ABSTRACT
Introduction
Extremity trauma is the most common battlefield injury, resulting in a high frequency of combat-related extremity wound infections (CEWIs). As these infections are associated with substantial morbidity and may impact wounded warriors long after initial hospitalization, CEWIs have been a focus of the Infectious Disease Clinical Research Program (IDCRP). Herein, we review findings of CEWI research conducted through the IDCRP and discuss future and ongoing analyses.
Methods
Military personnel with deployment-related trauma sustained between 2009 and 2014 were examined in retrospective analyses through the observational Trauma Infectious Disease Outcomes Study (TIDOS). Characteristics of wounded warriors with ≥1 open extremity wound were assessed, focusing on injury patterns and infection risk factors. Through a separate trauma-associated osteomyelitis study, military personnel with combat-related open fractures of the long bones (tibia, femur, and upper extremity) sustained between 2003 and 2009 were examined to identify osteomyelitis risk factors.
Results
Among 1,271 wounded warriors with ≥1 open extremity wound, 16% were diagnosed with a CEWI. When assessed by their most severe extremity injury (i.e., amputation, open fracture, or open soft-tissue wound), patients with amputations had the highest proportion of infections (47% of 212 patients with traumatic amputations). Factors related to injury pattern, mechanism, and severity were independent predictors of CEWIs during initial hospitalization. Having a non-extremity infection at least 4 days before CEWI diagnosis was associated with reduced likelihood of CEWI development. After hospital discharge, 28% of patients with extremity trauma had a new or recurrent CEWI during follow-up. Risk factors for the development of CEWIs during follow-up included injury pattern, having either a CEWI or other infection during initial hospitalization, and receipt of antipseudomonal penicillin for ≥7 days. A reduced likelihood for CEWIs during follow-up was associated with a hospitalization duration of 15-30 days. Under the retrospective osteomyelitis risk factor analysis, patients developing osteomyelitis had higher open fracture severity based on Gustilo–Anderson (GA) and the Orthopaedic Trauma Association classification schemes and more frequent traumatic amputations compared to open fracture patients without osteomyelitis. Recurrence of osteomyelitis was also common (28% of patients with open tibia fractures had a recurrent episode). Although osteomyelitis risk factors differed between the tibia, femur, and upper extremity groups, sustaining an amputation, use of antibiotic beads, and being injured in the earlier years of the study (before significant practice pattern changes) were consistent predictors. Other risk factors included GA fracture severity ≥IIIb, blast injuries, foreign body at fracture site (with/without orthopedic implant), moderate/severe muscle damage and/or necrosis, and moderate/severe skin/soft-tissue damage. For upper extremity open fractures, initial stabilization following evacuation from the combat zone was associated with a reduced likelihood of osteomyelitis.
Conclusions
Forthcoming studies will examine the effectiveness of common antibiotic regimens for managing extremity deep soft-tissue infections to improve clinical outcomes of combat casualties and support development of clinical practice guidelines for CEWI treatment. The long-term impact of extremity trauma and resultant infections will be further investigated through both Department of Defense and Veterans Affairs follow-up, as well as examination of the impact on comorbidities and mental health/social factors.
INTRODUCTION
Among combat casualties injured in support of operations in Iraq and Afghanistan, there was a high proportion of severe extremity trauma.1–9 Between 2001 and 2005, 82% of 1,566 combat casualties sustained extremity trauma with a total of 3,575 extremity wounds documented, of which 53% were soft-tissue wounds, 21% were open fractures, and 4% were closed fractures.3 The proportion of amputations during this period was 4%3; however, the rate substantially increased in 2010 following the surge of military personnel into Afghanistan as a result of encounters with improvised explosive devices (IEDs) while dismounted (foot patrol). Specifically, the rate of amputations increased from 3.5 per 100 trauma admissions (forward stabilization in combat zone and combat support hospitals) in 2010 to 14 per 100 trauma admissions in 2011.5 Some of these casualties sustained multiple extremity amputations. A frequently encountered pattern of trauma with multiple extremities was the “dismounted complex blast injury”, consisting of an amputation of a lower extremity (through or above knee), a severe injury or amputation to the contralateral lower extremity, and abdominal, pelvic, or urogenital injury.9
Infectious complications, including deep soft-tissue infections and osteomyelitis, are a frequent occurrence associated with combat-related extremity trauma. In an analysis of open tibia fractures sustained by U.K. combat casualties, 23% developed an infection. Although 72% of the patients were injured via a blast, neither injury severity nor mechanism was associated with the infections. Nevertheless, there was a higher proportion of segmental bone loss among patients who developed infections.8 Extremity trauma and associated infections also have long-term effects. Examination of 664 U.S. combat casualties admitted to orthopedic service found that 15% were diagnosed with osteomyelitis, of which 27% had recurrence, requiring re-hospitalization. The duration following initial infection to recurrence was a median of 128 days (range: 30-387 days).10 Open tibia fractures with infectious complications have also been associated with a lower likelihood of returning to duty after injury and a higher proportion of disability.11 Furthermore, patients who sustained severe lower extremity trauma and underwent late amputations (≥12 weeks post-injury) were more likely to be diagnosed with a deep infection or osteomyelitis before the amputation.12
As part of the Infectious Disease Clinical Research Program (IDCRP) based at the Uniformed Services University of the Health Sciences (USU), the Trauma Infectious Disease Outcomes Study (TIDOS) has conducted analyses to examine the short- and long-term infectious outcomes of deployment-related trauma sustained between June 2009 and December 2014.13,14 Due to the high morbidity, extremity wound infections (skin and soft-tissue infections [SSTIs] and osteomyelitis) are a research focus of TIDOS. A second protocol (the Trauma-Associated Osteomyelitis study) examined the characteristics of patients with osteomyelitis to identify risk factors for development of incident infections, as well as recurrences. Patients were eligible for inclusion in the Trauma-Associated Osteomyelitis study if they sustained a combat-related open fracture of the long bones (tibia, femur, or upper extremity) between March 2003 and December 2009.15 For both TIDOS and Trauma-Associated Osteomyelitis, the wounded military personnel underwent inter-theater evacuation from the combat zone to Landstuhl Regional Medical Center (LRMC) before being further evacuated to a participating military hospital in the USA. The participating hospitals were the Walter Reed Army Medical Center and National Naval Medical Center (merged to become Walter Reed National Military Medical Center in September 2011) in the National Capital Region and Brooke Army Medical Center in Texas. These protocols were approved by the Institutional Review Board of USU.
Herein, we summarize findings of our TIDOS and Trauma-Associated Osteomyelitis studies and discuss ongoing/upcoming research related to extremity wound infections. For a discussion of extremity wound infection microbiology, see Mende et al.16 within this supplement.
TIDOS EXTREMITY WOUND INFECTION ANALYSES
Polytrauma Characterization
Due to the complexity of data resulting from the high proportion of polytrauma (64% of wounded warriors with ≥1 open extremity wound),17 a methodology for wound characterization was devised to support a better understanding of the infection burden. Specifically, information from the Department of Defense (DoD) Trauma Registry injury narrative was processed by Tri-Code software (Digital Innovations, Inc., Forest Hill, MD) to produce an Abbreviated Injury Scale (AIS) score for each unique wound. After generating the AIS scores, wound locations were mapped to predefined anatomic TIDOS injury sites (upper arm, elbow, lower arm, wrist, hand, thigh, knee, lower leg, ankle, and foot), as well as side (left and right). Each unique wound was categorized as an amputation, open fracture, or other open soft-tissue wounds (e.g., degloving injuries, lacerations, and penetrating wounds).17 Among patients with polytrauma, there were often multiple wounds at the same anatomic injury site, precluding the direct match of an infection to a wound. As such, wounds located at the same anatomic site and side were collapsed to create unique wound groups defined by the most severe injury at each respective anatomic site.
Combat-Related Extremity Wound Infection Epidemiology
Military personnel with combat-related open extremity wounds sustained over a 3-year period (2009-2012) were assessed for infectious outcomes (i.e., SSTIs and osteomyelitis) in a retrospective analysis. Among 1,858 combat casualties admitted to participating U.S. military hospitals, 1,409 (76%) had ≥1 open extremity wound. The majority (81%) of patients were injured via a blast mechanism (primarily with an IED), resulting in severe injuries (median injury severity score [ISS] of 21). When classified by their most serious injury, 31% had an amputation, 36% an open fracture (no amputation), and 33% an open soft-tissue wound (no amputation or open fracture). Approximately 25% of patients developed a combat-related extremity wound infection (CEWI). Patients with amputations had the highest proportion of infections (57%), followed by patients with open fractures as their most severe injury (17%) and open soft-tissue injuries (4%) (Table I).
TABLE I.
Patients With Combat-related Extremity Wound Infections by Injury Pattern
| Patient injury pattern | ||||||||
|---|---|---|---|---|---|---|---|---|
| Wound group type | Patients with ≥1 amputation | Patients with ≥1 open fracture, no amputation | Patients with ≥1 open soft-tissue wounds only | Total patients | ||||
| No. of patients | No. of patients with ≥1 infection (%) | No. of patients | No. of patients with ≥1 infection (%) | No. of patients | No. of patients with ≥1 infection (%) | No. of patients | No. of patients with ≥1 infection (%) | |
| Amputation | 437 | 205 (46.9) | 437 | 205 (46.9) | ||||
| LE | 413 | 199 (48.2) | NA | NA | NA | NA | 413 | 199 (48.2) |
| UE | 79 | 20 (25.3) | 79 | 20 (25.3) | ||||
| Open Fracture | 192 | 43 (22.4) | 511 | 66 (12.9) | 703 | 109 (15.5) | ||
| LE | 86 | 16 (18.6) | 352 | 53 (15.1) | NA | NA | 438 | 69 (15.8) |
| UE | 127 | 27 (21.3) | 212 | 13 (6.1) | 339 | 40 (11.8) | ||
| Open Soft-Tissue Wounds | 341 | 33 (9.7) | 338 | 13 (3.8) | 461 | 14 (3.0) | 1,140 | 60 (5.3) |
| LE | 195 | 23 (11.8) | 273 | 11 (4.0) | 325 | 10 (3.1) | 793 | 44 (5.6) |
| UE | 266 | 10 (3.8) | 176 | 2 (1.1) | 285 | 4 (1.4) | 727 | 16 (2.2) |
| Totala | 437 | 248 (56.7) | 511 | 89 (17.4) | 461 | 17 (3.7) | 1,409 | 354 (25.1) |
Abbreviations: LE, lower extremity; NA, not applicable; UE, upper extremity.
Total does not reflect the summation of column numbers since patients can have multiple wounds and multiple infections.
Delayed or recurrent CEWIs may impact wounded warriors long after their discharge from the hospital. Therefore, as part of TIDOS, patients were given the opportunity during their initial hospitalization to enroll in a longitudinal follow-up cohort to collect infection-related data after hospital discharge.18 Between 2009 and 2012, 826 patients with ≥1 open extremity wound enrolled in the TIDOS follow-up cohort. Among these patients, 237 (29%) had a CEWI diagnosed during the initial hospitalization, while 589 (71%) did not (Fig. 1). Approximately 19% of the patients with prior inpatient CEWIs had a recurrent CEWI and 34% had a new, incident CEWI during follow-up. In addition, 22% of the patients who did not have a CEWI during the initial hospitalization developed an incident CEWI during follow-up. Overall, 28% of patients with extremity trauma had either a new or recurrent CEWI during follow-up. The duration from hospital discharge to infection diagnosis during follow-up was a median of 126 days for SSTIs and 107 days for osteomyelitis.18 Although these findings demonstrate the high burden of infections on wounded warriors, there is a need for further examination of the long-term impact of CEWIs, including clinical outcomes, comorbidities, mental health and social factors, and health economics related to surgical and medical care.
FIGURE 1.
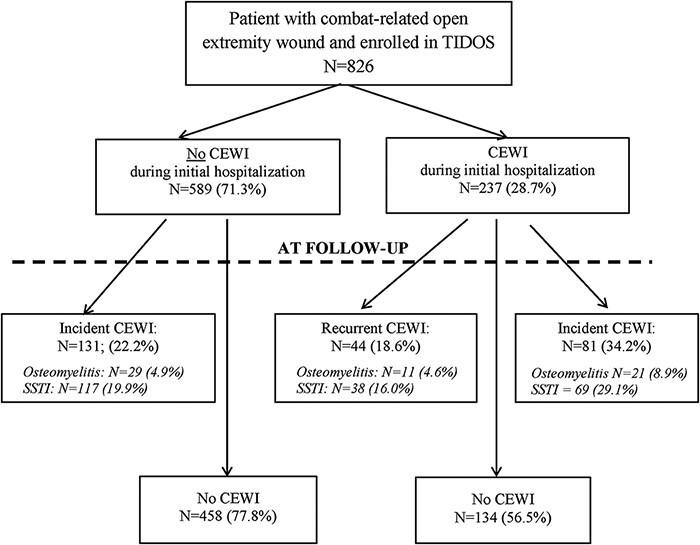
Flowchart for the occurrence of combat-related open extremity wound infections (CEWIs) during the initial hospitalization and follow-up. Recurrent CEWIs are defined by the infection occurring on the same site/side, while incident CEWIs occur on a different site/side. Patients may have both skin and soft-tissue infections (SSTI) and osteomyelitis, as well as both an incident and recurrent CEWI, so numbers may add to more than indicated totals. The total number of patients with a CEWI during initial hospitalization who had a CEWI during follow-up is 103. Figure and legend are reprinted from Tribble et al.18 with permission of Mary Ann Liebert, Inc.
CEWI Risk Factors
Due to the high morbidity and resource utilization associated with CEWIs, identification of early predictors is needed to improve patient clinical outcomes. In an analysis restricted to patients admitted to the U.S. hospitals within 7 days post-injury and had a traumatic amputation or open fractures as their most severe injury (N = 669), characteristics of patients with and without CEWIs were compared. Early predictors for the development of CEWIs during the initial trauma hospitalization included sustaining an amputation (Hazard Ratio [HR]: 1.79; 95% confidence interval [CI]: 1.25-2.56), being injured via an IED, first documented shock index of ≥0.80, receiving ≥10 units of blood within 24 h post-injury, and having >4 injury sites. Having a confirmed non-extremity infection (e.g., pneumonia and bloodstream infection) at least 4 days before the CEWI diagnosis was associated with a reduced likelihood of developing a CEWI (HR: 0.28; 95% CI: 0.17-0.46), suggesting that antibiotic exposure and other factors related to medical management may impact CEWI risk.17 The association of variables related to injury severity is consistent with prior studies that found an increased infection risk in more severely injured patients.19–22
To assess risk factors associated with the development of a CEWI after hospital discharge (delayed CEWI), characteristics of 234 patients with a CEWI during follow-up were compared to 592 patients without a CEWI. Sustaining an amputation (Relative Risk [RR]: 3.37; 95% CI: 1.56-7.25) or an open fracture (RR: 4.33; 95% CI: 2.06-9.12) were independent predictors of a delayed CEWI. Receipt of antipseudomonal penicillin for ≥7 days was also a risk factor and likely a surrogate for injury severity as 78% of the patients who received that antibiotic had an ISS ≥25. The occurrence of either an inpatient CEWI (RR: 2.25; 95% CI: 1.23-4.13) or other non-extremity infection (RR: 2.35; 95% CI: 1.30-4.27) were also associated with a higher likelihood of developing a delayed CEWI. When length of hospitalization was considered, a shorter length of stay (15-30 days) was associated with a decreased risk of a delayed CEWI (RR: 0.46; 95% CI: 0.23-0.89),18 and likely an indicator that the patient was less severely injured or did not experience complications resulting in an extended hospitalization.
Post-Trauma Antibiotic Prophylaxis
Clinical practice guidelines for the prevention of combat trauma-related infections were first published in 2008 and subsequently revised over the years.23–26 The DoD Joint Trauma System guidelines provide recommendations for post-trauma antibiotic prophylaxis with different injury patterns, including open fractures and extremity soft-tissue wounds. Following publication of a revised clinical practice guideline in 2011, which incorporated recommendations to support antibiotic stewardship and reduce use of unnecessary broad-spectrum antibiotics,23 there was a marked reduction in use of expanded Gram-negative coverage antibiotics (e.g., fluoroquinolones and aminoglycosides) as part of post-trauma prophylaxis with open fractures.27 This change in practice patterns allowed for an assessment of infectious outcomes following use of different antibiotic prophylactic regimens.
In a TIDOS analysis of 1,044 combat casualties with open fractures sustained over a 5-year period (2009-2014), infectious outcomes were examined based on receipt of DoD-recommended narrow-spectrum post-trauma antibiotic prophylaxis (i.e., cefazolin or clindamycin) with/without use of expanded Gram-negative coverage. Although use of a narrow-spectrum antibiotic regimen without expanded Gram-negative coverage had a slightly increased risk of extremity SSTIs (HR: 1.41; 95% CI: 1.09-1.83), there was no difference in osteomyelitis risk (HR: 0.99; 95% CI: 0.63-1.56). Moreover, use of expanded Gram-negative coverage was associated with the adverse effect of greater recovery of organisms that were resistant to aminoglycosides and/or fluoroquinolones (49% vs. 40% with only narrow-spectrum antibiotics; P < .001).28 Findings were similar when infectious outcomes following use of the differing post-trauma antibiotic prophylactic regimens with extremity soft-tissue wounds were examined.29 Overall, these two studies provide support for the current DoD recommendations related to use of narrow-spectrum antibiotics with open fractures and soft-tissue wounds.
TRAUMA-ASSOCIATED OSTEOMYELITIS
As part of the Trauma-Associated Osteomyelitis study, data from combat casualties with open fractures of the long bones (tibia, femur, and upper extremity) were examined in separate analyses. For each long bone site, risk factors for the development of osteomyelitis were assessed in case–control analyses, while characteristics of the osteomyelitis patients and risk of recurrence following resolution of the initial infection were examined in case–case comparisons. Patients were categorized in the osteomyelitis case group if they met standardized clinical diagnostic criteria for osteomyelitis.30 Controls were patients with open long bone fractures who did not meet diagnostic criteria for osteomyelitis. Cases and controls were independently verified by an infectious disease clinician and orthopedic surgeon. Fractures were assessed using a modified version of the Gustilo–Anderson (GA) classification to include traumatic amputations (i.e., transtibial, transfemoral, and transradial/transhumeral) as the highest level.31 The Orthopaedic Trauma Association Open Fracture Classification (OTA OFC) system was also used to characterize fracture severity.32,33
Incident Osteomyelitis
The study populations for the three bone groups were tibia (130 case patients and 85 controls), femur (103 case patients and 64 controls), and upper extremity fractures (64 case patients and 96 controls).15,34,35 Differences between the osteomyelitis cases and controls were similar across the three analyses. In particular, there was a higher proportion of amputations among patients with tibia osteomyelitis (32% vs. 15%; P < .001) and femur osteomyelitis (40% vs. 27%; P = .002) compared to the controls.15,34 Patients with upper extremity fractures and osteomyelitis had a higher proportion of fractures classified as GA-IIIc or were transhumeral/transradial amputations (27% vs. 5% with controls; P < .001). Among the upper extremity group, patients with osteomyelitis have a higher proportion of fractures of the humerus compared to the control patients (58% vs. 31%; P = .002).35
Using the OTA OFC system, skin, muscle, arterial, and bone injuries are classified based on level of severity (mild, moderate, or severe). Tibia, femur, and upper extremity osteomyelitis patients had a higher proportion of injuries classified at the severe level for skin (extensive degloving), muscle (dead muscle or loss of muscle function), and bone (segmental bone loss) compared to controls (Fig. 2; P < .005 for distribution across the different severity levels).15,34 Patients with femur and upper extremity osteomyelitis also had a higher proportion of arterial injuries with distal ischemia (P < .005 for distribution across the different severity levels).34,35
FIGURE 2.

Summary of Orthopaedic Trauma Association Open Fracture Classification (OTA OFC) severity indicators33 for tibia, femur, and upper extremity open fracture osteomyelitis cases and controls. The OTA OFC has mild, moderate, and severe levels for the skin, muscle, arterial, and bone classifications. Only the highest severity level rates are shown.
Patients with tibia osteomyelitis had a longer time to radiographic union (median of 210 days vs. 165 days with controls; P = .014).15 Although there was no significant difference in the timing of radiographic union between the groups in the femur and upper extremity analyses, a higher proportion of osteomyelitis patients in those studies had final stabilization occurring after 6 months compared to the controls (femur analysis: 22% vs. 8%; P = .033; upper extremity analysis: 27% vs. 6%; P = 0.005).34,35
Using standardized diagnostic criteria of the Centers for Disease Control and Prevention National Healthcare Safety Network, the osteomyelitis cases were classified as definite, probable, or possible based on the type of clinical evidence (e.g., positive bone culture, signs/symptoms, and local/systemic inflammation).30 Among the 130 patients with tibia osteomyelitis, 19% met criteria to be classified as definite/probable while 81% were classified as possible osteomyelitis. With the exception of greater localized swelling among cases classified as definite/probable compared to possible osteomyelitis (52% vs. 27%; P = 0.008), there were no significant differences between the osteomyelitis cases with regard to clinical presentation (i.e., localized tenderness, heat, redness, drainage, necrosis, spontaneous dehiscence, and abscess) and systemic inflammation (i.e., fever, leukocytosis, elevated C-reactive protein, and/or erythrocyte sedimentation rate). The timing of definitive orthopedic surgery was also comparable between the groups.15,36
Among the 103 patients with femur osteomyelitis, 31% were classified as definite/probable and 69% as possible osteomyelitis. Except for a higher proportion of purulent drainage or necrosis among the definite/probable cases (65% vs. 34% with possible osteomyelitis; P = .005), there were no significant differences between the classification groups.34 For patients with upper extremity osteomyelitis, 9% were classified as a definite/probable and 91% were possible. As with the femur population, the only significant difference between the classification groups was a higher proportion of purulent drainage or necrosis among the definite/probable cases (67% vs. 22% with possible osteomyelitis; P = .038).35 These findings indicate that the cases of osteomyelitis classified as possible should be managed similarly.
Osteomyelitis Recurrence
Tibial osteomyelitis recurrence was assessed among 112 patients after infection.36 Thirty-one (28%) patients were diagnosed with an osteomyelitis recurrence a median of 188 days after last antibiotics for initial treatment. There were no significant differences in osteomyelitis classification (definite/probable vs. possible), smoking history, or GA fracture classification between the patients with and without a recurrence. Organisms were isolated from cultures from 28 patients with a recurrence. Approximately 64% of the cultures from the recurrent infections were polymicrobial. In addition, 29% of recurrent infections had a Gram-negative organism in common with the initial infection and 25% had a Gram-positive organism in common. Although Acinetobacter spp. were frequently isolated from the initial infections (54%), there were no Acinetobacter spp. associated with the initial infections recovered from recurrent osteomyelitis.36
Osteomyelitis Risk Factors
Incident Osteomyelitis
Risk factors for the development of osteomyelitis following an open fracture have been assessed in the tibia, femur, and upper extremity case–control populations.15,34,35 Sustaining a traumatic amputation (highest level of modified GA fracture severity scale), use of antibiotic beads (surrogate for soft tissue injury severity), and being injured in the earlier years of the study (2003-2006) were independent predictors of osteomyelitis risk for all three study populations (Table II).15,34,35 In particular, transtibial amputation carried the highest risk in the tibia population (odds ratio [OR]: 15.10; 95% CI: 3.22-71.07).15 Occurrence of a foreign body at the fracture site (with/without orthopedic implant for tibia and with orthopedic impact for femur) was also associated with osteomyelitis risk, and like with amputations, primarily caused by a blast injury, which was also a risk factor in the tibia population.15,34
TABLE II.
Summary of Osteomyelitis Risk Factors Associated With Open Fractures of the Long Bonesa
| Risk factor | Tibia | Femur | Upper extremity |
|---|---|---|---|
| Gustilo-Anderson (GA) fracture severity | |||
| GA-IIIa | ✓ | ||
| GA-IIIb | ✓ | ✓ | |
| GA-IIIc | ✓ | ✓b | |
| Amputationc | ✓ | ✓ | ✓b |
| OTA OFC: Muscle | |||
| Loss of muscle but function remains | ✓ | ✓ | |
| Dead muscle/loss of function | ✓ | ✓ | |
| OTA OFC: Skin | |||
| Cannot be approximated | ✓ | ||
| Extensive degloving | ✓ | ||
| Use of antibiotic beads | ✓ | ✓ | ✓ |
| Time periodd | ✓ | ✓ | ✓ |
| Initial stabilization outside combat zone | Reduced risk | ||
| Foreign body | |||
| Fragment only | ✓ | ||
| Fragment plus orthopedic implant | ✓ | ✓ | |
| Implant only | |||
| Blast injury mechanism | ✓ |
Abbreviation: OTA OFC, Orthopaedic Trauma Association Open Fracture Classification.
As there was high correlation between the GA classification system and OTA OFC system, risk factors for each of the long bone groups were assessed in two separate models: one including GA and one for OTA OFC. Models were also rerun for the tibia and femur populations after excluding transtibial and transfemoral amputations, respectively. Findings from all models are included in the summary table. ✓ indicates increased risk.
Due to limited numbers, GA-IIIc and amputations were combined for examination as risk factors in the multivariate model.
Amputations include transtibial, transfemoral, and transradial/transhumeral for the tibia, femur, and upper extremity groups, respectively.
Sustaining an injury between the earlier years (2003-2006) was compared to 2007-2009 (used as the reference).
As the decision to use antibiotic beads is left to the discretion of the surgeon, this likely provides an explanation for the association with risk as beads are likely utilized more often when there was clinical suspicion of a greater infection risk due to wound clinical appearance, severity of soft-tissue and bone damage, as well as contamination. The association of risk with the earlier years of the study is likely due to changes in combat care clinical practice as negative pressure wound therapy became more common in 2006/2007 and use of high-pressure irrigation decreased.23,37 Although a recent Cochrane review found it uncertain whether negative pressure wound therapy reduced the risk of infections,38 a meta-analysis identified use of pulsatile lavage as a risk factor for infections complicating open fractures.39 Resuscitation practices also evolved, restricting use of crystalloid products and promoting a 1:1 ratio of packed red blood cells and plasma.40 This practice change reduced third space fluid, which may lessen the risk of infection; however, use of large-volume blood transfusions may also increase infection risk through immune suppression. Further investigation into the impact of practice changes on infectious outcomes is warranted.
For both the tibia and femur populations, having a muscle injury classified as moderate (i.e., loss of muscle, but function remains) or severe (i.e., dead muscle/loss of function) according to the OTA OFC was an osteomyelitis risk factor.15,34 Severe muscle injury was associated with the highest risk in the femur population (OR: 32.87; 95% CI: 5.43-199.08).34 This finding corresponds to a prior study which found an association between infection risk and severity of soft-tissue injury.41 Moderate (i.e., cannot be approximated) or severe (i.e., extensive degloving) skin injury as classified by the OTA OFC was an independent osteomyelitis predictor with open upper extremity fractures (Table II).35 The modified GA fracture classification was also associated with osteomyelitis risk (GA-IIIb/c/traumatic amputations with open tibia and upper extremity fractures),15,35 which corresponds to findings of civilian studies.41–43 Fractures classified as GA-IIIb carried the highest risk for osteomyelitis in the upper extremity population (OR: 22.20; 95% CI: 3.60-136.95).35 Only the GA-IIIa fracture and transfemoral amputation classifications were osteomyelitis risk factors in the open femur fracture population; however, the lack of association with the GA-IIIb/c levels may result from the low number of patients with those classifications.34
A reduced likelihood of osteomyelitis was identified in the upper extremity fracture population with initial stabilization following evacuation from the combat zone (OR: 0.16; 95% CI: 0.03-0.87 when stabilization occurred at LRMC; OR: 0.26; 95%: 0.09-0.79 for stabilization at a U.S. military hospital).35 Stabilization within the combat zone included hardware placement (primarily external fixation, but also internal fixation), as well as resuscitative care and wound decontamination (pulsatile lavage or negative pressure wound therapy). Use of high-pressure irrigation in the earlier study years may have resulted in wounds not being fully decontaminated before stabilization, resulting in a higher risk of infection.
Recurrent Osteomyelitis
Predictors for osteomyelitis recurrence were assessed among patients with osteomyelitis in the open tibia fracture group.36 Tibia osteomyelitis recurrence was significantly associated with fractures classified with moderate bone damage according to the OTA OFC (i.e., bone missing or devascularized with contact by proximal/distal fragments) in a univariate model (HR: 3.94; 95% CI: 1.12-13.81); however, there was no association between bone loss and osteomyelitis recurrence in a Kaplan–Meier survival plot (log rank Chi-square = 3.79; P = .052). As the limited number of patients with osteomyelitis recurrence prevented analysis with a multivariate model, these findings should be considered preliminary.36 Assessment of risk factors associated with femur and upper extremity osteomyelitis recurrence are being finalized.
Future and Ongoing Research
Examination of CEWI antibiotic management practices over a 3-year period was recently completed and substantial variation in prescribing patterns was noted,44 warranting further evaluation with regard to outcomes. With the goal of supporting the development of a Joint Trauma System clinical practice guideline for the treatment of combat-related infections, analyses focused on medical management approaches for the treatment of CEWIs are underway. In particular, the effectiveness of vancomycin regimens with deep soft-tissue infections is being examined. In addition, as part of a collaboration with the Veterans Affairs St. Louis Health Care System, forthcoming analyses will further assess the long-term impact of extremity trauma and subsequent infections with regard to comorbidities, mental health and social factors, and healthcare utilization.
ACKNOWLEDGMENTS
We are indebted to the Infectious Disease Clinical Research Program Wound Infections Research Area study team of clinical coordinators, microbiology technicians, data managers, clinical site managers, and administrative support personnel for their tireless hours to ensure the success of this project. Special thanks to Leigh Carson for her assistance in manuscript preparation.
Contributor Information
MAJ Joseph L Petfield, Landstuhl Regional Medical Center, Landstuhl 19180, Germany.
CDR Louis R Lewandowski, Walter Reed National Military Medical Center, Bethesda, MD 20852, USA.
Laveta Stewart, Infectious Disease Clinical Research Program, Preventive Medicine & Biostatistics Department, Uniformed Services University of the Health Sciences, Bethesda, MD 20814, USA; Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Bethesda, MD 20817, USA.
BG Clinton K Murray, Brooke Army Medical Center, JBSA Fort Sam Houston, TX 78234, USA.
David R Tribble, Infectious Disease Clinical Research Program, Preventive Medicine & Biostatistics Department, Uniformed Services University of the Health Sciences, Bethesda, MD 20814, USA.
FUNDING
This work was conducted by the Infectious Disease Clinical Research Program, a DoD program executed through the Uniformed Services University of the Health Sciences, Department of Preventive Medicine and Biostatistics through a cooperative agreement with The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. This project has been funded by the National Institute of Allergy and Infectious Diseases; the National Institute of Health (Inter-Agency Agreement Y1-AI-5072); the Defense Health Program; the U.S. DoD, under award HU0001190002; the Department of the Navy under the Wounded, Ill, and Injured Program; and the Defense Medical Research and Development Program.
CONFLICT OF INTEREST
None declared.
REFERENCES
- 1. Belmont PJ Jr, Goodman GP, Zacchilli M, Posner M, Evans C, Owens BD: Incidence and epidemiology of combat injuries sustained during “the surge” portion of Operation Iraqi Freedom by a U.S. Army Brigade Combat Team. J Trauma 2010; 68(1): 204–10. [DOI] [PubMed] [Google Scholar]
- 2. Belmont PJ, Owens BD, Schoenfeld AJ: Musculoskeletal injuries in Iraq and Afghanistan: epidemiology and outcomes following a decade of war. J Am Acad Orthop Surg 2016; 24(6): 341–8. [DOI] [PubMed] [Google Scholar]
- 3. Owens BD, Kragh JF Jr, Macaitis J, Svoboda SJ, Wenke JC: Characterization of extremity wounds in Operation Iraqi Freedom and Operation Enduring Freedom. J Orthop Trauma 2007; 21(4): 254–7. [DOI] [PubMed] [Google Scholar]
- 4. Schoenfeld AJ, Dunn JC, Bader JO, Belmont PJ Jr: The nature and extent of war injuries sustained by combat specialty personnel killed and wounded in Afghanistan and Iraq, 2003-2011. J Trauma Acute Care Surg 2013; 75(2): 287–91. [DOI] [PubMed] [Google Scholar]
- 5. Krueger CA, Wenke JC, Ficke JR: Ten years at war: comprehensive analysis of amputation trends. J Trauma Acute Care Surg 2012; 73(6 Suppl 5): S438–44. [DOI] [PubMed] [Google Scholar]
- 6. Chandler H, MacLeod K, Penn-Barwell JG: Extremity injuries sustained by the UK military in the Iraq and Afghanistan conflicts: 2003-2014. Injury 2017; 48(7): 1439–43. [DOI] [PubMed] [Google Scholar]
- 7. Penn-Barwell JG, Bennett PM, Fries CA, Kendrew JM, Midwinter MJ, Rickard RF: Severe open tibial fractures in combat trauma: management and preliminary outcomes. Bone Joint J 2013; 95-B(1): 101–5. [DOI] [PubMed] [Google Scholar]
- 8. Penn-Barwell JG, Bennett PM, Mortiboy DE, Fries CA, Groom AF, Sargeant ID: Factors influencing infection in 10 years of battlefield open tibia fractures. Strategies Trauma Limb Reconstr 2016; 11(1): 13–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ficke JR, Eastridge BJ, Butler F, et al. : Dismounted complex blast injury report of the army dismounted complex blast injury task force. J Trauma Acute Care Surg 2012; 73(6 Suppl 5): S520–34. [Google Scholar]
- 10. Yun HC, Branstetter JG, Murray CK: Osteomyelitis in military personnel wounded in Iraq and Afghanistan. J Trauma 2008; 64(2 Suppl): S163–8. [DOI] [PubMed] [Google Scholar]
- 11. Napierala MA, Rivera JC, Burns TC, et al. : Infection reduces return-to-duty rates for soldiers with Type III open tibia fractures. J Trauma Acute Care Surg 2014; 77(3 Suppl 2): S194–7. [DOI] [PubMed] [Google Scholar]
- 12. Huh J, Stinner DJ, Burns TC, Hsu JR, Late Amputation Study Team : Infectious complications and soft tissue injury contribute to late amputation after severe lower extremity trauma. J Trauma 2011; 71(1 Suppl): S47–51. [DOI] [PubMed] [Google Scholar]
- 13. Tribble DR, Conger NG, Fraser S, et al. : Infection-associated clinical outcomes in hospitalized medical evacuees after traumatic injury: Trauma Infectious Disease Outcome Study. J Trauma 2011; 71(1 Suppl): S33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tribble DR, Murray CK, Lloyd BA, et al. : After the battlefield: infectious complications among wounded warriors in the Trauma Infectious Disease Outcomes Study. Mil Med 2019; 184(Suppl 2): 18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tribble DR, Lewandowski LR, Potter BK, et al. : Osteomyelitis risk factors related to combat trauma open tibia fractures: a case-control analysis. J Orthop Trauma 2018; 32(9): e344–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mende K, Akers KS, Tyner S, et al. : Multidrug-Resistant and Virulent Organisms (MDR/VO) trauma infections: TIDOS initiative. Mil Med 2022; 187(Suppl 2): 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stewart L, Shaikh F, Bradley W, et al. : Combat-related extremity wounds: injury factors predicting early onset infections. Mil Med 2019; 184(Suppl 1): 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tribble DR, Krauss M, Murray CK, et al. : Epidemiology of trauma-related infections among a combat casualty cohort after initial hospitalization: the Trauma Infectious Disease Outcomes Study. Surg Infect (Larchmt) 2018; 19(5): 494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tribble DR, Li P, Warkentien TE, et al. : Impact of operational theater on combat and noncombat trauma-related infections. Mil Med 2016; 181(10): 1258–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Petersen K, Riddle MS, Danko JR, et al. : Trauma-related infections in battlefield casualties from Iraq. Ann Surg 2007; 245(5): 803–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Murray CK, Wilkins K, Molter NC, et al. : Infections complicating the care of combat casualties during Operations Iraqi Freedom and Enduring Freedom. J Trauma 2011; 71(1 Suppl): S62–73. [DOI] [PubMed] [Google Scholar]
- 22. Murray CK, Wilkins K, Molter NC, et al. : Infections in combat casualties during Operations Iraqi and Enduring Freedom. J Trauma 2009; 66(4 Suppl): S138–44. [DOI] [PubMed] [Google Scholar]
- 23. Hospenthal DR, Murray CK, Andersen RC, et al. : Guidelines for the prevention of infections associated with combat-related injuries: 2011 update: endorsed by the Infectious Diseases Society of America and the Surgical Infection Society. J Trauma 2011; 71(2 Suppl 2): S210–34. [DOI] [PubMed] [Google Scholar]
- 24. Hospenthal DR, Murray CK, Andersen RC, et al. : Guidelines for the prevention of infection after combat-related injuries. J Trauma 2008; 64(3): S211–20. [DOI] [PubMed] [Google Scholar]
- 25. Saeed O, Tribble D, Biever K, Kavanaugh M, Crouch H: Infection prevention in combat-related injuries (CPG ID: 24). Joint Trauma System. 2016. Available at https://jts.amedd.army.mil/assets/docs/cpgs/JTS_Clinical_Practice_Guidelines_(CPGs)/Infection_Prevention_08_Aug_2016_ID24.pdf; accessed November 16, 2018. [DOI] [PubMed]
- 26. Saeed O, Tribble DR, Biever KA, Crouch HK, Kavanaugh M: Infection prevention in combat-related injuries. Mil Med 2018; 183(Suppl 2): 137–41. [DOI] [PubMed] [Google Scholar]
- 27. Lloyd BA, Murray CK, Bradley W, et al. : Variation in postinjury antibiotic prophylaxis patterns over five years in a combat zone. Mil Med 2017; 182(S1): 346–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lloyd BA, Murray CK, Shaikh F, et al. : Early infectious outcomes following addition of fluoroquinolone or aminoglycoside to post-trauma antibiotic prophylaxis in combat-related open fracture injuries. J Trauma Acute Care Surg 2017; 83(5): 854–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lloyd BA, Murray CK, Shaikh F, et al. : Antimicrobial prophylaxis with combat-related open soft-tissue injuries. Mil Med 2018; 183(9-10): e260–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Centers for Disease Control and Prevention . CDC/NHSN surveillance definitions for specific types of infections. 2019. Available at https://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf; accessed November 5, 2020.
- 31. Gustilo RB, Anderson JT: Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. J Bone Joint Surg Am 1976; 58(4): 453–8. [PubMed] [Google Scholar]
- 32. Marsh JL, Slongo TF, Agel J, et al. : Fracture and dislocation classification compendium—2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma 2007; 21(10 Suppl): S1–133. [DOI] [PubMed] [Google Scholar]
- 33. Orthopaedic Trauma Association: Open Fracture Study Group : A new classification scheme for open fractures. J Orthop Trauma 2010; 24(8): 457–64. [DOI] [PubMed] [Google Scholar]
- 34. Lewandowski LR, Potter BK, Murray CK, et al. : Osteomyelitis risk factors related to combat trauma open femur fractures: a case-control analysis. J Orthop Trauma 2019; 33(4): e110–9. [DOI] [PubMed] [Google Scholar]
- 35. Warkentien TE, Lewandowski LR, Potter BK, et al. : Osteomyelitis risk factors related to combat trauma open upper extremity fractures: a case-control analysis. J Orthop Trauma 2019; 33(12): e475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Petfield JL, Tribble DR, Potter BK, et al. : Is bone loss or devascularization associated with recurrence of osteomyelitis in wartime open tibia fractures? Clin Orthop Relat Res 2019; 477(4): 789–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Murray CK, Hsu JR, Solomkin JS, et al. : Prevention and management of infections associated with combat-related extremity injuries. J Trauma 2008; 64(3 Suppl): S239–51. [DOI] [PubMed] [Google Scholar]
- 38. Iheozor-Ejiofor Z, Newton K, Dumville JC, Costa ML, Norman G, Bruce J: Negative pressure wound therapy for open traumatic wounds. Cochrane Database Syst Rev 2018; 7(7): CD012522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kortram K, Bezstarosti H, Metsemakers WJ, Raschke MJ, Van Lieshout EMM, Verhofstad MHJ: Risk factors for infectious complications after open fractures; a systematic review and meta-analysis. Int Orthop 2017; 41(10): 1965–82. [DOI] [PubMed] [Google Scholar]
- 40. Langan NR, Eckert M, Martin MJ: Changing patterns of in-hospital deaths following implementation of damage control resuscitation practices in US forward military treatment facilities. JAMA Surg 2014; 149(9): 904–12. [DOI] [PubMed] [Google Scholar]
- 41. Thakore RV, Francois EL, Nwosu SK, et al. : The Gustilo–Anderson classification system as predictor of nonunion and infection in open tibia fractures. Eur J Trauma Emerg Surg 2017; 43(5): 651–6. [DOI] [PubMed] [Google Scholar]
- 42. Bowen TR, Widmaier JC: Host classification predicts infection after open fracture. Clin Orthop Relat Res 2005; 433: 205–11. [DOI] [PubMed] [Google Scholar]
- 43. Westgeest J, Weber D, Dulai SK, Bergman JW, Buckley R, Beaupre LA: Factors associated with development of nonunion or delayed healing after an open long bone fracture: a prospective cohort study of 736 subjects. J Orthop Trauma 2016; 30(3): 149–55. [DOI] [PubMed] [Google Scholar]
- 44. Stewart L, Li P, Blyth DM, et al. : Antibiotic practice patterns for extremity wound infections among blast-injured subjects. Mil Med 2020; 185(Suppl 1): 628–36. [DOI] [PMC free article] [PubMed] [Google Scholar]


