ABSTRACT
Background
Hemodialysis patients are at high risk of Covid-19, though vaccination has significant efficacy in preventing and reducing the severity of infection. Little information is available on disease severity and vaccine efficacy since the dissemination of the Omicron variant.
Methods
In a multi-center study, during a period of the epidemic driven by the Omicron variant, all hemodialysis patients positive for SARS-CoV-2 were identified. Outcomes were analyzed according to predictor variables including vaccination status. Risk of infection was analyzed using a Cox proportional hazards model.
Results
SARS-CoV-2 infection was identified in 1126 patients including 200 (18%) unvaccinated, 56 (5%) post first dose, 433 (38%) post second dose, and 437 (39%) at least 7 days beyond their third dose. The majority of patients had a mild course but 160 (14%) were hospitalized and 28 (2%) died. In regression models adjusted for age and comorbidity, two-dose vaccination was associated with a 39% (95%CI: 2%–62%) reduction in admissions, but third doses provided additional protection, with a 51% (95%CI: 25%–69%) further reduction in admissions. Among 1265 patients at risk at the start of the observation period, SARS-CoV-2 infection was observed in 211 (17%). Two-dose vaccination was associated with a 41% (95%CI: 3%–64%) reduction in the incidence of infection, with no clear additional effect provided by third doses.
Conclusions
These data demonstrate lower incidence of SARS-CoV-2 infection after vaccination in dialysis patients during an Omicron dominant period of the epidemic. Among those developing infection, severe illness was less common with prior vaccination, particularly after third vaccine doses.
Keywords: clinical epidemiology, Covid-19, hemodialysis, vaccination
KEY LEARNING POINTS.
What is already known about this subject?
Patients receiving hemodialysis are both more likely to acquire SARS-CoV-2 infection, and more likely to experience severe Covid-19 outcomes, including death.
Although impaired immune responses have been reported, in clinical studies vaccination substantially reduces both the incidence and severity of infection in this group.
Severe Covid-19 can still occur in vaccinated hemodialysis patients, and vaccination may be less effective against the Omicron variant, which has become dominant in many regions.
What this study adds?
During an Omicron dominant period of the epidemic, vaccination remains associated with a lower incidence of infection in hemodialysis patients and less severe outcomes in those developing infection.
Compared to two-dose vaccination, third doses did not further reduce the incidence of infection but did provide significant additional protection from severe outcomes.
In this Omicron dominant period of the epidemic, severe Covid-19 was less common than in recent epidemics due to other variants, even in unvaccinated patients.
What impact this may have on practice or policy?
This study supports the continued promotion and prioritization of vaccination in hemodialysis patients.
This study encourages vaccine uptake, and third doses in particular, among hemodialysis patients.
The study suggests that additional doses of current vaccines may be helpful in the future, in protecting hemodialysis patients from emerging SARS-CoV-2 variants.
INTRODUCTION
Patients receiving in-center hemodialysis face a dual hazard from SARS-CoV-2, since dialysis attendance creates a greater likelihood of exposure to infection, and infection is more severe once acquired [1,2]. The development of vaccines has therefore been most welcome in this population, though as a group with comorbidity and impaired immune responses, there have been concerns that vaccination may be less efficacious.
Several studies have investigated either humoral [3–5] or cellular immune responses [6] to vaccination in dialysis patients, finding impaired but detectable responses in the majority, which weaken over time. Evidence of clinical effectiveness has also emerged, with two-dose vaccination associated with a much lower incidence of symptomatic infection [7,8]. Although immunogenicity is impaired, vaccination therefore remains clinically efficacious, though patients remain vulnerable compared to those without kidney disease.
Waning immunity and the emergence of new variants may alter these dynamics, and since Omicron became the dominant variant, many countries have seen further epidemic waves. Few studies have addressed infection severity or vaccine efficacy in this vulnerable population, but the clinical effectiveness of vaccination remains a pressing concern and is vital for supporting vaccine uptake [9]. This study aims to estimate the clinical efficacy of vaccination in preventing SARS-CoV-2 infection and severe disease in hemodialysis patients, during an epidemic wave driven by the Omicron variant.
MATERIALS AND METHODS
This cohort study of SARS-CoV-2 infections in prevalent hemodialysis patients included all patients with positive PCR on surveillance or otherwise indicated testing, between 6th December 2021 and 16th January 2022. Dates were chosen to include the first wave of infection due to the Omicron variant. The study was sponsored by St George's Hospital and received approval from the National Research Ethics Service (IRAS Ref 283130). The data underlying this article may be shared by request to the corresponding author.
In-center hemodialysis is provided to approximately 5500 patients in London across seven nephrology centers, with enhanced infection surveillance and isolation of cases during the pandemic, described elsewhere [2]. All London nephrology centers were included. The main study population included all prevalent in-center hemodialysis patients with SARS-CoV-2 infection, identified by positive PCR (Fig. 1). During the study period all centers had a policy of temperature/symptom screening at every dialysis session, SARS-CoV-2 PCR testing of all patients on a weekly basis, and additional PCR testing of contacts of cases. Cases otherwise identified, with testing triggered by contact with a case or symptoms, for example presenting to emergency services, were also included. Patients receiving home dialysis were excluded, as were those receiving short-term dialysis for recoverable kidney disease. SARS-CoV-2 infection date was defined by the date of the first positive PCR during the observation period. Prior infection was defined if there was previous positive PCR before the observation period.
Figure 1:
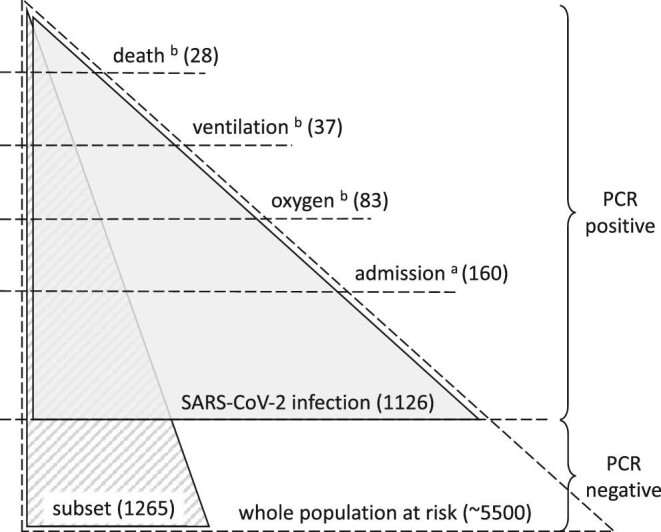
Study populations. The whole population at risk contains all those receiving hemodialysis (in-center) during the observation period at any of the seven London nephrology centers. Weekly PCR screening was carried out in this population, with additional PCR testing as indicated by symptoms or contact with a case. The main study population (gray shading) contains all SARS-CoV-2 infections, defined by positive PCR (in any setting) during the observation period, and is used to assess the risk of severe disease in those with infection. The supplementary study population (striped shading) contains a subset of the whole population at risk, comprising one nephrology center, for whom full vaccination data were available, and is only used to assess the risk of developing infection. aWithin 14 days of positive PCR. bWithin 28 days of positive PCR.
Clinical severity definitions included any hospital admission within 14 days (including a small number of infections acquired in patients already hospitalized), any period of sustained oxygen use within 28 days, any ventilatory support (including non-invasive methods) within 28 days, and death from any cause within 28 days (with or without hospital admission). These outcomes were defined hierarchically so that each category includes more severe Covid-19 outcomes. Hospital records were reviewed to determine the supportive treatment required and outcome. Immune suppression was defined if at the onset of infection patients were receiving steroids (equivalent to prednisolone > 10 mg daily), tacrolimus, mycophenolate or azathioprine, or if they had received cytotoxic chemotherapy or immunomodulating biologic agents within the last 6 months. Ethnicity-associated differences in Covid-19 outcomes have been reported so patients were grouped as Asian/other, Black or White, using ethnicity data extracted from electronic records.
Time period of infection was included as a predictor variable to account for secular trends, making 3 time periods of 2 weeks each. Third dose vaccination was administered during this period using either BNT162b2 (Pfizer-BioNTech) or mRNA-1273 (Moderna), with vaccination status considered to change after the 7th day post vaccine administration. Data were complete for comorbidity and clinical outcome, apart from two cases moving out of area, which were excluded from the analysis. The observation period ended on 16th January 2022, with 28-day outcome complete on 13th February 2022. Data collection took place during and after the observation period and was completed on 4th March 2022.
Covariates associated with clinical outcome were analyzed using mixed logistic regression models, with fixed effects including age, gender, ethnicity, diabetes, immune suppression, prior SARS-CoV-2 and time period, with nephrology center as a random effect. Effect sizes were expressed as odds ratios with 95% confidence interval, and estimated vaccine efficacy, in preventing each outcome after SARS-CoV-2 infection, was defined as 1–odds ratio. Vaccine effect was also analyzed as a linear (per dose) trend, and by months since the last dose. Sub-group analyses were performed to estimate the effect of age and immune suppression on vaccine efficacy, as well as the effect of time since the second or third vaccine dose. Sensitivity analyses were performed in which patients with prior SARS-CoV-2 infection were excluded, and the analysis restricted to individual time periods.
In a secondary analysis, a subgroup for whom full vaccination data were available (comprising one nephrology center) was defined from those at risk from the start of the observation period (Fig. 1), with the incidence of SARS-CoV-2 infection observed during the study period, defined by positive PCR. Variables associated with infection were analyzed using a Cox proportional hazards model with third dose vaccination as a time-varying covariate, considered to change 7 days after administration. This analysis was repeated using a period-rate model using 2-week intervals with dialysis unit as a random effect. SPSS v27.0 (IBM, New York) was used for modeling.
RESULTS
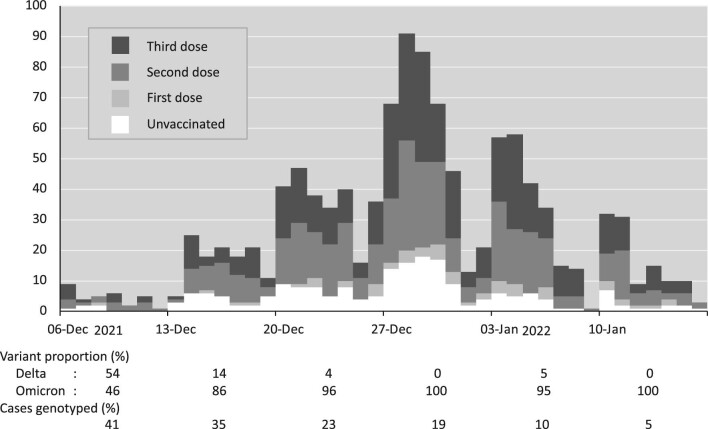
Between 6th December 2021 and 16th January 2022, SARS-CoV-2 infection was detected by PCR in 1126 hemodialysis patients (aged 19–94 years, 59% male, with ethnicity grouped as Asian/other 35%, Black 40% and White 25%) with a unimodal epidemic time course (Fig. 2).
Figure 2:
Epidemic time course. Number of new SARS-CoV-2 infections by date and vaccination status. The proportions of Delta and Omicron variants are provided as percentages (of those known) along with the percentage genotyped.
At the time of diagnosis, 200 patients (18%) were unvaccinated, 56 (5%) were at least 7 days beyond their first dose, 433 (38%) were at least 7 days beyond their second dose, and 437 (39%) were at least 7 days beyond their third dose. The majority of PCR samples were taken in the dialysis unit as part of weekly surveillance, or in response to exposure or symptoms, but 6% were taken on a Sunday. Immune suppressing treatments were taken by 185 patients (16%), of which the majority were on tacrolimus monotherapy. Further patient characteristics are given in Table 1.
Table 1.
Characteristics and outcome of patients with SARS-CoV-2 infection stratified by vaccination status
| Unvaccinated | First dose | Second dose | Third dose | Total | ||
|---|---|---|---|---|---|---|
| N | 200 | 56 | 433 | 437 | 1126 | |
| Days post dose, median(IQR) | 293 (158–344) | 252 (220–270) | 64 (51–80) | |||
| Age, median(IQR) | 55 (44–64) | 62 (45–73) | 60 (50–72) | 64 (54–75) | 61 (50–73) | |
| Gender | Male | 100 (50) | 34 (61) | 251 (58) | 282 (65) | 667 (59) |
| Ethnicity | Asian/other | 44 (22) | 20 (36) | 141 (33) | 186 (43) | 391 (35) |
| Black | 119 (59) | 27 (48) | 180 (42) | 128 (29) | 454 (40) | |
| White | 37 (19) | 9 (16) | 112 (26) | 123 (28) | 281 (25) | |
| Diabetes | 77 (39) | 24 (43) | 206 (48) | 202 (46) | 509 (45) | |
| Immune suppressiona | 31 (16) | 8 (14) | 74 (17) | 72 (16) | 185 (16) | |
| Prior SARS-CoV-2b | 40 (20) | 12 (21) | 67 (15) | 69 (16) | 188 (17) | |
| Outcome | Admissionc | 39 (20) | 9 (16) | 69 (16) | 43 (10) | 160 (14) |
| Oxygend | 19 (10) | 5 (9) | 35 (8) | 24 (5) | 83 (7) | |
| Ventilationd | 7 (4) | 3 (5) | 18 (4) | 9 (2) | 37 (3) | |
| Deathd | 5 (3) | 2 (4) | 14 (3) | 7 (2) | 28 (2) |
Except where stated data are N (%).
Clinical outcomes are ‘all cause’, not specifically due to Covid-19.
Vaccination status considered to change after the 7th post dose day.
Any immune suppression treatment including steroids, tacrolimus, mycophenolate, azathioprine, cytotoxic and biologic agents.
PCR positive at least 90 days prior to the current infection.
Within 14 days of positive PCR.
Within 28 days of positive PCR.
A mild course was observed in 966 patients (86%) who did not require admission, but 83 (7%) at least required oxygen and 28 (2%) died before 28 days. The association of clinical variables with disease severity is shown in Table 2: older age, diabetes and immune suppressing treatment were associated with greater illness severity. The Omicron variant accounted for around half of infections in the first week, but rapidly became dominant thereafter, accounting for 96% of infections in weeks 2–6 (Fig. 2). Severe outcomes appeared to be more frequent with the Delta variant, though the numbers were small, but there was no drift in severity over time (Supplementary data, Table S1). Hospitalized cases and those occurring earlier in the study period were more likely to be genotyped.
Table 2.
Factors associated with severe Covid-19 outcomes in patients with SARS-CoV-2 infection
| Odds ratio (95%CI) for severe Covid-19 outcomes | |||||
|---|---|---|---|---|---|
| Admissionh | Oxygeni | Ventilationi | Deathi | ||
| Age | /year | 1.03 (1.01–1.04) | 1.03 (1.01–1.05) | 1.02 (1.00–1.03) | 1.02 (1.00–1.04) |
| Gender | Male | 0.98 (0.68–1.40) | 0.76 (0.47–1.23) | 0.88 (0.51–1.53) | 0.87 (0.48–1.55) |
| Ethnicitya | Asian/other | 0.77 (0.49–1.21) | 0.71 (0.40–1.26) | 0.68 (0.34–1.34) | 0.76 (0.37–1.57) |
| Black | 0.59 (0.38–0.92) | 0.41 (0.22–0.75) | 0.59 (0.30–1.15) | 0.66 (0.32–1.35) | |
| Diabetes | 1.73 (1.20–2.48) | 2.17 (1.32–3.56) | 1.31 (0.75–2.29) | 1.16 (0.64–2.09) | |
| Immune suppressionb | 2.42 (1.55–3.77) | 2.74 (1.52–4.93) | 1.49 (0.74–3.01) | 1.17 (0.53–2.58) | |
| Prior SARS-CoV-2c | 0.64 (0.38–1.09) | 0.81 (0.41–1.62) | 1.24 (0.62–2.50) | 1.07 (0.50–2.31) | |
| Time periodd | Weeks 3–4 | 1.08 (0.64–1.81) | 1.00 (0.51–1.95) | 1.17 (0.50–2.73) | 1.12 (0.46–2.72) |
| Weeks 5–6 | 0.80 (0.44–1.44) | 0.60 (0.27–1.30) | 1.03 (0.41–2.61) | 1.03 (0.39–2.74) | |
| Vaccinatione | One | 0.64 (0.28–1.48) | 0.76 (0.26–2.24) | 1.20 (0.34–4.20) | 1.09 (0.27–4.34) |
| Two | 0.61 (0.38–0.98) | 0.62 (0.33–1.16) | 0.96 (0.44–2.08) | 0.99 (0.43–2.25) | |
| Three | 0.30 (0.17–0.50) | 0.34 (0.17–0.69) | 0.66 (0.29–1.51) | 0.72 (0.30–1.73) | |
| Three (ref Two) | 0.49 (0.31–0.75) | 0.56 (0.31–0.99) | 0.69 (0.36–1.30) | 0.73 (0.37–1.42) | |
| Vaccination (per dose)f | 0.69 (0.58–0.81) | 0.71 (0.57–0.89) | 0.86 (0.67–1.12) | 0.89 (0.68–1.17) | |
| Vaccination (months since)g | 1.06 (1.00–1.13) | 1.08 (1.00–1.18) | 1.04 (0.95–1.14) | 1.04 (0.94–1.14) | |
Odds ratio (95% CI) by multivariable logistic regression model, adjusted for all variables shown.
Clinical outcomes are ‘all cause’, not specifically due to Covid-19.
Vaccination status considered to change after the 7th post dose day.
Boldface indicates confidence interval not including 1.
Reference ethnicity White.
Any immune suppression treatment including steroids, tacrolimus, mycophenolate, azathioprine, cytotoxic and biologic agents.
PCR positive at least 90 days prior to the current infection.
Reference time period weeks 1–2.
Vaccination reference group: none (unvaccinated) except where stated.
Vaccination as number of doses (linear effect, 0 = unvaccinated).
Vaccination as time since last vaccine dose (unvaccinated excluded).
Within 14 days of positive PCR.
Within 28 days of positive PCR.
Compared to unvaccinated patients, severe Covid-19 outcomes were observed less often in patients testing positive for SARS-CoV-2 after vaccination, reaching around half the frequency after the third dose. In logistic regression models adjusted for demographics and comorbidity, both two-dose and three-dose vaccinations were associated with a lower risk of admission, and three-dose vaccination was associated with a lower requirement for oxygen treatment (Table 2). Compared to two doses, three-dose vaccination provided additional protection, with a 51% (95%CI: 25%–69%) further reduction in admissions, and 44% (95%CI: 1%–69%) further reduction in the requirement for oxygen. No clear protective effect of vaccination was seen from more severe outcomes including death, but with mortality at 2%, the numbers of severe outcomes were small compared with previous SARS-CoV-2 variants.
Similar protection from severe illness associated with vaccination was seen in patients over 65 years, and those receiving immune suppressive treatment (Supplementary data, Table S2). And in sensitivity analyses, very similar vaccine effects were seen when those with prior SARS-CoV-2 were excluded, or when the analysis was restricted to individual time periods (Supplementary data, Table S3). In vaccinated patients more severe outcomes were associated with greater time since the last vaccine dose, explained by a significant effect in the two-dose group (HR for admission 1.30 per month since the second dose, 95%CI 1.17–1.44) in whom infection was acquired at a median(IQR) of 252(220–270) days after the second vaccine dose (Supplementary data, Table S2).
In the secondary analysis of the subgroup of the patients at risk (Fig. 1), the incidence of SARS-CoV-2 infection was observed in 1265 patients (aged 19–94, 61% male) who were on hemodialysis on 6th December 2021, with baseline characteristics given in Table 3. During the observation period SARS-CoV-2 infection developed in 211 (17%). In a Cox proportional hazards model censored for transplantation, death or transfer to another center, both two-dose (HR 0.59, 95%CI: 0.36–0.97) and three-dose (HR 0.48, 95%CI: 0.31–0.75) vaccination were associated with a lower incidence of infection, but there was no clear additional protection from the third dose (Table 4). Modest protection was observed in the 464 (37%) with prior infection identified by positive PCR before the observation period (HR 0.62, 95%CI 0.45–0.84). Similar effects were seen using a period-rate model, but neither analysis was able to demonstrate clearly any decay over time in vaccine efficacy against infection.
Table 3.
Characteristics of subgroup patients (N = 1265) stratified by SARS-CoV-2 PCR status
| PCR positive | PCR negative | ||
|---|---|---|---|
| N | 211 | 1054 | |
| Age, median(IQR) | 62 (49–73) | 66 (55–45) | |
| Gender | Male | 123 (58) | 649 (62) |
| Ethnicity | Asian/other | 95 (45) | 492 (47) |
| Black | 73 (35) | 253 (24) | |
| White | 43 (20) | 309 (29) | |
| Diabetes | 94 (45) | 397 (38) | |
| Prior SARS-CoV-2 | 58 (27) | 406 (39) | |
| Vaccine a | Unvaccinated | 26 (12) | 52 (5) |
| First dose | 8 (4) | 30 (3) | |
| Second dose | 44 (21) | 166 (16) | |
| Third dose | 133 (63) | 806 (76) |
Except where stated data are N (%).
Vaccination status considered to change after the 7th post dose day.
Status at positive PCR, or end of observation in those with negative PCR.
Table 4.
Predictors of SARS-CoV-2 infection in a subgroup of the population at risk (N = 1265)
| Hazard ratio (95% CI) for SARS-CoV-2 infection | |||
|---|---|---|---|
| Proportional hazards modela | Period-rate modelb | ||
| Age | /year | 0.98 (0.98–0.99) | 0.98 (0.97–0.99) |
| Gender | Male | 0.93 (0.71–1.23) | 0.91 (0.68–1.22) |
| Ethnicityc | Asian/other | 1.33 (0.92–1.91) | 1.36 (0.93–1.98) |
| Black | 1.70 (1.15–2.50) | 1.78 (1.18–2.66) | |
| Diabetes | 1.48 (1.12–1.97) | 1.53 (1.14–2.07) | |
| Prior SARS-CoV-2d | 0.62 (0.45–0.84) | 0.60 (0.44–0.82) | |
| Vaccinatione | One | 0.79 (0.38–1.64) | 0.81 (0.37–1.80) |
| Two | 0.59 (0.36–0.97) | 0.52 (0.31–0.89) | |
| Three | 0.48 (0.31–0.75) | 0.46 (0.28–0.75) | |
| Three (ref Two) | 0.80 (0.57–1.14) | 0.88 (0.61–1.28) | |
| Vaccination (per dose)f | 0.78 (0.68–0.90) | 0.78 (0.67–0.91) | |
| Vaccination (months since)g | 1.04 (1.00–1.09) | 1.04 (0.98–1.09) | |
Cox proportional hazards model censored for transplantation, death, or transfer to another center.
Period-rate model using 2-week intervals with dialysis unit as random effect.
Reference ethnicity White.
PCR positive at least 90 days prior to the current infection.
Vaccination reference group: none (unvaccinated) except where stated.
Vaccination as number of doses (linear effect, 0 = unvaccinated).
Vaccination as time since last vaccine dose (unvaccinated excluded).
Vaccination status considered to change after the 7th post dose day.
Boldface indicates confidence interval not including 1.
DISCUSSION
In this multi-center study of hemodialysis patients with SARS-CoV-2 infection mostly due to Omicron variant, significant protection from severe disease was seen after vaccination, with hospitalizations 39% lower (95%CI: 2–62) after two doses, and 70% lower (95%CI: 50–83) after three doses. This suggests a substantial clinical benefit from vaccination in a population that is particularly vulnerable and highlights the significant additional protection offered by the third dose. Among unvaccinated hemodialysis patients with infection in this study, 20% required admission and mortality was 3%, contrasting with outcomes in unvaccinated hemodialysis patients with infection due to earlier strains of SARS-CoV-2, among whom 42% required admission and mortality was 14% [10]: independent of vaccination therefore, Omicron appeared to cause less severe infection than Delta or other previous strains of SARS-CoV-2, though outcomes remain poor when compared to the general population.
Although many studies have examined immunogenicity of vaccines in hemodialysis patients, few have attempted to estimate clinical efficacy. Those which have, report vaccine efficacy against symptomatic infection around 69%–78%, prior to the establishment of the Omicron variant as the dominant strain. For example, in a US study of over 12 000 hemodialysis patients receiving BNT162b2, the subsequent risk of symptomatic Covid-19 was substantially reduced compared to a matched unvaccinated cohort dialyzing at the same facilities (HR 0.22, 95% CI 0.13–0.35) [7]. Similarly, in a Canadian study of over 13 000 hemodialysis patients, two-dose vaccination was associated with lower rates of SARS-CoV-2 infection (HR 0.31, 95%CI 0.22–0.42) and hospitalization (HR 0.17, 95%CI 0.10–0.30) [8]. An early report, on a subset of this study population, found a lower incidence of Omicron infection after three-dose vaccination compared to unvaccinated individuals (HR 0.50, 95%CI 0.29–0.92) [11]. However, due to study size and possibly analytic limitations, no efficacy was demonstrated with fewer vaccine doses, and neither was any vaccine effect on disease severity observed. Without vaccination, outcomes are poor in hemodialysis patients [2], therefore, while substantially protected compared to their unvaccinated peers, vaccinated hemodialysis patients remain at high risk for severe Covid-19 outcomes when compared to individuals without kidney disease.
Alongside clinical efficacy, the likely effect of vaccination can also be inferred from immunogenicity: the ability of a vaccine to induce antibody and cellular immune responses in patients. Several studies have reported reduced antibody responses in dialysis patients, but impaired immunogenicity compared to healthy controls does not imply reduced clinical efficacy, which is defined by comparison with unvaccinated dialysis patients. In a meta-analysis of 32 studies comprising 4917 dialysis patients, mostly hemodialysis patients receiving two doses of BNT162b2, Chen reported detectable antibody responses in 86% of patients (95%CI 81%–89%) [12]. And after two-dose BNT162b2 vaccination, neutralizing antibody titers (to variants other than Omicron) similar to healthy controls have been observed, with a weaker effect following AZD1222 [6]. However, immunogenicity against Omicron is poorer after two-dose vaccination. Although neutralizing antibodies to Delta were detected in most patients after BNT162b2, the median neutralizing antibody titer against Omicron was below the limit of detection (<1:40), though after a third dose neutralizing antibodies were detectable in most patients [13].
This study clearly demonstrates additional protection following the third dose of vaccine, with severe outcomes halved compared to those developing infection after two doses, though the effect of the third dose on the incidence of infection was unclear. Two-dose vaccination was still associated with useful protection however, both in terms of incidence and severity of infection. However, dose number is confounded by time since vaccination: the last vaccination preceded infection by a median(IQR) of 64(51–80) days in the three-dose group, versus 252(220–270) days in the two-dose group. It is interesting therefore that an association was seen between time since vaccination and severity, which in subgroup analysis appears to be due to waning of the two-dose effect (Supplementary data, Table S2) with a reasonably large effect size (HR 1.30 per month). Third doses could therefore be described as restoring the two-dose effect, which has diminished over time, though it remains possible that protection after three doses exceeds that provided initially by two doses.
These results are relevant to vaccine uptake, and third doses in particular, which have become standard for vaccination in many countries. Vaccine hesitancy remains a problem in dialysis patients [14], but by emphasizing substantial clinical efficacy which persists despite the emergence of new variants, this study may be useful in reducing vaccine hesitancy in a group which remains vulnerable. In this regard it is noteworthy that similar vaccination efficacy was observed in older and younger patients, as well as in those taking immune suppressive treatment, though the smaller group sizes lead to wider confidence intervals.
An important limitation is that SARS-CoV-2 variant information was not available in the majority of cases. The proportion of infections known to be due to the Delta variant decreased rapidly during the study period, and though severe outcomes were more frequent with the Delta variant, the numbers were small, and not large enough to impact on severity or vaccine efficacy over time. Removing known Delta variant cases is not helpful, since Delta would also contribute to a small number of the non-genotyped cases. Conclusions therefore apply to a mixed epidemic, due mostly but not exclusively to the Omicron variant. This situation is similar to clinical risk in the real world: though one variant may be dominant, patients are still at risk of infection with other variants.
This study has several other important limitations, in particular the main study only addresses clinical severity once individuals are infected, with limited focus on the likelihood of acquiring infection, assessed in the secondary analysis only. Though weekly screening allows a consistent threshold for detection, the inclusion of mild cases may impair comparison with other studies. Only limited comorbidity data were available, and changes in clinical practice, for example as new treatments became available for non-hospitalized patients, may also have confounded the relationship between vaccination and severe Covid-19 outcomes.
This study, undertaken during an epidemic phase largely due to the Omicron variant, demonstrates that vaccination is associated with a lower incidence of SARS-CoV-2 infection, and a substantially lower risk of severe Covid-19 outcomes in hemodialysis patients who develop infection, particularly after the third vaccine dose. Although significant vulnerability remains, this population have much to gain from vaccination, regardless of age. These results support a policy of promoting and prioritizing vaccination, including third doses, in this vulnerable group.
Supplementary Material
ACKNOWLEDGEMENTS
The authors acknowledge the role of clinical nursing and medical staff who enabled this work.
APPENDIX
In addition to the authors, the pan-London Covid-19 renal audit group includes:
Omer Ali, Marilina Antonelou, Katy Bennet-Richards, Mark Blunden, John Booth, Rawya Charif, Saurabh Chaudhury, Andrea Cove-Smith, Hamish Dobbie, Phillippa Dodd, Gavin Dreyer, Neill Duncan, Catriona Goodlad, Megan Griffith, Sevda Hassan, Ulla Hemmilla, Heidy Hendra, Peter Hill, Ajith James, Daniel Jones, Anila Laurence, Marina Loucaidou, Gaetano Lucisano, Viyaasan Mahalingasivam, Bethia Manson, Daniel McGuiness, Adam McLean, Rosa Montero, Vasantha Muthuppalaniappan, Tom Oates, Andrew Palmer, Ravi Rajakariar, Emma Salisbury, Nasreen Samad, Eleanor Sandhu, Edward Stern, Damir Tandaric, James Tomlinson, Gisele Vajgel, Phil Webster, William White, Kate Wiles, David Wright, and Sajeda Yousef.
Contributor Information
Damien R Ashby, Renal and Transplant Centre, Hammersmith Hospital, Imperial College Healthcare NHS Trust, London, UK; Department of Immunology and Inflammation, Imperial College London, London, UK.
Ben Caplin, Department of Renal Medicine, University College London, London, UK.
Richard W Corbett, Renal and Transplant Centre, Hammersmith Hospital, Imperial College Healthcare NHS Trust, London, UK.
Elham Asgari, Kidney Services, Guy's and St. Thomas’ NHS Foundation Trust, London, UK.
Nicola Kumar, Kidney Services, Guy's and St. Thomas’ NHS Foundation Trust, London, UK.
Alexander Sarnowski, Renal and Transplantation Unit, St. George's University Hospitals NHS Foundation Trust, London, UK.
Richard Hull, Renal and Transplantation Unit, St. George's University Hospitals NHS Foundation Trust, London, UK.
David Makanjuola, South West Thames Renal and Transplantation Unit, Epsom and St. Helier University Hospitals NHS Trust, London, UK.
Nicholas Cole, South West Thames Renal and Transplantation Unit, Epsom and St. Helier University Hospitals NHS Trust, London, UK.
Jian Chen, Renal Service, Barts Health NHS Trust, London, UK.
Sofia Nyberg, Renal Service, Barts Health NHS Trust, London, UK.
Suzanne Forbes, Renal Service, Barts Health NHS Trust, London, UK.
Kieran McCafferty, Renal Service, Barts Health NHS Trust, London, UK.
Faryal Zaman, Department of Renal Medicine, King's College Hospital NHS Foundation Trust, London, UK.
Hugh Cairns, Department of Renal Medicine, King's College Hospital NHS Foundation Trust, London, UK.
Claire Sharpe, Department of Renal Medicine, King's College Hospital NHS Foundation Trust, London, UK.
Kate Bramham, Department of Renal Medicine, King's College Hospital NHS Foundation Trust, London, UK.
Reza Motallebzadeh, Royal Free London NHS Foundation Trust, London, UK.
Kashif Anwari, Royal Free London NHS Foundation Trust, London, UK.
Tayeba Roper, Royal Free London NHS Foundation Trust, London, UK.
Alan D Salama, Department of Renal Medicine, University College London, London, UK.
Debasish Banerjee, Renal and Transplant Centre, Hammersmith Hospital, Imperial College Healthcare NHS Trust, London, UK.
The pan-London Covid-19 renal audit groups:
Omer Ali, Marilina Antonelou, Katy Bennet-Richards, Mark Blunden, John Booth, Rawya Charif, Saurabh Chaudhury, Andrea Cove-Smith, Hamish Dobbie, Phillippa Dodd, Gavin Dreyer, Neill Duncan, Catriona Goodlad, Megan Griffith, Sevda Hassan, Ulla Hemmilla, Heidy Hendra, Peter Hill, Ajith James, Daniel Jones, Anila Laurence, Marina Loucaidou, Gaetano Lucisano, Viyaasan Mahalingasivam, Bethia Manson, Daniel McGuiness, Adam McLean, Rosa Montero, Vasantha Muthuppalaniappan, Tom Oates, Andrew Palmer, Ravi Rajakariar, Emma Salisbury, Nasreen Samad, Eleanor Sandhu, Edward Stern, Damir Tandaric, James Tomlinson, Gisele Vajgel, Phil Webster, William White, Kate Wiles, David Wright, and Sajeda Yousef
FUNDING
No funding was received for this study.
AUTHORS’ CONTRIBUTIONS
D.A., B.C., D.B., and A.S. conceived the study; all authors curated the data; D.A. and R.C. analyzed the data; D.A. drafted the paper which was modified by other authors; all authors approved the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
D. Banerjee reports receiving research funding from the British Heart Foundation; receiving grants from AstraZeneca and Kidney Research UK; and receiving honoraria from AstraZeneca, Pfizer, and Viforpharma. K. Bramham reports consultancy agreements with Alexion; receiving honoraria from Alexion and Otsuka; and serving as a scientific advisor or member of Alexion. B. Caplin reports consultancy agreements with LifeArc and receiving research funding from AstraZeneca and grants from Colt Foundation, Medical Research Council, and Royal Free Charity outside the submitted work. R. Hull reports consultancy agreements with AstraZeneca, Pharmocosmos UK Ltd, and Travere Pharmaceuticals; speakers bureau for Napp Phamaceuticals. K. McCafferty reports receiving research funding from AstraZeneca and receiving honoraria from Bayer, Napp, Pharmacosmos, and Vifor Fresenius. A. Salama reports receiving research funding from Chiesi and Natera; receiving honoraria from AnaptysBio, AstraZeneca, Hansa Medical, and Vifor Pharmaceuticals. C. Sharpe reports consultancy agreements with Novartis Pharmaceuticals; Travere Pharmaceuticals; and receives funding from AstraZenica. All remaining authors have nothing to disclose.
REFERENCES
- 1. Corbett RW, Blakey S, Nitsch Det al. . Epidemiology of COVID-19 in an urban dialysis center. J Am Soc Nephrol 2020; 31: 1815–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Caplin B, Ashby D, McCafferty Ket al. . Risk of COVID-19 disease, dialysis unit attributes, and infection control strategy among London in-center hemodialysis patients. Clin J Am Soc Nephrol 2021; 16: 1237–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carr EJ, Wu M, Harvey Ret al. . Neutralising antibodies after COVID-19 vaccination in UK haemodialysis patients. Lancet North Am Ed 2021; 398: 1038–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garcia P, Anand S, Han Jet al. . COVID19 vaccine type and humoral immune response in patients receiving dialysis. J Am Soc Nephrol 2022; 33: 33–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lacson E, Argyropoulos CP, Manley HJet al. . Immunogenicity of SARS-CoV-2 vaccine in dialysis. J Am Soc Nephrol 2021; 32: 2735–2742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thieme CJ, Blazquez-Navarro A, Safi Let al. . Impaired humoral but substantial cellular immune response to variants of concern B1.1.7 and B.1.351 in hemodialysis patients after vaccination with BNT162b2. J Am Soc Nephrol 2021; 32: 2725–2727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sibbel S, McKeon K, Luo Jet al. . Real-world effectiveness and immunogenicity of BNT162b2 and mRNA-1273 SARS-CoV2 vaccines in patients on hemodialysis. J Am Soc Nephrol 2022;33: 49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oliver MJ, Thomas D, Balamchi Set al. . Vaccine effectiveness against SARS-CoV-2 infection and severe outcomes in the maintenance dialysis population in Ontario. J Am Soc Nephrol 2022;33: 839–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kliger AS, Silberzweig J. COVID-19 and dialysis patients: unsolved problems in early 2021. J Am Soc Nephrol 2021;32: 1018–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ashby DR, Caplin B, Corbett RWet al. . Severity of COVID-19 after vaccination among hemodialysis patients: an observational cohort study. Clin J Am Soc Nephrol 2022;17: 843–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Spensley KJ, Gleeson S, Martin Pet al. . Comparison of vaccine effectiveness against the Omicron (B.1.1.529) variant in haemodialysis patients. Kidney Int Rep 2022;7: 1406–1409. doi: 10.1016/j.ekir.2022.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen JJ, Lee TH, Tian YCet al. . Immunogenicity rates after SARS-CoV-2 vaccination in people with end-stage kidney disease: a systematic review and meta-analysis. JAMA Netw Open 2021; 4: e2131749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carr EJ, Wu M, Harvey Ret al. . Omicron neutralising antibodies after COVID-19 vaccination in haemodialysis patients. Lancet North Am Ed 2022; 399: 800–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garcia P, Montez-Rath ME, Moore Het al. . SARS-CoV-2 vaccine acceptability in patients on hemodialysis: a nationwide survey. J Am Soc Nephrol 2021; 32: 1575–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.