Abstract
The design and baseline data of the PRECISION study, which evaluates the effect of the dual endothelin receptor antagonist aprocitentan on blood pressure (BP) in patients with resistant hypertension (RHT) are presented. The study is a blinded, randomized, parallel‐group Phase 3 study and its three‐part design assesses the short‐term and sustained long‐term effects of aprocitentan on BP. Results are expected in 2022.
Patients with uncontrolled BP (measured as unattended automated office BP) despite the use of three or more antihypertensive medications for at least 1 year were screened. They were switched to a single‐tablet triple fixed combination antihypertensive therapy for at least 4 weeks before entering a single‐blind placebo run‐in period. The 4‐week placebo run‐in period further excluded placebo responders. The randomization period consisted of three sequential parts: (1) a 4‐week double‐blind part with aprocitentan 12.5 mg, 25 mg, or placebo (1:1:1 ratio); (2) a 32‐week single‐blind part with aprocitentan 25 mg; and (3) a 12‐week randomized withdrawal part with aprocitentan 25 mg or placebo (1:1 ratio). The purpose was to demonstrate the BP lowering effect of aprocitentan in RHT (Part 1) and the persistence of this effect (Parts 2 and 3).
Out of 1965 screened patients, 730 were randomized resulting in an overall inclusion failure rate of 62.8%. The most common reason for exclusion (44.4% of all screened patients) was failure to meet the BP inclusion criteria. These results underline the high proportion of pseudoresistant hypertension among patients referred for RHT.
Keywords: aprocitentan, blood pressure, endothelin receptor antagonist, pseudoresistant hypertension
1. INTRODUCTION
Since the 1960s, hypertension awareness and availability of several antihypertensive drugs have substantially improved blood pressure (BP) control, which has nevertheless reached a plateau in high‐income countries in the past decade. 1 , 2 Patients having uncontrolled BP despite three antihypertensive treatments at optimal dose and from different classes including a diuretic, so called resistant hypertension (RHT), have an increased risk of cardiovascular mortality and morbidity. According to current guidelines, 3 , 4 recommended drug classes for the treatment of hypertension are those acting through the renin angiotensin aldosterone system, specifically angiotensin‐converting‐enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs), calcium channel blockers (CCBs), diuretics, and sympatholytics in case of cardiac morbidity, which are all pharmacological pathways described many decades ago. Availability of newer antihypertensive drug therapies with novel mechanisms of action would be a valuable addition to current treatment strategies, given the low rates of hypertension control, which do not exceed 50% worldwide. 5
The endothelin (ET) system plays an important role in hypertension, 6 especially in volume and salt‐dependent forms, which are common features in patients with RHT. 7 , 8 Aprocitentan is a dual endothelin receptor antagonist acting on both the ETA and ETB receptors involved in mediating the vasoconstricting, hypertrophic, proinflammatory and profibrotic effects of ET‐1 in hypertension (ETA:ETB inhibitory potency ratio 1:16). 9 In a Phase 2 clinical trial, 10 aprocitentan monotherapy (10, 25 and 50 mg, once daily) produced a significant reduction in BP in patients with mild‐to‐moderate hypertension. Endothelin dual receptor antagonism may represent a useful complementary approach to achieve BP control in RHT patients.
Phase 3 trials conducted over the past decade to investigate new therapies (eg, darusentan, 11 renal denervation 12 ) in RHT have failed to demonstrate a consistent treatment benefit. In retrospect, this may be attributed to the therapeutic approach being inefficient or to deficiencies in clinical trial design such as shortened screening periods to ascertain diagnosis, nonstandardized background medication and imperfect methodology used to measure BP resulting in the enrollment of patients with pseudo‐RHT, and absence of a control group in early trials. 13 In order to eliminate pseudo‐RHT, the selection of patients requires a strict working process 14 including a more appropriate methodology to measure BP, such as repeated unattended office blood pressure monitoring, optimal antihypertensive background therapy and good adherence to treatment. 4
With the above considerations in mind, the PRECISION study was designed to investigate the BP lowering effects of the endothelin receptor antagonist, aprocitentan, and its sustained effect in a prospective, blinded, randomized, parallel‐group Phase 3 clinical trial in patients with “true” RHT, excluding those with apparent/pseudo‐RHT, as per recent American Heart Association Scientific Statement. 4 Here we describe the objectives, design, and baseline characteristics of the PRECISION study as well as the measures implemented to ensure the appropriate selection of an RHT population.
2. METHODS
PRECISION is a multicenter, blinded, randomized, PaRallEl‐group, Phase 3 study with aproCItentan in Subjects with ResIstant HypertensiON (NCT03541174). This study is designed to evaluate whether aprocitentan, added to routine standard‐of‐care, reduces BP compared with placebo in patients with “true” RHT, and whether this effect is sustained for up to 48 weeks. Study enrollment (in Europe, North America, Asia and Australia) is completed, and results are expected in 2022.
2.1. Study patients
Eligible study participants are legal adults. They are required to have “true” RHT, that is, they should meet the following inclusion criteria throughout the screening, run‐in, and randomization process. They should have a history of uncontrolled BP despite taking at least three antihypertensive medications for at least 1 year before screening, and at least three antihypertensive drugs from different pharmacological classes for at least 4 weeks before screening. At screening, participants should have a sitting systolic blood pressure (SiSBP) ≥ 140 mm Hg assessed by measurement of unattended automated office blood pressure (uAOBP), in the absence of a secondary cause of hypertension. A trough SiSBP ≥ 140 mm Hg measured as uAOBP is also a requirement for starting standardized background therapy (SBT, see Treatments section); for entering the placebo run‐in period after 4 weeks of SBT; and for trial randomization. Patients complying with all these requirements are considered to have “true” RHT, while patients failing any of the SiSBP requirements are considered to have pseudo‐RHT and are not enrolled. This high threshold for uAOBP 15 (as compared with guideline‐reported values for AOBP [135 mm Hg] 3 ) is an important aspect of the study design and was chosen in order to increase the sensitivity of the study to treatment effects.
Patients with either confirmed severe hypertension (grade 3 3 ), recent (previous 6 months) major cardiovascular, renal, cerebrovascular medical complications, or heart failure (New York Heart Association stage III‐IV) are excluded, as are patients with N‐terminal probrain natriuretic peptide levels ≥ 500 pg/ml or an estimated glomerular filtration rate < 15 ml/min/1.73 m2. The complete lists of inclusion/exclusion criteria are provided in Tables S1–S3 in the Data Supplement.
All patients provided written informed consent prior to enrollment. The ethics committees or institutional review boards of all the participating sites approved the protocol. The study is conducted in full compliance with the International Conference on Harmonization Good Clinical Practice Guideline, the principles of the Declaration of Helsinki, and the laws and regulations of the country in which it is performed.
2.2. Study design
The study comprises four consecutive periods (Figure 1). Period 1) The screening period (4–12 weeks) is used to select patients with RHT and confirm the diagnosis of “true” RHT; patients fulfilling the screening criteria SiSBP ≥ 140 mm Hg are switched to SBT for 4 weeks to improve treatment adherence. Period 2) The single blind (SB) run‐in period (4 weeks), where placebo is added to SBT, is used to exclude placebo responders. Period 3) This treatment period (48 weeks) consists of three sequential parts: Part 1) a 4‐week double blind (DB), randomized, parallel‐group and placebo‐controlled part, where patients receive aprocitentan 12.5 mg, aprocitentan 25 mg, or placebo in a 1:1:1 ratio; Part 2) a 32‐week SB and single‐arm part, where patients receive aprocitentan 25 mg; and Part 3) a 12‐week DB, randomized, parallel‐group and placebo‐controlled withdrawal (DB‐WD) part, where patients are rerandomized to aprocitentan 25 mg or placebo in a 1:1 ratio. Lastly, Period 4) The safety follow‐up period covers the 30 days after the last dose of study treatment.
FIGURE 1.
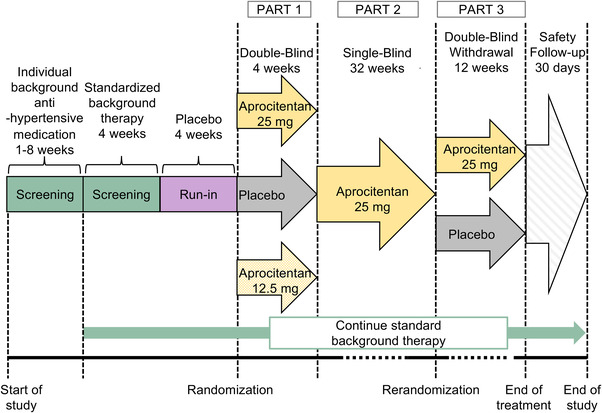
PRECISION study design
2.3. Randomization
Randomization is implemented by an independent Interactive Response Technology system. During the treatment period of the study, the rerandomization that occurs at the end of the SB part is stratified according to the randomized treatment used in the DB part.
2.4. 4 Treatments
Study treatment (aprocitentan/placebo) are provided as identical tablets of 12.5 and 25 mg aprocitentan or matching placebo.
The SBT is a single‐tablet triple fixed combination of a CCB (amlodipine), an ARB (valsartan), and a diuretic (hydrochlorothiazide); two dose strengths are available: 5/160/25 mg and 10/160/25 mg, respectively. The maximum tolerated dose strength is selected at the investigator's discretion during the treatment period of the study and must be kept stable for at least 1 week prior to randomization continuing through the end of the DB part and again during the DB‐WD part. Patients who are treated with a β‐blocker at screening will continue their treatment throughout the study.
Study treatment and SBT are to be taken every morning except on the morning of study visit days, where treatment is administered after the completion of the visit assessments and the measurement of BP.
Study treatment and SBT compliance are assessed throughout the study based on tablet counting. In addition, intake of SBT is monitored by assessing both participant urine via liquid chromatography with tandem mass spectrometric to detect valsartan, and by direct observed treatment intake, performed before start of the ambulatory BP monitoring (ABPM).
2.5. Study assessments
Trough uAOBP is measured at each visit with the same automated oscillometric sphygmomanometer (Microlife WatchBP Office), which records five sitting BP readings (one per minute, first value excluded from the average), with the patient resting undisturbed, alone (unattended) in a quiet place for 5 min.
ABPM is performed over a 24‐h period with the Mobil‐O‐Graph NG device at baseline, and Weeks 4, 36, and 40. Systolic BP and diastolic BP are measured every 20 min from 06:00 to 23:00 and every 30 min from 23:00 to 06:00. Monitoring is initiated between 06:00 and 11:00.
Adverse events are recorded throughout the study.
The schedule of visits and assessments is provided in Tables S4 and S5 in the Data Supplement.
2.6. Efficacy and safety endpoints
The primary efficacy endpoint is the change from baseline to Week 4 of DB treatment in mean trough SiSBP measured as uAOBP, where baseline is defined as the last available measurement before the start of DB treatment.
The key secondary efficacy endpoint is the change from DB‐WD baseline (Week 36 or last available measurement before Week 36) to Week 40 in mean trough SiSBP measured as uAOBP. Other secondary efficacy endpoints include changes from baseline to Week 4 and from DB‐WD baseline to Week 40 in mean trough sitting diastolic blood pressure (SiDBP) measured as uAOBP and in 24‐h systolic/diastolic BP, measured by ABPM.
Safety assessments include adverse events, vital signs, body weight, clinical laboratory findings, and 12‐lead electrocardiograms.
The complete list of study endpoints including pharmacokinetic and biomarker endpoints is provided in Tables S6‐S8 in the Data Supplement.
2.7. Statistical analyses
In this design report, continuous endpoints are summarized by descriptive statistics and categorical variables by numbers and percentages. Categorical variables were compared using the Cochran–Mantel–Haenszel test for ordered variables and the Chi‐square test for nominal variables. In addition, a stepwise multivariable logistic regression was performed for the patients with pseudo‐RHT and those who were randomized to identify predictors of pseudo‐RHT among the patient characteristics at screening (P < .05 for inclusion in the model).
In the final analysis, three null hypotheses will be tested (See Figure S1 in the Data Supplement). The first two null hypotheses, which stipulate there is no difference between aprocitentan and placebo in the DB part, in the mean change from baseline to Week 4 in mean trough SiSBP measured as uAOBP (H10 for aprocitentan 25 mg and H20 for aprocitentan 12.5 mg). These hypotheses will be tested at a two‐sided significance level of .025 using the Bonferroni correction. The third null hypothesis (H30) is that there is no difference between aprocitentan 25 mg and placebo in the DB‐WD part, in the mean change from DB‐WD baseline (Week 36) to Week 40 in mean trough SiSBP measured as uAOBP. This hypothesis will only be tested if H10 or H20 is rejected: at a two‐sided significance level of .05 if both H10 and H20 have been rejected and at a two‐sided significance level of .025 if only one of H10 and H20 has been rejected. In this way the overall type I error is protected at .05.
The primary analysis will include all randomized patients who have a baseline SiSBP measured as uAOBP. Changes from baseline to visits up to Week 4 will be analyzed using a mixed model with factors for treatment group, time, and treatment by time interaction, and covariates for baseline SiSBP and the interaction between baseline and time.
The key secondary analysis will include all patients who were rerandomized in the DB‐WD part and have a DB‐WD baseline (Week 36) SiSBP assessment. Changes from DB‐WD baseline (Week 36) to Week 40 will be analyzed with the same mixed model as described above but using the DB‐WD baseline and an additional factor for stratum (ie, randomized treatment in the DB part).
The sample size was driven by the power for the key secondary endpoint. The within‐group standard deviation for the change from DB‐WD baseline (Week 36) to Week 40 in mean trough SiSBP (measured as uAOBP) was expected to be around 15 mm Hg. 10 With a type I error of .05, the sample size needed for 90% power to detect a difference of 5 mm Hg between aprocitentan 25 mg and placebo was 380 patients (190 per groups in the DB‐WD part). To have 380 patients in the DB‐WD part, a total of 600 patients were to be randomized (200 in each of the three groups in the DB part, assuming a drop‐out rate of 37% between randomization and rerandomization). Ultimately, 730 patients were randomized. The overrunning was caused by the addition of sites to compensate for the lower recruitment due to the Covid‐19 pandemic.
Endpoint analyses are described in Table S9 and changes to the protocol occurring after study start in Table S10, in the Data Supplement.
3. RESULTS
Recruitment of patients for PRECISION began in June 2018 and was completed in January 2021. A total of 1965 individuals were screened at 193 sites in 22 countries. Most patients were recruited from Europe (52.1%) and North America (37.4%), and fewer from Asia and Australia (10.5%).
3.1. Patient disposition
Out of 1965 screened patients, 911 were included in the placebo‐run‐in period and 730 were randomized (Figure 2). Overall, 1235 (62.8%) patients were not randomized. Most of the inclusion failures (n = 1054) occurred during the screening period, whereas the number of failures in the placebo run‐in period was lower (n = 181). The most common reason for exclusion (44.4% of 1965) was failure to meet the SiSBP ≥ 140 mm Hg inclusion criteria.
FIGURE 2.
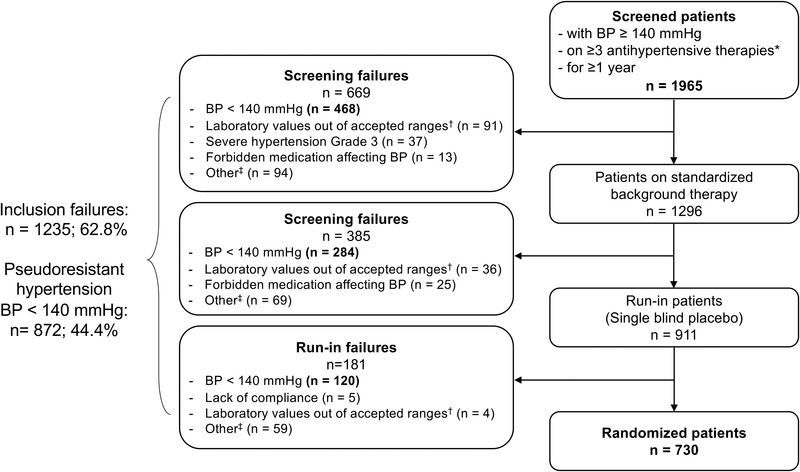
Patient disposition. *From different pharmacological classes for at least 4 weeks before screening. †Laboratory values out of accepted ranges included: alanine aminotransferase or aspartate aminotransferase >3 times the upper limit of normal, hemoglobin <100 g/L, estimated glomerular filtration rate <15 mL/min/1.73 m2, N‐terminal pro‐brain natiuretic peptide ≥500 pg/ml. ‡Causes for exclusion/non‐inclusion reported under 'other' relate to the inclusion/exclusion criteria provided in Tables S1–S3 in the Data Supplement. More than one cause of inclusion failure may apply per patient. BP indicates blood pressure
3.2. Patient characteristics
3.2.1. Randomized population
The randomized population was predominantly male (59.5%) and white (82.9%), and the mean age was 61.7 years (Table 1). Over two thirds were obese (body mass index [BMI] ≥ 30 to < 40 kg/m2) or severely obese (BMI ≥ 40 kg/m2) and a history of cardiovascular diseases was frequent. The average estimated glomerular filtration rate was 76.4 ± 21.9 ml/min/1.73 m2, and 22.2% patients had stage 3 or 4 chronic kidney disease at screening (estimated glomerular filtration rate ≥ 15 to < 60 ml/min/1.73 m2). The randomized participants were receiving < 3 (1.5%), 3 (36.4%), 4 (45.3%) or ≥ 5 (16.3%) antihypertensive medications at screening, with renin angiotensin system blockers, diuretics, and CCBs being the most common treatments; 61.2% patients received a β‐blocker (Figure 3). The mean SiSBP/SiDBP (measured as uAOBP) of the randomized patients was 156.9/88.5 mm Hg at screening and 153.3/87.6 mm Hg at baseline (Table 2).
TABLE 1.
Patient characteristics at screening
| Nonrandomized patients n = 1235 | P‐value | |||
|---|---|---|---|---|
| Characteristics | Pseudo‐ RHTa n = 872 | Non‐pseudo‐RHTa n = 363 | Randomized patients, n = 730 | Pseudo‐RHTa versus randomized |
| Age (years) | 62.2 ± 11.8 | 63.0 ± 12.5 | 61.7 ± 10.6 | .0541 |
| 18 ‐ < 65 | 463 (53.1) | 182 (50.1) | 409 (56.0) | |
| 65 ‐ < 75 | 290 (33.3) | 118 (32.5) | 249 (34.1) | |
| ≥75 | 119 (13.6) | 63 (17.4) | 72 (9.9) | |
| Sex | .8010 | |||
| Men | 513 (58.8) | 211 (58.1) b | 434 (59.5) | |
| Geographic area | <.0001 | |||
| Europe | 429 (49.2) | 146 (40.2) | 448 (61.4) | |
| North America | 316 (36.2) | 187 (51.5) | 232 (31.8) | |
| Asia/Australia | 127 (14.6) | 30 (8.3) | 50 (6.8) | |
| Race | <.0001 | |||
| White | 637 (73.1) | 250 (68.9) | 605 (82.9) | |
| Black/African American | 129 (14.8) | 81 (22.3) | 82 (11.2) | |
| Asian | 87 (10.0) | 22 (6.1) | 38 (5.2) | |
| Other or not reported | 19 (2.2) | 8 (2.2) | 5 (.7) | |
| Missing c | – | 2 (.6) | – | |
| BMI (kg/m2) | 32.2 ± 6.4 | 32.3 ± 6.9 | 33.7 ± 6.2 | .0001 |
| Low to overweight (< 30) | 315 (36.1) | 139 (38.3) | 225 (30.8) | |
| Obese (30‐ < 40) | 387 (44.4) | 157 (43.3) | 399 (54.7) | |
| Severely obese (≥40) | 85 (9.7) | 40 (11.0) | 106 (14.5) | |
| Missing c | 85 (9.7) | 27 (7.4) | – | |
| eGFR (ml/min/ 1.73 m2) | 74.8 ± 21.6 | 65.9 ± 26.1 | 76.4 ± 21.9 | .9188 |
| CKD stage 1–2 (≥60) | 541 (62.0) | 195 (53.7) | 568 (77.8) | |
| CKD stage 3a (45‐59) | 97 (11.1) | 56 (15.4) | 93 (12.7) | |
| CKD stage 3b‐4 (15‐44) | 65 (7.5) | 69 (19.0) | 69 (9.5) | |
| CKD stage 5 (< 15) | 1 (.1) | 4 (1.1) | – | |
| Missing c | 168 (19.3) | 39 (10.7) | – | |
| Medical history | ||||
| Diabetes mellitus | 372 (42.7) | 184 (50.7) | 389 (53.3) | <.0001 |
| Ischemic Heart Disease | 217 (24.9) | 123 (33.9) | 222 (30.4) | .0135 |
| Stroke | 140 (16.1) | 65 (17.9) | 167 (22.9) | .0006 |
| Congestive heart failure | 101 (11.6) | 66 (18.2) | 137 (18.8) | <.0001 |
| Sleep apnea syndrome | 114 (13.1) | 46 (12.7) | 103 (14.1) | .5461 |
Values are means (standard deviation) for continuous variables; n (%) for categorical variables.
aPatients with pseudo‐RHT did not meet the SiSBP ≥ 140 mm Hg inclusion criteria at screening, at switch to standardized background therapy, at run‐in entry, or at randomization. Patients with non‐pseudo‐RHT failed inclusion for a reason other than SiSBP < 140 mm Hg.
Missing in one non‐pseudo‐RHT patient.
Nonrandomized patients meeting the SiSBP exclusion criterion at screening may have an incomplete set of assessments.
Abbreviations: BMI, body mass index; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; RHT, resistant hypertension; SiSBP, sitting systolic blood pressure.
FIGURE 3.
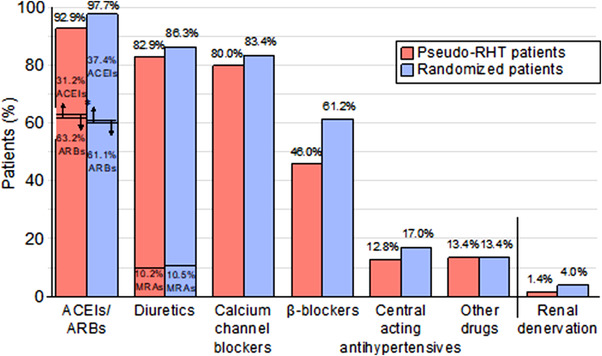
Main individual antihypertensive therapies of randomized participants at screening. ACEIs indicates angiotensin‐converting enzyme inhibitors; ARBs: angiotensin receptor blockers; RD renal denervation; MRAs: mineralocorticoid receptor antagonists
TABLE 2.
Sitting systolic and diastolic blood pressure measured as unattended automated office blood pressure at screening and baseline
| Nonrandomized patients, n = 1235 | Randomized patients, n = 730 | |||
|---|---|---|---|---|
| Characteristics | Pseudo RHTa n = 872 | Non‐pseudo‐RHTa n = 363 | ||
| At screening (n = 863) | At screening (n = 347) | At screening (n = 725) | At baseline (n = 730) | |
| SiSBP (mm Hg) | 141.3 ± 14.5 | 160.7 ± 16.1 | 156.9 ± 11.6 | 153.3 ± 8.9 |
| SiDBP (mm Hg) | 82.4 ± 12.0 | 88.0 ± 13.6 | 88.5 ± 10.6 | 87.6 ± 9.7 |
Values are mean ± standard deviation.
aPatients with pseudo‐RHT did not meet the SiSBP ≥ 140 mm Hg inclusion criteria at screening, at switch to standardized background therapy, at run‐in entry, or at randomization. Patients with non‐pseudo‐RHT failed inclusion for a reason other than SiSBP < 140 mm Hg.
Abbreviations: RHT, resistant hypertension; SiSBP, sitting systolic blood pressure; SiDBP, sitting diastolic blood pressure.
3.2.2. Pseudo‐RHT population (description at screening)
Participants who were not randomized because they failed any of the four SiSBP ≥ 140 mm Hg inclusion criteria, were considered to have pseudo‐RHT. These pseudo‐RHT patients were more likely to have been recruited in North America/Asia/Australia than the randomized patients (50.8% vs. 38.6%) and to be Black, African American, or Asian (24.8% vs. 16.4%). They tended to have a lower than the randomized patients (32.2 vs. 33.7 kg/m2) and were less likely to have a history of cardiovascular disease (Table 1). The pseudo‐RHT patients also tended to receive fewer antihypertensive treatments (3.3 ± 1.0 [n = 855] on average vs. 3.6 ± 1.1 [n = 728] in randomized patients) and to have lower SiSBP/SiDBP than the randomized patients (Table 2). In a multivariable logistic regression analysis including the patients with pseudo‐RHT and those who were randomized, black race, low BMI, and low SiSBP at screening were independent predictors of pseudo‐RHT, as was (absence of) history of diabetes (Table 3).
TABLE 3.
Multivariable logistic regression model to identify independent predictors for pseudoresistant hypertension
| Covariate | Class | Odds ratioa | 95% CI | P‐value | |
|---|---|---|---|---|---|
| Race | Asian | 1.377 | .839 | 2.259 | .9737 |
| Black or African American | 1.863 | 1.300 | 2.672 | .0309 | |
| White | 1 | ||||
| BMI | <30 kg/m2 | 1.453 | .981 | 2.151 | .0100 |
| 30– < 40 kg/m2 | 1.015 | .702 | 1.466 | .1802 | |
| ≥40 kg/m2 | 1 | ||||
| History of diabetes | Present | .702 | .553 | .892 | .0038 |
| Absent | 1 | ||||
| Systolic BP at screening | per mm Hg increase | .930 | .920 | .939 | <.0001 |
aOdds ratio < 1 indicates decreased risk for pseudoresistant hypertension as compared to the reference category (Odds ratio = 1).
Age group, sex, region, chronic kidney disease, diastolic BP at screening and history of ischemic heart disease, stroke, congestive heart failure and sleep apnea syndrome were not statistically significant at the .05 level in the stepwise procedure.
Based on 1385 patients who had pseudo RHT or were randomized; 217 patients were excluded due to missing values for the explanatory variables.
Abbreviations: BMI, body mass index; BP, blood pressure; CI, confidence interval.
Individual patient data illustrate the stability of SiSBP during the screening and run‐in periods for the patients with “true” RHT. These patients present minor increases or decreases in SiSBP from one visit to the next contributing to a limited overall change (Table 2), while most patients with pseudo‐RHT experience a drop in SiSBP during both periods (Figure 4).
FIGURE 4.
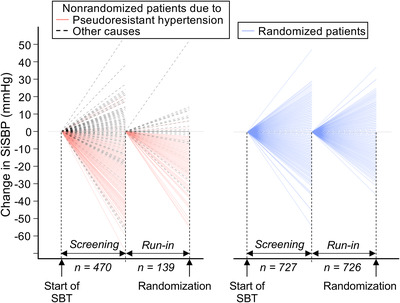
Illustration of the blood pressure lowering effect associated with study design (n: patients with assessments). SBT indicates standard background therapy; SiSBP: sitting systolic blood pressure
4. DISCUSSION
The PRECISION study investigates the effect of adding aprocitentan, an endothelin receptor antagonist, on top of guideline‐recommended antihypertensive medications, 3 , 4 thereby exploring the utility of dual endothelin antagonism as a complementary therapeutic approach for the treatment of RHT. The study was designed to overcome frequent methodological limitations that may result in the inclusion of patients who do not have “true” RHT. An analysis of the study inclusion failures shows that 44.4% of the screened patients considered as candidates for a supplementary treatment for RHT have pseudo‐RHT.
The main strength of the study design is the use of a long screening/run‐in period (at least 8 weeks) before randomization to select patients with “true” RHT. This period includes 4 weeks of SBT provided as a single‐tablet triple antihypertensive treatment combination, which should reduce medical inertia while improving adherence, 16 and 4 weeks of placebo run‐in to exclude placebo responders. To the best of our knowledge, this is the most rigorous study design yet employed to ensure inclusion of patients with “true” RHT within a clinical trial. Another strength of the design is a long treatment period extending over 48 weeks with 16 planned visits. The initial 4 weeks DB part is designed to confirm the BP treatment effect compared with placebo and the tolerability of the two dose strengths of aprocitentan (12.5 and 25 mg) selected from the previous Phase 2 dose‐finding study, 10 thereby demonstrating the short‐term effects and fast onset of BP lowering efficacy. In the dose‐finding study, at least 80% of the expected BP reduction was already observed within the first 2 weeks of treatment. 10 To demonstrate the long‐term BP lowering effect of aprocitentan, a randomized DB‐WD part was added following a long‐term SB active treatment part. This design has become standard at least in children to avoid long‐term placebo exposure. 17 Thus, the PRECISION design addresses both the BP lowering effect of aprocitentan and the persistence of this effect for up to 48 weeks in a single study.
Pseudo‐RHT exclusion is a crucial step in the investigation of RHT. 18 More than one third of the patients referred for RHT may not have a confirmed diagnosis. 19 , 20 The diagnosis of RHT, based on reliable BP measurement and the optimization of background antihypertensive therapies, has not always been optimal in previous RHT clinical trials. This may have affected the proper identification of those patients who may benefit from a new treatment for RHT. 11 , 13 In the PRECISION trial, pseudo‐RHT patients were excluded at four critical steps prior to randomization including screening, switch to SBT, run‐in entry, and randomization. These patients accounted for 44.4% of all screened patients, while 18.5% were excluded for a reason other than BP stabilization. The high inclusion failure rate highlights the importance of rigorous exclusion of pseudo‐RHT, which would likely reduce the variability of BP changes observed in past RHT trials.
The characteristics of the population enrolled in the PRECISION study underline the well known high‐risk profile of RHT patients with a high prevalence of comorbidities. Diabetes and previous stroke were considerably more frequent in the PRECISION study (53% and 22%, respectively) than in previous RHT trials (14‐47% and 8–13%, respectively), 8 , 11 , 21 , 22 , 23 , 24 while obesity and renal dysfunction were in the upper severity range reported in these studies. The low prevalence of sleep apnea (14% in PRECISION vs. 16–32% in the previously cited trials) may be explained by the absence of a proactive investigation in the PRECISION study and is similar to that reported in a Veterans Administration RHT cohort. 25 The best predictors for “true” RHT in the PRECISION study were higher BP and BMI at screening and white race.
To treat RHT, it is widely accepted that there is a medical need for additional pharmacological therapy acting on pathways different from those currently targeted. 4 , 26 Aprocitentan has a mechanism of action that fits the RHT pathophysiological profile 6 , 9 , 27 and is distinct from that of drugs targeting the renin angiotensin system or producing sodium depletion. 28 Therefore, it is hypothesized that aprocitentan can be combined with background therapy including ACEIs/ARBs, CCBs and diuretics to provide additional BP lowering potential in a susceptible population with difficult‐to‐control BP 9 and multiple pathologies and cardiovascular risk factors.
4.1. Study limitations
Office BP measurement has been the gold standard to evaluate the effect of BP decrease on morbidity/mortality from 1967 29 until recently (STEP trial), 30 but current guidelines recommend out‐of‐office BP measurement, such as ABPM, for confirmation of the diagnosis of RHT 3 , 4 to eliminate white‐coat effect. The choice of uAOBP for both the confirmation of the diagnosis and the evaluation of the patients may thus be seen as a limitation of the study but it is associated with less burden for the patients than ABPM, which requires repeat recording until validated. Moreover, like ABPM, uAOBP minimizes white coat effect 31 secondary to sympathetic activation 32 and may be closer to worldwide medical practice. 33
It should be noted that the description of the screen‐failure patients was not planned by study protocol. Furthermore, ineligible patients were not required to complete all study assessments and individual data may be missing.
5. CONCLUSIONS
Resistant hypertension is common and associated with significantly increased cardiovascular risk. Identification of “true” RHT requires specific emphasis on appropriate BP measurement techniques, confirmation of uncontrolled BP by out‐of‐office BP measurement, attention to potential secondary causes, and assessment/confirmation of patient adherence with prescribed medication, which should be optimal. The current study applies a rigorous work‐up to confirm “true” RHT and a design that includes a placebo‐controlled phase with two doses of aprocitentan to demonstrate the short‐term BP lowering efficacy in “true” RHT. Importantly, the study design also allows assessment of long‐term sustainability of BP lowering up to 48 weeks to confirm the durability of the effect. The baseline data from the PRECISION study presented here readily demonstrates the high rate of pseudo‐RHT in a patient population referred for RHT and the importance of thorough clinical workup as summarized above. Most importantly, findings from this study will inform on the utility of aprocitentan as add‐on therapy to established guideline‐recommended background medication to reduce BP in patients with “true” RHT and its capacity to achieve BP control. More broadly, the study will establish the usefulness of dual endothelin antagonism as a therapeutic principle in the treatment of RHT.
CONFLICT OF INTEREST
Parisa Danaietash, Pierre Verweij, and Marc Bellet are employees of Idorsia Pharmaceuticals Ltd. Ji‐Guang Wang has received lecture and consulting fees from Novartis, Omron and Viatris. George Dresser has received lecture fees from Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Medtronic, Pfizer, and Servier. Ilkka Kantola has received lecture, travel, and consulting fees from Amicus, Chiesi, Bayer, Boehringer‐Ingelheim, Sanofi‐Genzyme and Takeda‐Shire. Mary Katherine Lawrence has received funding to perform clinical trials for Idorsia. Krzysztof Narkiewicz has received lecture and consulting fees from Berlin‐Chemie/Menarini, Egis, Gedeon Richter, Krka, Medtronic, Merck, Polpharma, Recordati, Sandoz and Servier. Markus Schlaich has received lecture fees and consulting fees from Medtronic, Abbot, Merck, Boehringer Ingelheim, Servier, ReCor and has been supported by an NHMRC Senior Research Fellowship.
Supporting information
Supporting Information
ACKNOWLEDGEMENTS
We thank the investigators and staff at each study site (See Listing S1 in the Data Supplement). We also acknowledge Mouna Sassi‐Sayadi (Idorsia Pharmaceuticals Ltd.) and Thomas Müller (SDE Services AG) for their contribution to the review paper statistical analyses. Sylvie I. Ertel (Sundgau Medical Writers) provided medical writing support, which was funded by Idorsia Pharmaceuticals Ltd. The PRECISION study is sponsored by Idorsia Pharmaceuticals Ltd.
Danaietash P, Verweij P, Wang J‐G, et al. Identifying and treating resistant hypertension in PRECISION: A randomized long‐term clinical trial with aprocitentan. J Clin Hypertens. 2022;24:804–813. 10.1111/jch.14517
REFERENCES
- 1. Risk Factor Collaboration. Long‐term and recent trends in hypertension awareness, treatment, and control in 12 high‐income countries: an analysis of 123 nationally representative surveys. Lancet. 2019; 394: 639–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Egan BM, Li J, Sutherland SE, Rakotz MK, Wozniak GD. Hypertension control in the United States 2009 to 2018: factors underlying falling control rates during 2015 to 2018 across age‐ and race‐ethnicity groups. Hypertension (Dallas, Tex 1979). 2021; 78: 578–587. [DOI] [PubMed] [Google Scholar]
- 3. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018; 39: 3021–3104. [DOI] [PubMed] [Google Scholar]
- 4. Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension (Dallas, Tex : 1979). 2018; 72: e53–e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Risk Factor Collaboration. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population‐representative studies with 104 million participants. Lancet. 2021; 398: 957–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dhaun N, Goddard J, Kohan DE, Pollock DM, Schiffrin EL, Webb DJ. Role of endothelin‐1 in clinical hypertension: 20 years on. Hypertension (Dallas, Tex : 1979). 2008; 52: 452–459. [DOI] [PubMed] [Google Scholar]
- 7. Gaddam KK, Nishizaka MK, Pratt‐Ubunama MN, et al. Characterization of resistant hypertension: association between resistant hypertension, aldosterone, and persistent intravascular volume expansion. Arch Intern Med. 2008; 168: 1159–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Williams B, MacDonald TM, Morant S, et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug‐resistant hypertension (PATHWAY‐2): a randomised, double‐blind, crossover trial. Lancet. 2015; 386: 2059–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Trensz F, Bortolamiol C, Kramberg M, et al. Pharmacological characterization of aprocitentan, a dual endothelin receptor antagonist, alone and in combination with blockers of the renin angiotensin system, in two models of experimental hypertension. J Pharmacol Experim Therap. 2019; 368: 462–473. [DOI] [PubMed] [Google Scholar]
- 10. Verweij P, Danaietash P, Flamion B, Menard J, Bellet M. Randomized dose‐response study of the new dual endothelin receptor antagonist aprocitentan in hypertension. Hypertension (Dallas, Tex : 1979). 2020; 75: 956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bakris GL, Lindholm LH, Black HR, et al. Divergent results using clinic and ambulatory blood pressures: report of a darusentan‐resistant hypertension trial. Hypertension (Dallas, Tex : 1979). 2010; 56: 824–830. [DOI] [PubMed] [Google Scholar]
- 12. Bhatt DL, Bakris GL. Renal denervation for resistant hypertension. N Engl J Med. 2014; 371: 184. [DOI] [PubMed] [Google Scholar]
- 13. Kandzari DE, Mahfoud F, Bhatt DL, et al. Confounding factors in renal denervation trials: revisiting old and identifying new challenges in trial design of device therapies for hypertension. Hypertension (Dallas, Tex : 1979). 2020; 76: 1410–1417. [DOI] [PubMed] [Google Scholar]
- 14. Braam B, Taler SJ, Rahman M, et al. Recognition and management of resistant hypertension. Clin J Am Soc Nephrol. 2017; 12: 524–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Paini A, Bertacchini F, Stassaldi D, et al. Unattended versus attended blood pressure measurement: mean values and determinants of the difference. Int J Cardiol. 2019; 274: 305–310. [DOI] [PubMed] [Google Scholar]
- 16. Choudhry NK, Kronish IM, Vongpatanasin W, et al. American Heart Association Council on Hypertension Medication adherence and blood pressure control: a scientific statement from the American Heart Association. Hypertension (Dallas, Tex : 1979). 2021; 79: e1–e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Benjamin DK Jr, Smith PB, Jadhav P, et al. Pediatric antihypertensive trial failures: analysis of end points and dose range. Hypertension (Dallas, Tex : 1979). 2008; 51: 834–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burnier M, Wuerzner G. Ambulatory blood pressure and adherence monitoring: diagnosing pseudoresistant hypertension. Semin Nephrol. 2014; 34: 498–505. [DOI] [PubMed] [Google Scholar]
- 19. Bhatt H, Siddiqui M, Judd E, Oparil S, Calhoun D. Prevalence of pseudoresistant hypertension due to inaccurate blood pressure measurement. J Am Soc Hypertens. 2016; 10: 493–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de la SierraA, Segura J, Banegas JR, et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension (Dallas, Tex : 1979). 2011; 57: 898–902. [DOI] [PubMed] [Google Scholar]
- 21. Azizi M, Sapoval M, Gosse P, et al. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open‐label, randomised controlled trial. Lancet. 2015; 385: 1957–1965. [DOI] [PubMed] [Google Scholar]
- 22. Azizi M, Sanghvi K, Saxena M, et al. Ultrasound renal denervation for hypertension resistant to a triple medication pill (RADIANCE‐HTN TRIO): a randomised, multicentre, single‐blind, sham‐controlled trial. Lancet. 2021; 397: 2476–2486. [DOI] [PubMed] [Google Scholar]
- 23. Weber MA, Black H, Bakris G, et al. A selective endothelin‐receptor antagonist to reduce blood pressure in patients with treatment‐resistant hypertension: a randomised, double‐blind, placebo‐controlled trial. Lancet. 2009; 374: 1423–1431. [DOI] [PubMed] [Google Scholar]
- 24. Bhatt DL, Kandzari DE, O'Neill WW, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014; 370: 1393–1401. [DOI] [PubMed] [Google Scholar]
- 25. Fatemi O, Goa C, Faselis C, Kokkinos P, Papademetriou V. Improvement in all‐cause mortality with blood pressure control in a Group of US veterans with drug‐resistant hypertension. J Clin Hypertens (Greenwich). 2016; 18: 33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vemulapalli S, Ard J, Bakris GL, et al. Proceedings from Duke resistant hypertension think tank. Am Heart J. 2014; 167: 775–788. [DOI] [PubMed] [Google Scholar]
- 27. Clozel M. Aprocitentan and the endothelin system in resistant hypertension. Can J Physiol Pharmacol. 2022. 10.1139/cjpp-2022-0010. Online ahead of print. [DOI] [PubMed]
- 28. Davenport AP, Hyndman KA, Dhaun N, et al. Endothelin. Pharmacol Rev. 2016; 68: 357–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Veterans Administration Cooperative Study Group on Antihypertensive Agents . Effects of treatment on morbidity in hypertension. Results in patients with diastolic blood pressures averaging 115 through 129 mm Hg. JAMA. 1967;202:1028–1034. [PubMed] [Google Scholar]
- 30. Zhang W, Zhang S, Deng Y, et al. Trial of intensive blood‐pressure control in older patients with hypertension. N Engl J Med. 2021; 385: 1268–1279. [DOI] [PubMed] [Google Scholar]
- 31. Myers MG, Valdivieso M, Kiss A. Use of automated office blood pressure measurement to reduce the white coat response. J Hypertens. 2009; 27: 280–286. [DOI] [PubMed] [Google Scholar]
- 32. Grassi G, Quarti‐Trevano F, Seravalle G, et al. Sympathetic neural mechanisms underlying attended and unattended blood pressure measurement. Hypertension (Dallas, Tex : 1979). 2021; 78: 1126–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Desai R, Park H, Dietrich EA, Smith SM. Trends in ambulatory blood pressure monitoring use for confirmation or monitoring of hypertension and resistant hypertension among the commercially insured in the U.S., 2008–2017. Int J Cardiol Hypertens. 2020; 6: 100033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


