of the International Symposium on Nitrogen in Grapes and Wine. Seattle, Wash: American Society for Enology and Viticulture; 1991. pp. 224–227. [Google Scholar]
- 5.Conacher H B S, Page B D. Proceedings of Euro Food Tox II; Interdisciplinary Conference on Natural Toxicants in Food. Zürich, Switzerland. 1986. Ethyl carbamate in alcoholic beverages: a Canadian case history; pp. 237–242. [Google Scholar]
- 6.Davis C R, Wibowo D, Eschenbruch R, Lee T H, Fleet G H. Practical implications of malolactic fermentation: a review. Am J Enol Vitic. 1985;36:290–301. [Google Scholar]
- 7.de Man J C, Rogosa M, Sharpe M E. A medium for the cultivation of lactobacilli. J Appl Bacteriol. 1960;23:130–135. [Google Scholar]
- 8.Henick-Kling T. Malolactic fermentation. In: Fleet G H, editor. Wine microbiology and biotechnology. 1st ed. Chur, Switzerland: Harwood Academic Publishers; 1993. pp. 289–326. [Google Scholar]
- 9.Kodama S, Suzuki T, Fujinawa S, de la Teja P, Yotsuzuka F. Urea contribution to ethyl carbamate formation in commercial wines during storage. Am J Enol Vitic. 1994;45:17–24. [Google Scholar]
- 10.Lehtonen P. Determination of amines and amino acids in wine—a review. Am J Enol Vitic. 1996;47:127–133. [Google Scholar]
- 11.Liu S-Q. Arginine metabolism in malolactic wine lactic acid bacteria and its oenological implications. Ph.D. thesis. Palmerston North, New Zealand: Massey University; 1993. [Google Scholar]
- 12.Liu S-Q, Davis C R, Brooks J D. Growth and metabolism of selected lactic acid bacteria in synthetic wine. Am J Enol Vitic. 1995;46:166–174. [Google Scholar]
- 13.Liu S-Q, Pritchard G G, Hardman M J, Pilone G J. Citrulline production and ethyl carbamate (urethane) precursor formation from arginine degradation by wine lactic acid bacteria Leuconostoc oenos and Lactobacillus buchneri. Am J Enol Vitic. 1994;45:235–242. [Google Scholar]
- 14.Liu S-Q, Pritchard G G, Hardman M J, Pilone G J. Arginine catabolism in wine lactic acid bacteria: is it via the arginine deiminase pathway or the arginase-urease pathway? J Appl Bacteriol. 1996;81:486–492. [Google Scholar]
- 15.Lonvaud-Funel A. Lactic acid bacteria in the quality improvement and depreciation of wine. Antonie Leeuwenhoek. 1999;76:317–331. [PubMed] [Google Scholar]
- 16.Manca de Nadra C M, Pesce de Ruiz Holgado A A, Oliver G. Utilization of l-arginine in Lactobacillus buchneri: arginine deiminase. Milchwissenschaft. 1981;36:356–359. [Google Scholar]
- 17.Marquis R E, Bender G R, Murray D R, Wong A. Arginine deiminase system and bacterial adaptation to acid environments. Appl Environ Microbiol. 1987;53:198–200. doi: 10.1128/aem.53.1.198-200.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Micklus M J, Stein I M. The colorimetric determination of mono- and disubstituted guanidines. Anal Biochem. 1973;54:545–553. doi: 10.1016/0003-2697(73)90386-2. [DOI] [PubMed] [Google Scholar]
- 19.Mira de Orduña R, Liu S-Q, Patchett M L, Pilone G J. Ethyl carbamate precursor citrulline formation from arginine degradation by malolactic wine lactic acid bacteria. FEMS Microbiol Lett. 2000;183:31–35. doi: 10.1111/j.1574-6968.2000.tb08929.x. [DOI] [PubMed] [Google Scholar]
- 20.Mira de Orduña R, Liu S-Q, Patchett M L, Pilone G J. Kinetics of the arginine metabolism of malolactic wine lactic acid bacteria Lactobacillus buchneri CUC-3 and Oenococcus oeni Lo111. J Appl Microbiol. 2000;89:547–552. doi: 10.1046/j.1365-2672.2000.01135.x. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen J C, Richelieu M. Control of flavor development in wine during and after malolactic fermentation by Oenococcus oeni. Appl Environ Microbiol. 1999;65:740–745. doi: 10.1128/aem.65.2.740-745.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pilone G J, Kunkee R E, Webb A D. Chemical characterization of wines fermented with various malo-lactic bacteria. Appl Microbiol. 1966;14:608–615. doi: 10.1128/am.14.4.608-615.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poolman B, Driessen A J M, Konings W N. Regulation of arginine-ornithine exchange and the arginine deiminase pathway in Streptococcus lactis. J Bacteriol. 1987;169:5597–5604. doi: 10.1128/jb.169.12.5597-5604.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlatter J, Lutz W K. The carcinogenic potential of ethyl carbamate (urethane): risk assessment at human dietary exposure levels. Food Chem Toxicol. 1990;28:205–211. doi: 10.1016/0278-6915(90)90008-b. [DOI] [PubMed] [Google Scholar]
- 25.Spayd S E, Andersen-Bagge J. Free amino acid composition of grape juice from 12 Vitis vinifera cultivars in Washington. Am J Enol Vitic. 1996;47:389–402. [Google Scholar]
- 26.Spector L, Jones M E. Acetylglutamic acid. In: Colowick S P, Kaplan N O, editors. Methods in enzymology. London, United Kingdom: Academic Press; 1963. pp. 557–562. [Google Scholar]
- 27.Sponholz W R. Nitrogen compounds in grapes, must, and wine. In: Rantz J M, editor. Proceedings of the International Symposium on Nitrogen in Grapes and Wine. Seattle, Wash: American Society for Enology and Viticulture; 1991. pp. 67–77. [Google Scholar]
- 28.Thompson J. Ornithine transport and exchange in Streptococcus lactis. J Bacteriol. 1987;169:4147–4153. doi: 10.1128/jb.169.9.4147-4153.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson J, Harr R J, Donkersloot J A. N5-(l-1-carboxyethyl)-l-ornithine : NADP+ oxidoreductase in Streptococcus lactis: distribution, constitutivity, and regulation. Curr Microbiol. 1990;20:239–244. [Google Scholar]
- 30.Tourdot-Maréchal R, Fortier L-C, Guzzo J, Lee B, Diviès C. Acid sensitivity of neomycin-resistant mutants of Oenococcus oeni: a relationship between reduction of ATPase activity and lack of malolactic activity. FEMS Microbiol Lett. 1999;178:319–326. doi: 10.1111/j.1574-6968.1999.tb08694.x. [DOI] [PubMed] [Google Scholar]
- 31.Zimmerli B, Schlatter J. Ethyl carbamate: analytical methodology, occurrence, formation, biological activity and risk assessment. Mutat Res. 1991;259:325–350. doi: 10.1016/0165-1218(91)90126-7. [DOI] [PubMed] [Google Scholar]