Abstract
Manganese (Mn) is an essential trace metal element that is associated with diabetes; however, the results of previous studies are inconsistent. Furthermore, few studies have been conducted in a hypertensive population. The purpose of this study is to explore the relationship between manganese and diabetes in a population with hypertension. A cross‐sectional study was conducted, including 2575 hypertensive individuals from 14 provinces in China. Serum manganese concentrations were measured by the inductively coupled plasma mass spectrometry (ICP‐MS) method. And logistic regression models were used to analyze the association between serum manganese and the risk of diabetes. The prevalence of diabetes was 27.0% in this hypertensive population. In logistic regression models, the odds ratios (95% confidence interval) for diabetes in tertile subgroups were 1.40 (1.12, 1.76) and 1.32 (1.05, 1.65) for tertiles 1 and tertiles 3, respectively, compared to tertile 2 (reference). Additionally, an interaction between sex and manganese was observed. The odds ratios (95% confidence interval) for diabetes were 1.29 (0.95, 1.75) and 0.96 (0.70, 1.31) for tertiles 1 and tertiles 3 among males, and 1.44 (1.01, 2.04) and 1.81 (1.29, 2.55) for tertiles 1 and tertiles 3 among females, respectively, compared to tertile 2. In conclusion, a U‐shaped association between serum manganese and diabetes was observed in a Chinese population with hypertension, and the association was modified by sex.
Keywords: Chinese, diabetes, hypertensive population, serum manganese
1. INTRODUCTION
Chronic noncommunicable diseases (NCDs) are the leading causes of death and increasing challenge in public health globally. As a common NCD, diabetes is characterized by hyperglycemia resulting from abnormalities in insulin secretion, insulin action, or both. 1 In 2019, it was estimated that 463 million people were living with diabetes, and China has the largest diabetes epidemic. 2 , 3 Meanwhile, hypertension is another major NCD with a prevalence exceeding 1.3 billion worldwide. 4 Previous researches suggested that hypertensive patients were at higher risk of diabetes than general population. 5 Due to the high prevalence of hypertension worldwide and elevated susceptibility to diabetes, the prevention of diabetes in individuals with hypertension is of utmost importance.
Manganese is an essential trace metal element that is necessary for human health. 6 Not only does manganese play an important role in many biological functions, 7 but also the key component of manganese‐dependent enzymes in the body. 8 Besides, Mn is strongly associated with risks of many NCDs including cardiovascular disease, dyslipidemia, chronic kidney disease and diabetes. 8 , 9 , 10 , 11 , 12 However, as a potential toxicant, overexposure to manganese can also be dangerous because of its neurotoxicity, reproductive toxicity, and cardiovascular toxicity. 13 , 14 Additionally, excessive manganese can result in higher oxidative stress and inflammation levels by disrupting the antioxidative activity of MnSOD and promoting the production of ROS. 8 , 15 Therefore, manganese status should be maintained at an appropriate status for optimal health.
Previous studies have suggested that manganese status may be associated with the risk of diabetes. In vitro experiments as well as animal experiments have shown that low manganese levels could result in depressed pancreatic insulin synthesis, 16 enhanced degradation, 16 and reduced glucose transport and metabolism in adipose cells, 17 which are closely related to the development of diabetes. Several population‐based studies have reported an association of manganese status and diabetes, but the results have been inconsistent. 18 , 19 , 20 , 21 Moreover, these studies above were mostly conducted in general populations instead of hypertensive patients. Considering trace element imbalance often exists among hypertensive patients and the manganese status might be different with general populations according to previous studies, 22 , 23 , 24 its association with diabetes might change. Therefore, we performed a cross‐sectional study to investigate the association between serum manganese and diabetes in a representative sample of adults with hypertension from 14 provinces in China.
2. MATERIALS AND METHODS
2.1. Study population
The current study is a posthoc analysis and the population is from a multicentric epidemiological study for the identification, education and registration of a high‐risk population with hypertension in China, which was initiated in February 2017 with ongoing enrollment. Patients were recruited from the community through open recruitment rather than through random selection. The inclusion criteria included: (1) individuals with hypertension, defined as seated, resting systolic blood pressure of 140 mmHg or higher or, diastolic blood pressure of 90 mmHg or higher at the recruitment visit or, who were taking antihypertensive medication, according to the diagnostic criteria of the 2010 Chinese guidelines for the management of hypertension 25 ; (2) individuals who gave signed, written, informed consent. To ensure accuracy, participants were asked to sit in a quiet place for at least 15 minutes before the measurement starts. For each participant, blood pressure was measured for four times, with a 2‐minute break between each measurement.
Sample size was calculated using PASS 11 software (PASS, NCSS Statistical Software, Kaysville, UT, USA). In specific, confidence level and allowable error were set to 0.95 and 0.02 separately. Meanwhile, the proportion was set to 0.25 according to previous studies on Chinese hypertensive patients. 26 As a result, the minimum sample size was 1801 in current study.
Of the eligible participants of this ongoing epidemiological study cohort, we selected two subsamples without duplication, which were conducted at 10 and 16 months after study initiation, in December 2017 and in June 2018, respectively. First, 900 participants were randomly selected from nine provinces and stratified by province, who were enrolled from June to August 2017 and had complete screening records (physical exam, questionnaire, and biological samples). Second, 1709 participants were randomly selected from 14 provinces (including the nine provinces in the first sampling set plus an additional five provinces) and stratified by province, sex and age group, who were enrolled from February 2017 to May 2018 and had complete screening records. Finally, after combining the two subsamples, a total of 2575 participants from all 14 provinces without duplication, was included in the present study, after excluding 12 individuals with missing data on serum manganese concentration, two individuals with missing data on serum creatinine, and 20 with missing data on fasting blood glucose (Figure 1).
FIGURE 1.
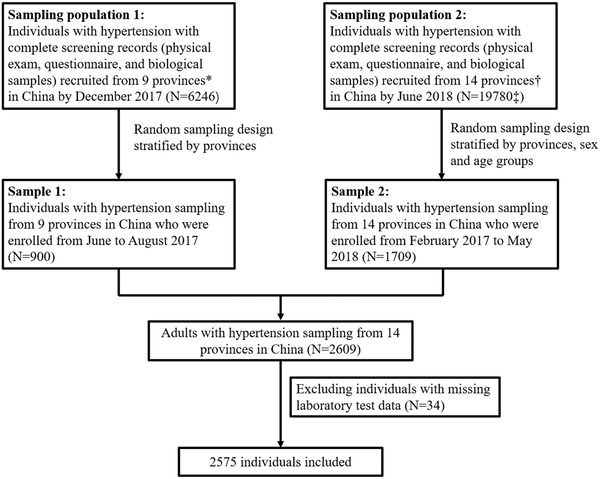
Flow chart of the study participants. * Sample 1 includes nine provinces in China (Gansu, Liaoning, Beijing, Hebei, Jiangsu, Shanxi, Sichuan, Guangxi, and Hunan). † Sample 2 includes 14 provinces in China (Gansu, Heilongjiang, Liaoning, Shandong, Anhui, Beijing, Hebei, Jiangsu, Ningxia, Yunnan, Shanxi, Sichuan, Guangxi, and Hunan). ‡ Individuals in Sampling population 1 were not included in Sampling population 2
The parent study and the current study were approved by the Ethics Committee of Peking University First Hospital, Beijing, China (Ethics code: 20161231). Written, informed consent was obtained from all participants.
2.2. Serum manganese measurements
A fasting, venous blood sample was obtained from each participant. Serum samples were separated within 30 minutes of collection and stored at ‐80°C. Serum manganese concentrations were measured by inductively coupled plasma mass spectrometry (ICP‐MS) using Thermo Fisher iCAP Q ICPMS, in a commercial lab (Beijing DIAN Medical Diagnostics Laboratory, China).
2.3. Definition of diabetes
Diabetes was defined as a fasting blood glucose level of ≥ 126 mg/dL (≥ 7.0 mmol/L) 27 ; or a self‐reported history of diabetes; or current diabetes medication use (treatment).
2.4. Statistical analysis
Data were presented as frequencies (percentages) for categorical variables and medians (interquartile ranges) for continuous variables. Differences in characteristics between males and females, and diabetes group and control group were compared using chi‐square tests for categorical variables, and Wilcoxon rank tests for continuous variables.
Odds ratios (OR) and 95% confidence intervals (95% CI) of serum manganese levels in association with diabetes were estimated using logistic regression models, without or with adjustment for potential confounding factors, including sex, age, body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), smoking status, drinking status, lipid indexes, levels of physical activity, and family history of diabetes. Interactions between serum manganese levels and selected demographic variables were tested by the addition of the cross‐product terms in the regression model. P‐values for linear trends were calculated using the tertiles of manganese concentrations.
Differences were considered statistically significant at a two‐tailed P‐value of < .05. All statistical analyses were performed using R statistical software (version 3.6.1, www.R‐project.org).
3. RESULTS
3.1. Characteristics of participants
As illustrated in the flow chart (Figure 1), after excluding 34 participants lacking data on serum manganese, serum creatinine and fasting blood glucose, a total of 2575 participants, 1380 (53.6%) males and 1195 (46.4%) females, were included. Overall, the age range of this study participants was from 25 to 97 years and median age was 63.6 years. Detailed participant characteristics according to sex are presented in Table 1. Compared with females, males were significantly younger and had lower systolic blood pressure, cholesterol, triglycerides, high‐density lipoprotein cholesterol (HDL‐C), low‐density lipoprotein cholesterol (LDL‐C); and significantly higher diastolic blood pressure, body mass index (BMI), serum creatinine, and prevalence of smoking and drinking (P < .05). However, no sex‐specific differences were observed in serum manganese concentrations, fasting blood glucose concentrations, and the prevalence of diabetes. Also, participant characteristics by the 14 study provinces are listed in Supplemental Table 1.
TABLE 1.
Descriptive characteristics of study participants by sex
| Characteristics | Total | Male | Female | P |
|---|---|---|---|---|
| No. | 2575 | 1380 | 1195 | |
| Age, y | 63.60 (52.99, 73.35) | 62.41 (52.03, 72.74) | 64.52 (54.08, 74.15) | <.001 |
| Age, categories, No. (%) | .014 | |||
| <60, y | 1046 (40.6) | 594 (43.0) | 452 (37.8) | |
| 60‐70, y | 659 (25.6) | 350 (25.4) | 309 (25.9) | |
| ≥70, y | 870 (33.8) | 436 (31.6) | 434 (36.3) | |
| Systolic Blood Pressure, mmHg | 141.00 (131.33, 153.33) | 140.67 (131.33, 151.33) | 141.67 (131.50, 154.67) | .017 |
| Diastolic Blood Pressure, mmHg | 87.00 (79.67, 94.00) | 88.00 (80.00, 95.00) | 85.33 (78.33, 92.67) | <.001 |
| BMI, kg/m2 | 24.84 (22.60, 27.34) | 24.91 (22.94, 27.43) | 24.84 (22.22, 27.13) | .014 |
| Fasting Blood Glucose, mmol/L | 5.53 (4.97, 6.54) | 5.52 (4.94, 6.54) | 5.55 (5.00, 6.54) | .524 |
| Cholesterol, mmol/L | 4.23 (3.55, 4.98) | 4.08 (3.42, 4.80) | 4.41 (3.71, 5.14) | <.001 |
| Triglycerides, mmol/L | 1.40 (1.01, 1.93) | 1.36 (0.97, 1.90) | 1.42 (1.06, 1.95) | .008 |
| HDL‐C, mmol/L | 1.23 (1.04, 1.45) | 1.17 (0.99, 1.38) | 1.29 (1.10, 1.51) | <.001 |
| LDL‐C, mmol/L | 2.58 (2.03, 3.12) | 2.50 (1.95, 3.04) | 2.67 (2.12, 3.18) | <.001 |
| Serum creatinine, μmol/L | 69.00 (58.00, 84.00) | 78.00 (67.00, 91.00) | 61.00 (52.00, 70.00) | <.001 |
| Serum Mn, μg/L | 1.56 (0.90, 2.77) | 1.60 (0.93, 2.84) | 1.51 (0.86, 2.67) | .060 |
| Smoking, No. (%) | <.001 | |||
| No | 2073 (80.5) | 904 (65.5) | 1169 (97.8) | |
| Yes | 502 (19.5) | 476 (34.5) | 26 (2.2) | |
| Drinking, No. (%) | <.001 | |||
| No | 2117 (82.2) | 948 (68.7) | 1169 (97.8) | |
| Yes | 458 (17.8) | 432 (31.3) | 26 (2.2) | |
| Use of antihypertensive medications, No. (%) | .117 | |||
| No | 834 (32.4) | 466 (33.8) | 368 (30.8) | |
| Yes | 1741 (67.6) | 914 (66.2) | 827 (69.2) | |
| Diabetes, No. (%) | .894 | |||
| No | 1879 (73.0) | 1005 (72.8) | 874 (73.1) | |
| Yes | 696 (27.0) | 375 (27.2) | 321 (26.9) |
For continuous variables, values are presented as median (IQR).
Abbreviations: BMI, body mass index; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; Mn, manganese.
Based on the World Health Organization criteria, 27 we identified 696 cases of diabetes in total. Detailed characteristics of participants according to diabetes status (patients without diabetes vs patients with diabetes) are summarized in Table 2. Compared with the patients without diabetes, patients with diabetes were older and had higher BMIs and triglycerides, but had lower diastolic blood pressure, cholesterol, high‐density lipoprotein cholesterol (HDL‐C), and low‐density lipoprotein cholesterol (LDL‐C). The medians (IQR) of serum manganese concentrations were 1.55 μg/L (0.86, 3.07) for the diabetes mellitus group and 1.57 μg/L (0.92, 2.69) for the control group. There were no statistical differences in sex, systolic blood pressure, prevalence of smoking and drinking, and serum manganese concentrations between the two groups.
TABLE 2.
Descriptive characteristics of the study participants by diabetic status
| Characteristics | Non‐diabetics | Diabetics | P |
|---|---|---|---|
| No. | 1879 | 696 | |
| Age, y | 62.44 (51.81, 72.84) | 66.05 (56.25, 74.13) | <.001 |
| Age, categories, No. (%) | <.001 | ||
| < 60, y | 829 (44.1) | 217 (31.2) | |
| 60‐70, y | 450 (23.9) | 209 (30.0) | |
| ≥70, y | 600 (31.9) | 270 (38.8) | |
| Sex, No. (%) | .894 | ||
| Male | 1005 (53.5) | 375 (53.9) | |
| Female | 874 (46.5) | 321 (46.1) | |
| Systolic Blood Pressure, mmHg | 141.00 (131.33, 153.00) | 141.33 (131.58, 154.67) | .575 |
| Diastolic Blood Pressure, mmHg | 87.67 (80.00, 94.67) | 85.00 (78.58, 91.67) | <.001 |
| BMI, kg/m2 | 24.77 (22.41, 27.18) | 25.30 (23.17, 27.68) | <.001 |
| Fasting Blood Glucose, mmol/L | 5.30 (4.81, 5.78) | 7.74 (6.78, 9.42) | <.001 |
| Cholesterol, mmol/L | 4.30 (3.61, 5.05) | 4.06 (3.38, 4.82) | <.001 |
| Triglycerides, mmol/L | 1.37 (0.99, 1.87) | 1.46 (1.07, 2.09) | <.001 |
| HDL‐C, mmol/L | 1.25 (1.06, 1.48) | 1.16 (0.98, 1.35) | <.001 |
| LDL‐C, mmol/L | 2.61 (2.07, 3.16) | 2.50 (1.93, 3.01) | <.001 |
| Serum creatinine, μmol/L | 69.00 (59.00, 83.00) | 69.00 (57.00, 85.00) | .674 |
| Serum Mn, μg/L | 1.57 (0.92, 2.69) | 1.55 (0.86, 3.07) | .963 |
| Smoking, No. (%) | .639 | ||
| No | 1508 (80.3) | 565 (81.2) | |
| Yes | 371 (19.7) | 131 (18.8) | |
| Drinking, No. (%) | .123 | ||
| No | 1531 (81.5) | 586 (84.2) | |
| Yes | 348 (18.5) | 110 (15.8) |
For continuous variables, values presented as median (IQR).
Abbreviations: BMI, body mass index; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; Mn, manganese.
3.2. The associations of serum manganese levels with diabetes
A generalized additive model (GAM) was first applied to identify the association between serum manganese concentrations and diabetes. As shown in Figure 2, after adjusting for covariates in the adjusted model, a U‐shaped, curved association was observed between serum manganese concentrations and diabetes.
FIGURE 2.
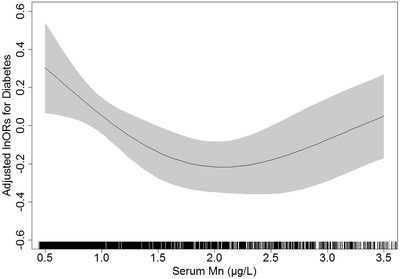
Adjusted odds ratios and 95% confidence intervals for diabetes*. * Adjusted for age, sex, BMI, SBP, DBP, drinking status, smoking status, TC, TG, HDL‐C, LDL‐C, serum creatinine, physical activity, use of antihypertensive medications, and family history of diabetes. Abbreviations: DBP, diastole blood pressure; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; Mn, manganese; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride
In order to further explore and calculate the risk of diabetes within different manganese levels, participants were divided into three groups according to serum manganese concentrations: tertile 1: < 1.1 μg/L (lowest), tertile 2: 1.1–2.2 μg/L (reference), and tertile 3: ≥ 2.2 μg/L (highest). Table 3 presents the results of the multivariate logistic regression. In the crude model, setting tertile 2 as the reference, significantly higher ORs for the risk of diabetes were observed in both tertile 1 (OR, 1.37, 95% CI:1.10 to 1.70) and tertile 3 (OR, 1.35, 95% CI:1.09 to 1.68). The ORs remained significant in the adjusted model. Compared with tertile 2, significantly higher ORs for the risk of diabetes were observed in participants with serum manganese levels in tertile 1 (OR, 1.40, 95% CI:1.12 to 1.76) and tertile 3 (OR, 1.32, 95% CI: 1.05 to 1.65). These results from the multivariate logistic regression were consistent with that of the smooth curve above.
TABLE 3.
Odds ratios (95% confidence interval) of the association between serum Mn tertiles and diabetes
| No. | Case (%) | Crude Model OR (95% CI) | P | Adjusted Model OR (95% CI) | P | |
|---|---|---|---|---|---|---|
| Manganese, μg/L | ||||||
| Total | ||||||
| T1 (< 1.1) | 858 | 250 (29.1) | 1.37 (1.10,1.70) | .004 | 1.40 (1.12,1.76) | .004 |
| T2 (1.1‐2.2) | 858 | 198 (23.1) | ref | NA | ref | NA |
| T3 (≥2.2) | 859 | 248 (28.9) | 1.35 (1.09,1.68) | .006 | 1.32 (1.05,1.65) | .017 |
| P for trend | .902 | .583 | ||||
| Male | ||||||
| T1 (< 1.1) | 460 | 137 (29.8) | 1.23 (0.92,1.64) | .162 | 1.29 (0.95,1.75) | .098 |
| T2 (1.1‐2.3) | 460 | 118 (25.7) | ref | NA | ref | NA |
| T3 (≥2.3) | 460 | 120 (26.1) | 1.02 (0.76,1.37) | .880 | 0.96 (0.70,1.31) | .796 |
| P for trend | .208 | .054 | ||||
| Female | ||||||
| T1 (< 1.0) | 398 | 109 (27.4) | 1.41 (1.02,1.95) | .039 | 1.44 (1.01,2.04) | .044 |
| T2 (1.0‐2.2) | 398 | 84 (21.1) | ref | NA | ref | NA |
| T3 (≥2.2) | 399 | 128 (32.1) | 1.77 (1.28,2.43) | <0.001 | 1.81 (1.29,2.55) | <0.001 |
| P for trend | 0.135 | 0.141 | ||||
Odds ratios were determined from logistic regression analyses for the tertiles of serum manganese.
Crude: No adjustment;.
Adjusted model: Adjusted for age, sex, BMI, SBP, DBP, drinking status, smoking status, TC, TG, HDLC, LDLC, serum creatinine, physical activity, use of antihypertensive drugs, and family history of diabetes.
Abbreviations: BMI, body mass index; CI, confidence interval; DBP, diastolic blood pressure; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; Mn, manganese; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides.
3.3. Subgroup analyses
To better understand other possible influencing factors in the relationship between serum manganese and diabetes among participants with hypertension, exploratory subgroup analyses were performed to assess the effect of serum manganese on the risk of diabetes in various subgroups. As shown in Table 4, a significant interaction was found between serum manganese levels and sex (P for interaction < .05). In general, the odds ratios for the risk of diabetes in females were higher than those in males in both low and high levels of serum manganese. For males, the association in tertile 1 was stronger than the association in tertile 3, whereas the opposite was observed in females. Specifically, the ORs in tertile 1 and tertile 3 were 1.29 (95% CI: 0.95 to 1.75) and 0.96 (95% CI: 0.70 to 1.31), respectively for males, while they were 1.44 (95% CI: 1.01 to 2.04) and 1.81 (95% CI: 1.29 to 2.55), respectively for females. Figure 3 shows similar U‐shaped curves between serum manganese levels and diabetes in both sexes. The serum manganese levels corresponding to the lowest odds ratios of diabetes were in the range of 2.4–2.6 μg/L for males and 1.4–1.6 μg/L for females.
TABLE 4.
Adjusted odds ratios (95% confidence interval) of the association between serum Mn tertiles and diabetes in subgroups
| Tertile 1 | Tertile 2 | Tertile 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | Cases (%) | Serum Mn Median (IQR) | No. | OR (95% CI) | No. | OR (95% CI) | No. | OR (95% CI) | P for interaction | |
| Sex | .021 | |||||||||
| Male | 1380 | 375 (27.2) | 1.60 (0.93, 2.84) | 460 | 1.29 (0.95,1.75) | 460 | ref | 460 | 0.96 (0.70,1.31) | |
| Female | 1195 | 321 (26.9) | 1.51 (0.86, 2.67) | 398 | 1.44 (1.01,2.04) | 398 | ref | 399 | 1.81 (1.29,2.55) | |
| BMI, kg/m 2 | .253 | |||||||||
| < 24 | 982 | 232 (23.6) | 1.56 (0.88, 2.70) | 327 | 1.77 (1.19,2.62) | 327 | ref | 328 | 1.75 (1.18,2.58) | |
| ≥24 | 1593 | 464 (29.1) | 1.56 (0.92, 2.81) | 531 | 1.27 (0.96,1.68) | 531 | ref | 531 | 1.13 (0.86,1.50) | |
| Age, y | .697 | |||||||||
| < 65 | 1391 | 324 (23.3) | 1.59 (0.92, 2.83) | 464 | 1.28 (0.93,1.78) | 463 | ref | 464 | 1.27 (0.91,1.76) | |
| ≥65 | 1184 | 372 (31.4) | 1.53 (0.88, 2.71) | 395 | 1.63 (1.18,2.25) | 394 | ref | 395 | 1.50 (1.09,2.07) | |
| Smoking | .422 | |||||||||
| Yes | 502 | 131 (26.1) | 1.71 (1.01, 3.04) | 167 | 1.52 (0.89,2.59) | 167 | ref | 168 | 1.21 (0.71,2.08) | |
| No | 2073 | 565 (27.3) | 1.52 (0.88, 2.71) | 691 | 1.40 (1.08,1.80) | 691 | ref | 691 | 1.39 (1.08,1.79) | |
| Drinking | .824 | |||||||||
| Yes | 458 | 110 (24.0) | 1.65 (0.98, 2.78) | 153 | 0.92 (0.52,1.64) | 152 | ref | 153 | 0.94 (0.52,1.67) | |
| No | 2117 | 586 (27.7) | 1.54 (0.89, 2.76) | 706 | 1.43 (1.12,1.84) | 705 | ref | 706 | 1.38 (1.07,1.76) | |
| Cholesterol, mmol/L | 0.433 | |||||||||
| < 5.18 | 2084 | 599 (28.7) | 1.60 (0.93, 2.83) | 695 | 1.54 (1.20,1.97) | 694 | ref | 695 | 1.33 (1.04,1.71) | |
| ≥5.18 | 491 | 97 (19.8) | 1.37 (0.83, 2.45) | 164 | 1.60 (0.87,2.96) | 163 | ref | 164 | 1.99 (1.08,3.66) | |
| Triglycerides, mmol/L | 0.099 | |||||||||
| < 1.7 | 1677 | 416 (24.8) | 1.57 (0.88, 2.75) | 559 | 1.72 (1.29,2.30) | 559 | ref | 559 | 1.39 (1.04,1.87) | |
| ≥1.7 | 898 | 280 (31.2) | 1.55 (0.92, 2.81) | 299 | 1.08 (0.74,1.58) | 299 | ref | 300 | 1.26 (0.87,1.83) | |
| HDL‐C status | .837 | |||||||||
| Poor | 969 | 312 (32.2) | 1.66 (0.92, 2.97) | 323 | 1.44 (1.01,2.03) | 323 | ref | 323 | 1.38 (0.97,1.96) | |
| Good | 1606 | 384 (23.9) | 1.52 (0.88, 2.67) | 535 | 1.51 (1.11,2.03) | 535 | ref | 536 | 1.36 (1.00,1.83) | |
| Blood pressure | .296 | |||||||||
| < 140/90 | 966 | 268 (27.7) | 1.61 (0.93, 2.86) | 322 | 1.21 (0.84,1.75) | 322 | ref | 322 | 1.44 (1.00,2.08) | |
| ≥140/90 | 1609 | 428 (26.6) | 1.52 (0.88, 2.72) | 536 | 1.53 (1.15,2.05) | 536 | ref | 537 | 1.27 (0.94,1.70) | |
Status of HDL‐C: Poor, < 1.0 mmol/L in men and < 1.3 mmol/L in women; Good, ≥1.0 mmol/L in men and ≥1.3 mmol/L in women.
Adjusted for age, sex, BMI, SBP, DBP, drinking status, smoking status, TC, TG, HDL‐C, LDL‐C, serum creatinine, physical activity, use of antihypertensive drugs, and family history of diabetes.
Abbreviations: BMI, body mass index; CI, confidence interval; DBP, diastolic blood pressure; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; Mn, manganese; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides.
FIGURE 3.
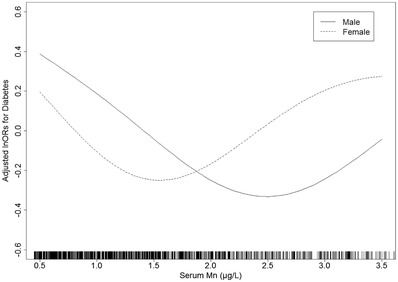
Adjusted odds ratios of the association between serum Mn and diabetes in males and females*. * Adjusted for age, BMI, SBP, DBP, drinking status, smoking status, TC, TG, HDL‐C, LDL‐C, serum creatinine, physical activity, use of antihypertensive medications, and family history of diabetes. Abbreviations: DBP, diastole blood pressure; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; Mn, manganese; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride
4. DISCUSSION
The objectives of our study were to investigate the association between serum manganese levels and diabetes in a Chinese population with hypertension and to explore possible factors. Our results suggest that there is a U‐shaped association between serum manganese and diabetes within this Chinese hypertensive population. In addition, the U‐shaped association was modified by sex.
It is quite plausible, within a certain range, that the risk of diabetes declines when concentrations of serum manganese increase. Manganese known to reduce oxidative stress and protect cells from damage caused by ROS through antioxidant enzyme systems and nonenzymatic pathways, 28 and therefore decrease the risk of insulin resistance and diabetes. Moreover, manganese also plays an important role in the synthesis and secretion of insulin, 29 and the metabolism of carbohydrates, fats, and proteins. 7 In animal models, it has been demonstrated that manganese deficiency can lead to impaired insulin secretion and glucose intolerance. 30 Although human manganese deficiency is exceedingly rare today, since manganese naturally presents in a variety of foods, including whole grains, nuts, teas, leafy vegetables, and even in water, higher risks of diabetes have been observed among individuals with low dietary manganese intake in both domestic studies 31 and those conducted overseas. 32 However, in our study, significantly higher odds ratios were also observed among participants with high levels of serum manganese. Correspondingly, higher levels of plasma manganese were also observed in diabetes patients when compared with healthy controls in several previous studies. 33 , 34 , 35 A possible reason for this could be that manganese is a potential toxicant and oxidant at high levels. Researches have shown that high levels of manganese can enhance oxidative stress by disrupting the antioxidative activity of the MnSOD complex within the mitochondria and promoting the production of ROS. 8 , 15 It appears that the risks of enhancing oxidative stress outweigh the benefits of detoxifying ROS under the condition of overexposure to manganese. Furthermore, excess in manganese could result in abnormal carbohydrate metabolism. Studies have shown that manganism patients have hypoglycemia following a glucose load, which is consistent with animal models. 36
Interestingly and consistent with our findings, a case‐control study conducted in Chinese population reported a similar U‐shaped association between plasma manganese and diabetes. 19 Another cross‐sectional study conducted in Tianjin also observed a U‐shaped trend between plasma manganese and diabetes despite of nonsignificant results, 37 while other studies of non‐Chinese populations only observed a positive linear association. 18 , 33 This discrepancy may be explained by differences in race, region, age, and/or dietary habits of the populations. Evidence shows that Asians, especially Chinese, are at higher risk of developing diabetes for multiple reasons. First, one study recently identified 61 loci newly implicated in predisposition to type 2 diabetes among east Asians. 38 Second, a Chinese diet incorporates higher intakes of rice, which is characterized by a high glycemic index, and may therefore, increase the risk of developing diabetes. 39 It is also worth noting that the median age in our study was 63 years. This population could have been exposed to the severe Chinese famine (1959‐1961) in their early years of life which could have, in turn, very possibly increased the risks of hyperglycemia and type 2 diabetes in this population, although this information was not collected. 40 Therefore, taking these differences into consideration, it seems reasonable that significantly higher risks of diabetes were observed among those Chinese individuals with low serum manganese levels in our study due to this susceptibility.
Another novel insight is that this study is the first to observe an interaction between sex and serum manganese in the risk of diabetes. Specifically, the risks of diabetes in tertile 1 and tertile 3 for females were higher than those for males when setting tertile 2 as the reference group. Additionally, the serum manganese concentration corresponding to the lowest odds ratios in males was higher than that for females. These differences in sex may be related to the higher manganese status and higher iron concentrations found in men. 41 A previous study reported slightly higher serum manganese levels in males than females, which is consistent with our study. 42 Research also demonstrates that there is much competition between manganese and iron for binding to MnSOD. Binding to iron not only inactivates the enzyme, but also gains peroxidase activity and has the potential to generate toxic oxygen radicals. 43 , 44 , 45 Since higher iron levels tend to be observed in men, men may require higher serum manganese concentrations in order to achieve the low odds ratios observed in the women in our study. Therefore, manganese status should be maintained in a moderate range for the minimal risk of diabetes, especially in Chinese populations with hypertension. Sexual difference should also be taken into consideration for the goal of precision nutrition.
Our study also has several limitations. First, since the current study was a cross‐sectional study, no causal conclusions can be drawn from our results. Second, the current study is a posthoc analysis. Although we adjusted for various confounding factors in the adjusted models, we cannot exclude the effects of other unmeasured potential confounders such as dietary intake and environmental exposure. Third, our participants were mostly middle‐aged and older adults with hypertension; the results may not apply to hypertensive patients of younger ages.
5. CONCLUSIONS
Our study revealed a U‐shaped association between serum manganese levels and diabetes in a representative Chinese population with hypertension and the association was modified by sex. Additional prospective studies are warranted to confirm our findings. Maintaining serum manganese at a moderate level might be beneficial in preventing diabetes.
CONFLICTS OF INTEREST
All authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AUTHOR CONTRIBUTIONS
Conceptualization, H.C., X.X., X.Q., Y.Y., and F.S.; methodology, X.X., X.Q., Z.Z., N.Z. and H.C.; software, L.L.; validation, X.Q. and L.L.; formal analysis, H.C., Z.C., W.L. and P.W.; investigation, J.W., Z.W., T.L., Y.S., L.L., P.C., B.W., and H.Z.; resources, X.X., X.Q., G.T., Y.D., X.H., Y.Y., and F.S.; data curation, L.L.; writing—original draft preparation, H.C., X.Q. and F.S.; writing‐review and editing, X.Q. and F.S.; supervision, X.X., X.Q., Y.Y., and F.S.; project administration, H.C. and X.X.; funding acquisition, X.X. All authors have read and agreed to the published version of the manuscript.
Supporting information
Supplementary information
ACKNOWLEDGMENTS
The authors acknowledge the contribution of all staff members who participated in this study as well as the study participants.
The study was supported by the National Key Research and Development Program [2016YFE0205400, 2018ZX09739010, 2018ZX09301034003]; Key R&D Projects, Jiangxi [20203BBGL73173], the National Natural Science Foundation of China [81960074, 81773534]; Project of Jiangxi Provincial Health Commission [202130440]. the Department of Science and Technology of Guangdong Province [2020B121202010]; the Science and Technology Planning Project of Guangzhou, China [201707020010]; the Science, Technology and Innovation Committee of Shenzhen [GJHS20170314114526143, JSGG20180703155802047]; the Economic, Trade and Information Commission of Shenzhen Municipality [20170505161556110, 20170505160926390, 201705051617070].
Chen H, Cui Z, Lu W, et al. Association between serum manganese levels and diabetes in Chinese adults with hypertension. J Clin Hypertens. 2022;24:918–927. 10.1111/jch.14520
Contributor Information
Xianhui Qin, Email: pharmaqin@126.com.
Fenglin Song, Email: songfl@gdpu.edu.cn.
REFERENCES
- 1. Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37:S81–S90. [DOI] [PubMed] [Google Scholar]
- 2. Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:10. [DOI] [PubMed] [Google Scholar]
- 3. Wang L, Gao P, Zhang M, et al. Prevalence and Ethnic Pattern of Diabetes and Prediabetes in China in 2013. JAMA. 2017;317(24):2515–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mills KT, Bundy JD, Kelly TN, et al. Global Disparities of Hypertension Prevalence and Control: a Systematic Analysis of Population‐Based Studies From 90 Countries. Circulation. 2016;134(6):441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Emdin CA, Anderson SG, Woodward M, Rahimi K. Usual Blood Pressure and Risk of New‐Onset Diabetes: evidence From 4.1 Million Adults and a Meta‐Analysis of Prospective Studies. J Am Coll Cardiol. 2015;66(14):1552–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aschner JL, Aschner M. Nutritional aspects of manganese homeostasis. Mol Aspects Med. 2005;26(4‐5):353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Erikson KM, Thompson K, Aschner J, Aschner M. Manganese neurotoxicity: a focus on the neonate. Pharmacol Ther. 2007;113(2):369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li L, Yang X. The Essential Element Manganese, Oxidative Stress, and Metabolic Diseases: links and Interactions. Oxid Med Cell Longev. 2018;2018:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kitada M, Xu J, Ogura Y, Monno I, Koya D. Manganese Superoxide Dismutase Dysfunction and the Pathogenesis of Kidney Disease. Front Physiol. 2020;11:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Luo X, Liu Z, Ge X, et al. High manganese exposure decreased the risk of high triglycerides in workers: a cross‐sectional study. BMC Public Health. 2020;20(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xiao S, Zhou Y, Liu T, et al. The association between manganese exposure with cardiovascular disease in older adults: nHANES 2011–2018. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2021;56(11):1221–1227. [DOI] [PubMed] [Google Scholar]
- 12. Meishuo O, Eshak ES, Muraki I, et al. Association between Dietary Manganese Intake and Mortality from Cardiovascular Disease in Japanese Population: the Japan Collaborative Cohort Study. J Atheroscler Thromb. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O'Neal SL, Zheng W. Manganese Toxicity Upon Overexposure: a Decade in Review. Curr Environ Health Rep. 2015;2(3):315–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang H, Wang J, Yang X, et al. Occupational manganese exposure, reproductive hormones, and semen quality in male workers: a cross‐sectional study. Toxicol Ind Health. 2019;35(1):53–62. [DOI] [PubMed] [Google Scholar]
- 15. Kaur G, Kumar V, Arora A, et al. Affected energy metabolism under manganese stress governs cellular toxicity. Sci Rep. 2017;7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baly DL, Curry DL, Keen CL, Hurley LS. Dynamics of insulin and glucagon release in rats: influence of dietary manganese. Endocrinology. 1985;116(5):1734–1740. [DOI] [PubMed] [Google Scholar]
- 17. Baly DL, Schneiderman JS, Garcia‐Welsh AL. Effect of manganese deficiency on insulin binding, glucose transport and metabolism in rat adipocytes. J Nutr. 1990;120(9):1075–1079. [DOI] [PubMed] [Google Scholar]
- 18. Cabral M, Kuxhaus O, Eichelmann F, et al. Trace element profile and incidence of type 2 diabetes, cardiovascular disease and colorectal cancer: results from the EPIC‐Potsdam cohort study. Eur J Nutr. 2021;60(6):3267–3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shan Z, Chen S, Sun T, et al. U‐Shaped Association between Plasma Manganese Levels and Type 2 Diabetes. Environ Health Perspect. 2016;124(12):1876–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Walter RM Jr, Uriu‐Hare JY, Olin KL, et al. Copper, zinc, manganese, and magnesium status and complications of diabetes mellitus. Diabetes Care. 1991;14(11):1050–1056. [DOI] [PubMed] [Google Scholar]
- 21. Yang J, Yang A, Cheng N, et al. Sex‐specific associations of blood and urinary manganese levels with glucose levels, insulin resistance and kidney function in US adults: national health and nutrition examination survey 2011–2016. Chemosphere. 2020;258:10. [DOI] [PubMed] [Google Scholar]
- 22. Gouaref I, Bellahsene Z, Zekri S, Alamir B, Koceir EA. The link between trace elements and metabolic syndrome/oxidative stress in essential hypertension with or without type 2 diabetes. Ann Biol Clin (Paris). 2016;74(2):233–243. [DOI] [PubMed] [Google Scholar]
- 23. Liu WM, Zhu ZG, Leng HX. Analysis of the contents of K, Na, Ca, Mg, Zn, Cu, Fe and Mn in serum of middle and old‐aged hypertension patients. Guang Pu Xue Yu Guang Pu Fen Xi. 2004;24(3):360–362. [PubMed] [Google Scholar]
- 24. Zhang Z, Zhao S, Wu H, et al. Cross‐sectional study: relationship between serum trace elements and hypertension. J Trace Elem Med Biol. 2022;69:8. [DOI] [PubMed] [Google Scholar]
- 25. Liu LS. 2010 Chinese guidelines for the management of hypertension. Zhonghua xin xue guan bing za zhi. 2011;39(7):579–615. [PubMed] [Google Scholar]
- 26. Liu J, Zhao D, Liu J, Qi Y, Sun J, Wang W. Prevalence of diabetes mellitus in outpatients with essential hypertension in China: a cross‐sectional study. BMJ Open. 2013;3(11):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553. [DOI] [PubMed] [Google Scholar]
- 28. Aguirre JD, Culotta VC. Battles with iron: manganese in oxidative stress protection. J Biol Chem. 2012;287(17):13541–13548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee SH, Jouihan HA, Cooksey RC, et al. Manganese supplementation protects against diet‐induced diabetes in wild type mice by enhancing insulin secretion. Endocrinology. 2013;154(3):1029–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baly DL, Curry DL, Keen CL, Hurley LS. Effect of manganese deficiency on insulin secretion and carbohydrate homeostasis in rats. J Nutr. 1984;114(8):1438–1446. [DOI] [PubMed] [Google Scholar]
- 31. Du S, Wu X, Han T, et al. Dietary manganese and type 2 diabetes mellitus: two prospective cohort studies in China. Diabetologia. 2018;61(9):1985–1995. [DOI] [PubMed] [Google Scholar]
- 32. Gong JH, Lo K, Liu Q, et al. Dietary Manganese, Plasma Markers of Inflammation, and the Development of Type 2 Diabetes in Postmenopausal Women: findings From the Women's Health Initiative. Diabetes Care. 2020;43(6):1344–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Anetor JI, Asiribo OA, Adedapo KS, Akingbola TS, Olorunnisola OS, Adeniyi FA. Increased plasma manganese, partially reduced ascorbate, 1 and absence of mitochondrial oxidative stress in type 2 diabetes mellitus: implications for the superoxide uncoupling protein 2 (UCP‐2) pathway. Biol Trace Elem Res. 2007;120(1‐3):19–27. [DOI] [PubMed] [Google Scholar]
- 34. Flores CR, Puga MP, Wrobel K, Garay Sevilla ME, Wrobel K. Trace elements status in diabetes mellitus type 2: possible role of the interaction between molybdenum and copper in the progress of typical complications. Diabetes Res Clin Pract. 2011;91(3):333–341. [DOI] [PubMed] [Google Scholar]
- 35. Ekin S, Mert N, Gunduz H, Meral I. Serum sialic acid levels and selected mineral status in patients with type 2 diabetes mellitus. Biol Trace Elem Res. 2003;94(3):193–201. [DOI] [PubMed] [Google Scholar]
- 36. Hurley LS, Keen CL, Baly DL. Manganese deficiency and toxicity: effects on carbohydrate metabolism in the rat. Neurotoxicology. 1984;5(1):97–104. [PubMed] [Google Scholar]
- 37. Wang X, Zhang M, Lui G, et al. Associations of Serum Manganese Levels with Prediabetes and Diabetes among ≥60‐Year‐Old Chinese Adults: a Population‐Based Cross‐Sectional Analysis. Nutrients. 2016;8(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Spracklen CN, Horikoshi M, Kim YJ, et al. Identification of type 2 diabetes loci in 433,540 East Asian individuals. Nature. 2020;582(7811):240–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hu EA, Pan A, Malik V, Sun Q. White rice consumption and risk of type 2 diabetes: meta‐analysis and systematic review. Bmj. 2012;344:e1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li Y, He Y, Qi L, et al. Exposure to the Chinese famine in early life and the risk of hyperglycemia and type 2 diabetes in adulthood. Diabetes. 2010;59(10):2400–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Institute of Medicine Panel on M . Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. National Academies Press, Washington, DC, US; 2001. [PubMed] [Google Scholar]
- 42. Filippini T, Michalke B, Grill P, et al. Determinants of serum manganese levels in an Italian population. Mol Med Rep. 2017;15(5):3340–3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yamakura F, Kobayashi K, Furukawa S, Suzuki Y. In vitro preparation of iron‐substituted human manganese superoxide dismutase: possible toxic properties for mitochondria. Free Radic Biol Med. 2007;43(3):423–430. [DOI] [PubMed] [Google Scholar]
- 44. Beyer WF Jr, Fridovich I. In vivo competition between iron and manganese for occupancy of the active site region of the manganese‐superoxide dismutase of Escherichia coli. J Biol Chem. 1991;266(1):303–308. [PubMed] [Google Scholar]
- 45. Ganini D, Petrovich RM, Edwards LL, Mason RP. Iron incorporation into MnSOD A (bacterial Mn‐dependent superoxide dismutase) leads to the formation of a peroxidase/catalase implicated in oxidative damage to bacteria. Biochim Biophys Acta. 2015;1850(9):1795–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information


