Abstract
Rationale
Many lung transplant recipients with cystic fibrosis (CF) have low preoperative body mass index (BMI); however, post-transplant BMI recovery is not well understood.
Objectives
To evaluate BMI recovery (⩾18.5 kg/m2) among CF lung transplant recipients with low preoperative BMI and to investigate the association of survival with BMI recovery.
Methods
The United Network for Organ Sharing and CF Foundation patient registries (June 2005–December 2016) were used to identify CF lung transplant recipients. Among recipients surviving ⩾1 year, Cox modeling compared post-transplant 1-year conditional survival between recipients with low (<17 and 17–18.49 kg/m2) versus normal preoperative BMI, stratified by BMI recovery.
Results
Of 1,977 CF lung transplant recipients, 272 (14%) and 449 (23%) had a preoperative BMI of <17 and 17–18.49 kg/m2, respectively. For subgroups with a BMI of <17 and 17–18.49 kg/m2, 29% versus 49%, respectively, of those alive at 1 year recovered their BMI. Among recipients with low preoperative BMI, adjusted post-transplant 1-year conditional survival was worse than that in those with preoperative BMI ⩾ 18.5 kg/m2; however, BMI recovery mitigated this. Preoperative BMI < 17 kg/m2 had an adjusted hazard ratio of 1.29 (95% confidence interval [CI], 0.92–1.81) with BMI recovery versus 1.57 (95% CI, 1.09–2.25) without recovery, and preoperative BMI 17–18.49 kg/m2 had an adjusted hazard ratio of 1.28 (95% CI, 1.02–1.61) with BMI recovery versus 1.72 (95% CI, 1.14–2.59) without recovery.
Conclusions
Patients with lower preoperative BMI were less likely to achieve BMI recovery within 1 year. However, for those who did, BMI recovery within 1 year after transplant was associated with longer survival.
Keywords: BMI, underweight, survival, forced expiratory volume in 1 second, lung function
Cystic fibrosis (CF) is an autosomal recessive disease that develops from mutations in the CFTR (cystic fibrosis transmembrane conductance regulator) gene (1). Although pulmonary complications are the primary driver of mortality, extrapulmonary manifestations, including malnutrition, are closely linked to morbidity and mortality (2). Promising therapeutics (e.g., CFTR modulators) can slow the decline in lung function and improve nutritional outcomes (3, 4), but lung transplant (LTx) continues to be required for many individuals with CF who experience progressive respiratory failure despite optimal medical management (5). Selecting candidates for LTx takes into account lung disease severity, as well as nutritional status and other medical comorbidities that correlate with post-transplant outcomes (6, 7).
Poor nutritional status as measured by body mass index (BMI) is an independent risk factor for pretransplant death in advanced cystic fibrosis lung disease (ACFLD), and low BMI is an indication for early referral to an LTx center (6, 8, 9). Underweight status has been shown to be common in ACFLD, with one study demonstrating BMI < 18.5 kg/m2 in 42% of CF transplant recipients (10). The mechanism of weight loss and malnutrition in ACFLD is multifactorial, driven by significant energy expenditure (increased work of breathing from ACFLD and repeated respiratory infections), gut inflammation and pancreatic insufficiency resulting in malabsorption, and poor appetite (11, 12). Although several studies describe the impact of preoperative malnutrition on pre- and post-transplant outcomes in CF (13–18), there are limited data about the effects of post-transplant nutritional status on patient outcomes.
The aim of this study was to evaluate the proportion of CF LTx recipients with low preoperative BMI who recover BMI (⩾18.5 kg/m2) after transplant and to determine whether post-transplant BMI recovery is associated with improved survival. Given that impaired nutrition is in part related to ACFLD, we hypothesized that a majority of malnourished individuals with CF who undergo LTx would have a normal BMI (⩾18.5 kg/m2) within 1 year of LTx. Furthermore, we hypothesized that those with low preoperative BMI who did not reach a normal BMI by 1 year would have worse post-transplant 1-year conditional survival and lung function recovery than individuals who achieved a normal BMI. Some of the results of this study have been previously reported in the form of an abstract (19).
Methods
Population
This retrospective cohort study included individuals with CF in the United States who had their first single-organ LTx between June 1, 2005, and December 31, 2015; participants were included if they initially underwent transplant during the study period even if they subsequently underwent repeat transplant, with follow-up time starting at the first transplant. United Network for Organ Sharing (UNOS) registry data were provided by the Organ Procurement and Transplantation Network (OPTN) and merged with data from the Cystic Fibrosis Foundation Patient Registry (CFFPR). CFFPR encounter-level data and UNOS thoracic data were available through December 31, 2016 (to allow at least 1 yr of post-transplant follow-up). Details of the linkage between UNOS and CFFPR records have been described previously (20). Recipients were excluded if they were <18 years old at transplant.
Data
Recipient characteristics at the time of LTx were obtained from the combined UNOS CFFPR dataset (see the Methods section of the online supplement). Patient BMI was documented by UNOS at the time of LTx. BMI categorization was based on the World Health Organization international classification of adult underweight status (BMI < 17 kg/m2, moderate to severe underweight; BMI 17–18.49 kg/m2, mild underweight; and BMI ⩾ 18.5 kg/m2, normal) (21). BMI recovery was defined as documentation of BMI ⩾ 18.5 kg/m2 and was recorded on the date of the first post-transplant CFFPR encounter with BMI ⩾ 18.5 kg/m2. BMI recovery could be documented within 1 year (⩽365 d), 2 years (⩽730 d), or ever during available follow-up.
Statistical Analysis
Descriptive statistics were used to compare the population of patients with and without post-transplant BMI data in CFFPR. We used a chi-square test to compare the proportion of recipients with lung function recovery (proportion with forced expiratory volume in 1 s [FEV1] > 80% of predicted or forced vital capacity > 80% of predicted within 1 yr after LTx) among those with low preoperative BMI (<17 and 17–18.49 kg/m2) with that in a reference group with preoperative BMI ⩾ 18.5 kg/m2.
We used multivariable Cox modeling to assess 1-year conditional survival among patients with low preoperative BMI, stratified by BMI recovery within 1 year (yes/no/unavailable), using preoperative BMI ⩾ 18.5 kg/m2 as the reference group. For those undergoing a repeat LTx, survival time included time after retransplant. We identified a minimal set of covariates a priori as potential confounders of the relationship between preoperative low BMI and post-transplant survival, including CF genotype, age at LTx, insurance status at LTx (private/Medicaid/other), lung allocation score (LAS) at LTx, transplant hospitalization length of stay ⩾ 28 days, any documented insulin use by 1 year post-transplant, and maximum FEV1 percent predicted by 1 year after LTx based on prior literature and clinical experience (22–24). All Cox models were stratified by LTx center, allowing center-specific baseline hazards and variable practices related to LTx in patients with CF with low BMI (25, 26). Sensitivity analyses repeated the Cox model for the subset of transplant centers that had post-transplant CFFPR encounter data within 1 year of LTx for at least 70% of CF recipients.
Tests of nonzero slope from regression of scaled Schoenfeld residuals on time found no evidence that the proportional hazards assumption was violated. All analyses were performed using Stata version 16 (StataCorp) with a two-sided significance value of 0.05. This study was approved by the University of Washington Institutional Review Board (7923).
Results
Recipient Characteristics at LTx
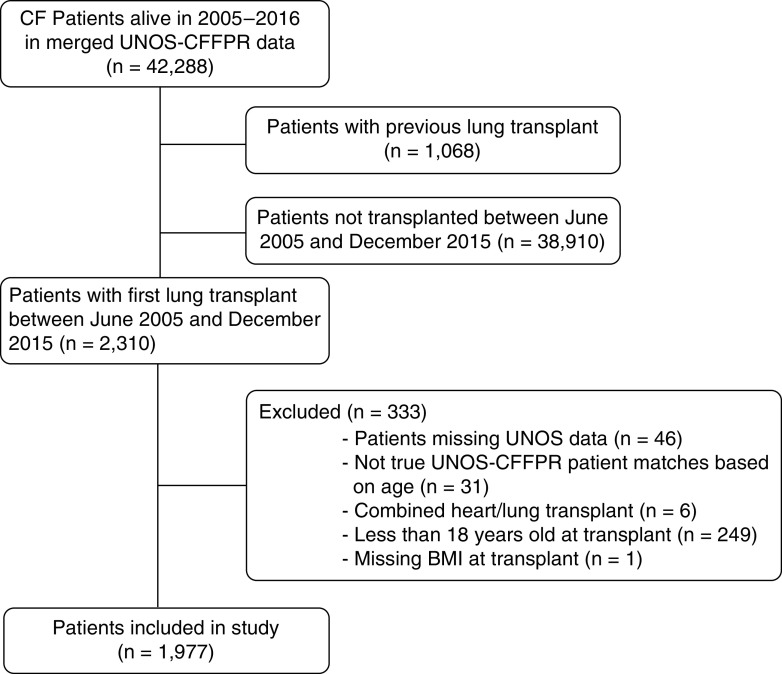
Our cohort included 1,977 adult recipients of single-organ first LTx (Figure 1). Of these, 1,256 (63%) had BMI ⩾ 18.5 kg/m2, 449 (23%) had BMI 17–18.49 kg/m2, and 272 (14%) had BMI < 17 kg/m2 preoperatively. Recipients with preoperative BMI < 17 kg/m2 were more often women and younger and had higher LASs (Table 1). Characteristics of the recipients who did not have CFFPR encounters with BMI recorded in the first year after LTx are available in Table E1 in the online supplement.
Figure 1.
Consolidated Standards of Reporting Trials diagram. BMI = body mass index; CF = cystic fibrosis; CFFPR = Cystic Fibrosis Foundation Patient Registry; UNOS = United Network for Organ Sharing.
Table 1.
Recipient characteristics at time of lung transplant
| Subgroups Defined by BMI at Transplant |
All (N = 1,977) | |||
|---|---|---|---|---|
| ⩾18.5 kg/m2 (n = 1,256) | 17–18.49 kg/m2 (n = 449) | <17 kg/m2 (n = 272) | ||
| Male sex, n (%) | 715 (57) | 226 (50) | 118 (43) | 1,059 (54) |
| Age, yr, median (IQR) | 31 (26–39) | 27 (23–33) | 25 (22–30) | 29 (24–37) |
| Race, n (%) | ||||
| White | 1,236 (98) | 438 (98) | 267 (98) | 1,942 (98) |
| African American | 14 (1) | 9 (2) | —* | 25 (1) |
| Hispanic | 46 (4) | 17 (4) | 6 (2) | 69 (3) |
| Other | 9 (1) | 6 (1) | 5 (2) | 20 (1) |
| Private insurance, n (%) | 732 (58) | 253 (56) | 163 (60) | 1,148 (58) |
| CF mutation class, n (%) | ||||
| 1–3, high risk | 888 (71) | 313 (70) | 195 (72) | 1,397 (71) |
| 4–5, low risk | 79 (6) | 11 (2) | 11 (4) | 101 (5) |
| Not yet classified | 141 (11) | 58 (13) | 39 (14) | 238 (12) |
| Other | 148 (12) | 67 (15) | 27 (10) | 242 (12) |
| Pancreatic insufficiency, n (%) | 1,233 (99) | 446 (100) | 268 (99) | 1,948 (99) |
| Height, cm, mean (SD) | 167 (9) | 166 (9) | 166 (8) | 167 (9) |
| Weight, kg, median (IQR) | 58 (52–66) | 49 (45–53) | 44 (41–48) | 54 (48–61) |
| BMI, kg/m2, median (IQR) | 21 (19–22) | 18 (17–18) | 16 (16–17) | 19 (18–21) |
| Functional status, n (%) | ||||
| Unknown | 11 (1) | 10 (2) | —* | 24 (1) |
| 10%: Moribund, fatal processes progressing rapidly | 57 (5) | 18 (4) | 13 (5) | 88 (4) |
| 20%: Very sick, hospitalization necessary: active treatment necessary | 168 (13) | 51 (11) | 54 (20) | 273 (14) |
| 30%: Severely disabled: hospitalization is indicated, death not imminent | 84 (7) | 25 (6) | 24 (9) | 133 (7) |
| 40%: Disabled: requires special care and assistance | 207 (16) | 90 (20) | 49 (18) | 346 (17) |
| 50%: Requires considerable assistance and frequent medical care | 185 (15) | 62 (14) | 43 (16) | 290 (15) |
| 60%: Requires occasional assistance but is able to care for needs | 224 (18) | 76 (17) | 29 (11) | 330 (17) |
| 70%: Cares for self: unable to carry on normal activity or active work | 178 (14) | 71 (16) | 31 (11) | 280 (14) |
| 80%: Normal activity with effort: some symptoms of disease | 99 (8) | 32 (7) | 15 (6) | 146 (7) |
| 90%: Able to carry on normal activity: minor symptoms of disease | 31 (2) | 10 (2) | 7 (3) | 48 (2) |
| 100%: Normal, no complaints, no evidence of disease | 12 (1) | 4 (1) | —* | 20 (1) |
| Diabetes status, n (%) | ||||
| None documented | 344 (28) | 117 (26) | 89 (33) | 550 (28) |
| Impaired glucose tolerance | 110 (9) | 43 (10) | 18 (7) | 172 (9) |
| Diabetes | 794 (64) | 289 (64) | 165 (61) | 1,248 (63) |
| Insulin status, n (%) | ||||
| No CFRD treatment | 362 (29) | 134 (30) | 89 (33) | 585 (30) |
| Dietary change or oral hypoglycemic agents | 49 (4) | 20 (4) | 12 (4) | 81 (4) |
| Intermittent insulin (e.g., with illness, steroids) | 50 (4) | 22 (5) | 15 (6) | 88 (4) |
| Chronic insulin | 787 (63) | 273 (61) | 156 (57) | 1,216 (62) |
| HbA1c, %, median (IQR) | 6.3 (5.7–7.1) | 6.3 (5.8–6.9) | 6.2 (5.7–7.3) | 6.3 (5.7–7.0) |
| Lung allocation score, median (IQR) | 40 (37–48) | 42 (38–50) | 45 (39–68) | 41 (37–50) |
| FEV1 percent predicted, median (IQR) | 22 (18–27) | 21 (18–25) | 20 (17–25) | 21 (18–26) |
| Length of stay from treatment to discharge, d, median (IQR) | 17 (12–26) | 16 (13–24) | 18 (14–29) | 17 (12–26) |
Definition of abbreviations: BMI = body mass index; CF = cystic fibrosis; CFRD = cystic fibrosis–related diabetes; FEV1 = forced expiratory volume in 1 second; HbA1c = hemoglobin A1c; IQR = interquartile range; SD = standard deviation.
Values < 5 were suppressed to avoid identification of individuals within the cohort.
Post-transplant BMI Recovery
Sixty-five percent of recipients had at least one CFFPR encounter with BMI data recorded within 1 year after LTx (67% for preoperative BMI ⩾ 18.5 kg/m2, 64% for BMI 17–18.49 kg/m2, and 57% for BMI < 17 kg/m2). Among recipients with low preoperative BMI who had BMI data available within 1 year after transplant, 49% of those with preoperative BMI 17–18.49 kg/m2 recovered to BMI ⩾ 18.5 kg/m2 compared with 29% of those with preoperative BMI < 17 kg/m2. By 2 years after transplant, BMI recovery occurred for 48% of those with preoperative BMI 17–18.49 kg/m2 versus 37% of those with preoperative BMI < 17 kg/m2 (Table 2). The median BMI change (difference between BMI at transplant and highest recorded BMI within the first year after transplant) was 1.7 kg/m2 (interquartile range [IQR], 0.3–3.3) for those who had a normal BMI (⩾18.5 kg/m2) at transplant compared with 2.2 kg/m2 (IQR, 1.0–3.7) for those with BMI 17–18.49 kg/m2 at transplant and 2.7 kg/m2 (IQR, 1.4–4.4) for those with BMI < 17 kg/m2 at transplant.
Table 2.
Recipient outcomes by 1 and 2 years after lung transplant
| Preoperative BMI ⩾ 18.5 kg/m2 (n = 1,256) |
Preoperative BMI 17–18.49 kg/m2 (n = 449) |
Preoperative BMI < 17 kg/m2 |
||||
|---|---|---|---|---|---|---|
| n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | |
| By 1 yr after transplant | ||||||
| Alive at 1 yr | 1,131 | 90% (88–92) | 388 | 86% (83–89) | 240 | 88% (84–92) |
| Alive, achieved BMI ⩾ 18.5 kg/m2 | — | — | 218 | 49% (44–53) | 80 | 29% (24–35) |
| Alive, did not achieve BMI ⩾ 18.5 kg/m2 | — | — | 47 | 10% (8–14) | 65 | 24% (19–29) |
| Alive but no BMI data | 329 | 26% (24–29) | 123 | 27% (23–32) | 95 | 35% (30–41) |
| Died by 1 yr | 125 | 10% (8,12) | 61 | 14% (11–17) | 32 | 12% (8–16) |
| Died before achieving BMI ⩾ 18.5 kg/m2 | — | — | 10 | 2% (1–4) | 5 | 2% (1–4) |
| Died after achieving BMI ⩾ 18.5 kg/m2 | — | — | 11 | 2% (1–4) | 4 | 1% (1–4) |
| Died but no BMI data | 91 | 7% (6–9) | 40 | 9% (7–12) | 23 | 8% (6–12) |
| By 2 yr after transplant | ||||||
| Alive at 2 yr | 1,031 | 82% (80–84) | 342 | 76% (72–80) | 212 | 78% (73–82) |
| Alive, achieved BMI ⩾ 18.5 kg/m2 | — | — | 217 | 48% (44–53) | 100 | 37% (31–43) |
| Alive, did not achieve BMI ⩾ 18.5 kg/m2 | — | — | 28 | 6% (4–9) | 44 | 16% (12–21) |
| Alive but no BMI data | 224 | 18% (16–20) | 97 | 22% (18–26) | 68 | 25% (20–30) |
| Died by 2 yr | 225 | 18% (16–20) | 107 | 24% (20–28) | 60 | 22% (18–27) |
| Died before achieving BMI ⩾ 18.5 kg/m2 | — | — | 19 | 4% (3–7) | 17 | 6% (4–10) |
| Died after achieving BMI ⩾ 18.5 kg/m2 | — | — | 37 | 8% (6–11) | 12 | 4% (3–8) |
| Died but no BMI data | 119 | 9% (8–11) | 51 | 11% (9–15) | 31 | 11% (8–16) |
Definition of abbreviations: BMI = body mass index; CI = confidence interval.
Posttransplant Graft Failure and Lung Function Recovery
Within 2 years after transplant, only 0–2% of recipients with a preoperative BMI of ⩾18.5 kg/m2 and 17–18.49 kg/m2 received a repeat transplant during the study period, whereas 9% of recipients with preoperative BMI < 17 kg/m2 underwent retransplant, none of whom had reached BMI ⩾ 18.5 kg/m2 (Table 3). Low preoperative BMI recipients who achieved normal BMI within 1 year after LTx had a similar proportion with lung function recovery compared with the group with normal preoperative BMI, whereas those who did not achieve normal BMI within 1 year were less likely to have recovered lung function (Table 3). Among recipients with low BMI at the time of transplant (<18.5 kg/m2), 26% had an episode of acute rejection within the first year, whereas 61% had no documented episode of rejection (14% were missing data about acute rejection). For LTx recipients who did not recover BMI by 1 year after LTx, 31% had an episode of acute rejection compared with 28% among those who did recover BMI.
Table 3.
Retransplant, BMI, and lung function after lung transplant for recipients with BMI data within 1 year after transplant
| Preoperative BMI ⩾ 18.5 kg/m2 (n = 836) | Preoperative BMI 17–18.5 kg/m2: Achieved BMI ⩾ 18.5 kg/m2 within 1 yr after LTx? |
Preoperative BMI < 17 kg/m2: Achieved BMI ⩾ 18.5 kg/m2 within 1 yr after LTx? |
|||
|---|---|---|---|---|---|
| Yes (n = 229) | No (n = 57) | Yes (n = 84) | No (n = 70) | ||
| Retransplant, n (%) | |||||
| Within 1 yr after LTx | 4 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (6) |
| Within 2 yr after LTx | 12 (1) | 4 (2) | 0 (0) | 0 (0) | 6 (9) |
| First BMI after LTx, kg/m2, median (IQR; range) | 21.1 (IQR, 19.5–22.9; range, 14.8–32.5) | 18.7 (IQR, 17.9–19.9; range, 14.6–26.1) | 17.3 (IQR: 16.4–18.1; range, 13.0–18.4) | 18.4 (IQR, 16.9–19.3; range, 14.6–23.9) | 16.2 (IQR, 15.2–17.0; range, 12.8–18.4) |
| Months from LTx to first BMI, median (IQR; range) | 2 (IQR, 1–4; range, 0–12) | 1 (IQR, 1–4; range, 0–12) | 2 (IQR, 1–5; range, 0–12) | 2 (IQR, 1–4; range, 0–11) | 3 (IQR, 1–5; range, 0–12) |
| Months from LTx to first lung function measurement, median (IQR; range) | 2 (IQR, 1–5; range, 0–64) | 2 (IQR, 1–4; range, 0–53) | 2 (IQR, 1–5; range, 0–12) | 2 (IQR, 1–4; range, 0–13) | 3 (IQR, 1–7; range, 1–35) |
| Lung function: FEV1% and FVC% | |||||
| Any recorded value within 1 yr after LTx, n (%) | 791 (95) | 212 (93) | 55 (96) | 82 (98) | 62 (89) |
| Any recorded value within 2 yr after LTx, n (%) | 805 (96) | 214 (93) | 55 (96) | 84 (100) | 62 (89) |
| Number of FEV1% measurements within 1 yr, median (IQR; range) | 4 (IQR, 2–7; range, 0–21) | 4 (IQR, 2–8; range, 0–18) | 2 (IQR, 1–4; range, 0–19) | 4 (IQR, 3–7; range, 0–16) | 3 (IQR, 1–5; range, 0–18) |
| First FEV1% after LTx, median (IQR; range) | 68 (IQR, 54–82; range, 11–128) | 61 (IQR, 48–78; range, 21–128) | 58 (IQR, 45–65; range, 29–97) | 66 (IQR, 49–78; range, 28–105) | 59 (IQR, 46–70; range, 22–101) |
| Maximum FEV1% in 1 yr after LTx, median (IQR; range) | 82 (IQR, 69–95; range, 17–146) | 82 (IQR, 70–93; range, 33–134) | 65 (IQR, 54–76; range, 29–102) | 79 (IQR, 70–89; range, 31–119) | 71 (IQR, 58–86; range, 22–103) |
| Minimum post-LTx FEV1% within 1 yr, median (IQR; range) | 65 (IQR, 49–78; range, 10–128) | 58 (IQR, 43–74; range, 21–121) | 54 (IQR, 44–65; range, 16–91) | 59 (IQR, 41–73; range, 21–99) | 57 (IQR, 43–69; range, 21–101) |
| Any FEV1% > 80 within 1 yr after LTx, n (%) | 435 (55) | 115 (54); P = 0.85* | 9 (16); P < 0.01* | 36 (44); P = 0.06* | 19 (31); P < 0.01* |
| First FVC% after LTx, median (IQR; range) | 66 (IQR, 52–78; range, 21–123) | 58 (IQR, 46–73; range, 26–111) | 55 (IQR, 46–65; range, 29–85) | 63 (IQR, 50–74; range, 26–112) | 57 (IQR, 45–71; range, 22–111) |
| Maximum FVC% in 1 yr after LTx, median (IQR; range) | 79 (IQR, 69–91; range, 24–133) | 78 (IQR, 67–88; range, 27–118) | 62 (IQR, 51–71; range, 31–97) | 77 (IQR, 71–88; range, 41–112) | 68 (IQR, 59–81; range, 22–111) |
| Minimum post-LTx FVC% within 1 yr, median (IQR; range) | 64 (IQR, 50–75; range, 19–123) | 56 (IQR, 45–70; range, 26–111) | 55 (IQR, 44–64; range, 27–83) | 62 (IQR, 48–71; range, 26–95) | 55 (IQR, 45–65; range, 20–101) |
| Any FVC% > 80 within 1 yr after LTx, n (%) | 382 (48) | 95 (45); P = 0.37* | 9 (16); P < 0.01* | 35 (43); P = 0.33* | 17 (27); P < 0.01* |
Definition of abbreviations: BMI = body mass index; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; IQR = interquartile range; LTx = lung transplant.
P values from chi-square test comparing proportion with reference group with BMI ⩾ 18.5 kg/m2 at LTx.
Posttransplant Survival
By 1 year after transplant, 10%, 14%, and 12% of recipients with a preoperative BMI of⩾18.5 kg/m2, 17–18.49 kg/m2, and <17 kg/m2, respectively, had died (Table 2). Among recipients who survived at least 1 year after LTx, those with preoperative BMI ⩾ 18.5 kg/m2 had median survival time of 10.4 years (95% confidence interval [CI], 9.5–12.2).
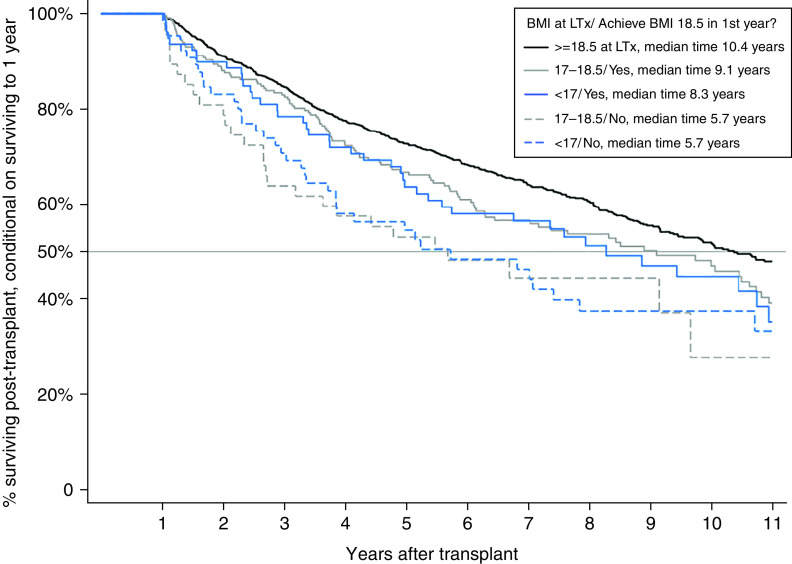
Among recipients with preoperative BMI 17–18.49 kg/m2 who survived at least 1 year after LTx, 1-year conditional survival time was 9.2 years (95% CI, 7.9–10.5), and the 5-year survival proportion was 0.67 (Table E2). When 1-year conditional survival was stratified by the presence or absence of BMI recovery within 1 year after transplant, among those with preoperative BMI 17–18.49 kg/m2, the median survival was 9.1 years (95% CI, 6.3–10.7) versus 5.7 years (95% CI, 2.7–9.7), respectively (Figure 2).
Figure 2.
Kaplan-Meier survival curves for subgroups defined by body mass index (BMI) at lung transplant (LTx) and within 1 year after transplant.
When recipients with preoperative BMI 17–18.49 kg/m2 were compared with those with preoperative BMI ⩾ 18.5 kg/m2, the adjusted hazard ratio (HR) for death conditional on surviving to 1 year was 1.28 (95% CI, 1.02–1.61) for those who achieved BMI ⩾ 18.5 kg/m2 within 1 year and 1.72 (95% CI, 1.41–2.59) for those who did not (Table 4). Among those with preoperative BMI <17 kg/m2, there was a higher risk of death for recipients, regardless of BMI recovery, but BMI recovery attenuated the risk (HR, 1.29 [95% CI, 0.92–1.81] versus 1.57 [95% CI, 1.09–2.25], respectively) (Table 4).
Table 4.
Adjusted* Cox modeling results for death, contingent on surviving to 1 year after LTx (reference group, BMI ⩾ 18.5 kg/m2 at LTx)
| Centers | HR | 95% CI | P Value |
|---|---|---|---|
| Including all centers, n = 1,733 with 783 deaths | |||
| 17–18.49 kg/m2 at LTx, BMI ⩾ 18.5 kg/m2 within 1 yr | 1.28 | (1.02–1.61) | 0.03 |
| 17–18.49 kg/m2 at LTx, no BMI ⩾ 18.5 kg/m2 in 1 yr | 1.72 | (1.14–2.59) | 0.01 |
| 17–18.49 kg/m2 at LTx, no BMI data in 1 yr | 0.62 | (0.44–0.86) | <0.01 |
| <17 kg/m2 at LTx, BMI ⩾ 18.5 kg/m2 within 1yr | 1.29 | (0.92–1.81) | 0.14 |
| <17 kg/m2 at LTx, no BMI ⩾ 18.5 kg/m2 in 1 yr | 1.57 | (1.09–2.25) | 0.02 |
| <17 kg/m2 at LTx, no BMI data in 1 yr | 0.93 | (0.66–1.31) | 0.69 |
| Sensitivity analysis omitting centers with post-transplant encounter data for <70% of recipients within 1 yr after LTx, n = 956 with 433 deaths | |||
| 17–18.49 kg/m2 at LTx, BMI ⩾ 18.5 kg/m2 within 1 yr | 1.15 | (0.88–1.52) | 0.31 |
| 17–18.49 kg/m2 at LTx, no BMI ⩾ 18.5 kg/m2 in 1 yr | 1.68 | (0.92–3.06) | 0.09 |
| 17–18.49 kg/m2 at LTx, no BMI data in 1 yr | 0.44 | (0.21–0.93) | 0.03 |
| <17 kg/m2 at LTx, BMI ⩾ 18.5 kg/m2 within 1 yr | 1.28 | (0.85–1.91) | 0.23 |
| <17 kg/m2 at LTx, no BMI ⩾ 18.5 kg/m2 in 1 yr | 1.48 | (0.94–2.32) | 0.09 |
| <17 kg/m2 at LTx, no BMI data in 1 yr | 1.10 | (0.65–1.87) | 0.72 |
Definition of abbreviations: BMI = body mass index; CI = confidence interval; HR = hazard ratio; LTx = lung transplant; N/A = not applicable.
Covariates include genotype (1–3, 4–5, N/A, other), age at transplant, insurance status at transplant (private, Medicaid, other), lung allocation score at transplant, transplant length of stay ⩾ 28 d, any documented insulin use by 1 year after LTx, and maximum recorded forced expiratory value in 1 second percentage during first year after LTx. Models stratified by LTx center.
Results of a sensitivity analysis omitting centers with post-transplant encounter data for <70% of recipients within 1 year after LTx were comparable to the primary results, but none reached statistical significance (Table 4). For LTx recipients who did not have BMI data available by 1 year, the adjusted HR showed a lower risk of death than that for recipients with BMI ⩾ 18.5 kg/m2 (Table 4).
Discussion
In this large cohort of patients with CF undergoing LTx in the United States, we found that less than half of those who underwent LTx with a low preoperative BMI were able to achieve a normal BMI by 1 year after transplant. Furthermore, at 2 years after transplant, there remained 56% of this cohort who had not yet achieved a normal BMI (⩾18.5 kg/m2). Strikingly, after adjustment for markers of disease severity at transplant and of early postoperative recovery, failure to recover BMI by 1 year was associated with poorer long-term survival and worsened post-transplant lung function recovery. Causal inference from observational data is limited, and early postoperative events (e.g., primary graft dysfunction, acute cellular rejection requiring hospitalization, serious infections) could explain the lack of BMI recovery and the worsened long-term outcomes, but these findings identify a potentially modifiable post-transplant condition (persistent low BMI) and its association with poorer lung function and survival.
Low preoperative BMI has been associated with worse post-transplant survival among patients with a variety of underlying lung conditions (18). However, underweight patients with CF have been shown to have a higher risk of death without LTx, highlighting the importance of low preoperative BMI as a marker of “transplant need” (10, 27, 28). In addition, recent research has found that LTx recipients with CF who have low preoperative BMI have post-transplant survival that is comparable to that of other common transplant cohorts (25). For our cohort, low preoperative BMI was associated with worse 1-year survival, but this risk varied on the basis of post-transplant BMI recovery. Existing studies suggest that weight gain after transplant may be associated with post-transplant survival (29), but only a small, single-center study by Levine and colleagues specifically looked at BMI recovery after LTx for CF (30). Our results would support this assertion, placing emphasis on the importance of nutrition in the post-transplant period. In a recent consensus statement about the post-transplant care of LTx recipients with CF, the CF Foundation recommended “ongoing consultation with a dietitian with CF expertise, to receive individualized nutritional therapy to achieve an established BMI or weight-for-length goal” (31). However, there are no specific, standardized nutritional guidelines or recommendations to aid in recovering BMI after LTx.
In addition to a potential relationship between BMI recovery and survival in the post-transplant period, improvements in nutritional status are also likely linked to lung function recovery (22). Poor lung function recovery early post-transplant, recently coined as “baseline lung allograft dysfunction,” is associated with post-transplant survival (32). An association between BMI and post-transplant lung function in LTx recipients with CF was suggested in a single-center study of 50 LTx recipients with CF in Israel, where the trend in FEV1 mirrored the trend in BMI over the first 2 years after LTx, but no statistical testing was performed (30). Our results show that low-BMI LTx recipients with CF who recovered BMI had lung function recovery (FEV1 > 80% and forced vital capacity > 80% of predicted) similar to that in those who started with a BMI ⩾ 18.5 kg/m2. Although lung function recovery is significantly associated with BMI recovery in LTx recipients with CF in our data, causal inference is limited. These findings support the importance of monitoring post-transplant BMI and supporting nutritional recovery after LTx. Even though pretransplant nutritional outcomes are improving with CFTR modulators, and even though there will likely be fewer individuals with malnutrition at the time of transplant, we hypothesize that the link between malnutrition and post-transplant lung function is likely to hold true in the era of highly effective modulators.
The present study has an important strength: It used a merged dataset with records from the CFFPR and the UNOS/OPTN registry, which yielded novel data about longitudinal BMI and lung function recovery for LTx recipients with CF in the United States. The present study also has limitations. First, national registry data do not take into account transplant center–specific policies, varying BMI requirements for LTx candidacy, or post-transplant care. Transplant centers that accept candidates with very low BMI may be different in meaningful ways from ones that do not. Analyses were stratified by transplant center, but residual confounding may remain. Second, missing data are informative for this cohort because recipients who did not have BMI data in the CFFPR appeared to have the extremes of health—either death within 1 year of LTx or a much lower risk of death overall. We postulate that low-BMI LTx recipients without post-transplant CFFPR BMI data who survive beyond 1 year have lower medical complexity or CF complications, which may lead them to remain away from their CF center for post-transplant care. Because of the potential for bias in the cohort of LTx recipients who returned to a CF center and have data in the CFFPR, sensitivity analyses were limited to transplant centers with high rates of capture of post-transplant data (>70% of recipients with CFFPR data). These sensitivity analyses showed similar results, although they had less power to show statistical significance with smaller numbers of events in the low-BMI subgroups. Third, in this study, BMI was used as a surrogate for nutritional status because the UNOS and CFF registries do not collect other variables that could more accurately reflect body composition and overall nutritional health. Finally, postoperative complications such as primary graft dysfunction, acute cellular rejection (large percentage missing), and infection are not reliably captured in the available registries and thus could bias the results. Analyses were adjusted for immediate postoperative hospitalization length of stay and LAS at the time of transplant to account for early postoperative complications, but other events that were not included in the analyses may explain poor recovery of both BMI and lung function for some recipients.
In conclusion, we present the largest study to describe post-transplant BMI recovery for LTx recipients with CF and examine the association between BMI recovery and survival of LTx recipients with CF. Optimal post-transplant management is evolving, and this study highlights the important role of an interdisciplinary shared model of post-transplant CF care that includes ongoing CF-specific nutritional education and interventions, because less than half of underweight recipients were alive with a normal BMI by 1 year after transplant. Having low preoperative BMI is a risk factor for poor outcome after LTx, but this study underscores the importance of attention to BMI recovery post-transplant. Future research should investigate whether approaches to augment weight gain after transplant improve outcomes, particularly among recipients with very low BMI at the time of transplant.
Acknowledgments
Acknowledgment
The authors thank the Cystic Fibrosis (CF) Foundation for the use of CF Foundation Patient Registry data to conduct this study. In addition, the authors thank the patients, care providers, and clinic coordinators at CF centers throughout the United States for their contributions to the CF Foundation Patient Registry. The data reported here have been supplied by the United Network for Organ Sharing as the contractor for the Organ Procurement and Transplantation Network (OPTN). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the OPTN or the U.S. government.
Footnotes
Supported by National Insitutes of Health (NIH) Clinical Center grant K23HL138154, Cystic Fibrosis Foundation grant RAMOS17A0, and a CHEST Foundation Cystic Fibrosis grant (K.J.R.); the National Heart, Lung, and Blood Institute of the NIH under award K23HL128793 and a grant from the Cystic Fibrosis Foundation (LONG17A0) (A.L.J.); NIH grants (UM1 HL119073, P30 DK089507, U01 HL114589, and UL1 TR000423), the Cystic Fibrosis Foundation, and the U.S. Food and Drug Administration (grants R01 FD003704 and R01 FD006848) (C.H.G.); and the Cystic Fibrosis Foundation (J.M.P. and M.L.A.).
Author Contributions: J.B.P., M.C.B., A.L.J., T.Y.H.W., J.M.P., S.G.K., M.L.A., C.H.G., and K.J.R. contributed to the design and implementation of the research study, to the analysis of the results, and to the writing of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Stoltz DA, Meyerholz DK, Welsh MJ. Origins of cystic fibrosis lung disease. N Engl J Med . 2015;372:351–362. doi: 10.1056/NEJMra1300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cohen-Cymberknoh M, Shoseyov D, Kerem E. Managing cystic fibrosis: strategies that increase life expectancy and improve quality of life. Am J Respir Crit Care Med . 2011;183:1463–1471. doi: 10.1164/rccm.201009-1478CI. [DOI] [PubMed] [Google Scholar]
- 3. Middleton PG, Mall MA, Dřevínek, Lands LC, McKone EF, Polineni D, et al. VX17-445-102 Study Group Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med . 2019;381:1809–1819. doi: 10.1056/NEJMoa1908639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heijerman HGM, McKone EF, Downey DG, Van Braeckel E, Rowe SM, Tullis E, et al. VX17-445-103 Trial Group Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet . 2019;394:1940–1948. doi: 10.1016/S0140-6736(19)32597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thabut G, Christie JD, Mal H, Fournier M, Brugière O, Leseche G, et al. Survival benefit of lung transplant for cystic fibrosis since lung allocation score implementation. Am J Respir Crit Care Med . 2013;187:1335–1340. doi: 10.1164/rccm.201303-0429OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ramos KJ, Smith PJ, McKone EF, Pilewski JM, Lucy A, Hempstead SE, et al. CF Lung Transplant Referral Guidelines Committee Lung transplant referral for individuals with cystic fibrosis: Cystic Fibrosis Foundation consensus guidelines. J Cyst Fibros . 2019;18:321–333. doi: 10.1016/j.jcf.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weill D, Benden C, Corris PA, Dark JH, Davis RD, Keshavjee S, et al. A consensus document for the selection of lung transplant candidates: 2014—an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant . 2015;34:1–15. doi: 10.1016/j.healun.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 8. Aaron SD, Stephenson AL, Cameron DW, Whitmore GA. A statistical model to predict one-year risk of death in patients with cystic fibrosis. J Clin Epidemiol . 2015;68:1336–1345. doi: 10.1016/j.jclinepi.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 9. Ramos KJ, Quon BS, Heltshe SL, Mayer-Hamblett N, Lease ED, Aitken ML, et al. Heterogeneity in survival in adult patients with cystic fibrosis with FEV1 <30% of predicted in the United States. Chest . 2017;151:1320–1328. doi: 10.1016/j.chest.2017.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lederer DJ, Wilt JS, D’Ovidio F, Bacchetta MD, Shah L, Ravichandran S, et al. Obesity and underweight are associated with an increased risk of death after lung transplantation. Am J Respir Crit Care Med . 2009;180:887–895. doi: 10.1164/rccm.200903-0425OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hirche TO, Knoop C, Hebestreit H, Shimmin D, Solé A, Elborn JS, et al. ECORN-CF Study Group Practical guidelines: lung transplantation in patients with cystic fibrosis. Pulm Med . 2014;2014:621342. doi: 10.1155/2014/621342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O’Sullivan BP, Freedman SD. Cystic fibrosis. Lancet . 2009;373:1891–1904. doi: 10.1016/S0140-6736(09)60327-5. [DOI] [PubMed] [Google Scholar]
- 13. Yen EH, Quinton H, Borowitz D. Better nutritional status in early childhood is associated with improved clinical outcomes and survival in patients with cystic fibrosis. J Pediatr . 2013;162:530–535.e1. doi: 10.1016/j.jpeds.2012.08.040. [DOI] [PubMed] [Google Scholar]
- 14. Goss CH, Sykes J, Stanojevic S, Marshall B, Petren K, Ostrenga J, et al. Comparison of nutrition and lung function outcomes in patients with cystic fibrosis living in Canada and the United States. Am J Respir Crit Care Med . 2018;197:768–775. doi: 10.1164/rccm.201707-1541OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kraemer R, Rüdeberg A, Hadorn B, Rossi E. Relative underweight in cystic fibrosis and its prognostic value. Acta Paediatr Scand . 1978;67:33–37. doi: 10.1111/j.1651-2227.1978.tb16273.x. [DOI] [PubMed] [Google Scholar]
- 16. Corey M, McLaughlin FJ, Williams M, Levison H. A comparison of survival, growth, and pulmonary function in patients with cystic fibrosis in Boston and Toronto. J Clin Epidemiol . 1988;41:583–591. doi: 10.1016/0895-4356(88)90063-7. [DOI] [PubMed] [Google Scholar]
- 17. Hollander FM, van Pierre DD, de Roos NM, van de Graaf EA, Iestra JA. Effects of nutritional status and dietetic interventions on survival in cystic fibrosis patients before and after lung transplantation. J Cyst Fibros . 2014;13:212–218. doi: 10.1016/j.jcf.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 18. Allen JG, Arnaoutakis GJ, Weiss ES, Merlo CA, Conte JV, Shah AS. The impact of recipient body mass index on survival after lung transplantation. J Heart Lung Transplant . 2010;29:1026–1033. doi: 10.1016/j.healun.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 19. Pryor J, Bradford M, Jennerich A, Hee Wai T, Kapnadak S, Aitken ML, et al. Body mass index recovery after lung transplant for cystic fibrosis [abstract] Am J Respir Crit Care Med . 2021;203:A2030. [Google Scholar]
- 20. Knapp EA, Fink AK, Goss CH, Sewall A, Ostrenga J, Dowd C, et al. The Cystic Fibrosis Foundation Patient Registry: design and methods of a national observational disease registry. Ann Am Thorac Soc . 2016;13:1173–1179. doi: 10.1513/AnnalsATS.201511-781OC. [DOI] [PubMed] [Google Scholar]
- 21.WHO Consultation on Obesity. Obesity: preventing and managing the global epidemic: report of a WHO consultation. Geneva, Switzerland: World Health Organization; 1999. [accessed 2021 Nov 12]. Available from: https://apps.who.int/iris/handle/10665/42330 [Google Scholar]
- 22. Steinkamp G, Wiedemann B. Relationship between nutritional status and lung function in cystic fibrosis: cross sectional and longitudinal analyses from the German CF quality assurance (CFQA) project. Thorax . 2002;57:596–601. doi: 10.1136/thorax.57.7.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gottlieb J. Lung allocation. J Thorac Dis . 2017;9:2670–2674. doi: 10.21037/jtd.2017.07.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Russo MJ, Iribarne A, Hong KN, Davies RR, Xydas S, Takayama H, et al. High lung allocation score is associated with increased morbidity and mortality following transplantation. Chest . 2010;137:651–657. doi: 10.1378/chest.09-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ramos KJ, Kapnadak SG, Bradford MC, Somayaji R, Morrell ED, Pilewski JM, et al. Underweight patients with cystic fibrosis have acceptable survival following lung transplantation: a United Network for Organ Sharing registry study. Chest . 2020;157:898–906. doi: 10.1016/j.chest.2019.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morrell MR, Pilewski JM. Lung transplantation for cystic fibrosis. Clin Chest Med . 2016;37:127–138. doi: 10.1016/j.ccm.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 27. Madill J, Gutierrez C, Grossman J, Allard J, Chan C, Hutcheon M, et al. Toronto Lung Transplant Program Nutritional assessment of the lung transplant patient: body mass index as a predictor of 90-day mortality following transplantation. J Heart Lung Transplant . 2001;20:288–296. doi: 10.1016/s1053-2498(00)00315-6. [DOI] [PubMed] [Google Scholar]
- 28. Culver DA, Mazzone PJ, Khandwala F, Blazey HC, Decamp MM, Chapman JT, CCF Lung Transplant Group Discordant utility of ideal body weight and body mass index as predictors of mortality in lung transplant recipients. J Heart Lung Transplant . 2005;24:137–144. doi: 10.1016/j.healun.2003.09.040. [DOI] [PubMed] [Google Scholar]
- 29. Singer LG, Brazelton TR, Doyle RL, Morris RE, Theodore J, International Lung Transplant Database Study Group Weight gain after lung transplantation. J Heart Lung Transplant . 2003;22:894–902. doi: 10.1016/s1053-2498(02)00807-0. [DOI] [PubMed] [Google Scholar]
- 30. Levine H, Prais D, Raviv Y, Rusanov V, Rosengarten D, Saute M, et al. Lung transplantation in cystic fibrosis patients in Israel: the importance of ethnicity and nutritional status. Clin Transplant . 2017;31:e13111. doi: 10.1111/ctr.13111. [DOI] [PubMed] [Google Scholar]
- 31. Shah P, Lowery E, Chaparro C, Visner G, Hempstead SE, Abraham J, et al. Cystic Fibrosis Foundation consensus statements for the care of cystic fibrosis lung transplant recipients. J Heart Lung Transplant . 2021;40:539–556. doi: 10.1016/j.healun.2021.04.011. [DOI] [PubMed] [Google Scholar]
- 32. Liu J, Jackson K, Weinkauf J, Kapasi A, Hirji A, Meyer S, et al. Baseline lung allograft dysfunction is associated with impaired survival after double-lung transplantation. J Heart Lung Transplant . 2018;37:895–902. doi: 10.1016/j.healun.2018.02.014. [DOI] [PubMed] [Google Scholar]