Abstract
Oral administration (gavage) of a second-generation probiotic, Lactobacillus reuteri, that releases Interleukin-22 (LR-IL-22) at 24 hrs after total body irradiation (TBI) mitigates damage to the intestine. We determined that LR-IL-22 also mitigates partial body irradiation (PBI), and whole abdomen irradiation (WAI). Irradiation can be an effective treatment for ovarian cancer, but its use is limited by intestinal toxicity. Strategies to mitigate toxicity are important and can revitalize this modality to treat ovarian cancer. In the present studies, we evaluated whether LR-IL-22 facilitates fractionated WAI in female C57BL/6 mice with disseminated ovarian cancer given single fraction 15.75 Gy or 19.75 Gy or 4 daily fractions of 6 Gy or 6.5 Gy WAI.
Mice receiving single or multiple administrations of LR-IL-22 during WAI showed improved intestinal barrier integrity (p=0.0167), reduced levels of radiation induced intestinal cytokines including KC/CXCL1 (p=0.002) and IFN-γ (p=0.0024), and reduced levels of plasma, Eotaxin/CCL11 (p=0.0088). LR-IL-22 significantly preserved the numbers of Lgr5+GFP+ intestinal stem cells (p = 0.0010) and improved survival (p <0.0343). Female C57BL/6MUC-1 mice with widespread abdominal syngeneic 2F8cis ovarian cancer that received LR-IL-22 during 6.5 Gy x 4 WAI had reduced tumor burden, less intestinal toxicity, and improved 30-day survival. Furthermore, LR-IL-22 facilitated WAI when added to Paclitaxel and Carboplatin chemotherapy and further increased survival. Oral administration (gavage) of LR-IL-22 is a potentially valuable intestinal radioprotector, which can facilitate therapeutic WAI for widespread intra-abdominal ovarian cancer.
Keywords: Lactobacillus reuteri-IL-22, radioprotection, intestine, radiation intestinal damage
Introduction:
There is a clear need for new therapeutic strategies to improve outcomes of patients with widespread diffuse ovarian cancer. Ovarian cancer is the most lethal gynecological cancer worldwide with an estimated yearly incidence of 239,000 new cases and 152,000 deaths (1). Late diagnosis accounts for its relatively poor prognosis, as most women have advanced disease with peritoneal dissemination (FIGO stage III) at initial presentation (2, 3). Despite optimal management with radical cytoreductive surgery and subsequent platinum/taxane-based chemotherapy agents (4, 5), most patients, including women who attain complete initial response, will suffer from recurrent disease within 18 months and develop increasing resistance to platinum-based chemotherapy (6-11). Whole abdomen irradiation (WAI) is a logical therapeutic approach for ovarian cancer, but there have been challenges.
The toxicity of whole abdomen irradiation is limited by acute intestinal injury (2-11), but there is also concern for toxicity to the liver, kidneys, and abdominal lymphoid organs including the spleen. Combinations of therapeutic WAI with chemotherapy agents used to treat widespread abdominal cancers include paclitaxel, carboplatin, and poly (ADP-ribose) polymerase (PARP) inhibitors (12-13), which can add to radiation toxicity to the intestines and other organs. Because of the concern for acute intestinal toxicity, recent clinical trials of WAI have used low radiation fraction sizes and yielded suboptimal results (9-10). A method by which to protect the intestine from irradiation damage is required for therapeutic fractionated whole abdomen irradiation (WAI), which may improve outcomes for treatment of disseminated ovarian cancer (15-21).
We evaluated gavage administration of a second-generation probiotic Lactobacillus reuteri engineered to synthesize and release interleukin-22 (LR-IL-22) (22) in a mouse model of WAI of ovarian cancer. We hypothesized that parenteral administration of IL-22 protein would not reach the irradiated intestinal crypts effectively due to microvascular swelling. In prior studies, intestinal gavage of E. coli-IL-22 also mitigated radiation-induced intestinal damage following TBI (22). We did not pursue the potential clinical translation of this other second-generation probiotic since some strains of E. coli are toxic and undesirable for humans. L. reuteri, as a platform is itself a probiotic, which can easily be adapted for use as LR-IL-22, either as a stand-alone drug or supplement to food making it accessible for patients receiving fractionated WAI (22).
We administered LR-IL-22 during single fraction or fractionated WAI alone or in combination with Paclitaxel and Carboplatin – chemotherapy. We measured the radioprotective effect of LR-IL-22 with respect to several established biomarkers of intestinal toxicity including intestinal barrier function, levels of stress response proteins, and preservation of intestinal stem cells. The results establish LR-IL-22 as a potentially valuable intestinal radioprotector, which may facilitate adding therapeutic WAI to new protocols for treating ovarian cancer.
Materials and Methods:
Mice and In Vivo Animal Model
Adult male and female C57BL/6 mice (Taconic Farms) and C57BL/6 with knockin allele-lgr5-EGFP-IRES creERTZ (23) (abbreviated lgr5+GFP+) and were housed 4 per cage and fed standard laboratory chow and deionized drinking water. MUC+/− mice (129 x SvJ) that are transgenic for the human mucin (MUC-1) antigen have been described (24-25). Mice received intraperitoneal injection of syngeneic 2F8cis ovarian cancer tumor cells and were sacrificed at specific time points or when body weight decreased over 20%. All mice were treated according to a University of Pittsburgh Institutional Animal Care and Use Committee regulations (IACUC) approved protocol.
Ovarian Cancer Cell Lines
The 2F8 and 2F8cis (Cis-platinum resistant) ovarian cancer cell line derived from murine orthotopic ovarian cancer tumors have been described previously (24-25).
Irradiation
Adult mice received either a single fraction of 9.25 Gy total body irradiation (TBI), 15 Gy of partial body irradiation (PBI) with one hind limb (5% of bone marrow shielded), or 19.75 Gy whole abdomen irradiation (WAI) using a True Beam Linear Accelerator (Varian Medical Systems, Palo Alto, CA). Radiation dosimetry and field accuracy and reproducibly were carried out using published methods (20). C57BL/6 mice with knockin allele-Lgr5-EGFP-IRES-creERTZ (23) biomarker, which detects intestinal stem cells (whether or not irradiated with WAI) were irradiated in single fraction or fractionated irradiation. In separate experiments, female C57BL/6 mice received 4 consecutive daily doses of WAI of 6 Gy or 6.5 Gy. For delivery of WAI, mice were placed in a 3 cm x 40 cm field with only the abdomen treated, shielding the neck, thoracic region, and lower limbs, then were irradiated at 600 monitor units (600 cGy) per minute using 6 MV photons at an SSD of 100 cm. All parameters of radiation followed published protocols (26-27).
Gavage Administration of LR-IL-22 or Parenteral Delivery of IL-22 Protein
The methods for LR-IL-22 and LR production as well as administration of IL-22 protein by intraperitoneal (I.P.) injections have been previously published (22). Briefly, 100 microliters containing numbers of bacteria (ranging from 106 to 109) of either LR-IL-22 or LR measured by optimal density were gavaged in 100 μl saline. Other mice received I.P. injections of 300 mg/kg IL-22 protein at the same time points as other groups (Pepro Tech, E. coli, 210-22). Mice were randomly divided into each of several groups: control mice or mice treated with intraoral gavage of either 1 x 109 control empty bacteria LR, or LR-IL-22 in 100 μl of saline, or I.P. injections of 20 μg/mL of IL-22 in 100 μl of saline. For initial TBI, PBI, or WAI studies, mice received a single LR-IL-22 therapy at 24 hrs after irradiation. For other WAI studies, LR-IL-22 treatments were administered at 3 times: 24 hrs and 3 hrs prior to, and 24 hrs following a single fraction of 19.75 Gy of WAI or at serial times during fractionated WAI. In some experiments, lower numbers of bacteria were used for gavage.
Administration of Chemotherapy
Paclitaxel (Millipore Sigma, St. Louis, MO) and Carboplatin (Millipore Sigma, St. Louis, MO) were administered as follows: Paclitaxel (40 mg/kg), and Carboplatin (10 mg/kg) were administered I.P. on day 3 after first radiation fraction.
Protein Analysis in Plasma and Small Intestine (Ileum) by Luminex Assay
Methods for preparation of intestinal tissues and plasma for Luminex assay, the production of Luminex chips for 33 inflammatory responses to stress associated proteins were utilized as published (26-27). The analysis of data and preparation of statistical evaluation were as published (26). Briefly, plasma was thawed and vortexed in order to remove particulate matter prior to dilution for the Luminex Protein Assay. A sample of 4 mg of intestine was dissected, weighed, and then homogenized in 1 mL of 0.1% Tween 80 in phosphate-buffered saline (PBS) to prevent protein clumping, and stored at −80° C. Prior to use in the Luminex Protein Assay, intestine homogenate was thawed to room temperature and centrifuged at 2000 rpm at 4° C for 10 min, and protein quantitated using the Biomed protein assay (Bio-Rad Laboratories, Hercules, CA).
Standardization of Plasma, and Intestine Protein Levels for Luminex Assays
A list of the 33 proteins assayed and known primary function is shown in Table 1. A Biorad protein assay was run for each tissue type (plasma and intestine) and experimental groups (For example, 19.75 Gy WAI, 19.75 Gy WAI + LR, 19.75 Gy WAI + IL-22, and 19.75 Gy WAI + LR-IL-22) using a Biotek Epoch microplate-spectrophotometer (Biotek Instruments, Inc., Winooski, VT). We utilized a TGF-β1 Single Plex Magnetic Bead Kit, as well as a 32 Multiplex Mouse Cytokine/Chemokine Magnetic Bead Panel (EMD Millipore, Billerica, MA, USA) that tested protein concentrations for each protein (Milliplex® for Luminex® xMAP® Technology). Each kit (MCYTOMAG-70K, TGFBMAG-64K-01) provided reagents for use in the Luminex Protein Immunoassay, which included 1 vial of Mouse Cytokine/Chemokine Standard, 2 vials Mouse Cytokine/Chemokine Quality Controls, 1 vial Serum Matrix, 96-Well Plate, 30 mL Assay Buffer, 60 mL 10X Wash Buffer, 3.2 mL bottle Mouse Cytokine Detection Antibodies, 1 3.2 mL bottle Streptavidin-Phycoerythrin. The 32 Multiplex kit (MCYTOMAG-70K) provided 1 3.5 mL pre-Mixed 32-plex Beads. The TGF-β1 kit (TGFBMAG-64K-01) provided the above reagents, as well as 5 mL Sample Diluent, 1.0 mL 1.0 N HCl, 1.0 mL 1 N NaOH, and 3.5 mL Anti-TGFB1 Beads.
Table 1:
List of 33 cytokines included in the plasma and intestine Luminex assay analysis and their respective function.
| Protein | Function |
|---|---|
| TGF-β | Inflammation, pro-fibrotic, controls proliferation and cellular differentiation |
| TNF-α | Inflammatory cytokine-stimulates necroptosis |
| IL-1α | Pro-Inflammatory |
| IL-1β | Pro-Inflammatory |
| IL-2 | Natural response to microbial infections; regulates activities of white blood cells |
| IL-3 | Stimulates hematopoietic stem cells to become myeloid progenitor cells |
| IL-4 | Stimulation of activated B-cell and T-cell proliferation, and stimulates B cells into plasma cells |
| IL-5 | Stimulates B cell growth and increases immunoglobulin secretion |
| IL-6 | Pro-Inflammatory |
| IL-7 | Stimulates hematopoietic stem cell differentiation into lymploid progenitor cells |
| IL-9 | Stimulates cell proliferation and prevents apoptosis |
| IL-10 | Anti-Inflammatory |
| IL-12 (p40) | Stimulates growth and function of T cells and stimulates IFN-γ and TNF-α |
| IL-12 (p70) | Stimulates growth and function of T cells and stimulates IFN-γ and TNF-α |
| IL-13 | Mediator of allergic inflammation and induces MMPs |
| IL-15 | Induces proliferation of natural killer cells |
| IL-17 | Recruits monocytes and neutrophils to sites of inflammation |
| IP10 | Chemoattractant for monocytes/macrophages, T cells, NK cells and dendritic cells |
| KC | Attracts neutrophils |
| LIF | Stem cell differentiation |
| LIX | Cell migration and activation of neutrophils |
| MCP-1 | Recruits monocytes, T cells and dendritic cells to sites of inflammation |
| M-CSF | Induces hematopoietic stem cells to differentiate into macrophages |
| MIG | T cell chemoattraction |
| MIP-1α | Recruitment and activation of granulocytes |
| MIP-1β | Chemoattractant for NK cells, monocytes and other immune cells |
| MIP-2 | Recruits neutrophils and lymphocytes in the intestine |
| RANTES | Recruits leukocytes into inflammatory sites |
| VEGF | Stimulates vasculogenesis and angiogenesis |
| Eotaxin | Recruits eosinophils |
| GM-CSF | Stimulates hematopoietic stem cells to produce granulocyes (neutrophils, eosinophils and basophils) and monocytes |
| G-CSF | Stimulates hematopoietic stem cells to produce granulocytes |
| IFN-γ | Activator of macrophages and inducer of class II major histocompatibility complex (MHC) molecule expression |
Preparation of Reagents and Standards for Immunoassay Beads, Quality Controls, Wash Buffer, and Serum Matrix
Antibody-immobilization beads were sonicated for 30 seconds and then vortexed for one minute prior to use. Quality Control 1 and 2 were reconstituted with 250 microliters of Assay Buffer. The 60 mL 10X Wash Buffer was diluted 1:10 with 540 mL deionized water. Thirty-four proteins were assayed by the Luminex method. TGF-β1 (the 33rd protein) was assayed by a different immunoassay technique. For the 32 Multiplex Assay, Serum Matrix for plasma samples was reconstituted in 2.0 mL of Assay Buffer, and allowed to sit at room temperature for 10 min.
Mouse Cytokine Standards were reconstituted in 250 microliters deionized water, inverted to mix, vortexed, and then transferred to a polypropylene microfuge tube and labeled as “Standard 6.” Serial dilutions were performed, by which 50 microliters of Standards 6, 5, 4, 3, and 2, was micro-pipetted to a microfuge tube containing either 200 microliters of Assay Buffer (for 32 Multiplex), or 150 microliters of Assay Buffer (for TGF-β1), until Standard 1 was obtained. Intestine homogenates were tested for protein concentration using the BioRad protein detection assay by diluting the detection solution 1:5 in deionized water. Each sample and standard (2 mg/mL, 1 mg/mL, 0.75mg/mL, 0.5mg/mL, 0.25mg/mL, 0.125mg/mL, and 0.0 mg/mL) was plated in triplicate on a 96-Flat Bottom Well Plate with 5 microliters of sample/standard combined as in previously published detailed methods (26).
Procedures for the 32-Multiplex Luminex Immunoassay
Subgroups of male and female C57BL/6 mice were sacrificed at serial times after irradiation according to an IACUC protocol using carbon dioxide inhalation. blood was retrieved in heparinized tubes and ileum were obtained from mice immediately after sacrifice and processed for the Luminex assay as described above. Beginning on the day of (but prior to) irradiation (day 0) and daily for the next several days after irradiation, specimens were collected for a total of 5-time points for each experiment. Intestine was washed free of blood prior to preparation for Luminex assay. Data was presented for each as picograms per milligram protein. All plasma and intestine samples were assayed in 96 well plates. The 96 well plate was conditioned by first adding 200 microliters of Wash Buffer to each well of the 96 well plate. The plate was then sealed and covered on a plate shaker for 10 minutes at room temperature. Wash buffer was then removed via suction.
As in prior publications (26-27), we reported the protein concentrations for plasma by Luminex assay in pg/ml. A protein concentration assay was run on each sample. For each intestine sample, the data from the Luminex assay for that sample was divided by the determined protein concentration to give standardized concentrations of pg/mg protein to facilitate comparison with levels in the intestine. Comparison of samples is reported as upregulated (red color) or downregulated (green color) based on p-values of significant differences.
TGF-β1 Immunoassay
Intestine samples for the TGF-β1 Immunoassay required an acidification step. 25 microliters of each tissue sample were plated onto a 96-Well Plate, treated with 2.0 microliters of 1.0 N HCl, and tested to ensure pH was below 3.0. The plate was sealed and covered with aluminum foil, and shaken at room temperature for 15 min. The acid-treated samples were then neutralized with 2 microliters of 1.0 N NaOH prior to addition to the sample well.
For the TGF-β1 assay of plasma, a Serum Matrix was reconstituted in 1.0 mL deionized water and 4.0 mL Assay Buffer was allowed to sit for 10 min at room temperature. Then, 0.1 mL of the reconstituted Serum Matrix (0.1 ml) was micro-pipetted and diluted in 0.5 mL Assay Buffer, for a final dilution of 1:30. The next procedures with the 96 well plate assay was then carried out as described above for the 32-multiplex Luminex immunoassay.
Measurement of Intestinal Barrier Function Integrity by Fluorochrome Bead Detection in Peripheral Blood
Male and female C57BL/6 mice received intraorally (gavage) 100 microliters of saline containing 1.0 μm diameter fluorochrome beads according to published methods (28) (F13083 FluoSpheres™ Polystyrene Microspheres, 1.0 μm, red fluorescent (580/605), for tracer studies). Peripheral blood was measured at days 2 and 5 after whole abdomen irradiation, and the number of beads per 100 μl of plasma was quantitated. Groups of 5 animals were sampled at each time point. To do so, 100 μL of blood was taken from each mouse and added to a 50 mL centrifuge tube in which 30 mL of ACK buffer were added at room temperature for 30 minutes. Samples were then centrifuged at 2000 G for 10 minutes, ACK buffer carefully poured out, and remaining sample transferred to 1.5 ml tubes containing 100 μL PBS. The final mix was eventually transferred to a black F-bottom 96 well plate (each well contained a unique sample) and beads were quantified with the BioTek SYNERGY H1 microplate reader (28).
All mice received 100 μL gavage of saline containing 1.0 μm diameter fluorochrome beads (F13083 FluoSpheres™ Polystyrene Microspheres, 1.0 μm, red fluorescent (580/605), for tracer studies) three hours prior to 15.75 Gy or 19.75 Gy WAI. Gavage techniques used in these mice were previously described (28). Peripheral blood was taken at days 2 and 5 after WAI, and systemic beads were quantified per 100 μl of plasma.
Measurement of Intestine Levels of Tight Junction Proteins Including I-CAM and Occludin
C57Bl/6NTac mice were divided into 3 groups (0 Gy, 15.75 Gy (WAI) or 15.75 Gy (WAI) + LR-IL-22). LR-IL-22 was administered 24 h after irradiation. On day 5 after irradiation, the mice were euthanized with the ileum excised, fecal contents removed by injection with 1 ml of phosphate buffered saline (PBS), fixed in 10% formalin, and sectioned. Primary antibody mixtures were prepared at manufacturer suggested dilutions in 5% Bovine Serum Albumin (BSA). The sections were incubated with primary antibody mixture overnight at 4 °C then washed with PBS three times for 5 minutes each. Secondary antibody solution was prepared at manufacturer suggested dilutions in PBS and added to the sections for 1 hour at room temperature. After washing with PBS three times for 5 minutes each, 0.5 μg/mL DAPI in PBS was added for 10-20 minutes at room temperature to label nuclei. Pictures were taken using fluorescence microscopes at University of Pittsburgh Center for Biologic Imaging. For each sample, 30 fields were subjected to analysis. There were 5 mice analyzed per data point. Data was presented as mean ± standard error of the percent of cells positive for target protein. The primary antibodies were anti- Occludin rabbit antibody (cat.no NBP1-87 402, NOVUS Biologicals, Littleton, CO) and anti-ICAM mouse antibody (Code No. 270415, SEIKAGAKU CORPORATION, Tokyo, Japan). The secondary antibodies were anti-mouse or anti-rabbit IgG Alexa 488 or 594 (Invitrogen/Fisher Scientific, Waltham, MA).
Assays for Intestinal Lgr5+ Stem Cells.
These assays were carried out using C57BL/6 mice with the knockin allele Lgr5-EGFP-IRES-creERTZ (23), which allows counting of green lgr5+ cells in intestinal crypts (22). The assays for GFP+ lgr5+ cells using antibodies and assay conditions according to published methods (23, 29-31).
Physics:
Linear accelerator based TBI and PBI (one leg shielded) will be carried out as described in detail in our previous publication (26) using a TrueBeam (Varian) linear accelerator with beam flatness and dose rate monitored daily for PBI, the dose under the shielded limb was confirmed by TLDs to be < 1.0% of the dose prescribed to the thoracic cavity. PBI was delivered on a Varian TrueBeam Linear Accelerator using animal immobilization, as described (20) and with adherence to all physics parameters reviewed in daily shielding of one hind limb with lead blocks and confirmation that dose to that limb by thermoluminescent dosimeters (TLDs) is <1.0% of dose to the thoracic cavity. Dose rate 600 cGy/min. Beam flatness was measured daily. Total body irradiation (TBI) was performed using a JL Shepherd and Associates Model 68A cesium irradiator at a dose rate of 298 cGy/minute. Partial body irradiation (PBI) was delivered on a Varian TrueBeam Linear accelerator. The mice were anesthetized with Nembutal and placed in a 30 cm x 30 cm field with the left leg pulled out of the field to preserve the bone marrow in the left leg to prevent bone marrow failure. The mice were irradiated with 6 MV photons at a dose rate of 600 monitor units per min at an SSD (source to surface distance) of 100 cm. A strict quality assurance and quality control program is in place to ensure that all beam parameters conform to the guidelines set by the American Association of Physicists in Medicine.
Statistics
For the cytokine data analysis, we first analyzed separately for each radiation type (TBI, PBI or WBI) for each of 34 cytokines and for each tissue (plasma or intestine). In these analyses, data were summarized for each subgroup with mean ± standard deviation (SD). At each day of measurement, we compared each treated group with the control. Also, for each treatment, we compared each day back to day 0. All these comparisons were done based on a two-way ANOVA model, followed by a t-test using ESTIMATE statement in Proc GLM of SAS 9.4 (SAS Institute Inc, Cary, NC). In this ANOVA model, treatment, day, and their interactions were factors.
For the bead leakage analysis assay and for the intestinal crypt stem cell lgr5+ cell analysis, we also summarized data with mean ± SD, and analyzed the data using two-way ANOVA followed by t-tests for interested comparisons. For the mouse survival data were carried out by Kaplan-Meier survival curves and were plotted for each group. Each treated group was compared to the control, using the two-sided log-rank test. In these analyses, p<0.05 was regarded as significant. As these were exploratory analysis, we did not adjust p-values for multiple comparisons.
Results:
Intraoral Administration (Gavage) of LR-IL-22 Improves Survival of Mice When Delivered After Total Body Irradiation (TBI), Whole Abdomen Irradiation (WAI), and Partial Body Irradiation (PBI).
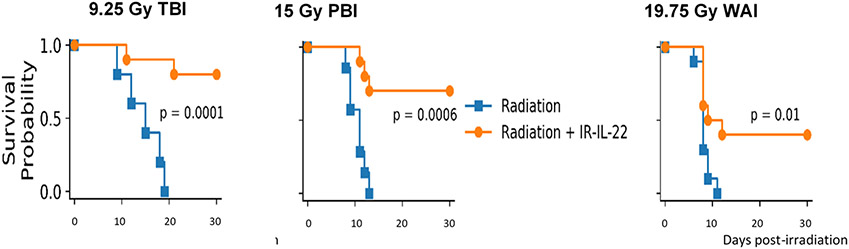
We first evaluated the effects of gavage of LR-IL-22 on survival of C57BL/6 female adult mice subjected to each of three techniques of irradiation: total body irradiation (TBI) delivering 9.25 Gy, PBI with 5% of bone marrow shielded (one hind) limb 15 Gy, and WAI carried out with the thoracic cavity, head and neck region shielded, and also the hind limbs (19.75 Gy). Mice were gavaged 24 hrs after irradiation with either 100 uL of regular saline (control) or 1×109 LR-IL-22 in 100 μL of regular saline. Mice receiving 15 Gy PBI had their right hind limb shielded from irradiation (32) providing marrow protection and facilitating survival past 30 days to allow study of radiation induced intestinal toxicity (32). The dose of 109 LR-IL-22 bacteria gavage was previously shown to induce a significant increase in survival following TBI in C57BL/6 mice (22). Here, we show that in all three radiation modalities, mice treated with LR-IL-22 delivered at 24 hrs post-irradiation have a significantly improved 30-day survival compared to control irradiated mice (Fig. 1). No significant improvement in survival was detected with mice undergoing oral gavage with control LR bacteria or IL-22 protein delivered I.P. at 24 hr after each irradiation protocol.
Figure 1: LR-IL-22 gavage improves 30-day survival in irradiated mice receiving TBI, PBI, or WAI.
C57BL/6 female mice were irradiated with either 9.25 Gy TBI, 15 By PBI, and 19.75 Gy WAI, and then gavaged 24 hrs later with 100 μL of regular saline or 100 μL of 1x109 LR-IL-22 bacteria. Mice were followed for 30 days and survival was monitored among different treatment modalities. (n = 15)
The results establish that mice receiving gavage of LR-IL-22 had improved survival compared to mice following TBI, PBI, or WAI alone.
Effects of LR-IL-22 On Biomarkers of Irradiation-Induced Intestinal Damage from WAI.
We evaluated the effect of each radiation modality on expression level of each of 34 proteins representing stress response gene products and inflammatory cytokines in the small intestine (ileum) compared to plasma (Table 1). These proteins have previously been shown to be useful biomarkers of successful application of radiation mitigators (26-27).
To assess the effect of LR-IL-22 compared to LR on irradiation, we measured irradiation-induced changes in intestine and plasma proteins. There was an irradiation-induced increase in levels of several proteins in intestine and in plasma following WAI and PBI. There was also a significant effect of LR-IL-22 gavage compared to gavage of LR at reducing levels of several inflammatory proteins (Fig. 2). In separate studies with female mice, we compared the effect of LR-IL-22 with that of LR or IL-22 protein delivered I.P. in mice receiving TBI, or PBI, compared to WAI. Increase in levels of several proteins in the plasma and intestine of animals was observed in mice receiving each of the three irradiation volumes: TBI, PBI, and WAI (Supplemental Figures 1-33).
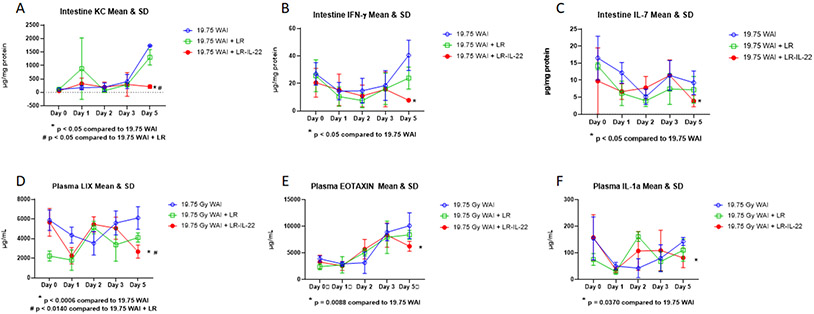
Figure 2: LR-IL-22 modulates intestinal and plasma levels of cytokine expression after 9.75 Gy WAI.
Three groups of mice (n = 4 – 5): control, intraoral 109 copies of LR in 100 μL of saline, and 109 copies of LR-IL-22 in 100 μL of saline. Mice received their respective treatment 24 hours and 3 hours prior to and 24 hours following 19.75 Gy WAI. Intestinal tissues and plasma were analyzed at indicated times (day) after WAI. (A–C) Intestinal mucosal expression of indicated cytokines. (D–F) Plasma expression of indicated cytokines. Values are Mean ±SEM.
The results at day 5 after 19.75 Gy WAI with 6 representative inflammatory cytokine proteins KC, IFNγ, and IL-7 in intestine and LIX, Eotaxin, and IL-1α in plasma showed significant downregulation by LR-IL-22 (Fig. 2). The results applied to day 5 only, but not earlier. The present results establish specific effects of LR-IL-22 on reducing the levels of intestinal IL-7, KC/CXCL1, IFN-γ, and plasma LIX/CXCL5, Eotaxin/CCL11, and IL-1α at day 5 after 19.75 Gy WAI (Fig. 2). These findings establish that WAI significantly changes the levels of expression of a select few cytokines both locally in the intestine and systemically in plasma at day after irradiation, and after single fraction WAI, there was a significant effect of LR-IL-22 on levels of IL-7, IFN-γ, KC in intestine and levels of LIX, eotaxin, and IL-1α in plasma (Fig. 2). Analysis of the changes in 33 proteins levels comparing TBI, PBI, and WAI is shown in Supplemental Figures 1-33.
The analysis of 33 proteins in plasma and intestine over the 5 days after each of 3 radiation modalities (TBI, PBI, WAI) was carried out, and details are shown in Supplemental Figures 1-33. In each of these figures, we compared significant increase (red) or decrease (green) in the levels of each protein over time and between each of the 3 modalities. Panel A in each supplemental figure shows results with each treatment delivered at 24 hrs after irradiation (LR, IL-22 protein, LR-IL-22, or no treatment) compared to the irradiated untreated group on that same day for days 1 through 5. Panel B shows the same data, but comparing results with each protein over the 5 days after irradiation with its own baseline pre-irradiation level at day 0.
We calculated protein level changes by two methods. Four patterns of protein increases in the intestine and plasma were observed. Increases relative to the level on the same day for each treatment compared to irradiation alone (Panels A) were seen with G-CSF, GM-CSF, and LIF. Increases relative to the level for that protein at day 0 (Panel B) were seen with G-CSF, Eotaxin, IFN-γ, IL-2, IL-5, IL-6, IL-7, IL-9, IL-10, IL-12 (p40), IL-12 (p70), LIF, IL-15, IP-10, KC, MCP-1, MIP-1α, MIP1β, MCSF, MIP-2, VEGF, TNF-α, TGF-β, and LIX. Increases in proteins in the intestine were seen with all 3 radiation modalities (TBI, PBI, and WAI). The results with plasma were similar with all modalities and reflect the contribution of abdominal organs to the plasma levels.
In contrast, there were decreases in other protein levels with irradiated groups when analyzed by the same two methods. Decreases relative to the same day for irradiated only groups (Panel A) compared to treatment groups were seen with IL-5, IL-6, IL-9, LIF, IL-17, MIP-1α, MIP1β, M-CSF, MIP-2, Rantes, TNF-α, and TGF-β. Protein decreases relative to each proteins’ own baseline level at day 0 (Panel B) were seen with IFN-γ, IL-1β, IL-3, IL-4, IL-5, IL-7, IL-15, IL-17, MIP1β, MIP-2, MIG, Rantes, and LIX. Decreases in these proteins in intestine were observed with all 3 radiation modalities.
The data show similarity of individual protein increases or decreases with TBI, PBI, and WAI, when each was compared to its own baseline level at day 0. There were some differences that were dependent on the volume of tissue irradiated, such as the increase in eotaxin for WAI and decrease in IL-1β, IL-17, and MPIβ with WAI and decreases in Rantes for PBI and WAI. As shown in Figure 2, the therapeutic effect of LR-IL-22 in the WAI group compared to control irradiated mice indicates that intestinal KC, IFN-γ, IL-7, and plasma LIX, Eotaxin, and IL-1α are potential biomarkers for a therapeutic effect of LR-IL-22. The data also indicate that plasma protein levels alone may not be reliable indicators of the effectiveness of a gavaged second-generation probiotic radioprotector or mitigator.
The higher dose of irradiation to the intestine in the WAI group produced a more robust and rapid increase in specific cytokine levels in the plasma and ileum such as Eotaxin (increase) (Supplemental Fig. 2), or IL-1β (decrease) in intestine (Supplemental Fig. 6). Elevated or decreased plasma protein levels in animals that received PBI or TBI were not as dramatic to that seen in those that received WAI. The data confirm the importance of volume of tissue treated, as well as, irradiation dose relative to levels of plasma proteins.
We observed that some groups of mice that received gavage of control LR (bacteria) also showed amelioration of levels of radiation induced protein biomarkers in ileum, and plasma. The data indicate that the time course of elevations or decreases in the levels of specific stress response gene products and inflammatory cytokines indicate a therapeutic effect of LR, as well as, LR-IL-22.
LR-IL-22 Gavage Ameliorates Irradiation-Induced Disruption of Intestinal Barrier Function.
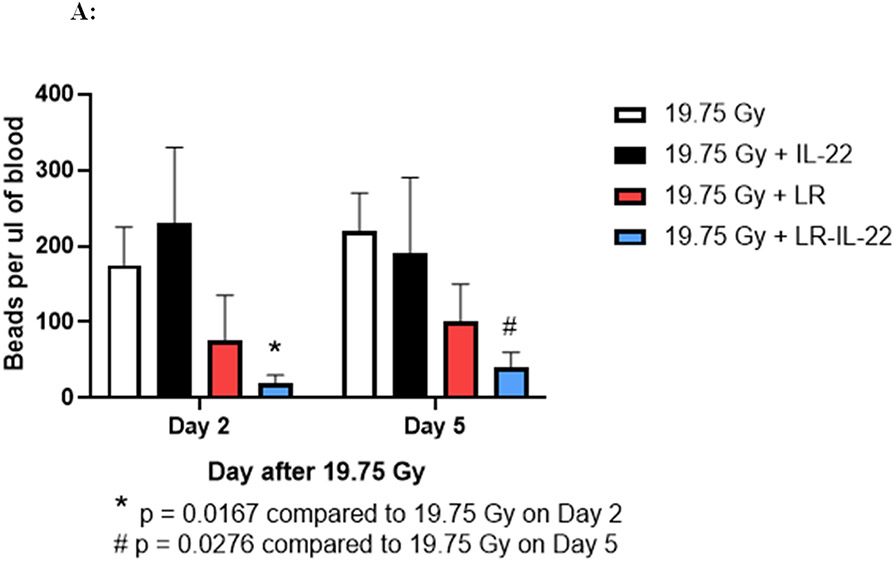
We next evaluated mice receiving 19.75 Gy WAI with respect to intestinal barrier function. Following irradiation, animals received intraoral administration of 4-micron diameter fluorescent beads at serial time points. Following whole abdomen irradiation (WAI), we analyzed the intestinal barrier permeability through the intraoral administration of fluorescent beads (F13083 FluoSpheres™ Polystyrene Microspheres, 1.0 μm, red fluorescent (580/605), for tracer studies. Blood plasma was collected and assayed for appearance of the beads in the plasma (28). This assay has been shown to be a reliable indication of intestinal barrier function breakdown leading to leakage of fluorochrome beads from the intestinal lumen into the plasma (28). The results shown in Figure 3A demonstrate significant protection against barrier breakdown with respect to fluorochrome bead leakage in the animals that received LR-IL-22, but not soluble IL-22. We observed that both groups of mice treated with LR, as well as LR-IL-22 had significantly lower levels of red fluorescent beads in their systemic circulation as of day 2 following WAI, while control irradiated mice and those treated with intraperitoneal injections of IL-22 had significantly elevated levels of fluorescent beads systemically (Fig. 3A).
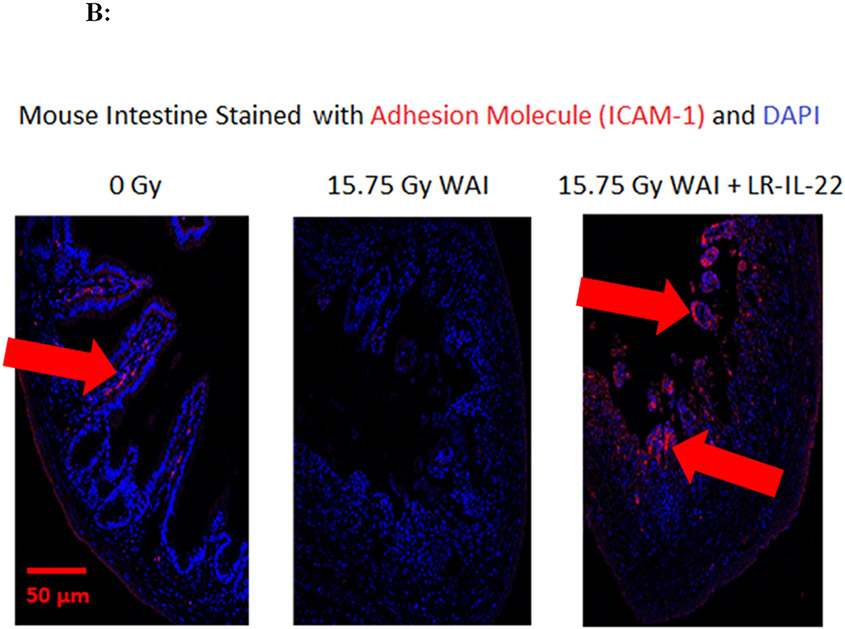
Figure 3: LR-IL-22 gavage reduces systemic leakage of intraluminal injected fluorescent beads at days 2 and 5 following WAI and preserves barrier integrity.
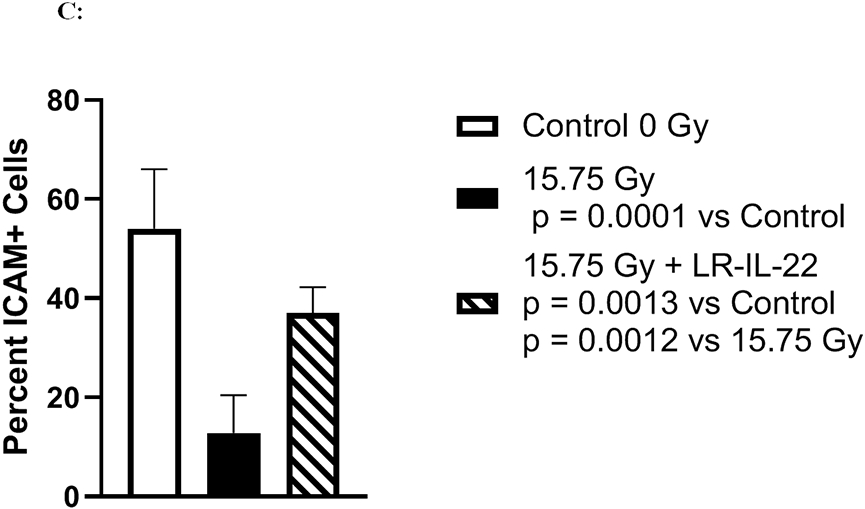
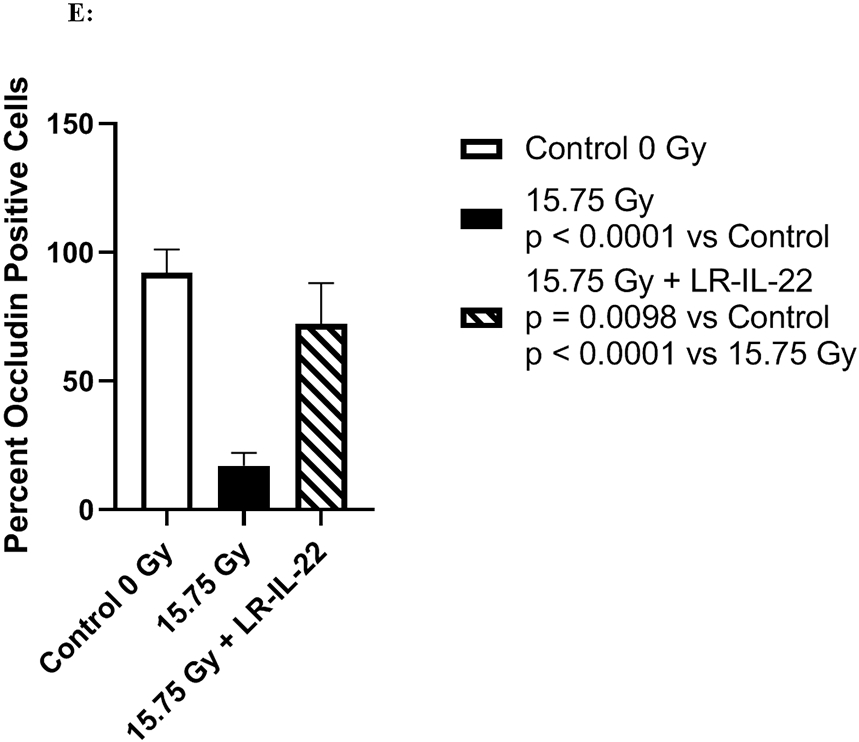
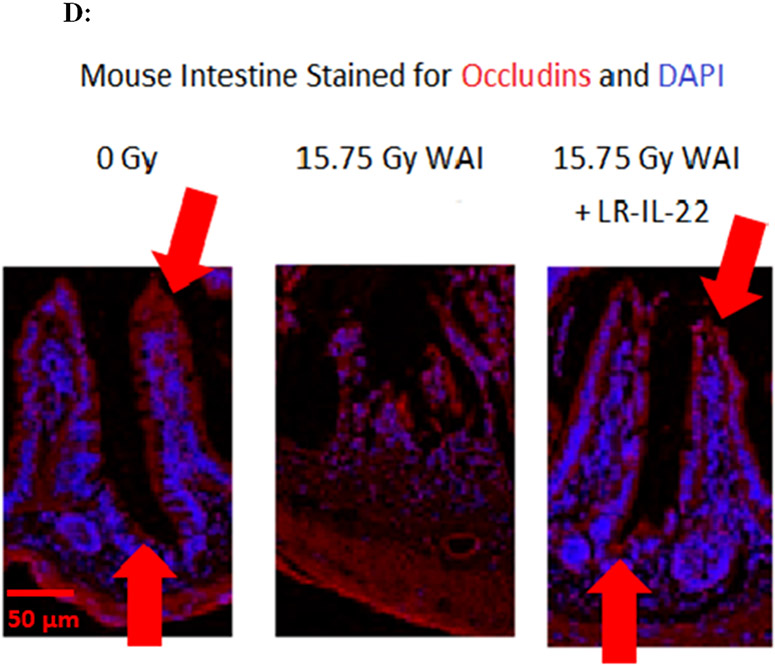
C57BL/6 male and female mice were divided into four different groups and each group received their respective treatment 24 hours and 3 hours prior to, and 24 hours after a single fraction of 19.75 Gy of WAI. A) Fluorescent beads (F13083 FluoSpheres™ Polystyrene Microspheres, 1.0 μm, red fluorescent (580/605), for tracer studies) were administered 3 hours prior to sacrifice on days 2 or 5. (n = 5) (t-Test: paired two samples for means). B) Immunostaining, as described in the methods, for ICAM levels in intestine at day 5 after 15.75 Gy WAI. Blue color is DAPI, red is I-CAM C) Quantitation of I-CAM. D) Immunostaining for levels of occludin at day 5 after 15.75 Gy WAI (x40 magnification). E) Quantitation of occludin in intestine (x 40 magnification). For quantification in Fig. 3C and 3E, 30 microscopic fields were scored per mouse with 5 mice per group. The percent positive cells was based on scoring 1000 cells per mouse for 5 mice per data point.
We measured the effect of irradiation and LR-IL-22 on other biomarkers of an intact intestinal barrier. WAI 15.75 Gy clearly induced a decrease in I-CAM (Figs. 3B-3C) and occludin (Figs. 3D-3E), which are two intercellular adhesion proteins the levels of which serve as biomarkers of an intact barrier, which normally prevents bacterial entry into the circulation. There was a restoration of levels of I-CAM and occludin in mice gavaged with LR-IL-22 at 24 hrs after irradiation further indicating an improved barrier function (Fig. 3B-3E). The results between mice that received empty L. reuteri and those that received the genetically engineered probiotic LR-IL-22 indicate that LR by itself helps maintain intestinal barrier integrity and limits the systemic dissemination of fluorescent beads (Fig. 3A); however, the restorative effect was less than that observed in mice treated with LR-IL-22.
The levels of I-CAM (Figs. 3B-8C), and occludin (Figs. 3D-3E) proteins decreased rapidly after WAI. In contrast, those animals receiving LR-IL-22 showed elevated levels of these proteins at day 5 (Figs. 3B-3E). These results establish that gavage of LR-IL-22 prior to WAI, stabilized intestinal barrier function as assayed by both leakage of fluorochrome beads from the intestinal lumen into the plasma, and levels of protein adhesion molecules necessary for intestinal barrier integrity.
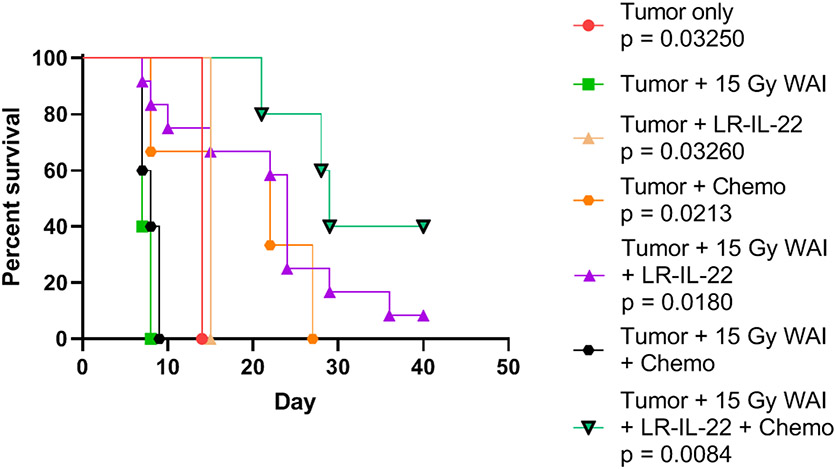
Figure 8: Improved survival in mice with widespread ovarian cancer by delivery of chemotherapy with WAI and LR-IL-22.
Mice with 2F8cis tumors received 15 Gy WAI, and paclitaxel (10 mg/kg), cisplatin (40 mg/kg) on day 3. Administration of LR-IL-22 gavage and WAI prior radiation fraction improves survival (p=0.0084). P values relative to tumor only. P<0.001 for ▼ compared to ■. Two-sided log-ranked test. ∧-Anti-PD-L1 alone after WAI. WAI was ineffective. (n=12 mice per group). Median survival for each group is shown in Table 4. Statistical evaluation was carried out using a two-sided log rank test.
LR-IL-22 Rescues and Preserves Critical Leucine-Rich Repeat-Containing G-Protein-Coupled Receptor 5 (Lgr5+) Cells in Ileum of Lgr5+ Green Fluorescent Protein (GFP)+ Mice from Fractionated Whole Abdomen Irradiation
The above results established that LR-IL-22 prevented the breakdown of intestinal barrier function after single fraction WAI. We next determined whether LR-IL-22 preserved intestinal crypt cell stem cell numbers, as another indication of radiation protection and mitigation during fractionated WAI of Lgr5+GFP+ mice. We tested several schedules of delivery of LR-IL-22 during fractionated WAI. We evaluated the effect of fractionated WAI on numbers of Lgr5+GFP+ intestinal stem cells. C57BL/6 mice were randomly divided into four different groups: 1) control non-irradiated group, 2) irradiated mice received 6.5 Gy x 4 WAI daily; 3) WAI daily plus LR-IL-22 daily; or 4) WAI daily plus LR daily. Mice were gavaged with 100 μl saline containing 109 bacteria or saline alone 3 hrs prior to each fraction of WAI.
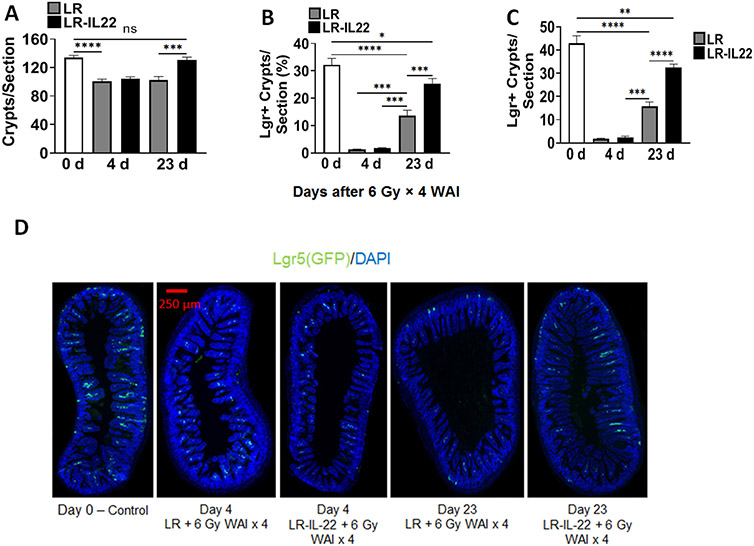
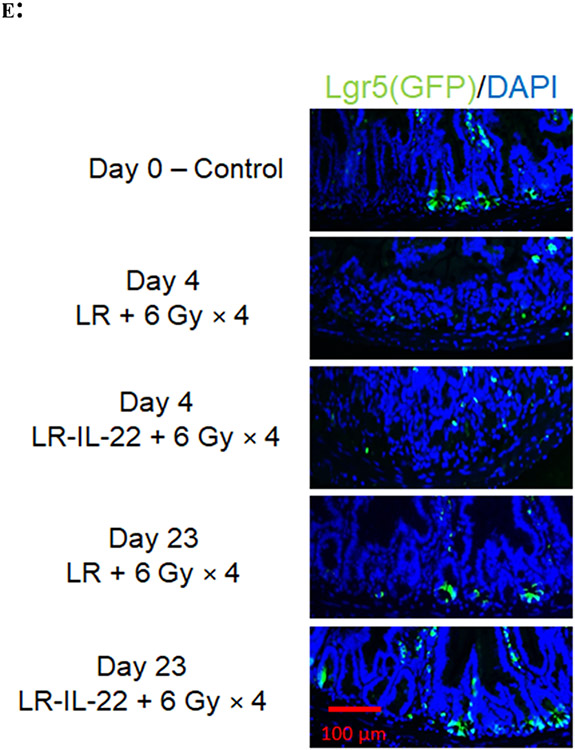
LR-IL-22 treated mice that were sacrificed at day 4 (n = 5) or day 23 (n = 6) following the fourth fraction of 6 Gy × 4 WAI had twice as many Lgr5+GFP+ crypt cells at day 23 following last fraction compared to mice solely treated with empty bacteria (p < 0.0001) (Figs. 4A-4E). Such a difference was not observed when intestinal crypt cells were counted on day 4 (p = 0.9982) following last fraction of WAI (Figs. 4A-4E). We confirmed the effectiveness of LR-IL-22 compared to LR gavage in lgr5+GFP+ mice with green fluorescence biomarker positive for lgr5+ intestinal stem cells in the ileum (Fig. 4D - 4E). Irradiated mice had a significant decrease in the intestinal stem cell population on day 4 following the last fraction of WAI. By day 23, Lgr5+ stem cells were observed to increase in both LR (p < 0.0001) and LR-IL-22 (p < 0.0001) treated mice, but more so in the latter group in which the increase was twice as much that observed in mice receiving empty L. reuteri (Fig. 4B-4C). Total crypt stem cell numbers were improved in LR-IL-22 treated mice (p = 0.0008) to a final level highly comparable to non-irradiated control mice (Figs. 4A-4D). Our results confirm and extend a prior publication demonstrating that gavage of LR-IL-22 after TBI preserved intestinal stem cell numbers (22) after irradiation and extend these results to fractionated WAI. These results also establish that intraoral gavage of LR-IL-22 preserved intestinal stem cell numbers following fractionated WAI; however, levels observed by day 23 were still lower than those in the control non-irradiated mice.
Figure 4: LR-IL-22 gavage and preserves numbers of lgr5+ intestinal crypt stem cells following 6 Gy x 4 WAI.
Lgr5+ GFP+ mice (n = 13) were randomly divided into two groups: 1) 6 Gy x 4 WAI plus LR; 2) 6 Gy x 4 WAI plus LR-IL-22. Mice received either LR or LR-IL-22 intraorally (gavage) 24 hours prior to the initial fraction and 3 hours prior to each subsequent fraction (total of 4 doses, each equal to 109 copies of bacteria in 100 μL of saline). Mice were then sacrificed at day 0 prior to irradiation (n = 2), and day 4 (n = 5) and day 23 (n = 6) following last fraction of WAI for intestinal crypt cell analysis. (A) Total intestinal crypt cell quantification per section. (B) Percentage of Lgr5+ crypt cells per section. (C) Quantification of Lgr5+ crypt cells per section. (D) GFP+ intestinal crypt cells at ×40 magnification. (E) GFP+ intestinal crypt cells at ×200 magnification (*** = p < 0.05). (F) Lgr5+GFP+ stem cell numbers in irradiated ileum at days 0, 4, 23 for LR-IL-22 compared to LR (x 40 magnification). There were 5 mice per group per time point with 1000 cells scored in each of 30 microscopic sections per mouse.
LR-IL-22 Protects the Intestine from Single Fraction Irradiation in Mice with Disseminated Intra-Abdominal Ovarian Cancer.
We next applied the therapeutic application of LR-IL-22 radioprotection of the intestine in a model of ovarian cancer. In these studies, we used MUC1 Tg mice that were challenged I.P. with a syngeneic, ovarian cancer cell line, which has been shown to be Cisplatinum resistant (2F8cis) (24-25). The 2F8cis tumors grow throughout the abdominal cavity with tumor nodules present in the mesentery, omentum, as well as, throughout the peritoneal cavity and abdominal wall. MUC1 is an oncoprotein that has been recently shown to play a role in the pathogenesis and treatment of epithelial ovarian cancer as 80% of which overexpress this specific glycoprotein involved in multiple signaling pathways essential for cell growth, proliferations and metastasis (33). 2F8cis cells show moderate resistance to Cisplatin making them a suitable model for recurrent ovarian cancer (33). When transgenic MUC1 mice receive intraperitoneal injection of 2F8cis cells, tumor growth occurs in a highly inflammatory environment, which promotes accelerated loco-regional tumor growth and dissemination (33).
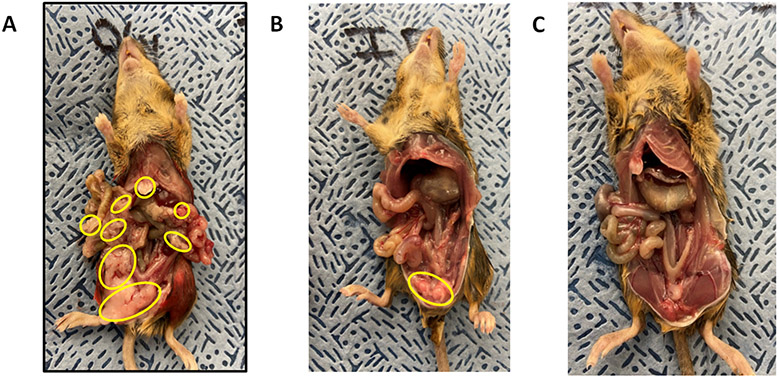
Single fraction WAI was first tested in mice with ovarian cancer. As shown in (Fig. 5A), mice injected I.P. with 107 ovarian cancer 2F8cis cells demonstrated diffuse miliary deposits of ovarian cancer by 5 days after injection. Transgenic female MUC1 mice with 2F8cis tumor cells received either a single fraction of 15.75 Gy WAI or a combined regimen of irradiation and additional LR-IL-22. Mice were then sacrificed at day 10 following tumor cell injection (day 5 after irradiation). Control non-irradiated mice had over 100 disseminated tumor nodules throughout their abdomen, while tumor burden was notably reduced in irradiated mice compared to the non-irradiated group (Fig. 5B). The control non-irradiated mice contained 54.0 ± 9.0 tumor nodules, while the irradiated control mice had 5.3 ± 1.5 nodules (p=0.0062 compared to non-irradiated mice). The mice irradiated and gavaged with LR-IL-22 had 0.7 ± 0.3 tumor nodules (p=0.0041 compared to non-irradiated mice). Mice with diffuse ovarian tumors that received single fraction 15.75 Gy alone displayed intestinal swelling, but there was significant tumor volume reduction (Fig. 5B). Mice that received LR-IL-22 had less than 10 tumor nodules (p=0.0471) (Fig. 5C). There were 12 mice per group, and the differential tumor burden was consistent in all mice in each group, represented by the images in Fig. 5. These results suggested that LR-IL-22 protection of the intestine would prolong survival during fractionated WAI of mice with diffuse ovarian cancer.
Figure 5: Gross pathologic appearance of mice with disseminated abdominal 2F8cis ovarian cancer.
C57BL/6/-MUC-1 mice were injected I.P. with 106 2F8cis cells, then on day 5 given single fraction 19.75 Gy WAI. Results are for day 5 after irradiation (10 days after tumor cell injection). A) No treatment; B) Radiation; and C) radiation preceded by gavage of 109 LR-IL-22 bacteria in 100 μl saline.
LR-IL-22 Improves Survival of Mice Receiving Fractionated WAI Therapy for Disseminated Ovarian Cancer
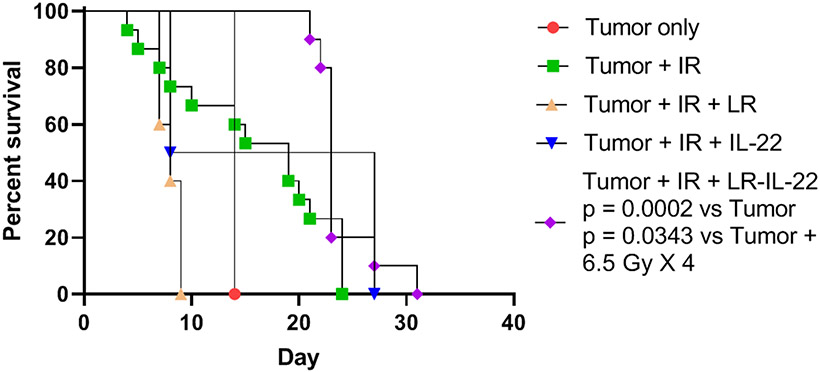
The above results establish the effectiveness of LR-IL-22 in protecting the intestine from single fraction 19.75 Gy WAI in mice harboring ovarian cancer. We next designed experiments to demonstrate improved tolerance to fractionated WAI (Fig. 6). We tested whether 4 fractions of 6.5 Gy WAI in mice with diffuse abdominal ovarian cancer displayed effective tumor reduction and also intestinal protection by multiple administrations of LR-IL-22. Mice were treated in three protocols: 1) 4 fractions of 6.5 Gy WAI daily for four days; 2) 6.5 Gy x 4 WAI plus LR gavage daily; or 3) 6.5 Gy daily WAI plus I.P. IL-22 protein; or 4) 6.5 Gy WAI daily for 4 days plus daily LR-IL-22 (Fig. 6). The median survival for groups represented in Fig. 6 is shown in Table 2. The criteria for sacrifice included >20% body weight loss or severe abdominal distension, as defined by IACUC Institutional policy. The data showed rapid death from tumor, or tumor plus WAI alone or with LR alone. There was little increase in survival in animals given WAI plus IL-22. There was very modest increase in survival comparing irradiation alone (20 days median survival to 23-25 days) for irradiation plus LR-IL-22. These results show that LR-IL-22 modestly improved survival of irradiated mice with 2F8cis ovarian cancer, who received 6.5 Gy x 4 WAI.
Figure 6: Effect on survival of daily LR-IL-22 delivered by gavage to mice with 2F8cis tumors and 6.5 Gy x 4.
Groups of 12 mice with disseminated 2F8cis ovarian cancer were prepared as follows: Intraperitoneal injection of 106 2F8cis cells on day 0, then 5 days later some received the first of 4 daily WAI fractions of 6.5 Gy. Subgroups of mice received daily gavage of 109 bacteria of LR, or daily gavage of LR-IL-22 (on the day of each irradiation fraction), or daily IL-22 protein injected I.P. Survival measured over the next 30 days. (p = 0.0002 for LR-IL-22 compared to tumor only or 0.0343 compared to tumor + 6.5 Gy X 4). Median survival of groups is shown in Table 2.
Table 2:
Effect on survival of daily LR-IL-22 delivered by gavage to mice with 2F8cis tumors and receiving 6.5 Gy x 4 WAI.
| Group | Median Survival (Day) |
|---|---|
| Tumor + 6.5 Gy X 4 | 19 |
| Tumor only | 14 |
| Tumor + 6.5 Gy X 4 + LR | 8 |
| Tumor + 6.5 Gy X 4 + IL-22 | 8 |
| Tumor + 6.5 Gy X 4 + LR-IL-22 | 23 (p = 0.0183) |
p value compares to group. Tumor + 6.5 Gy x 4, p value shown. Data relate to Fig. 6.
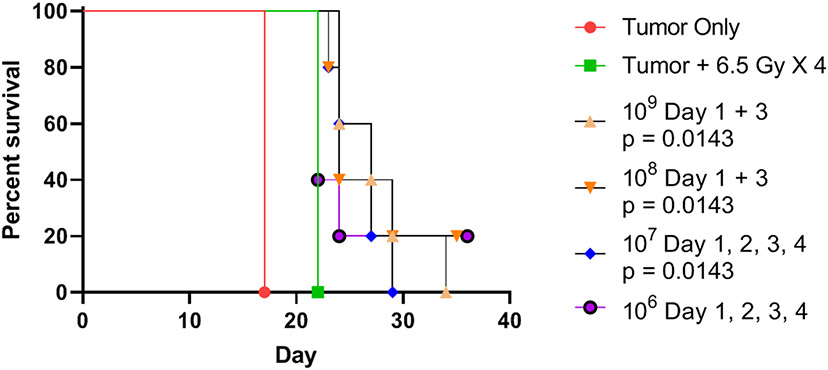
Optimal schedule of administration of LR-IL-22 during fractionated WAI.
We next compared several schedules of administration of LR-L-22, including different numbers of bacteria and daily or twice weekly gavage delivery. As shown in Figure 7 in each of the schedules of delivery of LR-IL-22 with WAI, there was a modest improvement in survival of mice with disseminated abdominal ovarian cancer compared to untreated mice or mice receiving WAI alone. Mice receiving 108 or 109 bacteria on days 1 and 3 showed some improved survival (Fig. 7) compared to animals receiving irradiation alone, who rapidly died from radiation toxicity. Median survival for the groups displayed in Fig. 7 is shown in Table 3. In each of the experimental protocols, untreated animals died from progression of ovarian cancer measured as >20% weight loss, or severe abdominal distension as defined by IACUC regulations.
Figure 7: Comparison of LR-IL-22 bacteria cell numbers and schedules of administration on survival of mice with 2F8cis tumors over a course of 4 days of 6.5 Gy fractionated WAI.
Groups of 12 mice with disseminated 2F8cis ovarian cancer, as described in the legend to Figure 6, received no treatment, WAI at 6.5 Gy per day x 4 days, or irradiation plus twice weekly or daily LR-IL-22 in the bacterial cell numbers shown and quantitated as published (19). Median survival of groups is shown in Table 3.
Table 3:
Effect on survival of varied LR-IL-22 bacteria cell numbers delivered by gavage to mice with 2F8cis tumors over a course of 4 days of daily 6.5 Gy fractionated WAI.
| Group | Median Survival |
|---|---|
| Tumor + 6.5 Gy X 4 | 22 |
| Tumor Only | 17 |
| 109 cells on day 1 and 3 | 27 (p = 0.0143) |
| 108 cells on Day 1 and 3 | 24 |
| 107 cells on Day 1, 2, 3, and 4 | 27 |
| 106 cells on Day 1, 2, 3 and 4 | 22 |
p values are comparison with the group with tumor plus 6.5 Gy x 4. Only significant p values are shown. Results relate to Fig. 7.
There was a clearly detectable radioprotective effect of IL-22 parenteral protein injection, but not to the magnitude of that seen with LR-IL-22 (Fig. 7). Mice receiving LR control bacteria gavage showed no significant improvement in survival.
LR-IL-22 Treatment Facilitates Fractionated WAI to Enhance Chemotherapy of Disseminated Ovarian Cancer
We next tested the effect of combination chemotherapy and WAI in mice with diffuse 2F8cis tumors. Groups of transgenic female MUC1 mice with 2F8cis tumor cells were randomly divided into seven different groups (n=12): 1) a control non-irradiated group; 2) a group that received chemotherapy (Carboplatin and Paclitaxel); and 3) a group that received LR-IL-22; There were 4 other groups that received WAI at 15 Gy single fraction: 1) WAI alone; 2) WAI plus intraoral gavage of LR-IL-22; 3) WAI plus gavage of LR; and 4) WAI, chemotherapy, and LR-IL-22. As shown in Fig. 8, untreated mice died from tumor. The irradiated mice that received 15 Gy WAI alone, LR-IL-22 alone, or WAI plus chemotherapy died rapidly. In contrast, survival was significantly improved in mice treated with chemotherapy alone (p=0.0213); or WAI and LR-IL-22 (p=0.0180). The best survival was observed in mice receiving WAI, LR-IL-22, and chemotherapy (p=0.0084) (Fig. 8). Median survival for each of the groups is shown in Table 4.
Table 4:
Chemotherapy or survival of tumor-bearing mice effect of LR-IL-22 gavage combined with irradiation.
| Group | Median Survival (Day) |
|---|---|
| Tumor + 15 Gy WAI | 7 |
| Tumor only | 14 (p = 0.0005) |
| Tumor + LR-IL-22 | 15 (p = 0.0013) |
| Tumor + Chemotherapy | 22 (p = 0.0029) |
| Tumor + 15 Gy WAI + LR-IL-22 | 24 (P = 0.0002) |
| Tumor + 15 Gy + Chemotherapy | 8 |
| Tumor + 15 Gy WAI + LR-IL-22 + Chemotherapy | 24 (p = 0.0013) |
p values are compared to group with tumor + 15 Gy WAI. Significant p values less than 0.05 are shown. Results relate to Fig. 8.
Discussion:
The present report demonstrates the effectiveness as an intestinal radioprotector of a genetically engineered second-generation probiotic, Lactobacillus reuteri, which releases IL-22 (LR-IL-22). This new radiation protector/mitigator facilitates delivery of therapeutic doses of fractionated whole abdomen irradiation (WAI). We delivered LR-IL-22 by intraoral gavage, which facilitates IL-22 transit through the stomach and release of IL-22 in the intestinal villi by bacteriophage lysis, which stabilizes the L. reuteri throughout gastrointestinal transit (22). This delivery method enables concentrated levels of IL-22 to arrive locally at the gastrointestinal crypts, where vulnerable radiation sensitive lgr5+ stem cells reside. In contrast, gavage of mice with control LR did not provide protection. Furthermore, mice treated with systemic injections of IL-22 protein were not protected as effectively as those receiving LR-IL-22. The success of LR-IL-22 as a therapeutic may be attributable to delivery of IL-22 locally to the intestinal crypts. Mechanistically, vascular swelling after each radiation fraction may have limited parenterally administered drug from reaching the intestinal villi.
LR-IL-22 treatment maintained intestinal barrier function, measured as reduced leakage of red fluorescent beads that were gavaged after LR-IL-22 treatment at either day 2 or 5 following WAI. Intestinal radioprotection was correlated with improved levels of biomarkers of radiation injury including intact intestinal epithelial tight junctions following radiation measured as levels of intestinal tight junction proteins I-CAM and occludin which correlated with the reduced fluorescent bead leakage from intestinal lumen into the blood (34). The therapeutic effect of LR-IL-22 was associated with modulation of irradiation induced levels of biomarkers of inflammation including intestinal KC, IFNγ, and IL-7, and plasma levels of LIX, eotaxin, and IL-1α biomarkers of intestinal damage (35-44).
Oral administration of LR-IL-22 prevented radiation induced changes in LIX/CXCL5 and Eotaxin/CCL11 consistent with their reported elevation during the irradiation inflammatory response. LIX is a known chemoattractant protein that regulates leukocyte migration, inflammation, and subsequent advancement of disease severity in IBD patients (40). A significant increase of levels of LIX systemically in IBD patients has been reported compared to healthy individuals and elevation has been correlated with intestinal inflammation and tissue damage in colitis (40). Elevation of eotaxin, which is an eosinophil chemotactic protein has been associated with radiation-induced intestinal damage including fibrosis (41). Inhibition of the eotaxin receptor CCR3 by neutralizing antibodies has been reported to suppress eosinophil recruitment to the intestinal submucosa after irradiation and the suppression reduces fibrosis (41). We also demonstrated that lgr5+ intestinal crypt stem cells were preserved by LR-IL-22 treatment after WAI compared to irradiated control mice followed out to day 23 following single fraction or fractionated (WAI) irradiation.
The present results established that LR-IL-22 treated mice with disseminated abdominal ovarian cancer better tolerated single fraction or fractionated WAI. We further established a protocol for optimized delivery of LR-IL-22 by gavage twice a week in a 4 fraction WAI regimen that improved survival.
There is a rationale for using WAI with chemotherapy and targeted therapies including immune checkpoint inhibitors (ICIs) in advanced or recurrent epithelial ovarian cancer. New data have highlighted a synergistic effect of combination therapies to eradicating other cancers by application of radiotherapy to induce in-situ vaccination of tumor cells and apoptosis of Treg lymphocytes, that could further enhance cell-mediated immune responses and cytotoxic T-cell activity (45-54). Several clinical studies have illustrated that radiotherapy is a potential monotherapy in oligometastatic disease, as the advent of IMRT, SBRT, and low dose hyper-fractionation have facilitated the administration of abdominal irradiation. However, intestinal toxicity has continued to remain a problem (7-10).
The present report describes a novel approach to intestinal radioprotection during WAI for widespread abdominal malignancy including Ovarian cancer. The mouse model we used is an established replica, which mimics human ovarian cancer. Transgenic mice expressing the human mucin 1 (MUC1), which is a glycoprotein that is essential for EOC metastasis and progression, develop aggressive intraperitoneal (IP) tumors following IP injections of 2F8cis cells which not only are cisplatin resistant, but have also shown to significantly induce tumor PD-L1 upregulation following chemotherapy compared to 2F8 cells which are platinum sensitive. Additionally, 2F8cis tumors show a much more significant increase in CD8 T cell infiltration, thus giving it an immunological “hot” phenotype that positively correlate with PD-L1 expression, yet anti-PD-L1 monotherapy in this model was also unsatisfactory (59). Fractionated WAI may upregulate PD-L1 and MUC1 receptors to increase the effectiveness of immunotherapies, but WAI has been limited by intestinal toxicity (6-7, 11-21). The present data support translational studies for the use of LR-IL-22 radioprotection in women suffering from advanced, recurrent or cisplatin resistant disease, who may benefit from WAI. The addition of LR-IL-22 to a combined treatment modality with fractionated WAI and systemic chemoimmunotherapy and immunotherapy (55-58) regimens may facilitate a safe and effective protocol to reduce tumor burden, increase survival, and improve the quality of life for patients with widespread abdominal Ovarian cancer.
Supplementary Material
Acknowledgements:
Supported by grant from NIAID/NIH, U19-AI068021.
References:
- 1.Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: a review. Cancer Biology & Medicine 2017; 14(1): 9–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haie C, Pejovic-Lenfant MH, George M, Michel G, Gerbaulet A, Prade M, et al. Whole abdominal irradiation following chemotherapy in patients with minimal residual disease after second look surgery in ovarian carcinoma. Int J Radiat Oncol Biol Phys 1989; 17(1): 15–9. [DOI] [PubMed] [Google Scholar]
- 3.Bolis G, Zanaboni F, Vanoli P, Russo A, Franchi M, Scarfone G, et al. The impact of whole-abdomen radiotherapy on survival in advanced ovarian cancer patients with minimal residual disease after chemotherapy. Gynecol Oncol 1990; 39(2): 150–4. [DOI] [PubMed] [Google Scholar]
- 4.Pickel H, Lahousen M, Petru E, Stettner H, Hackl A, Kapp K, et al. Consolidation radiotherapy after carboplatin-based chemotherapy in radically operated advanced ovarian cancer. Gynecol Oncol 1999; 72(2): 215–9. [DOI] [PubMed] [Google Scholar]
- 5.Dembo AJ. Epithelial ovarian cancer: the role of radiotherapy. Int J Radiat Oncol Biol Phys 1992; 22(5): 835–45. [DOI] [PubMed] [Google Scholar]
- 6.May LF, Belinson JL, Roland TA. Palliative benefit of radiation therapy in advanced ovarian cancer. Gynecol Oncol 1990; 37(3): 408–11. [DOI] [PubMed] [Google Scholar]
- 7.Faul C, Gerszten K, Edwards R, Land S, D’Angelo G, Kelley J III, et al. A phase I/II study of hypofractionated whole abdominal radiation therapy in patients with chemoresistant ovarian carcinoma: Karnofsky score determines treatment outcome. Int J Radiat Oncol Biol Phys 2000; 47(3): 749–54. [DOI] [PubMed] [Google Scholar]
- 8.Kunos C, Sill M, Buekers T, Walker J, Schilder J, Yamada S, et al. Low-dose abdominal radiation as a docetaxel chemosensitizer for recurrent epithelial ovarian cancer: a phase 1 study of the Gynecologic Oncology Group. Gynecol Oncol 2011; 120(2): 224–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ngoi N, Heong V, Tang JI, Choo BA, Kumarakulasinghe NB, Lim D, et al. Phase I study of low-dose fractionated whole abdominal radiation therapy in combination with weekly paclitaxel for platinum-resistant ovarian cancer (GCGS-01). Int J Radiat Oncol Biol Phys 2021; 109(3): 701–11. [DOI] [PubMed] [Google Scholar]
- 10.Arians N, Kieser M, Benner L, Rochet N, Schroder L, Katayama S, et al. Adjuvant intensity modulated whole-abdominal radiation therapy for high-risk patients with ovarian cancer FIGO stage III: final results of a prospective phase 2 study. Radiat Oncol 2019; 14(1): 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dembo AJ. The role of radiotherapy in ovarian cancer. Bull Cancer 1982; 69(3): 275–83. [PubMed] [Google Scholar]
- 12.Moore KN, Bookman M, Sehouli J, Miller A, Anderson C, Scambia G, et al. Atezolizumab, bevacizumab, and chemotherapy for newly diagnosed stage III or IV ovarian cancer: Placebo-controlled randomized phase III trial (IMagyn050/GOG 3015/ENGOT-OV39). J Clin Oncol 2021; 39: 1842–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konstantinopoulos PA, Cannistra SA. Immune checkpoint inhibitors in ovarian cancer: can we bridge the gap between IMagynation and reality? J Clin Oncol 2021; 39(17): 1833–1837. [DOI] [PubMed] [Google Scholar]
- 14.Mun G-I, Kim S, Choi E, Kim CS, Lee Y-S. Pharmacology of natural radioprotectors. Archives of Pharmacol Research 2018; 41: 1033–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mun S, Jang S-H, Ryu A. Early-stage ovarian carcinoma with symptoms mimicking tuberculous peritonitis in a postmenopausal woman. Medicine 2018; 97(40): e12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet 2014; 384(9951): 1376–1388. [DOI] [PubMed] [Google Scholar]
- 17.Perren TJ, Swart Am, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 2011; 365(26): 2484–96. [DOI] [PubMed] [Google Scholar]
- 18.Burger RA, Brady MR, Bookman MA, Fleming FG, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 2011; 365(26): 2473–83. [DOI] [PubMed] [Google Scholar]
- 19.Smith JP, Rutledge FN, Delclos L. Postoperative treatment of early cancer of the ovary: a random trial between postoperative irradiation and chemotherapy. Natl Cancer Inst Monogr 1975; 42: 149–53. [PubMed] [Google Scholar]
- 20.Carey MS, Dembo AJ, Simm JE, Fyles AW, Treger T, Bush RS. Testing the validity of a prognostic classification in patients with surgically optimal ovarian carcinoma: a 15-year review. Int J. Gynecol Cancer 1993; 3(1): 24–35. [DOI] [PubMed] [Google Scholar]
- 21.Grabosch S, Zeng F, Zhang L, Strange M, Brozick J, Edwards RP, et al. PD-L1 biology in response to chemotherapy in vitro and in vivo in ovarian cancer. J. Immunotherapy Cancer 2015; 3(Suppl. 2): P302. [Google Scholar]
- 22.Zhang X, Fisher R, Hou W, Shields D, Epperly MW, Wang H, et al. Second-generation probiotics producing IL-22 increase survival of mice after total body irradiation. In Vivo 2020; 34(1): 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barker N, van Es JH, Kulpers J, Kujala P, van den Born M, Cozijnsen M, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5+. Nature 2007; 449: 1003–1007. [DOI] [PubMed] [Google Scholar]
- 24.Budiu RA, Elishaev E, Brozick J, Lee M, Edwards RP, Kalinski P, et al. Immunobiology of human mucin 1 in a preclinical ovarian tumor model. Oncogene 2013; 32: 3664–3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mony JT, Zhang L, Ma T, Grabosch S, Tirodkar TS, Brozick J, et al. Anti-PD-L1 prolongs survival and triggers T cell but not humoral anti-tumor immune responses in a human MUC1-expressing preclinical ovarian cancer model. Cancer Immunol Immunother 2015; 64(9): 1095–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinman J, Epperly M, Hou W, Willis J, Wang H, Fisher R, et al. Improved total-body irradiation survival by delivery of two radiation mitigators that target distinct cell death pathways. Radiat Res 2018; 189(1): 68–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thermozier S, Hou W, Zhang X, Shields D, Fisher R, Bayir H, et al. Anti-ferroptosis drug, Baicalein, enhances total body irradiation mitigation by two other drugs that target apoptosis and necroptosis. Radiat Res 2020; 195: 435–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LeFevre ME, Boccio AM, Joel DD. Intestinal uptake of fluorescent microspheres in young and aged mice. Proc Soc Exp Biol Med 1989; 190(1): 23–27. [DOI] [PubMed] [Google Scholar]
- 29.Wei L, Leibowitz BJ, Wang X, Epperly M, Greenberger J, Zhang L, et al. Inhibition of CDK4/6 protects against radiation-induced intestinal injury in mice. J Clin Invest 2016; 126(11): 4076–4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei L, Leibowitz BJ, Epperly M, Bi C, Li A, Steinman J, et al. The GS-nitroxide JP4-039 improves intestinal barrier and stem cell recovery in irradiated mice. Scientific Rep 2018; 8: 2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leibowitz BJ, Zhao G, Wei L, Ruan H, Epperly M, Chen L, et al. Interferon β drives intestinal regeneration after radiation. Sci Adv 2021; 7: eabl5253, (1-12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valente M, Denis J, Grenier N, Arvers P, Foucher B, Desangles F, et al. Revisiting Biomarkers of Total-Body and Partial-Body Exposure in a Baboon Model of Irradiation. PLoS One 2015; 10(7): e0132194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grabosch S, Bulatovic M, Zeng F, Ma T, Zhang L, Ross M, et al. Cisplatin-induced immune modulation in ovarian cancer mouse models with distinct inflammation profiles. Oncogene 2019; 38(13): 2380–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Mumm JB, Herbst R, Kolbeck R, Wang Y. IL-22 Increases Permeability of Intestinal Epithelial Tight Junctions by Enhancing Claudin-2 Expression. J Immunol 2017; 199: 3316–3325. [DOI] [PubMed] [Google Scholar]
- 35.Yamazaki M, Yajima T, Tanabe M, Fukui K, Okada E, Okamoto R, et al. Mucosal T cells expressing high levels of IL-7 receptor are potential targets for treatment of chronic colitis. J Immunol 2003; 171: 1556–1563. [DOI] [PubMed] [Google Scholar]
- 36.Alzoghaibi MA, Al-Mofleh IA, Al-Jebreen AM. Neutrophil chemokines GCP-2 and GRO-alpha in patients with inflammatory bowel disease. J Dig Dis 2008; 9: 144–148. [DOI] [PubMed] [Google Scholar]
- 37.Mitsuyama K, Tsuruta O, Tomiyasu N, Takaki K, Suzuki A, Masuda J, et al. Increased circulating concentrations of growth-related oncogene (GRO)-alpha in patients with inflammatory bowel disease. Dig Dis Sci 2006; 51:173–177. [DOI] [PubMed] [Google Scholar]
- 38.Takashima S, Martin ML, Jansen SA, Fu Y, Bos J, Chandra D, et al. T cell-derived interferon-γ programs stem cell death in immune-mediated intestinal damage. Sci Immunol 2019; 4(42): eaay8556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eriguchi Y, Nakamura K, Yokoi Y, Sugimoto R, Takahashi S, Hashimoto D, et al. Essential role of IFN-γ in T cell–associated intestinal inflammation. JCI Insight 2018; 3(18): e121886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh UP, Singh NP, Murphy EA, Price RL, Fayad R, Nagarkatti M, et al. Chemokine and cytokine levels in inflammatory bowel disease patients. Cytokine 2016; 77: 44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takemura N, Kurashima Y, Mori Y, Okada K, Ogino T, Osawa H, et al. Eosinophil depletion suppresses radiation-induced small intestinal fibrosis. Sci Transl Med 2018; 10: eaan0333. [DOI] [PubMed] [Google Scholar]
- 42.Bersudsky M, Luski L, Fishman D, White RM, Ziv-Sokolovskaya N, Dotan S, et al. Non-redundant properties of IL-1α and IL-1β during acute colon inflammation in mice. Gut 2014; 63(4): 598–609. [DOI] [PubMed] [Google Scholar]
- 43.Scarpa M, Kessler S, Sadler T, West G, Homer C, McDonald C, et al. The epithelial danger signal IL-1α is a potent activator of fibroblasts and reactivator of intestinal inflammation. Am J Pathol 2015; 185(6): 1624–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Champiat S, Ileana E, Giaccone G, Besse B, Mountzios G, Eggermont A, et al. Incorporating immune-checkpoint inhibitors into systemic therapy of NSCLC. J Thorac Oncol 2014; 9(2): 144–153. [DOI] [PubMed] [Google Scholar]
- 45.Alme AK, Karir BS, Faltas BM, Drake CG. Blocking immune checkpoints in prostate, kidney, and urothelial cancer: An overview. Urol Oncol 2016; 34(4): 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD81 tumor-infiltrating lymphocytes and a high CD81/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA 2005; 102: 18538–18543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hwang WT, Adams SF, Tahirovic E, Hagemann IS, Coukos G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: A meta-analysis. Gynecol. Oncol 2012; 124: 192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.González-Martín A, Sánchez-Lorenzo L. Immunotherapy with checkpoint inhibitors in patients with ovarian cancer: Still promising? Cancer 2019; 125: 4616–4622. [DOI] [PubMed] [Google Scholar]
- 49.Zamarin D, Burger RA, Sill MW, Powell DJ Jr., Lankes HA, Feldman MA, et al. Randomized Phase II trial of Nivolumab versus Nivolumab and Ipilimumab for recurrent or persistent Ovarian cancer: an NRG Oncology Study. Proceedings of the 17th Biennial Meeting of the International Gynecologic Cancer Society; Kyoto, Japan. 14–16 September 2018, J Clin Oncol 2020; 38(16): 1814–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA 2007; 104: 3360–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu J, Gordon M, Veneris J, Braiteh F, Balmanoukian A, Eder JP, et al. Safety, clinical activity and biomarker assessments of atezolizumab from a Phase I study in advanced/recurrent ovarian and uterine cancers. Gynecol Oncol 2019; 154(2): 314–322. [DOI] [PubMed] [Google Scholar]
- 52.Disis ML, Taylor MH, Kelly K, Beck JT, Gordon M, Moore KM, et al. Efficacy and safety of avelumab for patients with recurrent or refractory ovarian cancer: Phase 1b results from the JAVELIN solid tumor trial. JAMA Oncol 2019; 5: 393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drew Y, De Jonge M, Hong SH, Park YH, Wolfer A, Brown J, et al. An open-label, phase II basket study of olaparib and durvalumab (MEDIOLA): Results in germline BRCA-mutated (gBRCAm) platinum-sensitive relapsed (PSR) ovarian cancer (OC) Gynecol. Oncol 2018; 149: 246–247. [Google Scholar]
- 54.Lee JM, Cimino MA, Peer CJ, Zimmer A, Lipkowitz S, Annunziata CM, et al. Safety and clinical activity of the programmed death-ligand 1 inhibitor Durvalumab in combination with poly (ADP-Ribose) polymerase inhibitor Olaparib or vascular endothelial growth factor receptor 1-3 inhibitor Cediranib in women’s cancers: A dose-escalation, Phase I Study. J. Clin. Oncol 2017; 35: 2193–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Herrera FG, Ronet C, Ochoa de Olza M, Barras D, Crespo I, Andreatta M, et al. Low dose radiotherapy reverses tumor immune desertification and resistance to immunotherapy. Cancer Discov 2021; Online ahead of print: DOI: 10.1158/2159-8290.CD-21-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ansaldo E, Belkaid Y. How microbiota improve immunotherapy. Science 2021; 373(6558): 966–967. [DOI] [PubMed] [Google Scholar]
- 57.Hu Y, Paris S, Barsoumian H, Abana CO, He K, Wasley M, et al. Radiation therapy enhanced by NBTXR3 nanoparticles overcomes anti-PD1 resistance and evokes abscopal effects. Int J Radiat Oncol Biol Phys 2021; 111(3): 647–657. [DOI] [PubMed] [Google Scholar]
- 58.Herrera FG, Irving M, Kandalaft LE, Coukos G. Rational combinations of immunotherapy with radiotherapy in ovarian cancer. Lancet Oncol 2019; 20: 417–433. [DOI] [PubMed] [Google Scholar]
- 59.Previs RA, Secord AA. Ovarian Cancer: clinical trial breakthroughs and impact on management. Obstet Gynecol Clin North Am 2019; 46(1): 67–88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.