What you need to know.
Bites from venomous snakes can result in bleeding, paralysis, long term disability, and death
Immobilise the bitten limb when transporting the patient to a medical facility; the universal use of pressure immobilisation is controversial, and tourniquets are not recommended
The 20-minute whole blood clotting test is a simple bedside test to screen for and monitor coagulopathy in resource-limited settings
Assess vital parameters and initiate resuscitation measures if the patient is clinically unstable with signs of bleeding, shock, paralysis, or respiratory distress
Intravenous antivenom is recommended in patients with systemic symptoms; the dose and type depend on likely snake species, local guidelines, and availability
Snakebite affects between 1.8 to 2.7 million people worldwide each year, and it is estimated to cause between 80 000 and 138 000 deaths.1 2 A mixture of toxins (venom) is injected into the body following bite by a venomous snake.3 Envenoming can be a highly dynamic clinical event. Symptoms can progressively worsen to a life-threatening emergency. Snakebites can have long term physical sequelae such as amputation, paralysis and disability, and psychological health consequences.4 5 6 7
Snakebite envenoming is more common in South and South-East Asia (2 million annually), sub-Saharan Africa (420 000) and Latin America (150 000).1 2 These regions also report a high burden of deaths from snakebite (100 000, 32 000, and 5000 deaths respectively) possibly due to poor access to medical aid.1 2 Delayed diagnosis and treatment can worsen prognosis.8 9 10 11 The World Health Organization recognised snakebite as a neglected tropical disease in 2017 and called for concerted global action to reduce deaths and disability.12
In this clinical update, we present an approach to evaluation and management of snakebites for primary care providers in resource-limited settings in endemic regions. The principles of management are broadly similar, but it is beyond the scope of this article to cover clinical syndromes and management for the varied snake species globally.
Sources and selection criteria.
We searched the Cochrane Library, Google, and PubMed, using the MeSH terms: “(snakebite, diagnosis and treatment guidelines) and (snake, scientific names of individual snake species, snakebite, envenoming, venom, and antivenom)”. Research papers and case reports from Latin America, South and South-East Asia, and sub-Saharan Africa were retrieved. There were no language restrictions. Additional articles were obtained by citation tracking of review and original articles. Although the search focused on key papers published in the past five years, older publications of importance have been included. The review also drew from the widely established African and South-East Asian regional guidelines on the treatment and prevention of snakebite envenoming.
Who is at risk of snakebite?
Rural communities in tropical countries are worst affected.13 14 15 16 Agricultural workers, hunter-gatherers, herders, fishermen, and rural families living in precarious housing conditions with outdoor toilets have a higher risk of snakebite. Their living environments intersect with snake habitats.12 Men between 10 and 40 years are more commonly affected.4 15 17 18 19 20 Non-mechanised farming techniques, barefoot farming, and sleeping on the floor further increase the risk.16 21 Bites are more common during wetter months, when agricultural activities and breeding season for snakes potentially converge.15 17 20 22 23
How does envenoming occur?
Most medically important snakes in these regions belong to two taxonomic families24 25 26 27:
Viperidae—African adders and bush vipers, Asian pit vipers, mamushis, habus, and New World rattlesnakes, moccasins, bushmasters, and lanceheads
Elapidae—African and Asian cobras, African mambas, African rinkhals, Asian kraits, Australian and Papuan venomous snakes, Asian and New World coral snakes and sea snakes
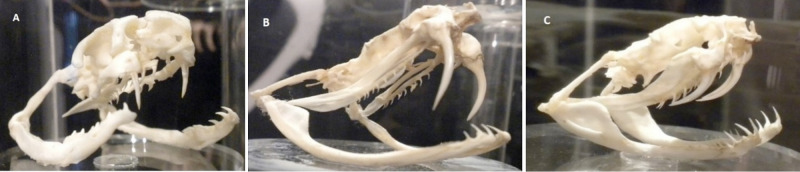
Venomous snakes inject venom during a bite using specialised grooved or hollow teeth called fangs. The fangs are connected to venom glands on each side of the upper jaw via a duct.28 Viperids usually have long foldable front-fangs, while those in elapids are short and fixed (fig 1).28 Depending on fang length, venom is introduced either subcutaneously or intramuscularly.3
Fig 1.
Difference in fangs between elapids and viperids. A) Elapids have short, fixed, front fangs. B&C) Vipers have much longer and retractable front fangs. (Photos courtesy of Ahmad Khaldun Ismail)
Some snakes with fangs towards the back of the mouth (“non-front-fanged colubroids” such as African boomslang and vine snakes, South American racers, and Asian yamakagashis) and the burrowing asps (or stiletto snakes) whose unique jaw kinesis coupled with exceptional neck flexure allow for a single protruding fang to be jabbed sideways or backwards, can also cause envenoming.3 28 29 Spitting cobras and rinkhals can eject venom over several metres, often delivering venom droplets into the eyes of the animal or human perceived to be a threat.30 31 32
What are the clinical effects of snakebite?
Not all people with a snakebite have clinical symptoms. Often bites are by non-venomous snakes. Sometimes venomous snakes do not inject venom during a bite.33
Clinical manifestations vary between species of snakes (see box 1).3 Some toxins in venom exert local effects such as swelling, blistering, bruising, and necrosis at the bite site.24 25 Other toxins can be distributed systemically through lymphatics and blood vessels and act at distant sites. See supplementary text for an overview of important sites and pathophysiology of venom-toxin action. Common systemic effects include bleeding, paralysis, generalised rhabdomyolysis, and acute kidney injury. Venom injection deep into a limb can cause tissue swelling in the tightly constrained space and compromise neurovascular function.54 55 This manifests as “acute compartment syndrome.”24 25 31
Box 1. Clinical effects of snakebite.
Local effects
Bite site—Swelling, blistering, bruising, necrosis (usual after bites by cobras and vipers, with some exceptions in each family, and burrowing asps)24 25
Acute compartment syndrome after deep bite into a limb—Intense pain, abnormal sensations, or a cold, pulseless, immobile limb24 25
Venom ophthalmia from entry of venom droplets or spray into the eyes—Intense pain, redness, blepharitis, blepharospasm, and corneal erosions31
Systemic effects
Vascular—Envenoming by most viperid and Australopapuan elapid species and some non-front-fanged colubroids can trigger clotting failure, platelet abnormalities, and vessel wall damage.34 35 36 Effects range from clotting test abnormalities to mild bite site or mucosal bleeds to severe spontaneous systemic or intracranial haemorrhage24 25
Shock—From bleeding or plasma extravasation systemically or into the swollen, bitten limb, myocardial dysfunction, pituitary bleeds, vasodilation, sepsis, and anaphylaxis29 36 37 38 39 40 41 42
Neuromuscular—Most elapid and some viperid venoms can cause paralysis by action at the nerve (presynaptic) or muscle fibre (postsynaptic) of the neuromuscular junction.24 25 Weakness of eye muscles initially present as ptosis, diplopia, and blurred vision. This is followed by sequential weakness of bulbar (dysphagia, dysphonia, and drooling), neck, respiratory, and limb muscles43
Generalised muscle destruction is caused by envenoming by sea snakes and some elapid and viperid species.24 25 This manifests as muscle pain and tenderness, especially of the neck, trunk, and proximal limbs with dark urine29
Acute kidney injury likely results from secondary effects such as hypotension, fibrin-platelet microthrombi in capillaries and arterioles, and immune or haem related tubular damage, or directly from effect of venom44 45 46 47 48 49 50 51 52 53
How do patients present?
Patients usually give a history of being bitten by a snake, except those who experience painless nocturnal bites by kraits while asleep.22 56 57 Patients are often fearful and anxious. Occasionally, painful bites may be mistaken for a puncture wound from a thorn or sharp stone and be ignored initially.58 Some patients, especially children, bitten by highly venomous snakes, may present with cardiovascular collapse, unconsciousness, bleeding, paralysis, or respiratory failure and may not provide a clear history of snakebite.44 59 It is important to consider envenoming in these situations in regions where snakebites are common.24 25
Nausea, vomiting, abdominal pain, and headache are non-specific symptoms but must be monitored as these may herald serious complications such as uraemia, acute pituitary or intracranial bleeds, and anaphylaxis.24
What first aid can be provided?
Reassure the person about prompt first aid and medical assistance to allay fears. Arrange for rapid transport to the nearest medical facility, preferably with access to antivenom and critical care support.60
Immobilise the person, and especially the bitten limb to slow venom spread.24 Remove rings and other tight objects around the limb.61 A systematic review identified pressure immobilisation with an elastic bandage or pad (at a comfortable pressure) at the bite site as an effective first aid measure to slow venom spread, but the quality of evidence was very low.62 63 Its use is variable, and it is discouraged in most practice and guidelines because of the uncertainty of benefit and possibility of worsening local tissue damage.15 29 62 64 65 66 67 However, pressure immobilisation is generally recommended for neurotoxic elapid bites in some regions.68 Its clinical efficacy and risk of worsening soft tissue injury in local envenoming have not been adequately assessed.24 29 66 69 A small study (15 patients) in Myanmar found that pressure pads were effective in reducing venom spread in Russell’s viper bite, and local effects after pad application were no more severe than those before treatment.70
Tourniquets can cause severe local damage and gangrene and must not be used.24 25 It is common for communities to resort to traditional therapies such as wound incisions, cauterisation, and application of herbs, minerals, or animal excrement. These can delay access to effective treatment and may cause more harm.15 71 72 Irrigate eyes with copious amounts of water if there is exposure to venom.31
What to cover on initial assessment?
Rural and remote primary care centres are often the first point of medical aid for people with a snakebite. Laboratory and intensive care services at such facilities are often limited. A competent clinical assessment is vital to guide management and referral decisions.14 73 74 75 76
Snakebite envenoming can quickly worsen into a life-threatening emergency. Assess vital parameters to identify if the patient is critical or at risk for shock, respiratory failure, and cardiac arrest.24 25 Published severity scores for snakebite are unreliable.3 The Glasgow coma scale score and pupillary reactions can be misleading in patients with advanced paralysis who are unable to open their eyes or respond to painful stimuli and should be avoided in these circumstances.24
History
Reassure clinically stable patients. Ask about their symptoms to determine the presence, nature, and extent of envenoming. Details about the site, circumstances, and timing of the bite can reflect distinctive features of epidemiology, habitats, and periods of activity of medically important snakes locally and help infer likely biting-species.3 24 25
Inquire about medications, substance use, and comorbidities as these can influence diagnosis and outcomes. Recent ethanol or recreational drug use may modify presenting symptoms. Antiplatelets or anticoagulants may worsen bleeding and interfere with key blood tests. Shock in patients with pre-existing coronary artery disease can precipitate a myocardial infarction.3
Examination
Bite site—Look for fang marks, retained fangs, bleeding, swelling, bruising, discoloration, and blisters.24 Fang marks do not confirm snakebite since bites by lizards, fish, rodents, large spiders, and some insects and thorns also leave paired punctures.3 Their absence does not preclude envenoming, as many snake species produce faint or undetectable bites.22 24 25 56 77 78 Raised vertical, red, tender streaks on the bitten limb suggest lymphangitis.3 24 25 Regional lymph nodes may be enlarged and tender with bruised overlying skin.3 24 25 Note any tourniquets, ligatures, wound incisions or cauterisation, and local traditional remedies as these may lead to specific complications requiring management.24 25 For instance, tourniquets and ligatures, if left on for long, can cause severe local damage including ischaemia, necrosis, and gangrene. Similarly, incisions and local applications can lead to local bacterial infections, sepsis, and tetanus.18 79 80
Systemic examination—Look for signs of coagulopathy such as sub-conjunctival, retinal, nasal, and gingivobuccal bleeds, ecchymoses and internal haemorrhage (such as intracranial, pericardial, pleural, and retroperitoneal).24 25 Assess extraocular movements, bulbar function, and muscle power.24 25 Look for ptosis, muscle tenderness, and jaw stiffness.24 25 Jaw stiffness is a prominent but often overlooked feature in sea snake envenoming that, unlike trismus, can be reduced by sustained pressure on the lower jaw.81
Identifying snake species—Occasionally patients or accompanying persons may bring the killed snake for identification or have a picture of it. A herpetologist can be consulted to help identify the species.29 Identification of snakes based on description by victims or recognition from pictures is often unreliable.29 82 83 Identifying biting-species helps avoid unnecessary antivenom in patients bitten by non-venomous snakes or by species whose venoms are not neutralised by available products. It can help select appropriate antivenom in countries with products specific against single species and anticipate clinical progression. However, delaying emergency treatment until the species is identified is unnecessary.
Knowledge of local snake species, comparison of clinical effects in the patient against established species-specific syndromes, and consideration of the circumstances and timing of the incident can help infer likely biting species.29 82 This approach is widely used to guide treatment with polyspecific antivenom in endemic areas of Africa and Asia.82 84 Snake identification tests based on venom antigen are valuable research tools but are currently unavailable for routine clinical use except in Australia.85
What tests can be performed?
Perform a baseline 20-minute whole blood clotting test (20WBCT) to screen for coagulopathy in patients without overt bleeding. The 20WBCT is a simple, rapid, and inexpensive bedside test to screen for and monitor coagulopathy in areas with limited access to emergency laboratory facilities.29 86 87 Collect a sample of venous blood from the patient and place a few millilitres into a clean dry test tube. Leave it undisturbed for 20 minutes at ambient temperature. Unclotted blood that runs out or a friable clot that readily breaks down on tipping the tube once at 20 minutes indicates a possible clotting disorder (fig 2).24 25
Fig 2.
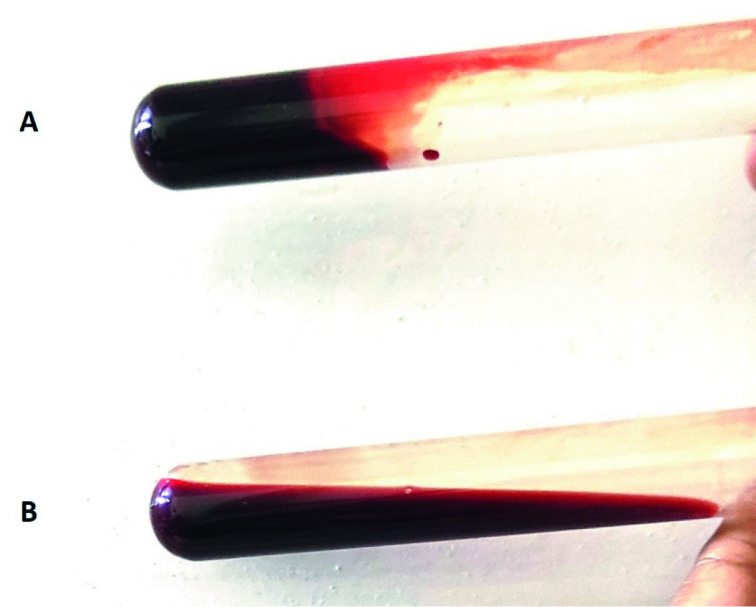
Examples of the 20-minute whole blood clotting test. A) With normal blood, a clot is seen at 20 minutes after sample collection. B) Incoagulable blood at 20 minutes, from a patient with Russell’s viper envenoming
Most clinical validation studies on the 20WBCT report a sensitivity of 82-89% and specificity of 82-98%. One study indicated that the test might potentially miss one of every five coagulopathic patients.88 89 A recent systematic review and meta-analysis of the 20WBCT to evaluate its accuracy in detecting coagulopathy (defined as INR >1.4 or fibrinogen <100 mg/dL) revealed an 84% sensitivity and 91% specificity using the international normalised ratio (INR) as reference standard and 72% sensitivity and 94% specificity using plasma fibrinogen as reference standard.90 The test was less sensitive in detecting milder coagulopathy (median INR for patients with a false negative 20WBCT was 1.9 (IQR 1.6 to 12, skewness of 1.06 and kurtosis of −0.83) and resolution of coagulopathy following antivenom administration (sensitivity 5% to 67%).
In settings with laboratory support, additional tests might include a complete blood count, coagulation studies, and biochemical assays including creatinine phosphokinase (CPK), serum creatinine, blood urea, and electrolytes.3 A low haematocrit usually occurs with blood loss. Higher than normal values may indicate haemoconcentration from systemic plasma extravasation. Peripheral neutrophilic leucocytosis represents a general inflammatory response and confirms systemic envenoming. Severe thrombocytopenia contributes to bleeding diatheses. It may indicate microangiopathic haemolysis when accompanied by schistocytes in the blood film and acute kidney injury. Prothrombin and activated partial thromboplastin times, D dimer, fibrinogen, and fibrin degradation products are more sensitive indicators of venom induced clotting disturbances. Blood urea, serum creatinine, and electrolyte concentrations help screen and monitor acute kidney injury. CPK levels above 10 000 units/L indicate severe rhabdomyolysis. Unexplained hypoglycaemia (venous blood glucose <55 mg/dL) can be an important clue to acute hypopituitarism following snake envenoming.91
How is snakebite managed?
Resuscitation and supportive care
Admit all snakebite patients for observation for a minimum of 24 hours. The onset of symptoms may be delayed but can worsen rapidly. Inform patients and/or their relatives about potential complications, treatment, and critical-care measures using simple language, after emergency medical stabilisation. If required, explain the need for referral clearly.
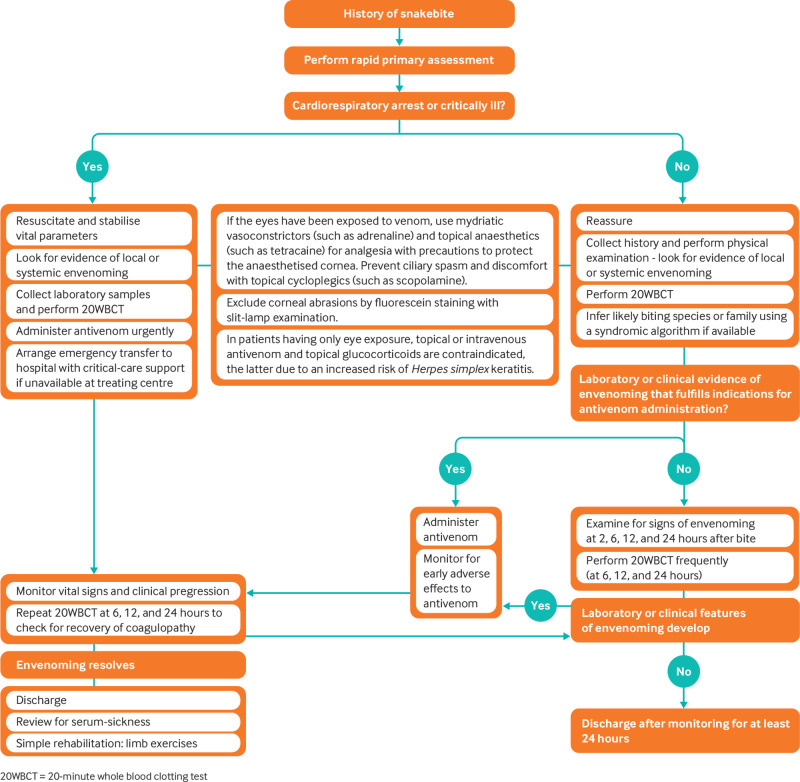
Promptly manage airway obstruction, respiratory paralysis, and shock by restoring airway, oxygen, intubation, and assisted ventilation as needed, and intravenous fluids. Figure 3 summarises the management of snakebite. Choose sites of venous access such as the hands, wrists, and in some cases the feet where haemostasis by external pressure is most likely to succeed.24 Avoid central venous or arterial punctures before establishing a negative 20WBCT.24 Ensure that an intravenous line and resuscitation facilities are in place before releasing tourniquets, since this may trigger pronounced clinical deterioration.92 Avoid aspirin or other NSAIDs to control pain as they can exacerbate bleeding diathesis.3
Fig 3.
Flowchart for the management of snakebite
Monitor vital parameters and urine output at regular intervals in all patients. The 20WBCT can be repeated as it is sometimes negative initially, and coagulopathy may be detected later.24 25
Antivenom
Guidelines from the WHO recommend antivenom treatment for patients with shock, spontaneous systemic bleeding, uncoagulable blood, neurotoxicity, black urine, acute kidney injury, rapidly progressive local swelling, and bites by species known to cause local necrosis and digital bites.24 25
Antivenoms are whole or fragmented immunoglobulins fractionated from the plasma of domesticated animals hyper-immunised with venom from one or more snake species over variable periods.93 They are highly specific and will neutralise only the venoms used in their production and those of a few closely related species.93 Polyspecific antivenoms are raised against a mixture of venoms from more than one species. Antivenoms raised against venom from a single species are monospecific.93
Early administration of antivenom prevents or limits haemodynamic alterations, progression of coagulopathy to clinically overt bleeding, postsynaptic neurotoxicity, myotoxicity, acute kidney injury, and local tissue damage.24 25 94 Physiological levels of clotting factors are at least partially restored within a median of six hours with sufficient doses of specific antivenoms.45 95 96 97 98 99 100 101
Robust clinical data on the safe and effective initial dose of antivenom are lacking for most products.102 Clinicians often rely on manufacturers’ recommendations provided as package inserts or labels, but these can be unreliable.103 104 We suggest following national protocols or standard regional guidelines for dose.24 25 Administration is always intravenous, as bolus or diluted in saline solution over 10-60 minutes, at the same dose for adults and children.24 Repeat administration of antivenom if bleeding persists, if weakness or cardiovascular signs worsen within two hours, or if a 20WBCT is positive at six hours after antivenom administration.3
The effectiveness of antivenoms in treating established neurotoxicity, soft tissue damage, and acute kidney injury is not established.24 25 94 105 106 Additional treatments are indicated for these.
Other treatments
Neostigmine with atropine is a potentially useful adjunct in patients bitten by snakes such as some cobras with postsynaptic neurotoxins in their venom. Its use must never delay or preclude antivenom treatment or intubation.24
Administer a tetanus toxoid booster in all patients except in those with coagulopathy, in which case injection is postponed until haemostasis is achieved.3 Aspirate large tense bullae to facilitate nursing the bitten limb, pre-empt spontaneous rupture, and prevent secondary infection. Broad spectrum antibiotics are indicated only if the wound has been incised or there are signs of necrosis, wound infection, or abscess formation.3 Surgical debridement or amputation of gangrenous digits or limbs and skin grafting may be needed.24 25 Fasciotomies are rarely justified since compartment pressures usually remain within normal limits.3 107
Risk of adverse reactions with antivenom
Monitor patients for adverse reactions in the first two hours of antivenom administration (box 2).29 Anaphylaxis or pyrogenic reactions occur early (within minutes or hours). Mechanistic studies suggest that most events are not IgE mediated and thus cannot be accurately predicted by skin tests for immediate hypersensitivity.109 However, their incidence and severity can be reduced by a prophylactic subcutaneous injection of low dose adrenaline.108 110 Pyrogenic reactions result from product contamination during manufacture.111
Box 2. Clinical features and frequency of adverse reactions to antivenom.
Anaphylaxis symptoms
Itching
Urticaria
Nausea, vomiting, abdominal pain, and diarrhoea
Life threatening shock, bronchospasm, and angioedema108
Pyrogenic reactions
These present with fever, rigors, and vasodilation with or without hypotension
Late or serum-sickness type reactions24
Fever
Nausea, vomiting, diarrhoea
Itching or recurrent wheals
Joint and muscle aches or joint swelling
Lymph node enlargement
Proteinuria and kidney disease
Depending on the dose, speed of administration, and product quality, the risk of any early reaction varies from 3% to more than 80% in studies from Latin America and South Asia. About 5-10% of such events are associated with life threatening consequences.29 98 108 110 112 The incidence of fatal reactions is unclear because of confusion with symptoms of envenoming, but some have been reported.112
Treat anaphylaxis at the earliest sign.24 25 Suspend antivenom administration and inject adrenaline intramuscularly, ideally into the upper lateral thigh.24 25 Additional treatment includes intravenous antihistamines and glucocorticoids and inhaled bronchodilators for bronchospasm.24 25 Anaphylaxis can recur, and glucocorticoids do not prevent recurrence.112 On resolution of the episode, cautiously resume antivenom in patients with a definite indication for continued treatment.24 25 Treat pyrogenic reactions with physical cooling, antipyretics, and intravenous fluids.24 25
Late reactions may manifest a week after administration.108 111 113 Their incidence varies widely from 5% to 56% in observational studies and trials using differing diagnostic criteria.108 WHO guidelines recommend a five-day course of oral antihistamines for those with serum-sickness type late reactions, and a five-day course of prednisolone in those who fail antihistamine therapy after the first two days.24 25
Whom to refer?
Patients with persistent bleeding despite repeated antivenom treatment or having respiratory and renal failure may require urgent supportive measures such as blood transfusion, mechanical ventilation, and renal replacement therapy respectively.24 25 If these are not available, arrange for transfer to a specialised centre. Patients with substantial bleeding, worsening paralysis, dropping urine output, refractory shock, anaphylaxis non-responsive to adrenaline, or compartment syndrome may also require specialist management and intensive care.24
Having contact details of emergency transport and the referral centre readily available can avoid delays. Inform the receiving hospital about the patient’s condition over the phone and send a referral letter with details of assessment and treatment.114
What to cover on discharge and follow-up?
Patients who are clinically stable or asymptomatic with persistently negative 20WBCT after 24 hours may be discharged. Educate patients and their families on snakebite prevention and first aid, preferably using printed leaflets with clear visually represented information and minimal reliance on text.3 12
Inform patients who have received antivenom to report late adverse reactions. Arrange a follow-up after two weeks to review late reactions and sequelae.
What are the long-term sequelae of snakebite?
There is insufficient data on long term sequelae after a snakebite.6 Amputations following snakebite-related soft tissue injuries range from 5908 to 14 614 annually in sub-Saharan Africa, based on a meta-analysis of data published between 1970 and 2010.4 Even in patients not requiring amputations, tissue loss may result in chronic ulcers, malignant transformation, and scarring.29 Musculoskeletal sequelae such as contractures, wasting, and joint stiffness affected up to 3% of snakebite survivors in a study of 816 patients in Sri Lanka.115 Cerebrovascular accidents result in persisting limb weakness and visual or cognitive impairment.29 Eye exposure to venom can result in blindness.31 Some patients with acute kidney injury may progress to chronic renal failure.116 117 118 119 120 Limited data from case reports and observational studies from South Asia indicate that chronic hypopituitarism, a sequel of acute pituitary haemorrhage, can present as fatigue, arrested puberty, amenorrhoea, and hypothyroidism as late as 10 years after the bite.37 91 121 122 123 Snakebite survivors also have higher rates of post-traumatic stress disorder and depression compared with matched controls.5 7 Ensuring access to physiotherapy, psychological services, and specialists, as needed, can be essential in managing long term sequelae of snake bite.
Areas for future research.
High-quality randomised controlled clinical trials evaluating the feasibility and effectiveness of pressure immobilisation in bites by different snake species
Large, well designed clinical trials to establish safety, effectiveness, and optimal dose of antivenoms. The risks of anaphylaxis and sensitisation to animal proteins in antivenoms place ethical limitations on conventional phase I/II designs.124 Some literature suggests that model based adaptive designs, as used to test anticancer drugs, could be safer alternatives for antivenom testing95 102 124
Studies on envenoming syndromes to establish species-syndrome correlation and aid early identification of snake species
Clinical trials to evaluate the effectiveness and safety of adjunctive treatment options including small molecular therapeutics such as secretory phospholipase A2 inhibitors (varespladib/varespladib-methyl), matrix metalloproteinase inhibitors (batimastat and marimastat), and peptide and oligomer based technologies such as toxin-specific monoclonal antibodies and aptamers125 126
Observational studies with long post-discharge follow-up to determine the prevalence, severity, clinical progression, and risk factors of long term sequelae from snakebite
Additional resources.
Information on the spatial ecology of medically important venomous snakes, antivenom products, and the location of antivenom manufacturers by country or region can be obtained from the World Health Organization’s Snakebite Information and Data Platform https://www.who.int/teams/control-of-neglected-tropical-diseases/snakebite-envenoming/snakebite-information-and-data-platform.
-
Information on envenoming profiles of different snakes and their management can be obtained from the following sites:
African snakebites—WHO. Guidelines for the prevention and clinical management of snakebite in Africa. 2010. https://apps.who.int/iris/handle/10665/204458
South-East Asian snakebites—WHO. Guidelines for the management of snakebites. 2nd ed. 2016. https://apps.who.int/iris/handle/10665/249547
Global snakes database—University of Adelaide. Clinical toxicology resources: Snakes. www.toxinology.com
Education into practice.
Think about the last time you treated a patient with snakebite. Reflect on the challenges you encountered while diagnosing and treating envenoming. What changes, if any, might you make to your approach next time?
How do you review a snakebite patient as an outpatient discharge? Think about common persisting physical and psychological problems that you might have noticed during review. To what extent was the patient able to share difficulties in returning to routine life? How might you involve the patient in making decisions about long term?
How patients were involved in the creation of this article.
No patients were involved in the creation of this article.
Acknowledgments
We thank Abdulrazaq Habib, Bayero University, Kano, Nigeria; Colin J Forsyth, Drugs for Neglected Diseases initiative-North America; Ahmad Khaldun Ismail, Profesor Madya, Department of Emergency Medicine, Faculty of Medicine, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia; José María Gutiérrez, Instituto Clodomiro Picado, Facultad de Microbiología, Universidad de Costa Rica, Costa Rica; and Scott A Weinstein, Women’s and Children’s Hospital, North Adelaide, Australia for their instructive comments on this manuscript.
Supplementary illustrations on the pathophysiology of venom-toxin action were created with BioRender.com
Web Extra.
Extra material supplied by the author
Supplementary text: How do snake venoms act?
Contributors: RR formulated and wrote the initial draft. All authors searched the literature, framed manuscript content, contributed to critical revisions and approved of the final version. RR is the guarantor of this article.
The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. The authors are fully responsible for the contents and editorial decisions for this manuscript.
Competing interests: We have read and understood BMJ policy on declaration of interests and have no relevant interests to declare.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1. Chippaux JP. Snake-bites: appraisal of the global situation. Bull World Health Organ 1998;76:515-24. [PMC free article] [PubMed] [Google Scholar]
- 2. Kasturiratne A, Wickremasinghe AR, de Silva N, et al. The global burden of snakebite: a literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med 2008;5:e218. 10.1371/journal.pmed.0050218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gutiérrez JM, Calvete JJ, Habib AG, Harrison RA, Williams DJ, Warrell DA. Snakebite envenoming. Nat Rev Dis Primers 2017;3:17063. 10.1038/nrdp.2017.63. [DOI] [PubMed] [Google Scholar]
- 4. Chippaux J-P. Estimate of the burden of snakebites in sub-Saharan Africa: a meta-analytic approach. Toxicon 2011;57:586-99. 10.1016/j.toxicon.2010.12.022 [DOI] [PubMed] [Google Scholar]
- 5. Williams SS, Wijesinghe CA, Jayamanne SF, et al. Delayed psychological morbidity associated with snakebite envenoming. PLoS Negl Trop Dis 2011;5:e1255. 10.1371/journal.pntd.0001255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Waiddyanatha S, Silva A, Siribaddana S, Isbister GK. Long-term effects of snake envenoming. Toxins (Basel) 2019;11:E193. 10.3390/toxins11040193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Habib ZG, Salihu AS, Hamza M, et al. Posttraumatic stress disorder and psycho-social impairment following snakebite in Northeastern Nigeria. Int J Psychiatry Med 2021;56:97-115. 10.1177/0091217420913400 [DOI] [PubMed] [Google Scholar]
- 8. Ameade EPK, Bonney I, Boateng ET. Health professionals’ overestimation of knowledge on snakebite management, a threat to the survival of snakebite victims—A cross-sectional study in Ghana. PLoS Negl Trop Dis 2021;15:e0008756. 10.1371/journal.pntd.0008756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Michael GC, Grema BA, Aliyu I, et al. Knowledge of venomous snakes, snakebite first aid, treatment, and prevention among clinicians in northern Nigeria: a cross-sectional multicentre study. Trans R Soc Trop Med Hyg 2018;112:47-56. 10.1093/trstmh/try028 [DOI] [PubMed] [Google Scholar]
- 10. Sapkota S, Pandey DP, Dhakal GP, Gurung DB. Knowledge of health workers on snakes and snakebite management and treatment seeking behavior of snakebite victims in Bhutan. PLoS Negl Trop Dis 2020;14:e0008793. 10.1371/journal.pntd.0008793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Taieb F, Dub T, Madec Y, et al. Knowledge, attitude and practices of snakebite management amongst health workers in Cameroon: Need for continuous training and capacity building. PLoS Negl Trop Dis 2018;12:e0006716. 10.1371/journal.pntd.0006716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Snakebite envenoming–A strategy for prevention and control. 2019. https://www.who.int/publications/i/item/9789241515641. [DOI] [PubMed]
- 13. Kasturiratne A, Pathmeswaran A, Wickremasinghe AR, et al. The socio-economic burden of snakebite in Sri Lanka. PLoS Negl Trop Dis 2017;11:e0005647. 10.1371/journal.pntd.0005647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cruz LS, Vargas R, Lopes AA. Snakebite envenomation and death in the developing world. Ethn Dis 2009;19(Suppl 1):S1-42, 6. [PubMed] [Google Scholar]
- 15. Alirol E, Sharma SK, Bawaskar HS, Kuch U, Chappuis F. Snake bite in South Asia: a review. PLoS Negl Trop Dis 2010;4:e603. 10.1371/journal.pntd.0000603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harrison RA, Hargreaves A, Wagstaff SC, Faragher B, Lalloo DG. Snake envenoming: a disease of poverty. PLoS Negl Trop Dis 2009;3:e569. 10.1371/journal.pntd.0000569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chippaux J-P. Incidence and mortality due to snakebite in the Americas. PLoS Negl Trop Dis 2017;11:e0005662. 10.1371/journal.pntd.0005662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pochanugool C, Wildde H, Bhanganada K, et al. Venomous snakebite in Thailand. II: Clinical experience. Mil Med 1998;163:318-23. 10.1093/milmed/163.5.318 [DOI] [PubMed] [Google Scholar]
- 19. Aye K-P, Thanachartwet V, Soe C, et al. Clinical and laboratory parameters associated with acute kidney injury in patients with snakebite envenomation: a prospective observational study from Myanmar. BMC Nephrol 2017;18:92. 10.1186/s12882-017-0510-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Laohawiriyakamol S, Sangkhathat S, Chiengkriwate P, Patrapinyokul S. Surgery in management of snake envenomation in children. World J Pediatr 2011;7:361-4. 10.1007/s12519-011-0282-8 [DOI] [PubMed] [Google Scholar]
- 21. Ralph R, Sharma SK, Faiz MA, et al. The timing is right to end snakebite deaths in South Asia. BMJ 2019;364:k5317. 10.1136/bmj.k5317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tongpoo A, Sriapha C, Pradoo A, et al. Krait envenomation in Thailand. Ther Clin Risk Manag 2018;14:1711-7. 10.2147/TCRM.S169581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ochoa-Avilés A, Heredia-Andino OS, Escandón SA, et al. Viperidae snakebites in Ecuador: A review of epidemiological and ecological aspects. Toxicon X 2020;7:100051. 10.1016/j.toxcx.2020.100051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. Guidelines for the management of snakebites, 2nd ed. 2016. https://apps.who.int/iris/handle/10665/249547.
- 25.World Health Organization. Guidelines for the prevention and clinical management of snakebite in Africa. 2010. https://apps.who.int/iris/handle/10665/204458.
- 26. Hsiang AY, Field DJ, Webster TH, et al. The origin of snakes: revealing the ecology, behavior, and evolutionary history of early snakes using genomics, phenomics, and the fossil record. BMC Evol Biol 2015;15:87. 10.1186/s12862-015-0358-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pyron RA, Burbrink FT, Wiens JJ. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evol Biol 2013;13:93. 10.1186/1471-2148-13-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kerkkamp HMI, Casewell NR, Vonk FJ. Evolution of the snake venom delivery system. In: Malhotra A, Gopalakrishnakone P, eds. Evolution of venomous animals and their toxins. Springer Netherlands, 2017: 303-16, 10.1007/978-94-007-6458-3_11 . [DOI] [Google Scholar]
- 29. Warrell DA. Snake bite. Lancet 2010;375:77-88. 10.1016/S0140-6736(09)61754-2 [DOI] [PubMed] [Google Scholar]
- 30. Young BA, Dunlap K, Koenig K, Singer M. The buccal buckle: the functional morphology of venom spitting in cobras. J Exp Biol 2004;207:3483-94. 10.1242/jeb.01170 [DOI] [PubMed] [Google Scholar]
- 31. Chu ER, Weinstein SA, White J, Warrell DA. Venom ophthalmia caused by venoms of spitting elapid and other snakes: Report of ten cases with review of epidemiology, clinical features, pathophysiology and management. Toxicon 2010;56:259-72. 10.1016/j.toxicon.2010.02.023 [DOI] [PubMed] [Google Scholar]
- 32. Westhoff G, Tzschätzsch K, Bleckmann H. The spitting behavior of two species of spitting cobras. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 2005;191:873-81. 10.1007/s00359-005-0010-8 [DOI] [PubMed] [Google Scholar]
- 33. Pucca MB, Knudsen C, S Oliveira I, et al. Current knowledge on snake dry bites. Toxins (Basel) 2020;12:E668. 10.3390/toxins12110668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Isbister GK, Scorgie FE, O’Leary MA, Seldon M, Brown SG, Lincz LF, ASP Investigators . Factor deficiencies in venom-induced consumption coagulopathy resulting from Australian elapid envenomation: Australian Snakebite Project (ASP-10). J Thromb Haemost 2010;8:2504-13. 10.1111/j.1538-7836.2010.04050.x [DOI] [PubMed] [Google Scholar]
- 35. Rucavado A, Soto M, Escalante T, Loría GD, Arni R, Gutiérrez JM. Thrombocytopenia and platelet hypoaggregation induced by Bothrops asper snake venom. Toxins involved and their contribution to metalloproteinase-induced pulmonary hemorrhage. Thromb Haemost 2005;94:123-31. 10.1160/TH05-02-0112 [DOI] [PubMed] [Google Scholar]
- 36. Gutiérrez JM, Escalante T, Rucavado A, Herrera C. Hemorrhage caused by snake venom metalloproteinases: a journey of discovery and understanding. Toxins (Basel) 2016;8:93. 10.3390/toxins8040093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tun-Pe, Phillips RE, Warrell DA, et al. Acute and chronic pituitary failure resembling Sheehan’s syndrome following bites by Russell’s viper in Burma. Lancet 1987;2:763-7. 10.1016/S0140-6736(87)92500-1 [DOI] [PubMed] [Google Scholar]
- 38. Slagboom J, Kool J, Harrison RA, Casewell NR. Haemotoxic snake venoms: their functional activity, impact on snakebite victims and pharmaceutical promise. Br J Haematol 2017;177:947-59. 10.1111/bjh.14591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Péterfi O, Boda F, Szabó Z, Ferencz E, Bába L. Hypotensive snake venom components-a mini-review. Molecules 2019;24:E2778. 10.3390/molecules24152778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Camargo ACM, Ianzer D, Guerreiro JR, Serrano SMT. Bradykinin-potentiating peptides: beyond captopril. Toxicon 2012;59:516-23. 10.1016/j.toxicon.2011.07.013 [DOI] [PubMed] [Google Scholar]
- 41. Silva A, Pilapitiya S, Siribaddana S. Acute myocardial infarction following a possible direct intravenous bite of Russell’s viper (Daboia russelli). BMC Res Notes 2012;5:500. 10.1186/1756-0500-5-500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jeevagan V, Katulanda P, Gnanathasan CA, Warrell DA. Acute pituitary insufficiency and hypokalaemia following envenoming by Russell’s viper (Daboia russelii) in Sri Lanka: Exploring the pathophysiological mechanisms. Toxicon 2013;63:78-82. 10.1016/j.toxicon.2012.11.012 [DOI] [PubMed] [Google Scholar]
- 43. Ranawaka UK, Lalloo DG, de Silva HJ. Neurotoxicity in snakebite--the limits of our knowledge. PLoS Negl Trop Dis 2013;7:e2302. 10.1371/journal.pntd.0002302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Allen GE, Brown SGA, Buckley NA, et al. ASP Investigators . Clinical effects and antivenom dosing in brown snake (Pseudonaja spp.) envenoming--Australian snakebite project (ASP-14). PLoS One 2012;7:e53188. 10.1371/journal.pone.0053188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Myint-Lwin, Warrell DA, Phillips RE, Tin-Nu-Swe, Tun-Pe, Maung-Maung-Lay. Bites by Russell’s viper (Vipera russelli siamensis) in Burma: haemostatic, vascular, and renal disturbances and response to treatment. Lancet 1985;2:1259-64. 10.1016/S0140-6736(85)91550-8 [DOI] [PubMed] [Google Scholar]
- 46. Alves EC, Sachett JAG, Sampaio VS, et al. Predicting acute renal failure in Bothrops snakebite patients in a tertiary reference center, Western Brazilian Amazon. PLoS One 2018;13:e0202361. 10.1371/journal.pone.0202361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pinho FMO, Zanetta DMT, Burdmann EA. Acute renal failure after Crotalus durissus snakebite: a prospective survey on 100 patients. Kidney Int 2005;67:659-67. 10.1111/j.1523-1755.2005.67122.x [DOI] [PubMed] [Google Scholar]
- 48. Johnston CI, Ryan NM, Page CB, et al. The Australian Snakebite Project, 2005-2015 (ASP-20). Med J Aust 2017;207:119-25. 10.5694/mja17.00094 [DOI] [PubMed] [Google Scholar]
- 49. Isbister GK, O’Leary MA, Elliott M, Brown SGA. Tiger snake (Notechis spp) envenoming: Australian Snakebite Project (ASP-13). Med J Aust 2012;197:173-7. 10.5694/mja11.11300 [DOI] [PubMed] [Google Scholar]
- 50. Sitprija V. Snakebite nephropathy. Nephrology (Carlton) 2006;11:442-8. 10.1111/j.1440-1797.2006.00599.x [DOI] [PubMed] [Google Scholar]
- 51. Kularatne SA. Epidemiology and clinical picture of the Russell’s viper (Daboia russelii russelii) bite in Anuradhapura, Sri Lanka: a prospective study of 336 patients. Southeast Asian J Trop Med Public Health 2003;34:855-62. [PubMed] [Google Scholar]
- 52. Kanjanabuch T, Sitprija V. Snakebite nephrotoxicity in Asia. Semin Nephrol 2008;28:363-72. 10.1016/j.semnephrol.2008.04.005 [DOI] [PubMed] [Google Scholar]
- 53. Hung D-Z, Wu M-L, Deng J-F, Lin-Shiau S-Y. Russell’s viper snakebite in Taiwan: differences from other Asian countries. Toxicon 2002;40:1291-8. 10.1016/S0041-0101(02)00137-X [DOI] [PubMed] [Google Scholar]
- 54. Garfin SR, Castilonia RR, Mubarak SJ, Hargens AR, Russell FE, Akeson WH. Rattlesnake bites and surgical decompression: results using a laboratory model. Toxicon 1984;22:177-82. 10.1016/0041-0101(84)90018-7 [DOI] [PubMed] [Google Scholar]
- 55. Gold BS, Barish RA, Dart RC, Silverman RP, Bochicchio GV. Resolution of compartment syndrome after rattlesnake envenomation utilizing non-invasive measures. J Emerg Med 2003;24:285-8. 10.1016/S0736-4679(02)00762-X [DOI] [PubMed] [Google Scholar]
- 56. Kularatne SA. Common krait (Bungarus caeruleus) bite in Anuradhapura, Sri Lanka: a prospective clinical study, 1996-98. Postgrad Med J 2002;78:276-80. 10.1136/pmj.78.919.276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Saini RK, Singh S, Sharma S, Rampal V, Manhas AS, Gupta VK. Snake bite poisoning presenting as early morning neuroparalytic syndrome in jhuggi dwellers. J Assoc Physicians India 1986;34:415-7. [PubMed] [Google Scholar]
- 58. Bawaskar HS, Bawaskar PS. Snake bite poisoning. J Mahatma Gandhi Inst Med Sci. 2015;20:5-14 10.4103/0971-9903.151717 . [DOI] [Google Scholar]
- 59. Gutiérrez JM. Current challenges for confronting the public health problem of snakebite envenoming in Central America. J Venom Anim Toxins Incl Trop Dis 2014;20:7. 10.1186/1678-9199-20-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sharma SK, Bovier P, Jha N, Alirol E, Loutan L, Chappuis F. Effectiveness of rapid transport of victims and community health education on snake bite fatalities in rural Nepal. Am J Trop Med Hyg 2013;89:145-50. 10.4269/ajtmh.12-0750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mallik S, Singh SR, Sahoo S, Mohanty MK. Ornament induced complications in snake bites: Revisiting the “Do it RIGHT” approach. J Family Med Prim Care 2016;5:474-6. 10.4103/2249-4863.192351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Anker RL, Straffon WG, Loiselle DS, Anker KM. Retarding the uptake of “mock venom” in humans: comparison of three first-aid treatments. Med J Aust 1982;1:212-4. 10.5694/j.1326-5377.1982.tb132272.x [DOI] [PubMed] [Google Scholar]
- 63. Avau B, Borra V, Vandekerckhove P, De Buck E. The treatment of snake bites in a first aid setting: a systematic review. PLoS Negl Trop Dis 2016;10:e0005079. 10.1371/journal.pntd.0005079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sutherland SK, Coulter AR, Harris RD. Rationalisation of first-aid measures for elapid snakebite. Lancet 1979;1:183-6. 10.1016/S0140-6736(79)90580-4 [DOI] [PubMed] [Google Scholar]
- 65. Helden DFV, Dosen PJ, O’Leary MA, Isbister GK. Two pathways for venom toxin entry consequent to injection of an Australian elapid snake venom. Sci Rep 2019;9:8595. 10.1038/s41598-019-45022-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Seifert SA, White J, Currie BJ. Commentary: pressure bandaging for North American snake bite? No! J Med Toxicol 2011;7:324-6. 10.1007/s13181-011-0188-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bush SP, Green SM, Laack TA, Hayes WK, Cardwell MD, Tanen DA. Pressure immobilization delays mortality and increases intracompartmental pressure after artificial intramuscular rattlesnake envenomation in a porcine model. Ann Emerg Med 2004;44:599-604. 10.1016/j.annemergmed.2004.06.007 [DOI] [PubMed] [Google Scholar]
- 68. Isbister GK, Brown SG, Page CB, McCoubrie DL, Greene SL, Buckley NA. Snakebite in Australia: a practical approach to diagnosis and treatment. Med J Aust 2013;199:763-8. 10.5694/mja12.11172 [DOI] [PubMed] [Google Scholar]
- 69. Parker-Cote J, Meggs WJ. First aid and pre-hospital management of venomous snakebites. Trop Med Infect Dis 2018;3:E45. 10.3390/tropicalmed3020045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tun-Pe, Aye-Aye-Myint, Khin-Ei-Han, Thi-Ha, Tin-Nu-Swe. Local compression pads as a first-aid measure for victims of bites by Russell’s viper (Daboia russelii siamensis) in Myanmar. Trans R Soc Trop Med Hyg 1995;89:293-5. 10.1016/0035-9203(95)90547-2 [DOI] [PubMed] [Google Scholar]
- 71. Abubakar SB, Habib AG, Mathew J. Amputation and disability following snakebite in Nigeria. Trop Doct 2010;40:114-6. 10.1258/td.2009.090266 [DOI] [PubMed] [Google Scholar]
- 72. Michael GC, Thacher TD, Shehu MIL. The effect of pre-hospital care for venomous snake bite on outcome in Nigeria. Trans R Soc Trop Med Hyg 2011;105:95-101. 10.1016/j.trstmh.2010.09.005 [DOI] [PubMed] [Google Scholar]
- 73. Iliyasu G, Tiamiyu AB, Daiyab FM, Tambuwal SH, Habib ZG, Habib AG. Effect of distance and delay in access to care on outcome of snakebite in rural north-eastern Nigeria. Rural Remote Health 2015;15:3496. 10.22605/RRH3496 [DOI] [PubMed] [Google Scholar]
- 74. George M. The role of basic laboratory services in strengthening primary health centres. Indian J Med Ethics 2011;8:161-3. 10.20529/IJME.2011.064 [DOI] [PubMed] [Google Scholar]
- 75. Means R, Cabrera J, Moreno X, Amini R. Remote South American snakebite with extensive myonecrosis. Clin Pract Cases Emerg Med 2017;1:47-9. 10.5811/cpcem.2016.11.31220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Jain R, Rao B. Role of laboratory services in primary health center (PHC) outpatient department performance: an Indian case study. Prim Health Care Res Dev 2019;20:e112. 10.1017/S1463423619000537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lalloo DG, Trevett AJ, Korinhona A, et al. Snake bites by the Papuan taipan (Oxyuranus scutellatus canni): paralysis, hemostatic and electrocardiographic abnormalities, and effects of antivenom. Am J Trop Med Hyg 1995;52:525-31. 10.4269/ajtmh.1995.52.525 [DOI] [PubMed] [Google Scholar]
- 78. Bucaretchi F, Capitani EMD, Vieira RJ, et al. Coral snake bites (Micrurus spp.) in Brazil: a review of literature reports. Clin Toxicol (Phila) 2016;54:222-34. 10.3109/15563650.2015.1135337 [DOI] [PubMed] [Google Scholar]
- 79. Amaral CF, Campolina D, Dias MB, Bueno CM, Rezende NA. Tourniquet ineffectiveness to reduce the severity of envenoming after Crotalus durissus snake bite in Belo Horizonte, Minas Gerais, Brazil. Toxicon 1998;36:805-8. 10.1016/S0041-0101(97)00132-3 [DOI] [PubMed] [Google Scholar]
- 80. Habib AG. Tetanus complicating snakebite in northern Nigeria: clinical presentation and public health implications. Acta Trop 2003;85:87-91. 10.1016/S0001-706X(02)00234-6 [DOI] [PubMed] [Google Scholar]
- 81. Reid HA. Sea-snake bites. BMJ 1956;2:73-8. 10.1136/bmj.2.4984.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ariaratnam CA, Sheriff MHR, Arambepola C, Theakston RDG, Warrell DA. Syndromic approach to treatment of snake bite in Sri Lanka based on results of a prospective national hospital-based survey of patients envenomed by identified snakes. Am J Trop Med Hyg 2009;81:725-31. 10.4269/ajtmh.2009.09-0225 [DOI] [PubMed] [Google Scholar]
- 83. Harris JB, Faiz MA, Rahman MR, et al. Snake bite in Chittagong Division, Bangladesh: a study of bitten patients who developed no signs of systemic envenoming. Trans R Soc Trop Med Hyg 2010;104:320-7. 10.1016/j.trstmh.2009.12.006 [DOI] [PubMed] [Google Scholar]
- 84. Blaylock RS. The identification and syndromic management of snakebite in South Africa. S Afr Fam Pract 2005;47:48-53 10.1080/20786204.2005.10873288 . [DOI] [Google Scholar]
- 85. Theakston RDG, Laing GD. Diagnosis of snakebite and the importance of immunological tests in venom research. Toxins (Basel) 2014;6:1667-95. 10.3390/toxins6051667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Warrell DA, Davidson NMcD, Greenwood BM, et al. Poisoning by bites of the saw-scaled or carpet viper (Echis carinatus) in Nigeria. Q J Med 1977;46:33-62. [PubMed] [Google Scholar]
- 87. Sano-Martins IS, Fan HW, Castro SC, et al. Butantan Institute Antivenom Study Group . Reliability of the simple 20 minute whole blood clotting test (WBCT20) as an indicator of low plasma fibrinogen concentration in patients envenomed by Bothrops snakes. Toxicon 1994;32:1045-50. 10.1016/0041-0101(94)90388-3 [DOI] [PubMed] [Google Scholar]
- 88. Isbister GK, Maduwage K, Shahmy S, et al. Diagnostic 20-min whole blood clotting test in Russell’s viper envenoming delays antivenom administration. QJM 2013;106:925-32. 10.1093/qjmed/hct102 [DOI] [PubMed] [Google Scholar]
- 89. Wedasingha S, Isbister G, Silva A. Bedside coagulation tests in diagnosing venom-induced consumption coagulopathy in snakebite. Toxins (Basel) 2020;12:E583. 10.3390/toxins12090583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lamb T, Abouyannis M, de Oliveira SS, et al. The 20-minute whole blood clotting test (20WBCT) for snakebite coagulopathy-A systematic review and meta-analysis of diagnostic test accuracy. PLoS Negl Trop Dis 2021;15:e0009657. 10.1371/journal.pntd.0009657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Naik BN, Bhalla A, Sharma N, et al. Pituitary dysfunction in survivors of Russell’s viper snake bite envenomation: A prospective study. Neurol India 2018;66:1351-8. 10.4103/0028-3886.241378 [DOI] [PubMed] [Google Scholar]
- 92. Watt G, Padre L, Tuazon L, Theakston RDG, Laughlin L. Bites by the Philippine cobra (Naja naja philippinensis): prominent neurotoxicity with minimal local signs. Am J Trop Med Hyg 1988;39:306-11. 10.4269/ajtmh.1988.39.306 [DOI] [PubMed] [Google Scholar]
- 93.World Health Organization. WHO guidelines for the production, control and regulation of snake antivenom immunoglobulins. WHO Tech Rep Ser 2018. https://www.who.int/bloodproducts/AntivenomGLrevWHO_TRS_1004_web_Annex_5.pdf.
- 94. Alfred S, Bates D, White J, et al. Acute kidney injury following Eastern Russell’s viper (Daboia siamensis) snakebite in Myanmar. Kidney Int Rep 2019;4:1337-41. 10.1016/j.ekir.2019.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Abubakar SB, Abubakar IS, Habib AG, et al. Nigeria-UK EchiTab Study Group . Pre-clinical and preliminary dose-finding and safety studies to identify candidate antivenoms for treatment of envenoming by saw-scaled or carpet vipers (Echis ocellatus) in northern Nigeria. Toxicon 2010;55:719-23. 10.1016/j.toxicon.2009.10.024 [DOI] [PubMed] [Google Scholar]
- 96. Warrell DA, Looareesuwan S, Theakston RD, et al. Randomized comparative trial of three monospecific antivenoms for bites by the Malayan pit viper (Calloselasma rhodostoma) in southern Thailand: clinical and laboratory correlations. Am J Trop Med Hyg 1986;35:1235-47. 10.4269/ajtmh.1986.35.1235 [DOI] [PubMed] [Google Scholar]
- 97. Cardoso JL, Fan HW, França FO, et al. Randomized comparative trial of three antivenoms in the treatment of envenoming by lance-headed vipers (Bothrops jararaca) in São Paulo, Brazil. Q J Med 1993;86:315-25. [PubMed] [Google Scholar]
- 98. Smalligan R, Cole J, Brito N, et al. Crotaline snake bite in the Ecuadorian Amazon: randomised double blind comparative trial of three South American polyspecific antivenoms. BMJ 2004;329:1129. 10.1136/bmj.329.7475.1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Isbister GK, Duffull SB, Brown SGA, ASP Investigators . Failure of antivenom to improve recovery in Australian snakebite coagulopathy. QJM 2009;102:563-8. 10.1093/qjmed/hcp081 [DOI] [PubMed] [Google Scholar]
- 100. Tanos PP, Isbister GK, Lalloo DG, Kirkpatrick CMJ, Duffull SB. A model for venom-induced consumptive coagulopathy in snake bite. Toxicon 2008;52:769-80. 10.1016/j.toxicon.2008.08.013 [DOI] [PubMed] [Google Scholar]
- 101. Gulati A, Isbister GK, Duffull SB. Effect of Australian elapid venoms on blood coagulation: Australian Snakebite Project (ASP-17). Toxicon 2013;61:94-104. 10.1016/j.toxicon.2012.11.001 [DOI] [PubMed] [Google Scholar]
- 102. Watson JA, Lamb T, Holmes J, et al. A Bayesian phase 2 model based adaptive design to optimise antivenom dosing: Application to a dose-finding trial for a novel Russell’s viper antivenom in Myanmar. PLoS Negl Trop Dis 2020;14:e0008109. 10.1371/journal.pntd.0008109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Calvete JJ, Arias AS, Rodríguez Y, et al. Preclinical evaluation of three polyspecific antivenoms against the venom of Echis ocellatus: Neutralization of toxic activities and antivenomics. Toxicon 2016;119:280-8. 10.1016/j.toxicon.2016.06.022 [DOI] [PubMed] [Google Scholar]
- 104. Harrison RA, Oluoch GO, Ainsworth S, et al. Preclinical antivenom-efficacy testing reveals potentially disturbing deficiencies of snakebite treatment capability in East Africa. PLoS Negl Trop Dis 2017;11:e0005969. 10.1371/journal.pntd.0005969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Silva A, Hodgson WC, Isbister GK. Antivenom for neuromuscular paralysis resulting from snake envenoming. Toxins (Basel) 2017;9:E143. 10.3390/toxins9040143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Gutiérrez JM, León G, Rojas G, Lomonte B, Rucavado A, Chaves F. Neutralization of local tissue damage induced by Bothrops asper (terciopelo) snake venom. Toxicon 1998;36:1529-38. 10.1016/S0041-0101(98)00145-7 [DOI] [PubMed] [Google Scholar]
- 107. Darracq MA, Cantrell FL, Klauk B, Thornton SL. A chance to cut is not always a chance to cure- fasciotomy in the treatment of rattlesnake envenomation: A retrospective poison center study. Toxicon 2015;101:23-6. 10.1016/j.toxicon.2015.04.014 [DOI] [PubMed] [Google Scholar]
- 108. de Silva HA, Ryan NM, de Silva HJ. Adverse reactions to snake antivenom, and their prevention and treatment. Br J Clin Pharmacol 2016;81:446-52. 10.1111/bcp.12739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Stone SF, Isbister GK, Shahmy S, et al. Immune response to snake envenoming and treatment with antivenom; complement activation, cytokine production and mast cell degranulation. PLoS Negl Trop Dis 2013;7:e2326. 10.1371/journal.pntd.0002326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Premawardhena AP, de Silva CE, Fonseka MM, Gunatilake SB, de Silva HJ. Low dose subcutaneous adrenaline to prevent acute adverse reactions to antivenom serum in people bitten by snakes: randomised, placebo controlled trial. BMJ 1999;318:1041-3. 10.1136/bmj.318.7190.1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. León G, Herrera M, Segura Á, Villalta M, Vargas M, Gutiérrez JM. Pathogenic mechanisms underlying adverse reactions induced by intravenous administration of snake antivenoms. Toxicon 2013;76:63-76. 10.1016/j.toxicon.2013.09.010 [DOI] [PubMed] [Google Scholar]
- 112. Sharma SK, Alirol E, Ghimire A, et al. Acute severe anaphylaxis in Nepali patients with neurotoxic snakebite envenoming treated with the VINS polyvalent antivenom. J Trop Med 2019;2019:2689171. 10.1155/2019/2689171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Dart RC, McNally J. Efficacy, safety, and use of snake antivenoms in the United States. Ann Emerg Med 2001;37:181-8. 10.1067/mem.2001.113372 [DOI] [PubMed] [Google Scholar]
- 114. Ramanayake RPJC, Ranasingha S, Lakmini S. Management of emergencies in general practice: role of general practitioners. J Family Med Prim Care 2014;3:305-8. 10.4103/2249-4863.148089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Jayawardana S, Gnanathasan A, Arambepola C, Chang T. Chronic musculoskeletal disabilities following snake envenoming in Sri Lanka: A population-based study. PLoS Negl Trop Dis 2016;10:e0005103. 10.1371/journal.pntd.0005103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Herath HMNJ, Wazil AWM, Abeysekara DTDJ, et al. Chronic kidney disease in snake envenomed patients with acute kidney injury in Sri Lanka: a descriptive study. Postgrad Med J 2012;88:138-42. 10.1136/postgradmedj-2011-130225 [DOI] [PubMed] [Google Scholar]
- 117. Golay V, Roychowdhary A, Pandey R, Singh A, Pasari A, Abraham A. Acute interstitial nephritis in patients with viperine snake bite: single center experience of a rare presentation. Saudi J Kidney Dis Transpl 2012;23:1262-7. [DOI] [PubMed] [Google Scholar]
- 118. Waikhom R, Sircar D, Patil K, Bennikal M, Gupta SD, Pandey R. Long-term renal outcome of snake bite and acute kidney injury: a single-center experience. Ren Fail 2012;34:271-4. 10.3109/0886022X.2011.647297 [DOI] [PubMed] [Google Scholar]
- 119. Pulimaddi R, Parveda AR, Brahmanpally B, Kalakanda PM, Ramakrishna K, Chinnapaka VRD. Incidence & prognosis of acute kidney injury in individuals of snakebite in a tertiary care hospital in India. Indian J Med Res 2017;146:754-8. 10.4103/ijmr.IJMR_1581_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Priyamvada PS, Jaswanth C, Zachariah B, Haridasan S, Parameswaran S, Swaminathan RP. Prognosis and long-term outcomes of acute kidney injury due to snake envenomation. Clin Kidney J 2019;13:564-70. 10.1093/ckj/sfz055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Antonypillai CN, Wass JA, Warrell DA, Rajaratnam HN. Hypopituitarism following envenoming by Russell’s vipers (Daboia siamensis and D. russelii) resembling Sheehan’s syndrome: first case report from Sri Lanka, a review of the literature and recommendations for endocrine management. QJM 2011;104:97-108. 10.1093/qjmed/hcq214 [DOI] [PubMed] [Google Scholar]
- 122. Srinivasan KG, Srividya S, Usha Nandhini KP, Ramprabananth S. Chronic pituitary failure resembling Sheehan’s syndrome following a bite of Russell’s viper. A case report. Neuroradiol J 2010;23:38-41. 10.1177/197140091002300106 [DOI] [PubMed] [Google Scholar]
- 123. Shivaprasad C, Aiswarya Y, Sridevi A, et al. Delayed hypopituitarism following Russell’s viper envenomation: a case series and literature review. Pituitary 2019;22:4-12. 10.1007/s11102-018-0915-1 [DOI] [PubMed] [Google Scholar]
- 124. Williams DJ, Habib AG, Warrell DA. Clinical studies of the effectiveness and safety of antivenoms. Toxicon 2018;150:1-10. 10.1016/j.toxicon.2018.05.001 [DOI] [PubMed] [Google Scholar]
- 125. Ascoët S, De Waard M. Diagnostic and therapeutic value of aptamers in envenomation cases. Int J Mol Sci 2020;21:E3565. 10.3390/ijms21103565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Williams HF, Layfield HJ, Vallance T, et al. The urgent need to develop novel strategies for the diagnosis and treatment of snakebites. Toxins (Basel) 2019;11:E363. 10.3390/toxins11060363 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary text: How do snake venoms act?