Abstract
Background
Little was known about US parental attitudes, beliefs, and intentions surrounding coronavirus disease 2019 (COVID-19) vaccines for children before their introduction.
Methods
An online cross-sectional nationally representative survey of US parents/guardians of children < 18 years old via Ipsos KnowledgePanel, fielded from October 26, 2021 to November 30, 2021.
Results
Response rate was 64.2% (3230/5034). For children ages 0–4 years, 51.5% of parents were likely to have their children vaccinated, and for ages 5–11 and 12–17, 54.0% and 69.7% of parents, respectively, reported they were likely to vaccinate or had already vaccinated their children. Among respondents with unvaccinated children, 25.2% (ages 0–4) and 22.0% (ages 5–11) reported they would seek COVID-19 vaccination for their children as soon as authorization occurred. Factors associated with willingness to have children receive a COVID-19 vaccine were: belief in benefits of COVID-19 vaccination (odds ratio [OR] = 6.44, 5.68, 4.57 in ages 0–4, 5–11, and 12–17 respectively), acceptance of routine childhood vaccines (OR = 6.42, 5.48, 1.76), parental COVID-19 vaccination (OR = 1.85, 3.70, 6.16), perceptions that pediatric COVID-19 is severe (OR = 1.89, 1.72, 1.35), Hispanic ethnicity (OR = 2.07, 2.29, 2.60), influenza vaccine acceptance (OR = 1.07, 0.88, 1.62), presence of children of another age group in the household (OR = 0.71, 0.71, 0.65), and attitudinal barriers to COVID-19 vaccination (OR = 0.30, 0.26, 0.49).
Conclusions
Belief in the benefits of COVID-19 vaccination and acceptance of routine childhood vaccines are the strongest predictors of intention to vaccinate children. Further research is needed to track how parental attitudes change as more data about pediatric COVID-19 vaccines become available and how intentions translate into pediatric vaccine uptake.
Keywords: COVID-19, immunization, paediatrics, SARS-CoV-2, vaccines, vaccine hesitancy, vaccine acceptance
In a November 2021 nationally representative survey of US parents, >50% were likely to vaccinate/had already vaccinated their children. The most important predictors of child COVID-19 vaccine intention were parental COVID-19 vaccination and belief in the benefits of COVID-19 vaccination.
INTRODUCTION
As of May 10, 2022, the United States (US) has reported >81 million coronavirus disease 2019 (COVID-19) cases and 990 000 COVID-19-related deaths, with >12.6 million cases and 1500 deaths in children <18 years old [1]. Pediatric severe acute respiratory syndrome coronavirus two (SARS-CoV-2)-related disease ranges from mild cold-like symptoms to severe manifestations, such as multi-organ failure and multisystem inflammatory syndrome in children (MIS-C) [2]. US COVID-19 pediatric deaths have exceeded pre-pandemic annual pediatric influenza deaths, and the pediatric COVID-19 death rate is higher pre-vaccination than recorded era rates for many now vaccine-preventable diseases [3, 4]. Three COVID-19 vaccines have been authorized or licensed by the US Food and Drug Administration (FDA) for adults [5]. Through early May 2022, pediatric use has been limited to the Pfizer-BioNTech vaccine, which the FDA authorized for ages ≥ 16 years in December 2020, 12–15 years in May 2021, and 5–11 years on October 29, 2021[6, 7].
While others have characterized factors contributing to COVID-19 vaccine hesitancy among US adults, less is known regarding parental opinions about COVID-19 vaccination for children. Our objectives were to assess parental attitudes and beliefs about SARS-CoV-2-related disease and COVID-19 vaccines, gauge parental acceptance of COVID-19 vaccines for children, characterize parents who reported willingness to vaccinate their children against COVID-19, and evaluate factors that may influence willingness to vaccinate.
METHODS
From October 26 to November 30, 2021, we surveyed a nationally representative sample of noninstitutionalized US adults who are medical decision-makers for children <18 years old (hereafter, parents). All affiliated Institutional Review Boards determined the study to be nonhuman subject research.
Instrument Development
The survey instrument was developed in collaboration with the US Centers for Disease Control and Prevention (CDC). We measured parental COVID-19 vaccine acceptance metrics for children of different age groups (0–4, 5–11, and 12–17 years). Parents with children in multiple age groups were asked questions for each applicable age group. We asked parents with children ages 0–4: “If a safe, effective COVID-19 vaccine were available for children 4 and younger, how likely would you be to get your child[ren] aged 0 to 4 vaccinated against COVID-19?” Responses were “[My child]/[At least one of my children] already got vaccinated as part of a trial,” “very likely,” “somewhat likely,” “somewhat unlikely,” and “very unlikely.” We asked parents with children ages 5–11 and 12–17: “[Has your child]/[Have any of your children] aged [5 to 11]/[12 to 17] received at least one dose of a COVID-19 vaccine?” with responses of “Yes” and “No.” For parents who responded “No” or refused, we asked: “How likely are you to get your child[ren] aged [5 to 11]/[12 to 17] vaccinated against COVID-19?” Responses were “very likely,” “somewhat likely,” “somewhat unlikely,” and “very unlikely.” A modified version of the World Health Organization’s Vaccine Hesitancy Scale (VHS) was used to measure baseline vaccine hesitancy (Supplement 1, Q1.1–1.10) [8, 9]. To reduce neutral response bias, we converted the VHS to a 4-point bipolar Likert scale, eliminating the neutral option [10]. For consistency with other studies using the VHS, we recoded responses as strongly agree = 1, somewhat agree = 2, somewhat disagree = 4, and strongly disagree = 5 [9]. Additional original 4-point Likert scale and multiple-choice items measured beliefs related to routine childhood immunizations and COVID-19 vaccines. Respondents were asked about seasonal influenza vaccine uptake, personal experiences with SARS-CoV-2-related disease, and their own COVID-19 vaccination status. We designed survey questions based on the Health Belief Model [11], focusing on four domains: disease susceptibility, disease severity, benefits of vaccination, and barriers to vaccination. We used a 4-point unipolar Likert scale to evaluate predictors of vaccination intent and a 5-point bipolar Likert scale to assess factors that may change respondents’ willingness to vaccinate their children against SARS-CoV-2. The final instrument was translated into Spanish (Supplement 1 for English instrument; Spanish instrument available upon request).
Ipsos Survey Panel
We conducted the survey on Ipsos’s KnowledgePanel, a probability-based web panel designed to be representative of the US population (Supplement 2). Ipsos uses an address-based sampling (ABS) recruitment methodology based on the US Postal Service’s Delivery Sequence File. Stratified random sampling ensures the geodemographic composition mimics the US adult population [12]. The Ipsos KnowledgePanel supplements traditional ABS using dual-frame random-digit-dialing sampling to recruit a Spanish-language-dominant Hispanic sample [13]. All panel members are provided privacy and confidentiality protections. If needed, Ipsos provides a web-enabled device and free internet service.
Sample Selection
These analyses use a composite sample constructed from (1) a longitudinal sample with Wave 1 collected in February 2021 and Wave 2 in October/November 2021 and (2) an add-on sample of respondents who did not complete the Wave 1 survey, collected October/November 2021. Both samples were selected using the equal probability selection method. We sampled panel members expected to meet inclusion criteria based on their KnowledgePanel profiles.
Survey Administration
We fielded the survey in English and Spanish. Eligible panel members received an email invitation followed by reminders with a $5–10 incentive upon survey completion.
Data Cleaning
We cleaned from the final data set all responses completed in <25% of the median survey completion time, responses wherein panel members skipped ≥50% of eligible questions, and responses wherein reported age and sex did not match panel enrollment demographics. Seven responses were excluded due to responses on screening questions about child age that triggered incorrect skip logic for the remainder of the survey. Overall, 40 respondents were removed before weighting, leaving a final sample of 3042 respondents.
Weighting
Design weights for the longitudinal and add-on samples were produced separately and then combined into a final weight for the pooled cross-sectional sample. The design weights were produced using an iterative proportional fitting (raking) procedure and parent demographic benchmarks from the 2019 American Community Survey and the 2020 March Supplement of the Current Population Survey. The longitudinal and add-on samples were combined based on their effective sample sizes and raked to the population geodemographic distributions of parents who are healthcare decision-makers for children <18 years old. The resulting weights were trimmed and scaled to add up to the total number of qualified respondents to produce final weights for these analyses (detailed weighting methods in Supplement 2).
Statistical Methods
All analyses reported in this paper are cross-sectional and represent the Wave 2 responses of the combined longitudinal and add-on samples using the final weights. We summarized respondent characteristics by counts, weighted counts, weighted proportions, and corresponding linearized standard errors for categorical variables. We reverse-coded negatively worded questions as applicable.
The primary outcomes were parental COVID-19 vaccine acceptance for children ages 0–4, 5–11, and 12–17, which we dichotomized as “already received or very/somewhat likely” versus “somewhat/very unlikely” for each age group. The VHS was included as a single variable using a composite score; the average of answers for each participant was calculated, and a score of >3 was deemed “not accepting” [9]. We calculated the weighted Cronbach’s alpha (α) to ensure additional and edited questions did not compromise the internal consistency of the VHS.
We examined responses to items designed to reflect the prespecified Health Belief Model domains and used weighted Cronbach’s α to evaluate internal consistency for each domain. An average composite score was calculated for the domains with Cronbach’s α ≥ 0.7 for use in subsequent analyses.
For each age group, the bivariate analysis examined the unadjusted association between each predictor of interest and the primary binary outcome. Three separate series of weighted multiple logistic regressions identified statistically significant, independently predictive factors for parental willingness for COVID-19 vaccination of children ages 0–4, 5–11, and 12–17 using a common set of predictors. The final set of common predictors by age group (0–4, 5–11, and 12–17) was determined using a multi-step process. First, we identified a best-fitting model for each age group using a base set of 27 predictors. We dropped variables showing a high level of multicollinearity (variance inflation factor ≥ 10) and then used backward stepwise model selection based on the Bayesian information criterion. Finally, predictors identified in one or more of the final age-specific best-fitting models were combined into a set of cross-group predictors. All analyses were conducted in R-4.1.2 for Windows using packages “survey” and “MASS” [14–17].
RESULTS
Response rate was 64.2% (3230/5034); 3042 responses qualified for analysis after data cleaning (1511 longitudinal sample respondents and 1571 add-on sample respondents). The sample size for the primary outcome differed with 950, 1613, and 1620 responses for parents with children ages 0–4, 5–11, and 12–17, respectively. Two respondents did not complete the primary outcome questions for children ages 5–11 and were excluded. Table 1 presents respondent characteristics (Supplemental Table 1 includes nonrespondents). The modified VHS had Cronbach’s α = 0.91.
Table 1.
Survey Respondent Characteristics
| Characteristic | N a | Weighted n | Weighted % (SE) |
|---|---|---|---|
| Age (years) | |||
| 18–34 | 608 | 908.3 | 29.9 (1.1) |
| 35–44 | 1390 | 1254.5 | 41.2 (1.0) |
| 45–54 | 835 | 714.4 | 23.5 (0.8) |
| ≥ 55 | 209 | 164.7 | 5.4 (0.4) |
| Age(s) of child(ren) (years)b | |||
| 0–4 | 950 | 1109.7 | 36.5 (1.1) |
| 5–11 | 1613 | 1622.4 | 53.3 (1.1) |
| 12–17 | 1620 | 1523.0 | 50.1 (1.1) |
| Presence of child(ren) of other age group(s) in household | |||
| No | 1994 | 1941.8 | 63.8 (1.0) |
| Yes | 1048 | 1100.2 | 36.2 (1.0) |
| Number of children in household | |||
| 1 | 1230 | 1226.4 | 40.3 (1.0) |
| 2 | 1179 | 1149.9 | 37.8 (1.0) |
| 3 | 436 | 458.1 | 15.1 (0.8) |
| ≥ 4 | 197 | 207.6 | 6.8 (0.6) |
| Sex | |||
| Male | 1326 | 1363.6 | 44.8 (1.1) |
| Female | 1716 | 1678.4 | 55.2 (1.1) |
| Race | |||
| White | 2508 | 2330.9 | 76.6 (1.0) |
| Black | 230 | 362.3 | 11.9 (0.8) |
| American Indian/Alaskan Native | 27 | 42.5 | 1.4 (0.3) |
| Asian/Native Hawaiian/Pacific Islander | 161 | 221.8 | 7.3 (0.6) |
| ≥2 races | 116 | 84.5 | 2.8 (0.3) |
| Ethnicity | |||
| Not Spanish, Hispanic, or Latino | 2521 | 2381.1 | 78.3 (0.9) |
| Spanish, Hispanic, or Latino | 521 | 660.9 | 21.7 (0.9) |
| Education level | |||
| Less than high school degree | 195 | 324.5 | 10.7 (0.8) |
| High school degree | 525 | 690.4 | 22.7 (1.0) |
| Some college or Associate degree | 751 | 892.4 | 29.3 (1.0) |
| Bachelor’s degree or higher | 1571 | 1134.7 | 37.3 (1.0) |
| Employment status | |||
| Employed | 2392 | 2335.1 | 76.8 (0.9) |
| Unemployed | 650 | 706.9 | 23.2 (0.9) |
| Healthcare worker | |||
| No | 2715 | 2741.5 | 90.4 (0.6) |
| Yes | 317 | 290.1 | 9.6 (0.6) |
| Nurse | 79 | 73.6 | 25.9 (3.0)c |
| Advanced practice provider (ie, NP, PA) | 18 | 13.3 | 4.7 (1.2)c |
| Physician | 17 | 15.3 | 5.4 (1.4)c |
| Other | 198 | 182.1 | 64.0 (3.2)c |
| Annual household income | |||
| < $25 000 | 288 | 268.3 | 8.8 (0.6) |
| $25 000–$74 999 | 871 | 972.3 | 32.0 (1.0) |
| ≥ $75 000 | 1883 | 1801.3 | 59.2 (1.1) |
| Census region | |||
| Northeast | 463 | 501.0 | 16.5 (0.8) |
| Midwest | 763 | 641.8 | 21.1 (0.8) |
| South | 1041 | 1158.3 | 38.1 (1.1) |
| West | 775 | 740.9 | 24.4 (0.9) |
| Urbanicity | |||
| Urban | 945 | 968.6 | 31.9 (1.0) |
| Rural | 520 | 507.1 | 16.7 (0.8) |
| Suburban | 1575 | 1564.7 | 51.5 (1.1) |
| Child with chronic health condition | |||
| No | 2694 | 2716.6 | 89.6 (0.6) |
| Yes | 337 | 315.6 | 10.4 (0.6) |
| Heard of MIS-C | |||
| No | 1951 | 2080.7 | 68.5 (1.0) |
| Yes | 1087 | 957.1 | 31.5 (1.0) |
| Acceptance of routine childhood vaccinesd | |||
| Non-accepting | 390 | 411.5 | 13.5 (0.7) |
| Accepting | 2652 | 2630.5 | 86.5 (0.7) |
| Influenza vaccine uptake for childrene | |||
| No | 1239 | 1311.3 | 43.1 (1.1) |
| Yes | 1802 | 1728.6 | 56.9 (1.1) |
| Parental vaccination status | |||
| Unvaccinated | 802 | 879.6 | 28.9 (1.0) |
| Vaccinated | 2238 | 2160.1 | 71.1 (1.0) |
| Experience of COVID-19 in adultsf | |||
| No experience/no or mild symptoms | 708 | 796.0 | 26.2 (1.0) |
| Moderate-severe symptoms/death | 2333 | 2245.7 | 73.8 (1.0) |
| Experience of COVID-19 in childreng | |||
| No experience/no or mild symptoms | 2430 | 2491.9 | 82.7 (0.8) |
| Moderate-severe symptoms/death | 584 | 519.7 | 17.3 (0.8) |
Abbreviations: n, sample size; MIS-C, multisystem inflammatory syndrome in children; NP, nurse practitioner; PA, physician assistant; SE, standard error.
The sum of the cell frequencies for sub-categories within each category may not add up to the total respondent sample size (n = 3042) when respondents failed to answer all survey questions.
Respondents could have children in more than one age group.
Weighted proportion among healthcare workers.
Based on World Health Organization Vaccine Hesitancy Scale responses, with non-accepting defined as a score > 3 and accepting defined as a score ≤ 3.
Based on reporting that one or more of their children received the influenza vaccine for the 2019–2020 season.
Included respondents’ reported experiences in self and/or other adults they know.
Included respondents’ reported experiences with their own children or other children they know.
Parental Attitudes and Beliefs About SARS-CoV-2-Related Disease and COVID-19 Vaccines
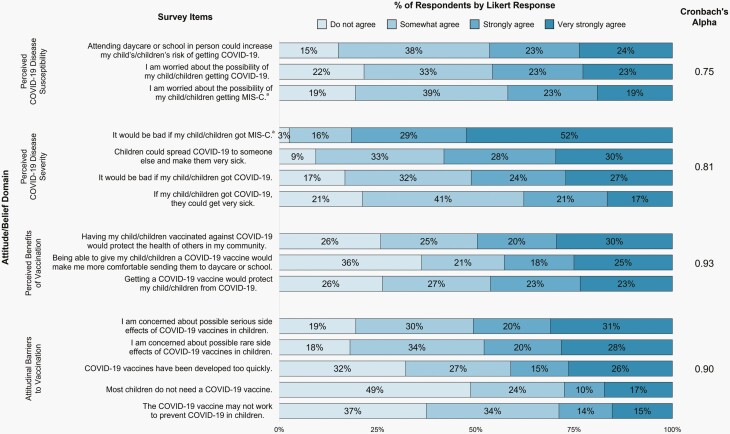
Supplemental Table 2 presents respondents’ attitudes and beliefs about SARS-CoV-2-related disease and COVID-19 vaccines. 83.1% of parents agreed it would be bad if their children got COVID-19, and 81.2% of parents were concerned about how new variants could affect children. Of the 31.5% who had heard of MIS-C, 96.3% agreed it would be bad if their children got MIS-C, while 80. 3% were worried about the possibility of their children getting MIS-C. 89.4% agreed their children’s healthcare provider is a reliable and trustworthy source of information about COVID-19 vaccines. 80.1% and 81.5% agreed they were worried about serious and rare vaccine side effects, respectively. 67.5% agreed that COVID-19 vaccines were developed too quickly. 51.1% agreed that most children do not need a COVID-19 vaccine, and 62.1% agreed the vaccines might not work to prevent COVID-19 in children. Composite scores grouping questions by attitude/ belief domains were confirmed to be internally reliable (Figure 1).
Figure 1.
Domain analysis for composite predictors. aMIS-C questions were only presented to and answered by those respondents who indicated that they had heard of MIS-C on a screening question.
Parental Acceptance of COVID-19 Vaccines for Children
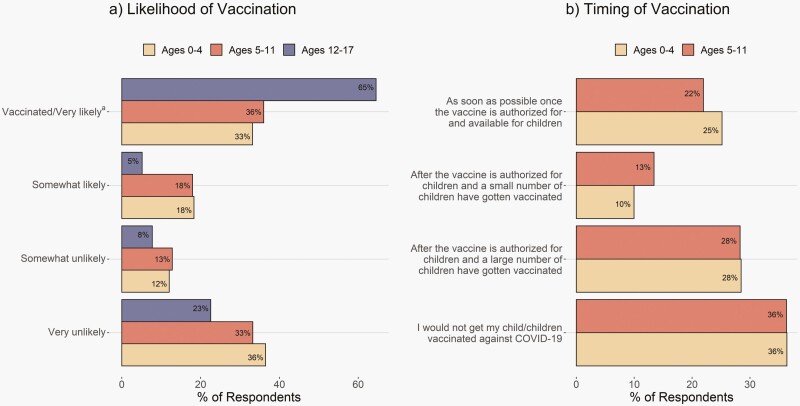
The percentage of parents who were very/somewhat likely to have their children vaccinated or whose children were already vaccinated against COVID-19 was 51.5% for ages 0–4, 54.0% for 5–11, and 69.7% for 12–17 (Figure 2a). Among respondents with an unvaccinated child < 12 years, 25.2% (ages 0–4) and 22.0% (ages 5–11) reported they would seek COVID-19 vaccination for their children as soon as pediatric authorization occurred, and larger proportions of respondents reported wanting to wait until other children were vaccinated or not being comfortable letting their child receive a COVID-19 vaccine (Figure 2b).
Figure 2.
Respondents’ willingness to have their children receive a COVID-19 vaccine and timing relative to pediatric vaccine authorization. a60.6% of children ages 12–17 were vaccinated, 9.9% of children ages 5–11 were vaccinated, and 0.2% of children ages 0–4 were vaccinated (as part of a trial).
Characteristics of Parents Who Reported Willingness to Vaccinate Their Child Against COVID-19
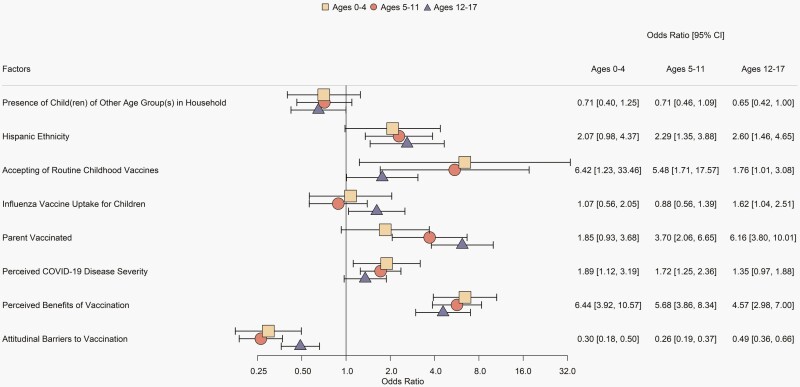
Our final multivariable models contained eight predictors that were significant in at least one of the age-specific best-fitting models (Figure 3; see Supplemental Table 3 for bivariate associations, Supplemental Table 4 and Supplemental Figure 1 for age-specific best-fitting models, and Supplemental Table 5 for all predictors included in the final models). The strongest positive predictors of parental likelihood to vaccinate children across all age groups were belief in the benefits of COVID-19 vaccination and acceptance of routine childhood vaccinations. Positive predictors also included perception that pediatric COVID-19-related disease is severe for children ages 0–4 and 5–11, Hispanic ethnicity and parental COVID-19 vaccination for ages 5–11 and 12–17, and 2019–2020 influenza vaccine uptake for ages 12–17. The strongest negative predictor across all age groups was attitudinal barriers to COVID-19 vaccination. Having children of another age group in the household was a negative predictor for children ages 12–17.
Figure 3.
Predictors of respondents’ willingness to have their children receive a COVID-19 vaccine. Forest plot for odds ratio of having a child vaccinated against COVID-19 with 95% confidence intervals based on weighted multivariable logistic regression. Figure includes effects that were statistically significant (P < .05) for at least one age group.
Factors That May Influence Willingness to Vaccinate
Overall, respondents most frequently reported that pediatric vaccines receiving full FDA approval (rather than Emergency Use Authorization) would make them more likely to vaccinate their children (Table 2 and Supplemental Table 6). The second most frequently reported factor that would increase the likelihood of vaccination for children was school vaccine requirements for respondents very/somewhat unlikely to vaccinate their children across all age groups. For respondents somewhat likely to vaccinate their children, the second most frequently reported factor that would increase the likelihood of vaccination differed by age group: for ages 0–4, it was a recommendation from their child’s healthcare provider; for ages 5–11, it was knowing a lot of other children who received a COVID-19 vaccine; and for ages 12–17, it was school requirements. If COVID-19 vaccines were expected to cause more severe side effects than routine vaccines, 53.0% of all respondents indicated they would be less likely to vaccinate their children, including 32.2%, 31.7%, and 51.7% of respondents who initially reported they were very likely to have children ages 0–4, 5–11, and 12–17, respectively, receive a COVID-19 vaccine.
Table 2.
Factors Parents Report Would Make Them More or Less Likely to Have Children Receive a COVID-19 Vaccine Among Parents Somewhat Unlikely to Accept COVID-19 Vaccination for Their Childrena
| Change in Intent, Weighted n (%, SE) | |||||
|---|---|---|---|---|---|
| Factor | Much More Likely | Somewhat More Likely | No More or Less Likely | Somewhat Less Likely | Much Less Likely |
| Ages 0–4 | |||||
| Vaccine received full FDA approval | 17.4 (13.2, 3.9) | 48.0 (36.4, 5.4) | 55.9 (42.3, 5.9) | 9.4 (7.2, 2.9) | 1.3 (1.0, 0.7) |
| Required to return to school or daycare | 7.6 (5.8, 2.4) | 47.2 (35.7, 5.3) | 57.0 (43.2, 5.9) | 13.5 (10.3, 3.6) | 6.7 (5.0, 1.9) |
| A lot of children I know have gotten it | 10.2 (7.7, 2.8) | 41.2 (31.2, 5.1) | 68.7 (52.1, 5.8) | 7.5 (5.7, 2.6) | 4.4 (3.3, 1.5) |
| Causes the same or fewer short-term side effectsb | 6.0 (4.6, 2.0) | 40.8 (31.0, 5.4) | 68.8 (52.4, 5.8) | 10.3 (7.8, 2.9) | 5.6 (4.2, 2.3) |
| Child(ren)’s healthcare provider recommends it | 4.5 (3.4, 2.0) | 39.4 (29.8, 5.2) | 81.1 (61.4, 5.6) | 5.7 (4.3, 2.3) | 1.3 (1.0, 0.7) |
| Required to travel | 2.4 (1.9, 1.4) | 39.5 (30.6, 5.3) | 69.1 (53.6, 5.8) | 12.2 (9.5, 3.2) | 5.8 (4.5, 1.6) |
| A lot of people of all ages I know have gotten it | 6.5 (4.9, 2.4) | 33.7 (25.6, 4.9) | 69.3 (52.5, 5.8) | 16.4 (12.4, 4.2) | 6.0 (4.6, 2.5) |
| Given at same time as a routine vaccine | 0.0 (0.0, 0.0) | 25.0 (18.9, 4.4) | 95.1 (72.0, 5.0) | 7.7 (5.9, 2.7) | 4.2 (3.2, 1.4) |
| Different type of vaccine becomes available for children | 3.2 (2.5, 1.6) | 14.8 (11.5, 3.7) | 97.2 (75.4, 4.9) | 7.0 (5.5, 2.4) | 6.7 (5.2, 2.3) |
| Free childcare assistance | 2.3 (1.8, 1.1) | 9.8 (7.6, 3.1) | 101.5 (79.5, 4.6) | 9.2 (7.2, 3.1) | 4.9 (3.9, 1.9) |
| Encouraged by local religious/community leaders | 1.5 (1.1, 0.8) | 7.4 (5.8, 2.2) | 86.0 (66.7, 5.4) | 17.5 (13.6, 3.8) | 16.5 (12.8, 4.1) |
| Paid time off work | 0.0 (0.0, 0.0) | 7.7 (5.9, 2.4) | 106.4 (82.6, 4.0) | 7.3 (5.6, 2.4) | 7.5 (5.8, 2.5) |
| Free transportation | 1.1 (0.9, 0.9) | 4.5 (3.5, 1.8) | 97.9 (76.0, 5.0) | 15.2 (11.8, 4.2) | 10.2 (7.9, 2.9) |
| Causes more severe side effectsb | 0.6 (0.4, 0.4) | 1.6 (1.2, 0.9) | 42.7 (32.4, 5.5) | 41.5 (31.5, 5.4) | 45.6 (34.5, 5.6) |
| Ages 5–11 | |||||
| Vaccine received full FDA approval | 24.2 (11.9, 2.4) | 72.6 (35.7, 4.0) | 87.0 (42.8, 4.3) | 12.3 (6.1, 2.4) | 7.2 (3.6, 1.4) |
| Required to return to school or daycare | 21.9 (10.7, 2.4) | 65.8 (32.1, 3.9) | 86.5 (42.2, 4.3) | 13.8 (6.7, 2.5) | 17.1 (8.4, 2.0) |
| A lot of children I know have gotten it | 13.5 (6.6, 1.8) | 70.1 (34.4, 3.9) | 101.7 (49.9, 4.3) | 8.4 (4.1, 1.6) | 10.0 (4.9, 2.1) |
| Child(ren)’s healthcare provider recommends it | 11.2 (5.5, 1.7) | 66.2 (32.3, 3.9) | 106.0 (51.7, 4.3) | 14.5 (7.1, 2.5) | 7.1 (3.5, 1.5) |
| A lot of people of all ages I know have gotten it | 7.8 (3.8, 1.5) | 57.0 (28.0, 3.6) | 119.9 (58.9, 4.1) | 12.7 (6.2, 2.4) | 6.3 (3.1, 1.4) |
| Required to travel | 11.4 (5.6, 1.6) | 50.2 (24.6, 3.6) | 111.0 (54.3, 4.2) | 16.6 (8.1, 2.7) | 15.3 (7.5, 2.0) |
| Causes the same or fewer short-term side effectsb | 6.3 (3.1, 1.2) | 49.3 (24.5, 3.5) | 120.3 (59.8, 4.2) | 17.8 (8.9, 2.8) | 7.6 (3.8, 1.5) |
| Different type of vaccine becomes available for children | 2.7 (1.3, 0.6) | 30.5 (15.0, 3.4) | 143.7 (70.9, 4.0) | 11.4 (5.6, 1.8) | 14.5 (7.1, 2.4) |
| Given at same time as a routine vaccine | 2.1 (1.0, 0.5) | 23.3 (11.5, 2.4) | 144.9 (71.6, 3.8) | 12.4 (6.1, 2.3) | 19.8 (9.8, 2.6) |
| Paid time off work | 4.8 (2.3, 1.2) | 19.0 (9.2, 2.7) | 154.7 (74.7, 3.9) | 16.4 (7.9, 2.7) | 12.3 (5.9, 1.7) |
| Free childcare assistance | 6.4 (3.1, 1.3) | 14.7 (7.2, 2.6) | 159.7 (78.4, 3.4) | 7.3 (3.6, 1.3) | 15.6 (7.6, 2.0) |
| Free transportation | 3.2 (1.6, 0.9) | 5.9 (2.9, 1.3) | 156.7 (76.0, 3.6) | 22.6 (11.0, 3.0) | 17.7 (8.6, 2.0) |
| Encouraged by local religious/community leaders | 0.9 (0.4, 0.4) | 4.2 (2.1, 0.9) | 152.8 (75.0, 3.6) | 20.8 (10.2, 2.4) | 25.1 (12.3, 2.8) |
| Causes more severe side effectsb | 2.6 (1.3, 0.8) | 2.2 (1.1, 0.8) | 68.3 (33.9, 4.1) | 56.1 (27.8, 3.8) | 72.6 (36.0, 4.1) |
| Ages 12–17 | |||||
| Vaccine received full FDA approval | 6.1 (5.3, 2.1) | 31.0 (26.8, 4.7) | 59.5 (51.5, 5.3) | 11.4 (9.9, 3.1) | 7.6 (6.6, 2.8) |
| Required to return to school or daycare | 8.8 (7.5, 2.6) | 25.8 (22.1, 4.4) | 64.5 (55.2, 5.2) | 4.7 (4.1, 1.7) | 13.0 (11.2, 3.3) |
| Causes the same or fewer short-term side effectsb | 3.6 (3.1, 1.9) | 26.2 (22.6, 4.5) | 71.5 (61.6, 5.2) | 7.5 (6.5, 2.6) | 7.2 (6.2, 2.4) |
| Child(ren)’s healthcare provider recommends it | 2.2 (1.9, 1.4) | 24.5 (21.0, 4.3) | 77.8 (66.6, 5.0) | 6.8 (5.8, 2.4) | 5.5 (4.7, 2.2) |
| Required to travel | 3.9 (3.4, 1.6) | 21.0 (18.2, 4.1) | 74.4 (64.5, 5.0) | 10.1 (8.7, 2.9) | 5.9 (5.1, 2.1) |
| A lot of children I know have gotten it | 0.0 (0.0, 0.0) | 17.1 (14.8, 3.5) | 81.8 (70.6, 4.7) | 5.3 (4.6, 2.3) | 11.6 (10.0, 3.0) |
| A lot of people of all ages I know have gotten it | 0.0 (0.0, 0.0) | 15.7 (13.5, 3.2) | 81.4 (69.7, 4.7) | 10.8 (9.2, 2.9) | 8.9 (7.6, 2.8) |
| Different type of vaccine becomes available for children | 0.8 (0.7, 0.5) | 10.9 (9.3, 3.0) | 84.4 (72.3, 4.6) | 10.5 (9.0, 2.9) | 10.2 (8.7, 2.9) |
| Given at same time as a routine vaccine | 0.0 (0.0, 0.0) | 8.9 (7.6, 2.9) | 90.1 (77.2, 4.4) | 7.4 (6.3, 2.4) | 10.3 (8.8, 2.9) |
| Paid time off work | 0.7 (0.6, 0.6) | 4.9 (4.2, 2.4) | 90.1 (76.5, 4.5) | 5.5 (4.6, 2.2) | 16.7 (14.2, 3.6) |
| Free transportation | 0.9 (0.8, 0.6) | 4.0 (3.4, 2.4) | 86.4 (74.0, 4.6) | 5.5 (4.8, 2.3) | 19.9 (17.1, 3.7) |
| Encouraged by local religious/community leaders | 0.0 (0.0, 0.0) | 3.7 (3.1, 2.0) | 88.5 (75.1, 4.5) | 7.5 (6.4, 2.6) | 18.1 (15.4, 3.6) |
| Free childcare assistance | 0.7 (0.6, 0.6) | 2.3 (2.0, 1.7) | 91.8 (78.6, 4.2) | 7.0 (6.0, 2.4) | 15.0 (12.8, 3.3) |
| Causes more severe side effectsb | 0.0 (0.0, 0.0) | 2.6 (2.2, 1.8) | 57.1 (49.1, 5.3) | 18.6 (16.0, 3.7) | 38.0 (32.7, 4.9) |
Data for parents who were “very unlikely,” “somewhat likely,” and “very likely” at baseline to accept COVID-19 vaccination for their children are available in Supplemental Table 2.
Compared to routine vaccines.
DISCUSSION
In this nationally representative sample of US parents, more than half were likely to accept COVID-19 vaccination for their children. Roughly 40% of parents with children 0–11 years old wanted to “wait and see” before vaccination, and another 36% would not let their children get a COVID-19 vaccine in this survey conducted when COVID-19 vaccination for 5–11-year-olds was first recommended and no vaccine was available for younger children. Belief in COVID-19 vaccination benefits and acceptance of routine childhood vaccines were the strongest positive predictors of intention to have children vaccinated across all age groups. The strongest negative predictor was attitudinal barriers to COVID-19 vaccination. Among all respondents, the most frequently cited factor that would increase their likelihood of having children vaccinated was full FDA approval; all groups also responded they would be less likely to vaccinate their children if side effects were worse than those experienced with routine vaccines.
Our finding of >50% parental acceptance of COVID-19 vaccination for children is higher than early studies of US parental acceptance of COVID-19 vaccines for children but consistent with more recent surveys [18–26]. Our rates of parents reporting they themselves and their teenage children had been vaccinated were consistent with CDC’s vaccination statistics at the time the survey was conducted, and subsequent data have shown uptake among children 5–11 consistent with our projected timeframes for adoption [27–29]. Parents were 2–6 times more likely to report acceptance of child COVID-19 vaccination if they had received a COVID-19 vaccine themselves; however, this effect was not statistically significant for parents of children 0–4 years old, which may reflect uncertainty about the vaccine not yet having been authorized for that age group. Increasing overall COVID-19 vaccine confidence in US adults will play an important role in achieving high COVID-19 vaccine uptake in children.
Parents may balance perceived risks of COVID-19 against perceived risks of vaccination. Although fewer children experience severe disease than adults, “long COVID” and MIS-C are important sources of morbidity in children, and parental perception that pediatric COVID-19 disease is severe was a significant positive predictor of vaccination intention for children 0–4 and 5–11 [28, 30]. Even among respondents very likely to vaccinate children ages 0–4 and 5–11 against COVID-19, nearly one-third indicated that if side effects were more severe than those experienced with routine vaccines, they would be less likely to vaccinate their children, and baseline acceptance of other routine childhood immunizations was a significant positive predictor of parental COVID-19 vaccine acceptance across all age groups. Clear messaging from public health entities and healthcare providers about the magnitude and severity of the risks associated with COVID-19 vaccination relative to other childhood vaccines and SARS-CoV-2-related diseases is needed to help parents make informed decisions about COVID-19 vaccines for their children.
Vaccination against influenza the season before the COVID-19 pandemic was significantly associated with parental willingness to vaccinate teenage children against COVID-19. In recent years, pediatric influenza vaccine uptake has been lower than routine childhood vaccine uptake [31–34]. Because we do not know what the periodicity of COVID-19 vaccination will be, it will be important for public health messaging to address factors that influence reluctance around seasonal vaccines.
Earlier studies showed lower COVID-19 vaccine confidence among Hispanic populations, but in our study, Hispanic parents reported higher COVID-19 vaccine acceptance for their children than non-Hispanic parents [24, 35]. Our finding is consistent with more recent studies of COVID-19 vaccine acceptance and uptake among Hispanic populations [26, 27, 29]. These changes in attitudes may be due to targeted community outreach over the course of the pandemic and the cumulative burden of disease suffered by the Hispanic community [1, 35].
Given our finding that many parents want to “wait and see” before getting their children a COVID-19 vaccine, we expect slow pediatric COVID-19 vaccine uptake with a potential surge in vaccination once full FDA approval of pediatric vaccines is granted, although a similar effect has not been observed in adults [36]. School vaccination requirements may encourage vaccine uptake for some children, including among those whose parents were very unlikely to accept COVID-19 vaccination; however, school requirements are just one approach along a continuum of policy options [37–41]. We expect parents who were very unlikely to accept COVID-19 vaccination for their children at baseline will be less likely to change their decisions relative to parents who were somewhat unlikely at baseline, but a combination of strategies that incorporate structural interventions like vaccine requirements with interventions centered on interpersonal communication with healthcare providers and peers may encourage some parents to accept vaccination.
This study represents parental attitudes when COVID-19 vaccines for US children ages 5–11 were first authorized and no vaccines were available for children <5 years, and attitudes may have changed since we conducted our survey. COVID-19 vaccine authorization for children 5–11 years occurred during our survey administration period, meaning some respondents completed the survey before and some after this announcement. The rapid release of information and speed of changes in public opinion pose challenges not only for survey design and data interpretation for the 5–11-year age group but also for keeping public health messaging current and relevant. Our findings are not generalizable outside of the US or for non-English- or Spanish-speaking US populations. The attitudes, beliefs, and intentions measured in this study represent factors that influence planned behaviors but may not represent what parents ultimately decide when a COVID-19 vaccine becomes available to their children.
CONCLUSION
In this nationally representative survey of US parents of children <18 years conducted very early in the availability of COVID-19 vaccines for children ages 5–11, most parents reported intention to vaccinate their children. Belief in the benefits of COVID-19 vaccination and acceptance of routine childhood vaccines were the most important predictors of COVID-19 vaccine acceptance for their children. Efforts to ensure pediatric COVID-19 vaccine uptake should include messaging to increase overall confidence in COVID-19 vaccines among US adults. Further work is needed to track changes in attitudes and intentions as more data become available about COVID-19 vaccines in children and adolescents and to study whether intention to vaccinate translates into vaccination.
Supplementary Material
Notes
Financial support. E.A.H. was supported by the United States National Institutes of Health [NIH grant T32A1007524] during the preparation and writing of this manuscript. Funding for this study was generously provided by the United States Centers for Disease Control and Prevention (CDC). There were no other sources of funding for this study.
Role of funder/sponsor. The NIH had no role in the design or conduct of this study. The CDC contributed to the early-stage design of the study but had no role in the conduct of the study, review of the data, or manuscript preparation.
Potential conflicts of interest . E.A.H. and J.D.C. are investigators on clinical trials of COVID-19 vaccines sponsored by Moderna and Novavax but receive no personal financial support for these activities; their institution is provided support for study implementation. E.A.H. has received compensation for serving as an endpoint adjudicator for COVID-19 vaccine trials sponsored by Oxford University and AstraZeneca.
Contributor Information
E Adrianne Hammershaimb, Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore, Maryland, USA; Department of Pediatrics, University of Maryland School of Medicine, Baltimore, Maryland, USA.
Lyndsey D Cole, Department of Pediatrics, University of Colorado Anschutz Medical Campus, Aurora, Colorado, USA.
Yuanyuan Liang, Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore, Maryland, USA; Department of Epidemiology and Public Health, University of Maryland School of Medicine, Baltimore, Maryland, USA.
Megan A Hendrich, Ipsos Public Affairs, Washington DC, USA.
Dhiman Das, Ipsos Public Affairs, Washington DC, USA.
Robert Petrin, Ipsos Public Affairs, Washington DC, USA.
Jessica R Cataldi, Department of Pediatrics, University of Colorado Anschutz Medical Campus, Aurora, Colorado, USA; Adult and Child Consortium for Health Outcomes Research and Delivery Science, University of Colorado Anschutz Medical Campus Aurora, Colorado, USA; Children’s Hospital Colorado, Aurora, Colorado, USA.
Sean T O’Leary, Department of Pediatrics, University of Colorado Anschutz Medical Campus, Aurora, Colorado, USA; Adult and Child Consortium for Health Outcomes Research and Delivery Science, University of Colorado Anschutz Medical Campus Aurora, Colorado, USA; Children’s Hospital Colorado, Aurora, Colorado, USA.
James D Campbell, Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore, Maryland, USA; Department of Pediatrics, University of Maryland School of Medicine, Baltimore, Maryland, USA.
References
- 1. Centers for Disease Control and Prevention. Demographic Trends of COVID-19 Cases and Deaths in the US Reported to CDC. Accessed May 10, 2022. https://covid.cdc.gov/covid-data-tracker/#demographics.
- 2. Centers for Disease Control and Prevention. Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19) ; 2020. Accessed March 10, 2022. https://emergency.cdc.gov/han/2020/han00432.asp.
- 3. Centers for Disease Control and Prevention. 2019–20 Season’s Pediatric Flu Deaths Tie High Mark Set During 2017–18 Season. Accessed April 8, 2021. https://www.cdc.gov/flu/spotlights/2019-2020/2019-20-pediatric-flu-deaths.htm.
- 4. Anderson EJ, Campbell JD, Creech CB, et al. Warp speed for COVID-19 vaccines: why are children stuck in neutral? Clin Infect Dis 2020; 73:336–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. U.S. Food and Drug Administration. COVID-19 Vaccines. Accessed March 4, 2022. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines.
- 6. Wallace M, Woodworth KR, Gargano JW, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 Vaccine in Adolescents Aged 12-15 Years - United States, May 2021. MMWR Morb Mortal Wkly Rep 2021; 70:749–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Woodworth K, Moulia D, Collins J.et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Pfizer-BioNTech COVID-19 vaccine in children aged 5–11 years—United States, November 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1579–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Larson HJ, Jarrett C, Schulz WS, et al. Measuring vaccine hesitancy: the development of a survey tool. Vaccine 2015; 33:4165–75. [DOI] [PubMed] [Google Scholar]
- 9. Kempe A, Saville AW, Albertin C, et al. Parental hesitancy about routine childhood and influenza vaccinations: a national survey. Pediatrics 2020; 146:e20193852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chyung SY, Roberts K, Swanson I, Hankinson A.. Evidence-based survey design: the use of a midpoint on the Likert scale. Perform Improv 2017; 56:15–23. [Google Scholar]
- 11. Rosenstock IM, Strecher VJ, Becker MH.. Social learning theory and the Health Belief Model. Health Educ Q 1988; 15:175–83. [DOI] [PubMed] [Google Scholar]
- 12. Fahimi M, Kulp D.. Address-based sampling may provide alternatives for surveys that require contacts with representative samples of households. Quirk’s Mark Res Rev 2009. https://www.quirks.com/articles/address-based-sampling-may-provide-alternatives-for-surveys-that-require-contacts-with-representative-samples-of-households. [Google Scholar]
- 13. Fahimi M. Practical guidelines for dual-frame RDD survey Methodology (now that the dust is settling). Survey Practice 2014; 7(2):1–13. doi: 10.29115/SP-2014-0011.26451335 [DOI] [Google Scholar]
- 14. R Core Team. R: A Language and Environment for Statistical Computing (Version 4.1.2). R Foundation for Statistical Computing; 2021. https://www.R-project.org/. [Google Scholar]
- 15. Lumley T. Survey: Analysis of Complex Survey Samples (R Package Version 4.0) ; 2020. https://cran.r-project.org/package=survey
- 16. Lumley T. Complex Surveys: A Guide to Analysis Using R. Hoboken, NJ: John Wiley & Sons; 2010. [Google Scholar]
- 17. Venables WN, Ripley BD.. Modern Applied Statistics With S. 4th ed. Springer, NY: Springer. 2002. [Google Scholar]
- 18. Szilagyi PG, Shah MD, Delgado JR, et al. Parents’ intentions and perceptions about COVID-19 vaccination for their children: results from a national survey. Pediatrics 2021; 148:e2021052335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goldman RD, Krupik D, Ali S, et al. Caregiver willingness to vaccinate their children against COVID-19 after adult vaccine approval. Int J Environ Res Public Health; 2021; 18:10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Teherani M, Banskota S, Camacho-Gonzalez A, et al. Intent to Vaccinate SARS-CoV-2 Infected Children in US Households: A Survey. Vaccines 2021; 9:1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hamel L, Lopes L, Sparks G, Stokes M, Brodie M.. KFF COVID-19 Vaccine Monitor: April 2021 ; 2021. Accessed March 10, 2022. https://www.kff.org/coronavirus-covid-19/poll-finding/kff-covid-19-vaccine-monitor-april-2021/.
- 22. Teasdale CA, Borrell LN, Kimball S, et al. Plans to vaccinate children for COVID-19: a survey of United States parents. J Pediatr. 2021; 237:292–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ruggiero KM, Wong J, Sweeney CF, et al. Parents’ intentions to vaccinate their children against COVID-19. J Pediatr Health Care. 2021; 35:509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rane MS, Robertson MM, Westmoreland DA, Teasdale CA, Grov C, Nash D.. Intention to Vaccinate Children Against COVID-19 Among Vaccinated and Unvaccinated US Parents. JAMA Pediatr 2022; 176:201–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen F, He Y, Shi Y.. Parents’ and Guardians’ Willingness to Vaccinate Their Children against COVID-19: A Systematic Review and Meta-Analysis. Vaccines (Basel) 2022; 10:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hamel L, Lopes L, Sparks G, et al. KFF COVID-19 Vaccine Monitor: October 2021; 2021. https://www.kff.org/coronavirus-covid-19/poll-finding/kff-covid-19-vaccine-monitor-october-2021/. Accessed March 10, 2022.
- 27. Centers for Disease Control and Prevention. Demographic Trends of People Receiving COVID-19 Vaccinations in the United States . Accessed March 10, 2022. https://covid.cdc.gov/covid-data-tracker/#vaccination-demographics-trends.
- 28. Centers for Disease Control and Prevention. Pediatric Data . Accessed May 11, 2022. https://covid.cdc.gov/covid-data-tracker/#pediatric-data.
- 29. Trujillo KL, Lazer D, Simonson MD, et al. The COVID States Project #71: Childhood COVID-19 Vaccine Uptake and Intentions. OSF Preprints. doi: 10.31219/osf.io/cu4jz. [DOI] [Google Scholar]
- 30. Fainardi V, Meoli A, Chiopris G, et al. Long COVID in children and adolescents. Life 2022; 12:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Elam-Evans LD, Yankey D, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2019. MMWR Morb Mortal Wkly Rep 2020; 69:1109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hill HA, Yankey D, Elam-Evans LD, Singleton JA, Pingali SC, Santibanez TA.. Vaccination coverage by age 24 months among children born in 2016 and 2017—National Immunization Survey-child, United States, 2017–2019. MMWR Morb Mortal Wkly Rep 2020; 69:1505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Seither R, McGill MT, Kriss JL, et al. Vaccination coverage with selected vaccines and exemption rates among children in kindergarten—United States, 2019–20 school year. MMWR Morb Mortal Wkly Rep 2021; 70:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Centers for Disease Control and Prevention. Flu Vaccination Coverage, United States, 2019–2020 Influenza Season; 2020. [Google Scholar]
- 35. Khubchandani J, Macias Y.. COVID-19 vaccination hesitancy in Hispanics and African-Americans: a review and recommendations for practice. Brain Behav Immun Health 2021; 15:100277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cohen E. Full FDA Approval of Pfizer’s Covid-19 Vaccine Had Only a Modest Impact on Uptake. Here’s What Mattered More. CNN; 2021. Accessed March 6, 2022. https://amp.cnn.com/cnn/2021/10/04/health/pfizer-covid-19-vaccine-approval-bump/index.html. [Google Scholar]
- 37. Greyson D, Vriesema-Magnuson C, Bettinger JA.. Impact of school vaccination mandates on pediatric vaccination coverage: a systematic review. CMAJ Open 2019; 7:E524–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brewer NT. What works to increase vaccination uptake. Acad Pediatr 2021; 21:S9–S16. [DOI] [PubMed] [Google Scholar]
- 39. Braun C and O’Leary ST. Recent advances in addressing vaccine hesitancy. Curr Opin Pediatr 2020; 32:601–9. [DOI] [PubMed] [Google Scholar]
- 40. Brewer NT, Buttenheim AM, Clinton CV, et al. Incentives for COVID-19 vaccination. Lancet Reg Health Am 2022; 8:100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Omer SB, Benjamin RM, Brewer NT, et al. Promoting COVID-19 vaccine acceptance: recommendations from the Lancet Commission on Vaccine Refusal, Acceptance, and Demand in the USA. Lancet 2021; 398:2186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.