Abstract
An enantioselective Michael addition of malonates to α,β-unsaturated para-nitrophenyl esters was achieved using the Lewis basic isothiourea HyperBTM, giving excellent levels of product enantioselectivity (up to >99:1 enantiomeric ratio) in good yields and with complete regioselectivity (>20:1 regioselectivity ratio) in the presence of alternative (phenyl ketone and ethyl ester) Michael acceptors. Density functional theory calculations indicate that N-acylation is rate-limiting. This constitutes a rare example of a highly enantioselective addition of simple, readily available malonates to α,β-unsaturated esters.
The asymmetric Michael reaction is a powerful method for stereoselective C–C bond formation. While enantioselective catalytic Michael addition of carbon nucleophiles to α,β-unsaturated aldehydes, ketones, and alkylidene malonates are well-established,1 analogous enantioselective addition to α,β-unsaturated esters are rare. This is likely due to the low inherent electrophilicity of the carboxylic acid oxidation state compared to alternative Michael acceptors2 combined with the lack of enantiofacial discrimination. Despite these issues, several useful catalytic enantioselective additions have been achieved with highly reactive nucleophilic partners, including silyl ketene acetals,3 dihydropyrazol-3-ones,4 aryl boronic acids,5 thiols and amines,6 and Grignard reagents.7 However, the addition of less reactive, stabilized carbon nucleophiles, such as malonates, remains an unsolved challenge. The current state of the art was demonstrated by Nakamura and co-workers in 2016, who employed a chiral lithium binaphtholate complex 1 to promote the highly enantioselective addition of malonates to symmetric maleic esters,8 but this was limited by the lack of variability at the β position of the Michael acceptor (Scheme 1A). As a result of the importance of this bond disconnection, alternative enantioselective methods with a broad scope would be a welcome addition to the synthetic toolbox.
Scheme 1. Selected Examples of Chiral Amine-Catalyzed Michael Reaction/Cyclization Cascades with Malonate Derivatives and Comparison to This Work.
Chiral tertiary amines, such as chiral 4-dimethylaminopyridine (DMAP) derivatives,9 cinchona alkaloids,10 and isothioureas,11 are effective organocatalysts for inducing asymmetry in a variety of transformations with α,β-unsaturated carboxylic acid derivatives via chiral α,β-unsaturated N-acylammonium intermediates.12 This technique is frequently employed with bis-nucleophile coupling partners that rely upon an initial stereoselective conjugate addition followed by a second nucleophilic addition to achieve turnover of the chiral tertiary amine catalyst. Using this strategy, several methods have been developed employing an asymmetric Michael reaction with malonate derivatives followed by cyclization to release the organocatalysts, with instructive examples highlighted in Scheme 1B.
Romo and co-workers developed an elegant cinchona alkaloid 2-catalyzed Michael reaction/proton transfer/lactamization cascade to provide lactams from aminomalonates and α,β-unsaturated acid chlorides (top left).10 The isothioureas, HBTM 4 and HyperBTM 5, have been employed in cascade reactions, where an initial Michael reaction with β-ketoesters10a,13 (top right) and β-ketomalonates14 (bottom left) was followed by a cyclization event to release the catalyst and deliver δ- and β-lactones in high enantioselectivity, respectively. Building on this precedent and previous work that demonstrated the multifunctional nature of electron-deficient phenoxides as a leaving group and as a secondary nucleophile to achieve catalytic turnover in isothiourea catalysis,15 we posited that α,β-unsaturated p-nitrophenyl (PNP) esters would be able to perform the Michael addition reaction without the need for a pendent secondary nucleophile to achieve catalytic turnover. This PNP turnover strategy has previously been employed to promote the enantioselective nitronate addition to α,β-unsaturated PNP esters;15a however, this process required nitroalkane to be used as a solvent or highly reactive silyl nitronates to be used as stoichiometric nucleophiles.15b,16 The use of dihydropyrazol-3-ones and 3-substituted oxindoles as N-heterocyclic enolates was also achieved through the aryloxide catalytic turnover.4 Herein, we report the HyperBTM-catalyzed addition of simple malonates and related derivatives to α,β-unsaturated aryl esters possessing a variety of electron-withdrawing β substituents under mild reaction conditions.
An examination to determine the most suitable reaction parameters began with an analysis of solvents and bases (Table 1). β-Trifluoromethyl α,β-unsaturated PNP ester 6 was reacted with dimethyl malonate 7 in the presence of 20 mol % HyperBTM 5 and 1 equiv of diisopropylethylamine in CH2Cl2 to provide the desired product with promising 62:38 enantiomeric ratio (er) (entry 1). Moving to more polar solvents, acetonitrile (MeCN) and N,N-dimethylformamide (DMF), provided higher yields (58 and 65%) and enantioselectivity (70:30 and 89:11) (entries 2 and 3). Gratifyingly, cooling the reaction in DMF to 0 °C increased the er to 90:10 (entry 4). Performing the reaction in the absence of external base at 0 °C (entry 5) provided high levels of enantioinduction (>99:1 er). Lowering the catalyst loading of compound 5 to 10 mol % (entry 6) resulted in similar enantioselectivity but a decreased yield. Attempting the reaction with (R)-BTM 9 furnished the desired Michael adduct in only 8% yield but with high 95:5 er (entry 7), while (S)-tetramisole 10 provided no desired product under the optimized reaction conditions (entry 8).
Table 1. Reaction Optimizationa.
| entry | solvent | catalyst (mol %) | base | T (°C) | yield (%) | er |
|---|---|---|---|---|---|---|
| 1 | CH2Cl2 | 5 (20) | iPr2NEt | rt | 30 | 62:38 |
| 2 | MeCN | 5 (20) | iPr2NEt | rt | 58 | 70:30 |
| 3 | DMF | 5 (20) | iPr2NEt | rt | 65 | 89:11 |
| 4 | DMF | 5 (20) | iPr2NEt | 0 | 73 | 90:10 |
| 5 | DMF | 5 (20) | 0 | 66 | >99:1 | |
| 6 | DMF | 5 (10) | 0 | 47 | >99:1 | |
| 7 | DMF | 9 (20) | 0 | 8 | 96:4 | |
| 8 | DMF | 10 (20) | 0 | 0 |
All yields are isolated yields after purification by column chromatography. Enantiomeric ratios are determined by high-performance liquid chromatography (HPLC) analysis on a chiral stationary phase. PNP = p-nitrophenyl.
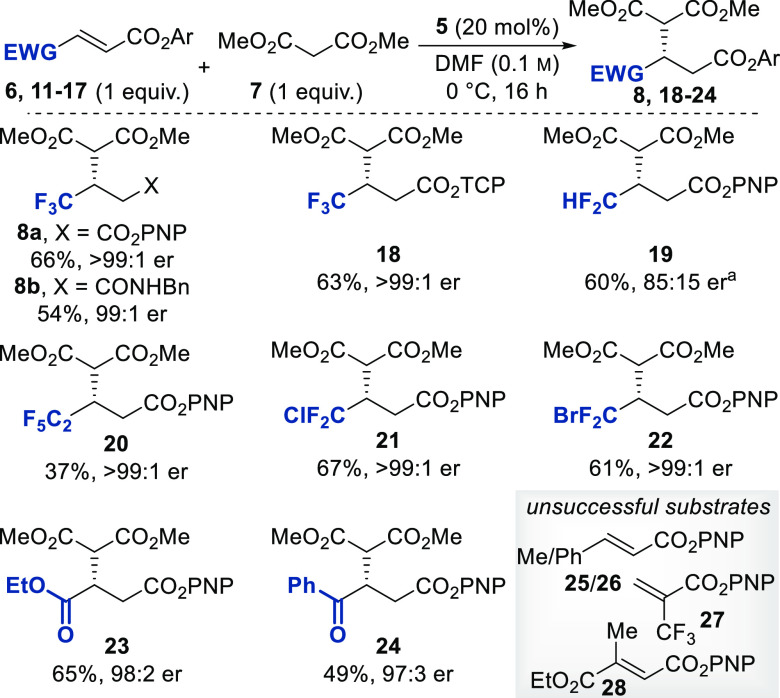
With the optimized conditions in hand, the steric and electronic parameters of the process were investigated. A variety of α,β-unsaturated aryl esters with electron-withdrawing β substituents were subjected to the optimized reaction conditions, with the results presented in Scheme 2.
Scheme 2. Scope and Limitations of Dimethyl Malonate Addition to α,β-Unsaturated Esters.
(2R,3S)-HyperBTM was used, and the product has the opposite absolute configuration to that shown.
All yields are isolated yields after purification by column chromatography. Enantiomeric ratios are determined by HPLC analysis on a chiral stationary phase. PNP, p-nitrophenyl; TCP, 2,4,6-trichlorophenyl.
Model β-trifluoromethyl α,β-unsaturated p-nitrophenyl ester 6 and β-trifluoromethyl α,β-unsaturated 2,4,6-trichlorophenyl (TCP) ester 11 performed similarly in the reaction conditions, providing 66 and 63% yields, respectively, with complete enantioselectivity (>99:1 er) in both cases. This suggests that p-nitrophenoxide and 2,4,6-trichlorophenoxide are both capable of facilitating catalyst turnover to propagate the reaction. The reaction can be performed on a gram scale (3.8 mmol) to provide compound 8a in a 60% yield and 99:1 er. To demonstrate the utility of p-nitrophenyl esters,15c compound 8a was derivatized in situ via the addition of benzylamine to produce the amide 8b. The absolute configuration within compound 22 was unambiguously determined by single-crystal X-ray analysis to be the S enantiomer, with the configuration of all other examples assigned by analogy. Extension of this protocol to the use of alternative β-substituted a,β-unsaturated PNP esters gave product yields ranging from 37 to 67% with high levels of enantioselectivity (from 85:15 to >99:1 er). The enantioselectivity was complete for all β-perhalogenated examples 20–22 (β-C2F5, β-CF2Cl, and β-CF2Br), with lower enantioselectivity observed for β-ester 23 (98:2 er), β-ketone 24 (97:3 er), and β-CHF219 (85:15 er). Because CHF2 functions as a bioisostere for an alcohol,18 the hydrogen-bonding abilities of these three substrates may contribute to the slightly diminished enantiomeric ratios. The ethyl ester 23 constitutes the first highly enantioselective addition of malonate to unsymmetric fumaric ester and proceeds with complete regioselectivity [20:1 regioselectivity ratio (rr)]. Additionally, the labile PNP ester offers the opportunity for facile differentiation between the two ester moieties of fumaric ester. Interestingly, full regioselectivity is also observed for the aryl ketone substrate 24. This highlights that the activated electrophilic α,β-unsaturated acyl isothiouronium intermediate can override the inherent selectivity of the parent molecule to provide exclusive Michael addition to the α,β-unsaturated PNP ester. Although promising, limitations of the methodology include the requirement of an activating β-electron-withdrawing substituent. Alternative substrates, such as β-methyl and β-phenyl α,β-unsaturated PNP esters 25 and 26 did not provide the desired Michael addition product, returning only starting material. The β,β-disubstituted fumaric ester 27 also provided no desired product, and the incorporation of a strongly withdrawing trifluoromethyl substituent in the α position for compound 28 instead of the β position was not supported.
The variability of the nucleophilic partner was then explored, commencing with the alkyl malonate series (Scheme 3). Gratifyingly, in addition to dimethyl malonate, dimethyl fluoromalonate provided the desired fluorinated tetrasubstituted carbon-containing product 37 in 82% yield and 98:2 er; however, dimethyl methylmalonate provided no desired product likely as a result of steric hindrance at the nucleophilic site. Ethyl, isopropyl, benzyl, and tert-butyl malonates were then examined and showed a decrease in yield correlating with increasing steric bulk within the nucleophile: ethyl (43%) 38, isopropyl (32%) 39, and tert-butyl (0%) malonates, while the 2-fluorobenzyl malonate and benzyl malonate gave the desired products 40 and 41 in 81 and 72% yields, respectively. The relatively high yields obtained when using benzyl malonates may result from π-stacking interactions with the α,β-unsaturated acyl ammonium complex. All examples 37–44 provided complete enantioselectivity of >99:1 er. With the performance of the reaction in MeCN and addition of catalytic diisopropylethylamine, malononitrile could be used, giving compound 42 in 48% yield with >99:1 er. Dithiomalonates are valuable substrates as a result of their ability to be converted into aldehydes and ketones more easily than their ester counterparts.19 Odorless S,S-bis(4-tert-butyl)benzyl)propanebis(thiolate) in MeCN with catalytic diisopropylethylamine gave the desired product 43 as a precipitate after 3 h in 58% yield and >99:1 er. To the best of our knowledge, these represent the first example of malononitrile or dithiomalonate addition in an enantioselective fashion to an α,β-unsaturated ester. Finally, β-ketoesters have been previously demonstrated to provide access to dihydropyrans in HyperBTM-catalyzed reactions of homoanhydrides. This reaction also proceeded smoothly with α,β-unsaturated PNP ester to provide compound 44 in 66% yield and 99:1 er. This example does not use the ability of p-nitrophenoxide to reform the ester, with turnover instead facilitated by the nascent enolate. In comparison to the use of a homoanhydride substrate, use of the ester starting material represents better atom economy with p-nitrophenol as the only byproduct and does not require an excess of the isothiouronium precursor.
Scheme 3. Scope and Limitations of the Addition of Nucleophiles to β-Trifluoromethyl α,β-Unsaturated PNP Ester.
(2R,3S)-HyperBTM was used, and the product has the opposite absolute configuration to that shown.
In MeCN, and 10% diisopropylethyl amine was added.
Complete in 5 h.
Complete in 3 h.
All yields are isolated yields after purification by column chromatography. Enantiomeric ratios are determined by HPLC analysis on a chiral stationary phase.
On the basis of prior investigations15b and in combination with density functional theory (DFT) studies [M06-2X/6-31G(d,p)/IEFPCM optimized, see Supporting Information for details] based on methodology introduced by Wang et al.,20 the proposed catalytic cycle for the transformation is illustrated in Scheme 4. Acylation of HyperBTM 5 by the α,β-unsaturated PNP ester and displacement of p-nitrophenoxide were calculated to be rate-limiting (ΔG⧧ = 52.8 kJ mol–1), forming the corresponding α,β-unsaturated isothiouronium ion pair. This electrophilic complex is then engaged by the malonate anion in a stereoselective Michael addition through transition state 49. Within this transition state, the isothiouronium adopts a s-cis conformation, with an stabilizing syn-coplanar 1,5-S···O chalcogen bond (no to σ*S–C)19−23 providing a conformational lock. To minimize 1,2 strain, the aryl stereodirecting unit adopts a pseudo-axial orientation, promoting facial selectivity in the Michael addition. This transition state is further stabilized by two weak CH···O interactions between ortho-C–H of the stereodirecting phenyl substituent and C–H α to positively charged nitrogen of acylated HyperBTM with the anionic malonate. Malonate addition to the electrophile is computed to be irreversible, and anti addition to the stereodirecting phenyl group is favored over the corresponding diastereomeric transition state by ΔΔG⧧ = 17.5 kJ mol–1 (Table S1, Supporting Information). This leads to preferential formation of the (S)-enantiomer of the product and is consistent with the level of enantioselectivity observed experimentally (>99:1 er). Resultant isothiouronium enolate is protonated, presumably by p-nitrophenol, providing p-nitrophenoxide necessary to complete catalytic turnover15 and generate the Michael addition product.
Scheme 4. Proposed Catalytic Cycle [M06-2X/6-31G(d,p)/IEFPCM Optimized]: TS to (S)-Product Enantiomer.
To conclude, the isothiourea-catalyzed addition of malonates and malonate derivatives to α,β-unsaturated p-nitrophenyl esters is disclosed. The reaction exploits the multifunctional nature of p-nitrophenoxide as a (1) leaving group, (2) proton shuttle, and (3) secondary nucleophile to provide catalytic turnover without the need for a pendent nucleophile within malonate. A variety of α,β-unsaturated aryl ester electrophiles containing β-electron-withdrawing substituents and malonate nucleophiles were tolerated in good yield and excellent enantioselectivity (typically >99:1 er). Exquisite regioselectivity was observed in examples with competing Michael addition reaction sites. Finally, DFT studies identified Michael addition of malonate to the chiral isothiouronium ion intermediate to be stereodetermining, consistent with experimental observations.
Acknowledgments
Michael Bühl thanks EaStCHEM and the School of Chemistry for support. Computations were performed on a local high-performance computing (HPC) cluster maintained by Dr. H. Früchtl. Gregory R. Boyce is grateful to Florida Gulf Coast University for a sabbatical award.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.2c01486.
Full experimental procedures, characterization data, nuclear magnetic resonance (NMR) spectra, and HPLC chromatograms (PDF)
xyz coordinates of all optimized DFT structures in the manuscript (TXT)
The research data supporting this publication can be accessed at https://doi.org/10.17630/807cc4de-3e6c-43d6-a091-54f073849543.
The authors declare no competing financial interest.
Supplementary Material
References
- For recent reviews on the enantioselective organocatalytic Michael reaction, see; a Parella R.; Jakkampudi S.; Zhao J. C.-G. Recent Applications of Asymmetric Organocatalytic Methods in Total Synthesis. ChemistrySelect 2021, 6, 2252–2280. 10.1002/slct.202004196. [DOI] [Google Scholar]; b Scheffler U.; Mahrwald R. Recent Advances in Organocatalytic Methods for Asymmetric C–C Bond Formation. Chem.—Eur. J. 2013, 19, 14346–14396. 10.1002/chem.201301996. [DOI] [PubMed] [Google Scholar]; c Zhang Y.; Wang W. Recent Advances in Organocatalytic Asymmetric Michael Reactions. Catal. Sci. Technol. 2012, 2, 42–53. 10.1039/C1CY00334H. [DOI] [Google Scholar]
- Allgäuer D. S.; Jangra H.; Asahara H.; Li Z.; Chen Q.; Zipse H.; Ofial A. R.; Mayr H. Quantification and Theoretical Analysis of the Electrophilicities of Michael Acceptors. J. Am. Chem. Soc. 2017, 139 (38), 13318–13329. 10.1021/jacs.7b05106. [DOI] [PubMed] [Google Scholar]
- Gatzenmeier T.; Kaib P. S. J.; Lingnau J. B.; Goddard R.; List B. The Catalytic Asymmetric Mukaiyama-Michael Reaction of Silyl Ketene Acetals with α,β-Unsaturated Methyl Esters. Angew. Chem., Int. Ed. 2018, 57, 2464–2468. 10.1002/anie.201712088. [DOI] [PubMed] [Google Scholar]
- Shu C.; Liu H.; Slawin A. M. Z.; Carpenter-Warren C.; Smith A. D. Isothiourea-Catalysed Enantioselective Michael Addition of N-Heterocyclic Pronucleophiles to α,β-Unsaturated Aryl Esters. Chem. Sci. 2020, 11, 241–247. 10.1039/C9SC04303A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Takaya Y.; Senda T.; Kurushima H.; Ogasawara M.; Hayashi T. Rhodium-catalyzed Asymmetric 1,4-Addition of Arylboron Reagents to α,β-Unsaturated Esters. Tetrahedron: Asymmetry 1999, 10, 4047–4056. 10.1016/S0957-4166(99)00417-6. [DOI] [Google Scholar]; b Sakuma S.; Sakai M.; Itooka R.; Miyaura N. Asymmetric Conjugate 1,4-Addition of Arylboronic Acids to α,β-Unsaturated Esters Catalyzed by Rhodium(I)/(S)-Binap. J. Org. Chem. 2000, 65, 5951–5955. 10.1021/jo0002184. [DOI] [PubMed] [Google Scholar]
- a Yang J.; Farley A. J. M.; Dixon D. J. Enantioselective Bifunctional Iminophosphorane Catalyzed Sulfa-Michael Addition of Alkyl Thiols to Unactivated β-Substituted-α,β-Unsaturated Esters. Chem. Sci. 2017, 8, 606–610. 10.1039/C6SC02878K. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Lin Y.; Hirschi W. J.; Kunadia A.; Paul A.; Ghiviriga I.; Abboud K. A.; Karugu R. W.; Vetticatt M. J.; Hirschi J. S.; Seidel D. A Selenourea-Thiourea Brønsted Acid Catalyst Facilitates Asymmetric Conjugate Additions of Amines to α,β-Unsaturated Esters. J. Am. Chem. Soc. 2020, 142, 5627–5635. 10.1021/jacs.9b12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harutyunyan S. R.; den Hartog T.; Geurts K.; Minnaard A. J.; Feringa B. L. Catalytic Asymmetric Conjugate Addition and Allylic Alkylation with Grignard Reagents. Chem. Rev. 2008, 108, 2824–2852. 10.1021/cr068424k. [DOI] [PubMed] [Google Scholar]
- a Sakamoto M.; Kaneko T.; Orito Y.; Nakajima M. Enantioselective Michael Addition of a Malonic Ester to a Maleic Ester Catalyzed by Lithium Binaphtholate. Synlett 2016, 27, 2477–2480. 10.1055/s-0035-1562691. [DOI] [Google Scholar]; b Sakamoto M.; Kaneko T.; Orito Y.; Shimoda Y.; Nakajima M. Lithium Binaphtholate-Catalyzed Michael Reaction of Malonates with Maleates and Its Application to the Enantioselective Synthesis of Tricarboxylic Acid Derivatives. Chem. Pharm. Bull. 2019, 67, 452–460. 10.1248/cpb.c18-00993. [DOI] [PubMed] [Google Scholar]
- Bappert E.; Müller P.; Fu G. C. Asymmetric [3 + 2] Annulations Catalyzed by a Planar-Chiral Derivative of DMAP. Chem. Commun. 2006, 2604–2606. 10.1039/B603172B. [DOI] [PubMed] [Google Scholar]
- a Vellalath S.; Van K. N.; Romo D. Direct Catalytic Asymmetric Synthesis of N-Heterocycles from Commodity Acid Chlorides by Employing α,β-Unsaturated Acylammonium Salts. Angew. Chem., Int. Ed. 2013, 52, 13688–13693. 10.1002/anie.201306050. [DOI] [PubMed] [Google Scholar]; b Kang G.; Yamagami M.; Vellalath S.; Romo D. Enantioselective Synthesis of Medium-Sized Lactams via Chiral α,β-Unsaturated Acylammonium Salts. Angew. Chem., Int. Ed. 2018, 57, 6527–6531. 10.1002/anie.201802483. [DOI] [PubMed] [Google Scholar]
- Bitai J.; Westwood M. T.; Smith A. D. α,β-Unsaturated Acyl Ammonium Species as Reactive Intermediates in Organocatalysis: An Update. Org. Biomol. Chem. 2021, 19, 2366–2384. 10.1039/D0OB02208J. [DOI] [PubMed] [Google Scholar]
- Vellalath S.; Romo D. Asymmetric Organocatalysis: The Emerging Utility of α,β-Unsaturated Acylammonium Salts. Angew. Chem., Int. Ed. 2016, 55, 13934–13943. 10.1002/anie.201602217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson E. R. T.; Fallan C.; Simal C.; Slawin A. M. Z.; Smith A. D. Anhydrides as α,β-Unsaturated Acyl Ammonium Precursors: Isothiourea-Promoted Catalytic Asymmetric Annulation Processes. Chem. Sci. 2013, 4, 2193–2200. 10.1039/c3sc50199j. [DOI] [Google Scholar]
- Liu G.; Shirley M. E.; Van K. N.; McFarlin R. L.; Romo D. Rapid Assembly of Complex Cyclopentanes employing Chiral, α,β-Unsaturated Acylammonium Intermediates. Nat. Chem. 2013, 5, 1049–1057. 10.1038/nchem.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For selected examples of the aryloxide rebound strategy, see; a Schwarz K. J.; Amos J. L.; Klein J. C.; Do D. T.; Snaddon T. N. Uniting C1-Ammonium Enolates and Transition Metal Electrophiles via Cooperative Catalysis: The Direct Asymmetric α-Allylation of Aryl Acetic Acid Esters. J. Am. Chem. Soc. 2016, 138, 5214–5217. 10.1021/jacs.6b01694. [DOI] [PubMed] [Google Scholar]; b Matviitsuk A.; Greenhalgh M. D.; Antunez D. B.; Slawin A. M. Z.; Smith A. D. Aryloxide-Facilitated Catalyst Turnover in Enantioselective α,β-Unsaturated Acyl Ammonium Catalysis. Angew. Chem., Int. Ed. 2017, 56, 12282–12287. 10.1002/anie.201706402. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Fyfe J. W. B.; Kabia O. M.; Pearson C. M.; Snaddon T. N. Si-Directed Regiocontrol in Asymmetric Pd-Catalyzed Allylic Alkylations using C1-Ammonium Enolate Nucleophiles. Tetrahedron 2018, 74, 5383–5391. 10.1016/j.tet.2018.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Scaggs W. R.; Snaddon T. N. Enantioselective α-Allylation of Acyclic Esters Using B(pin)-Substituted Electrophiles: Independent Regulation of Stereocontrol Elements through Cooperative Pd/Lewis Base Catalysis. Chem.—Eur. J. 2018, 24, 14378–14381. 10.1002/chem.201803543. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Scaggs W. R.; Scaggs T. D.; Snaddon T. N. An Enantioselective Synthesis of α-Alkylated Pyrroles via cooperative Isothiourea/Palladium Catalysis. Org. Biomol. Chem. 2019, 17, 1787–1790. 10.1039/C8OB02600A. [DOI] [PMC free article] [PubMed] [Google Scholar]; For a review of this reactivity, see; f Godemert J.; Oudeyer S.; Levacher V. Chiral Ammonium Aryloxides: Efficient Multipurpose Basic Organocatalysts. ChemCatChem 2016, 8, 74–85. 10.1002/cctc.201500616. [DOI] [Google Scholar]
- Matviitsuk A.; Greenhalgh M. D.; Taylor J. E.; Nguyen X. B.; Cordes D. B.; Slawin A. M. Z.; Lupton D. W.; Smith A. D. Unanticipated Silyl Transfer in Enantioselective α,β-Unsaturated Acyl Ammonium Catalysis using Silyl Nitronates. Org. Lett. 2020, 22, 335–339. 10.1021/acs.orglett.9b04404. [DOI] [PubMed] [Google Scholar]
- a Erickson J. A.; McLoughlin J. I. Hydrogen Bond Donor Properties of the Difluoromethyl Group. J. Org. Chem. 1995, 60, 1626–1631. 10.1021/jo00111a021. [DOI] [Google Scholar]; b Sessler C. D.; Rahm M.; Becker S.; Goldberg J. M.; Wang F.; Lippard S. J. CF2H, a Hydrogen Bond Donor. J. Am. Chem. Soc. 2017, 139, 9325–9332. 10.1021/jacs.7b04457. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Zafrani Y.; Sod-Moriah G.; Yeffet D.; Berliner A.; Amir D.; Marciano D.; Elias S.; Katalan S.; Ashkenazi N.; Madmon M.; Gershonov E.; Saphier S. CF2H, a Functional Group-Dependent Hydrogen-Bond Donor: Is It a More or Less Lipophilic Bioisostere of OH, SH, and CH3?. J. Med. Chem. 2019, 62, 5628–5637. 10.1021/acs.jmedchem.9b00604. [DOI] [PubMed] [Google Scholar]
- a Tokuyama H.; Yokoshima S.; Yamashita T.; Fukuyama T. A Novel Ketone Synthesis by a Palladium-Catalyzed Reaction of Thiol Esters and Organozinc Reagents. Tetrahedron Lett. 1998, 39, 3189–3192. 10.1016/S0040-4039(98)00456-0. [DOI] [Google Scholar]; b Liebeskind L. S.; Srogl J. Thiol Ester–Boronic Acid Coupling. A Mechanistically Unprecedented and General Ketone Synthesis. J. Am. Chem. Soc. 2000, 122, 11260–11261. 10.1021/ja005613q. [DOI] [Google Scholar]; c Miyazaki T.; Han-ya Y.; Tokuyama H.; Fukuyama T. New Odorless Protocols for the Synthesis of Aldehydes and Ketones from Thiol Esters. Synlett 2004, 477–480. 10.1055/s-2004-815427. [DOI] [Google Scholar]; d Mori Y.; Seki M. A Practical Synthesis of Multifunctional Ketones through the Fukuyama Coupling Reaction. Adv. Synth. Catal. 2007, 349, 2027–2038. 10.1002/adsc.200600610. [DOI] [Google Scholar]; e Cherney A. H.; Reisman S. E. Pd-Catalyzed Fukuyama Cross-Coupling of Secondary Organozinc Reagents for the Direct Synthesis of Unsymmetrical Ketones. Tetrahedron 2014, 70, 3259–3265. 10.1016/j.tet.2013.11.104. [DOI] [Google Scholar]
- Wang C.; Li S.-J.; Zhang Q.-C.; Wei D.; Ding L. Insights into isothiourea-catalyzed asymmetric [3 + 3] annulation of alpha,beta-unsaturated aryl esters with 2-acylbenzazoles: Mechanism, origin of stereoselectivity and switchable chemoselectivity. Catal. Sci. Technol. 2020, 10, 3664–3669. 10.1039/D0CY00295J. [DOI] [Google Scholar]
- For a review of 1,5-chalcogen bonding in organoselenium chemistry, see; a Mukherjee A. J.; Zade S. S.; Singh H. B.; Sunoj R. B. Organoselenium Chemistry: Role of Intramolecular Interactions. Chem. Rev. 2010, 110, 4357–4416. 10.1021/cr900352j. [DOI] [PubMed] [Google Scholar]; For selected examples in catalysis, see; b Fujita K.; Iwaoka M.; Tomoda S. Chem. Lett. 1994, 23, 923–926. 10.1246/cl.1994.923. [DOI] [Google Scholar]; c Fujita K. I.; Murata K.; Iwaoka M.; Tomoda S. Asymmetric intramolecular selenoetherification and selenolactonization using an optically active diaryl diselenide derived from d-mannitol. J. Chem. Soc., Chem. Commun. 1995, 1641–1642. 10.1039/c39950001641. [DOI] [Google Scholar]; d Fujita K.-i.; Murata K.; Iwaoka M.; Tomoda S. Asymmetric methoxyselenenylation of olefins using an optically active diaryl diselenide derived from d-mannitol. Tetrahedron Lett. 1995, 36, 5219–5222. 10.1016/0040-4039(95)00976-J. [DOI] [Google Scholar]; e Wirth T. Asymmetric Reaction of Arylalkenes with Diselenides. Angew. Chem., Int. Ed. 1995, 34, 1726–1728. 10.1002/anie.199517261. [DOI] [Google Scholar]; f Wirth T.; Häuptli S.; Leuenberger M. Catalytic Asymmetric Oxyselenenylation–Elimination Reactions using Chiral Selenium Compounds. Tetrahedron: Asymmetry 1998, 9, 547–550. 10.1016/S0957-4166(98)00031-7. [DOI] [Google Scholar]; g Tiecco M.; Testaferri L.; Santi C.; Tomassini C.; Marini F.; Bagnoli L.; Temperini A. New Nitrogen Containing Chiral Diselenides: Synthesis and Asymmetric Addition Reactions to Olefins. Tetrahedron: Asymmetry 2000, 11, 4645–4650. 10.1016/S0957-4166(00)00469-9. [DOI] [Google Scholar]; h Tiecco M.; Testaferri L.; Santi C.; Tomassini C.; Marini F.; Bagnoli L.; Temperini A. Preparation of a New Chiral Non-Racemic Sulfur-Containing Diselenide and Applications in Asymmetric Synthesis. Chem.—Eur. J. 2002, 8, 1118–1124. . [DOI] [PubMed] [Google Scholar]
- For an early theoretical investigation of chalcogen bonding, see; a Bleiholder C.; Gleiter R.; Werz D. B.; Köppel H. Theoretical Investigations on Heteronuclear Chalcogen-Chalcogen Interactions: On the Nature of Weak Bonds between Chalcogen Centers. Inorg. Chem. 2007, 46, 2249–2260. 10.1021/ic062110y. [DOI] [PubMed] [Google Scholar]; For a recent review, see; b Kolb S.; Oliver G. A.; Werz D. B. Chemistry Evolves, Terms Evolve, but Phenomena do Not Evolve: From Chalcogen-Chalcogen Interactions to Chalcogen Bonding. Angew. Chem., Int. Ed. 2020, 59, 22306–22310. 10.1002/anie.202007314. [DOI] [PubMed] [Google Scholar]
- For discussions on the S···O interaction in isothiourea catalysis, see; a Birman V. B.; Li X.; Han Z. Nonaromatic Amidine Derivatives as Acylation Catalysts. Org. Lett. 2007, 9, 37–40. 10.1021/ol0623419. [DOI] [PubMed] [Google Scholar]; b Liu P.; Yang X.; Birman V. B.; Houk K. N. Origin of Enantioselectivity in Benzotetramisole-Catalyzed Dynamic Kinetic Resolution of Azlactones. Org. Lett. 2012, 14, 3288–3291. 10.1021/ol301243f. [DOI] [PubMed] [Google Scholar]; c Abbasov M. E.; Hudson B. M.; Tantillo D. J.; Romo D. Acylammonium Salts as Dienophiles in Diels–Alder/Lactonization Organocascades. J. Am. Chem. Soc. 2014, 136, 4492–4495. 10.1021/ja501005g. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Robinson E. R. T.; Walden D. M.; Fallan C.; Greenhalgh M. D.; Cheong P. H.-Y.; Smith A. D. Chem. Sci. 2016, 7, 6919–6927. 10.1039/C6SC00940A. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Greenhalgh M. D.; Smith S. M.; Walden D. M.; Taylor J. E.; Brice Z.; Robinson E. R. T.; Fallan C.; Cordes D. B.; Slawin A. M. Z.; Richardson H. C.; Grove M. A.; Cheong P. H.-Y.; Smith A. D. A C=O···Isothiouronium Interaction Dictates Enantiodiscrimination in Acylative Kinetic Resolutions of Tertiary Heterocyclic Alcohols. Angew. Chem., Int. Ed. 2018, 57, 3200–3206. 10.1002/anie.201712456. [DOI] [PubMed] [Google Scholar]; f Young C. M.; Elmi A.; Pascoe D. J.; Morris R. K.; McLaughlin C.; Woods A. M.; Frost A. B.; de la Houpliere A.; Ling K. B.; Smith T. K.; Slawin A. M. Z.; Willoughby P. H.; Cockroft S. L.; Smith A. D. The Importance of 1,5-Oxygen···Chalcogen Interactions in Enantioselective Isochalcogenourea Catalysis. Angew. Chem., Int. Ed. 2020, 59, 3705–3710. 10.1002/anie.201914421. [DOI] [PubMed] [Google Scholar]; For a discussion on the origin of these interactions, see; g Pascoe D. J.; Ling K. B.; Cockroft S. L. The Origin of Chalcogen-Bonding Interactions. J. Am. Chem. Soc. 2017, 139, 15160–151. 10.1021/jacs.7b08511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.