Abstract
BACKGROUND:
Although satisfactory volume reduction in secondary unilateral lower limb lymphedema after lymphaticovenous anastomosis (LVA) in the affected limb has been well reported, alleviation of muscle edema and the impact of LVA on the contralateral limb have not been investigated.
STUDY DESIGN:
This retrospective cohort study enrolled patients who underwent supermicrosurgical LVA between November 2015 and January 2017. Pre- and post-LVA muscle edema were assessed using fractional anisotropy (FA) and apparent diffusion coefficient (ADC). The primary endpoint was changes in limb/subfascial volume assessed with magnetic resonance volumetry at least 6 months after LVA.
RESULTS:
Twenty-one patients were enrolled in this study. Significant percentage reductions in post-LVA muscle edema were found in the affected thigh (83.6% [interquartile range = range of Q1 to Q3; 29.8–137.1] [FA], 53.3% [27.0–78.4] [ADC]) as well as limb (21.7% [4.4–26.5]) and subfascial (18.7% [10.7–39.1]) volumes. Similar findings were noted in the affected lower leg: 71.8% [44.0–100.1] (FA), 59.1% [45.8–91.2] (ADC), 21.2% [6.8–38.2], and 28.2% [8.5–44.8], respectively (all p < 0.001). Significant alleviation of muscle edema was also evident in the contralateral limbs (thigh: 25.1% [20.4–57.5] [FA]; 10.7% [6.6–17.7] [ADC]; lower leg: 47.1% [35.0–62.8] [FA]; 14.6% [6.5–22.1] [ADC]; both p < 0.001), despite no statistically significant difference in limb and subfascial volumes.
CONCLUSIONS:
Our study found significant reductions in muscle edema and limb/subfascial volumes in the affected limb after LVA. Our findings regarding edema in the contralateral limb were consistent with possible lymphedema-associated systemic influence on the unaffected limb, which could be surgically relieved.
Significant reduction of muscle edema and subfascial volume in the affected limbs and edema reduction in the contralateral limbs after lymphaticovenous anastomosis have not been discussed previously. Our findings suggest possible interconnection between the superficial and deep lymphatic systems, as well as possible lymphedema-associated systemic influence on the contralateral limbs.
Lymphedema is a chronic debilitating disease that affects as many as 1 in 30 people worldwide.1 According to the World Health Organization database in 2020, more than 2.2 million patients with breast cancer and 600,000 patients with cervical cancer were newly registered worldwide. Among them, 20% to 40% will eventually develop lymphedema.2,3 Supermicrosurgical lymphaticovenous anastomosis (LVA) is a bypass procedure that not only improves lymphedema by channeling stagnant lymph from the lymphatic vessels (LVs) via the recipient veins in the lymphedematous limbs, but has also been shown to improve both mild and severe lymphedema.4-7
Because the tissue swelling in lymphedema is believed to be mainly epifascial, particularly in the highly compliant subcutis,8-11 muscle edema has rarely been discussed. Previous studies have suggested that muscle edema can also be caused by deep vein thrombosis and venous insufficiency.12-14 The issue of muscle edema may also be due to a lack of visual cues and measuring parameters, as well as the difficulty in quantifying muscle swelling because of the tight enveloping fascia.15 However, the importance of muscle edema is highlighted by the fact that interstitial fluid drainage into the microlymphatic system is 2 to 3 times faster in muscle than in subcutis,15-19 and the finding that a high intramuscular lymphatic flow with subsequent lymphatic failure could be an early sign of lymphedema.20-22
Diffusion tensor imaging (DTI) is a MRI technique used to evaluate well-organized microstructures, such as nerves and muscles, by assessing the 3-dimensional movements of water molecules within.23 Fractional anisotropy (FA) and apparent diffusion coefficient (ADC) are the 2 main parameters used to quantify architectural changes in muscle edema. Because a reduction in FA and an increase in ADC are associated with restricted water molecule diffusion in pathologic conditions such as muscle edema,24 DTI enables the early detection of injury-induced abnormalities.24-26 Previous studies have also reported its high sensitivity for discerning pathophysiologic muscle changes related to exercise, ischemia, dermatomyositis, trauma, denervation, and polymyositis,27,28 with a good reproducibility in skeletal muscles.28-33 However, DTI has not been used for the evaluation of lymphedema-induced muscle edema.
In addition, previous clinical34 and experimental studies35,36 have demonstrated lymphedema-related elevations in oxidative stress and inflammatory cytokines, highlighting its proinflammatory nature. Such an increase in oxidative stress has been found to be relieved after LVA.37 An association between a reduction in free radical generation and alleviation of lower limb swelling after femoropopliteal bypass surgery has been reported,38 suggesting a positive association between oxidative stress and limb edema. Therefore, the aim of this study was to investigate the changes in muscle edema and limb/subfascial volumes in both the affected and contralateral limbs after LVA is performed on the affected limb.
METHODS
Protocol and patient population
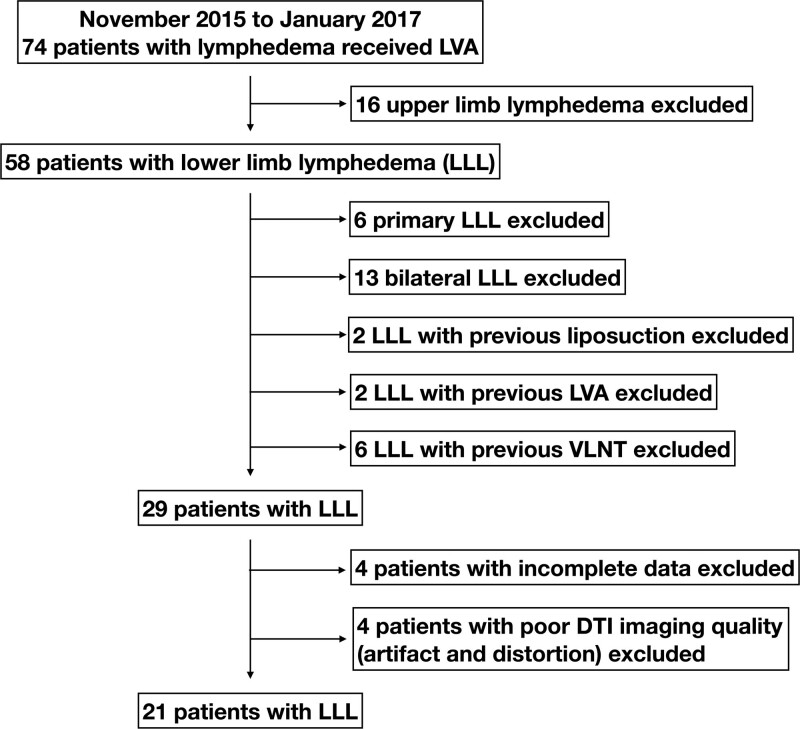
The current longitudinal cohort study was approved by the institutional review board of our institution (approval number 202001420B0). All adult patients (age >18 years) who received LVA for secondary unilateral lower limb lymphedema at a tertiary medical center from November 2015 to January 2017 were retrospectively reviewed. Patients with a diagnosis of primary lymphedema or bilateral lower limb lymphedema, deep vein thrombosis, and a history of previous LVA, lymph node transfer, liposuction, or excisional therapy such as the Charles procedure, and those lost to follow-up or with incomplete data were excluded (Fig. 1). Lymphedema was confirmed with lymphoscintigraphy and indocyanine green (ICG) lymphangiography before surgery in all patients (Fig. 2A).
Figure 1.
Inclusion and exclusion criteria for patient selection. DTI, diffusion tensor imaging; LLL, lower limb lymphedema; LVA, lymphaticovenous anastomosis; VLNT, vascularized lymph node transfer.
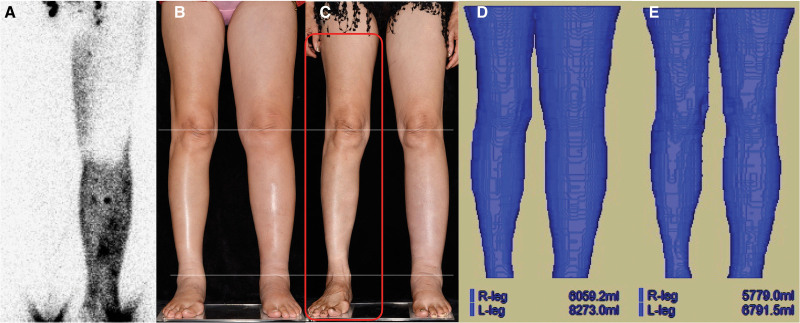
Figure 2.
A 65-year-old woman with BMI 22.8 kg/m2, who had received endometrial cancer ablation and lymph node dissection 5 years ago with postoperative radiotherapy, suffered from stage II to III left lower limb lymphedema for 4 years with 2 cellulitis episodes. A total of 8 lymphaticovenous anastomoses (LVA) were performed. (A) Lymphoscintigraphy showing marked radiocolloid stasis in her left lower (affected) limb without evidence of lymphedema in her right lower (contralateral) limb. (B) Pre-LVA appearance of lower limbs demonstrating prominent swelling in the affected limb. (C) Appearance of lower limbs at 2 years and 6 months post-LVA follow-up with notable improvements in both legs and contralateral ankle. (D) Pre-LVA magnetic resonance (MR) volumetry showing notably increased volume of the affected limb (affected limb 8,273.0 mL; contralateral limb 6,059.2 mL). (E) Post-LVA percentage volume reductions assessed with MR volumetry at 2 years and 6 months follow-up of her affected and contralateral lower limbs were 63.4% [(8,273.0 mL – 6,691.5 mL)/(8,273.0 mL – 5,779.0 mL) × 100%] and 4.6% [(5,779.0 mL – 6,059.2 mL)/6,059.2 mL × 100%], respectively.
Definitions
The severity of lymphedema can be classified based on the International Society of Lymphology staging system (mild: stages 0 to I; moderate-to-severe: stages II to III) and leg dermal backflow stage39,40 (stage 0: no dermal backflow pattern; stage I: splash pattern around the groin region; stage II: stardust pattern extended proximal to the superior border of the patella; stage III: stardust pattern extended distal to the superior border of patella; stage IV: stardust pattern extended to the whole limb; and stage V: existence of diffuse pattern with stardust pattern in the background). All patients underwent supermicrosurgical LVA performed by a single senior surgeon with 11-0 nylon sutures (Ethilon, Ethicon, NJ) using a high-power surgical microscope (Pentero 900, Carl Zeiss AG, Oberkochen, Germany). The positivity of ICG and flow of an LV were defined based on microscopic observations. ICG-positive LVs were defined as those that were positive for fluorescence observed at an excitation peak of 789 nm and an emission peak of 814 nm with a microscope-integrated near-infrared imaging system. Flow(+) LVs referred to those with microscopically discernible lymphatic flow from the distal opening of a transected LV. The diameters of the LVs were determined with a precision of 0.01 mm. The classification of lymphosclerosis was based on intraoperative findings of 4 criteria: wall thickness, appearance, wall expandability, and lumen. This evaluation was based on a previous study41 in which all LVs were classified into 4 categories: s0 (very thin, translucent, expandable with identifiable lumen), s1 (thin, white, expandable with identifiable lumen), s2 (thick, white, not expandable with identifiable lumen), and s3 (very thick, white, not expandable with unidentifiable lumen), in which s0 and s1 were considered ideal, s2 suboptimal, and s3 not suitable for LVA.
Parameters and procedures
Demographic data including sex, age, cause of lymphedema, International Society of Lymphology staging, BMI, presence of diabetes mellitus and hypertension, the side of the affected lower limb, adjuvant chemotherapy and radiotherapy, duration of lymphedema, and the incidence of cellulitis episode were also recorded via chart review.
Intraoperative findings under the surgical microscope were recorded, including the total number of LVs, incisions per patient, and LVs per patient, as well as the diameter of LVs, LVA performed per patient (either end-to-end or end-to-side orientation), number and diameter of ICG-positive LVs, number and diameter of ICG-negative/flow-positive LVs, lymphosclerosis classification, total number and median diameter of the recipient veins, and number of recipient veins per patient.
For those who did not routinely wear compression stockings before LVA, they were asked to wear them for at least 1 month before LVA and resume compression 1 week after LVA. Postoperatively, the compression garment was recommended to be worn at least during the daytime with periodic revisions when necessary. No compression was used on the contralateral limb. Written consent was obtained from all patients for the use of preoperative and postoperative photographs.
Operative technique
ICG (0.1 mL) was injected intradermally into each of the first and third toe web spaces in addition to the medial and lateral malleoli. The normal linear pattern of the LVs was detected with a handheld near-infrared imaging device (Fluobeam, FluoOptic, Grenoble, France) and marked as the basis for incision placement. For patients with a diffuse dermal backflow pattern, incisions were placed along the anatomical location of the great saphenous vein. The incision was usually 3 cm in length and extended when necessary. The operative techniques have been described previously.5
Magnetic resonance imaging protocol
All patients underwent MRI studies (3.0 T Siemens MAGNETOM Skyra scanner) before and 6 months after LVA with a combination of 2 anterior 18-channel body matrix coils and one 32-channel spine coil (Siemens Healthcare, Erlangen, Germany) to cover the bilateral lower limbs. The patients were placed feet-first in a supine position. DTI and T1-weighted images for anatomic reference were acquired for water diffusion (edema) and volumetric evaluation, respectively.
The DTI acquisition consisted of one b value (Supplemental Digital Content 1, http://links.lww.com/JACS/A73) = 0 sec/mm2 image and 6 gradient directions with b value = 500 sec/mm2. To avoid chemical shift artifacts, frequency-selective suppression and gradient reversal were used to suppress the fat signal. The image parameters were as follows: repetition time/echo time = 4500/62 ms; field of view = 420 × 300 mm2; generalized auto-calibrating partially parallel acquisitions (GRAPPA) = 2; 50 adjacent axial slices of 5-mm thickness; voxel size = 5.5 × 5.5 × 5 mm3; time of acquisition = 3 minutes 34 seconds. The scan range was divided into 2 sections: thigh and lower leg. Anatomical T1-weighted images were acquired using a coronal 3-dimensional sampling perfection with the application of optimized contrasts using different values of flip angle evolution (SPACE; repetition time/echo time = 500/11 ms; field of view = 420 × 420 mm2; size of matrix = 320 × 320; voxel size = 1.3 × 1.3 × 3.0 mm3; 65 contiguous slices without interslice gap; time of acquisition = 2 minutes 52 seconds). The scan range was from the pelvis to the feet and divided into 3 sections: thigh, knee, and leg. Finally, the 3 individual section stacks were merged to create 1 set for volume measurement. A senior radiologist reviewed all magnetic resonance scans to exclude any organic disorders other than lymphedema. Magnetic resonance volumetry has been used to measure muscle volume.42-45
Quantitative evaluation of muscle edema by diffusion tensor imaging
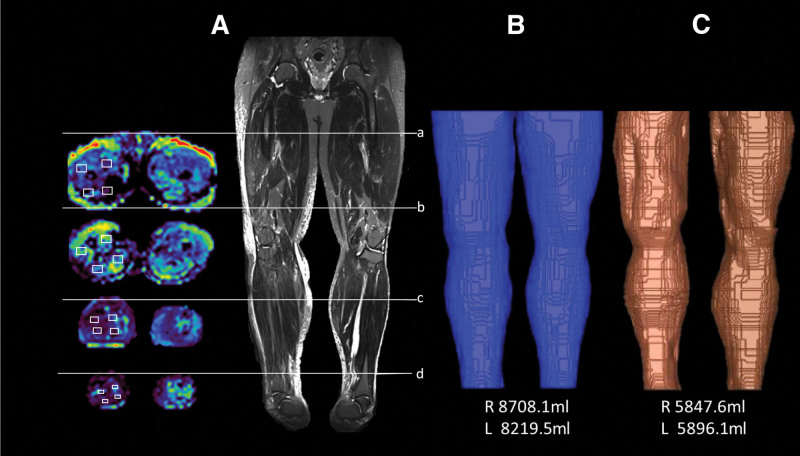
After in-plane correction of deformations of the acquired DTI images induced by field inhomogeneities, the FA and ADC values were automatically calculated and measured offline using a region-of-interest technique by a senior radiologist. Regions of interest were drawn at the proximal/distal thigh and proximal/distal lower leg of the affected (lymphedematous) limb and contralateral limb. An illustration of this approach in a male patient with lymphedema is shown in Figure 3A. Four regions of interest within the muscular structure were drawn at each level from the FA and ADC maps and expressed as the average value of the 4 measurements. To investigate potential impacts of irradiation on the degree of muscle edema of the affected limb, the participants were divided into irradiated and nonirradiated groups.
Figure 3.
Preoperative magnetic resonance diffusion tensor imaging (DTI) and volumetry acquisition for a 52-year-old man with a BMI of 34.0 kg/m2 suffering from stage III right lower limb lymphedema. (A) Computation of fractional anisotropy (FA) and apparent diffusion coefficient (ADC) through selecting 4 sets of regions of interest (ROI; small white rectangles) in the muscle compartment by a senior radiologist at 4 different levels (a: proximal thigh; b: distal thigh; c: proximal lower leg; d: distal lower leg) of the affected (lymphedematous) and contralateral limbs. Preoperative volumetric calculations of limb volume (B) and subfascial volume (C).
Limb/subfascial volume measurement by magnetic resonance volumetry
The volume of the lower limbs was calculated using commercialized AZE VirtualPlace software (AZE Ltd., Tokyo, Japan). The area on each layer image was measured using the free-HAD mode and autothreshold function, and the total volume of the extremity was automatically calculated. For the lower limbs, to avoid miscalculation by including pelvic soft tissue in the volumetric analysis, the upper margin was set at 20 cm above the knee joint surface of the distal femoral condyle, and the lower margin was set at the ankle articular surface of the inferior tibia (Figs. 2D, 2E, 3B). In addition to lower limb volume, the subfascial volume, which is mainly composed of muscle and bone, was also measured (Fig. 3C).
Calculations for pre– and post–lymphaticovenous anastomosis percentage reduction in muscle edema, limb/subfascial volume
To accurately evaluate the improvements in muscle edema and limb/subfascial volume after LVA, percentage reduction was used instead of the differences before and after LVA. The calculations for the contralateral limbs (ContraLimb) and affected limbs (AffectLimb) were different. The post-LVAContraLimb improvement, expressed as a reduction in DTI or a decrease in limb or subfascial volume, was calculated using the following equation:
Absolute values were used for subsequent analyses. Because the post-LVAContraLimb values theoretically represent the healthy condition in which the influence of muscle edema and pathologic increases in limb/subfascial volume are minimal, they were used as references instead of the pre-LVAContraLimb for measuring the post-LVAAffectLimb improvements. Therefore, the percentage reduction in
Absolute values were used for subsequent comparisons.
Statistical analysis
The minimal number of patients to be included to achieve statistical significance assessed with the G*Power software by setting parameters including alpha error probability = 0.05, effect size dz = 0.7, and a power (1 – beta error probability) of 0.8 was 19. All values are summarized as mean ± standard deviation or median (interquartile range 25% to 75%). Chi-square tests were used for comparisons of categorical variables. For continuous variables, 2 sample t-tests were used to compare the differences between the means for the normal distribution groups. The Kruskal–Wallis rank sum test was used to statistically evaluate the differences between 2 or more groups for a variable with continuous or ordinal values. Mann–Whitney Wilcoxon tests were used for nonnormally distributed continuous variables or for small sample sizes. Paired comparison analysis was used for pre- vs postprocedure comparisons. Statistical analysis was performed using the SPSS Version 22.0 software package. A p < 0.05 was considered statistically significant.
RESULTS
Demographic data
A total of 21 (20 females/1 male) patients were recruited for the current study (more than the minimal number of 19 assessed with the G*Power software) with unilateral lower limb lymphedema were enrolled with a mean age of 63.9 ± 10.8 years (range 35 to 80). The majority of patients had gynecologic cancer (95.2%) with International Society of Lymphology stage II to III (90.5%) and leg dermal backflow stage IV (47.6%) and V (23.8%). The median BMIs before and after LVA were 25.8 [24.3 to 28.8] kg/m2 vs 25.8 [23.9 to 28.0] kg/m2, respectively (p < 0.001) (Table 1). Nineteen percent of the patients were diabetic and 42.9% were hypertensive. More than half of the patients experienced lower limb lymphedema on the left side (66.7%). Less than half of the patients had received adjuvant chemotherapy (38.1%) or radiotherapy (38.1%). The median duration of lymphedema was 7.3 [5.9 to 10.4] years. The median time from the previous operation to the onset of lymphedema was 5.0 [1.0 to 11.0] years. The median number of episodes of cellulitis was 1 [0 to 2] per year (Table 1). Consistent with the findings of a previous study of LVA,46 no LVA-related morbidity was observed in the current investigation.
Table 1.
Patient Demographics of 21 Patients with Unilateral Lower Limb Lymphedema
| Demographic | Total (n = 21) | p Value |
|---|---|---|
| Sex, n (%) | — | |
| Male | 1 (4.8) | |
| Female | 20 (95.2) | |
| Age, y, mean ± SD | 63.9 ± 10.8 | — |
| Etiology, n (%) | ||
| Gynecologic cancer* | 20 (95.2) | — |
| Nongynecologic cancer† | 1 (4.8) | — |
| ISL stage (0–I), n (%) | 2 (9.5) | — |
| Stage (II–III), n (%) | 19 (90.5) | — |
| Leg dermal backflow stage, n (%) | ||
| Contralateral limbs | ||
| 0 | 21 (100) | |
| I | 0 (0) | |
| II | 0 (0) | |
| III | 0 (0) | |
| IV | 0 (0) | |
| V | 0 (0) | |
| Affected limbs | ||
| 0 | ||
| I | 0 (0) | |
| II | 2 (9.5) | |
| III | 4 (19.0) | |
| IV | 10 (47.6) | |
| V | 5 (23.8) | |
| BMI, median [IQR] | <0.001‡ | |
| Before LVA, kg/m2 | 25.8 [24.3–28.8] | |
| After LVA, kg/m2 | 25.8 [23.9–28.0] | |
| DM, yes, n (%) | 4 (19.0) | — |
| HTN, yes, n (%) | 9 (42.9) | — |
| Affected limb, n (%) | — | |
| Left | 14 (66.7) | |
| Right | 7 (33.3) | |
| Chemotherapy, yes, n (%) | 8 (38.1) | — |
| Radiotherapy, yes, n (%) | 8 (38.1) | — |
| Duration of LE, y, median [IQR] | 7.3 [5.9–10.4] | — |
| Duration between previous operation to LE onset, y, median [IQR] | 5.0 [1.0–11.0] | — |
| Cellulitis episodes/y, median [IQR] | 1 [0–2] | — |
p Value was obtained with Mann-Whitney Wilcoxon test.
Gynecologic cancer cases included: cervical cancer, endometrial cancer, and ovarian cancer
Nongynecologic cancer included one patient with cellulitis-induced lymphedema.
Significant reduction in BMI before and after LVA.
DM, diabetes mellitus; HTN, hypertension; ISL, International Lymphology Society; IQR, interquartile range; LE, lymphedema; LVA, lymphaticovenous anastomosis.
Intraoperative findings
A total of 154 LVs were identified, with a median of 5 incisions [5 to 5]. The median number of LVs was 6 [6 to 8] per patient, with a median LV diameter of 0.5 [0.4 to 0.6] mm. The median number of LVAs performed per patient was 8 [6 to 10]. The total number of ICG(+) LVs was 121 (78.6%) with a median diameter of 0.5 [0.4 to 0.6] mm. The total number of ICG(–) but flow(+) LVs was 29 (18.8%) with a median diameter of 0.5 [0.4 to 0.6] mm. LVs that were ICG(+) and ICG(–)/flow(+) were considered functional. Based on the lymphosclerosis classification, 14 s0 LVs (9.1%), 91 s1 LVs (59.1%), 48 s2 LVs (31.2%), and 1 s3 LV (0.6%) were identified. The total number of recipient veins was 120, with a median of 5 [4 to 6] recipient veins per patient and a median diameter of 0.8 [0.5 to 0.9] mm. The median post-LVA follow-up was 6.0 [5.0 to 6.0] months (Table 2).
Table 2.
Intraoperative Findings and Post-LVA Outcomes
| Intraoperative finding | Total (n = 21) |
|---|---|
| Total LV found, n | 154 |
| Incisions per patient, median [IQR] | 5 [5–5] |
| LV found per patient, median [IQR] | 6 [6–8] |
| Diameter of LV, mm, median [IQR] | 0.5 [0.4–0.6] |
| LVA performed per patient, median [IQR] | 8 [6–10] |
| Total no. of ICG(+)/LV, n/n (%) | 121/154 (78.6) |
| Diameter, mm, median [IQR] | 0.5 [0.4–0.6] |
| Total number of ICG(–)/flow(+) LV, n/n (%) | 29/154 (18.8) |
| Diameter, mm, median [IQR] | 0.5 [0.4–0.6] |
| Lymphosclerosis classification, n, (%) | |
| s0 (ideal for LVA) | 14 (9.1) |
| s1 (ideal for LVA) | 91 (59.1) |
| s2 (suboptimal for LVA) | 48 (31.2) |
| s3 (not suitable for LVA) | 1 (0.6) |
| Total no. of recipient veins, n | 120 |
| Recipient veins per patient, median [IQR] | 5 [4–6] |
| Diameter, mm, median [IQR] | 0.8 [0.5–0.9] |
| Length of follow-up, post-LVA, mo, median [IQR] | 6.0 [5.0–6.0] |
ICG(+), indocyanine green-positive; IQR, interquartile range; LV, lymphatic vessels; LVA, lymphaticovenous anastomosis.
Changes in muscle edema, post–lymphaticovenous anastomosis
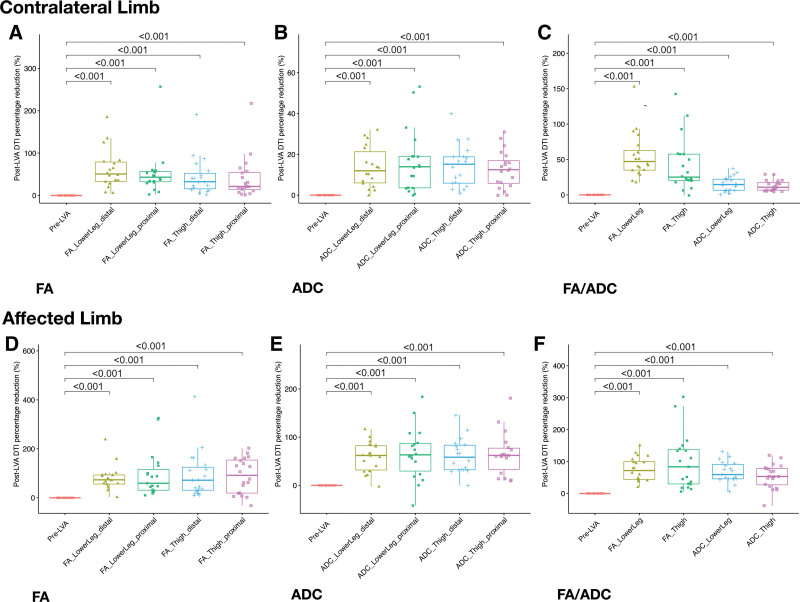
For the contralateral limbs, based on FA measurement, significant median percentage muscle edema reduction was noted in all 4 regions: proximal thigh (21.3% [13.7 to 54.0], p < 0.001); distal thigh (32.5% [15.7 to 52.3], p < 0.001); proximal lower leg (43.1% [32.4 to 56.7], p < 0.001); and distal lower leg (50.7% [33.3 to 78.9], p < 0.001; Fig. 4A). The ADC measurement also revealed similar findings: proximal thigh (12.5% [5.8 to 16.9], p < 0.001); distal thigh (15.2% [5.9 to 18.8], p < 0.001); proximal lower leg (13.9% [3.6 to 19.0], p < 0.001); and distal lower leg (12.0% [6.0 to 21.3], p < 0.001; Fig. 4B). Although both FA and ADC results indicated a higher percentage of edema reduction in the lower leg than in the thigh, ADC appeared to exhibit more consistent results than those of FA. Overall, in the contralateral limb after LVA, greater muscle edema percentage reduction was evident in the lower leg region than in the thigh region, as proved by both FA (47.1% vs 25.1%, respectively) and ADC (14.6% vs 10.7%, respectively; Fig. 4C; Supplemental Digital Content 2 and 3, http://links.lww.com/JACS/A73).
Figure 4.
Percentage reduction in muscle edema after lymphaticovenous anastomosis (LVA) assessed with fractional anisotropy (FA) and apparent diffusion coefficient (ADC). In the contralateral limbs, (A) and (B) both demonstrated significant post-LVA reductions at all 4 different levels (distal/proximal lower leg and distal/proximal thigh; both †‡p < 0.001). In the affected limbs, (D) and (E) followed the same trend (both †‡p < 0.001). (C) The average values of FA and ADC both demonstrated significant post-LVA reductions in the regions of lower leg and thigh of the contralateral, and (F) the affected limbs (both †‡p < 0.001). Statistical analyses were performed with Kruskal−Wallis rank sum test and Mann–Whitney Wilcoxon test which were represented by † and ‡, respectively.
For the affected limbs, with reference to the post-LVAContraLimb FA value, significant muscle edema reduction was found in all 4 regions: proximal thigh (91.6% [19.2 to 154.1], p < 0.001); distal thigh (71.7% [30.9 to 124.6], p < 0.001); proximal lower leg (59.3% [31.0 to 115.7], p < 0.001); and distal lower leg (73.4% [56.1 to 93.3], p < 0.001; Fig. 4D). With the use of the post-LVAContraLimb ADC value as a reference, significant muscle edema reduction was also noted in the 4 regions: proximal thigh (62.2% [33.1 to 77.2], p < 0.001); distal thigh (58.4% [32.5 to 83.3], p < 0.001); proximal lower leg (63.4% [29.7 to 87.2], p < 0.001); and distal lower leg (62.1% [31.9 to 82.2], p < 0.001; Fig. 4E). ADC appeared to yield results with fewer fluctuations in all 4 regions than those of FA. Overall, in the affected limbs, analysis of the degree of post-LVA muscle edema reduction showed mixed results, but FA indicated a greater edema reduction in the thigh region than that in the lower leg (83.6% vs 71.8%, respectively). ADC demonstrated less edema reduction in the thigh than in the lower leg (53.3% vs 59.1%, respectively; Fig. 4F; Supplemental Digital Content 2 and 3, http://links.lww.com/JACS/A73). Regarding the irradiated and nonirradiated groups, no intergroup difference was found in muscle edema in the affected limbs before LVA as well as post-LVA percentage reduction in muscle edema (Supplemental Digital Content 4, http://links.lww.com/JACS/A73).
Post–lymphaticovenous anastomosis changes in limb and subfascial volumes
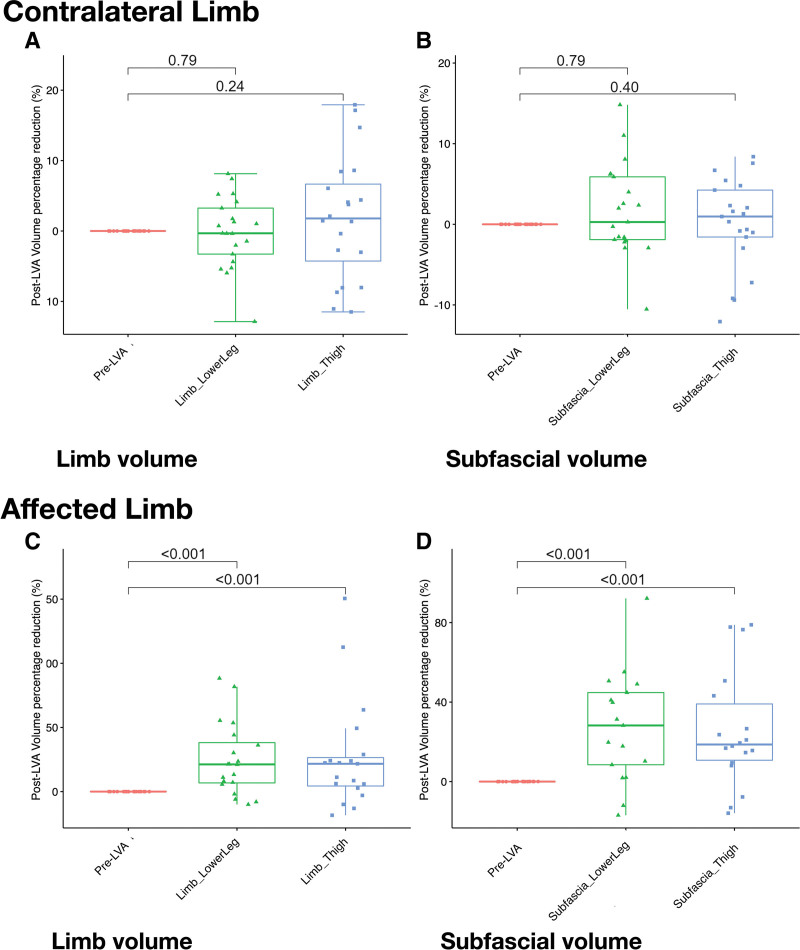
For the contralateral limbs, analysis of limb volume revealed nonsignificant volume reduction in the regions of the lower leg and thigh (p = 0.24 and p = 0.79, respectively; Fig. 5A). Nonsignificant decreases in the subfascial volume were also found in the regions of the lower leg and thigh (p = 0.40 and p = 0.79, respectively; Fig. 5B; Supplemental Digital Content 2 and 3, http://links.lww.com/JACS/A73). For the affected limbs, significant reductions were evident in both regions of the lower leg and thigh (p < 0.001 and p < 0.001, respectively; Fig. 5C). Changes in the subfascial volume of the thigh and lower leg also followed the same trend (p < 0.001 and p < 0.001, respectively; Fig. 5D; Supplemental Digital Content 2 and 3, http://links.lww.com/JACS/A73). The findings are summarized in Table 3.
Figure 5.
Percentage reductions in limb and subfascial volumes after lymphaticovenous anastomosis (LVA) quantified by magnetic resonance (MR) volumetry. Nonsignificant post-LVA reductions in (A) limb volume [lower leg and thigh (†p = 0.79 and 0.24, respectively] (‡p = 0.53), and (B) subfascial volume (†p = 0.79 and 0.40, respectively; ‡p = 0.82) of the contralateral limbs. Significant reductions in post-LVA (C) limb, and (D) subfascial volumes in lower leg and thigh of the affected limb (all †‡p < 0.001). Statistical analyses were performed with Kruskal−Wallis rank sum test and Mann–Whitney Wilcoxon test that were represented by † and ‡, respectively.
Table 3.
Summary of Pre– and Post–Lymphaticovenous Anastomosis Reduction in Muscle Edema, Limb, and Subfascial Volumes of Contralateral and Affected Limbs
| Variable | Post-LVA | |||
|---|---|---|---|---|
| Contralateral limb | Affected limb | |||
| % | p Value | % | p Value | |
| Reduction in muscle edema | ||||
| FA | ||||
| Thigh | 25.1 | <0.001 | 83.6 | <0.001 |
| Lower leg | 47.1 | <0.001 | 71.8 | <0.001 |
| ADC | ||||
| Thigh | 10.7 | <0.001 | 53.3 | <0.001 |
| Lower leg | 14.6 | <0.001 | 59.1 | <0.001 |
| MR volumetry volume reduction | ||||
| Limb volume | ||||
| Thigh | 1.8 | 0.24 | 21.7 | <0.001 |
| Lower leg | 0.3 | 0.79 | 21.2 | <0.001 |
| Subfascial volume | ||||
| Thigh | 1 | 0.40 | 21.7 | <0.001 |
| Lower leg | 0.3 | 0.79 | 21.2 | <0.001 |
ADC, apparent diffusion coefficient; FA, fractional anisotropy; LVA, lymphaticovenous anastomosis.
DISCUSSION
Although previous studies have investigated the improvements in lymphedema of the lower limb by assessing the changes in limb volume after supermicrosurgical LVA, no study has focused on the alleviation of muscle edema. In addition, no previous report has evaluated the impact of LVA on the contralateral unaffected limb. Using imaging analyses, the present study is the first to address these issues and shed light on some pathophysiologic aspects of the disease. Our results validated the alleviation of muscle edema of the affected limb, indicating communication between superficial lymphatics and those in the muscle compartment. The findings of the current study further revealed significant reductions in both limb and subfascial volumes of the affected limb after surgery. DTI of the contralateral limb showed a significant postoperative decrease in muscle edema compatible with a reduction in water content if lymphedema was absent on preoperative lymphoscintigraphy.
A previous study reported an association between a reduction in free radical generation (ie oxidative stress) after femoropopliteal bypass and an improvement in lower limb swelling.38 In concert with this finding, previous investigations have demonstrated that chronic lymphedema is associated with a systemic increase in free radicals and oxidative stress.34,37,47 The decrease in oxidative stress has consistently been shown to be related to a reduction in lymphedematous limb volume after LVA.37 The current study revealed a postoperative reduction in the volume of the contralateral limb, thereby providing a healthy reference for the assessment of improvements in both the affected and contralateral limbs. Our findings may support the concept that localized lymphedema in the lower limbs may have systemic deleterious effects, such as edematous changes in the contralateral leg. Whether this systemic effect can lead to unnoticed and undetected generalized edema warrants further investigation. On the other hand, there may be other mechanisms by which lymphedema is alleviated after LVA. For instance, previous studies have demonstrated that a more active lifestyle and positive mood swing after improvement in lymphedema may help reduce edema.48,49
Previous studies on lymphedema focused mainly on the changes in limb volume and epifascial tissue, including fibrotic changes and adipose tissue deposition,10,50-53 but the subfascial compartment in lower limb lymphedema has rarely been discussed.51,52,54Changes in muscle edema and subfascial volume after LVA have not been investigated. Muscle edema, which is often overlooked because of the lack of quantitative measurement, might be the cause of pain and limited range of motion among patients with lymphedema.55,56 After LVA, our results revealed that both affected and contralateral limbs showed significant reductions in muscle edema, despite the fact that the contralateral limbs were free of preoperative lymphedema. Therefore, the findings suggest that the preoperative edematous subfascial compartment in the contralateral limb may be attributable to water retention. Overall, our results implied that ADC might be a more suitable parameter for assessing muscle edema than FA because of the higher data consistency of the former. The reduction in subfascial volume in the affected limb, together with the finding of a nonsignificant decrease in the subfascial volume of the contralateral limb despite a significantly reduced muscle edema in the latter after LVA, suggest an increased stretchability of muscle fascia due to prolonged pressure in the limb affected with chronic lymphedema compared to that in the contralateral limb. Previous studies have suggested that muscle edema can also be caused by venous diseases.12-14 However, in this study, preoperative Doppler ultrasound confirmed negative deep vein thrombosis in all patients; therefore, our results suggest that lymphedema can also lead to muscle edema.
Regarding the mechanism underlying the reduction of muscle edema in the affected limb after LVA, previous anatomical studies have demonstrated no communication between the superficial and deep lymphatic systems under normal circumstances.57,58 However, the deep lymphatic system serves as a reservoir for the superficial lymphatics to arrest the progression of lymphedema in patients experiencing this condition.58 Despite the identification of interconnections between the superficial and deep lymphatic systems in patients with upper limb lymphedema,59 such communication in lower limb lymphedema has not been reported. In the present study, because all LVAs were performed on the superficial lymphatics of the affected limb, the marked postoperative reduction in muscle edema may be explained by interconnections between the 2 lymphatic systems and a possible flow reversal. This finding contradicts the concept that LVA can only improve lymphedema in the epifascial compartment.54 The current study may offer the first indirect evidence supporting the existence of interconnections between the superficial and deep lymphatic systems in lower limb lymphedema. Identification of such communication routes, as well as the absence or presence of dysfunctional valves in these interconnections that led to this flow reversal, warrant further investigation. In addition, whether these findings can be applied to upper limb lymphedema remains to be elucidated.,59
Consistent with the findings of a previous study of LVA, no LVA-related morbidity was observed in the current investigation.
There are several limitations to this study. First, the number of patients is relatively small. However, by setting parameters including alpha error probability = 0.05, effect size dz = 0.7, and total sample size = 21, the power (1 – beta error probability) obtained was 0.845 (G*Power software), which was higher than the required value of 0.8. Following the same rationale, previous studies have achieved the statistical significance of their outcomes based on a small number of patients.60-62 Second, because FA and ADC are directly dependent on the setup and acquisition parameters, which are sensitive to both demographics and transient conditions, no normal reference values for FA and ADC are available. Nevertheless, DTI is still a suitable research tool for group comparisons in longitudinal and prospective studies.28 Third, epifascial DTI was not measured due to artifacts that caused a large variation. Finally, the degree of oxidative stress or presence of inflammatory markers was not quantified in the present study for comparison.
CONCLUSION
This is the first report to demonstrate lymphedema-associated muscle edema and its reduction in the affected and contralateral limbs after LVA. The postoperative alleviation of muscle edema in the affected limb suggests the presence of interconnections between superficial and deep lymphatic systems. The postoperative improvement in muscle edema in the contralateral limb suggest a systemic impact of localized lymphedema. ADC was a more suitable parameter for assessing the degree of muscle edema compared to FA.
Author Contributions
Study conception and design: Yang, Wu, Lin
Acquisition of data: Wang, Luo, Kuo, Chien, Tsai
Analysis and interpretation of data: Wang, Luo Chien, Hsieh
Drafting of manuscript: Yang, Wu, Lin
Critical revision: Yang, Wu, Lin
Acknowledgment:
Special thanks to Sherry Hsin-Miao Shih, Hsiu-Ling Wu, RN, Lili Su, RN, Shu-Hsia Chang, RN, Yi-Chun Lin, RN, Shu-Hui Peng, RN, and all our colleagues for their help.
Supplementary Material
Abbreviations and Acronyms
- ADC=
- apparent diffusion coefficient
- DTI=
- diffusion tensor imaging
- FA=
- fractional anisotropy
- ICG=
- indocyanine green
- LV=
- lymphatic vessel
- LVA=
- lymphaticovenous anastomosis
Disclosure Information: Nothing to disclose.
Supplemental digital content is available for this article.
REFERENCES
- 1.Rockson SG, Rivera KK. Estimating the population burden of lymphedema. Ann N Y Acad Sci. 2008;1131:147–154. [DOI] [PubMed] [Google Scholar]
- 2.DiSipio T, Rye S, Newman B, et al. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14:500–515. [DOI] [PubMed] [Google Scholar]
- 3.Kuroda K, Yamamoto Y, Yanagisawa M, et al. Risk factors and a prediction model for lower limb lymphedema following lymphadenectomy in gynecologic cancer: a hospital-based retrospective cohort study. BMC Womens Health. 2017;17:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang JC, Yen YH, Wu SC, et al. Supermicrosurgical lymphaticovenous anastomosis as an alternative treatment option for patients with lymphorrhea. Plast Reconstr Surg. 2019;144:1214–1224. [DOI] [PubMed] [Google Scholar]
- 5.Yang JC, Wu SC, Lin WC, et al. Supermicrosurgical lymphaticovenous anastomosis as alternative treatment option for moderate-to-severe lower limb lymphedema. J Am Coll Surg. 2020;230:216–227. [DOI] [PubMed] [Google Scholar]
- 6.Cha HG, Oh TM, Cho MJ, et al. Changing the paradigm: lymphovenous anastomosis in advanced stage lower extremity lymphedema. Plast Reconstr Surg. 2021;147:199–207. [DOI] [PubMed] [Google Scholar]
- 7.Hara H, Mihara M. Lymphaticovenous anastomosis for advanced-stage lower limb lymphedema. Microsurgery. 2021;41:140–145. [DOI] [PubMed] [Google Scholar]
- 8.Collins CD, Mortimer PS, D’Ettorre H, et al. Computed tomography in the assessment of response to limb compression in unilateral lymphoedema. Clin Radiol. 1995;50:541–544. [DOI] [PubMed] [Google Scholar]
- 9.Mellor RH, Bush NL, Stanton AW, et al. Dual-frequency ultrasound examination of skin and subcutis thickness in breast cancer-related lymphedema. Breast J. 2004;10:496–503. [DOI] [PubMed] [Google Scholar]
- 10.Brorson H, Ohlin K, Olsson G, et al. Adipose tissue dominates chronic arm lymphedema following breast cancer: an analysis using volume rendered CT images. Lymphat Res Biol. 2006;4:199–210. [DOI] [PubMed] [Google Scholar]
- 11.Brorson H, Ohlin K, Olsson G, et al. Breast cancer-related chronic arm lymphedema is associated with excess adipose and muscle tissue. Lymphat Res Biol. 2009;7:3–10. [DOI] [PubMed] [Google Scholar]
- 12.Liu PT, Ilaslan H. Unicompartmental muscle edema: an early sign of deep venous thrombosis. Skeletal Radiol. 2003;32:41–45. [DOI] [PubMed] [Google Scholar]
- 13.Useche JN, de Castro AM, Galvis GE, et al. Use of US in the evaluation of patients with symptoms of deep venous thrombosis of the lower extremities. Radiographics. 2008;28:1785–1797. [DOI] [PubMed] [Google Scholar]
- 14.List-Hellwig E, Meents H. Magnetic resonance imaging and computed tomography in advanced chronic venous insufficiency. Curr Probl Dermatol. 1999;27:109–113. [DOI] [PubMed] [Google Scholar]
- 15.Stanton AW, Mellor RH, Cook GJ, et al. Impairment of lymph drainage in subfascial compartment of forearm in breast cancer-related lymphedema. Lymphat Res Biol. 2003;1:121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gersh I, Still MA. Blood vessels in fat tissue. Relation to problems of gas exchange. J Exp Med. 1945;81:219–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knight AD, Levick JR. The density and distribution of capillaries around a synovial cavity. Q J Exp Physiol. 1983;68:629–644. [DOI] [PubMed] [Google Scholar]
- 18.Modi S, Stanton AW, Mellor RH, et al. Regional distribution of epifascial swelling and epifascial lymph drainage rate constants in breast cancer-related lymphedema. Lymphat Res Biol. 2005;3:3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stanton AW, Modi S, Mellor RH, et al. A quantitative lymphoscintigraphic evaluation of lymphatic function in the swollen hands of women with lymphoedema following breast cancer treatment. Clin Sci (Lond). 2006;110:553–561. [DOI] [PubMed] [Google Scholar]
- 20.Mortimer PS, Levick JR. Chronic peripheral oedema: the critical role of the lymphatic system. Clin Med (Lond). 2004;4:448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Modi S, Stanton AW, Svensson WE, et al. Human lymphatic pumping measured in healthy and lymphoedematous arms by lymphatic congestion lymphoscintigraphy. J Physiol. 2007;583(pt 1):271–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stanton AW, Modi S, Bennett Britton TM, et al. Lymphatic drainage in the muscle and subcutis of the arm after breast cancer treatment. Breast Cancer Res Treat. 2009;117:549–557. [DOI] [PubMed] [Google Scholar]
- 23.Basser PJ, Jones DK. Diffusion-tensor MRI: theory, experimental design and data analysis - a technical review. NMR Biomed. 2002;15:456–467. [DOI] [PubMed] [Google Scholar]
- 24.Budzik JF, Balbi V, Verclytte S, et al. Diffusion tensor imaging in musculoskeletal disorders. Radiographics. 2014;34:E56–E72. [DOI] [PubMed] [Google Scholar]
- 25.Van Donkelaar CC, Kretzers LJ, Bovendeerd PH, et al. Diffusion tensor imaging in biomechanical studies of skeletal muscle function. J Anat. 1999;194 (pt 1):79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heemskerk AM, Drost MR, van Bochove GS, et al. DTI-based assessment of ischemia-reperfusion in mouse skeletal muscle. Magn Reson Med. 2006;56:272–281. [DOI] [PubMed] [Google Scholar]
- 27.Budzik JF, Le Thuc V, Demondion X, et al. In vivo MR tractography of thigh muscles using diffusion imaging: initial results. Eur Radiol. 2007;17:3079–3085. [DOI] [PubMed] [Google Scholar]
- 28.Oudeman J, Nederveen AJ, Strijkers GJ, et al. Techniques and applications of skeletal muscle diffusion tensor imaging: a review. J Magn Reson Imaging. 2016;43:773–788. [DOI] [PubMed] [Google Scholar]
- 29.Galbán CJ, Maderwald S, Uffmann K, et al. Diffusive sensitivity to muscle architecture: a magnetic resonance diffusion tensor imaging study of the human calf. Eur J Appl Physiol. 2004;93:253–262. [DOI] [PubMed] [Google Scholar]
- 30.Galbán CJ, Maderwald S, Uffmann K, et al. A diffusion tensor imaging analysis of gender differences in water diffusivity within human skeletal muscle. NMR Biomed. 2005;18:489–498. [DOI] [PubMed] [Google Scholar]
- 31.Sinha S, Sinha U, Edgerton VR. In vivo diffusion tensor imaging of the human calf muscle. J Magn Reson Imaging. 2006;24:182–190. [DOI] [PubMed] [Google Scholar]
- 32.Kermarrec E, Budzik JF, Khalil C, et al. In vivo diffusion tensor imaging and tractography of human thigh muscles in healthy subjects. AJR Am J Roentgenol. 2010;195:W352–W356. [DOI] [PubMed] [Google Scholar]
- 33.Damon BM, Froeling M, Buck AK, et al. Skeletal muscle diffusion tensor-MRI fiber tracking: rationale, data acquisition and analysis methods, applications and future directions. NMR Biomed. 2017;30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siems WG, Brenke R, Beier A, et al. Oxidative stress in chronic lymphoedema. QJM. 2002;95:803–809. [DOI] [PubMed] [Google Scholar]
- 35.Tabibiazar R, Cheung L, Han J, et al. Inflammatory manifestations of experimental lymphatic insufficiency. PLoS Med. 2006;3:e254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang TC, Uen YH, Chou CH, et al. The role of cyclooxygenase-derived oxidative stress in surgically induced lymphedema in a mouse tail model. Pharm Biol. 2013;51:573–580. [DOI] [PubMed] [Google Scholar]
- 37.Yang JC, Huang LH, Wu SC, et al. Lymphaticovenous anastomosis supermicrosurgery decreases oxidative stress and increases antioxidant capacity in the serum of lymphedema patients. J Clin Med. 2021;10:1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soong CV, Young IS, Lightbody JH, et al. Reduction of free radical generation minimises lower limb swelling following femoropopliteal bypass surgery. Eur J Vasc Surg. 1994;8:435–440. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto T, Matsuda N, Doi K, et al. The earliest finding of indocyanine green lymphography in asymptomatic limbs of lower extremity lymphedema patients secondary to cancer treatment: the modified dermal backflow stage and concept of subclinical lymphedema. Plast Reconstr Surg. 2011;128:314e–321e. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto T, Narushima M, Doi K, et al. Characteristic indocyanine green lymphography findings in lower extremity lymphedema: the generation of a novel lymphedema severity staging system using dermal backflow patterns. Plast Reconstr Surg. 2011;127:1979–1986. [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto T, Yamamoto N, Yoshimatsu H, et al. Factors associated with lymphosclerosis: an analysis on 962 lymphatic vessels. Plast Reconstr Surg. 2017;140:734–741. [DOI] [PubMed] [Google Scholar]
- 42.Mandić M, Rullman E, Widholm P, et al. Automated assessment of regional muscle volume and hypertrophy using MRI. Sci Rep. 2020;10:2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Norte GE, Knaus KR, Kuenze C, et al. MRI-based assessment of lower-extremity muscle volumes in patients before and after ACL reconstruction. J Sport Rehabil. 2018;27:201–212. [DOI] [PubMed] [Google Scholar]
- 44.Pons C, Borotikar B, Garetier M, et al. Quantifying skeletal muscle volume and shape in humans using MRI: A systematic review of validity and reliability. PLoS One. 2018;13:e0207847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huber FA, Del Grande F, Rizzo S, et al. MRI in the assessment of adipose tissues and muscle composition: how to use it. Quant Imaging Med Surg. 2020;10:1636–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Forte AJ, Khan N, Huayllani MT, et al. Lymphaticovenous anastomosis for lower extremity lymphedema: a systematic review. Indian J Plast Surg. 2020;53:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beier A, Siems W, Brenke R, et al. [Increased formation of free radicals in chronic lymphedema]. Z Lymphol. 1994;18:8–11. [PubMed] [Google Scholar]
- 48.Salgarello M, Mangialardi ML, Pino V, et al. A prospective evaluation of health-related quality of life following lymphaticovenular anastomosis for upper and lower extremities lymphedema. J Reconstr Microsurg. 2018;34:701–707. [DOI] [PubMed] [Google Scholar]
- 49.Phillips GSA, Gore S, Ramsden A, et al. Lymphaticovenular anastomosis in the treatment of secondary lymphoedema of the legs after cancer treatment. J Plast Reconstr Aesthet Surg. 2019;72:1184–1192. [DOI] [PubMed] [Google Scholar]
- 50.Borri M, Gordon KD, Hughes JC, et al. Magnetic resonance imaging-based assessment of breast cancer-related lymphoedema tissue composition. Invest Radiol. 2017;52:554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoffner M, Peterson P, Månsson S, et al. Lymphedema leads to fat deposition in muscle and decreased muscle/water volume after liposuction: a magnetic resonance imaging study. Lymphat Res Biol. 2018;16:174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trinh L, Peterson P, Brorson H, et al. Assessment of subfascial muscle/water and fat accumulation in lymphedema patients using magnetic resonance imaging. Lymphat Res Biol. 2019;17:340–346. [DOI] [PubMed] [Google Scholar]
- 53.Dayan JH, Wiser I, Verma R, et al. Regional patterns of fluid and fat accumulation in patients with lower extremity lymphedema using magnetic resonance angiography. Plast Reconstr Surg. 2020;145:555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chu SY, Wang SC, Chan WH, et al. Volumetric differences in the suprafascial and subfascial compartments of patients with secondary unilateral lower limb lymphedema. Plast Reconstr Surg. 2020;145:1528–1537. [DOI] [PubMed] [Google Scholar]
- 55.Stuiver MM, ten Tusscher MR, Agasi-Idenburg CS, et al. Conservative interventions for preventing clinically detectable upper-limb lymphoedema in patients who are at risk of developing lymphoedema after breast cancer therapy. Cochrane Database Syst Rev. 2015:CD009765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koehler LA, Hunter DW, Blaes AH, et al. Function, shoulder motion, pain, and lymphedema in breast cancer with and without axillary web syndrome: an 18-month follow-up. Phys Ther. 2018;98:518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suami H, Scaglioni MF. Anatomy of the lymphatic system and the lymphosome concept with reference to lymphedema. Semin Plast Surg. 2018;32:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suami H. Anatomical theories of the pathophysiology of cancer-related lymphoedema. Cancers (Basel). 2020;12:E1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suami H, Pan WR, Taylor GI. Changes in the lymph structure of the upper limb after axillary dissection: radiographic and anatomical study in a human cadaver. Plast Reconstr Surg. 2007;120:982–991. [DOI] [PubMed] [Google Scholar]
- 60.Jaimes C, Berman JI, Delgado J, et al. Diffusion-tensor imaging of the growing ends of long bones: pilot demonstration of columnar structure in the physes and metaphyses of the knee. Radiology. 2014;273:491–501. [DOI] [PubMed] [Google Scholar]
- 61.Stavres J, Wang J, Sica CT, et al. Diffusion tensor imaging indices of acute muscle damage are augmented after exercise in peripheral arterial disease. Eur J Appl Physiol. 2021;121:2595–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zivadinov R, Bergsland N, Hagemeier J, et al. Effect of teriflunomide on gray and white matter brain pathology in multiple sclerosis using volumetric and diffusion-tensor imaging MRI measures. J Neurol Sci. 2018;388:175–181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.