Abstract
Irritable bowel syndrome with diarrhoea (IBS‐D) and functional diarrhoea (FDr) are the two major functional bowel disorders characterized by diarrhoea. In spite of their high prevalence, IBS‐D and FDr are associated with major uncertainties, especially regarding their optimal diagnostic work‐up and management. A Delphi consensus was performed with experts from 10 European countries who conducted a literature summary and voting process on 31 statements. Quality of evidence was evaluated using the grading of recommendations, assessment, development, and evaluation criteria. Consensus (defined as >80% agreement) was reached for all the statements. The panel agreed with the potential overlapping of IBS‐D and FDr. In terms of diagnosis, the consensus supports a symptom‐based approach also with the exclusion of alarm symptoms, recommending the evaluation of full blood count, C‐reactive protein, serology for coeliac disease, and faecal calprotectin, and consideration of diagnosing bile acid diarrhoea. Colonoscopy with random biopsies in both the right and left colon is recommended in patients older than 50 years and in presence of alarm features. Regarding treatment, a strong consensus was achieved for the use of a diet low fermentable oligo‐, di‐, monosaccharides and polyols, gut‐directed psychological therapies, rifaximin, loperamide, and eluxadoline. A weak or conditional recommendation was achieved for antispasmodics, probiotics, tryciclic antidepressants, bile acid sequestrants, 5‐hydroxytryptamine‐3 antagonists (i.e. alosetron, ondansetron, or ramosetron). A multinational group of European experts summarized the current state of consensus on the definition, diagnosis, and management of IBS‐D and FDr.
Keywords: abdominal pain, clinical practice guidelines, diarrhea, FDr, functional bowel disorders, functional diarrhea, IBS‐D, irritable bowel syndrome
INTRODUCTION
Functional gastrointestinal disorders, now termed disorders of gut‐brain interaction (DGBI), are chronic conditions characterized by persistent and recurring gastrointestinal symptoms. 1 , 2 , 3 Among these, the two major functional bowel disorders characterized by diarrhoea are irritable bowel syndrome with diarrhoea (IBS‐D) and functional diarrhoea (FDr). 1 According to the Rome IV criteria, the primary factor that differentiates these two conditions is the presence and frequency of abdominal pain. 1 Accordingly, abdominal pain must be present, on average, 1 day per week in the last 3 months for the diagnosis of IBS (Table 1). 1 Although abdominal pain can be present in patients with FDr, it should not be the predominant symptom. 1 As these conditions should be viewed as a continuous disease spectrum, it may be not easy to differentiate IBS‐D from FDr.
TABLE 1.
Rome IV diagnostic criteria for IBS‐D and FDr
| Rome IV IBS‐D diagnostic criteria | Rome IV FDr diagnostic criteria |
|---|---|
| 1. Recurrent abdominal pain, on average, at least 1 day per week in the last 3 months and associated with two or more or the following: | 1. Loose or watery stools, without predominant abdominal pain or bothersome bloating, occurring in >25% of stools. |
| a. Related to defecation | 2. Criteria fulfilled for the last 3 months with symptom onset at least 6 months prior to diagnosis. |
| b. Associated with a change in frequency of stool | 3. Patients meeting criteria for IBS‐D should be excluded |
| c. Associated with a change in stool form, with the IBS‐D subtype identified with: > 25% Bristol stool types 6 or 7 and <25% Bristol stool types 1 or 2 | ‐ |
| 2. Criteria fulfilled for the last 3 months with symptom onset at least 6 months prior to diagnosis | ‐ |
Abbreviations: FDr, functional diarrhoea; IBS, Irritable bowel syndrome.
In a recent multinational online survey in 54,127 individuals from 26 countries, 4 the prevalence of Rome IV confirmed FDr was 4.7% (4.5%–4.9%), while it was 1.2% (1.0%–1.3%) in a household survey sample of 18,949 individuals from 9 countries. Using the same surveys and the same criteria, the prevalence of IBS‐D was 1.2% (1.1%–1.3%) and 0.4% (0.3%–0.5%), respectively. Likewise, a recent meta‐analysis showed a pooled prevalence of Rome IV‐defined IBS‐D of 1.4% (95% CI 0.9%–1.9%). 5 Despite their high prevalence, IBS‐D and FDr are associated with major uncertainties, especially regarding their optimal diagnostic work‐up and their targeted and more appropriate management.
Consequently, the United European Gastroenterology (UEG) and the European Society for Neurogastroenterology and Motility (ESNM) identified the need to develop updated clinical practical guidelines to increase the awareness of these disorders and support clinicians in the diagnosis and management of patients, in order to optimize clinical outcomes.
METHODS
The ESNM initiated a Delphi process, to develop consensus statements on different aspects of functional bowel disorders with diarrhoea in collaboration with other European societies. The Delphi approach, which combines the principles of evidence‐based medicine, supported by systematic literature reviews and a voting process, aims to determine consensus for complex problems in medicine for which evidence from controlled trials is lacking. 6
The principal steps in the process were: (1) selection of a Working Group of six ESNM members with expertise in functional disorders and/or Delphi consensus processes; (2) identification of 31 clinical questions to answer using the patient, intervention, control, and outcome (PICO) process; (3) selection of an European Consensus Group consisting of experts in DGBI from different European countries, recruited within the ESNM board and UEG sister societies; (4) systematic literature review to answer each PICO and drafting of statements with a summary of the evidence; (5) two rounds of repeated voting of the statements and (6) grading of the strength using accepted criteria.
For the Consensus Group, ESNM board members nominated experts from their respective national societies for participation, and the UEG sister societies (Società Italiana di Gastroenterologia ed Endoscopia (SIGE), Romanian Society of Gastroenterology & Hepatology (RSGH) and Turkish Society of Gastroenterology (TSG)) nominated additional experts. A total of 30 experts from 10 European countries agreed to participate. Members had a background of expertise in gastroenterology, general practice, and gastrointestinal motility. All members submitted a conflict‐of‐interest statement by September 2019.
The six‐member Core Group identified 29 clinical questions to answer using the PICO process (Appendix 1). A systematic review of the literature was carried out for each question using MEDLINE (accessed via PubMed), EMBASE, and the Cochrane Database of Systematic Reviews (Cochrane Library) until 30 December 2020, with no language restrictions. The literature review and references were made available on a share‐point server, accessible to all members. Moreover, the grading of recommendations, assessment, development, and evaluation (GRADE) methodology (https://www.gradeworkinggroup.org/) to assess the quality of evidence of statements/recommendations was applied, and the recommendations for the different clinical scenarios were classified into four categories: strong recommendation for an intervention (implying it should definitely be done), weak recommendation for an intervention (implying it should probably be done), weak against an intervention (implying it should probably not be done) and strong against an intervention (implying it should definitely not be done). The strength of recommendation (Grade of recommendation (GR): strong or weak) using the GRADE approach was only given for studies on the accuracy of diagnostic procedures and on the assessment of the treatment efficacy (Table 2). The level of evidence (LE) was classified in four categories: high, moderate, low, or very low quality, based on the strict assessment of the quality of the evidence. The quality of the evidence could be downgraded as a result of limitations in the study design or in its implementation, imprecision of estimates, variability in the results, indirectness of the evidence or publication bias; or upgraded because of a very large magnitude of effects, a dose‐response gradient, or if all the plausible biases would reduce an apparent treatment effect. Moreover, the recommendations were also based on some other factors, such as desirable and undesirable consequences of alternative management strategies, variability in values and preferences and the use of resources, including costs.
TABLE 2.
Six‐point Likert scale
| Point | Description |
|---|---|
| A+ | Agree strongly |
| A | Agree with minor reservation |
| A‐ | Agree with major reservation |
| D‐ | Disagree with major reservation |
| D | Disagree with minor reservation |
| D+ | Disagree strongly |
The finalized list of statements with the summary of evidence was evaluated in a first voting round by all members in May 2021, where each member indicated the level of agreement for the statement using a 6‐point Likert scale (Table 1). Participants were blinded to the votes of other participants and gave feedback on clarity of the statement and made suggestions for adapting or splitting the statements into two or more questions, or for adding additional statements on a given topic. After the first‐round voting, the statements and recommendations were revised by the Core Group, followed by another round of statement review, blinded voting and, finally, Core Group revision. When 80% of the Consensus Group agreed with a statement (A+ or A), this was defined as consensus. In the final version, each statement and recommendation are accompanied by the LE (high, moderate, low, very low), grade of recommendation, result of the vote (percentage of the agreement with endorsement). After the final voting round (summarized in Table 3), the manuscript was drafted and reviewed by participants for final approval. The references cited in this chapter are only a selection of the articles reviewed in each area, chosen to clarify the discussion.
TABLE 3.
All statements with endorsement, level of evidence, grade of recommendation and agreement
| Section and number | Statement/recommendation | Endorsement | Level of evidence | Grade of recommendation | Agreement |
|---|---|---|---|---|---|
| Section 1 | Diagnosis | ‐ | ‐ | ‐ | ‐ |
| Section 1.1 | UEG/ESNM recognize IBS‐D and FDr as two potentially overlapping conditions. | Yes | NA | Consensus | 96% |
| Section 1.2 | UEG/ESNM recommends FOR a symptom‐based approach as compared with a diagnostic strategy of exclusion, but minimal diagnostic assessment is mandatory due to the multitude of conditions causing chronic diarrhoea. | Yes | NA | Consensus | 81% |
| Section 1.3 | UEG/ESNM recognize that there is a relationship between IBS‐D and psychosocial factors but that such an association with FDr is affected by limited scientific evidence. | Yes | NA | Consensus | 100% |
| Section 1.4 | UEG/ESNM recommends FOR questioning all patients with chronic diarrhoea about faecal incontinence with appropriate phrasing for it. | Yes | NA | Consensus | 96% |
| Section 1.5 | UEG/ESNM recommends FOR limited blood testing in patients with suspected IBS‐D or FDr in the absence of alarm features, including a full blood count, C‐reactive protein, and serologic testing to rule out coeliac disease. | Yes | Moderate | Strong | 96% |
| Section 1.6 | UEG/ESNM recommends FOR coeliac disease‐associated antibody testing in patients with suspected IBS‐D or FDr in order to exclude coeliac disease. | Yes | Moderate | Strong | 96% |
| Section 1.7 | UEG/ESNM recommends AGAINST routine stool testing for enteric pathogens in all patients with IBS‐D or FDr | Yes | Low | Weak | 93% |
| Section 1.8 | UEG/ESNM recommends FOR faecal calprotectin evaluation in patients with suspected IBS‐D or FDr in order to exclude the presence of inflammatory bowel disease. | Yes | Moderate | Strong | 100% |
| Section 1.9 | UEG/ESNM recommends FOR colonoscopy in patients with suspected IBS‐D or FDr older than 50 years, according to the colorectal cancer‐screening programme, and in those with alarm features in order to perform a correct differential diagnosis. | Yes | Moderate | Strong | 96% |
| Section 1.10 | UEG/ESNM recommends FOR biopsies during colonoscopy in all patients with suspected IBS‐D or FDr, which should be performed in both the right and left colon to exclude microscopic colitis. | Yes | Moderate | Strong | 88% |
| Section 1.11 | UEG/ESNM recommends FOR the use of video capsule endoscopy in a small group of patients with suspected IBS‐D or FDr who have persistently severe or aggravating symptoms, or who have symptoms refractory to standard medical therapy. | Yes | Low | Weak | 81% |
| Section 1.12 | UEG/ESNM recommends FOR the use of device‐assisted enteroscopy in patients with suspected IBS‐D or FDr only for targeted lesions identified by small bowel imaging or video capsule endoscopy, requiring further endoscopic diagnostic or therapeutic intervention. | Yes | NA | Consensus | 85% |
| Section 1.13 | UEG/ESNM recommends AGAINST intestinal transit studies in the work‐up of patients with suspected IBS‐D or FDr. | Yes | Very low | Weak | 92% |
| Section 1.14 | UEG/ESNM recommends AGAINST the use of breath tests in patients with suspected IBS‐D or FDr to identify carbohydrate malabsorption. | Yes | Low | Strong | 89% |
| Section 1.15 | UEG/ESNM recommends FOR considering the diagnosis of bile acid diarrhoea, and testing with SeHCAT or other biomarkers if available, or if not, a trial of treatment, in all patients with unexplained chronic diarrhoea. | Yes | High | Strong | 93% |
| Section 1.16 | UEG/ESNM recommends AGAINST routine diagnostic testing for small intestinal bacterial overgrowth in all patients with suspected IBS‐D or FDr, but testing should be considered in selected cases with strong clinical suspicion based on the presence of predisposing conditions (e.g. gastrointestinalmotility diseases, gastrointestinal anatomical abnormalities, hypochlorhydria, various immune deficiency conditions, signs of malabsorption). | Yes | Moderate | Strong | 96% |
| Section 1.17 | UEG/ESNM recommends AGAINST microbiota testing in patients with IBS‐D or FDr, as at this stage, the clinical relevance of its testing remains unclear. | Yes | Low | Strong | 100% |
| Section 2 | Treatment | Yes | ‐ | ‐ | ‐ |
| Section 2.1 | UEG/ESNM recommends FOR the use of antispasmodic agents in patients with IBS‐D, but there is no data for FDr. | Yes | Low | Weak | 96% |
| Section 2.2 | UEG/ESNM recommends FOR the use of loperamide in patients with IBS‐D or FDr. | Yes | Low | Strong | 89% |
| Section 2.3 | UEG/ESNM recommends FOR the use of rifaximin in patients with IBS‐D, although the therapeutic gain over placebo could be limited. There is limited evidence of efficacy of rifaximin in the treatment of FDr. | Yes | High | Strong | 96% |
| Section 2.4 | UEG/ESNM recommends FOR the use of probiotics that may improve overall symptoms and diarrhoea in some patients with IBS‐D, but there is no evidence for FDr. | Yes | Low | Conditional | 93% |
| Section 2.5 | UEG/ESNM recommends AGAINST the use of mesalazine in patients with IBS‐D or FDr. | Yes | Moderate | Strong | 93% |
| Section 2.6 | UEG/ESNM recommends FOR the use of bile acid sequestrants in patients with proven bile acid diarrhoea. If testing is not available, a trial of a bile acid sequestrant should be considered in patients with persistent unexplained chronic diarrhoea. | Yes | Moderate | Moderate | 93% |
| Section 2.7 | UEG/ESNM recommends FOR the short‐term usefulness of a low FODMAPs diet in patients with IBS‐D when other measures have failed, but there is no evidence for FDr. | Yes | Low | Strong | 100% |
| Section 2.8 | UEG/ESNM recommends AGAINST a gluten free diet for patients with IBS‐D, but there is no evidence for FDr. | Yes | Low | Strong | 100% |
| Section 2.9 | UEG/ESNM recommends FOR gut‐directed psychological therapies as an alternative treatment in patients with IBS‐D, but there is no evidence for FDr. | Yes | Low | Strong | 89% |
| Section 2.10 | UEG/ESNM recommends AGAINST the use of faecal microbiota transplantation in patients with IBS‐D or FDr. | Yes | Low | Strong | 100% |
| Section 2.11 | UEG/ESNM recommends FOR eluxadoline for treating patients with IBS‐D, but there is no evidence for FDr. | Yes | High | Strong | 96% |
| Section 2.12 | UEG/ESNM recommends FOR the use of TCAs for treating patients with IBS‐D, but there is no evidence for FDr. | Yes | NA | Consensus | 70% |
| Section 2.13 | UEG/ESNM recommends AGAINST the use of SSRIs for treating patients with IBS‐D or FDr. | Yes | Very low | Conditional | 100% |
| Section 2.14 | UEG/ESNM recommends FOR the use of 5‐HT3 antagonists (alosetron, ondansetron, ramosetron) in treating patients with IBS‐D to improve IBS symptoms, but there is no evidence for FDr | Yes | Moderate | Strong | 96% |
Abbreviations: FDr, functional diarrhoea; IBS‐D, irritable bowel syndrome with diarrhoea; NA, not available: unable to assess using GRADE methodology; UEG, United European Gastroenterology.
RESULTS
Section 1: Diagnosis
Statement 1.1: UEG/ESNM recognize IBS‐D and FDr as two potentially overlapping conditions.
Statement endorsed, overall agreement: 96%: A+ 59%, A 37%, A‐ 0%, D‐ 0%, D 0%, D+ 4%.
LE: unable to assess using GRADE methodology; GR: consensus recommendation.
Summary of evidence: It is well‐recognized that almost half of the general population will meet criteria for a DGBI at any given time, and that these conditions frequently overlap with each other. 2 , 4 , 7 A cross‐sectional survey published in 2014 by Ford et al. found that the degree of overlap between IBS‐D and FDr was 27.6% based on Rome III criteria. 8 In a survey which used Rome IV criteria people with IBS‐D were significantly younger than FDr patients. 6 Also, using Rome III criteria there were significantly more IBS‐D patients who were female, met criteria for anxiety, and reported high levels of somatization‐type behaviour. 8
Singh et al. 9 compared patients with FDr (n = 48) with IBS‐D (n = 49) based on Rome IV criteria. As expected, a significantly lower proportion of patients with FDr reported abdominal pain (77.1%) than patients with IBS‐D (100%, p < 0.001). In addition, the presence of abdominal bloating, its severity, and the proportion of bowel movements with diarrhoea present did not differ significantly (p = 0.54). However, significantly higher levels of faecal urgency‐related distress were reported by patients with IBS‐D. 9 The proportion of patients with anxiety, depression, or sleep disturbance and their severities did not differ significantly between the two groups. Based on these results, the authors concluded that there was a significant overlap in gastrointestinal and psychological symptoms among FDr and IBS‐D patients, suggesting these entities seem to exist on a continuum. 9
Statement 1.2: UEG/ESNM recommend FOR a symptom‐based approach as compared with a diagnostic strategy of exclusion, however minimal diagnostic assessment is mandatory due to the multitude of conditions causing chronic diarrhoea.
Statement endorsed, overall agreement: 81%: A+ 59%, A 22%, A‐ 19%, D‐ 0%, D 0%, D+ 0%.
LE: unable to assess using GRADE methodology; GR: consensus recommendation.
Summary of evidence: Rome IV questionnaires for DGBI and the Bristol stool form scale are the most commonly used diagnostic criteria for IBS‐D and FDr. 1 , 10 Part of the positive symptom‐based diagnostic criteria for DGBI include the exclusion of alarm features (unintentional weight loss, nocturnal diarrhoea, tenesmus, haematochezia, presumed high‐volume diarrhoea, a very high number of bowel movements, suggestion or evidence of malnutrition, or a family history of colorectal neoplasia). The presence of these features should all prompt further investigation. 10 In the absence of these alarm features, a careful clinical history, focused on key abdominal and diarrhoeal symptoms, combined with a physical examination and minimal diagnostic testing (see below), is sufficient as a positive diagnostic strategy for IBS‐D and FDr. 10
Nevertheless, chronic diarrhoea can be caused by a multitude of organic diseases affecting the gastrointestinal tract, as well as several systemic diseases. These disorders include, but are not limited to, coeliac disease, Crohn's disease, food allergies, carbohydrate maldigestion, bile acid diarrhoea, small intestinal bacterial overgrowth (SIBO), reactions to a variety of drugs, and hyperthyroidism. 10 Hence, beside symptom based criteria, personalized additional investigations are indicated in selected cases. Taking a dietary history may help to identify the ingestion of large amounts of poorly absorbable carbohydrates, and travel history can help to elucidate risk of important infections.
The Rome consensus recommends checking full blood count and C‐reactive protein (CRP) in all patients with chronic diarrhoea, and a thyroid profile if there is some clinical suspicion of hyperthyroidism. 1 In addition, serum electrolytes, serology for coeliac disease, stool analysis for parasites (if endemic), and faecal calprotectin should be analysed. 1 Giardiasis (and tropical sprue) should be excluded, especially when there is a history of acute onset diarrhoea. 1
For patients with persistent symptoms, more sophisticated tests can be considered. Stool specimens can be analysed for faecal pancreatic elastase or for fat content. Colonoscopy should be reserved for those with abnormal tests, with alarm features, or risk factors (age above screening threshold for polyps). If colonoscopy is performed, biopsies should be obtained from both the right and left colon to rule out microscopic colitis. 10
Bile acid diarrhoea is under‐recognized and may account for up to one third of presumed cases of IBS‐D and FDr. 11 Testing can be done via 75Se‐homocholic acid taurine (SeHCAT) or 7‐alpha‐hydroxy‐5‐cholesten‐3‐one (C4) plasma level determination, but these are not available in some countries. Breath tests for carbohydrate malabsorption and bacterial overgrowth can be considered. 12 In conclusion, there are a multitude of conditions that can cause chronic diarrhoea and hence minimal testing is highly recommended. 12 Routine full blood count, CRP, electrolytes, thyroid function testing, and faecal calprotectin should be considered in the vast majority of patients. 12 Additional tests can be considered case‐by‐case according to specific patient (age, family history) and local (prevalence of coeliac disease, dietary habits, onset of colorectal cancer screening) factors and by the (absence of) response to initial (symptomatic) therapies. 12
Statement 1.3: UEG/ESNM recognize that there is a relationship between IBS‐D and psychosocial factors but that such an association with FDr is affected by limited scientific evidence.
Statement endorsed, overall agreement: 100%: A+ 89%, A 11%, A‐ 0%, D‐ 0%, D 0%, D+ 0%.
LE: unable to assess using GRADE methodology; GR: consensus recommendation.
Summary of evidence: The prevalence of psychiatric illnesses in IBS patients is controversial, but IBS consulters often complain of psychological and somatoform symptoms. 13 Van Tilburg et al. 14 showed that the two most important variables associated with IBS severity were catastrophizing and somatization. Moreover, somatization could explain the extraintestinal manifestations often reported by IBS patients, such as urinary and sexual symptoms, headaches, and fatigue. 15 In a large UK community study, those free of IBS that reported all psychological markers of somatization at baseline, were significantly more likely to develop IBS in the short‐term when compared with those who were exposed to none. 16 After adjustment for confounding variables, high levels of illness behaviour, anxiety, sleep disturbances, and somatic symptoms were independent predictors of IBS onset. 16 Moreover, there is increasing evidence that somatization, per se, more than the severity of IBS symptoms, influences the way patients perceive their illness. 17 , 18 Patients with IBS with predominant constipation (IBS‐C) and IBS‐D subtypes have more anxiety, however depression was more common only in IBS‐D, a finding confirmed in three studies (standardized mean differences 1.75, 95% CI 0.20–3.31, p = 0.027). 19 There are few data on psychological features in FDr patients. 20 Chronic diarrhoea was more common in patients with moderate or severe depression (15.53%; 95% CI, 11.34%–20.90%) compared with non‐depressed patients (6.05%; 5.24%–6.98% CI; p < 0.0001). 20
Recently, Singh et al. compared 48 FDr patients with 49 IBS‐D patients using validated questionnaires. 9 Abdominal pain and urgency where significantly more severe in IBS‐D compared with FDr patients. However, the proportions of patients with anxiety, depression, or sleep disturbance, and their severities, did not differ significantly between groups. 9 In a meta‐analysis, Fond et al. reported on the associations of IBS subtypes with anxiety and/or depression. 19 IBS patients had significantly higher anxiety and depression levels than controls. This significant difference was confirmed for patients with both IBS‐C and IBS‐D for anxiety, but only in IBS‐D for depression. 19 Moreover, a disordered bowel habit seems to be more frequently reported in depressed subjects than in non‐depressed ones. Using data from the National Health and Nutrition Examination Survey, 495 depressed and 4709 non‐depressed subjects were identified and studied to evaluate the relationship between mood and bowel habits by validated questionnaires. A higher proportion of depressed individuals reported disordered bowel function than non‐depressed individuals. Chronic diarrhoea was strongly associated with depression, thus supporting a relationship between mood and specific bowel habits. 20
Statement 1.4: UEG/ESNM recommend FOR questioning all patients with chronic diarrhoea about faecal incontinence with appropriate phrasing for it.
Statement endorsed, overall agreement: 96%: A+ 74%, A 22%, A‐ 4%, D‐ 0%, D 0%, D+ 0%.
LE: unable to assess using GRADE methodology; GR: consensus recommendation.
Summary of evidence: Faecal incontinence refers to recurring, uncontrolled passage of solid or liquid stool for a period of at least 3 months in an individual with a developmental age of at least 4 years 21 Soiling or staining of underwear is included in this definition, even if no significant amount of solid or liquid stool is passed, according to the Rome IV criteria. 21 Unintended passage of gas is not included in the definition of faecal incontinence because it occurs frequently in most people. 22 The term “faecal incontinence” is used by caregivers to communicate with each other, but it is either misunderstood or avoided by patients because of embarrassment. 22 , 23 Many patients prefer the term “accidental bowel leakage” making the diagnosis often cumbersome. 23 Faecal incontinence is a prevalent disorder with symptoms reported in up to 8.4% of non‐institutionalized U.S. adults and no significant difference between women (9.4%) and men (7.3%). 24 However, a recent review reported a median prevalence of faecal incontinence in up to 42.8% of care home residents, including both nursing and residential care. 25 Continence depends on multiple mechanisms, both pelvic and bowel‐related, and because of this redundancy, a deficit in any one of these mechanisms may not result in faecal incontinence. 21 , 26 However, chronic diarrhoea (e.g., frequent and loose stools) has been reported repeatedly in prospective studies to be a relevant predisposing factor for faecal incontinence. 22 , 23 , 24 , 25 , 27 In a recent Rome IV criteria‐based Internet survey in a multinational sample of 5931 subjects in Canada, the UK, and the USA, the strongest factors associated with faecal incontinence were diarrhoea, urgency to defaecate, and abdominal pain. 27 Moreover, a diet low in fermentable oligo‐, di‐, and mono‐saccharides and polyols (FODMAPs) has been reported to benefit both stool consistency and faecal incontinence in chronic diarrhoea patients. 28
Notwithstanding these data, the prevalence of faecal incontinence in chronic diarrhoea patients seems hard to assess as it may not be reported by patients due to embarrassment and therefore underestimated. In one study, Leigh and co‐workers reported on 76 chronic diarrhoea patients seen at a referral centre. 29 Half of the patients reported faecal incontinence when specifically questioned about this symptom, but less than 50% of those affected volunteered the symptom. 29 Several subsequent studies on larger samples have confirmed the “stigma” perception of faecal incontinence in the general population and reported on rates and reasons for not seeking care in patient populations, with the most common being fear of being considered unhygienic and embarrassment. 22 , 23 , 24 , 30 Moreover, the “don't ask, don't tell” policy of health care providers is another major factor of underestimation of the condition. A recent cross‐sectional electronic survey of 154 US primary care providers to ascertain beliefs, attitudes, and behaviour regarding faecal incontinence reported a more than two times higher screening rate for urinary than for faecal incontinence (75% vs. 35%, p < 0.001). 31 Physicians believed that both urinary incontinence and faecal incontinence screening were important but felt better informed to treat urinary incontinence (p < 0.001). Again, using adequate phrasing about the symptom (e.g., accidental bowel leakage) seemed critical to pursue a diagnosis of faecal incontinence. 31
Statement 1.5: UEG/ESNM recommend FOR limited blood testing in patients with suspected IBS‐D or FDr in the absence of alarm features, including a full blood count, CRP, and serologic testing to rule out coeliac disease.
Statement endorsed, overall agreement: 96%: A+ 81%, A 15%, A‐ 4%, D‐ 0%, D 0%, D+ 0%.
LE: Moderate; GR: Strong.
Summary of evidence: In the absence of alarm features, the Rome IV criteria recommend making a positive clinical diagnosis of IBS aided by limited diagnostic testing. 1 Several organic disorders, including coeliac disease, inflammatory bowel disease, or microscopic colitis, may present with diarrhoea or IBS‐like symptoms, and limited investigations are, therefore, indicated to distinguish these conditions. 1 , 32
A complete blood count should be performed to identify alarm features such as anaemia or leucocytosis deserving further investigation. 1 , 32 Among the available tests performed to rule out inflammatory bowel disease in patients fulfilling Rome IV criteria for IBS‐D and FDr, CRP should be measured. A systematic review and meta‐analysis showed that a CRP level ≤0.5 mg/dl essentially excludes Inflammatory Bowel Disease in patients with diarrhoeal symptoms, with a 1% or lower likelihood of having inflammatory bowel disease. 33 Erythrocyte sedimentation rate has little clinical utility, and none of these serological biomarkers reliably distinguish IBS from healthy controls. 33 The diagnostic role of faecal calprotectin (and/or faecal lactoferrin) will be assessed in an ad hoc recommendation.
Serologic tests for coeliac disease, including immunoglobulin A (IgA) tissue transglutaminase and quantitative IgA levels, should be performed in patients with DGBI with diarrhoea, particularly if they fail initial therapy. 1 , 34 The medical literature clearly indicates an increased likelihood of positive endomysial antibodies and/or IgA tissue transglutaminase (2.75, 95% CI 1.35–5.61) or biopsy‐proven coeliac disease (4.48, 95% CI 2.33–4.60) in patients with diarrhoea and IBS‐like symptoms as compared with controls, suggesting the need to rule out coeliac disease in these patients. 34 All these aspects will be fully assessed in an ad hoc recommendation.
Routine thyroid tests are not required in all patients with IBS‐D and FDr, but they can be assessed when clinically indicated. 1
Although anti‐cytolethal distending toxin B and anti‐vinculin antibodies have been proposed in the workup of chronic diarrhoea, further studies are needed to clarify their role as potential biomarkers to discriminate functional from organic bowel disorders with diarrhoea. 35
Similarly, interesting but preliminary data suggest that serum zonulin may be a useful biomarker to discriminating IBS‐D from gluten related disorders, including coeliac disease and non‐coeliac gluten sensitivity. 36 However, further validation studies are required.
Statement 1.6: UEG/ESNM recommend FOR coeliac disease‐associated antibody testing in patients with suspected IBS‐D or FDr in order to exclude coeliac disease.
Statement endorsed, overall agreement: 96%: A+ 85%, A 11%, A‐ 0%, D‐ 4%, D 0%, D+ 0%.
LE: Moderate; GR: Strong.
Summary of evidence: A number of prospective case‐control studies, 37 , 38 , 39 systematic reviews and meta‐analyses 34 , 40 , 41 , 42 , 43 have examined the clinical utility and the cost‐effectiveness of testing for coeliac disease in patients who meet Rome criteria for DGBI with diarrhoea. All except one 38 support the concept that coeliac disease ‐associated antibody testing should be recommended in patients with FDr and IBS‐D. As these patients are an at‐risk group for coeliac disease with an expected prevalence ranging from 2.1% to 5.2%, 43 they should undergo enzyme‐linked immunosorbent assay determination of anti‐tissue transglutaminase antibodies of IgA class, while eating a gluten‐containing diet. Anti‐tissue transglutaminase antibodies offer the best combination of sensitivity, specificity, and positive and negative likelihood ratios. Of note, weak anti‐tissue transglutaminase antibody positivity should be confirmed by immunofluorescent anti‐endomysial antibody detection, and testing for total IgA should be part of the serological search for coeliac disease. IgG‐based tests should only be performed in the case of IgA deficiency. 44 The cost‐effectiveness of this case‐finding strategy in patients affected by DGBI with diarrhoea is less clear, as it varies depending on the prevalence of coeliac disease in the target population, and the costs of diagnostic tests and proposed therapies for IBS. 41 Upper gastrointestinal endoscopy with duodenal biopsies should be performed in cases of positive serology in adult patients. 45
Statement 1.7: UEG/ESNM recommend AGAINST routine stool testing for enteric pathogens in all patients with IBS‐D or FDr.
Statement endorsed, overall agreement: 93%: A+ 63%, A 30%, A‐ 0%, D‐ 7%, D 0%, D+ 0%.
LE: Low; GR: Conditional recommendation.
Summary of evidence: Although bacterial and viral gastroenteritis are generally self‐limiting, protozoan infections, including giardiasis and amoebiasis, are more likely to result in chronic infections. 46 Testing for stool ova and parasites is more frequently performed among community gastroenterologist and primary care physicians, as compared with IBS experts. The latter are more conservative in light of poor evidence demonstrating a role of chronic parasitic infection in changing the diagnosis or outcomes. 47 One study failed to detect ova and parasites in faecal samples in a series of 170 patients with IBS. 48 In addition, data combined from two large multinational studies of patients with IBS reported positive faecal ova and parasite tests in less than 2% (19/1154) of patients. 49 The limited reported detection rates of these tests suggest that they should not be performed routinely in these patients. However, it is well‐known that intestinal parasites are more likely to affect the poorest and most deprived areas in tropical and subtropical regions. 50 Therefore, testing for these pathogens should be considered in patients with chronic diarrhoea who live or who have travelled to developing countries. 50
Animal studies demonstrate an association between surrogate indicators of IBS symptoms and the development of visceral hypersensitivity, activation of nociceptive signalling pathways, increased intraepithelial lymphocytes and mast cells within the jejunum, and disruption of the intestinal barrier after Giardiasis. 51 Therefore, in patients with risk factors for Giardiasis (e.g. travel to endemic areas, poor water quality, camping, day‐care exposure), testing is indicated and should be performed through immunoassays (sensitivity: from 82.2% to 100%; specificity: from 91.5% to 100%) or polymerase chain reaction (sensitivity: from 13% to 100%; specificity: from 74.7% to 100%). 52
Statement 1.8: UEG/ESNM recommend FOR faecal calprotectin assessment in patients with suspected IBS‐D or FDr in order to exclude the presence of inflammatory bowel disease.
Statement endorsed, overall agreement: 100%: A+ 85%, A 15%, A‐ 0%, D‐ 0%, D 0%, D+ 0%.
LE: Moderate; GR: Strong.
Summary of evidence: Calprotectin is a 36 kDa calcium and zinc binding protein, which represents about 60% of soluble proteins of the cytoplasm of granulocytes. T is released when inflammatory processes occur, due to the degranulation of neutrophil granulocytes. 53 In a recent review of the literature, different cut‐offs of faecal calprotectin were evaluated to discriminate between the presence of organic or functional gastrointestinal diseases. 43 Faecal calprotectin values ranging between 100 and 164 mcg/mg correctly identified 64% of patients with organic disease, while 90% of patients without organic disease will be identified correctly as negative by using this cut‐off (sensitivity, 0.64; 95% CI 0.49–0.77; specificity 0.90; 95%CI, 0.72–0.97). Using a cut‐off of 50 mcg/mg, the performance of the test was higher with a sensitivity of 0.81 (96% IC, 0.75–0.86) and an insignificant loss in specificity (0.87, 95%CI, 0.78–0.92). This cut‐off seems to be the most useful in clinical practice as patients with a positive calprotectin are six times more likely to have inflammatory bowel disease (positive likelihood ratio, 6.0; 95% CI, 3.0–9.5). The most important role of faecal calprotectin is to exclude the presence of inflammatory bowel disease, given its high negative predictive value, while a positive result requires further investigation. 43 A meta‐analysis, comparing faecal calprotectin with endoscopy, showed a sensitivity and specificity of faecal calprotectin for inflammatory bowel disease of 93% (CI 85%–97%) and 96% (79%–99%), respectively. 54 Therefore, faecal calprotectin can be considered a useful screening tool for identifying those patients who are most likely to need endoscopy for inflammatory bowel disease.
Regarding the role of faecal calprotectin to discriminate IBS from inflammatory bowel disease, it has been reported that the pre‐test probability of inflammatory bowel disease in IBS is 0.5%–1.2%. 40 , 55 This prevalence becomes very low in the absence of alarm features. However, the incidence of inflammatory bowel disease has been reported as being up to five times higher in patients with IBS than in controls after 5 years of symptoms. 56 , 57
Statement 1.9: UEG/ESNM recommend FOR colonoscopy in patients with suspected IBS‐D or FDr older than 50 years, according to the colorectal cancer‐screening programmes, and in those with alarm features.
Statement endorsed, overall agreement: 96%: A+ 85%, A 11%, A‐ 4%, D‐ 0%, D 0%, D+ 0%.
LE: Moderate; GR: Strong.
Summary of evidence: Colonoscopy is useful to exclude organic gastrointestinal diseases that might be responsible for diarrhoea in selected patients, such as inflammatory bowel disease, microscopic colitis, or colorectal cancer. The presence of alarm symptoms (unintentional weight loss, haematochaezia, melaena, older age of onset of symptoms, family history of inflammatory bowel disease, family history of colorectal cancer, persistent watery stools, or family history of other significant gastrointestinal diseases), in patients with suspected IBS‐D and FDr, is suggestive of an organic disorder. 58 However, most of the structural lesions found during colonoscopy in patients with suspected non‐constipation‐predominant IBS are not the cause of diarrhoea (adenomas, angiodysplasia). 59 As for the risk of malignancy, there are studies suggesting that a change in bowel habit is a poor guide to the presence of neoplasia unless advanced to the point of disease. 60 In a Japanese study on 4528 subjects undergoing colonoscopy, the authors identified 60 patients with IBS‐D, 65 with IBS‐C, 47 with IBS with mixed bowel habits (IBS‐M) and 10 with IBS unclassified (IBS‐U). Colorectal cancer was found in five IBS‐C patients, whereas ulcerative colitis and other non‐specific inflammatory lesions were found in nine IBS‐D patients. 61 Therefore, all patients should remain up‐to‐date with colon cancer screening independently from IBS symptoms.
Statement 1.10: UEG/ESNM recommend FOR taking mucosal biopsies from the right and left colon in all patients who undergo colonoscopy for suspected IBS‐D or FDr to exclude microscopic colitis.
Statement endorsed, overall agreement: 88%: A+ 81%, A 7%, A‐ 8%, D‐ 0%, D 4%, D+ 0%.
LE: Moderate; GR: Strong.
Summary of evidence: Microscopic colitis is a chronic inflammatory bowel disease characterized by normal or almost normal endoscopic appearance of the colon, and chronic watery, non‐bloody diarrhoea. There are three distinct histological subtypes: collagenous colitis, lymphocytic colitis, and incomplete microscopic colitis. 62 Even though some laboratory markers (i.e. auto‐antibodies, faecal short chain fatty acids, calprotectin, and lactoferrin) can be altered in up to 50% of patients with microscopic colitis, these are neither sensitive nor specific for the disease. 63 , 64 Currently, the only proven diagnostic approach to exclude microscopic colitis with an acceptable degree of confidence is colonoscopy with biopsy. 62 In a meta‐analysis of studies of patients meeting criteria for IBS‐D, the prevalence of microscopic colitis was 9.8% (95% CI 4.4%–17.1%). 65 These forms are more common in women and the mean age at presentation is around 60. However, microscopic colitis can present in much younger patients in around one‐quarter of cases. 66 , 67 During endoscopic examination, the colonic mucosa is usually unremarkable or, in some cases, may show minor nonspecific changes such as oedema or erythema. The histological findings can be patchy rather than continuous and, therefore, it is currently recommended to obtain multiple biopsy samples from different colonic segments to establish or exclude the diagnosis. 68 The recent UEG guidelines for microscopic colitis recommend taking biopsies from at least the right and left colon. 62
Statement 1.11: UEG/ESNM recommend FOR the use of video‐capsule endoscopy (VCE) in a small group of patients with suspected IBS‐D or FDr who have persistently severe or aggravating symptoms, or who have symptoms refractory to standard medical therapy.
Statement endorsed, overall agreement: 81%: A+ 41%, A 40%, A‐ 15%, D‐ 0%, D 4%, D+ 0%.
LE: Low; GR: Weak.
Summary of evidence: Ohlsson et al. demonstrated that small bowel abnormalities can be observed during VCE in up to one‐quarter of patients with IBS (1), although without a clear correlation between findings and symptoms. The authors concluded that use of VCE should be recommended in patients with persistently severe or aggravating IBS symptoms, rather than used routinely. 69 Another study by Kalla et al. 70 evaluated patients with IBS‐D (n = 151, 103 female, mean age 39 years) using VCE and demonstrated subtle mucosal changes in 30% of patients (n = 45), including erosions in 47% (n = 21/45) and petechiae and ulcers in 53% (n = 24/45) (2). Recently, VCE was used to detect small bowel mucosal abnormalities in patients with IBS‐D refractory to standard medical treatment and functional abdominal pain. 71 Clinically significant lesions were detected via VCE in over 50% of the patients in the diarrhoea group. Villous atrophy (4/22, 18.2%) and Crohn's disease related ulcer (8/22, 36.4%) were the most frequent lesions identified in 15% of patients in the refractory IBS‐D group. The authors concluded that routine use of VCE in patients with IBS should not be recommended but that in patients with refractory conditions, it may identify abnormalities.
Statement 1.12: UEG/ESNM recommend FOR the use of device‐assisted enteroscopy in patients with suspected IBS‐D or FDr only for targeted lesions identified by small bowel imaging or VCE, requiring further endoscopic diagnostic or therapeutic intervention.
Statement endorsed, overall agreement: 85%: A+ 63%, A 22%, A‐ 0%, D‐ 11%, D 4%, D+ 0%.
LE: unable to assess using GRADE methodology; GR: consensus recommendation.
Summary of evidence: Device‐assisted enteroscopy (double balloon, single balloon or spiral) should be reserved for targeting lesions (and obtaining histology) or therapeutic intervention when abnormalities are identified by small bowel imaging or VCE in patients with persistently severe or aggravating symptoms, or who have symptoms refractory to standard medical therapy, as above. 72 , 73
Statement 1.13: UEG/ESNM recommend AGAINST intestinal transit studies in the work‐up of patients with suspected IBS‐D or FDr.
Statement endorsed, overall agreement: 92%: A+ 85%, A 7%, A‐ 0%, D‐ 4%, D 4%, D+ 0%.
LE: Very Low; GR: Moderate.
Summary of evidence: The diagnosis of diarrhoea is mainly clinical. A recent study evaluating total and segmental colonic transit time, assessed using radiopaque markers, in 359 patients with IBS, found that stool form and frequency as assessed by the Bristol stool form scale were correlated with total colonic transit time. 74
Transit time studies may be indicated in patients with rapid transit diarrhoea 75 and diarrhoea associated with SIBO, where there is a suspicion of an underlying intestinal neuropathy (i.e. patients with scleroderma or patients with diabetics neuropathy). 76 However, in these circumstances gastrointestinal manometry should be the test of choice to diagnose intestinal neuropathy, 77 although no specific manometric patterns have been found in patients with IBS‐D. 78 In a recent review of 137 small bowel manometries performed during 6 years, only six patients had this investigation performed for diarrhoea, and there was an intestinal neuropathy in only one of these patients. 79 Hence, in the clinical setting, intestinal motility studies are only indicated in selected patients with chronic intestinal pseudo‐obstruction, or other concomitant diseases that raise the suspicion of intestinal motility disorders as the cause of diarrhoea.
Statement 1.14: UEG/ESNM recommend AGAINST the use of breath tests in patients with suspected IBS‐D or FDr to identify carbohydrate malabsorption.
Statement endorsed, overall agreement: 89%: A+ 63%, A 26%, A‐ 0%, D‐ 4%, D 0%, D+ 7%.
LE: Low; GR: Strong.
Summary of evidence: Breath tests have been an attractive non‐invasive method to identify carbohydrate malabsorption or SIBO. The lactose breath test measures the excretion of hydrogen in expired air after an oral challenge with a standard dose of lactose. As hydrogen is not produced by mammalian enzymes, its presence indicates contact of the sugar with bacteria, indicating lactose malabsorption, although SIBO cannot be excluded. A hydrogen‐non‐producing microbiota can lead to a false‐negative hydrogen breath test. 80
In a recent meta‐analysis of 175 papers including 62,910 participants from 89 countries, the global prevalence of lactose malabsorption in adults was 74% using genotyping data (C/T‐13910) only, whereas it was 55% using lactose tolerance test data, and 57% using lactose hydrogen breath test data. 81 Moreover, in a meta‐analysis of 10 case control studies, including 2008 subjects, there was no significant difference in the prevalence of lactose malabsorption in IBS patients compared with controls without gastrointestinal symptoms. 82 Furthermore, the few studies that have assessed the efficacy of lactose avoidance in improving symptoms in IBS patients showed conflicting results. 83 , 84 , 85 , 86 , 87
Information about the daily fructose dose tolerated in the healthy population are lacking. 88 Thus, the appropriate fructose dose for the fructose breath test for discriminating between normal and pathological conditions remains disputed. 89 Furthermore, the sensitivity and specificity of the breath test to detect fructose malabsorption is unknown. 90 A recent study evaluating patients with IBS and functional dyspepsia highlighted that there was no correlation between fructose ingestion and breath gas concentrations and, consequently, no evidence of an association between these markers of absorption. 91 Thus, its use is not advised.
Sorbitol, is an osmotic sugar alcohol widespread in plants, and mainly found in fruits and juice, as well as in some liquid pharmaceutical preparations. Sorbitol hydrogen breath testing mirrors a reduction in absorption surface and is sensitive in detecting small bowel damage, but is not specific. Therefore, its use is not recommended in clinical practice.
Lactulose and glucose breath tests have been widely utilized to detect SIBO by non‐invasively detecting hydrogen‐producing bacteria in the small intestine. Nonetheless, they have not been validated and their diagnostic accuracy is rather poor. In particular, in many patients, the lactulose breath test may reflect rapid transit rather than SIBO, particularly in patients with diarrhoea, and therefore its use has been questioned. The glucose breath test is more sensitive, although since glucose is rapidly absorbed in the duodenum and proximal jejunum, it will not detect SIBO in the distal jejunum and ileum. In a meta‐analysis of 24 case series, including 2698 patients with all subtypes of IBS, a glucose hydrogen breath test detected a prevalence of SIBO of 25% (95% CI, 19%–32%). However, there was a significant heterogeneity between studies and use of a variety of non‐validated cut‐offs. 82 Furthermore, there is a paucity of studies that address the efficacy of antibiotic therapy for improving symptoms in patients in a controlled fashion (i.e., individuals with a positive vs. a negative breath test). 92 , 93 Therefore, the diagnostic accuracy of breath tests to detect SIBO is still debated and the contribution of SIBO symptom generation in IBS patients is not fully recognized, and routine use of breath testing in IBS is not supported by the evidence.
Statement 1.15: UEG/ESNM recommend FOR considering the diagnosis of bile acid diarrhoea, and testing with SeHCAT or other biomarkers if available, or if not, a trial of treatment, in all patients with unexplained chronic diarrhoea.
Statement endorsed, overall agreement: 93%: A+ 60%, A 33%, A‐ 7%, D‐ 0%, D 0%, D+ 0%.
LE: High; GR: Strong.
Summary of evidence: Bile acid diarrhoea occurs when excessive amounts of bile acids enter the colon, having failed to be absorbed in the ileum. Bile acids stimulate water and electrolyte loss in the colon, producing diarrhoea, urgency, and sometimes abdominal pain and incontinence. Bile acid diarrhoea may be secondary to ileal resection or Crohn's disease, and is also commonly found after cholecystectomy or abdominal radiotherapy. Primary bile acid diarrhoea, also known as idiopathic bile acid/salt malabsorption, is frequently misdiagnosed as IBS‐D or FDr, and is thought to be due to overproduction of bile acids following impaired negative feedback. 94 There is often a considerable delay in making the correct diagnosis. 95
Bile acid diarrhoea can be diagnosed by the SeHCAT test, a nuclear medicine test which measures the retention of a 75Se‐labelled bile acid over 7 days. Alternative tests include measuring the fasting serum levels of the bile acid precursor 7α‐OH‐4‐cholesten‐3‐one, or the regulatory hormone FGF19 (fibroblast growth factor 19), but these have limited availability and are usually measured only at a single time‐point. Faecal collections for total or primary bile acids also appear to be useful. 96
Multiple studies have shown a high prevalence of bile acid diarrhoea in patients with chronic FDr thought to be due to IBS‐D, diagnosed by SeHCAT or by other biomarkers. These have been the subject of several systematic reviews. 11 , 97 , 98 With combined numbers of patients in the thousands, the prevalence of Bile Acid Diarrhea in IBS‐D, diagnosed by SeHCAT was 28% (95% CI: 23%–34%), 11 with similar figures resulting from use of other biomarkers. 98 In patients with Rome IV criteria for IBS‐D, bile acid diarrhoea was the most common organic disease diagnosed. 99 A trial of therapy with colestyramine was suggestive for bile acid diarrhoea in 28% of patients with chronic diarrhoea. 100
We concur with consensus guidelines developed independently, which recommend testing to exclude bile acid diarrhoea, with SeHCAT or other biomarkers, as available, in all patients with unexplained chronic diarrhoea. 12 , 101 , 102
Statement 1.16: UEG/ESNM recommend AGAINST routine diagnostic testing for SIBO in all patients with suspected IBS‐D or FDr, but testing should be considered in selected cases with strong clinical suspicion based on the presence of predisposing conditions (e.g. gastrointestinal motility diseases, gastrointestinal anatomical abnormalities, hypochlorhydria, various immune deficiency conditions, signs of malabsorption).
Statement endorsed, overall agreement: 96%: A+ 89%, A 7%, A‐ 4%, D‐ 0%, D 0%, D+ 0%.
LE: Moderate; GR: Strong.
Summary of evidence: SIBO is defined by excessive and/or an abnormal type of bacteria in the small bowel. 103 This condition may be overrepresented in patients with IBS and other functional bowel disorders. The presence of >105 colony‐forming units per millilitre (cfu/ml) of colonic‐type bacteria in cultures of jejunal aspirates has been considered to define SIBO but, recently, lower cut off have been proposed (>103 CFU/ml coliforms on fresh aspirate culture). However, in clinical practice, culture‐based techniques using aspirates have largely been replaced by breath tests, as they are simple and non‐invasive. These tests measure hydrogen and methane in expired air after intake of carbohydrates, predominantly glucose or lactulose. The sensitivity and specificity of these tests are poor, 103 , 104 which reduces the clinical usefulness, and complicates the interpretation of the SIBO literature in IBS‐D and FDr, which is largely based on studies using these tests.
The symptoms that are traditionally linked to SIBO include bloating, diarrhoea, and abdominal pain/discomfort, while steatorrhea may be seen in more severe cases. There are a number of diseases and conditions that are linked to SIBO, such as severe diseases of gastrointestinal motility, gastrointestinal anatomical abnormalities, hypochlorhydria, and various immune deficiency conditions, but the link with IBS‐D and FDr remains unclear. 103 A recent systematic review suggested a link between SIBO and IBS, and the association was stronger in IBS‐D compared with IBS‐C. 105 However, the authors stated that the quality of the evidence was low, due to clinical heterogeneity of included studies and poor performance of diagnostic tests, in particular breath tests. Moreover, the clinical usefulness of an abnormal test result in IBS and other DGBI remains unclear, even though one recent study suggested that a positive lactulose hydrogen breath test predicted a higher likelihood of a positive clinical response to rifaximin in IBS‐D. 106
More studies with larger sample sizes are needed to demonstrate convincingly that the result on a breath test in patients with DGBI influences clinical management. Furthermore, in a recent study it was demonstrated that SIBO based on duodenal aspirate culture reflects an overgrowth of anaerobes, but does not correspond with patient symptoms, and may rather be a result of dietary preferences. 107 Hence, based on the existing evidence, routine diagnostic testing SIBO in patients with diarrhoea with a clinical suspicion of a DGBI and with no underlying predisposing conditions or diseases predisposing to SIBO (e.g. abnormal small intestinal motility, anatomical abnormalities, hypochlorhydria, immune deficiency, signs of malabsorption), cannot be recommended since the specificity and sensitivity of these tests are poor, limiting their clinical usefulness.
Statement 1.17: UEG/ESNM recommend AGAINST microbiota testing in patients with IBS‐D or FDr, as at this stage, the clinical relevance of its testing remains unclear.
Statement endorsed, overall agreement: 100%: A+ 89%, A 11%, A‐ 0%, D‐ 0%, D 0%, D+ 0%.
LE: Low; GR: Strong.
Summary of evidence: There are now several studies suggesting alterations in gut microbiota composition and function in patients with IBS and other DGBI. Overall there is substantial heterogeneity among available studies regarding a link between specific microbiota alterations and IBS subtypes and symptom patterns, 108 even though more recent studies suggest an association with IBS symptom severity 109 and specific subtypes when using a longitudinal multi‐omics approach. 110 However, the clinical relevance of alterations in gut microbiota composition and function remains unclear, and studies demonstrating that findings from microbiota studies can predict treatment and management are lacking. There are also studies demonstrating that alterations in gut microbiota composition in patients with unexplained gastrointestinal symptoms compatible with functional bowel disorders may be reflective of dietary changes rather than being the sole and direct explanation of symptoms. 107 Moreover, a recently published study identified potentially pathogenic spirochetes, Brachyspira species, in a substantial proportion of IBS subjects, and in particular in IBS‐D. 111
Ongoing studies will determine the clinical relevance of intestinal spirochaetosis, treatment options, and prevalence in IBS and other DGBI. As treatment with metronidazole, the currently recommended antibiotic for intestinal spirochaetosis, paradoxically resulted in relocation of the Brachyspira into goblet cell secretory granules, this cannot be recommended as a generally viable treatment option. Furthermore, if and how Brachyspira causes symptoms in IBS remains incompletely understood. Hence, more studies are needed before a causative role of the microbiota on gastrointestinal symptoms in patients with IBS and other functional bowel disorders can be determined. Therefore, it was logical that American Gastroenterological Association in their recent guidelines for laboratory evaluation of patients with chronic diarrhoea only recommended testing for certain chronic infections, such as chronic giardiasis, but not routine testing for microbiota composition. 102 These tests remain valuable research tools until the cause‐and‐effect question regarding microbiota composition and function, and specific gastrointestinal symptoms, has been more clearly answered.
Section 2: Treatment
Statement 2.1: UEG/ESNM recommend FOR the use of antispasmodic agents in patients with IBS‐D, but there are no data for FDr.
Statement endorsed, overall agreement: 96%: A+ 81%, A 15%, A‐ 4%, D‐ 0%, D 0%, D+ 0%.
LE: Low; GR: Weak.
Summary of evidence: Antispasmodic agents may improve abdominal pain by decreasing intestinal smooth muscle contraction. These compounds are generally well tolerated and are associated with few adverse side effects. 112
Peppermint oil is a direct smooth muscle relaxant agent. However, its efficacy in patients with IBS is controversial. A meta‐analysis of 12 randomized controlled trials in 835 patients with IBS showed that peppermint oil is a well‐tolerated and effective therapy for pain and global symptoms in adults with IBS. 113 Another recent network meta‐analysis 114 found that peppermint oil and other antispasmodic drugs were significantly more effective than placebo after 4–12 weeks of treatment for the improvement of global IBS symptoms. Moreover, through indirect comparisons across studies peppermint oil ranked first for efficacy for global symptoms. 114 However, many early studies were limited by study design, inconsistencies in methodology with a lack of evidence for adverse outcomes and assessment of risk profile. 112 PERSUADE is a recent well‐designed placebo‐controlled study involving 190 IBS patients randomized to receive small‐intestinal release or ileocolonic release peppermint oil therapy or placebo for 8 weeks 115 Using strict Food and Drug Administration (FDA) and European Medicines Agency (EMA) endpoints no significant reduction in overall symptom relief or abdominal pain was noted for either peppermint oil preparation. However, the small intestinal release therapy was found to significantly reduce abdominal discomfort and abdominal pain, and improve IBS symptom severity. 115
Mebeverine is a direct smooth muscle relaxant which is well tolerated with few adverse effects. Non‐placebo‐controlled trials of mebeverine in combination with cognitive behavioural therapy (CBT), 116 or in comparison with 5‐hydroxytrytamine‐3 (5‐HT3) receptor antagonists, 117 found that it is helpful in terms of symptom relief and stool consistency. However, these findings have not been reproduced in placebo‐controlled studies. A systematic review of eight randomized trials found no statistically significant improvement in global symptoms of IBS when compared with placebo for clinical improvement or relief of abdominal pain. 118 Furthermore, no significant additional benefit was found by using a higher dose of 200 mg compared with 135 mg dosing. 118
Otilonium bromide is a quaternary ammonium derivative with an excellent safety profile which has been widely used to treat abdominal pain, particularly in patients with IBS. The spasmolytic action of otilonium bromide is mainly secondary to calcium channel blockade in smooth muscle cells. However, this compound also exerts tachykinin receptor antagonism on smooth muscle cells and primary sensory neurons. In a double‐blind, randomised, placebo‐controlled phase IV study including 356 patients with IBS, otilonium bromide (40 mg t.d.s.) was more effective than placebo in reducing the frequency of abdominal pain, severity of abdominal bloating, and preventing symptom relapse. 119 The effect on pain was evident after 10 weeks of treatment. 119 A pooled analysis, including a total of 883 patients with IBS, showed that otilonium bromide was more effective than placebo in improving abdominal pain. 120 Therapeutic benefits were significant after 10 weeks and maximal after 15 weeks of treatment.
Hyoscine butylbromide is an anticholinergic and antimuscarinic agent. A meta‐analysis published in 2008 showed efficacy on global IBS symptoms based on three trials including 426 patients. 121
Pinaverium bromide is a calcium channel blocker which showed promising results in improving abdominal pain and Bristol stool form scale scores in a randomized trial from China, reaching response rate of up to 77.5% of patients at 4 weeks of treatment. 122
Alverine citrate is a non‐atropinic papaverine‐like musculotropic antispasmodic agent which may provide benefit in some patients with DGBI. Although, an earlier study failed to show efficacy of alverine citrate in patients with IBS, 123 a randomised controlled trial in 412 IBS patients observed that this antispasmodic agent significantly reduced abdominal pain (P = 0.047). 124 , 125
Statement 2.2: UEG/ESNM recommend FOR the use of loperamide in patients with IBS‐D or FDr.
Statement endorsed, overall agreement: 89%: A+ 63%, A 26%, A‐ 11%, D‐ 0%, D 0%, D+ 0%.
LE: Low, GR: Strong.
Summary of evidence: Anti‐diarrhoeal drugs can be broadly characterized as agents that reduce the symptoms of diarrhoea by decreasing stool frequency, improving stool consistency, or reducing stool weight. The best studied anti‐diarrhoeal agents up to now are loperamide, diphenoxylate, and dioctahedral smectite.
Loperamide and diphenoxylate are phenylpiperidine derivatives and act as opiate receptor agonists by binding to μ ‐opioid receptors in the enteric nervous system and sensory afferents leading to reduction of peristalsis and intestinal transit, as well as inhibition of intestinal secretion. In a randomized controlled study by Hovdenak et al., loperamide‐treated patients experienced an improvement in stool frequency and stool consistency compared with placebo‐treated patients, without improvement in abdominal pain (p < 0.01). 126 A randomized controlled study by Lavoe et al., showed that loperamide given at bedtime was effective in the treatment of IBS‐D. 127 However, pooled analyses of these studies showed no efficacy in improving global IBS‐D symptoms. 82 , 128 In a randomized controlled study by Cann et al., loperamide improved daily stool frequency compared with placebo after 5 weeks of treatment (1.3 vs. 1.9 stools/day, respectively). 129 Also, patients reported a significant reduction in the percentage of loose stools (P < 0.01), and incidence of urgency (P < 0.001). However, there was no significant modification in stool weight. 129 A recent analysis, including 2428 patients from two randomized controlled studies evaluating the efficacy of eluxadoline in IBS‐D, found that 36.0% of patients reported loperamide use prior to the start of eluxadoline trial. Of these patients, 61.8% stated inadequate IBS‐D symptom control with loperamide. 130 Some caution should be taken in the use of loperamide as this drug may induce constipation, abdominal pain, and prolonged QTc when the drug is used at high dosages. 131 Moreover, there is no evidence that loperamide is effective for treating abdominal pain and bloating in IBS‐D. Dioctahedral smectite (diosmectite) is a natural silicate of aluminium and magnesium used as an intestinal adsorbent in the treatment of infectious and non‐infectious acute and chronic diarrhoea. However the published studies include a small number of patients and limited data are available to supporting its continuous use. 132 , 133
Film‐forming agents capable of protecting the intestinal mucosal barrier, such as xyloglucan, have been reported to be effective for the treatment of acute diarrhoea. 134 Recently, a xyloglucan and xylo‐oligosaccharides containing medical device was used in a randomized controlled cross‐over study including 60 patients with IBS‐D. At day 28, a significantly higher proportion of patients starting this treatment reported normal stools (Bristol stool form scale type 3 and 4), other than reporting a subjective improvement in abdominal pain, bloating, quality of life, and general health. 135 However, this latter medical device is commercialized in only a limited number of European countries (i.e., Andorra, France, Italy, Portugal and Spain).
Promising results have been reported for the treatment of acute diarrhoea with racecadotril, also known as scetorphan. 136 This compound is an enkephalinase inhibitor acting as an antisecretory agent. However, studies in FDr are lacking.
Statement 2.3: UEG/ESNM recommend FOR the use of rifaximin in patients with IBS‐D, although the therapeutic gain over placebo could be limited. There is limited evidence of efficacy of rifaximin in the treatment of FDr.
Statement endorsed, overall agreement: 96%: A+ 78%, A 18%, A‐ 4%, D‐ 0%, D 0%, D+ 0%.
LE: High; GR: Strong.
Summary of evidence: Rifaximin is a non‐absorbed antibiotic first licenced in Italy in 1985 for the treatment of acute bacterial diarrhoea and portal systemic encephalopathy and subsequently approved in 33 countries under different tradenames. Rifaximin has been approved subsequently by the FDA for the treatment of patients with IBS‐D. The rationale behind the use of rifaximin in patients with IBS is based on the hypothesis that a proportion of patients with IBS‐D have an abnormal microbiome and low‐grade intestinal inflammation. In this perspective, rifaximin has been shown to exert: (1) antibiotic activities through inhibition of bacterial RNA synthesis by irreversible binding to the α‐subunit (RpoB) of bacterial DNA‐dependent RNA polymerase 137 ; (2) ‘eubiotic’ effects, exerted through growth of commensal bacteria with beneficial properties 138 and (3) anti‐inflammatory effects via a gut‐specific activation of pregnane X receptor. 139
The clinical use of this drug is supported by several clinical trials. In two identically designed, phase 3, placebo‐controlled studies, the efficacy of rifaximin, at a dose of 550 mg three times daily for 2 weeks, was assessed in determining significant relief of IBS global symptoms, bloating, abdominal pain, and loose or watery stools. Pooled results from both trials showed that 40.8% of rifaximin treated patients had a statistically significant improvement in both abdominal pain and stool consistency compared with 31.7% in patients treated with placebo (P < 0.001). 140 A subsequent trial assessed the efficacy of rifaximin retreatment. 106 The initial open label trial of this study showed that rifaximin, administered to 1074 patients improved symptoms in 44% of subjects. After 18 weeks 64% of the initial responders to rifaximin relapsed and were then randomized to receive up to two courses of rifaximin or placebo, each for 2 weeks. The percentage of responders to FDA combined endpoints was significantly greater with rifaximin than with placebo. The efficacy and safety of rifaximin for the treatment of IBS‐D is supported by pooling of data from five randomized controlled trials in a recent meta‐analysis,141with a significant symptom benefit of rifaximin over placebo with a number needed to treat of 9. In a post‐hoc analysis of a previous trial, 141 that rifaximin was efficacious in improving abdominal pain in adults with IBS‐D. 142 The use of antibiotics for the treatment of a benign condition such as IBS have raised safety concerns. Nonetheless, several studies showed a high safety profile by virtue of negligible systemic absorption, no substantial modification of microbiome structure, 143 rare bacterial chromosomal mutation, rare development of C. difficile colitis, rare antibiotic resistance with intermittent use, and quick disappearance of resistant bacterial strains within 12 weeks after rifaximin discontinuation. 144 , 145 In a summary of four studies, rifaximin showed a favourable safety profile with a number needed to harm of almost 9000. 146
Statement 2.4: UEG/ESNM recommend FOR the use of probiotics that may improve overall symptoms and diarrhoea in some patients with IBS‐D, but there is no evidence for FDr.
Statement endorsed, overall agreement: 93%: A+ 63%, A 30%, A‐ 7%, D‐ 0%, D 0%, D+ 0%.
LE: Low; GR: Conditional recommendation.
Summary of evidence: A previous systematic review with meta‐analysis on the efficacy of probiotics in IBS, 144 and a systematic review on probiotics in the management of lower gastrointestinal symptoms, 147 suggested that probiotics, when grouped together, may improve global as well as some specific symptoms in patients with IBS. An updated evidence‐based international consensus, indicated that specific probiotics help to reduce overall symptoms, as assessed in 495 patients with IBS‐D, but do not improve diarrhoea in patients with IBS. 147
Since these studies have been published, a number of new randomized controlled trials have been performed, including more than 2000 patients with DGBI with diarrhoea or without constipation. Of these studies, some were negative or showed mixed results without a clear effect on IBS symptoms, indicating that specific probiotics were ineffective in improving IBS symptoms or diarrhoea. 148 , 149 , 150 Of these, only one study was performed in more than 100 patients, failing to demonstrate superiority of a mixture of probiotics over placebo. 150 On the other hand, the majority of new published trials reported positive outcomes. Of these, four studies were performed in more than 100 patients. 151 , 152 In particular: (1) a study performed in 313 Rome IV IBS patients showed that specific probiotics improved abdominal pain and symptom severity scores with a corresponding normalization of bowel habits 151 ; (2) a study performed in 200 Rome III IBS‐D patients indicated that a specific probiotic improved overall symptoms, quality of life and stool frequency 152 ; (3) a study performed in 104 Rome III patients without constipation showed adequate symptom relief by using a multi‐strain preparation as compared with placebo 153 ; (4) a recently published study performed in 445 Rome III IBS patients, of whom 177 had IBS‐D and 34 IBS‐M, showed that a specific strain of heat‐inactivated probiotic significantly improved IBS symptoms fulfilling the primary composite endpoint (i.e., the combination of at least 30% improvement of abdominal pain and adequate relief of overall IBS symptoms for at least 50% of weeks during treatment) as recommended by the EMA. 154
However, different strains, formulations, or mixtures of probiotics were assessed, the trial designs vary with different comparators, inclusion criteria, comorbidity (e.g., anxiety and depression), outcomes, and endpoints, and there was heterogeneity among studies. 144 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 Finally, although probiotics may improve diarrhoea symptoms in IBS‐D, they have not been specifically tested in patients with FDr.
Statement 2.5: UEG/ESNM recommend AGAINST the use of mesalazine in patients with IBS‐D or FDr.
Statement endorsed, overall agreement: 93%: A+ 89%, A 4%, A‐ 7%, D‐ 0%, D 0%, D+ 0%.
LE: Moderate; GR: Strong.
Summary of evidence: The rationale behind the use of mesalazine in patients with DGBI with diarrhoea is based on the evidence that subsets of patients with IBS have an increased number of inflammatory cells in the duodenal, ileal, and colonic mucosa. 155 This low‐grade inflammatory process is several‐fold less than that observed in inflammatory bowel disease and is likely induced by different factors, such as genetic predisposition, impaired mucosal permeability, dysbiosis, stress, and atopy. 156 There are several small uncontrolled studies of mesalazine in patients with IBS, particularly in the subset with diarrhoea, overall, providing contradictory results. 157 , 158 , 159 , 160 Two large multicentre, double‐blind, randomised, placebo‐controlled trials by Barbara et al 161 and Lam et al. 162 assessed the effect of mesalazine for 12 weeks in patients with Rome III‐confirmed IBS and IBS‐D, respectively. Both studies showed that mesalazine was no better than placebo in relieving IBS symptoms, abdominal pain, or changes in bowel habit, although the study by Lam et al. showed that a subgroup of patients who developed IBS symptoms after infection (i.e., post‐infection IBS) tended to improve their clinical picture. A subsequent small size randomized placebo‐controlled double‐blind trial in IBS‐D patients confirmed that mesalazine was not superior to placebo in reducing abdominal pain and bowel habit changes. 163 A recent meta‐analysis of the pooled data from the available randomised controlled trials confirmed that mesalazine was not superior to placebo in relieving abdominal pain, bloating, or defaecation frequency in patients with IBS, although the analysis showed high heterogeneity among studies. 164
Statement 2.6: UEG/ESNM recommend FOR the use of bile acid sequestrants in patients with proven bile acid diarrhoea. If testing is not available, a trial of a bile acid sequestrant should be considered in patients with persistent unexplained chronic diarrhoea.
Statement endorsed, overall agreement: 93%: A+ 67%, A 26%, A‐ 7%, D‐ 0%, D 0%, D+ 0%.
LE: Moderate; GR: Moderate.
Summary of evidence: Bile acid sequestrants include colestyramine, colestipol, and colesevelam. They bind bile acids in the intestine and were developed initially to increase bile acid excretion and so lower cholesterol. They were first shown to be useful in relieving diarrhoea in patients with bile acid diarrhoea due to ileal resection. 165 Their use in primary bile acid diarrhoea/idiopathic bile salt malabsorption followed. 166
Patients with FDr or IBS‐D with abnormal SeHCAT tests were shown to be more likely to respond to colestyramine in several studies conducted in the 1980s. 167 , 168 In a systemic review of multiple studies, about 70% of patients responded, 169 and in an earlier review, patients with the most severe disease (SeHCAT <5% 7d‐retention), had a greater likelihood of response (96%) than those with less severe disease. 170 Similarly, in post‐cholecystectomy bile acid diarrhoea, data show a greater response rate (81%) in those with severe disease. 171 The only randomised clinical trial of colestyramine showed major decreases in number of total and watery stools, but also included an active comparator, hydroxypropyl cellulose. 172
Colestipol is an alternative and has been shown to be of use in studies of bile acid diarrhoea. 173 Colesevelam is a particularly effective sequestrant as demonstrated in a randomised controlled trial in Crohn's disease. 174
Colestyramine has remained the usual first choice drug but is often poorly tolerated, sometimes because the drug is not introduced slowly, or because of its formulation. 12 Colesevelam may be more acceptable in those who fail colestyramine. 170 , 175 Long‐term use of sequestrants is affected by their poor tolerability. 176 However, an initial response can indicate the presence of bile acid diarrhoea, in the absence of a suitable diagnostic test. 177
Statement 2.7: UEG/ESNM recommend FOR the short‐term usefulness of a low FODMAPs diet in patients with IBS‐D when other measures have failed, but there is no evidence for FDr.
Statement endorsed, overall agreement: 100%: A+ 67%, A 33%, A‐ 0%, D‐ 0%, D 0%, D+ 0%.
LE: Low; GR: Strong.
Summary of evidence: Many IBS patients report that intake of food induces or aggravates symptoms. 178 FODMAPs are short‐chain carbohydrates that are incompletely absorbed in the small intestine. Fermentable oligo‐, di‐, and mono‐saccharides and polyols enter the colon where they are fermented, causing production of gas and since they are osmotically active, they can lead to increased water content in the intestinal lumen. 179 This process is thought to be amplified in the presence of intestinal dysbiosis. This may cause symptoms such as abdominal pain, diarrhoea, flatulence, and bloating. Three meta‐analyses have demonstrated that a low FODMAP diet improves global symptoms in IBS patients, when compared with various and heterogeneous dietary interventions. 180 , 181 , 182 These improvements were investigated mostly in patients with IBS‐D. Due to study heterogeneity, Dionne et al. 181 performed separate analyses comparing a low FODMAP diet with various control interventions. The authors showed that there was a trend for a low FODMAP diet to reduce global IBS symptoms, as compared with alternative diets, including the National Institute for Health and Care Excellence (NICE) diet (relative risk (RR) = 0.82; 95% CI 0.66–1.02), and a statistically significant effect when compared with a usual diet (RR = 0.46; 95% CI 0.25–0.84). 183 More recently, a randomized controlled study from Iran, including 101 IBS‐D patients, found a low FODMAP diet was significantly superior to traditional dietary advice in improving overall gastrointestinal symptom scores, stool frequency, and stool consistency at 6 weeks. 184 A non‐randomized clinical trial of adult patients with IBS of Mediterranean origin, 185 compared a low FODMAP diet to a standard diet according to the British Dietetic Association's guidelines. Completion of 4 weeks of diet resulted in improvement of symptoms as well as quality of life in both groups. However, the low FODMAP diet led to higher rates of symptom relief, primarily with respect to abdominal pain and diarrhoea. In support of this, another prospective Italian study comparing low FODMAP diet, gluten‐free diet, and a Mediterranean diet, showed only the low‐FODMAP diet leading to the normalisation of stool consistency. 186
The above‐mentioned studies have mainly addressed the short‐term effectiveness (up to 4–6 weeks) of the low‐FODMAP diet. An initial restriction diet for a short period should be followed by a gradual reintroduction of food items containing FODMAPs in order to identify individual FODMAPs that should be restricted in the long‐term. 187 A recent randomized controlled study, including only patients with IBS‐D, evaluated the efficacy and acceptability of short‐term strict low FODMAP diet and of a long‐term “modified” FODMAP diet compared with traditional dietary advice, and showed that both, strict and modified low FODMAP diet, are acceptable and lead to significant improvement in symptoms and quality of life. 188 However, the valid threshold for reduction of FODMAPs in the short and long‐term has not been identified. 189
Sustained symptom relief with “adapted” low FODMAPs has been demonstrated by several long‐term observational studies. 190 Extensive long‐term restrictions are not recommended due to the risk of dietary inadequacy related to the exclusion of many nutrient‐rich foods, difficulties in adherence, costs, and social difficulties. Studies have shown a reduction of Bifidobacteria and other microbial changes as well as iron and calcium deficiency that may negatively impact patients' health. The complexity of the diet requires the involvement of experienced dieticians. 187 , 188 , 189 , 190
Statement 2.8: UEG/ESNM recommend AGAINST a gluten free diet for patients with IBS‐D, but there is no evidence for FDr.
Statement endorsed, overall agreement: 100%: A+ 67%, A 33%, A‐ 0%, D‐ 0%, D 0%, D+ 0%.
LE: Low; GR: Strong.
Summary of evidence: The majority of IBS patients consider their symptoms to be related to food and often automatically avoid gluten without medical consultation. 191 Gluten is a complex of proteins of wheat, mainly gliadins and glutenins, commonly used as an additive in processed foods for improved texture, moisture retention, and flavour. The effect of gluten restriction in IBS‐D is unclear. 192 Aziz et al., evaluated symptom response in 41 patients with IBS‐D who underwent a 6‐week gluten‐free diet. A clinical improvement was observed in 71% of patients. 193 A randomized controlled 4‐week trial reported the results of 45 patients with IBS‐D who were randomized to gluten‐free diet versus gluten‐containing diet. 194 The authors found that the daily stool frequency was higher in the patients consuming gluten compared with those who followed a gluten‐free diet and that the positive effect was more pronounced in patients with the human leucocyte antigen‐DQ2 or ‐DQ8 haplotype. 194
A recent meta‐analysis on the role of a gluten‐free diet in IBS identified only two randomized controlled studies including 111 participants. 181 Both studies recruited patients who reported an improvement of their symptoms on a gluten‐free diet and then randomised them to a gluten challenge or to continue with a gluten‐free diet. Both trials reported an advantage for the gluten‐free diet. However, when the two trials were pooled, the results were not statistically significant (RR = 0.42; 95% CI 0.11–1.55, I 2 = 88%). Two additional studies suggested that any benefit of a gluten‐free diet in IBS may not be related to gluten itself, but rather to decreased wheat‐related FODMAPs, particularly fructan. 195 , 196
Statement 2.9: UEG/ESNM recommend FOR gut‐directed psychological therapies as an alternative treatment in patients with IBS‐D, but there is no evidence for FDr.
Statement endorsed, overall agreement: 89%: A+ 78%, A 11%, A‐ 11%, D‐ 0%, D 0%, D+ 0%.
LE: Low; GR: Strong.
Summary of evidence: The pathophysiology of IBS is multifactorial, and psychological alterations are common and considered important for symptom generation in IBS. 197 In particular, fear of symptoms, pain catastrophizing, attentional bias/hypervigilance, somatization, and stress sensitivity play a major role in this context. Moreover, having multiple psychological alterations has been cumulatively associated with reporting more severe gastrointestinal symptoms in patients with IBS, 198 and appears to lead to a worse prognosis. 199 Indeed, previous studies suggest that gut‐directed psychological therapies, alone or adjunctive to medical therapies, including IBS‐specific CBT, relaxation, gut‐directed hypnotherapy, mindfulness‐based stress reduction, stress management, and psychodynamic therapy may be effective in the treatment of core symptoms of IBS such as abdominal pain, altered bowel habit, and quality of life. 200 , 201 , 202 , 203 Of note, the majority of these studies included patients with IBS‐D (about 30%–50% of the various populations included in the studies), without providing outcome data in this specific patient group.
A recent network meta‐analysis comparing different psychological therapies in patients with IBS, concluded that CBT and gut‐directed hypnotherapy were more efficacious than either education and/or support or routine care. Moreover, CBT via the telephone, contingency management, CBT via the Internet and dynamic psychotherapy were all superior to routine care. Further, psychological therapy trials have methodological limitations (i.e. inability to blind patients or the investigators as to treatment assignment, the difficulty of identifying and devising a control treatment, different measurement of treatment fidelity, differences in control for time, attention, and the therapist‐patient relationship). Nevertheless, a meta‐analysis revealed that psychological therapies appear to be effective as treatment for IBS, with a number needed to treat of 4 when the validated IBS symptom severity scale is used as a primary outcome measure. 200 , 201 Furthermore, adverse events were poorly reported in trials of these various different therapies. In summary, we suggest the use of psychological therapies in conjunction with other IBS therapies. As to the type and duration, a qualified provider will likely base this decision on patient preference, cost, ease of use, and presence of contraindications.
Statement 2.10: UEG/ESNM recommend AGAINST the use of faecal microbiota transplantation (FMT) in patients with IBS‐D or FDr.
Statement endorsed, overall agreement: 100%: A+ 81%, A 19%, A‐ 0%, D‐ 0%, D 0%, D+ 0%.
LE: Low; GR: Strong.
Summary of evidence: Dysbiosis has been suggested to play a major role in the pathogenesis of DGBI with diarrhoea. Thus, different agents able to modulate the gut microbiota such as probiotics, prebiotics, or antibiotics have been proposed as treatment options for IBS. More recently, the use of FMT, defined as the transfer of gastrointestinal microbiota from a healthy donor into the gastrointestinal tract of patients with dysbiosis, has been proposed and evaluated in IBS. Two recent meta‐analyses, including five randomized controlled studies with 267 patients enrolled, failed to observe a benefit of FMT in relieving symptoms in patients with IBS. 204 , 205 Of note, over 90% of patients had IBS‐D or IBS‐M. In the first meta‐analysis, Myneedu et al. failed to observe significant improvements with FMT over control (RR = 0.93; 95% CI 0.50–1.75) or changes in the IBS severity scoring system or IBS‐QoL. In the second meta‐analysis by Ianiro et al., the RR of IBS symptoms not improving was 0.98 (95% CI 0.58–1.66). The authors observed that FMT from donor stool delivered via colonoscopy was superior to autologous stool in two pooled Randomised Controlled Trial (RCTs) (RR = 0.63; 95% CI 0.43–0.93). Faecal microbiota transplantation from donor stool via naso‐jejunal tube showed a trend towards a benefit over autologous stool in one trial (RR = 0.69; 95% CI 0.46–1.02). However, several limitations of these studies have been underlined, which preclude any firm conclusions being drawn. Finally, a recent randomized controlled study in 165 patients with IBS found that FMT carried out using a well‐defined donor with a normal dysbiosis index and favourable specific microbial signature was an effective treatment for patients with IBS. 206 Future studies should test FMT in IBS to understand its efficacy, determine what or who is the optimal donor (e.g., fresh vs. frozen; random donor vs. universal donor), and which is the best technique (e.g., nasojejunal vs. colonoscopy vs. oral capsule).
Statement 2.11: UEG/ESNM recommend FOR the use of eluxadoline for treating patients with IBS‐D, but there is no evidence for FDr.
Statement endorsed, overall agreement: 96%: A+ 70%, A 26%, A‐ 4%, D‐ 0%, D 0%, D+ 0%.
LE: High; GR: Strong.
Summary of evidence: Eluxadoline is a phenylimidazole with mixed opioid receptor activity, acting as a μ‐opioid receptor agonist, a δ‐opioid receptor antagonist, and a κ‐opioid receptor agonist. 207 Two multicentre, double‐blind, placebo‐controlled, phase 3 studies, were conducted in 2428 patients with IBS‐D applying FDA and EMA endpoints. 207 , 208 These were 26 week studies followed by a 26 week follow‐up period and a 2 week post‐treatment follow‐up period in one trial and by a 4‐week withdrawal period in the other trial. These studies demonstrated that 100 mg eluxadoline was more effective than placebo in treating IBS‐D symptoms. 207 A significant effect was also seen for 75 mg eluxadoline, but in this case only according to FDA, not EMA, endpoints. Both doses showed superiority to placebo for stool consistency, frequency, urgency, adequate relief of IBS symptoms, global symptom scores, and scores on IBS‐quality of life questionnaires. However, eluxadoline was not superior to placebo when only the percentage of patients who reported an improvement of at least 30% in their worst abdominal pain was considered. Considering that its main effect is on bowel habit, it could be expected to be useful in patients with FDr.
The most common adverse events with eluxadoline were nausea, constipation, and abdominal pain. 207 However, a more serious side effect of pancreatitis was reported in some patients participating in the pivotal trials (five out of 1666 patients who received eluxadoline in the phase 3 trials, two on 75 mg and three on 100 mg) and there have been 120 reports of pancreatitis or death in patients receiving eluxadoline made to the FDA via the Federal Adverse Events Reporting System, a publicly accessible reporting system. 208 As these cases were more frequent in patients with previous cholecystectomy, the FDA declared previous cholecystectomy a contraindication to the use of eluxadoline in line with a previous recommendation by the EMA. 208 Other contraindications are conditions associated with increased risk of pancreatitis such as alcoholism, excessive alcohol use, and sphincter of Oddi spasm. 208 A recent multicentre phase IV trial conducted in USA and Canada in 346 adults with IBS‐D (Rome III criteria) randomly assigned to placebo or eluxadoline 100 mg twice daily for 12 weeks has demonstrated that eluxadoline is effective and safe in treating patients with an intact gallbladder reporting inadequate relief with prior loperamide use. In particular, rates of adverse events were similar between placebo and eluxadoline and no treatment‐related serious adverse event, cases of sphincter of Oddi spasm, or pancreatitis were reported. 209 Even though the drug was approved by the FDA, EMA, and NICE for the treatment of IBS‐D patients who have not responded to other pharmacological treatments, eluxadoline is currently only marketed in the USA and Canada.
Statement 2.12: UEG recommends FOR the use of TCAs for treating patients with IBS‐D, but there is no evidence for FDr.
Statement endorsed, overall agreement: 96%: A+ 70%, A 26%, A‐ 4%, D‐ 0%, D 0%, D+ 0%.
LE: unable to assess using GRADE methodology; GR: Consensus recommendation.
Summary of evidence: Tricyclic anti‐depressants (TCA) are a class of agents, now commonly referred to as neuromodulators, which include amitriptyline, nortriptyline, imipramine, and desipramine. They act through 5‐HT and nor‐adrenaline reuptake inhibition, therefore they are believed to improve visceral pain and central pain in addition to reduce psychological distress. 210 , 211 Moreover, they exert some anticholinergic effects, and therefore slow transit and have anti‐diarrhoeal actions. 200 , 210 , 211 In a meta‐analysis of 12 RCTs including 787 patients, TCAs were superior to placebo for global IBS symptoms or abdominal pain (RR 0.65; 95% CI 0.55–0.77) and for abdominal pain alone (RR 0.59; 95% CI 0.42–0.83). 200 A more recent systematic review with network meta‐analysis aimed to compare and rank the efficacy of soluble fibre, antispasmodic drugs, and gut–brain neuromodulators in patients with IBS, identified 10 out of 40 RCTs evaluating the efficacy and safety of TCAs at improving global IBS symptoms, with only three at low risk of bias and without heterogeneity between studies. 114 This network meta‐analysis highlighted that TCAs in IBS are significantly more efficacious than placebo after 4–12 weeks of treatment (0.66, 0.53–0.83; P‐score 0.77) and they were ranked second compared to antispasmodics and soluble fibres at improving global IBS symptoms. 114 Further, TCAs were ranked first in terms of efficacy at improving abdominal pain alone after 4–12 weeks of treatment from four out of 25 RCTs, with none of them at low risk of bias, but enrolling only 92 patients (RR 0.53, 95% CI 0.34–0.83; P‐score 0.87). 114 However, these studies are of limited sample size and in terms of control of stool pattern, most do not provide details on diarrhoea. Indeed, only one study was carried out in patients with IBS‐d, another one involved all IBS subtypes, while the other studies did not describe the proportion of IBS subtypes enrolled. In particular, in the former study, a placebo‐controlled trial of amitriptyline in 54 patients with IBS‐D, this compound was superior to placebo in decreasing the number of loose stools, feeling of incomplete defecation and complete response, defined as loss of all symptoms. 212 In an uncontrolled study in patients with faecal incontinence, amitriptyline prolonged colonic transit time, improved stool consistency, suppressed rectal motor events, and improved faecal incontinence. 213 Finally, the safety profile of TCAs for the treatment of IBS has been reviewed in several publications, observing that AEs, most commonly drowsiness and dry mouth, occurred at a significantly greater rate with TCAs than placebo (RR 1.59; 95% CI 1.23–2.06). 200 Other AEs reported included insomnia, constipation, urinary retention, flushing, cardiac effects such as palpitations, and decreased appetite.
In conclusion, while the evidence for TCAs in controlling abdominal pain is robust, an anti‐diarrhoeal effect of TCAs is limited although transit and motility studies show that TCAs inhibit bowel motility and slow transit. Moreover, data are not available to recommend one specific TCA and caution should be adopted when using them in order to minimize side effects. It should also be stressed that these drugs need be used at low doses for their pain modulatory properties and should be taken in the evening, before bedtime, due to their sedating effects. Finally, larger RCTs, potentially using different TCAs and specifically performed in patients with IBS‐d and FDr, are necessary to improve our understanding on the role of TCAs in functional bowel disorders with diarrhoea.
Statement 2.13: UEG/ESNM recommend AGAINST the use of Selective serotonin reuptake inhibitors (SSRIs) for treating patients with IBS‐D and FDr.
Statement endorsed, overall agreement: 100%: A+ 67%, A 33%, A‐ 0%, D‐ 0%, D 0%, D+ 0%.
LE: Very Low; GR: Conditional recommendation.
Summary of evidence: SSRIs, are a class of agents which include citalopram, fluoxetine, paroxetine. They increase the bioavailability of tissue ‐5HT by reducing its reuptake by epithelial cells, thus augmenting 5‐HT prokinetic and prosecretory effects. 210 , 211 In a meta‐analysis of 7 RCTs of SSRI, recruiting 356 patients, these drugs were superior to placebo for global IBS symptoms or abdominal pain (RR 0.68; 95% CI 0.51–0.91), but not abdominal pain alone. Moreover, there was significant heterogeneity among the seven trials. 200 A more recent systematic review with network meta‐analysis aimed to compare and rank the efficacy of soluble fibre, antispasmodic drugs, and gut–brain neuromodulators in patients with IBS, identified six trials of SSRIs, with three of them at low risk of bias but significant heterogeneity between studies. 114 This network meta‐analysis highlighted that SSRIs in IBS are not significantly more efficacious than placebo after 4–12 weeks of treatment (0.81, 0.59–1.11; P‐score 0.42) and they were ranked fifth and fourth for global symptoms and abdominal pain (RR 0.82, 95% CI 0.58–1.16; P‐score 0.37), respectively, compared to antispasmodics and soluble fibres. 114 The main limitation of these studies is the small sample size and the unknown effect of these drugs on stool pattern, since most do not provide details on diarrhoea or IBS subtypes. Finally, the safety profile of SSRIs for the treatment of IBS has been reviewed in a recent meta‐analysis including two studies, observing that AEs occurred at a significantly greater rate with SSRIs than placebo (RR 1.36; 95% CI 0.70–2.66). 200
In conclusion, there is limited evidence for SSRIs in controlling abdominal pain and diarrhoea in IBS. Moreover, transit and motility studies did not show that SSRIs may inhibit bowel motility and slow transit. Thus, although controlled trials show that SSRIs are able to improve overall IBS symptoms, there is a lack of reporting outcomes in patients with diarrhoea specifically, limiting their role in treating patients with functional bowel disorders with diarrhoea.
Statement 2.14: UEG/ESNM recommend FOR the use of 5‐HT 3 antagonists (i.e., alosetron, ondansetron, ramosetron) in treating patients with IBS‐D to improve IBS symptoms, but there is no evidence for FDr.
Statement endorsed, overall agreement: 96%: A+ 78%, A 18%, A‐ 4%, D‐ 0%, D 0%, D+ 0%.
LE: Moderate; GR: Strong.
Summary of evidence: 5‐HT receptors are classified into seven subtypes, and 5‐HT3 receptors are known to be localized on intestinal nervous plexuses, sensory nerves, and sympathetic and parasympathetic nerves. 5‐HT acts on 5‐HT3 receptors on the parasympathetic ganglia to cause smooth muscle contraction and increased intestinal secretion by stimulating nerve terminal acetylcholine release. Different 5‐HT3 receptor antagonists have been employed in patients with IBS. 214
Ondansetron was the first 5‐HT3 antagonist tested in functional bowel disorders. 215 , 216 , 217 This drug is licenced for treatment of chemotherapy‐induced nausea and vomiting and has a well‐established safety profile. A small crossover trial of ondansetron (titrated from 4 mg o.d. to a maximum of 8 mg t.i.d.) demonstrated a greater effect as compared with placebo on urgency and diarrhoea, but not abdominal pain. 218 A more recent RCT of a 12 mg o.d. bimodal release formulation of ondansetron also reported a greater effect over placebo on diarrhoea, but again not abdominal pain. 219 The most common side effect appears to be constipation. However, at the present time, the drug is not licenced for IBS‐D. A radomized parallel placebo controlled multicenter study to confirm the efficacy and safety of ondansetron in IBS‐D is currently ongoing. 220 Considering the predominant effect of ondansetron on diarrhoea, the drug could also be useful in FDr.
The 5‐HT3 antagonist alosetron was tested in IBS‐D. At a dosage of 1 mg bid it was t more effective than placebo in treating both pain and diarrhoea and it was FDA‐approved for IBS‐D. Adverse events more common than with placebo included constipation, nausea, and headache. 221 , 222 , 223 In 2001, the drug was withdrawn from the market, because of reported cases of ischaemic colitis. 224 A subsequent re‐evaluation of post‐marketing safety demonstrated that the risk of ischaemic colitis in patients treated with alosetron was no different from that of female patients with IBS. 224 Alosetron was reintroduced in the USA, under the risk evaluation and mitigation strategy, and is currently licenced at a dose of 0.5 mg b.i.d. only in women with severe IBS‐D. 224
Ramosetron, a more recently developed 5‐HT3 receptor antagonist, was tested in IBS‐D and has been demonstrated to be superior to placebo in treating both diarrhoea and abdominal pain. 225 The drug is currently licenced only in Asia at a dosage of 2.5mcg o.d. in women and 5mcg o.d. in men. No cases of ischaemic colitis have been reported and the only adverse event more common with ramosetron, as compared with placebo, was constipation. 226
In a recent network meta‐analysis, alosetron was ranked first while ramosetron was the second most effective drug, as compared with eluxadoline and rifaximin. 226 This network meta‐analysis included three RCTs of alosetron 1 mg b.i.d., with a total of 787 patients and a RR of remaining symptomatic of 0.69 (95% CI 0.60–0.80), and one RCT of ramosetron 2.5mcg o.d., with a total of 348 patients and a RR of remaining symptomatic of 0.78 (95% CI 0.67–0.91). 226 Both alosetron and ramosetron have not been tested in patients with FDr, but there are no reasons why they should not work as well as in IBS‐D.
SUMMARY OF RECOMMENDATIONS
Questions, statements, and recommendations on the functional bowel disorders with diarrhoea guidelines by the UEG/ESNM are detailed in Table 3.
Figure 1 schematically summarizes the findings regarding the diagnosis of functional bowel disorders with diarrhoea. Target patients are those with chronic (i.e. more than 3 months) abdominal pain or related symptoms and/or abnormal bowel movements, whereas patients with acute gastrointestinal symptoms are not included in this diagnostic algorithm. The consensus supports a positive diagnosis based on a symptom‐based approach rather than a diagnosis of exclusion. However, establishing a reasonably safe diagnosis of functional bowel disorders with diarrhea cannot be based on symptoms alone. The consensus supports a thorough history taking, a symptom‐based approach in conjunction with the exclusion of alarm symptoms, and minimal testing including full blood count, CRP, serology for coeliac disease, and faecal calprotectin. The initial assessment should direct the clinician to phenotype the condition (IBS‐D or FDr) and start an appropriate treatment, although the vast majority of options are not supported for a specific subgroup. In the case of persistent, severe and/or aggravating symptoms or in case of refractoriness to therapy, further testing should be recommended. In particular, colonoscopy with random biopsies in both the right and left colon is recommended in patients older than 50 years and in the presence of relevant risk factors, alarm features, and/or abnormal routine examination results to exclude colorectal cancer and inflammation. Moreover, VCE followed by enteroscopy upon identification of abnormalities, can be considered to better investigate the small bowel in patients refractory to medical therapy. SeHCAT testing, or other biomarkers, is recommended to identify bile acid diarrhoea. On the other hand, there was no consensus on the benefit of additional examinations including stool testing for enteric infections, apart from patients who live, or have travelled to, developing countries, breath testing for carbohydrate malabsorption, routine diagnostic testing for SIBO, and microbiota testing.
FIGURE 1.
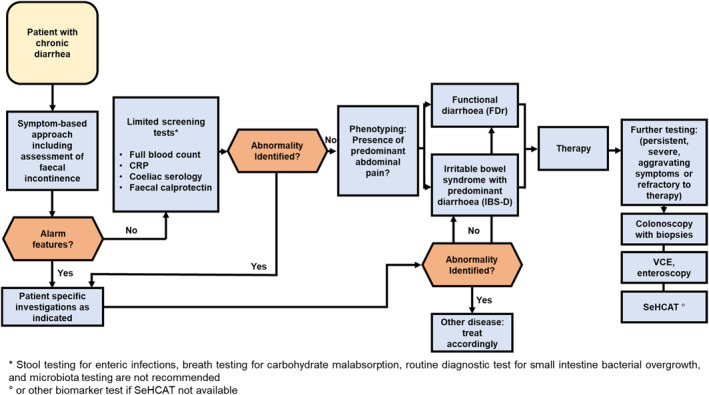
Diagnostic approach for IBS‐D and FDr. CRP, C‐reactive protein; FDr, functional diarrhoea; IBS‐D, irritable bowel syndrome with diarrhoea; SeHCAT, 75Se‐homocholic acid taurine; VCE, video‐capsule endoscopy
Figure 2 illustrates recommendations regarding treatment schematically. A strong consensus was achieved for the use of a low FODMAP diet, gut‐directed psychological therapies, rifaximin, bile acid sequestrants, loperamide and eluxadoline. In particular, bile acid sequestrants were recommended in patients with proven bile acid diarrhoea or, in case of unavailability of SeHCAT testing (or other biomarkers), as an initial trial in patients with persistent unexplained chronic diarrhoea. A weak or conditional recommendation was also achieved for antispasmodics, probiotics, and 5‐HT3 antagonists (i.e., alosetron, ondansetron, ramosetron). Moreover, there was further consensus on the lack of benefit of a gluten‐free diet, mesalazine, FMT, and SSIRs. Finally, no consensus was reached on the use of TCAs in functional bowel disorders with diarrhoea, although a relevant number of the experts were in favour of their use. Of note, most of the evidence for the use of the above‐mentioned treatments come from studies enrolling patients with IBS‐D, and there is a lack of, or only limited, data in patients with FDr.
FIGURE 2.
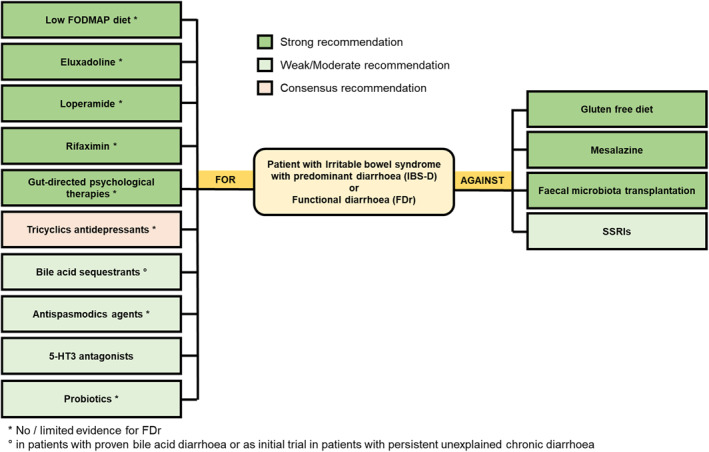
Therapeutic approach for IBS‐D and FDr. 5‐HT3, 5‐hydroxytryptamine‐3; FDr, functional diarrhoea; FODMAPs, fermentable oligo‐, di‐, monosaccharides and polyols; IBS‐D, irritable bowel syndrome with diarrhoea; SSRIs, selective serotonin reuptake inhibitor
CONCLUSION
Functional bowel disorders with diarrhoea are highly prevalent and relevant clinical conditions, with a great impact on physical and psychological status. This multinational and multidisciplinary group of European experts applied a Delphi process to summarize and grade the current state of consensus on the diagnosis and treatment of these conditions. The Consensus Group voted on various statements that may guide clinicians in the management of functional bowel disorders with diarrhoea in clinical practice, and also provide areas in need of future research to improve the quality of care of such challenging disorders.
AUTHOR CONTRIBUTIONS
All the authors contributed with data collection and analysis, writing of the manuscript, approving final version.
CONFLICT OF INTEREST
Edoardo Savarino: has served as speaker for Abbvie, AGPharma, Alfasigma, EG Stada Group, Fresenius Kabi, Grifols, Janssen, Innovamedica, Malesci, Pfizer, Reckitt Benckiser, Sandoz, SILA, Sofar, Takeda, Unifarco; has served as consultant for Alfasigma, Amgen, Biogen, Bristol‐Myers Squibb, Celltrion, Diadema Farmaceutici, Falk, Fresenius Kabi, Janssen, Merck & Co, Reckitt Benckiser, Regeneron, Sanofi, Shire, SILA, Sofar, Synformulas GmbH, Takeda, Unifarco; he received research support from Reckitt Benckiser, SILA, Sofar, Unifarco. Fabiana Zingone: Lecture fees from Sofar, Norgine, Janssen, Takeda, EG. Brigida Barberio: Lecture fees from Alfasigma, Janssen, Sofar, Takeda. Giovanni Marasco: Lecture fees from Echosens, SMEDA Medical, Bromatech. Consultation fees from AlfaSigma. Filiz Akyuz: none. Oana Barboi: none. Giorgia Bodini: Lecture fees from Sandoz, Janssen, Takeda. Serhat Bor: none. Giuseppe Chiarioni: Consultation fees from Takeda, Alfasigma, Kyowa‐Kirin. Gheorghe Cristian: none. Maura Corsetti: Consultation fees from Allergan. Antonio Di Sabatino: none. Anca Mirela Dimitriu: none. Vasile Drug: Lecture fee from: Takeda, Sandoz, KRKA, Reckitt Benckiser; Consultation fees from Bayer; Educational sponsorship from Johnson and Johnson. Dan L. Dumitrascu: Consultation fees for Alfasigma, Menarini Berlin Chemie, Abbvie, Abbott, Takeda, Bayer, Vedra, Terapia. Alexander C. Ford: none. Goran Hauser: Lecture fees from Abbott, Fresenius. Radislav Nakov: Lecture fees from Takeda, Pfizer. Nisha Patel: none. Daniel Pohl: none. Cătălin Sfarti: Lecture fees from Abbvie, Takeda, BMS. Jordi Serra: Research support from Bayer, Salvat, Almirall, Zespiri. Lecture or consultation fees from Norgine, Reckit Benkiser, Cassen‐Recordati, Bayer, Zespiri, Allergan. Magnus Simrén: Research support/collaboration: Glycom, Danone Nutricia Research, Ironwood; Consultant/Advisory Board member: Biocodex, Genetic Analysis AS, DSM, Tillotts, Takeda, Arena, Kyowa Kirin, Adnovate, and Atnahs Pharma.; Lecture fees: Tillotts, Kyowa Kirin, Takeda, Biocodex, AlfaSigma, Sanofi, Janssen Immunology, Pfizer, and Falk Foundation. Alina Suciu: none. Jan Tack: has given Scientific advice to Adare, AlfaWassermann, Arena, Bayer, Christian Hansen, Clasado, Danone, Devintec, Falk, FitForMe, Grünenthal, Ironwood, Janssen, Kiowa Kirin, Menarini, Mylan, Neurogastrx, Neutec, Novartis, Nutricia, Reckitt Benckiser, Recordati, Shionogi, Takeda, Truvion, Tsumura, Zealand and Zeria pharmaceuticals, has received research support from Biohit, Shire, Sofar and Takeda, and has served on the Speaker bureau for Abbott, Allergan, AstraZeneca, FitForMe, Janssen, Kyowa Kirin, Mayoly, Menarini, Mylan, Novartis, Schwabe Parmaceuticals, Takeda, Wellspect and Zeria. Murat Toruner: Lecture and Consultation fees from Abbvie, Takeda, Janssen, MSD, Sandoz. Advisory Board fees from Pfizer, Takeda, Janssen. Julian Walters: Research support from: Enyo Pharmaceuticals, Intercept Pharmaceutical. Advisory board, lecture or consultation fees from GE Healthcare, Metacrine Pharmaceuticals, Pendopharm Pharmaceuticals. Cesare Cremon: Lecture fees from Sofar, Interalia Pharma; Consultation fees from Alfasigma. Giovanni Barbara has been a consultant, served on the advisory board, or received speaker's bureau fees and/or research support from Cadigroup, Falkpharma, Menarini, Parmalat, Sofar, Zespri, Danone, Yakult, Malesci, Noos, Synergy, Alfasigma, Bromatech, Biocodex, AstraZeneca, Devintec, Formedica, GE Healthcare, Mayoly, Sanofi, Eurekol.
ACKNOWLEDGMENT
This consensus was supported by a grant from United European Gastroenterology. These guidelines were developed with the support of a UEG Activity Grant (UEG Grant/Award: no number). This article is published simultaneously in United European Gastroenterology Journal and Neurogastroenterology and Mobility.
Savarino E, Zingone F, Barberio B, Marasco G, Akyuz F, Akpinar H, et al. Functional bowel disorders with diarrhoea: Clinical guidelines of the United European Gastroenterology and European Society for Neurogastroenterology and Motility. United European Gastroenterol J. 2022;10(6):556–84. 10.1002/ueg2.12259
Edoardo Savarino and Fabiana Zingone share co‐first authorship.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Lacy BE, Mearin F, Chang L, Chey WD, Lembo AJ, Simren M, et al. Bowel disorders. Gastroenterology. 2016;150(6):1393–407.e5. 10.1053/j.gastro.2016.02.031 [DOI] [PubMed] [Google Scholar]
- 2. Black CJ, Drossman DA, Talley NJ, Ruddy J, Ford AC. Functional gastrointestinal disorders: advances in understanding and management. Lancet. 2020;396(10263):1664–74. 10.1016/s0140-6736(20)32115-2 [DOI] [PubMed] [Google Scholar]
- 3. Barberio B, Houghton L, Yiannakou Y, Savarino EV, Black CJ, Ford AC. Symptom stability in Rome IV vs Rome III irritable bowel syndrome. Am J Gastroenterol. 2021;116(2):362–71. 10.14309/ajg.0000000000000946 [DOI] [PubMed] [Google Scholar]
- 4. Sperber AD, Bangdiwala SI, Drossman DA, Ghoshal UC, Simren M, Tack J, et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome foundation global study. Gastroenterology. 2021;160(1):99–114. 10.1053/j.gastro.2020.04.014 [DOI] [PubMed] [Google Scholar]
- 5. Oka P, Parr H, Barberio B, Black CJ, Savarino EV, Ford AC. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: a systematic review and meta‐analysis. Lancet Gastroenterol Hepatol. 2020;5(10):908–17. 10.1016/s2468-1253(20)30217-x [DOI] [PubMed] [Google Scholar]
- 6. Mullen PM. Delphi: myths and reality. J Health Organisat Manag. 2003;17(1):37–52. 10.1108/14777260310469319 [DOI] [PubMed] [Google Scholar]
- 7. Aziz I, Palsson OS, Törnblom H, Sperber AD, Whitehead WE, Simren M. The prevalence and impact of overlapping Rome IV‐diagnosed functional gastrointestinal disorders on somatization, quality of life, and healthcare utilization: a cross‐sectional general population study in three countries. Am J Gastroenterol. 2018;113(1):86–96. 10.1038/ajg.2017.421 [DOI] [PubMed] [Google Scholar]
- 8. Ford AC, Bercik P, Morgan DG, Bolino C, Pintos‐Sanchez MI, Moayyedi P. Characteristics of functional bowel disorder patients: a cross‐sectional survey using the Rome III criteria. Aliment Pharmacol Ther. 2014;39(3):312–21. 10.1111/apt.12573 [DOI] [PubMed] [Google Scholar]
- 9. Singh P, Lee HN, Rangan V, Ballou S, Lembo J, Katon J, et al. Similarities in clinical and psychosocial characteristics of functional diarrhea and irritable bowel syndrome with diarrhea. Clin Gastroenterol Hepatol. 2020;18(2):399–405.e1. 10.1016/j.cgh.2019.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schiller LR, Pardi DS, Sellin JH. Chronic diarrhea: diagnosis and management. Clin Gastroenterol Hepatol. 2017;15(2):182–93.e3. 10.1016/j.cgh.2016.07.028 [DOI] [PubMed] [Google Scholar]
- 11. Slattery SA, Niaz O, Aziz Q, Ford AC, Farmer AD. Systematic review with meta‐analysis: the prevalence of bile acid malabsorption in the irritable bowel syndrome with diarrhoea. Aliment Pharmacol Ther. 2015;42(1):3–11. 10.1111/apt.13227 [DOI] [PubMed] [Google Scholar]
- 12. Sadowski DC, Camilleri M, Chey WD, Leontiadis GI, Marshall JK, Shaffer EA, et al. Canadian association of gastroenterology clinical practice guideline on the management of bile acid diarrhea. Clin Gastroenterol Hepatol. 2020;18(1):24–41.e1. 10.1016/j.cgh.2019.08.062 [DOI] [PubMed] [Google Scholar]
- 13. Van Oudenhove L, Levy RL, Crowell MD, Drossman DA, Halpert AD, Keefer L, et al. Biopsychosocial aspects of functional gastrointestinal disorders. Gastroenterology. 2016;150:1355–67.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Van Tilburg MAL, Palsson OS, Whitehead WE. Which psychological factors exacerbate irritable bowel syndrome? Development of a comprehensive model. J Psychosom Res. 2013;74(6):486–92. 10.1016/j.jpsychores.2013.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kamp KJ, Weaver KR, Sherwin LAB, Barney P, Hwang SK, Yang PL, et al. Effects of a comprehensive self‐management intervention on extraintestinal symptoms among patients with IBS. J Psychosom Res. 2019;126:109821. 10.1016/j.jpsychores.2019.109821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nicholl BI, Halder SL, Macfarlane GJ, Thompson DG, O’Brien S, Musleh M, et al. Psychosocial risk markers for new onset irritable bowel syndrome – results of a large prospective population‐based study. Pain. 2008;137(1):147–55. 10.1016/j.pain.2007.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hod K, Ringel Y, van Tilburg MAL, Ringel‐Kulka T. Bloating in irritable bowel syndrome is associated with symptoms severity, psychological factors, and comorbidities. Dig Dis Sci. 2019;64(5):1288–95. 10.1007/s10620-018-5352-5 [DOI] [PubMed] [Google Scholar]
- 18. Lackner JM, Gudleski GD, Thakur ER, Stewart TJ, Iacobucci GJ, Spiegel BM. The impact of physical complaints, social environment, and psychological functioning on IBS patients’ health perceptions: looking beyond GI symptom severity. Am J Gastroenterol. 2014;109(2):224–33. 10.1038/ajg.2013.410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fond G, Loundou A, Hamdani N, Boukouaci W, Dargel A, Oliveira J, et al. Anxiety and depression comorbidities in irritable bowel syndrome (IBS): a systematic review and meta‐analysis. Eur Arch Psychiatr Clin Neurosci. 2014;264(8):651–60. 10.1007/s00406-014-0502-z [DOI] [PubMed] [Google Scholar]
- 20. Ballou S, Katon J, Singh P, Rangan V, Lee HN, McMahon C, et al. Chronic diarrhea and constipation are more common in depressed individuals. Clin Gastroenterol Hepatol. 2019;17(13):2696–703. 10.1016/j.cgh.2019.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rao SSC, Bharucha AE, Chiarioni G, Felt‐Bersma R, Knowles C, Malcolm A, et al. Functional anorectal disorders. Gastroenterology. 2016;150(6):1430–42.e4. 10.1053/j.gastro.2016.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Whitehead WE, Borrud L, Goode PS, Meikle S, Mueller ER, Tuteja A, et al. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology. 2009;137(2):512–17.e2. 10.1053/j.gastro.2009.04.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brown HW, Wexner SD, Segall MM, Brezoczky KL, Lukacz ES. Accidental bowel leakage in the mature women’s health study: prevalence and predictors. Int J Clin Pract. 2012;66(11):1101–8. 10.1111/ijcp.12018 [DOI] [PubMed] [Google Scholar]
- 24. Ditah I, Devaki P, Luma HN, Ditah C, Njei B, Jaiyeoba C, et al. Prevalence, trends, and risk factors for fecal incontinence in United States adults, 2005‐2010. Clin Gastroenterol Hepatol. 2014;12(4):636–643.e2. 10.1016/j.cgh.2013.07.020 [DOI] [PubMed] [Google Scholar]
- 25. Musa MK, Saga S, Blekken LE, Harris R, Goodman C, Norton C. The prevalence, incidence, and correlates of fecal incontinence among older people residing in care homes: a systematic review. J Am Med Dir Assoc. 2019;20(8):956–62.e8. 10.1016/j.jamda.2019.03.033 [DOI] [PubMed] [Google Scholar]
- 26. Bharucha AE, Rao SSC. An update on anorectal disorders for gastroenterologists. Gastroenterology. 2014;146(1):37–45.e2. 10.1053/j.gastro.2013.10.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Whitehead WE, Simren M, Busby‐Whitehead J, Heymen S, van Tilburg MA, Sperber AD, et al. Fecal incontinence diagnosed by the Rome IV criteria in the United States, Canada, and the United Kingdom. Clin Gastroenterol Hepatol. 2020;18(2):385–91. 10.1016/j.cgh.2019.05.040 [DOI] [PubMed] [Google Scholar]
- 28. Menees SB, Chandhrasekhar D, Liew EL, Chey WD. A low FODMAP diet may reduce symptoms in patients with fecal incontinence. Clin Transl Gastroenterol. 2019;10(7):e00060. 10.14309/ctg.0000000000000060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Leigh RJ, Turnberg LA. Faecal incontinence: the unvoiced symptom. Lancet. 1982;1(8285):1349–51. 10.1016/s0140-6736(82)92413-8 [DOI] [PubMed] [Google Scholar]
- 30. Johanson J, Lafferty J. Epidemiology of fecal incontinence: the silent affliction ‐ PubMed. Am J Gastroenterol. 1996;91(1):33–6. [PubMed] [Google Scholar]
- 31. Brown HW, Guan W, Schmuhl NB, Smith PD, Whitehead WE, Rogers RG. If we don’t ask, they won’t tell: screening for urinary and fecal incontinence by primary care providers. J Am Board Fam Med. 2018;31(5):774–82. 10.3122/jabfm.2018.05.180045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Begtrup LM, Engsbro AL, Kjeldsen J, Larsen PV, Schaffalitzky de Muckadell O, Bytzer P, et al. A positive diagnostic strategy is noninferior to a strategy of exclusion for patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2013;11(8):956–62.e1. 10.1016/j.cgh.2012.12.038 [DOI] [PubMed] [Google Scholar]
- 33. Menees SB, Powell C, Kurlander J, Goel A, Chey WD. A meta‐analysis of the utility of C‐reactive protein, erythrocyte sedimentation rate, fecal calprotectin, and fecal lactoferrin to exclude inflammatory bowel disease in adults with IBS. Am J Gastroenterol. 2015;110(3):444–54. 10.1038/ajg.2015.6 [DOI] [PubMed] [Google Scholar]
- 34. Irvine AJ, Chey WD, Ford AC. Screening for celiac disease in irritable bowel syndrome: an updated systematic review and meta‐analysis. Am J Gastroenterol. 2017;112(1):65–76. 10.1038/ajg.2016.466 [DOI] [PubMed] [Google Scholar]
- 35. Pimentel M, Morales W, Rezaie A, Marsh E, Lembo A, Mirocha J, et al. Development and validation of a biomarker for diarrhea‐predominant irritable bowel syndrome in human subjects. PLoS One. 2015;10(5):e0126438. 10.1371/journal.pone.0126438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barbaro MR, Cremon C, Morselli‐Labate AM, Di Sabatino A, Giuffrida P, Corazza GR, et al. Serum zonulin and its diagnostic performance in non‐coeliac gluten sensitivity. Gut. 2020;69(11):1966–74. 10.1136/gutjnl-2019-319281 [DOI] [PubMed] [Google Scholar]
- 37. Sanders DS, Carter MJ, Hurlstone DP, Pearce A, Ward AM, McAlindon ME, et al. Association of adult coeliac disease with irritable bowel syndrome: a case‐control study in patients fulfilling ROME II criteria referred to secondary care. Lancet. 2001;358(9292):1504–8. 10.1016/s0140-6736(01)06581-3 [DOI] [PubMed] [Google Scholar]
- 38. Cash BD, Rubenstein JH, Young PE, Gentry A, Nojkov B, Lee D, et al. The prevalence of celiac disease among patients with nonconstipated irritable bowel syndrome is similar to controls. Gastroenterology. 2011;141(4):1187–93. 10.1053/j.gastro.2011.06.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sánchez‐Vargas LA, Thomas‐Dupont P, Torres‐Aguilera M, Azamar‐Jacome AA, Ramirez‐Ceervanes KL, Aedo‐Garces MR, et al. Prevalence of celiac disease and related antibodies in patients diagnosed with irritable bowel syndrome according to the Rome III criteria. A case‐control study. Neuro Gastroenterol Motil. 2016;28(7):994–1000. 10.1111/nmo.12799 [DOI] [PubMed] [Google Scholar]
- 40. Cash BD, Schoenfeld P, Chey WD. The utility of diagnostic tests in irritable bowel syndrome patients: a systematic review. Am J Gastroenterol. 2002;97(11):2812–19. 10.1111/j.1572-0241.2002.07027.x [DOI] [PubMed] [Google Scholar]
- 41. Spiegel BMR, DeRosa VP, Gralnek IM, Wang V, Dulai GS. Testing for celiac sprue in irritable bowel syndrome with predominant diarrhea: a cost‐effectiveness analysis. Gastroenterology. 2004;126(7):1721–32. 10.1053/j.gastro.2004.03.012 [DOI] [PubMed] [Google Scholar]
- 42. Ford AC, Chey WD, Talley NJ, Malhotra A, Spiegel BMR, Moayyedi P. Yield of diagnostic tests for celiac disease in individuals with symptoms suggestive of irritable bowel syndrome: systematic review and meta‐analysis. Arch Intern Med. 2009;169(7):651–8. 10.1001/archinternmed.2009.22 [DOI] [PubMed] [Google Scholar]
- 43. Carrasco‐Labra A, Lytvyn L, Falck‐Ytter Y, Surawicz CM, Chey WD. AGA technical review on the evaluation of functional diarrhea and diarrhea‐predominant irritable bowel syndrome in adults (IBS‐D). Gastroenterology. 2019;157(3):859–80. 10.1053/j.gastro.2019.06.014 [DOI] [PubMed] [Google Scholar]
- 44. Di Sabatino A, Biagi F, Lenzi M, Frulloni L, Lenti MV, Giuffrida P, et al. Clinical usefulness of serum antibodies as biomarkers of gastrointestinal and liver diseases. Dig Liver Dis. 2017;49(9):947–56. 10.1016/j.dld.2017.06.010 [DOI] [PubMed] [Google Scholar]
- 45. Al‐Toma A, Volta U, Auricchio R, Castillejo G, Sanders DS, Cellier C, et al. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten‐related disorders. United Eur Gastroenterol J. 2019;7(5):583–613. 10.1177/2050640619844125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Klem F, Wadhwa A, Prokop LJ, Sundt WJ, Farrugia G, Camilleri M, et al. Prevalence, risk factors, and outcomes of irritable bowel syndrome after infectious enteritis: a systematic review and meta‐analysis. Gastroenterology. 2017;152(5):1042–54.e1. 10.1053/j.gastro.2016.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Spiegel BMR, Farid M, Esrailian E, Talley J, Chang L. Is irritable bowel syndrome a diagnosis of exclusion?: a survey of primary care providers, gastroenterologists, and IBS experts. Am J Gastroenterol. 2010;105(4):848–58. 10.1038/ajg.2010.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tolliver B, Herrera J, DiPalma J. Evaluation of patients who meet clinical criteria for irritable bowel syndrome ‐ PubMed. Am J Gastroenterol. 1194;89(2):176–8. [PubMed] [Google Scholar]
- 49. Lee JH, Salem R, Aslanian H, Chacho M, Topazian M. Endoscopic ultrasound and fine‐needle aspiration of unexplained bile duct strictures. Am J Gastroenterol. 2004;99(6):1069–73. 10.1111/j.1572-0241.2004.30223.x [DOI] [PubMed] [Google Scholar]
- 50. Speich B, Croll D, Fürst T, Utzinger J, Keiser J. Effect of sanitation and water treatment on intestinal protozoa infection: a systematic review and meta‐analysis. Lancet Infect Dis. 2016;16(1):87–99. 10.1016/s1473-3099(15)00349-7 [DOI] [PubMed] [Google Scholar]
- 51. Halliez MCM, Motta JP, Feener TD, Guerin G, LeGoff L, Francois A, et al. Giardia duodenalis induces paracellular bacterial translocation and causes postinfectious visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol. 2016;310(8):G574–85. 10.1152/ajpgi.00144.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Soares R, Tasca T. Giardiasis: an update review on sensitivity and specificity of methods for laboratorial diagnosis. J Microbiol Methods. 2016;129:98–102. 10.1016/j.mimet.2016.08.017 [DOI] [PubMed] [Google Scholar]
- 53. Rodrigo L. Fecal calprotectin. Rev Esp Enferm Dig. 2007;12:683–8. [DOI] [PubMed] [Google Scholar]
- 54. Van Rheenen PF, Van De Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta‐analysis. BMJ. 2010;341(jul15 1):188. 10.1136/bmj.c3369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Whitehead WE, Palsson OS, Feld AD, Levy RL, Von Korff M, Turner MJ, et al. Utility of red flag symptom exclusions in the diagnosis of irritable bowel syndrome. Aliment Pharmacol Ther. 2006;24(1):137–46. 10.1111/j.1365-2036.2006.02956.x [DOI] [PubMed] [Google Scholar]
- 56. Canavan C, Card T, West J. The incidence of other gastroenterological disease following diagnosis of irritable bowel syndrome in the UK: a cohort study. PLoS One. 2014;9:e106478. 10.1371/journal.pone.0106478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Porter CK, Cash BD, Pimentel M, Akinseye A, Riddle MS. Risk of inflammatory bowel disease following a diagnosis of irritable bowel syndrome. BMC Gastroenterol. 2012;12(1):55. 10.1186/1471-230x-12-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Patel P, Bercik P, Morgan DG, Bolino C, Pintos‐Sanchez MI, Moayyedi P, et al. Prevalence of organic disease at colonoscopy in patients with symptoms compatible with irritable bowel syndrome: cross‐sectional survey. Scand J Gastroenterol. 2015;50(7):816–23. 10.3109/00365521.2015.1007079 [DOI] [PubMed] [Google Scholar]
- 59. Chey WD, Nojkov B, Rubenstein JH, Dobhan RR, Greenson JK, Cash BD. The yield of colonoscopy in patients with non‐constipated irritable bowel syndrome: results from a prospective, controlled US trial. Am J Gastroenterol. 2010;105(4):859–65. 10.1038/ajg.2010.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Neugut A, Garbowski G, Waye J, Forde KA, Treat MR, Tsai JL, et al. Diagnostic yield of colorectal neoplasia with colonoscopy for abdominal pain, change in bowel habits, and rectal bleeding. Am J Gastroenterol. 1993;88(8):1179–83. [PubMed] [Google Scholar]
- 61. Ishihara S, Yashima K, Kushiyama Y, Izumi A, Kawashima K, Fujishiro H, et al. Prevalence of organic colonic lesions in patients meeting Rome III criteria for diagnosis of IBS: a prospective multi‐center study utilizing colonoscopy. J Gastroenterol. 2012;47(10):1084–90. 10.1007/s00535-012-0573-4 [DOI] [PubMed] [Google Scholar]
- 62. Miehlke S, Guagnozzi D, Zabana Y, Tontini GE, Kanstrup Fiehn A, Wildt S, et al. European guidelines on microscopic colitis: United European Gastroenterology and European Microscopic Colitis Group statements and recommendations. United Eur Gastroenterol J. 2021;9(1):13–37. 10.1177/2050640620951905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Roth B, Gustafsson RJ, Ohlsson B. Auto‐antibodies and their association with clinical findings in women diagnosed with microscopic colitis. PLoS One. 2013;8(6):e66088. 10.1371/journal.pone.0066088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wildt S, Nordgaard‐Lassen I, Bendtsen F, Rumessen JJ. Metabolic and inflammatory faecal markers in collagenous colitis. Eur J Gastroenterol Hepatol. 2007;19(7):567–74. 10.1097/meg.0b013e328058ed76 [DOI] [PubMed] [Google Scholar]
- 65. Guagnozzi D, Arias LAJ, Lucendo AJ. Systematic review with meta‐analysis: diagnostic overlap of microscopic colitis and functional bowel disorders. Aliment Pharmacol Ther. 2016;43(8):851–62. 10.1111/apt.13573 [DOI] [PubMed] [Google Scholar]
- 66. Pardi DS, Kelly CP. Microscopic colitis. Gastroenterology. 2011;140(4):1155–65. 10.1053/j.gastro.2011.02.003 [DOI] [PubMed] [Google Scholar]
- 67. Rasmussen MA, Munck LK. Systematic review: are lymphocytic colitis and collagenous colitis two subtypes of the same disease ‐ microscopic colitis? Aliment Pharmacol Ther. 2012;36(2):79–90. 10.1111/j.1365-2036.2012.05166.x [DOI] [PubMed] [Google Scholar]
- 68. Tong J, Zheng Q, Zhang C, Lo R, Shen J, Ran Z. Incidence, prevalence, and temporal trends of microscopic colitis: a systematic review and meta‐analysis. Am J Gastroenterol. 2015;110(2):265–76. 10.1038/ajg.2014.431 [DOI] [PubMed] [Google Scholar]
- 69. Ohlsson B, Bengtsson M, Nielsen J, Toth E. A prospective evaluation of the diagnostic value of video capsule endoscopy in patients initially classified as irritable bowel syndrome. Eur J Intern Med. 2009;20(1):48–52. 10.1016/j.ejim.2008.04.018 [DOI] [PubMed] [Google Scholar]
- 70. Kalla R, McAlindon ME, Sanders DS, Sidhu R. Subtle mucosal changes at capsule endoscopy in diarrhoea predominant Irritable Bowel Syndrome. Med Hypotheses. 2012;79(3):423. 10.1016/j.mehy.2012.05.038 [DOI] [PubMed] [Google Scholar]
- 71. Valero M, Bravo‐Velez G, Oleas R, Puga‐Tejada M, Soria‐Alcivar M, Escobar HA, et al. Capsule endoscopy in refractory diarrhea‐predominant irritable bowel syndrome and functional abdominal pain. Clin Endosc. 2018;51(6):570–5. 10.5946/ce.2018.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pennazio M, Spada C, Eliakim R, Keuchel M, May A, Mulder C, et al. Small‐bowel capsule endoscopy and device‐assisted enteroscopy for diagnosis and treatment of small‐bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2015;47(04):352–76. 10.1055/s-0034-1391855 [DOI] [PubMed] [Google Scholar]
- 73. Landi B, Tkoub M, Gaudric M, Guimbaud R, Cervoni JP, ChauSSade S, et al. Diagnostic yield of push‐type enteroscopy in relation to indication. Gut. 1998;42(3):421–5. 10.1136/gut.42.3.421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Törnblom H, Van Oudenhove L, Sadik R, Abrahamsson H, Tack J, Simren M. Colonic transit time and IBS symptoms: what’s the link? Am J Gastroenterol. 2012;107(5):754–60. 10.1038/ajg.2012.5 [DOI] [PubMed] [Google Scholar]
- 75. Manabe N, Wong BS, Camilleri M, burton D, mckinzie S, zinsmeister AR. Lower functional gastrointestinal disorders: evidence of abnormal colonic transit in a 287 patient cohort. Neuro Gastroenterol Motil. 2010;22(3):293–e82. 10.1111/j.1365-2982.2009.01442.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bures J, Cyrany J, Kohoutova D, Förstl M, Rejchrt S, Kvetina J, et al. Small intestinal bacterial overgrowth syndrome. World J Gastroenterol. 2010;16(24):2978–90. 10.3748/wjg.v16.i24.2978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Downes TJ, Cheruvu MS, Karunaratne TB, De Giorgio R, Farmer AD. Pathophysiology, diagnosis, and management of chronic intestinal pseudo‐obstruction. J Clin Gastroenterol. 2018;52(6):477–89. 10.1097/mcg.0000000000001047 [DOI] [PubMed] [Google Scholar]
- 78. Gorard DA, Libby GW, Farthing MJ. Ambulatory small intestinal motility in “diarrhoea” predominant irritable bowel syndrome. Gut. 1994;35(2):203–10. 10.1136/gut.35.2.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ang D, Pannemans J, Vanuytsel T, Tack J. A single‐center audit of the indications and clinical impact of prolonged ambulatory small intestinal manometry. Neuro Gastroenterol Motil. 2018;30(9):e13357. 10.1111/nmo.13357 [DOI] [PubMed] [Google Scholar]
- 80. Misselwitz B, Butter M, Verbeke K, Fox MR. Update on lactose malabsorption and intolerance: pathogenesis, diagnosis and clinical management. Gut. 2019;68(11):2080–91. 10.1136/gutjnl-2019-318404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Storhaug CL, Fosse SK, Fadnes LT. Country, regional, and global estimates for lactose malabsorption in adults: a systematic review and meta‐analysis. lancet Gastroenterol Hepatol. 2017;2(10):738–46. 10.1016/s2468-1253(17)30154-1 [DOI] [PubMed] [Google Scholar]
- 82. Moayyedi P, Andrews CN, MacQueen G, Korownyk C, Marsiglio M, Graff L, et al. Canadian association of gastroenterology clinical practice guideline for the management of irritable bowel syndrome (IBS). J Can Assoc Gastroenterol. 2019;2(1):6–29. 10.1093/jcag/gwy071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tolliver BA, Jackson MS, Jackson KL, Barnett ED, Chastang JF, DiPalma JA. Does lactose maldigestion really play a role in the irritable bowel? J Clin Gastroenterol. 1996;23(1):15–17. 10.1097/00004836-199607000-00005 [DOI] [PubMed] [Google Scholar]
- 84. Vernia P, Ricciardi M, Frandina C, Bilotta T, Frieri G. Lactose malabsorption and irritable bowel syndrome. Effect of a long‐term lactose‐free diet ‐ PubMed. Ital J Gastroenterol. 1995;27(3):117–21. [PubMed] [Google Scholar]
- 85. Parker TJ, Woolner JT, Prevost AT, Tuffnell Q, Shorthouse M, Hunter JO. Irritable bowel syndrome: is the search for lactose intolerance justified? Eur J Gastroenterol Hepatol. 2001;13(3):219–25. 10.1097/00042737-200103000-00001 [DOI] [PubMed] [Google Scholar]
- 86. Bozzani A, Penagini R, Velio P, Camboni G, Corbellini A, Quatrini M, et al. Lactose malabsorption and intolerance in Italians. Clinical implications. Dig Dis Sci. 1986;31(12):1313–16. 10.1007/bf01299809 [DOI] [PubMed] [Google Scholar]
- 87. Böhmer CJM, Tuynman HARE. The clinical relevance of lactose malabsorption in irritable bowel syndrome. Eur J Gastroenterol Hepatol. 1996;8(10):1013–16. 10.1097/00042737-199610000-00015 [DOI] [PubMed] [Google Scholar]
- 88. Rao SSC, Attaluri A, Anderson L, Stumbo P. Ability of the normal human small intestine to absorb fructose: evaluation by breath testing. Clin Gastroenterol Hepatol. 2007;5(8):959–63. 10.1016/j.cgh.2007.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Helwig U, Koch AK, Koppka N, Holtmann S, Langhorst J. The predictive value of the hydrogen breath test in the diagnosis of fructose malabsorption. Digestion. 2019;99(2):140–7. 10.1159/000489877 [DOI] [PubMed] [Google Scholar]
- 90. Kyaw MH, Mayberry JF. Fructose malabsorption: true condition or a variance from normality. J Clin Gastroenterol. 2011;45(1):16–21. 10.1097/mcg.0b013e3181eed6bf [DOI] [PubMed] [Google Scholar]
- 91. Wilder‐Smith C, Lee SH, Olesen SS, Low JY, Kioh DYQ, Ferraris R, et al. Fructose intolerance is not associated with malabsorption in patients with functional gastrointestinal disorders. Neuro Gastroenterol Motil. 2021;33(12). 10.1111/nmo.14150 [DOI] [PubMed] [Google Scholar]
- 92. Ghoshal UC, Shukla R, Ghoshal U. Small intestinal bacterial overgrowth and irritable bowel syndrome: a bridge between functional organic dichotomy. Gut Liver. 2017;11(2):196–208. 10.5009/gnl16126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol. 2000;95(12):3503–6. 10.1111/j.1572-0241.2000.03368.x [DOI] [PubMed] [Google Scholar]
- 94. Hofmann AF, Mangelsdorf DJ, Kliewer SA. Chronic diarrhea due to excessive bile acid synthesis and not defective ileal transport: a new syndrome of defective FGF19 release. Clin Gastroenterol Hepatol. 2009;7(11):1151–4. 10.1016/j.cgh.2009.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bannaga A, Kelman L, O’connor M, Pitchford C, Walters JRF, Arasaradnam RP. How bad is bile acid diarrhoea: an online survey of patient‐reported symptoms and outcomes. BMJ open Gastroenterol. 2017;4(1):e000116. 10.1136/bmjgast-2016-000116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Vijayvargiya P, Camilleri M. Current practice in the diagnosis of bile acid diarrhea. Gastroenterology. 2019;156(5):1233–8. 10.1053/j.gastro.2018.11.069 [DOI] [PubMed] [Google Scholar]
- 97. Wedlake L, A’Hern R, Russell D, Thomas K, Walters JRF, Andreyev HJN. Systematic review: the prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea‐predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2009;30(7):707–17. 10.1111/j.1365-2036.2009.04081.x [DOI] [PubMed] [Google Scholar]
- 98. Valentin N, Camilleri M, Altayar O, Vijayvargiya P, Acosta A, Nelson AD, et al. Biomarkers for bile acid diarrhoea in functional bowel disorder with diarrhoea: a systematic review and meta‐analysis. Gut. 2016;65(12):1951–9. 10.1136/gutjnl-2015-309889 [DOI] [PubMed] [Google Scholar]
- 99. Black CJ, Craig O, Gracie DJ, Ford AC. Comparison of the Rome IV criteria with the Rome III criteria for the diagnosis of irritable bowel syndrome in secondary care. Gut. 2021;70(6):1110–16. 10.1136/gutjnl-2020-322519 [DOI] [PubMed] [Google Scholar]
- 100. Costa S, Gattoni S, Nicolardi ML, Costetti M, Maimaris S, Schiepatti A, et al. Prevalence and clinical features of bile acid diarrhea in patients with chronic diarrhea. J Dig Dis. 2021;22(2):108–12. 10.1111/1751-2980.12969 [DOI] [PubMed] [Google Scholar]
- 101. Arasaradnam RP, Brown S, Forbes A, Fox MR, Hungin P, Kelman L, et al. Guidelines for the investigation of chronic diarrhoea in adults: British Society of Gastroenterology. Gut. 2018;67(8):1380–99. 10.1136/gutjnl-2017-315909.3rd ed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Smalley W, Falck‐Ytter C, Carrasco‐Labra A, Wani S, Lytvyn L, Falck‐Ytter Y. AGA clinical practice guidelines on the laboratory evaluation of functional diarrhea and diarrhea‐predominant irritable bowel syndrome in adults (IBS‐D). Gastroenterology. 2019;157(3):851–4. 10.1053/j.gastro.2019.07.004 [DOI] [PubMed] [Google Scholar]
- 103. Quigley EMM, Murray JA, Pimentel M. AGA clinical practice update on small intestinal bacterial overgrowth: expert review. Gastroenterology. 2020;159(4):1526–32. 10.1053/j.gastro.2020.06.090 [DOI] [PubMed] [Google Scholar]
- 104. Rezaie A, Buresi M, Lembo A, Lin H, McCallum R, Rao S, et al. Hydrogen and methane‐based breath testing in gastrointestinal disorders: the North American consensus. Am J Gastroenterol. 2017;112(5):775–84. 10.1038/ajg.2017.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Shah A, Talley NJ, Jones M, Kendall BJ, Koloski N, Walker MM, et al. Small intestinal bacterial overgrowth in irritable bowel syndrome: a systematic review and meta‐analysis of case‐control studies. Am J Gastroenterol. 2020;115(2):190–201. 10.14309/ajg.0000000000000504 [DOI] [PubMed] [Google Scholar]
- 106. Rezaie A, Heimanson Z, McCallum R, Pimentel M. Lactulose breath testing as a predictor of response to rifaximin in patients with irritable bowel syndrome with diarrhea. Am J Gastroenterol. 2019;114(12):1886–93. 10.14309/ajg.0000000000000444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Saffouri GB, Shields‐Cutler RR, Chen J, Yang Y, Lekatz HR, Hale VL, et al. Small intestinal microbial dysbiosis underlies symptoms associated with functional gastrointestinal disorders. Nat Commun. 2019;10(1). Available at: 10.1038/s41467-019-09964-7 https://pubmed.ncbi.nlm.nih.gov/31043597/ Accessed 2 December 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Pittayanon R, Lau JT, Yuan Y, Leontiadis GI, Tse F, Surette M, et al. Gut microbiota in patients with irritable bowel syndrome‐A systematic review. Gastroenterology. 2019;157(1):97–108. 10.1053/j.gastro.2019.03.049 [DOI] [PubMed] [Google Scholar]
- 109. Tap J, Derrien M, Törnblom H, Brazeilles R, Cools‐Portier S, Dore J, et al. Identification of an intestinal microbiota signature associated with severity of irritable bowel syndrome. Gastroenterology. 2017;152(1):111–23.e8. 10.1053/j.gastro.2016.09.049 [DOI] [PubMed] [Google Scholar]
- 110. Mars RAT, Yang Y, Ward T, Houtti M, Priya S, Lekatz HR, et al. Longitudinal multi‐omics reveals subset‐specific mechanisms underlying irritable bowel syndrome. Cell. 2020;182(4):1460–73.e17. 10.1016/j.cell.2020.10.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Jabbar KS, Dolan B, Eklund L, Wising C, Ermund A, Johansson A, et al. Association between Brachyspira and irritable bowel syndrome with diarrhoea. Gut. 2021;70(6):1117–29. 10.1136/gutjnl-2020-321466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lesbros‐Pantoflickova D, Michetti P, Fried M, Beglinger C, Blum AL. Meta‐analysis: the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2004;20(11‐12):1253–69. 10.1111/j.1365-2036.2004.02267.x [DOI] [PubMed] [Google Scholar]
- 113. Alammar N, Wang L, Saberi B, Nanavati J, Holtmann G, Shinohara RT, et al. The impact of peppermint oil on the irritable bowel syndrome: a meta‐analysis of the pooled clinical data. BMC Compl Alternative Med. 2019;19(1):21. 10.1186/s12906-018-2409-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Black CJ, Yuan Y, Selinger CP, Camilleri M, Quigley EMM, Moayyedi P, et al. Efficacy of soluble fibre, antispasmodic drugs, and gut–brain neuromodulators in irritable bowel syndrome: a systematic review and network meta‐analysis. Lancet Gastroenterol Hepatol. 2020;5(2):117–31. 10.1016/s2468-1253(19)30324-3 [DOI] [PubMed] [Google Scholar]
- 115. Zsa Z, Weerts RM, Masclee AAM, Witteman BJM, Clemens CHM, Winkens B, et al. Efficacy and safety of peppermint oil in a randomized, double‐blind trial of patients with irritable bowel syndrome; 2020. [DOI] [PubMed] [Google Scholar]
- 116. Kennedy T, Jones R, Darnley S, Seed P, Wessely S, Chalder T. Cognitive behaviour therapy in addition to antispasmodic treatment for irritable bowel syndrome in primary care: randomised controlled trial. BMJ. 2005;331(7514):435–7. 10.1136/bmj.38545.505764.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Lee KJ, Kim NY, Kwon JK, Huh KC, Lee OY, Lee JS, et al. Efficacy of ramosetron in the treatment of male patients with irritable bowel syndrome with diarrhea: a multicenter, randomized clinical trial, compared with mebeverine. Neuro Gastroenterol Motil. 2011;23(12):1098–104. 10.1111/j.1365-2982.2011.01771.x [DOI] [PubMed] [Google Scholar]
- 118. Darvish‐Damavandi M, Nikfar S, Abdollahi M. A systematic review of efficacy and tolerability of mebeverine in irritable bowel syndrome. World J Gastroenterol. 2010;16(5):547. 10.3748/wjg.v16.i5.547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Clavé P, Acalovschi M, Triantafillidis JK, Uspensky YP, Kalayci C, Shee V, et al. Randomised clinical trial: otilonium bromide improves frequency of abdominal pain, severity of distention and time to relapse in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2011;34(4):432–42. 10.1111/j.1365-2036.2011.04730.x [DOI] [PubMed] [Google Scholar]
- 120. Clavé P, Tack J. Efficacy of otilonium bromide in irritable bowel syndrome: a pooled analysis. Therap Adv Gastroenterol. 2017;10(3):311–22. 10.1177/1756283x16681708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ford AC, Talley NJ, Spiegel BMR, Foxx‐Orenstein AE, Schiller L, Quigley EMM, et al. Effect of fibre, antispasmodics, and peppermint oil in the treatment of irritable bowel syndrome: systematic review and meta‐analysis. BMJ. 2008;337(nov13 2):1388–92. 10.1136/bmj.a2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Zheng L, Lai Y, Lu W, Li B, Fan H, Yan Z, et al. Pinaverium reduces symptoms of irritable bowel syndrome in a multicenter, randomized, controlled trial. Clin Gastroenterol Hepatol. 2015;13(7):1285–92.e1. 10.1016/j.cgh.2015.01.015 [DOI] [PubMed] [Google Scholar]
- 123. Mitchell SA, Mee AS, Smith GD, Palmer KR, Chapman RW. Alverine citrate fails to relieve the symptoms of irritable bowel syndrome: results of a double‐blind, randomized, placebo‐controlled trial. Aliment Pharmacol Ther. 2002;16(6):1187–95. 10.1046/j.1365-2036.2002.01277.x [DOI] [PubMed] [Google Scholar]
- 124. Ford AC, Moayyedi P, Lacy BE, Lembo AJ, Saito YA, Schiller LR, et al. American college of gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109:S2–S26. 10.1038/ajg.2014.187 [DOI] [PubMed] [Google Scholar]
- 125. Wittmann T, Paradowski L, DucrottÉ P, Bueno L, Andro Delestrain MC. Clinical trial: the efficacy of alverine citrate/simeticone combination on abdominal pain/discomfort in irritable bowel syndrome‐‐a randomized, double‐blind, placebo‐controlled study. Aliment Pharmacol Ther. 2010;31(6):615–24. 10.1111/j.1365-2036.2009.04216.x [DOI] [PubMed] [Google Scholar]
- 126. Hovdenak N. Loperamide treatment of the irritable bowel syndrome. Scand J Gastroenterol Suppl. 1987;130(Suppl 130):81–4. 10.3109/00365528709091004 [DOI] [PubMed] [Google Scholar]
- 127. Lävo B, Stenstam M, Nielsen AL. Loperamide in treatment of irritable bowel syndrome‐‐a double‐blind placebo controlled study. Scand J Gastroenterol Suppl. 1987;130(Suppl 130):77–80. 10.3109/00365528709091003 [DOI] [PubMed] [Google Scholar]
- 128. Li X, Li B, Zhang J, Chen T, Wu H, Shi X, et al. Efficacy of opioid receptor modulators in patients with irritable bowel syndrome: a systematic review and meta‐analysis. Medicine (Baltim). 2021;100(4). 10.1097/md.0000000000024361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Cann PA, Read NW, Holdsworth CD, Barends D. Role of loperamide and placebo in management of irritable bowel syndrome (IBS). Dig Dis Sci. 1984;29(3):239–47. 10.1007/bf01296258 [DOI] [PubMed] [Google Scholar]
- 130. Lacy BE, Chey WD, Cash BD, Lembo AJ, Dove LS, Covington PS. Eluxadoline efficacy in IBS‐D patients who report prior loperamide use. Am J Gastroenterol. 2017;112(6):924–32. 10.1038/ajg.2017.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Whittaker G, Newman J. Loperamide: an emerging drug of abuse and cause of prolonged QTc. Clin Med. 2021;21(2):150–2. 10.7861/clinmed.2020-1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Dumitrascu D, Stanculete M, Mitrea I, Dumitrascu DM, Farcas A. The effect of two antidiarrhoeal drugs on the psychosocial adjustment to illness in chronic functional diarrhoea. Rom J Intern Med. 2004;42(1):191–7. [PubMed] [Google Scholar]
- 133. Chang FY, Lu CL, Chen CY, Luo JC. Efficacy of dioctahedral smectite in treating patients of diarrhea‐predominant irritable bowel syndrome. J Gastroenterol Hepatol. 2007;22(12):2266–72. 10.1111/j.1440-1746.2007.04895.x [DOI] [PubMed] [Google Scholar]
- 134. Gnessi L, Bacarea V, Marusteri M, Pique N. Xyloglucan for the treatment of acute diarrhea: results of a randomized, controlled, open‐label, parallel group, multicentre, national clinical trial. BMC Gastroenterol. 2015;15(1):153. 10.1186/s12876-015-0386-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Trifan A, Burta O, Tiuca N, Petrisor DC, Lenghel A, Santos J. Efficacy and safety of Gelsectan for diarrhoea‐predominant irritable bowel syndrome: a randomised, crossover clinical trial. United Eur Gastroenterol J. 2019;7(8):1093–101. 10.1177/2050640619862721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Prado D. A multinational comparison of racecadotril and loperamide in the treatment of acute watery diarrhoea in adults. Scand J Gastroenterol. 2002;37(6):656–61. 10.1080/00365520212495 [DOI] [PubMed] [Google Scholar]
- 137. Hartmann G, Honikel KO, Knüsel F, Nuesch J. The specific inhibition of the DNA‐directed RNA synthesis by rifamycin. Biochim Biophys Acta. 1967;145(3):843–4. 10.1016/0005-2787(67)90147-5 [DOI] [PubMed] [Google Scholar]
- 138. Ponziani FR, Scaldaferri F, Sienade M, Mangiola F, Matteo M, Pecere S, et al. Increased Faecalibacterium abundance is associated with clinical improvement in patients receiving rifaximin treatment. Benef Microbes. 2020;11(6):519–25. 10.3920/bm2019.0171 [DOI] [PubMed] [Google Scholar]
- 139. Ma X, Shah YM, Guo GL, Wang T, Krausz KW, Idle JR, et al. Rifaximin is a gut‐specific human pregnane X receptor activator. J Pharmacol Exp Therapeut. 2007;322(1):391–8. 10.1124/jpet.107.121913 [DOI] [PubMed] [Google Scholar]
- 140. Pimentel M, Lembo A, Chey WD, Zakko S, Ringel Y, Yu J, et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364(1):22–32. 10.1056/nejmoa1004409 [DOI] [PubMed] [Google Scholar]
- 141. Lembo A, Pimentel M, Rao SS, Schoenfeld P, Cash B, Weinstock LB, et al. Repeat treatment with rifaximin is safe and effective in patients with diarrhea‐predominant irritable bowel syndrome. Gastroenterology. 2016;151(6):1113–21. 10.1053/j.gastro.2016.08.003 [DOI] [PubMed] [Google Scholar]
- 142. Lembo A, Rao SSC, Heimanson Z, Pimentel M. Abdominal pain response to rifaximin in patients with irritable bowel syndrome with diarrhea. Clin Transl Gastroenterol. 2020;11(3):e00144. 10.14309/ctg.0000000000000144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Pimentel M, Cash BD, Lembo A, Wolf RA, Israel RJ, Schoenfeld P. Repeat rifaximin for irritable bowel syndrome: No clinically significant changes in stool microbial antibiotic sensitivity. Dig Dis Sci. 2017;62(9):2455–63. 10.1007/s10620-017-4598-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Ford AC, Harris LA, Lacy BE, Quigley EM, Moayyedi P. Systematic review with meta‐analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48(10):1044–60. 10.1111/apt.15001 [DOI] [PubMed] [Google Scholar]
- 145. Schoenfeld P, Pimentel M, Chang L, Lembo A, Chey WD, Yu J, et al. Safety and tolerability of rifaximin for the treatment of irritable bowel syndrome without constipation: a pooled analysis of randomised, double‐blind, placebo‐controlled trials. Aliment Pharmacol Ther. 2014;39(10):1161–8. 10.1111/apt.12735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Shah E, Kim S, Chong K, Lembo A, Pimentel M. Evaluation of harm in the pharmacotherapy of irritable bowel syndrome. Am J Med. 2012;125(4):381–93. 10.1016/j.amjmed.2011.08.026 [DOI] [PubMed] [Google Scholar]
- 147. Hungin APS, Mitchell CR, Whorwell P, Mulligan C, Cole O, Agreus L, et al. Systematic review: probiotics in the management of lower gastrointestinal symptoms ‐ an updated evidence‐based international consensus. Aliment Pharmacol Ther. 2018;47(8):1054–70. 10.1111/apt.14539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Maria IP‐S, Geoffrey BH, Kathy G, Nardelli A, Bolino C, Lau JT, et al. Probiotic bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with irritable bowel syndrome. Gastroenterology. 2017;153(2):448–59.e8. 10.1053/j.gastro.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 149. Cremon C, Guglielmetti S, Gargari G, Taverniti V, Castellazzi AM, Valsecchi C, et al. Effect of Lactobacillus paracasei CNCM I‐1572 on symptoms, gut microbiota, short chain fatty acids, and immune activation in patients with irritable bowel syndrome: a pilot randomized clinical trial. United Eur Gastroenterol J. 2018;6(4):604–13. 10.1177/2050640617736478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Hod K, Sperber AD, Ron Y, Boaz M, Dickman R, Berliner S, et al. A double‐blind, placebo‐controlled study to assess the effect of a probiotic mixture on symptoms and inflammatory markers in women with diarrhea‐predominant IBS. Neuro Gastroenterol Motil. 2017;29(7):e13037. 10.1111/nmo.13037 [DOI] [PubMed] [Google Scholar]
- 151. Martoni CJ, Srivastava S, Leyer GJ. Lactobacillus acidophilus DDS‐1 and bifidobacterium lactis UABla‐12 improve abdominal pain severity and symptomology in irritable bowel syndrome: randomized controlled trial. Nutrients. 2020;12(2):363. 10.3390/nu12020363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Sun YY, Li M, Li YY, Li LX, Zhai WZ, Wang P, et al. The effect of Clostridium butyricum on symptoms and fecal microbiota in diarrhea‐dominant irritable bowel syndrome: a randomized, double‐blind, placebo‐controlled trial. Sci Rep. 2018;8(1):2964. 10.1038/s41598-018-21241-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Staudacher HM, Lomer MCE, Farquharson FM, Louis P, Fava F, Franciosi E, et al. A diet low in FODMAPs reduces symptoms in patients with irritable bowel syndrome and A probiotic restores bifidobacterium species: a randomized controlled trial. Gastroenterology. 2017;153(4):936–47. 10.1053/j.gastro.2017.06.010 [DOI] [PubMed] [Google Scholar]
- 154. Andresen V, Gschossmann J, Layer P. Heat‐inactivated Bifidobacterium bifidum MIMBb75 (SYN‐HI‐001) in the treatment of irritable bowel syndrome: a multicentre, randomised, double‐blind, placebo‐controlled clinical trial. lancet Gastroenterol Hepatol. 2020;5(7):658–66. 10.1016/s2468-1253(20)30056-x [DOI] [PubMed] [Google Scholar]
- 155. Bashashati M, Moossavi S, Cremon C, Barbaro MR, Moraveji S, Talmon G, et al. Colonic immune cells in irritable bowel syndrome: a systematic review and meta‐analysis. Neuro Gastroenterol Motil. 2018;30(1):e13192. 10.1111/nmo.13192 [DOI] [PubMed] [Google Scholar]
- 156. Barbara G, Cremon C, Carini G, Bellacosa L, Zecchi L, Giorgio RD, et al. The immune system in irritable bowel syndrome. J Neurogastroenterol Motil. 2011;17(4):349–59. 10.5056/jnm.2011.17.4.349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Bafutto M, De Almeida JR, Leite NV, Costa MBG, Oliveira EC, Resende‐Filho J. Treatment of diarrhea‐predominant irritable bowel syndrome with mesalazine and/or Saccharomyces boulardii. Arq Gastroenterol. 2013;50(4):304–9. 10.1590/s0004-28032013000400012 [DOI] [PubMed] [Google Scholar]
- 158. Hanevik K, Dizdar V, Langeland N, Eide GE, Hausken T. Tolerability and effect of mesalazine in postinfectious irritable bowel syndrome. Aliment Pharmacol Ther. 2011;34(2):259–60. 10.1111/j.1365-2036.2011.04715.x [DOI] [PubMed] [Google Scholar]
- 159. Tuteja AK, Fang JC, Al‐Suqi M, Stoddard GJ, Hale DC. Double‐blind placebo‐controlled study of mesalamine in post‐infective irritable bowel syndrome‐‐a pilot study. Scand J Gastroenterol. 2012;47(10):1159–64. 10.3109/00365521.2012.694903 [DOI] [PubMed] [Google Scholar]
- 160. Dorofeyev AE, Kiriyan EA, Vasilenko IV, Rassokhina OA, Elin AF. Clinical, endoscopical and morphological efficacy of mesalazine in patients with irritable bowel syndrome. Clin Exp Gastroenterol. 2011;4:141–53. 10.2147/ceg.s18381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Barbara G, Cremon C, Annese V, Basilisco G, Bazzoli F, Bellini M, et al. Randomised controlled trial of mesalazine in IBS. Gut. 2016;65(1):82–90. 10.1136/gutjnl-2014-308188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Lam C, Tan W, Leighton M, Hastings M, Lingaya M, Falcone Y, et al. A mechanistic multicentre, parallel group, randomised placebo‐controlled trial of mesalazine for the treatment of IBS with diarrhoea (IBS‐D). Gut. 2016;65(1):91–9. 10.1136/gutjnl-2015-309122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Ghadir MR, Poradineh M, Sotodeh M, Ansari R, Kolahdoozan S, Hormati A, et al. Mesalazine has No effect on mucosal immune biomarkers in patients with diarrhea‐dominant irritable bowel syndrome referred to shariati hospital: a randomized double‐blind, placebo‐controlled trial. Middle East J Dig Dis. 2017;9(1):20–5. 10.15171/mejdd.2016.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Zhang FM, Li S, Ding L, Xiang SH, Zhu HT, Yu JH, et al. Effectiveness of mesalazine to treat irritable bowel syndrome: A meta‐analysis, 2019;98. Medicine (Baltim). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Hofmann AF, Poley JR, Rydell K. Role of bile acid malabsorption in pathogenesis of diarrhea and steatorrhea in patients with ileal resection: I. Response to cholestyramine or replacement of dietary long chain triglyceride by medium chain triglyceride. Gastroenterology. 1972;62(5):918–34. 10.1016/s0016-5085(72)80109-4 [DOI] [PubMed] [Google Scholar]
- 166. Hess Thaysen E, Pedersen L. Idiopathic bile acid catharsis. Gut. 1976;17(12):965–70. 10.1136/gut.17.12.965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Sciarretta G, Fagioli G, Furno A, Cecchetti L, Grigolo B, Verri A et al. 75Se HCAT test in the detection of bile acid malabsorption in functional diarrhoea and its correlation with small bowel transit. Gut. 1987;28(8):970–5. 10.1136/gut.28.8.970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Williams AJK, Merrick MV, Eastwood MA. Idiopathic bile acid malabsorption‐‐a review of clinical presentation, diagnosis, and response to treatment. Gut. 1991;32(9):1004–6. 10.1136/gut.32.9.1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Wilcox C, Turner J, Green J. Systematic review: the management of chronic diarrhoea due to bile acid malabsorption. Aliment Pharmacol Ther. 2014;39(9):923–39. 10.1111/apt.12684 [DOI] [PubMed] [Google Scholar]
- 170. Wedlake L, Thomas K, Lalji A, Anagnostopoulos C, Andreyev HJN. Effectiveness and tolerability of colesevelam hydrochloride for bile‐acid malabsorption in patients with cancer: a retrospective chart review and patient questionnaire. Clin Therapeut. 2009;31(11):2549–58. 10.1016/j.clinthera.2009.11.027 [DOI] [PubMed] [Google Scholar]
- 171. Ruiz‐Campos L, Gisbert JP, Ysamat M, Arau B, Loras C, Esteve M, et al. Systematic review with meta‐analysis: the prevalence of bile acid malabsorption and response to colestyramine in patients with chronic watery diarrhoea and previous cholecystectomy. Aliment Pharmacol Ther. 2019;49(3):242–50. 10.1111/apt.15099 [DOI] [PubMed] [Google Scholar]
- 172. Fernández‐Bañares F, Rosinach M, Piqueras M, Ruiz‐Cerulla A, Modolell I, Zabana Y, et al. Randomised clinical trial: colestyramine vs. hydroxypropyl cellulose in patients with functional chronic watery diarrhoea. Aliment Pharmacol Ther. 2015;41(11):1132–40. 10.1111/apt.13193 [DOI] [PubMed] [Google Scholar]
- 173. Bajor A, Törnblom H, Rudling M, Ung KA, Simren M. Increased colonic bile acid exposure: a relevant factor for symptoms and treatment in IBS. Gut. 2015;64(1):84–92. 10.1136/gutjnl-2013-305965 [DOI] [PubMed] [Google Scholar]
- 174. Beigel F, Teich N, Howaldt S, Lammert F, Maul J, Breiteneicher S, et al. Colesevelam for the treatment of bile acid malabsorption‐associated diarrhea in patients with Crohn’s disease: a randomized, double‐blind, placebo‐controlled study. J Crohns Colitis. 2014;8(11):1471–9. 10.1016/j.crohns.2014.05.009 [DOI] [PubMed] [Google Scholar]
- 175. Orekoya O, McLaughlin J, Leitao E, Johns W, Lal S, Paine P. Quantifying bile acid malabsorption helps predict response and tailor sequestrant therapy. Clin Med. 2015;15(3):252–7. 10.7861/clinmedicine.15-3-252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Lin S, Sanders DS, Gleeson JT, Osborne C, Messham L, Kurien M. Long‐term outcomes in patients diagnosed with bile‐acid diarrhoea. Eur J Gastroenterol Hepatol. 2016;28(2):240–5. 10.1097/meg.0000000000000541 [DOI] [PubMed] [Google Scholar]
- 177. Schiller LR. Good news about BAD. Clin Gastroenterol Hepatol. 2020;18(1):45–7. 10.1016/j.cgh.2019.10.031 [DOI] [PubMed] [Google Scholar]
- 178. Böhn L, Störsrud S, Törnblom H, Bengtsson U, Simren M. Self‐reported food‐related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am J Gastroenterol. 2013;108(5):634–41. 10.1038/ajg.2013.105 [DOI] [PubMed] [Google Scholar]
- 179. Barrett JS, Gearry RB, Muir JG, Irving PM, Rose R, Rosella O, et al. Dietary poorly absorbed, short‐chain carbohydrates increase delivery of water and fermentable substrates to the proximal colon. Aliment Pharmacol Ther. 2010;31:874–82. 10.1111/j.1365-2036.2010.04237.x [DOI] [PubMed] [Google Scholar]
- 180. Schumann D, Klose P, Lauche R, Dobos G, Langhorst J, Cramer H. Low fermentable, oligo‐di‐mono‐saccharides and polyol diet in the treatment of irritable bowel syndrome: a systematic review and meta‐analysis. Nutrition. 2018;45:24–31. 10.1016/j.nut.2017.07.004 [DOI] [PubMed] [Google Scholar]
- 181. Dionne J, Ford AC, Yuan Y, Chey WD, Lacy BE, Saito YA, et al. A systematic review and meta‐analysis evaluating the efficacy of a gluten‐free diet and a low FODMAPs diet in treating symptoms of irritable bowel syndrome. Am J Gastroenterol. 2018;113(9):1290–300. 10.1038/s41395-018-0195-4 [DOI] [PubMed] [Google Scholar]
- 182. Lanenvan AS, de Bree A, Greyling A. Efficacy of a low‐FODMAP diet in adult irritable bowel syndrome: a systematic review and meta‐analysis. Eur J Nutr. 2021;60:3505–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. McKenzie YA, Bowyer RK, Leach H, Gulia P, Horobin J, O'Sullivan NA, et al. British Dietetic Association systematic review and evidence‐based practice guidelines for the dietary management of irritable bowel syndrome in adults (2016 update). J Hum Nutr Diet. 2016;29(5):549–75. 10.1111/jhn.12385 [DOI] [PubMed] [Google Scholar]
- 184. Zahedi MJ, Behrouz V, Azimi M. Low fermentable oligo‐di‐mono‐saccharides and polyols diet versus general dietary advice in patients with diarrhea‐predominant irritable bowel syndrome: a randomized controlled trial. J Gastroenterol Hepatol. 2018;33(6):1192–9. 10.1111/jgh.14051 [DOI] [PubMed] [Google Scholar]
- 185. Guerreiro MM, Santos Z, Carolino E, Correa J, Cravo M, Augusto F, et al. Effectiveness of two dietary approaches on the quality of life and gastrointestinal symptoms of individuals with irritable bowel syndrome. J Clin Med. 2020;9(1):125. 10.3390/jcm9010125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Paduano D, Cingolani A, Tanda E, Usai P. Effect of three diets (Low‐FODMAP, gluten‐free and balanced) on irritable bowel syndrome symptoms and health‐related quality of life. Nutrients. 2019;11(7):1566. 10.3390/nu11071566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Whelan K, Martin LD, Staudacher HM, Lomer MCE. The low FODMAP diet in the management of irritable bowel syndrome: an evidence‐based review of FODMAP restriction, reintroduction and personalisation in clinical practice. J Hum Nutr Diet. 2018;31(2):239–55. 10.1111/jhn.12530 [DOI] [PubMed] [Google Scholar]
- 188. Goyal O, Batta S, Nohria S, Kishore H, Goyal P, Sehgal R, et al. Low fermentable oligosaccharide, disaccharide, monosaccharide, and polyol diet in patients with diarrhea‐predominant irritable bowel syndrome: a prospective, randomized trial. J Gastroenterol Hepatol. 2021;36(8):2107–15. 10.1111/jgh.15410 [DOI] [PubMed] [Google Scholar]
- 189. Rej A, Sanders DS, Buckle R, Buckle RL, AzIz I, Shaw C. What is the optimal FODMAP threshold in IBS? J Gastroenterol Hepatol. 2021;36(6):1723–5. 10.1111/jgh.15470 [DOI] [PubMed] [Google Scholar]
- 190. Rej A, Aziz I, Tornblom H, Sanders DS, Simren M. The role of diet in irritable bowel syndrome: implications for dietary advice. J Intern Med. 2019;286(5):490–502. 10.1111/joim.12966 [DOI] [PubMed] [Google Scholar]
- 191. Pauls RN, Max JB. Symptoms and dietary practices of irritable bowel syndrome patients compared to controls: results of a USA national survey. Minerva Gastroenterol Dietol. 2019;65:1–10. 10.23736/s1121-421x.18.02518-7 [DOI] [PubMed] [Google Scholar]
- 192. Singh P, Nee J. Role of diet in diarrhea‐predominant irritable bowel syndrome. J Clin Gastroenterol. 2021;55(1):25–9. 10.1097/mcg.0000000000001445 [DOI] [PubMed] [Google Scholar]
- 193. Aziz I, Trott N, Briggs R, North JR, Hadjivassiliou M, Sanders DS. Efficacy of a gluten‐free diet in subjects with irritable bowel syndrome‐diarrhea unaware of their HLA‐DQ2/8 genotype. Clin Gastroenterol Hepatol. 2016;14(5):696–703.e1. 10.1016/j.cgh.2015.12.031 [DOI] [PubMed] [Google Scholar]
- 194. Vazquez‐Roque MI, Camilleri M, Smyrk T, Murray JA, Marietta E, O'Neill J, et al. A controlled trial of gluten‐free diet in patients with irritable bowel syndrome‐diarrhea: effects on bowel frequency and intestinal function. Gastroenterology. 2013;144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Biesiekierski JR, Peters SL, Newnham ED, Rosella O, Muir JG, Gibson PR. No effects of gluten in patients with self‐reported non‐celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short‐chain carbohydrates. Gastroenterology. 2013;145(2):320–8.e1. 10.1053/j.gastro.2013.04.051 [DOI] [PubMed] [Google Scholar]
- 196. Skodje GI, Sarna VK, Minelle IH, Rolfsen KL, Muir JG, Gibson PR, et al. Fructan, rather than gluten, induces symptoms in patients with self‐reported non‐celiac gluten sensitivity. Gastroenterology. 2018;154(3):529–39.e2. 10.1053/j.gastro.2017.10.040 [DOI] [PubMed] [Google Scholar]
- 197. Simrén M, Törnblom H, Palsson OS, Van Oudenhove L, Whitehead WE, Tack J. Cumulative effects of psychologic distress, visceral hypersensitivity, and abnormal transit on patient‐reported outcomes in irritable bowel syndrome. Gastroenterology. 2019;157(2):391–402.e2. 10.1053/j.gastro.2019.04.019 [DOI] [PubMed] [Google Scholar]
- 198. Midenfjord I, Borg A, Törnblom H, Simren M. Cumulative effect of psychological alterations on gastrointestinal symptom severity in irritable bowel syndrome. Am J Gastroenterol. 2021;116(4):769–79. 10.14309/ajg.0000000000001038 [DOI] [PubMed] [Google Scholar]
- 199. Goodoory VC, Mikocka‐Walus A, Yiannakou Y, Houghton LA, Black CJ, Ford AC. Impact of psychological comorbidity on the prognosis of irritable bowel syndrome. Am J Gastroenterol. 2021;116(7):1485–94. 10.14309/ajg.0000000000001247 [DOI] [PubMed] [Google Scholar]
- 200. Ford AC, Lacy BE, Harris LA, Quigley EM, Moayyedi P. Effect of antidepressants and psychological therapies in irritable bowel syndrome: an updated systematic review and meta‐analysis. Am J Gastroenterol. 2019;114(1):21–39. 10.1038/s41395-018-0222-5 [DOI] [PubMed] [Google Scholar]
- 201. Laird KT, Tanner‐Smith EE, Russell AC, Hollon SD, Walker LS. Comparative efficacy of psychological therapies for improving mental health and daily functioning in irritable bowel syndrome: a systematic review and meta‐analysis. Clin Psychol Rev. 2017;51:142–52. 10.1016/j.cpr.2016.11.001 [DOI] [PubMed] [Google Scholar]
- 202. Everitt HA, Landau S, O’Reilly G, Sibelli A, Hughes S, Windgassen S, et al. Assessing telephone‐delivered cognitive–behavioural therapy (CBT) and web‐delivered CBT versus treatment as usual in irritable bowel syndrome (ACTIB): a multicentre randomised trial. Gut. 2019;68:1613–23. 10.1136/gutjnl-2018-317805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Everitt HA, Landau S, O’Reilly G, Sibelli A, Hughes S, Windgassen S, et al. Cognitive behavioural therapy for irritable bowel syndrome: 24‐month follow‐up of participants in the ACTIB randomised trial. Lancet Gastroenterol Hepatol. 2019;4(11):863–72. 10.1016/s2468-1253(19)30243-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Ianiro G, Eusebi LH, Black CJ, Gasbarrini A, Cammarota G, Ford AC. Systematic review with meta‐analysis: efficacy of faecal microbiota transplantation for the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2019;50(3):240–8. 10.1111/apt.15330 [DOI] [PubMed] [Google Scholar]
- 205. Myneedu K, Deoker A, Schmulson MJ, Bashashati M. Fecal microbiota transplantation in irritable bowel syndrome: a systematic review and meta‐analysis. United Eur Gastroenterol J. 2019;7(8):1033–41. 10.1177/2050640619866990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. El‐Salhy M, Hatlebakk JG, Gilja OH, Brathen Kristoffersen A, Hausken T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double‐blind, placebo‐controlled study. Gut. 2020;69(5):859–67. 10.1136/gutjnl-2019-319630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Lembo AJ, Lacy BE, Zuckerman MJ, Schey R, Dove LS, Andrae DA, et al. Eluxadoline for irritable bowel syndrome with diarrhea. N Engl J Med. 2016;374(3):242–53. 10.1056/nejmoa1505180 [DOI] [PubMed] [Google Scholar]
- 208. Chedid V, Vijayvargiya P, Camilleri M. Advantages and limitations of the federal adverse events reporting system in assessing adverse event reporting for eluxadoline. Clin Gastroenterol Hepatol. 2018;16(3):336–8. 10.1016/j.cgh.2017.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Brenner DM, Sayuk GS, Gutman CR, Jo E, Elmes SJR, Liu LWC, et al. Efficacy and safety of eluxadoline in patients with irritable bowel syndrome with diarrhea who report inadequate symptom control with loperamide: RELIEF phase 4 study. Am J Gastroenterol. 2019;114(9):1502–11. 10.14309/ajg.0000000000000327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Drossman DA, Toner BB, Whitehead WE, Diamant NE, Dalton CB, Duncan S, et al. Cognitive‐behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology. 2003;125(1):19–31. 10.1016/s0016-5085(03)00669-3 [DOI] [PubMed] [Google Scholar]
- 211. Gorard DA, Libby GW, Farthing MJG. Influence of antidepressants on whole gut and orocaecal transit times in health and irritable bowel syndrome. Aliment Pharmacol Ther. 1994;8(2):159–66. 10.1111/j.1365-2036.1994.tb00273.x [DOI] [PubMed] [Google Scholar]
- 212. Vahedi H, Merat S, Momtahen S, Kazzazi AS, Ghaffari N, Olfati G, et al. Clinical trial: the effect of amitriptyline in patients with diarrhoea‐predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2008;27(8):678–84. 10.1111/j.1365-2036.2008.03633.x [DOI] [PubMed] [Google Scholar]
- 213. Santoro GA, Eitan BZ, Pryde A, Bartolo DC. Open study of low‐dose amitriptyline in the treatment of patients with idiopathic fecal incontinence. Dis Colon Rectum. 2000;43(12):1676–81. 10.1007/bf02236848 [DOI] [PubMed] [Google Scholar]
- 214. Marciani L, Wright J, Foley S, Hoad CL, Totman JJ, Bush D, et al. Effects of a 5‐HT(3) antagonist, ondansetron, on fasting and postprandial small bowel water content assessed by magnetic resonance imaging. Aliment Pharmacol Ther. 2010;32:655–63. 10.1111/j.1365-2036.2010.04395.x [DOI] [PubMed] [Google Scholar]
- 215. Steadman CJ, Talley NJ, Phillips SF, Zinsmeister AR. Selective 5‐hydroxytryptamine type 3 receptor antagonism with ondansetron as treatment for diarrhea‐predominant irritable bowel syndrome: a pilot study. Mayo Clin Proc. 1992;67(8):732–8. 10.1016/s0025-6196(12)60797-6 [DOI] [PubMed] [Google Scholar]
- 216. Hammer J, Phillips SF, Talley NJ, Camilleri M. Effect of a 5HT3‐antagonist (ondansetron) on rectal sensitivity and compliance in health and the irritable bowel syndrome. Aliment Pharmacol Ther. 1993;7(5):543–51. 10.1111/j.1365-2036.1993.tb00131.x [DOI] [PubMed] [Google Scholar]
- 217. Maxton DG, Morris J, Whorwell PJ. Selective 5‐hydroxytryptamine antagonism: a role in irritable bowel syndrome and functional dyspepsia? Aliment Pharmacol Ther. 1996;10(4):595–9. 10.1046/j.1365-2036.1996.30172000.x [DOI] [PubMed] [Google Scholar]
- 218. Garsed K, Chernova J, Hastings M, Lam C, Marciani L, Singh G, et al. A randomised trial of ondansetron for the treatment of irritable bowel syndrome with diarrhoea. Gut. 2014;63(10):1617–25. 10.1136/gutjnl-2013-305989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Plasse TF, Barton G, Davidson E, Abramson D, Kalfus I, Fathi R, et al. Bimodal release ondansetron improves stool consistency and symptomatology in diarrhea‐predominant irritable bowel syndrome: a randomized, double‐blind, trial. Am J Gastroenterol. 2020;115(9):1466–73. 10.14309/ajg.0000000000000727 [DOI] [PubMed] [Google Scholar]
- 220. Gunn D, Fried R, Lalani R, Farrin A, Holloway I, Morris T, et al. Treatment of irritable bowel syndrome with diarrhoea using titrated ondansetron (TRITON): study protocol for a randomised controlled trial. Trials. 2019;20(1):517. 10.1186/s13063-019-3562-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Chang L, Chey WD, Harris L, Olden K, Surawicz C, Schoenfeld P. Incidence of ischemic colitis and serious complications of constipation among patients using alosetron: systematic review of clinical trials and post‐marketing surveillance data. Am J Gastroenterol. 2006;101(5):1069–79. 10.1111/j.1572-0241.2006.00459.x [DOI] [PubMed] [Google Scholar]
- 222. Barberio B, Savarino EV, Black CJ, Ford AC. Adverse events in trials of licensed drugs for irritable bowel syndrome with constipation or diarrhea: systematic review and meta‐analysis. Neuro Gastroenterol Motil. 2021;34(6):e14279. 10.1111/nmo.14279 [DOI] [PubMed] [Google Scholar]
- 223. Barberio B, Savarino EV, Black CJ, Ford AC. Placebo response rates in trials of licensed drugs for irritable bowel syndrome with constipation or diarrhea: meta‐analysis. Clin Gastroenterol Hepatol. 2021;20(5):e923–44. 10.1016/j.cgh.2021.08.025 [DOI] [PubMed] [Google Scholar]
- 224. Cole JA, Cook SF, Sands BE, Ajene AN, Miller DP, Walker AM. Occurrence of colon ischemia in relation to irritable bowel syndrome. Am J Gastroenterol. 2004;99(3):486–91. 10.1111/j.1572-0241.2004.04097.x [DOI] [PubMed] [Google Scholar]
- 225. Min YW, Rhee PL. The clinical potential of ramosetron in the treatment of irritable bowel syndrome with diarrhea (IBS‐D). Therap Adv Gastroenterol. 2015;8(3):136–42. 10.1177/1756283x15572580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Black CJ, Burr NE, Camilleri M, Earnest DL, Quigley EM, Moayyedi P, et al. Efficacy of pharmacological therapies in patients with IBS with diarrhoea or mixed stool pattern: systematic review and network meta‐analysis. Gut. 2020;69(1):74–82. 10.1136/gutjnl-2018-318160 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


