Abstract
The majority of patients with irritable bowel syndrome (IBS) experiences food‐related symptoms, which are associated with high symptom burden, reduced quality of life, increased healthcare consumption and reduced intake of certain nutrients. In this review we aimed to describe a clinically useful approach for physicians, by presenting the latest progress in knowledge and its translation to management in IBS patients with food‐related symptoms, as well as the underlying mechanisms involved. The research tools currently available that can be used in the future for a better characterization of this subgroup of patients are also discussed. Working towards this approach could lead to a more individualised work‐up and management of IBS patients with food‐related symptoms.
Keywords: food‐related symptoms, future directions, high symptom burden, IBS, individualized management of IBS, irritable bowel syndrome, quality of life, research tools
It has becoming increasingly obvious that food‐related gastrointestinal (GI) symptoms are common in the society 1 and among patients with irritable bowel syndrome (IBS). 2 , 3 An Asian study has even proposed that meal‐associated GI disorders should be considered as a diagnostic entity on its own. 4 Currently, IBS is defined by the presence of recurrent abdominal pain associated with abnormal bowel habits according to the Rome IV criteria 5 (Figure 1). It is likely that the importance of food‐related symptoms will be central and widely acknowledged in the upcoming Rome V criteria, but above all this aspect has to be considered in the management strategies of patients with IBS and other disorders of gut‐brain interaction (DGBI).
FIGURE 1.
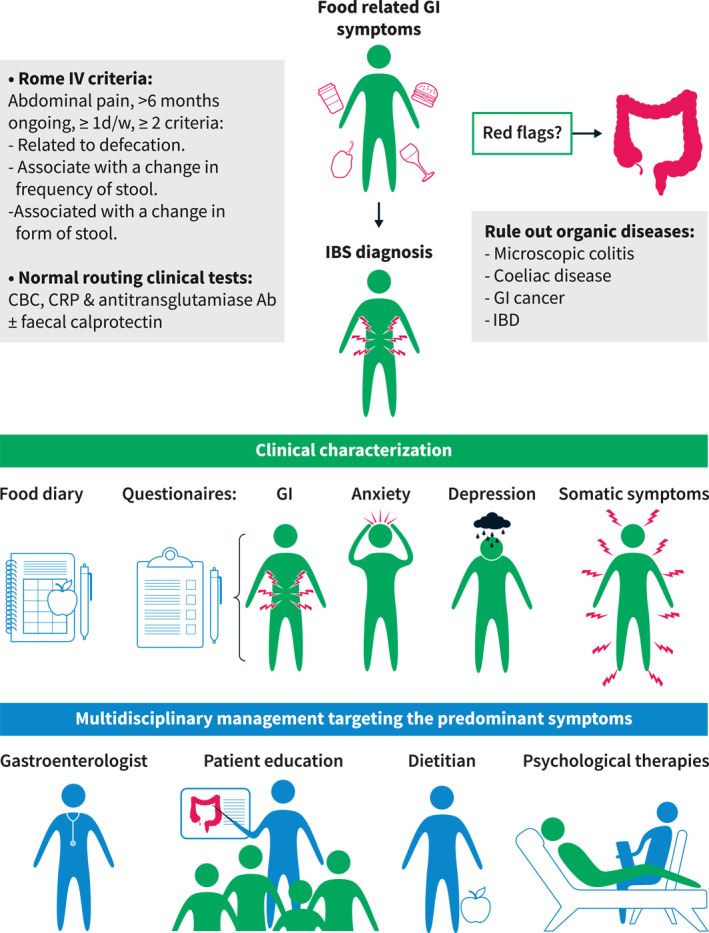
Clinical approach in irritable bowel syndrome (IBS) patients with food‐related gastrointestinal (GI) symptoms
Therefore, the aims of this review are to discuss the underlying mechanisms, the clinical management approach, and the currently used research tools in IBS with food‐related symptoms that could help guide us towards a practical approach for gastroenterologists who treat these patients. In turn, this could lead to a more individualised work‐up and management of patients in the future.
CLINICAL VIGNETTE
A 33‐year‐old female is referred to the gastroenterology outpatient clinic by her primary care physician. She is experiencing food‐related GI symptoms that began a few years ago. On average, she has two bowel movements per day, mostly Bristol stool form 6 (i.e., fluffy pieces with ragged edges, a mushy stool), associated with the presence of abdominal pain and bloating. More recently, her symptoms started to affect her social life. She is becoming more and more anxious by the frequent onset of abdominal pain and bloating after meal intake, which is why she avoids going to restaurants and to attend dinner parties. Furthermore, she has reduced her overall food intake with a normal body mass index (BMI = 20). Test results are normal, including normal blood cell count, C‐reactive protein (CRP), and thyroid‐stimulating hormone, as well as a negative tissue transglutaminase antibody test. The patient has already had different treatments, such as antispasmodics and loperamide without any success. Therefore, she has ordered a food immunoglobin (Ig) G test (her own initiative without involvement of her treating physician), and is IgG positive for yeast, wheat, egg white, cow milk, apple, and corn. She is currently avoiding these food items.
SEVERAL QUESTIONS ARE HIGHLIGHTED BY THIS CLINICAL CASE:
What is the optimal clinical work‐up leading to an IBS diagnosis for patients with food‐related symptoms?
Which symptoms are associated with food‐intake and what is the impact of food‐related symptoms?
Which food items can trigger symptoms and what are the associated underlying mechanisms behind this?
What are the current management strategies for IBS patients with food‐related symptoms?
Which research tools can currently help us to make the clinical management of IBS patients with food‐related symptoms more personalized and effective?
WHAT IS THE OPTIMAL CLINICAL WORK‐UP LEADING TO AN IRRITABLE BOWEL SYNDROME DIAGNOSIS FOR PATIENTS WITH FOOD‐RELATED SYMPTOMS?
The optimal clinical work‐up for IBS patients with food‐related symptoms is currently not different from other IBS patients, who do not report food triggers. Normal findings on basic laboratory tests, including blood (complete blood count, CRP, celiac serology) and stool analysis (faecal calprotectin when diarrhoea is present) are expected. This normal clinical work‐up can substantiate the IBS diagnosis and is sufficient in the majority of subjects fulfilling the diagnostic criteria and without alarm symptoms. Other diagnostic tests, such as an endoscopy with biopsies, can be recommended according to the clinical presentation, that is, in presence of red flags/alarm symptoms, such as family history of colon cancer, unintended weight loss, rectal bleeding (not of anal origin), abdominal mass, and age >50 years old at symptom onset, or to rule out celiac disease and microscopic colitis in patients with diarrhoea. In case of persistent diarrhoea or refractory symptoms, other tests could be added to the clinical work‐up, such as tests for bile acid diarrhoea (based on local availability) and stool tests (parasites). 6
When food is identified as a trigger of GI symptoms, and if it is one of the main concerns of the patient, high consumption of specific foods that can provoke IBS symptoms should be excluded or reduced (e.g., fibre, fatty food, caffeine, spicy food and alcohol). 7 After this first step and in case of refractory symptoms, lactose intolerance and carbohydrates malabsorption should be assessed through a detailed clinical history and potentially breath tests when available and if the clinical suspicion remains after the thorough clinical history. 6 , 8 IgE‐mediated food allergies are unlikely to be the cause of food‐related symptoms in most patients with typical IBS symptoms, while patients that suffer from IgE‐mediated food allergies typically experience type I hypersensitivity symptoms with rapid onset, for example, urticaria, angioedema, cutaneous manifestations, and pruritus after ingestion of the culprit food. 9 Moreover, testing for the presence of IgG antibodies against food items, as done by our patient, is unfortunately not useful in clinical practice. The presence of anti‐food IgG only reveals that there has been contact between the food item and the immune system, but does not provide information on food allergies, and based on current clinical experience and the scientific evidence cannot guide dietary exclusion reliably. However, ongoing research studies may lead to different recommendations in the future. Apart from the organic diseases that have been previously excluded by the standard tests, there are two other rare diseases that might be associated with food‐related symptoms: eosinophilic gastroenteritis and indolent systemic mastocytosis. Severe abdominal pain and diarrhoea are common in these rare diseases, and GI biopsies can confirm these diagnoses.
WHICH SYMPTOMS ARE ASSOCIATED WITH FOOD‐INTAKE AND WHAT IS THE IMPACT OF FOOD‐RELATED SYMPTOMS?
Our patient reported an association between food intake and abdominal pain and bloating, but she was not able to identify which food items were responsible for her symptoms. She also reported that she reduced food intake and social activities because of these symptoms. Food‐related GI symptoms are reported by up to 84% of IBS patients and when patients can identify specific food triggers, the number of food items reported is correlated with the overall GI symptom severity. 2 Common symptoms that IBS patients report to be food‐related are abdominal pain and bloating, as well as loose stools and urgency, less common symptoms include constipation or alternation/mixed bowel habits. 2 , 3 , 10 Not only factors locally in the gut can be of relevance for food‐related GI symptoms, but also depression and somatization influence the severity of food‐related GI symptoms in IBS, highlighting the importance of gut‐brain interactions. 11 Furthermore, a recent study reported a global prevalence of frequent meal‐related abdominal pain of 11%, and found that having meal‐related abdominal pain was associated with presence of a DGBI diagnosis, younger age, female sex, psychological distress, high healthcare utilization, and poor quality of life. 1 Most DGBI can be associated with meal‐related abdominal pain, but it is more common in IBS, functional dyspepsia (FD), functional dysphagia, and proctalgia fugax. 1 Additionally, IBS patients with food‐related symptoms are at risk to develop nutritional deficiencies due to inadequate food intake, as food avoidance and restriction is associated with reduced intake of certain nutrients. 12
WHICH FOOD ITEMS CAN TRIGGER SYMPTOMS AND WHAT ARE THE ASSOCIATED UNDERLYING MECHANISMS BEHIND THIS?
Our patient avoids food items based on presence of IgG antibodies to food items. Since the scientific and clinical support for this approach is insufficient, we would suggest the patient to reintroduce the food items gradually and only exclude foods permanently based on advice from a certified dietician, who provides recommendations in regards to nutritional replacements.
Among food items associated with food‐related GI symptoms, fatty food, coffee, alcohol, and hot spices are some of the most frequently reported. 2 , 3 , 10 These lead to GI symptoms via different mechanisms, including, effects on GI motility, visceral hypersensitivity, and intestinal permeability. 8 Despite the close link that is observed clinically between food intake and dyspeptic symptoms, the mechanisms underlying food‐related symptoms in FD are still incompletely understood, but gastric sensorimotor dysfunction, duodenal abnormalities, immune mechanisms and gastric hormones are likely to be involved. 13 Recent findings have also led to the focus on exclusion of specific food items, in particular foods rich in fermentable oligo‐, di‐, monosaccharides and polyols (FODMAPs), 14 and gluten. 15 The carbohydrates included in the FODMAPs concept are poorly absorbed in the small intestine and osmotically active, which leads to an increased water content in the intestines. When FODMAPs reach the colon, they are fermented by the microbiota causing gas production. The increased water content, in combination with gas production leads to luminal distension and altered motility in both IBS and healthy volunteers, but mostly IBS patients reach the GI symptom threshold, supporting the importance of visceral hypersensitivity. 16 There are also findings that suggest that the effect of FODMAPs on gut microbiota is of relevance, such as a decrease in the relative abundance of some bacteria. 17 However, the importance of these mechanisms for symptom generation is incompletely understood. Large mechanistic studies are needed to improve our understanding of the underlying mechanisms leading to symptom generation after FODMAP intake.
Gluten is a protein found in cereals and is central in coeliac disease, an enteropathy with a well‐characterized immune reactivity to gluten after ingestion. It has been suggested that gluten could also play a role in IBS, as some IBS patients report GI and extraintestinal symptoms after the ingestion of gluten. 15 Besides gluten, wheat contains fructan which is a FODMAP, and Skodje et al found that fructan, rather than gluten, elicit GI symptoms in patients with self‐reported non‐coeliac gluten sensitivity. 18 Therefore, the clinical role of gluten per se is questioned in IBS patients, while symptoms that are proposed to be gluten‐related could be explained by the presence of fructan in wheat and other cereals. Studies have assessed the efficacy of a gluten‐free diet in IBS, but with heterogeneous results. A possible explanation for this could be that the gluten‐free diet is effective only in a subgroup of IBS patients. Although associations between GI symptoms and intake of gluten are conflicting, IBS patients with more severe symptoms tend to avoid gluten and also have lower caloric intake. 8 , 19
WHAT ARE THE CURRENT MANAGEMENT STRATEGIES FOR IRRITABLE BOWEL SYNDROME PATIENTS WITH FOOD‐RELATED SYMPTOMS?
Currently, the clinical approach and treatments do not differ in IBS patients with or without food‐related symptoms. The recommended treatment approach is based on the predominant symptoms and bowel habit. 6 Ideally, a multidisciplinary care model should be advocated, where pharmacotherapy, dietary modification, and behavioural interventions are targeting the predominant symptoms and are all considered as equal partners. 20 These treatment strategies should be discussed with the appropriate healthcare professional, that is, gastroenterologist, certified dietician, psychiatrist, and psychologist. Moreover, the treatment choice should also consider the patient's beliefs for example, efficacy of previous therapy, patient preference and expectation to avoid unsatisfactory treatment results. 6 The management strategy should include realistic stepwise goals and should be based upon shared decision making.
Primary care treatments for IBS patients are well described in the National Institute for Health and Care Excellence guidelines. 7 These guidelines recommend that primary care physicians should encourage IBS patients to improve lifestyle factors, such as physical activity and follow simple dietary advice, such as sufficient intake of fluids, eating regular meals, avoid skipping meals, and limit simple and well‐known dietary triggers as describe a priori (i.e. alcohol, caffeine, etc.). In patients who do not respond to the lifestyle and dietary modifications, add‐on medication, behavioural treatment options, or the low FODMAP diet are valid alternatives. 6 The gluten‐free diet is currently not recommended in IBS patients.
In general, there is no evidence that dietary adjustments are more effective in patients with food‐related symptoms, even though this seems plausible. Nevertheless, the presence of food‐related symptoms is, for seemingly logical reasons, often used as a criterion to guide patients to dietary interventions. 6 , 14 When considering dietary adjustments in patients with IBS, physicians need to be aware of the fact that patients frequently self‐eliminate food items in order to improve their symptoms, 10 , 21 and that this restriction may lead to reduced energy intake and decreased macro‐ and micronutrients intake. 12 The early involvement of a trained dietician is highly recommended when considering dietary treatments (Figure 1). 7 , 22 Note that the presence of eating disorders such as avoidant/restrictive food intake disorder should excluded, as well as the risk of developing food‐related anxiety. 23
Clinical and biological factors that can reliably predict the response to different treatment options are unavailable in this patient population. However, recent studies suggested that combinations of psychological, nutritional, and microbial factors might predict the response to diet in IBS. 24 , 25 , 26 , 27 In the future, we will hopefully be able to integrate new tools to guide clinicians to choose the best available treatment options for their patients.
WHICH RESEARCH TOOLS CAN CURRENTLY HELP US TO MAKE THE CLINICAL MANAGEMENT OF IRRITABLE BOWEL SYNDROME PATIENTS WITH FOOD‐RELATED SYMPTOMS MORE PERSONALIZED AND EFFECTIVE?
A food‐symptom diary, currently used both in research settings and in clinical practice, is the most straightforward measure to assess food‐related symptoms 28 , 29 (Figure 1).
There are more techniques, currently used in research, that might guide the management of IBS with food‐related symptoms in the future. Standardised meal testing, assessing postprandial symptoms, is one of the techniques that can characterize food‐related GI symptom patterns. This test measures simultaneously objective markers, which provide physiologic information, and reflect real life situations. A standardised FODMAP‐rich meal has for example, been shown to increase hydrogen production and induce GI symptoms in IBS in association with metabolome and microbiota alterations. 17 , 30 Furthermore, different nutrient challenge tests combined with breath testing as a measure of fermentation, might help to better understand the mechanisms involved in symptom generation and guide dietary interventions. 25 , 31
A population‐based study showed an increased risk of IBS in subjects with asthma, food hypersensitivity, and eczema in childhood, 32 and it has been noted that IBS patients with atopy have higher total serum IgE levels and more frequently report food‐related symptoms. 33 On the other hand, atopy is also common in subjects without IBS, therefore systemic allergy cannot on its own explain IBS symptoms. Instead, these findings can suggest that local allergy‐like reactions to food in the GI tract might be relevant in the pathophysiology of food‐related GI symptoms in IBS. There are now observational studies supporting the relevance of local allergy‐like reactions in the gut in the absence of systemic allergy. First, Fritscher‐Ravens et al. demonstrated that a non‐IgE food reaction with immediate intestinal barrier alterations might be involved in IBS pathophysiology. 34 Second, Aguilera‐Lizarraga et al. have demonstrated that loss of tolerance to dietary antigens after a GI infection may trigger food‐induced abdominal symptoms. An IgE‐ and mast‐cell‐dependent reaction limited to the intestine induced visceral pain via sensitization of afferent neurons in preclinical models of IBS, and was supported by demonstration of similar mechanisms being present in a small number of IBS patients. 35 Currently, there are two novel endoscopic methods that have been used to investigate these mechanisms, the confocal laser endomicroscopy (CLE) and the colonoscopic allergen provocation (COLAP) test, 34 , 36 and could in the future be used clinically to identify atypical reactions to foods in the gut (Figure 2). However, these techniques should still be considered research tools until the clinical relevance and applicability of these findings are better understood in regards to food‐induced symptoms.
FIGURE 2.
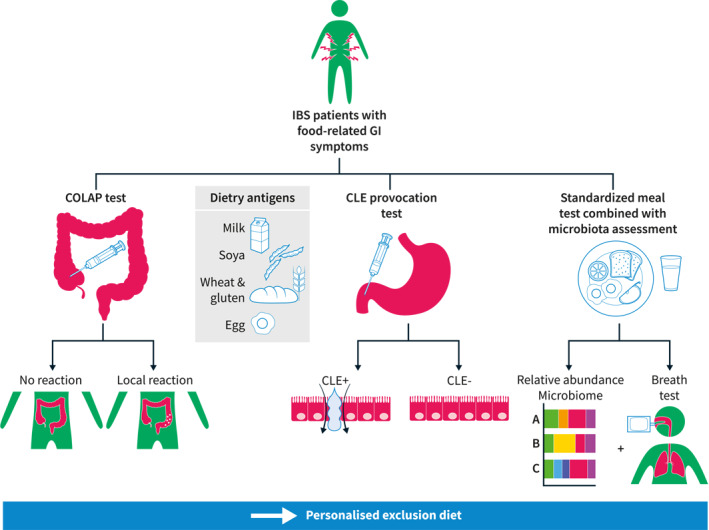
Future tools in the clinical approach of irritable bowel syndrome (IBS) patients with food‐related symptoms
The COLAP test is a local allergen provocation test, where dietary antigens are injected into the colonic mucosa (similar to the skin prick test used for clinical allergy testing). The CLE is a probe‐based endoscopic technique to assess disruption of the small intestinal barrier in the duodenum upon exposure to food antigens after intravenous injection of fluorescein. This technique can visualize fluid extravasation through mucosal epithelial leaks in real time. Currently, those two tests are only used in research and therefore not reimbursed, but it is likely that in the future, those tests might guide elimination diets in IBS.
CONCLUSION
Food‐related GI symptoms in IBS patients are common and associated with a higher symptom burden, a reduced quality of life, and a lower energy intake. Current treatment recommendations in IBS focus on targeting the predominant symptom and include dietary adjustments with involvement of a dietician when recommending more complex interventions to avoid nutritional deficiencies. The subgroup of IBS patients with food‐related symptoms needs to be better identified and characterised in the future. Recently developed tools to investigate GI reactions to food will probably allow us to soon move towards more personalized treatment recommendations.
Created with BioRender.com. AB: antibodies, CBC: complete blood count, CRP: C‐reactive protein, GI: gastrointestinal, IBD: inflammatory bowel disease, IBS: irritable bowel syndrome.
Created with BioRender.com. CLE: confocal laser endomicroscopy, COLAP: colonoscopic allergen provocation, GI: gastrointestinal, IBS: irritable bowel syndrome.
AUTHOR CONTRIBUTION
Chloé Melchior and Magnus Simrén designed the review. Chloé Melchior, Joost Algera and Esther Colomier drafted and wrote the review. Magnus Simrén and Hans Törnblom revised the articles. All authors approved the final version of the manuscript to be published.
CONFLICT OF INTEREST
Chloé Melchior has served as a consultant/advisory board member for Kyowa Kirin, Norgine, Biocodex, Mayoly Spindler, Tillots, and Ipsen. Magnus Simrén has received unrestricted research grants from Danone Nutricia Research, and Glycom; served as a consultant/advisory board member for Danone Nutricia Research, Ironwood, Menarini, Biocodex, GeneticAnalysis AS, Glycom, Arena, and Adnovate and been on the Speakers' bureau for Tillotts, Menarini, Kyowa Kirin, Takeda, Shire, Biocodex, Alimentary Health, Alfasigma, and Falk Foundation. Joost Algera, Esther Colomier and Hans Törnblom have no conflicts of interest to declare.
ACKNOWLEDGEMENTS
We are grateful to United European Gastroenterology (UEG) for the UEG Research Award 2020 that allowed Chloé Melchior to stay one year at Gothenburg University.
Melchior C, Algera J, Colomier E, Törnblom H, Simrén M. Irritable bowel syndrome with food‐related symptoms: future directions in the clinical management. United European Gastroenterol J. 2022;10(6):594–600. 10.1002/ueg2.12265
DATA AVAILABILITY STATEMENT
Data sharing not applicable–no new data generated.
REFERENCES
- 1. Colomier E, Melchior C, Algera JP, Hreinsson JP, Storsrud S, Tornblom H, et al. Global prevalence and burden of meal‐related abdominal pain. BMC Med. 2022;20(1):71. 10.1186/s12916-022-02259-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bohn L, Storsrud S, Tornblom H, Bengtsson U, Simren M. Self‐reported food‐related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am J Gastroenterol. 2013;108(5):634–41. 10.1038/ajg.2013.105 [DOI] [PubMed] [Google Scholar]
- 3. Simren M, Mansson A, Langkilde AM, Svedlund J, Abrahamsson H, Bengtsson U, et al. Food‐related gastrointestinal symptoms in the irritable bowel syndrome. Digestion. 2001;63(2):108–15. 10.1159/000051878 [DOI] [PubMed] [Google Scholar]
- 4. Ghoshal UC, Gwee KA, Chen M, Gong XR, Pratap N, Hou X, et al. Development, translation and validation of enhanced Asian Rome III questionnaires for diagnosis of functional bowel diseases in major Asian languages: a Rome foundation‐Asian Neurogastroenterology and motility association working team report. J Neurogastroenterol Motil. 2015;21(1):83–92. 10.5056/jnm14045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lacy, BE, Mearin F , Chang L, Chey WD, Lembo AJ, Simren M, et al. Bowel disorders. Gastroenterology; 2016. [DOI] [PubMed] [Google Scholar]
- 6. Moayyedi P, Mearin F, Azpiroz F, Andresen V, Barbara G, Corsetti M, et al. Irritable bowel syndrome diagnosis and management: a simplified algorithm for clinical practice. United European Gastroenterol J. 2017;5(6):773–88. 10.1177/2050640617731968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McKenzie YA, Bowyer RK, Leach H, Gulia P, Horobin J, O'Sullivan NA, et al. British Dietetic Association systematic review and evidence‐based practice guidelines for the dietary management of irritable bowel syndrome in adults (2016 update). J Hum Nutr Diet. 2016;29(5):549–75. 10.1111/jhn.12385 [DOI] [PubMed] [Google Scholar]
- 8. Algera J, Colomier E, Simren M. The dietary management of patients with irritable bowel syndrome: a Narrative review of the existing and emerging evidence. Nutrients. 2019;11(9):2162. 10.3390/nu11092162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Anvari S, Miller J, Yeh CY, Davis CM. IgE‐mediated food allergy. Clin Rev Allergy Immunol. 2019;57(2):244–60. 10.1007/s12016-018-8710-3 [DOI] [PubMed] [Google Scholar]
- 10. Lee HJ, Kim HJ, Kang EH, Jung KW, Myung SJ, Min YW, et al. Self‐reported food intolerance in Korean patients with irritable bowel syndrome. J Neurogastroenterol Motil. 2019;25(2):222–32. 10.5056/jnm18125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Van Oudenhove L, Tornblom H, Storsrud S, Tack J, Simren M. Depression and somatization are associated with increased postprandial symptoms in patients with irritable bowel syndrome. Gastroenterology. 2016;150(4):866–74. 10.1053/j.gastro.2015.11.010 [DOI] [PubMed] [Google Scholar]
- 12. Melchior C, Algera J, Colomier E, Tornblom H, Simren M, Storsrud S. Food avoidance and restriction in irritable bowel syndrome: relevance for symptoms, quality of life and nutrient intake. Clin Gastroenterol Hepatol. 2021. [DOI] [PubMed] [Google Scholar]
- 13. Tack J, Tornblom H, Tan V, Carbone F. Evidence based and emerging dietary approaches to upper Disorders of Gut‐Brain Interaction. Am J Gastroenterol. 2022;117(6):965–72. 10.14309/ajg.0000000000001780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gibson PR, Shepherd SJ. Evidence‐based dietary management of functional gastrointestinal symptoms: the FODMAP approach. J Gastroenterol Hepatol. 2010;25(2):252–8. 10.1111/j.1440-1746.2009.06149.x [DOI] [PubMed] [Google Scholar]
- 15. Dale HF, Biesiekierski JR, Lied GA. Non‐coeliac gluten sensitivity and the spectrum of gluten‐related disorders: an updated overview. Nutr Res Rev. 2019;32(1):28–37. 10.1017/s095442241800015x [DOI] [PubMed] [Google Scholar]
- 16. Major G, Pritchard S, Murray K, Alappadan JP, Hoad CL, Marciani L, et al. Colon hypersensitivity to distension, rather than excessive gas production, produces Carbohydrate‐related symptoms in Individuals with irritable bowel syndrome. Gastroenterology. 2017;152(1):124–33.e2. 10.1053/j.gastro.2016.09.062 [DOI] [PubMed] [Google Scholar]
- 17. McIntosh K, Reed DE, Schneider T, Dang F, Keshteli AH, De Palma G, et al. FODMAPs alter symptoms and the metabolome of patients with IBS: a randomised controlled trial. Gut. 2017;66(7):1241–51. 10.1136/gutjnl-2015-311339 [DOI] [PubMed] [Google Scholar]
- 18. Skodje GI, Sarna VK, Minelle IH, Rolfsen KL, Muir JG, Gibson PR, et al. Fructan, rather than gluten, induces symptoms in patients with self‐reported non‐celiac gluten sensitivity. Gastroenterology. 2018;154(3):529–39.e2. 10.1053/j.gastro.2017.10.040 [DOI] [PubMed] [Google Scholar]
- 19. Algera JP, Storsrud S, Lindstrom A, Simren M, Tornblom H. Gluten and fructan intake and their associations with gastrointestinal symptoms in irritable bowel syndrome: a food diary study. Clin Nutr. 2021;40(10):5365–72. 10.1016/j.clnu.2021.09.002 [DOI] [PubMed] [Google Scholar]
- 20. Chey WD, Keefer L, Whelan K, Gibson PR. Behavioral and diet therapies in integrated care for patients with irritable bowel syndrome. Gastroenterology. 2021;160(1):47–62. 10.1053/j.gastro.2020.06.099 [DOI] [PubMed] [Google Scholar]
- 21. Monsbakken KW, Vandvik PO, Farup PG. Perceived food intolerance in subjects with irritable bowel syndrome‐‐ etiology, prevalence and consequences. Eur J Clin Nutr. 2006;60(5):667–72. 10.1038/sj.ejcn.1602367 [DOI] [PubMed] [Google Scholar]
- 22. Mutsekwa RN, Larkins V, Canavan R, Ball L, Angus RL. A dietitian‐first gastroenterology clinic results in improved symptoms and quality of life in patients referred to a tertiary gastroenterology service. Clin Nutr ESPEN. 2019;33:188–94. 10.1016/j.clnesp.2019.05.016 [DOI] [PubMed] [Google Scholar]
- 23. Simons M, Taft TH, Doerfler B, Ruddy JS, Bollipo S, Nightingale S, et al. Narrative review: risk of eating disorders and nutritional deficiencies with dietary therapies for irritable bowel syndrome. Neuro Gastroenterol Motil. 2022;34(1):e14188. 10.1111/nmo.14188 [DOI] [PubMed] [Google Scholar]
- 24. Colomier E, Van Oudenhove L, Tack J, Bohn L, Bennet S, Nybacka S, et al. Predictors of symptom‐specific treatment response to dietary interventions in irritable bowel syndrome. Nutrients. 2022;14(2):397. 10.3390/nu14020397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schindler V, Giezendanner S, Van Oudenhove L, Murray FR, Buehler J, Bordier V, et al. Better response to low FODMAP diet in disorders of gut‐brain interaction patients with pronounced hydrogen response to a nutrient challenge test. J Gastroenterol Hepatol. 2021;36(12):3322–8. 10.1111/jgh.15573 [DOI] [PubMed] [Google Scholar]
- 26. Rossi M, Aggio R, Staudacher HM, Lomer MC, Lindsay JO, Irving P, et al. Volatile organic compounds in feces associate with response to dietary intervention in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2018;16(3):385–91.e1. 10.1016/j.cgh.2017.09.055 [DOI] [PubMed] [Google Scholar]
- 27. Zhang Y, Feng L, Wang X, Fox M, Luo L, Du L, et al. Low fermentable oligosaccharides, disaccharides, monosaccharides, and polyols diet compared with traditional dietary advice for diarrhea‐predominant irritable bowel syndrome: a parallel‐group, randomized controlled trial with analysis of clinical and microbiological factors associated with patient outcomes. Am J Clin Nutr. 2021;113(6):1531–45. 10.1093/ajcn/nqab005 [DOI] [PubMed] [Google Scholar]
- 28. Posserud I, Strid H, Storsrud S, Tornblom H, Svensson U, Tack J, et al. Symptom pattern following a meal challenge test in patients with irritable bowel syndrome and healthy controls. United European Gastroenterol J. 2013;1(5):358–67. 10.1177/2050640613501817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wright‐McNaughton M, Ten Bokkel Huinink S, Frampton CMA, McCombie AM, Talley NJ, Skidmore PML, et al. Measuring diet intake and gastrointestinal symptoms in irritable bowel syndrome: validation of the food and symptom times diary. Clin Transl Gastroenterol. 2019;10(12):e00103. 10.14309/ctg.0000000000000103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ong DK, Mitchell SB, Barrett JS, Shepherd SJ, Irving PM, Biesiekierski JR, et al. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J Gastroenterol Hepatol. 2010;25(8):1366–73. 10.1111/j.1440-1746.2010.06370.x [DOI] [PubMed] [Google Scholar]
- 31. Le Neve B, Brazeilles R, Derrien M, Tap J, Guyonnet D, Ohman L, et al. Lactulose challenge determines visceral sensitivity and severity of symptoms in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2016;14(2):226–33.e1‐3. 10.1016/j.cgh.2015.09.039 [DOI] [PubMed] [Google Scholar]
- 32. Sjolund J, Kull I, Bergstrom A, Jaras J, Ludvigsson JF, Tornblom H, et al. Allergy‐related diseases in childhood and risk for abdominal pain‐related functional gastrointestinal disorders at 16 years‐a birth cohort study. BMC Med. 2021;19(1):214. 10.1186/s12916-021-02069-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nybacka S, Ohman L, Storsrud S, Mybeck M, Bohn L, Wilpart K, et al. Neither self‐reported atopy nor IgE‐mediated allergy are linked to gastrointestinal symptoms in patients with irritable bowel syndrome. Neuro Gastroenterol Motil. 2018;30(10):e13379. 10.1111/nmo.13379 [DOI] [PubMed] [Google Scholar]
- 34. Fritscher‐Ravens A, Pflaum T, Mosinger M, Ruchay Z, Rocken C, Milla PJ, et al. Many patients with irritable bowel syndrome have atypical food allergies not associated with Immunoglobulin. Gastroenterol. 2019;157(1):109–18.e5. 10.1053/j.gastro.2019.03.046 [DOI] [PubMed] [Google Scholar]
- 35. Aguilera‐Lizarraga J, Florens MV, Viola MF, Jain P, Decraecker L, Appeltans I, et al. Local immune response to food antigens drives meal‐induced abdominal pain. Nature. 2021;590(7844):151–6. 10.1038/s41586-020-03118-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bischoff SC, Mayer J, Wedemeyer J, Meier PN, Zeck‐Kapp G, Wedi B, et al. Colonoscopic allergen provocation (COLAP): a new diagnostic approach for gastrointestinal food allergy. Gut. 1997;40(6):745–53. 10.1136/gut.40.6.745 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable–no new data generated.


