Abstract
Osteocalcin (OCN) is a bone‐derived and vitamin K dependent hormone that affects energy metabolism and vascular calcification. The relationship between serum OCN and vascular function in patients with chronic kidney disease (CKD) is uncertain. This study investigated the association between serum OCN and vascular function as expressed with reactive hyperemia index (RHI) and augmentation index (AIx) measured by Endo‐PAT 2000 device. This cross‐sectional analysis was based on 256 pre‐dialysis CKD patients who had completed the Endo‐PAT 2000 test and serum OCN at the First Center of Chinese PLA Hospital from November 2017 to December 2019. Based on whether the RHI was less than 1.67, the patients were divided into endothelial dysfunction and normal endothelial function groups. Multiple logistic and linear regression were used to analyze the association between OCN and vascular function. Subgroup analyses were performed to examine the effects of OCN on vascular function in different CKD populations. After multivariate adjustment, CKD with low OCN were more likely to have endothelial dysfunction (OR: 0.794; 95%CI: 0.674‐0.934; P = .006); on the contrary, patients with high OCN had a higher degree of arterial stiffness (standardized β: 0.174; P = .003). Subgroup analyses showed that higher OCN was associated with severe arterial stiffness but a better endothelial function in young (age < 65 years, P RHI/P AIx@75 = .027/.011), male (P RHI/P AIx@75 = .040/.016), patients with a history of hypertension (P RHI/P AIx@75 = .004/.009) or diabetes (P RHI/P AIx@75 = .005/.005), and in early CKD (P RHI/P AIx@75 = .014/.015). In conclusion, serum OCN correlates with vascular function in CKD patients: beneficial for endothelial function but detrimental to arterial stiffness.
Keywords: arterial stiffness, Endo‐PAT 2000, endothelial dysfunction, osteocalcin, vascular function
1. INTRODUCTION
Osteocalcin (OCN), a vitamin K dependent hormone derived from osteoblasts, plays a role in bone mineralization, implicates the activity of osteoblasts and osteoclasts, and regulates the energy metabolism of glucose and lipids. 1 As a marker of bone turnover, increasing studies suggest that OCN is also associated with vascular health, including endothelial function and vascular calcification. 2 , 3 , 4 Uremia and abnormal mineral metabolism are significant factors affecting vascular health, which precede the onset of cardiovascular disease (CVD) and lead to morbidity and mortality of chronic kidney disease (CKD). 5 , 6 However, recent studies exploring the effects of OCN on blood vessels, including endothelial function and vessel wall properties, have yielded significantly different results. Several studies have reported that higher circulating total OCN indirectly mediates vascular health via regulating metabolic markers, 2 , 7 and daily supplements of OCN can benefit vascular endothelial function. 8 Nevertheless, others have suggested that OCN correlates with indicators of arterial stiffness such as pulse wave velocity (PWV) and may play a vital role in the phenotype conversion of vascular smooth muscle cells (VSMCs) to bone‐forming cells. 9 , 10 , 11 , 12 OCN comes in two forms, uncarboxylated OCN (ucOCN) and carboxylated OCN (cOCN), with different physiological functions. 13 Animal studies have shown that the major pathway for the catabolism of circulating OCN is kidney filtration and degradation. 14 Elevated OCN is typical in CKD patients 15 ; however, the association between elevated OCN with vascular function in CKD patients remains unknown. Given the importance of vascular function in renal disease, it is imperative to investigate whether serum OCN is associated with vascular function in CKD patients and whether these effects are beneficial or detrimental.
Endo‐PAT 2000 (Itamar Medical, Israel) test is a noninvasive, automated, and recognized technique for measuring vascular function that simultaneously assesses both the reactive hyperemia index (RHI), which is indicative of endothelial vasodilator function, and the digital augmentation index (AIx) to indicate arterial stiffness. 16 Endo‐PAT diagnostic system records peripheral arterial tension (PAT) to determine the fingertips pulses volume change before and after unilateral blood flow occlusion by comparing it with the contralateral arm signal.
In light of previous evidence linking OCN to vascular health, we hypothesized that OCN had a relationship with vascular function in CKD patients. We tested this hypothesis in non‐dialysis CKD patients.
2. METHODS
2.1. Participants
Patients aged ≥ 18 years with the diagnosis of CKD stage 1–5 (non‐dialysis dependent) who were referred to our center were invited to participate in this study from November 2017 to December 2019. The diagnoses of CKD conformed to the KDIGO (Kidney Disease: Improving Global Outcomes) guidelines 17 and were confirmed by the research staff before enrollment. The exclusion criteria included patients who were unsuitable for the assessment of Endo‐PAT device, including those who had finger wounds; Reynolds phenomenon; and patients with arteriovenous fistula. We also excluded other unstable conditions when the research staff considered the patients unsuitable for participation.
This cross‐sectional study was approved by the Ethics Committee of the Chinese People's Liberation Army General Hospital (No. S2017‐038‐01). All participants provided written informed consent before enrollment.
2.2. General data collection
Patients were interviewed by research staff to obtain their general demographic and medical information. The available demographics include age, sex, height, weight, smoking status (current/non‐current smoking), and previous medical history of hypertension and diabetes. Body mass index (BMI) was calculated by dividing the weight in kilograms by the square of height in meters. Researchers also validated all disease conditions via medical records or kin of patients following the clinical diagnosis criteria. Given that medications affect vascular function, angiotensin‐converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB), statins, and aspirin may benefit endothelial vasodilator function 16 ; we searched the medical record system or interviewed patients to record the medications the patient was taking. Drugs of interest included ACEI/ARB, antiplatelet (aspirin and clopidogrel), and statins. Blood pressure (BP) was measured using an automated oscillometric device (Omron, Kyoto, Japan) within 5 minutes before the Endo‐PAT 2000 test. Estimated GFR (eGFR) was calculated according to the CKD Epidemiology Collaboration (CKD‐EPI) creatinine equation. 18
2.3. Osteocalcin and laboratory test
All biochemical analyses were conducted by the Clinical Diagnostic Laboratory of the Chinese PLA general hospital. Total cholesterol (TC), triglycerides (TG), high‐density lipoprotein (HDL), low‐density lipoprotein (LDL), fasting blood glucose (FBG), and urine protein were collected. Concentrations of OCN combined with other bone turnover markers, including parathyroid hormone (PTH), 25‐hydroxyvitamin D (25(OH)D), were measured by an automatic electrochemical luminescence method (Roche, Mannheim, Germany; [coefficient of variation, 10%]). This technique measured OCN concentration at 0.5–300 ng/mL.
2.4. Vascular function measurement
The Endo‐PAT 2000 device (Itamar Medical, Caesarea, Israel) assessed RHI and AIx, representing endothelial function and arterial stiffness. The test was performed once per patient by skilled investigators following the protocol instructions. 19 Participants were tested at a temperature of 21°C to 24°C, with dimmed light and a quiet environment, and all were in the supine position. The occluded cuff was placed on one upper arm, and finger probes were placed on the index finger of each hand to record the signal. The process consisted of 5‐minute baseline, 5‐minute occlusion, and 5‐minute post‐occlusion signal records. Occlusion pressure, the inflation pressure of the blood cuff, was 60 mmHg above SBP measured before the test or 200 mmHg; at a pressure of that, complete occlusion was achieved as judged by the device. Both RHI and AIx values were automatically generated, including the natural logarithm RHI (LnRHI) and AIx@75, which meant AIx corrected for heart rate 75 since wave reflections were easily affected by heart rate. RHI is the ratio of the amplitude of the PAT signal (fingertips pulses volume change) after cuff deflation divided by amplitude before cuff inflation, adjusted to the contralateral arm. Higher RHI indicates better endothelial vasodilator function and vascular reactivity. Based on previous studies, patients with an RHI below 1.67 were considered endothelial dysfunction. 20
2.5. Statistical analysis
Numerical variables were presented as mean ± SD or median (IQR) based on distributions. Dichotomous variables were presented as frequency (%). Correlation coefficients between variables and OCN were analyzed by Spearman correlation. We divided the patients into two groups, one with RHI values below 1.67 indicating endothelial dysfunction and other values corresponding to normal or enhanced endothelial function. We explored the association between OCN with endothelial dysfunction and AIx@75 with binary logistic regression and linear regression models. We also analyzed OCN concentrations in endothelial function groups and AIx@75 tertiles and compared the differences. Additionally, subgroup analyses stratified by age, gender, hypertension, diabetes, and eGFR were performed to analyze the effects of OCN on vascular function in different CKD populations. We completed the analyses with IBM SPSS 22.0 software (SPSS Institute; IBM).
3. RESULTS
3.1. Baseline characteristics
Patient baseline characteristics are summarized in Table 1. A total of 256 non‐dialysis patients with serum OCN and Endo‐PAT 2000 measurement records were enrolled in the current study. The median age and eGFR were 58 (45‐64) years and 51.00 (30.33‐79.55) mL/min/1.73 m2, respectively. The OCN values were skewed, with a 15.15 (9.91‐23.12) ng/mL median. The proportion of patients with endothelial dysfunction (RHI < 1.67) was 44.1%. The mean AIx@75 was 10 ± 17%.
TABLE 1.
Clinical characteristics of participants
| Variables | All (no. = 256) |
|---|---|
| Age (years) | 58 (45‐64) |
| Male sex (%) | 166 (64.8) |
| BMI (kg/m2) | 25.26 ± 3.60 |
| Official BP | |
| Systolic BP (mmHg) | 127 (115‐137) |
| Diastolic BP (mmHg) | 78 (70‐84) |
| Current smoker (%) | 52 (20.3) |
| Hypertension (%) | 184 (71.9) |
| Diabetes (%) | 88 (34.4) |
| FBG (mmol/L) | 4.64 (4.17‐5.38) |
| TC (mmol/L) | 4.49 (3.70‐5.45) |
| TG (mmol/L) | 1.76 (1.16‐2.78) |
| HDL (mmol/L) | 1.10 (0.86‐1.42) |
| LDL (mmol/L) | 2.80 (1.93‐3.68) |
| Osteocalcin (ng/mL) | 15.15 (9.91‐23.12) |
| 25(OH)D (ng/mL) | 10.7 (5.95‐15.70) |
| PTH (pg/mL) | 35.05 (23.55‐62.46) |
| eGFR (mL/min/1.73 m2) | 51.00 (30.33‐79.55) |
| Urine protein (g/day) | 2.22 (0.70‐4.51) |
| Endo‐PAT 2000 parameters | |
| RHI | 1.72 (1.47‐2.09) |
| RHI < 1.67 (%) | 113 (44.1) |
| LnRHI | 0.54 (0.39‐0.74) |
| AIx@75 (%) | |
| Tertile 1 (range) | −32 to 2 |
| Tertile 2 (range) | 3 to 16 |
| Tertile 3 (range) | 17 to 70 |
| Medications | |
| ACEI/ARB (%) | 139 (54.3) |
| Antiplatelet (%) | 59 (23.0) |
| Statins (%) | 122 (47.7) |
Note: Categorical characteristics were presented as number (percentage), and continuous characteristics were presented as mean ± standard deviation or median (25th‐75th percentile). Antiplatelet included aspirin and clopidogrel.
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitors; AIx@75, augmentation index standardized to a heart rate of 75; ARB, angiotensin receptor blockers; BP, blood pressure; eGFR, estimated glomerular filtration rate; FBG, fasting blood glucose; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; LnRHI, the natural logarithm RHI; PTH, parathyroid hormone; RHI, reactive hyperemia index; TC, total cholesterol; TG, triglyceride.
3.2. Relationships between serum osteocalcin level and variables
Table 2 summarizes the correlation coefficients between OCN concentration and the baseline characteristics of CKD patients. There was a positive but modest correlation between OCN and vascular function including RHI and AIx@75 (r = 0.203, P = .001; r = 0.193, P = .002, Table 2). In addition, OCN positively correlated with SBP and PTH but negatively with BMI, TC, HDL, and eGFR. In our study, there was no relationship between OCN and FBG. Patients with endothelial dysfunction (RHI < 1.67, Figure 1) had significantly lower levels of OCN (P < .001). In arterial stiffness (expressed by AIx@75) groups, OCN concentration was the highest in tertile three of AIx@75 (P = .011).
TABLE 2.
Correlation coefficients between serum osteocalcin and baseline characteristics of CKD patients
| r | P | |
|---|---|---|
| Age (years) | −0.088 | .161 |
| BMI (kg/m2) | −0.127 | .042 |
| Systolic BP (mmHg) | 0.200 | .001 |
| Diastolic BP (mmHg) | 0.119 | .057 |
| FBG (mmol/L) | 0.031 | .625 |
| TC (mmol/L) | −0.172 | .006 |
| TG (mmol/L) | −0.112 | .076 |
| HDL (mmol/L) | −0.369 | <.001 |
| LDL (mmol/L) | −0.099 | .116 |
| RHI | 0.203 | .001 |
| AIx@75 (%) | 0.193 | .002 |
| 25(OH)D (ng/mL) | 0.024 | .697 |
| PTH (pg/mL) | 0.644 | <.001 |
| eGFR (mL/min/1.73 m2) | −0.595 | <.001 |
| Urine protein (g/day) | 0.096 | .127 |
Note: Spearman correlation for variables was used to analyze correlation coefficients.
FIGURE 1.
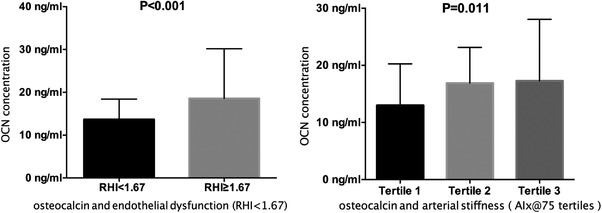
Comparison of osteocalcin in different vascular function groups. Tertile 1 of AIx@75 (range): ‐32 to 2; tertile 2 (range): 3 to 16; tertile 3 (range): 17 to 70.
3.3. Association between variables including osteocalcin and endothelial dysfunction (RHI < 1.67)
We investigated the association between variables, including OCN (per 10 ng/mL) and RHI < 1.67 (Table 3). Univariate logistic regression analysis showed a protective association between OCN and endothelial dysfunction, with an OR of 0.789 (95%CI: 0.670‐0.928, P = .004). Moreover, multivariate logistic regression analysis, including age, sex, and other factors selected by univariate analysis based on P < .1, identified that OCN (OR: 0.794; 95%CI: 0.674‐0.934; P = .006), with age and sex, was significantly associated with endothelial dysfunction.
TABLE 3.
Binary logistic regression analyses of variables contributing to endothelial dysfunction (RHI < 1.67) in CKD patients
| Univariate logistic regression analysis | Multiple logistic regression analysis | |||||
|---|---|---|---|---|---|---|
| Wald χ 2 | P | OR (95% CI) | Wald χ 2 | P | OR (95% CI) | |
| Age (per 10 years) | 7.454 | .006 | 1.311 (1.079‐1.592) | 9.399 | .009 | 1.389 (1.126‐1.714) |
| Female | 3.652 | .056 | 0.604 (0.360‐1.013) | 5.038 | .025 | 0.536 (0.311‐0.924) |
| Current smoker | 0.107 | .743 | 0.903 (0.490‐1.664) | |||
| BMI (kg/m2) | 1.554 | .213 | 0.957 (0.892‐1.026) | |||
| SBP (mmHg) | 3.041 | .081 | 0.986 (0.971‐1.002) | |||
| DBP (mmHg) | 0.168 | .682 | 0.995 (0.973‐1.018) | |||
| Diabetes | 0.326 | .568 | 1.163 (0.693‐1.953) | |||
| eGFR (per 10 mL/min/1.73 m2) | 5.734 | .017 | 1.105 (1.018‐1.198) | |||
| Urine protein (g/day) | 1.733 | .188 | 0.935 (0.845‐1.034) | |||
| FBG (mmol/L) | 0.195 | .659 | 0.966 (0.829‐1.126) | |||
| TC (mmol/L) | 0.128 | .721 | 1.030 (0.878‐1.208) | |||
| TG (mmol/L) | 1.040 | .308 | 0.915 (0.771‐1.086) | |||
| HDL (mmol/L) | 3.207 | .073 | 1.666 (0.953‐2.913) | |||
| LDL (mmol/L) | 0.407 | .523 | 0.899 (0.885‐1.272) | |||
| Osteocalcin (per 10 ng/mL) | 8.170 | .004 | 0.789 (0.670‐0.928) | 7.707 | .006 | 0.794 (0.674‐0.934) |
| 25(OH)D (ng/mL) | 0.379 | .538 | 1.061 (0.978‐1.043) | |||
| PTH (pg/mL) | 5.251 | .022 | 0.992 (0.986‐0.999) | |||
| ACEI/ARB use | 0.678 | .900 | 1.191 (0.548‐1.478) | |||
| Antiplatelet use | 0.779 | .377 | 0.769 (0.429‐1.378) | |||
| Statins use | 1.679 | .195 | 0.721 (0.439‐1.183) | |||
Note: Age, sex, and other factors with P < .1 in univariate logistic regression were included in multiple logistic forward regression.
3.4. Association between variables including osteocalcin and arterial stiffness (AIx@75)
We also explored the role of OCN in arterial stiffness (Table 4). A positive association between OCN and AIx@75 was detected in univariate linear regression (β = 0.182, P = .003); and, even after adjusting for age, blood pressure, diabetes, eGFR, and PTH, OCN (β = 0.174, P = .003) was still associated with arterial stiffness.
TABLE 4.
Linear regression analyses of variables contributing to AIx@75 in CKD patients
| Univariate linear regression | Multiple linear regression | |||
|---|---|---|---|---|
| Standardized β | P | Standardized β | P | |
| Age (per 10 years) | 0.251 | <.001 | 0.201 | .001 |
| Female | 0.071 | .256 | ||
| Current smoker | 0.006 | .930 | ||
| BMI (kg/m2) | −0.067 | .286 | ||
| SBP (mmHg) | 0.310 | <.001 | 0.246 | <.001 |
| DBP (mmHg) | 0.167 | .007 | ||
| Diabetes | 0.137 | .028 | ||
| eGFR (per 10 mL/min/1.73 m2) | −0.249 | <.001 | ||
| Urine protein (g/day) | 0.085 | .177 | ||
| Fasting blood glucose (mmol/L) | −0.024 | .707 | ||
| TC (mmol/L) | −0.050 | .426 | ||
| TG (mmol/L) | −0.012 | .846 | ||
| HDL (mmol/L) | −0.079 | .207 | ||
| LDL (mmol/L) | −0.046 | .466 | ||
| Osteocalcin (ng/mL) | 0.182 | .003 | 0.174 | .003 |
| 25(OH)D (ng/mL) | −0.023 | .715 | ||
| PTH (pg/mL) | 0.179 | .004 | ||
| ACEI/ARB use | 0.040 | .522 | ||
| Antiplatelet use | 0.058 | .354 | ||
| Statins use | 0.046 | .734 | ||
Note: Age, eGFR, and other factors with P < .1 in univariate linear regression were included in this multiple linear stepwise regression.
3.5. Subgroup analyses of the association between osteocalcin and vascular function
Based on age (< 65 years or ≥ 65 years), gender, hypertension, diabetes, and eGFR level (≥ 60 mL/min/1.73 m2 or eGFR < 60 mL/min/1.73 m2), we further performed subgroup analyses of the association between OCN and vascular function. In Table 5, the associations of OCN and vascular function were relatively stable across subgroups and were more pronounced in young (age < 65 years, P RHI/P AIx@75 = .027/.011), male (P RHI/P AIx@75 = .040/.016), patients with a history of hypertension (P RHI/P AIx@75 = .004/.009) or diabetes (P RHI/P AIx@75 = .005/.005), and in early CKD (P RHI/P AIx@75 = .014/.015). In these populations, high levels of OCN were associated with high levels of RHI and AIx@75, which was consistent with the results in the general population.
TABLE 5.
Subgroup analyses of the relationship between OCN (per 10 ng/mL) and vascular function
| Endothelial function (RHI) | Arterial stiffness (AIx@75) | |||
|---|---|---|---|---|
| Groups | OR (95%CI) | P | Standardized β | P |
| Total | 0.794 (0.674‐0.934) | .006 | 0.174 | .003 |
| Age | ||||
| < 65 years | 0.828 (0.700‐0.978) | .027 | 0.159 | .011 |
| ≥ 65 years | 0.729 (0.466‐1.139) | .165 | 0.200 | .164 |
| Gender | ||||
| Male | 0.780 (0.615‐0.989) | .040 | 0.178 | .016 |
| Female | 0.822 (0.663‐1.020) | .075 | 0.164 | .106 |
| Hypertension | ||||
| Yes | 0.722 (0.579‐0.901) | .004 | 0.186 | .009 |
| No | 1.165 (0.830‐1.634) | .377 | 0.079 | .503 |
| Diabetes | ||||
| Yes | 0.569 (0.385‐0.841) | .005 | 0.299 | .005 |
| No | 0.874 (0.734‐1.041) | .130 | 0.115 | .111 |
| eGFR | ||||
| ≥ 60 mL/min/1.73 m2 | 0.791 (0.655‐0.954) | .014 | 0.201 | .015 |
| < 60 mL/min/1.73 m2 | 0.808 (0.446‐1.465) | .483 | −0.068 | .423 |
Note: Subgroup analyses were based on multivariate analysis, including statistically significant covariates in multiple logistic regression or multiple linear regression: associations between osteocalcin and endothelial function adjusted for age and sex; osteocalcin and arterial stiffness adjusted for age and systolic blood pressure.
4. DISCUSSION
Previous studies reported that serum OCN seemed to have an important influence on bone and muscle strength, 21 energy metabolism, 22 brain development, 23 vascular function, 2 and male fertility. 24 It appears to be an important endocrine hormone that acts throughout the body. This CKD study found that OCN correlated with lipid profile, kidney function, and vascular function. We discovered that OCN was inversely associated with total cholesterol, which confirmed the involvement of OCN in energy regulation. However, the negative correlation between OCN and HDL should be noted. This finding is different from most previous studies reporting a positive correlation between serum OCN levels and HDL levels, and the difference may be partly due to the prevalence of lipid metabolism disorders in patients with renal disease, which requires more research to explore and confirm. We also detected a negative correlation between OCN and renal function, mainly caused by reduced glomerular filtration capacity. Notably, there was no relationship between OCN and vitamin D in our study, which may not support the observation that vitamin D is related to OCN synthesis. 25 Our results suggested that OCN had beneficial effects on endothelial function but detrimental effects on arterial stiffness. Next, we will focus on this discrepancy and discuss the underlying mechanisms.
4.1. Osteocalcin and endothelial function
The endothelium produces vasodilator and vasoconstrictor molecules and plays a fundamental role in regulating vascular tone and activity. Endothelial dysfunction is diagnosed as impaired endothelium‐dependent vasodilation by reducing the bioavailability of nitric oxide (NO). 26 , 27 Increasing evidence suggests a link between OCN and endothelial dysfunction. Qi Huan Lv and associates 28 reported that lower OCN was negatively associated with femoral intima‐media thickness in type 2 diabetes mellitus patients without renal dysfunction. Tawar Qaradakhi and associates 4 reported that ucOCN in vitro incubation could improve the impaired endothelium‐dependent vasodilation in rabbit abdominal aorta on an atherogenic diet for 4 weeks. Whether the improvement in vascular function by OCN is due to the lowering of blood sugar and lipids or the direct effect of OCN on vascular function is still uncertain. In an age and sex‐matched case‐control study, 61 male participants underwent coronary artery bypass grafting (CABG), and 61 were controls. In persons with CABG, the serum ucOCN level was decreased compared with those in the controls, regardless of energy metabolism status and renal function. 29 Furthermore, in our multivariate logistic regression, metabolic indicators such as BMI, blood lipids, and glucose were not associated with endothelial function, suggesting that the protective effect of OCN on endothelial function in CKD may not be affected by energy metabolism. OCN was expressed in all vascular layers, including endothelium, VSMCs, and adventitia. 4 , 30 Chang Hee Jung and associates 31 reported that, in human aortic endothelial cells, daily ucOCN treatment increased NO secretion from endothelial cells and partially reversed the inhibitory effect of free fatty acid on insulin signaling, exerting an anti‐apoptotic influence on vascular endothelial cells via the regulation of the phosphoinositide 3‐kinase/protein kinase B/endothelial NO synthase (PI3K/Akt/eNOs) signaling pathway. Phosphorylation of Akt/eNOS by ucOCN was also inhibited by the addition of Wortmannin, an inhibitor of PI3K. In addition, evidence from in vivo and in vitro experiments suggests that OCN could inhibit autophagy and endoplasmic reticulum stress and improve insulin signaling in vascular tissues and cells in the setting of insulin resistance via PI3K/Akt/NFκB/mTOR signaling pathway, 32 , 33 which plays a role in protecting vascular function. The receptors of OCN in the vasculature are yet to be identified. GPRC6A (G‐protein coupled receptor family C group 6 member A) was demonstrated to mediate the response of OCN in pancreatic beta‐cell to promote insulin secretion. Previous studies also have identified GPRC6A as an OCN receptor in mouse testis 34 and skeletal muscle. 35 Using the proximity ligation assays and immunohistochemistry, Tawar Qaradakhi's group 4 demonstrated the presence of ucOC and GPRC6A in human and diseased rabbit arteries and determined their physical proximity (<40 nm); OCN binding to GPRC6A in endothelial cells may exert vasoprotective effects, and help to provide a potential target for improving vascular health.
Anna Bar and associates 36 conducted a study to explore the effect of Vitamin K (the synthase of OCN) on vascular endothelial function. In ApoE/LDLR−/− mice with or without atherosclerotic plaques, treatment with a low (0.03 mg/kg) or high (10 mg/kg) dose of vitamin K2 improved acetylcholine and flow‐induced endothelium‐dependent vasodilation and increased in plasma nitrate concentration. Following a 12‐week high‐fat diet with daily injections of vehicle or OCN (30 ng/g), the dilation of the thoracic aorta was improved by 20% in the OCN‐treated mice compared to the vehicle‐treated mice. 8 Additionally, vascular endothelial function was measured in 24 atrial fibrillation patients who had been treated with warfarin, a vitamin K antagonist, for at least 12 months and 18 patients without warfarin. Those patients who received warfarin treatment had significant lower RHI values compared to those in non‐warfarin group (1.48 ± 0.11 vs 1.88 ± 0.12 ng/mL; P = .017). 37 However, some studies have found the opposite. Alexander Tacey and associates 38 reported that ucOCN acute treatment had no positive effect on endothelial vasodilation or markers of vasodilation in rabbit aorta ex vivo and human vascular cells in vitro in the presence or absence of high glucose; and the clinical study part found no association between ucOCN and brachial artery flow‐mediated dilation (FMD), another noninvasive measure of endothelial function, in healthy elderly patients with an average age of 73 years. Hiroyuki Sumino and associates 39 performed the Pearson correlation analysis of OCN with FMD in 85 postmenopausal women and reported no correlation. Above conflicting results may be due to inconsistencies in population, OCN modalities, or the location and size of measured vessels.
4.2. Osteocalcin and arterial stiffness
Vascular calcification is a pivotal cause and manifestation of arterial stiffness. It was considered a passive hydroxyapatite deposition in the vessel wall for many years; recently, understanding the phenotypic transformation of VSMCs into bone or chondroid cells has become increasingly accepted. Our finding of a positive correlation between serum OCN and digital AIx@75 is consistent with previous studies demonstrating an association between OCN and arterial stiffness. It suggests that elevated OCN in CKD may play a role in arterial wall remodeling and lead to a high incidence of cardiovascular disease in patients with CKD. Besides, the correlation between OCN and AIx@75 was independent of age, eGFR, blood pressure, diabetes, and PTH.
Currently, the pathophysiological mechanism of OCN on the development of arteriosclerosis is not fully known. 2 Some researchers assumed that OCN could mediate the vasculature via the metabolic effects 7 ; however, as mentioned before, our results showed that these glucose and lipid parameters did not contribute to arterial stiffness in multivariate analysis. Some researchers consider that OCN can bind to the hydroxyapatite mineral of bone with high affinity and promote bone mineralization, and in this context, it is reported that OCN is involved in calcifying VSMCs. Other researchers have suggested that OCN is involved in vascular calcification, possibly due to the transformation of VSMCs into an osteogenic phenotype. The fact that OCN levels are related to an increased risk of arteriosclerosis contrasts with the possible beneficial effects on endothelial function. OCN is increased in patients with cardiovascular disease and is thought to be associated with vascular calcification and cardiovascular disease. 30 , 40 , 41 Among patients undergoing coronary bypass surgery, their blood OCN concentrations correlated with OCN levels in coronary atherosclerotic plaques and were higher in patients with calcified coronary plaques; in addition, OCN content increased with the size of the calcification deposits in plaque. 42 Recent studies have reported that OCN‐expressing endothelial progenitor cells may involve vessel stiffness; they are generated in the bone marrow and secreted into vascular regions impaired by ischemia or endothelial dysfunction and mediate abnormal repair. 43 , 44 In vivo rat model of vitamin D induced vascular calcification revealed a correlation between elevated OCN levels. It increased hypoxia‐inducible factor‐1 alpha (HIF‐1α) expression in aortas, demonstrating a potential mechanism by which OCN stimulates expression of osteochondrogenic transcription factors in VSMCs via a HIF‐1α dependent manner which plays a crucial role in cartilage formation and survival. 45 In contrast, some studies supported no relationship between OCN and vascular calcification in CKD and non‐CKD patients and no evidence of an active role of ucOCN in arterial stiffness. 46 , 47 Not only that, carotid‐femoral pulse wave velocity (cf‐PWV), the gold standard for assessing arterial stiffness, was shown to be inversely correlated with OCN in hemodialysis patients, 9 implying that OCN may have a protective effect on vascular structures. However, this study also found that PWV was negatively associated with metabolic wastes (creatinine, urea nitrogen, phosphate, potassium, and PTH), for which the authors did not provide a clear explanation. Given these conflicting findings, further extensive research is needed on the link between OCN and atherosclerosis.
Some limitations in this study need to note. First, as mentioned before, we only investigated serum total OCN concentration; further research is warranted for the relations of ucOCN and cOCN with vascular function in CKD. Second, this was a cross‐sectional study, and thus we could not determine the causality between OCN and vascular function change. Third, many of CKD patients had glomerulonephritis with dyslipidemia, which may interfere with the analysis of the relationship between OCN and lipids.
In conclusion, OCN may provide new insights into the kidney‐bone‐cardiovascular axis; in patients with CKD, serum OCN is related to vascular function as measured by Endo‐PAT 2000. Higher OCN is associated with more severe arterial stiffness but better endothelial function.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
Xinru Guo: Substantial contributions to the conception, design, analysis, drafting of the work, and revising it critically for important intellectual content. Yisha Li: Contributions to collecting data for the work and revising it critically for important intellectual content. Yena Zhou: Contributions to collecting data and revising it critically for important intellectual content. Chun Zhang: Contributions to collecting data and analysis for the work. Shuang Liang: Contributions to collecting data and analysis for the work. Ying Zheng: Contributions to revising it critically for important intellectual content. Xiangmei Chen: Contributions to carrying out study and providing a research platform. Guangyan Cai: Substantial contributions to the conception, design, and revision of it critically for important intellectual content and final approval of the version to be published.
ACKNOWLEDGMENTS
The authors would like to thank all the participants in this study.
This study was supported by the National Key Technology R&D Program of China (2018YFA0108803), the National Key Technology Support Program of China (2015BAI12B06).
Guo X, Li Y, Zhou Y, et al. Osteocalcin association with vascular function in chronic kidney disease. J Clin Hypertens. 2022;24:928–936. 10.1111/jch.14523
REFERENCES
- 1. Wei J, Karsenty G. An overview of the metabolic functions of osteocalcin. Rev Endocr Metab Disord. 2015;16(2):93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tacey A, Qaradakhi T, Brennan‐Speranza T, Hayes A, Zulli A, Levinger I. Potential role for osteocalcin in the development of atherosclerosis and blood vessel disease. Nutrients. 2018;10(10):1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tacey A, Hayes A, Zulli A, Levinger I. Osteocalcin and vascular function: is there a cross‐talk? Mol Metab. 2021;49:101205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Qaradakhi T, Gadanec L, Tacey A, et al. The effect of recombinant undercarboxylated osteocalcin on endothelial dysfunction. Calcif Tissue Int. 2019;105(5):546–556. [DOI] [PubMed] [Google Scholar]
- 5. Reiss A, Miyawaki N, Moon J, et al. CKD, arterial calcification, atherosclerosis and bone health: inter‐relationships and controversies. Atherosclerosis. 2018;278:49–59. [DOI] [PubMed] [Google Scholar]
- 6. Kyriakidis N, Cobo G, Dai L, Lindholm B, Stenvinkel P. Role of uremic toxins in early vascular ageing and calcification. Toxins (Basel). 2021;13(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang L, Yang L, Luo L, Wu P, Yan S. Osteocalcin improves metabolic profiles, body composition and arterial stiffening in an induced diabetic rat model. Exp Clin Endocrinol Diabetes. 2017;125(4):234–240. [DOI] [PubMed] [Google Scholar]
- 8. Dou J, Li H, Ma X, et al. Osteocalcin attenuates high fat diet‐induced impairment of endothelium‐dependent relaxation through Akt/eNOS‐dependent pathway. Cardiovasc Diabetol. 2014;13:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Csiky B, Sági B, Peti A, Lakatos O, Prémusz V, Sulyok E. The impact of osteocalcin, osteoprotegerin and osteopontin on arterial stiffness in chronic renal failure patients on hemodialysis. Kidney Blood Press Res. 2017;42(6):1312–1321. [DOI] [PubMed] [Google Scholar]
- 10. Yun S, Kim M, Choi B, Park K, Park K, Kim Y. Low level of osteocalcin is related with arterial stiffness in Korean adults: an inverse J‐shaped relationship. J Clin Endocrinol Metab. 2016;101(1):96–102. [DOI] [PubMed] [Google Scholar]
- 11. Lin X, Li S, Wang Y, et al. Exosomal Notch3 from high glucose‐stimulated endothelial cells regulates vascular smooth muscle cells calcification/aging. Life Sci. 2019;232:116582. [DOI] [PubMed] [Google Scholar]
- 12. Chi P, Lin Y, Tasi J, et al. Osteocalcin and carotid‐femoral pulse wave velocity in patients on peritoneal dialysis. Ci Ji Yi Xue Za Zhi. 2019;31(1):23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tangseefa P, Martin S, Fitter S, Baldock P, Proud C, Zannettino A. Osteocalcin‐dependent regulation of glucose metabolism and fertility: skeletal implications for the development of insulin resistance. J Cell Physiol. 2018;233(5):3769–3783. [DOI] [PubMed] [Google Scholar]
- 14. Hauschka PV, Lian JB, Cole DE, Gundberg CM. Osteocalcin and matrix Gla protein: vitamin K‐dependent proteins in bone. Physiol Rev. 1989;69(3):990–1047. [DOI] [PubMed] [Google Scholar]
- 15. Kratz M, Zelnick L, Trenchevska O, et al. Relationship between chronic kidney disease, glucose homeostasis, and plasma osteocalcin carboxylation and fragmentation. J Ren Nutr. 2021;31(3):248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Axtell A, Gomari F, Cooke J. Assessing endothelial vasodilator function with the Endo‐PAT 2000. J Vis Exp. 2010(44):2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. PE S, A L. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–830. [DOI] [PubMed] [Google Scholar]
- 18. Levey A, Stevens L, Schmid C, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li Y, Yang Y, Wang W, et al. Peripheral arterial stiffness is correlated with intrarenal arteriolosclerosis according to biopsies from patients with kidney disease. Nephrology (Carlton). 2020;25(5):371–378. [DOI] [PubMed] [Google Scholar]
- 20. Koo B, Chung W, Moon M. Peripheral arterial endothelial dysfunction predicts future cardiovascular events in diabetic patients with albuminuria: a prospective cohort study. Cardiovasc Diabetol. 2020;19(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mohammad Rahimi G, Niyazi A, Alaee S. The effect of exercise training on osteocalcin, adipocytokines, and insulin resistance: a systematic review and meta‐analysis of randomized controlled trials. Osteoporos Int. 2021;32(2):213–224. [DOI] [PubMed] [Google Scholar]
- 22. Al‐Suhaimi E, Al‐Jafary M. Endocrine roles of vitamin K‐dependent‐osteocalcin in the relation between bone metabolism and metabolic disorders. Rev Endocr Metab Disord. 2020;21(1):117–125. [DOI] [PubMed] [Google Scholar]
- 23. Oury F, Khrimian L, Denny C, et al. Maternal and offspring pools of osteocalcin influence brain development and functions. Cell. 2013;155(1):228–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oury F, Sumara G, Sumara O, et al. Endocrine regulation of male fertility by the skeleton. Cell. 2011;144(5):796–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Giudici K, Fisberg R, Marchioni D, Peters B, Martini L. Crosstalk between bone and fat tissue: associations between vitamin D, osteocalcin, adipokines, and markers of glucose metabolism among adolescents. J Am Coll Nutr. 2017;36(4):273–280. [DOI] [PubMed] [Google Scholar]
- 26. Medina‐Leyte D, Zepeda‐García O, Domínguez‐Pérez M, González‐Garrido A, Villarreal‐Molina T, Jacobo‐Albavera L. Endothelial dysfunction, inflammation and coronary artery disease: potential biomarkers and promising therapeutical approaches. Int J Mol Sci. 2021;22(8):3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu S, Ilyas I, Little P, et al. Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: from mechanism to pharmacotherapies. Pharmacol Rev. 2021;73(3):924–967. [DOI] [PubMed] [Google Scholar]
- 28. Lv Q, Zhou J, Liu J, Kang D, Zhang H. Serum osteocalcin is inversely associated with lower extremity atherosclerotic disease in Chinese patients with type 2 diabetes mellitus. Endocr J. 2021;68(2):137–144. [DOI] [PubMed] [Google Scholar]
- 29. Kim K, Lim S, Moon J, et al. Lower uncarboxylated osteocalcin and higher sclerostin levels are significantly associated with coronary artery disease. Bone. 2016;83:178–183. [DOI] [PubMed] [Google Scholar]
- 30. Gössl M, Mödder U, Atkinson E, Lerman A, Khosla S. Osteocalcin expression by circulating endothelial progenitor cells in patients with coronary atherosclerosis. J Am Coll Cardiol. 2008;52(16):1314–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jung C, Lee W, Hwang J, et al. The preventive effect of uncarboxylated osteocalcin against free fatty acid‐induced endothelial apoptosis through the activation of phosphatidylinositol 3‐kinase/Akt signaling pathway. Metabolism. 2013;62(9):1250–1257. [DOI] [PubMed] [Google Scholar]
- 32. Zhou B, Li H, Liu J, et al. Intermittent injections of osteocalcin reverse autophagic dysfunction and endoplasmic reticulum stress resulting from diet‐induced obesity in the vascular tissue via the NFκB‐p65‐dependent mechanism. Cell Cycle. 2013;12(12):1901–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guo Q, Li H, Xu L, Wu S, Sun H, Zhou B. Undercarboxylated osteocalcin reverts insulin resistance induced by endoplasmic reticulum stress in human umbilical vein endothelial cells. Sci Rep. 2017;7(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Karsenty G, Oury F. Regulation of male fertility by the bone‐derived hormone osteocalcin. Mol Cell Endocrinol. 2014;382(1):521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mera P, Laue K, Ferron M, et al. osteocalcin signaling in myofibers is necessary and sufficient for optimum adaptation to exercise. Cell Metab. 2016;23(6):1078–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bar A, Kus K, Manterys A, et al. Vitamin K‐MK‐7 improves nitric oxide‐dependent endothelial function in ApoE/LDLR mice. Vascul Pharmacol. 2019:106581. [DOI] [PubMed] [Google Scholar]
- 37. Namba S, Yamaoka‐Tojo M, Hashikata T, et al. Long‐term warfarin therapy and biomarkers for osteoporosis and atherosclerosis. BBA Clin. 2015;4:76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tacey A, Millar S, Qaradakhi T, et al. Undercarboxylated osteocalcin has no adverse effect on endothelial function in rabbit aorta or human vascular cells. J Cell Physiol. 2021;236(4):2840–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sumino H, Ichikawa S, Kasama S, et al. Relationship between brachial arterial endothelial function and lumbar spine bone mineral density in postmenopausal women. Circ J. 2007;71(10):1555–1559. [DOI] [PubMed] [Google Scholar]
- 40. Al‐Hijji M, Narula N, Go J, et al. Circulating osteogenic progenitor cells in mild, moderate, and severe aortic valve stenosis. Mayo Clin Proc. 2019;94(4):652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Soriano S, Carmona A, Triviño F, et al. Endothelial damage and vascular calcification in patients with chronic kidney disease. Am J Physiol Renal Physiol. 2014;307(11):F1302–1311. [DOI] [PubMed] [Google Scholar]
- 42. Polonskaya Y, Kashtanova E, Murashov I, et al. The influence of calcification factors and endothelial‐dysfunction factors on the development of unstable atherosclerotic plaques. Diagnostics (Basel). 2020;10(12):1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zampetaki A, Kirton JP, Xu Q. Vascular repair by endothelial progenitor cells. Cardiovasc Res. 2008;78(3):413–421. [DOI] [PubMed] [Google Scholar]
- 44. Toya T, Ozcan I, Corban M, et al. Compositional change of gut microbiome and osteocalcin expressing endothelial progenitor cells in patients with coronary artery disease. PLoS One. 2021;16(3):e0249187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Idelevich A, Rais Y, Monsonego‐Ornan E. Bone Gla protein increases HIF‐1alpha‐dependent glucose metabolism and induces cartilage and vascular calcification. Arterioscler Thromb Vasc Biol. 2011;31(9):e55–71. [DOI] [PubMed] [Google Scholar]
- 46. Millar S, John S, Mcintyre C, Ralevic V, Anderson S, O'sullivan S. An investigation into the role of osteocalcin in human arterial smooth muscle cell calcification. Front Endocrinol (Lausanne). 2020;11:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tacey A, Smith C, Woessner M, et al. Undercarboxylated osteocalcin is associated with vascular function in female older adults but does not influence vascular function in male rabbit carotid artery ex vivo. PLoS One. 2020;15(11):e0242774. [DOI] [PMC free article] [PubMed] [Google Scholar]


