Abstract
The marine phage ΦHSIC has been previously reported to enter into a lysogenic relationship with its host, HSIC, identified as Listonella pelagia. This phage produces a variety of plaques on its host, including turbid and haloed plaques, from which lysogens were previously isolated. These lysogens were unstable during long-term storage at −80° C and were lost. When HSIC was reinfected with phage ΦHSIC, pseudolysogen-like interactions between the phage and its host were observed. The cells (termed HSIC-2 or HSIC-2e) produced high viral titers (1011 ml−1) in the absence of inoculating phage and yet reached culture densities of nearly 109 ml−1. Prophages were not induced by mitomycin C or the polyaromatic hydrocarbon naphthalene in cells harboring such infections. However, such cells were homoimmune to superinfection. Colonies hybridized strongly with a gene probe from a 100-bp fragment of the ΦHSIC genome, while the host did not. Analysis of chromosomal DNA preparations suggested the presence of a chromosomally integrated prophage. Phage adsorption experiments suggested that HSIC-2 was adsorption impaired. Because of the chromosomal prophage integration and homoimmunity, we interpret these results to indicate that ΦHSIC establishes a lysogenic relationship with its host that involves an extremely high level of spontaneous induction. This could be caused by a weak repressor of phage production. Additionally, poor phage adsorption of HSIC-2 compared to the wild type probably helped maintain this pseudolysogen-like relationship. In many ways, pseudolysogenic phage-host interactions may provide a paradigm for phage-host interactions in the marine environment.
The study of marine virus-host interactions is essential in order to understand the role that viruses play in the marine environment. Viruses can interact with bacterial host cells in at least three distinct ways, resulting in lytic, lysogenic, and pseudolysogenic relationships. The majority of the research on virus-host interactions has focused on lytic infection, although studies of lysogenic and pseudolysogenic interactions are becoming more prevalent (4, 5, 9, 10, 11, 12, 18, 22, 23, 25).
A lysogenic infection occurs when the viral genome becomes integrated into one of the host cellular replicons (chromosome, plasmid, or another phage genome). The prophages replicate along with the host cell and are passed onto daughter cells. Freifelder (7) claimed that greater than 90% of known bacteriophages are temperate, while Ackermann and Dubow (1) suggested that 50% of 1,200 bacterial strains contained inducible prophages. Prophages remain dormant until the lytic cycle is induced by any number of physical or chemical agents, such as mitomycin C, hydrogen peroxide, polyaromatic hydrocarbons, UV radiation, temperature, and pressure (9, 22, 25). The expression of lytic genes following damage to the host DNA by any of the above mechanisms is a viral strategy that has evolved to ensure viral propagation when conditions for host survival are compromised (16).
Little is known about induction mechanisms in marine lysogens. In coliphage lambda, induction activates the RecA protein, which subsequently cleaves and inactivates the repressor to initiate the lytic process (8). It is hypothesized that the RecA protein is activated by binding to damaged DNA during DNA repair (1). The introduction of an inducing agent to a population of lysogens may result in the activation of the RecA protein, ultimately resulting in the active replication of the viral genome and the subsequent release of viral particles through lysis of the host cell.
Lysogens gain specific advantages from their relationship with phage that improve their overall fitness. These effects may occur through unspecified mechanisms or the process of conversion, whereby prophage genes are expressed in the lysogens. Conversion can result in expanded metabolic capabilities, antibiotic resistance, and toxin production, but usually always in homoimmunity (13). Homoimmunity provides the resistance to superinfection by the same or similar strains of phage. Immunity observed in the halophilic archaebacterium Halobacterium salinarium to its phage ΦH is mediated by a phage repressor gene, rep (20).
In order for virulent phage to replicate and persist in the environment, the rate of host-phage encounters and production must exceed the rate of virus destruction and inactivation. Conversely, the persistence of temperate phages in the environment is dependent not on host cell density but on a certain percentage of sensitive host cells and the occasional induction and lysis of host cells (28). Freifelder (6) suggested that lysogeny might be a viral survival strategy to endure periods of low host density during nutrient starvation. Wilson and Mann (27) presented evidence that low nutrient concentrations coupled with a high virus/host cell ratio tended to favor lysogeny. This strategy would ensure that the genetic material of the temperate phage is passed on through host cell replication and division.
The prevalence of lysogeny in the marine environment is a topic of considerable debate. Jiang and Paul (9) found that 43% of the bacterial population from a series of different marine environments contained prophage that were inducible by mitomycin C and UV light. Cochran et al. (5) found that two-thirds of the marine environments sampled in the Gulf of Mexico contained inducible prophage. A seasonal investigation into the abundance of lysogens in Tampa Bay, Fla., revealed that 52.2% of the samples displayed prophage induction (4). On the other side of the debate, evidence provided by Weinbauer and Suttle (24) and Wilcox and Fuhrman (26) indicated that lysogeny is not an important source of phage production or bacterial mortality in coastal waters. Weinbauer and Suttle (24) found that an average of only 3% of total bacterial mortality resulted from the induction of lysogenic cells. Further evidence has suggested that lysogeny is more prevalent in oligotrophic waters than in coastal waters (22, 24, 26).
Another poorly studied interaction between bacteria and viruses that may be important in the marine environment is pseudolysogeny. Pseudolysogeny has been described by Ackermann and Dubow (1) as a phenomenon where there is a constant production of phage in the presence of high host cell abundance. That is, phage lysis results not in culture death but rather in a state whereby a high abundance of phage coexists with exponential host growth. This might be the result of a mixture of sensitive and resistant host cells and/or a mixture of temperate and virulent phages. Following infection, bacteriophage can either enter a dormant intracellular phase or proceed with lytic infection (28). In this respect, pseudolysogeny resembles true lysogeny. Unlike in true lysogeny, however, the phage genome does not integrate into host cellular replicons. Ripp and Miller (17, 18) suggested that pseudolysogeny was an environmental condition in which starved bacterial cells coexist in an unstable relationship with infective viruses. Under these conditions, host cells do not provide enough energy in order for phage to enter into a true lysogenic or lytic condition. Phage become either virulent or temperate upon the addition of sufficient nutrient concentrations (18). Although this hypothesis for pseudolysogenic existence appears to be a plausible explanation for the sustained production of viroplankton under conditions of low host density and nutrient depletion, Moebus (14) did not find that the release of phage in starving bacteria was delayed until sufficient nutrients became available.
We have investigated the relationship between ΦHSIC and its host, HSIC, a marine bacterium isolated from Mamala Bay, Oahu, Hawaii, in order to determine if the phage enters into a lysogenic or pseudolysogenic state with its host. HSIC is most closely related to Listonella pelagia, and the phage belongs to the Siphoviridae family. We reported previously that the interaction between ΦHSIC and its host could be described as lysogenic due to the confirmation of homoimmunity and phage production in the presence of mitomycin C (12). However, this original lysogen was unstable and was lost. Attempts to reestablish this interaction resulted in a pseudolysogen-like relationship. This relationship is inherently unstable and is characterized by high host abundance concurrent with a high level of spontaneous induction.
MATERIALS AND METHODS
Lysogenization of HSIC-2 and HSIC-2e.
The lysogen described as L-HSIC by Jiang et al. (12) was unstable upon freezing at −80°C, and was lost. We therefore attempted to reestablish the lysogenic interaction between the HSIC host and ΦHSIC. Reestablishment of the lysogenic interaction by the turbid-plaque method yielded strain HSIC-2. One milliliter of HSIC host was inoculated into 10 ml of the artificial seawater nutrient broth ASWJP+PY (15). The culture was incubated at 28°C for 2 h with shaking. One hundred microliters of phage lysate was diluted (10−1 to 10−8) and added to a melted-agar-overlay tube with 1 ml of HSIC host. The mixture was poured onto a bottom-agar plate and incubated at 28°C overnight. Following incubation, turbid plaques were lifted from the plate using a sterile Pasteur pipette and placed in 500 μl of ASWJP+PY nutrient broth. One hundred microliters of this solution was spread plated onto an ASWJP+PY agar plate and incubated overnight at 28°C. Representative colonies of all morphologies were streaked for isolation.
Reestablishment of the lysogenic interaction through the plate survival method yielded strain HSIC-2e. An overlay plate was prepared with undiluted phage lysate by the same procedure as that used above. Completely clear plates at 24 h of incubation indicated lysis of sensitive host cells, yet a few resistant colonies appeared within 2 days. The colonies were isolation streaked to purity. In order to screen for the presence of phage, random colonies were resuspended in 500 μl of sterile artificial seawater, ASWJP. Cells were pelleted, and a wet mount was created with 12.5 μl of the supernatant. The sample was stained for 12 min with 2.5 μl of a 1:10 dilution of the nucleic acid stain SYBER gold (Molecular Probes, Eugene, Oreg.). Slides prepared in this fashion were examined for the presence of phage using epifluorescence microscopy.
Assessment of colony morphology.
Colonies of HSIC, HSIC-2, and HSIC-2e were restreaked for isolation on fresh ASWJP+PY plates once a month. Plates were incubated at 28°C overnight. Colony morphology was noted as either smooth (typically indicative of the wild type) or crinkled and sticky (typically indicative of HSIC-2 and HSIC-2e).
Growth curves for HSIC host, HSIC-2, and HSIC-2e.
Cells of overnight cultures of HSIC-2 and HSIC-2e were harvested at 3,020 × g in a Beckman centrifuge and washed two times with sterile ASWJP in order to remove unattached phage. Cultures were diluted with ASWJP+PY to reach an initial optical density (OD) at 600 nm of 0.05 on the spectrophotometer. OD measurements were taken hourly for 10 h and once again at 24 h. Overlay plates were prepared as described above using 100 μl of diluted HSIC-2 and HSIC-2e cultures. To assess phage production by the cultures, HSIC-2 and HSIC-2e were diluted from 10−1 to 10−8 for use in overlays, with HSIC serving as the host. Plates were incubated at 28°C overnight, and PFU were enumerated on the following day.
Attempted induction of HSIC-2e with mitomycin C.
Cells from an overnight culture of HSIC-2e were harvested and washed as described above and diluted in fresh ASWJP+PY to an OD at 600 nm of 0.05. When the culture reached an OD at 600 nm of 0.6 to 0.8, mitomycin C (0.5 μg ml−1) was added. OD readings were taken hourly. Overlay plates were prepared as described above with dilutions of HSIC-2e (10−1 to 10−8). Plates were incubated at 28°C overnight, and PFU were enumerated on the following day.
Attempted induction of HSIC-2e with naphthalene.
Ten milligrams of the aromatic hydrocarbon naphthalene (Aldrich, Milwaukee, Wis.) was dissolved in 10 ml of benzene to make a 1-mg ml−1 stock solution. The organic solvent was evaporated overnight in a fume hood after the desired amount was added to sterile amber glass bottles. Cells from an overnight culture were harvested, washed, and diluted as described above. When the culture reached an OD of 0.6 to 0.8 at 600 nm, the correct volume of culture was added to the chemical-containing amber bottles to achieve a final concentration of 50 μg ml−1. OD readings were taken hourly. Overlay plates were prepared as described above with dilutions of HSIC-2e (10−1 to 10−8). Plates were incubated at 28°C overnight, and PFU were enumerated on the following day.
Homoimmunity of HSIC-2e.
An overlay plate was prepared as described above using 1 ml of HSIC-2e log-phase culture or HSIC to create the lawn. The plate was incubated at 28°C overnight. One microliter of purified phage lysate was dotted onto the plate, and the plate was incubated at 28°C overnight and examined for phage formation.
Adsorption of phage to HSIC-2 and the HSIC host.
Overnight cultures (0.5 ml)of HSIC-2 and HSIC host were inoculated into 50 ml of ASWJP+PY and incubated at 28°C with shaking until an OD at 600 nm of 0.3 was reached. Cells were harvested at 3,020 × g in a Beckman centrifuge for 15 min at 4°C. Cells were washed two times with sterile ASWJP. Cells were diluted with ASWJP+PY to 2 × 106. One-milliliter samples of each culture were taken, the cells were pelleted, and the supernatant was diluted with sterile ASWJP. Overlays were prepared with 100 μl of the diluted supernatant. Phage was added to the cultures at a final concentration of 106 ml−1. Samples were taken at 2-min intervals and prepared for use in overlays as described above. The protocol developed by Adams (2) was used in order to determine adsorption kinetics. The adsorption constant (time−1) was determined through multiplication of the slope of a semilog plot of the percentage of free viruses remaining in the supernatant versus time by 2.3 (21).
Colony lifts of HSIC-2e and the HSIC host.
Colony lifts of the HSIC host and HSIC-2e were prepared using 100-mm-diameter MAGNA charged nylon filters (Micron Separations, Inc., Westboro, Mass.) as described by Jiang and Paul (11) with minor modifications. DNA was cross-linked to the filters using a UV cross-linker (FB-UVXL-1000; Fisher Scientific). The colony lifts were probed for the presence of phage using a 100-bp gene probe derived from an AccI restriction fragment of the phage genome as described by Jiang and Paul (11). DNA was hybridized at 42°C overnight. Filters were washed at high stringencies as described by Jiang and Paul (11).
Integration of the ΦHSIC genome in cellular replicons.
Chromosomal DNA was extracted from the HSIC host and HSIC-2e using the protocol specified in the Promega (Madison, Wis.) genomic DNA purification kit. Plasmid DNA was extracted from washed cells using a modified alkaline lysis (miniprep) extraction method (19). Phage DNA was extracted using the protocol specified in the Promega lambda DNA purification kit. Chromosomal and plasmid DNA extracts from the HSIC host and HSIC-2e and phage DNA were digested with HindIII for 3 h at 37°C. Digested and undigested DNA preparations were run on a 1% agarose gel at 70 V. DNA preparations were Southern transferred to a charged nylon filter using a standard protocol (19). The DNA was cross-linked to the filter using a UV cross-linker (FB-UVXL-1000). The filter was probed for the presence of phage with a 100-bp gene probe derived from ΦHSIC as previously described (11).
RESULTS
Growth and phage production by lysogen HSIC-2e and the uninfected HSIC host.
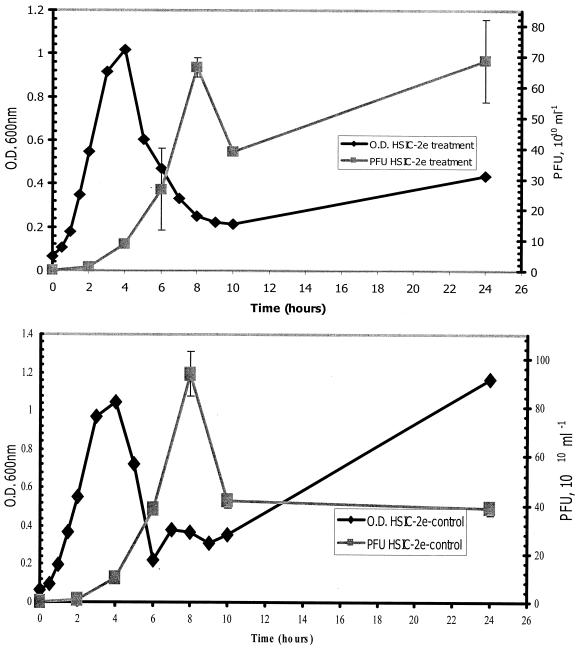
The results of growth experiments with HSIC-2e and wild-type HSIC are shown in Fig. 1. The attempt to reestablish the lysogenic interaction between the HSIC host and ΦHSIC through the plate survival method resulted in cultures that produced high numbers of both host cells and phage. No phage were produced during normal growth of the uninfected HSIC host (Fig. 1A). HSIC-2e grew exponentially for approximately 4 h, followed by a sharp decrease in host cell abundance and an increase in phage production to approximately 9 × 1011 phage (Fig. 1B). The initial growth rates for both HSIC-2e and the HSIC host were nearly identical (the generation time was 0.3 h for both the wild type and the lysogen).
FIG. 1.
Growth of the HSIC host (A) and growth and phage production of the lysogen HSIC-2e (B). Error bars indicate the range of PFU for duplicate plates.
Colony morphology.
The colony morphology of HSIC differed from the colony morphologies of HSIC-2 and HSIC-2e. The colonies of the host were smooth and round and did not appear to possess any stickiness. In contrast, the colonies of HSIC-2 and HSIC-2e had a crinkled appearance and were sticky. The crinkled and sticky characteristics of HSIC-2 and HSIC-2e were indicative of the presence of phage within the colonies. These characteristics were lost when segregation of the phage occurred following serial isolation streaking. The colony morphologies of HSIC-2 and HSIC-2e then reverted to that of the uninfected host.
Homoimmunity of HSIC-2e.
When phage ΦHSIC was spotted on lysogen HSIC-2e, no clearing of the bacterial lawn was observed following 12 h of incubation, indicating homoimmunity of HSIC-2e to the phage. Control overlays with HSIC showed a high efficacy of plaquing when challenged with ΦHSIC.
Attempted prophage induction of HSIC-2e with mitomycin C.
The results of attempts to induce prophage from HSIC-2e are shown in Fig. 2. The presence or absence of induction by mitomycin C was measured through the production of PFU by HSIC-2e. HSIC-2e treated with mitomycin C produced 6.6 × 1011 PFU ml−1 at 8 h and then showed a decrease in PFU to 3.9 × 1011 at 10 h. A second increase in PFU was observed at 24 h, reaching a peak value of 6.9 × 1011 PFU ml−1. HSIC-2e left untreated (control) experienced a peak in phage production at 8 h, producing 9.4 × 1011 PFU ml−1. The control also underwent a decrease in phage production at 10 h, producing 4.2 × 1011 PFU ml−1, with phage production remaining approximately the same (3.9 × 1011 PFU ml−1) at 24 h. Since phage were produced at high numbers in the presence or absence of mitomycin C, these results indicate that a significant induction of prophage did not occur. Previous attempts at induction of HSIC-2 resulted in no difference in phage production (data not shown).
FIG. 2.
Growth and phage production of HSIC-2e in the presence (top) or absence (bottom) of mitomycin C. Error bars indicate the range of PFU for duplicate plates.
Attempted prophage induction of HSIC-2e with naphthalene.
HSIC-2e was either treated or left untreated as a control with the polyaromatic hydrocarbon naphthalene as an alternate induction method. The HSIC host was also either treated or left untreated as a negative control with naphthalene. Treatment of HSIC-2e with naphthalene resulted in a rapid decrease in the OD, indicating its high level of toxicity for HSIC-2e cells. HSIC-2e cells treated with naphthalene exhibited an OD at 600 nm of 0.48 at 10.5 h, while HSIC-2e cells left untreated exhibited a significantly higher OD of 1.3 at 10 h (data not shown). The treated culture also produced fewer PFU at 10 h (6.80 × 109 ml−1) than the untreated culture at 10.5 h (4.78 × 1010 ml−1) (data not shown). Treatment of the uninfected HSIC host did not result in a rapid decrease in the OD. HSIC host cells treated with naphthalene exhibited an OD of 1.8 at 10 h, while HSIC host cells left untreated exhibited an OD of 1.5. Neither treated nor untreated HSIC host cultures produced any PFU. These results indicate that no induction of prophage occurred upon treatment with naphthalene.
Adsorption of phage to the lysogen HSIC-2 and the HSIC host.
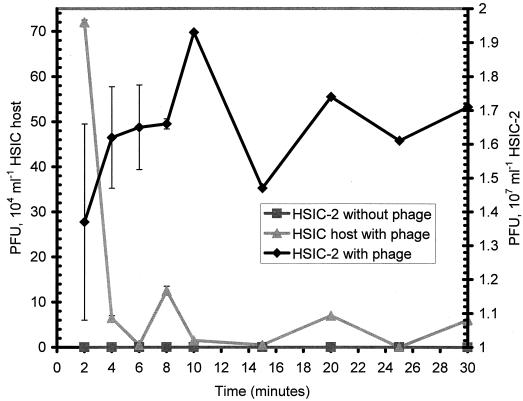
Adsorption experiments were conducted with HSIC-2 and the HSIC host in order to determine adsorption kinetics. When phage were added to HSIC-2, no detectable adsorption occurred; rather, constant phage production was noted. PFU per milliliter remained relatively constant over the course of 30 min (Fig. 3). On the other hand, the additional phage were rapidly removed from the medium by the HSIC host within the first 5 min, yielding an adsorption coefficient of 1.05 × 10−6 ml−1 min−1. This adsorption coefficient is relatively fast in comparison to values determined for bacteriophages, yet it still falls within the range of adsorption rates observed for other phage groups (1).
FIG. 3.
Adsorption of phage to the lysogen HSIC-2 (diamonds) and the HSIC host (triangles). For comparison, phage production in the absence of additional phage (squares) is shown. Error bars indicate the range of PFU for duplicate plates.
Colony lifts.
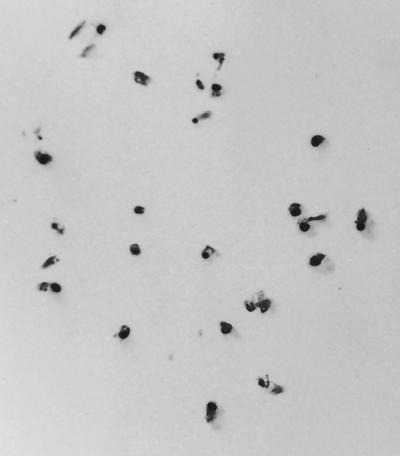
Colony lifts of both HSIC-2e and the HSIC host were probed with 35S-labeled phage ΦHSIC DNA as a rapid means to detect potential prophage integration (Fig. 4). Colonies of HSIC-2e strongly hybridized with ΦHSIC DNA, indicating the presence of phage DNA within the colonies. Colonies of the HSIC host failed to hybridize with the gene probe, indicating the absence of any phage DNA associated with the colonies.
FIG. 4.
Colony lift of the lysogen HSIC-2e probed with a 100-bp gene probe derived from the ΦHSIC phage.
Integration of the ΦHSIC genome in cellular replicons.
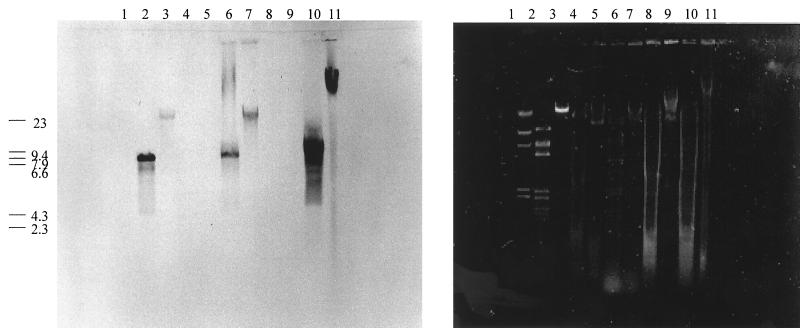
Southern hybridization of viral, plasmid, and chromosomal preparations of ΦHSIC, HSIC, and HSIC-2e is shown in Fig. 5. Molecular probing of the plasmid and chromosomal DNA preparations resulted in hybridization in both fractions. HindIII digestion of the purified phage genome, plasmid DNA, and chromosomal DNA of HSIC-2e (Fig. 5, lanes 2, 6, and 10) resulted in a common band which hybridized to the ΦHSIC probe and possessed a molecular size of 7.9 kb. The undigested plasmid preparation (Fig. 5, lane 7) comigrated with the purified phage DNA preparation (lane 3), near the 23-kb molecular size marker. The undigested chromosomal preparation (Fig. 5, lane 11) hybridized to the probe at a location in the gel indicative of a molecule of a much larger molecular size and corresponded to the expected position for chromosomal DNA in the gel. This band is at the same location in the gel as the chromosomal DNA bands (Fig. 5). This result suggests that a significant proportion of the phage DNA was integrated into the chromosome of HSIC-2e.
FIG. 5.
Southern transfer (left) and agarose gel (right) of the HindIII λ standard (lane 1), digested and undigested purified ΦHSIC DNA (lanes 2 and 3, respectively), digested and undigested plasmid minipreparation of the HSIC host (lanes 4 and 5, respectively), digested and undigested plasmid minipreparation of HSIC-2e (lanes 6 and 7, respectively), digested and undigested chromosomal preparation of the HSIC host (lanes 8 and 9, respectively), and digested and undigested chromosomal preparation of HSIC-2e (lanes 10 and 11, respectively). Numbers at left are sizes in kilobases.
DISCUSSION
Infection of the HSIC host with ΦHSIC resulted in a virus-host interaction that displayed properties of lysogeny and pseudolysogeny. The interaction resembled pseudolysogeny because of a high rate of phage production concurrent with high host cell abundance and segregation upon plating. Additionally, there was a lack of induction with mitomycin C or naphthalene coupled with the very low rate of adsorption of phage to host cells.
Pseudolysogens are often characterized by the sustained production of phage along with a thriving population of host cells (1). Another indicative characteristic of pseudolysogeny is the failure to induce prophage into a lysis cycle through the introduction of a mutagenic agent, such as mitomycin C (28). This result is caused by the lack of chromosomal integration. HSIC-2e thus exhibited some features that are consistent with pseudolysogeny. Wommack and Colwell (28) suggested an additional explanation for the existence of pseudolysogeny: the mutation of the host cell to an adhesion-deficient or -impaired state. The adsorption experiment conducted with HSIC-2 indicated a very low rate of adsorption of phage to host cells, a result that is consistent with pseudolysogeny.
Lysogeny is characterized by homoimmunity to superinfection and integration of the phage genome into the host genome (1). Homoimmunity in phage λ is often the result of excess repressor molecules that render the lysogenic bacterium immune to superinfection (1). The continual synthesis of repressor molecules maintains the phage genome as a prophage and prevents the transcription of phage genes, leading to the initiation of the lysogenic cycle (1). The relationship found in this study can be characterized as a lysogenic interaction because of homoimmunity and integration of the phage genome into the host chromosome. Hybridization of the gene probe to colonies of HSIC-2e indicated the potential for phage integration into the host chromosome. The colonies were clonally derived from washed cells, indicating that hybridization was not solely due to the attachment of virus to the outside of the cells. Probing of the plasmid and chromosomal DNA preparations provided evidence that prophage integration into the host chromosome had occurred. However, hybridization to the plasmid fraction was probably caused by the high level of lytic infections occurring in the culture. We suggest that this effect explains the comigration of the undigested plasmid DNA preparation with the purified phage DNA.
We conclude that ΦHSIC enters into a lysogenic relationship with the HSIC host that resembles pseudolysogeny. This interaction may be explained by two factors. First, we hypothesize that the lysogen may exhibit certain characteristics of pseudolysogeny due to weak or poor repression of phage DNA transcription. This effect may be caused by a partially defective repressor protein or by conditions of growth not permitting tight repressor binding. The pseudolysogenic characteristics displayed include high host cell abundance concomitant with a high rate of phage production and the failure of mitomycin C or the polyaromatic hydrocarbon naphthalene to induce prophage. According to Ackermann and Dubow (1), an unstable repressor would allow for a high rate of spontaneous induction along with high host cell abundance. When the repressor is not being actively synthesized, transcription of the phage genes occurs, ultimately leading to the initiation of the lytic cycle. When the repressor is being actively synthesized, transcription of the phage genes is repressed and the induction of prophage does not occur. The latter might explain the failure of mitomycin C or naphthalene to induce prophage. In order for prophage induction to occur in phage λ, a DNA-damaging agent must activate the RecA protein, which subsequently cleaves and inactivates the repressor (1). If our hypothesis is correct and the repressor in the HSIC-2e system poorly inhibits phage synthesis, then significant induction of prophage would not occur. Second, we propose that the homoimmunity observed might have been the result of a change in the cell surface of the lysogen which resulted in very poor phage adsorption (23, 28). Colonies of HSIC-2e can be described as crinkled and sticky. In contrast, wild-type colonies are smooth and not sticky. This alteration of colony morphology may be due to conversion by phage genes. This colony morphology persists as long as the prophage genes exist within the cellular replicons. When prophage genes are lost through segregation, colony morphology returns to that of the uninfected host. The combination of a high rate of prophage excision and poor adsorption has resulted in the observed pseudolysogen-like interaction.
The characteristics observed in this lysogenic interaction may be shared by many marine bacteria. For example, Chiura (3) has observed phage particles in many cultures of marine bacterial isolates. In fact, most marine environments contain a constant supply of host cells (106 ml−1) and relatively high phage particle abundance (107 or more per ml), fitting the criterion for pseudolysogeny of Ackermann and Dubow (1). Such a steady state may be the result of many individual pseudolysogenic interactions. Alternatively, the relationship between phages and bacteria in the marine environment may be the result of a mixture of sensitive and resistant host cells and/or a mixture of virulent and temperate phages. This explanation is also a definition of pseudolysogeny given by Ackermann and Dubow (1). This research has demonstrated the potential for such interactions between phages and bacteria in the marine environment.
ACKNOWLEDGMENTS
This research was supported by a grant from the National Science Foundation (OCE 9811319) and by Florida sea grant R/LR-MB-3.
REFERENCES
- 1.Ackermann H W, Dubow M S. Viruses of prokaryotes. 1. General properties of bacteriophages. Boca Raton, Fla: CRC Press, Inc.; 1987. [Google Scholar]
- 2.Adams M K. Bacteriophages. New York, N.Y: Interscience Publications; 1959. [Google Scholar]
- 3.Chiura H X. Generalized gene transfer by virus-like particles from marine bacteria. Aquat Microb Ecol. 1997;13:75–83. [Google Scholar]
- 4.Cochran P K, Paul J H. Seasonal abundance of lysogenic bacteria in a subtropical esturary. Appl Environ Microbiol. 1998;64:2308–2312. doi: 10.1128/aem.64.6.2308-2312.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cochran P K, Kellog C A, Paul J H. Prophage induction of indigenous marine lysogenic bacteria by environmental pollutants. Mar Ecol Prog Ser. 1998;164:125–133. [Google Scholar]
- 6.Freifelder D. Molecular biology. Boston, Mass: Jones & Bartlett Inc.; 1987. [Google Scholar]
- 7.Freifelder D. Molecular biology: a comprehensive introduction to prokaryotes and eukaryotes. Boston, Mass: Science Books International; 1983. [Google Scholar]
- 8.Gottesman M, Oppenheim A. Lysogeny and prophage. In: Webster R G, Granoff A, editors. Encyclopedia of virology. London, England: Academic Press Ltd.; 1994. pp. 814–823. [Google Scholar]
- 9.Jiang S C, Paul J H. Occurrence of lysogenic bacteria in marine microbial communities as determined by prophage induction. Mar Ecol Prog Ser. 1996;142:27–28. [Google Scholar]
- 10.Jiang S C, Paul J H. Significance of lysogeny in the marine environment: studies with isolates and a model of lysogenic phage production. Microb Ecol. 1997;35:235–243. doi: 10.1007/s002489900079. [DOI] [PubMed] [Google Scholar]
- 11.Jiang S C, Paul J H. Gene transfer by transduction in the marine environment. Appl Environ Microbiol. 1998;64:2780–2787. doi: 10.1128/aem.64.8.2780-2787.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang S C, Kellog C A, Paul J H. Characterization of marine temperate phage-host systems isolated from Mamala Bay, Oahu, Hawaii. Appl Environ Microbiol. 1998;64:535–542. doi: 10.1128/aem.64.2.535-542.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levin B R, Lenski R E. Coevolution in bacteria and their viruses and plasmids. In: Futuyma D J, Slatkin M, editors. Coevolution. Sunderland, Mass: Sinauer; 1983. pp. 99–127. [Google Scholar]
- 14.Moebus K. Marine bacteriophage reproduction under nutrient-limited growth in host bacteria. I. Investigations with six phage-host systems. Mar Ecol Prog Ser. 1996;144:1–12. [Google Scholar]
- 15.Paul J H. The use of Hoechst dyes 33258 and 33342 for the enumeration of attached and pelagic bacteria. Appl Environ Microbiol. 1982;43:939–949. doi: 10.1128/aem.43.4.939-944.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramirez E, Villaverde A. Viral spread within ageing bacterial populations. Gene. 1997;202:147–149. doi: 10.1016/s0378-1119(97)00467-8. [DOI] [PubMed] [Google Scholar]
- 17.Ripp S, Miller R. The role of pseudolysogeny in bacteriophage-host interactions in a natural freshwater environment. Microbiology. 1997;143:2065–2070. doi: 10.1099/00221287-143-6-2065. [DOI] [PubMed] [Google Scholar]
- 18.Ripp S, Miller R. Dynamics of the pseudolysogenic response in slowly growing cells of Pseudomonas aeruginosa. Microbiology. 1998;144:2225–2232. doi: 10.1099/00221287-144-8-2225. [DOI] [PubMed] [Google Scholar]
- 19.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 20.Stolt P, Zillig W. Transcription of the halophage ΦH repressor gene is abolished by transcription from an inversely oriented lytic promoter. FEBS Lett. 1994;344:125–128. doi: 10.1016/0014-5793(94)00347-5. [DOI] [PubMed] [Google Scholar]
- 21.Suttle C A, Chan A M. Marine cyanophages infecting oceanic and coastal strains of Synechococcus: abundance, morphology, cross-infectivity, and growth characteristics. Mar Ecol Prog Ser. 1993;92:99–109. [Google Scholar]
- 22.Tapper M A, Hicks R E. Temperate viruses and lysogeny in Lake Superior bacterioplankton. Limnol Oceanogr. 1998;43:95–103. [Google Scholar]
- 23.Thompson B J, Domingo E, Warner R C. Pseudolysogeny of Azotobacter phages. Virology. 1980;102:267–277. doi: 10.1016/0042-6822(80)90094-x. [DOI] [PubMed] [Google Scholar]
- 24.Weinbauer M G, Suttle C A. Potential significance of lysogeny to bacteriophage production and bacterial mortality in coastal waters of the Gulf of Mexico. Appl Environ Microbiol. 1996;62:4374–4380. doi: 10.1128/aem.62.12.4374-4380.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weinbauer M G, Suttle C A. Lysogeny and prophage induction in coastal and offshore bacterial communities. Aquat Microb Ecol. 1999;18:217–225. [Google Scholar]
- 26.Wilcox R M, Fuhrman J A. Bacterial viruses in coastal seawater: lytic rather than lysogenic production. Mar Ecol Prog Ser. 1994;114:35–45. [Google Scholar]
- 27.Wilson W H, Mann N H. Lysogenic and lytic viral production in marine microbial communities. Aquat Microb Ecol. 1997;13:95–100. [Google Scholar]
- 28.Wommack K E, Colwell R R. Virioplankton: viruses in aquatic ecosystems. Microbiol Mol Biol Rev. 2000;64:69–114. doi: 10.1128/mmbr.64.1.69-114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]