Abstract
Background:
Confirmatory tests are recommended for diagnosing primary aldosteronism, but the supporting evidence is unclear.
Methods:
We searched Medline, EMBASE, and the Cochrane Central Register of Controlled Trials. Studies evaluating any guideline-recommended confirmatory test (ie, saline infusion test, salt loading test, fludrocortisone suppression test, and captopril challenge test), compared with a reference standard were included. The Quality Assessment of Diagnostic Accuracy Studies-2 tool was used to assess the risk of bias. Meta-analyses were conducted using hierarchical summary receiver operating characteristic models.
Results:
Fifty-five studies were included, comprising 26 studies (3654 participants) for the recumbent saline infusion test, 4 studies (633 participants) for the seated saline infusion test, 2 studies (99 participants) for the salt loading test, 7 studies (386 participants) for the fludrocortisone suppression test, and 25 studies (2585 participants) for the captopril challenge test. Risk of bias was high, affecting more than half of studies, and across all domains. Studies with case-control sampling overestimated accuracy by 7-fold (relative diagnostic odds ratio, 7.26 [95% CI, 2.46–21.43]) and partial verification or use of inconsistent reference standards overestimated accuracy by 5-fold (5.12 [95% CI, 1.48–17.77]). There were large variations in how confirmatory tests were conducted, interpreted, and verified. Under most scenarios, confirmatory testing resulted in an excess of missed cases. The certainty of evidence underlying each test (Grading of Recommendations, Assessment, Development, and Evaluations) was very low.
Conclusions:
Recommendations for confirmatory testing in patients with abnormal screening tests and high probability features of primary aldosteronism are based on very low-quality evidence and their routine use should be reconsidered.
Keywords: aldosterone, captopril, fludrocortisone, phenotype, prevalence
Novelty and Relevance.
What Is New?
There are large variations in how confirmatory tests are conducted, interpreted, and verified.
Verification bias and spectrum bias are very common, leading to overestimation of test accuracy by 5- to 7-fold.
What Is Relevant?
Under most clinical scenarios, use of confirmatory tests results in an excess of missed cases, such that many patients with primary aldosteronism would be overlooked for targeted treatment.
Clinical/Pathophysiological Implications?
The use of confirmatory tests in patients with abnormal screening and high probability features of primary aldosteronism is based on very low-quality evidence. Current recommendations for confirmatory testing may need revision.
Primary aldosteronism (PA) is the most common cause of remediable hypertension,1 yet <1% of affected people are diagnosed and treated.2 PA poses a major public health problem, not only because of its high prevalence, but also due to the excess risk of cardiovascular, metabolic, and kidney disease if untreated.3–5
In the absence of an extreme phenotype, it is recommended that at least one of 4 confirmatory tests (ie, saline infusion test [SIT], oral salt loading test [SLT], fludrocortisone suppression test [FST], or captopril challenge test [CCT]) be used to confirm PA in individuals with positive screening before proceeding to further investigations or treatment.6 An elevated aldosterone following any of these tests is purported to be diagnostic of PA, whereas a suppressed aldosterone is believed to rule-out disease.
Most studies evaluating confirmatory testing have been limited by case-control selection and inconsistent use of a gold standard for verification (ie, individuals with normal testing did not proceed to surgery or targeted medical therapy), scenarios that bias towards inflated diagnostic yields.7,8 In light of these limitations, the purpose of this study was to assess the characteristics of confirmatory tests for PA and to interpret these in the context of study design and potential risks of bias.
Methods
The authors declare that all supporting data are available within the article.
Data Sources and Searches
This study was registered with PROSPERO (CRD42021258919). We searched Medline, EMBASE, and the Cochrane Central Register of Controlled Trials (inception to June 1, 2021; see Supplemental Material for detailed search strategy and methods).
Study Selection
Original studies evaluating any guideline-recommended confirmatory test for PA were eligible if they included comparison to a reference standard. Two reviewers independently screened titles and abstracts for eligibility and selected articles for further review if the study reported original data on confirmatory testing for PA. Full-text articles were reviewed in duplicate. Studies were selected for final inclusion if a 2×2 diagnostic accuracy table could be extracted (or reconstructed). Discrepancies were resolved by consensus.
Data Extraction and Quality Assessment
We collected information related to testing conditions, reference standards, and study design (eg, single-gate design where the entire sample was drawn from a single clinical population suspected to have PA versus 2- or multi-gate designs where cases and controls were sampled from 2 or more distinct source populations, such that cases were known or strongly suspected to have PA, but controls had an alternative diagnosis, like essential hypertension, or were healthy participants never at risk of having PA).8 The Quality Assessment of Diagnostic Accuracy Studies-2 tool was used to evaluate the risk of bias and concerns of applicability.9 Data extraction and assessment of study quality were performed in duplicate.
Statistical Analysis
We used coupled forest plots and the receiver operating characteristic (ROC) space to visualize variation between studies. Meta-analyses were conducted using hierarchical summary ROC models.10 We explored for sources of heterogeneity using meta-regression, considering differences in methodological quality and clinical characteristics between studies.11 To quantify differences, we calculated the relative diagnostic odds ratio, which is a summary measure of the relative accuracy.7 Because summary statistics are only interpretable when studies share a similar threshold (but thresholds varied considerably in our current review), we estimated the sensitivities at discrete points on the summary ROC curve corresponding to the lower quartile, median, and upper quartile of the reported specificities to facilitate comparisons.11 We calculated the number of missed cases and over-diagnosed cases per 1000 patients and presented these in a summary of findings table.12,13 Please see Supplemental Material for details.
Results
Included Studies
There were 55 studies included (Supplemental Material), comprising 26 studies (3654 participants) for the recumbent SIT,14–42 4 studies (633 participants) for the seated SIT,34,35,41,43–45 2 studies (99 participants) for the SLT,46,47 7 studies (386 participants) for the FST,28,48–53 and 25 studies (2585 participants) for the CCT (see Supplemental Material).24,26,29,32,33,37,44,54–74
Quality Assessment
Risk of bias was high, affecting more than half of studies, and across all domains (Supplemental Material). Half of studies (47.3%) had 2- or multi-gate study designs with unclear sampling or case-control selection of patients (50.9%), such that confirmatory tests were applied to people who were never suspected of having PA, leading to a high risk of spectrum bias. Less than two-thirds of studies were prospective (61.8%) and interpretation of tests were commonly performed post hoc without blinding (72.7%). In the majority of studies (67.3%), a confirmatory test was used as a reference standard,18,22–25,28,29,31,32,34,36–42,44,47,50,53,55–59,61,63–67,69–74 and in nearly a quarter (23.6%), the same test was used as part of its own reference standard.23,28,29,31,36–39,41,42,53,71,74 Few studies applied complete verification with the same reference standard to all participants (25.5%) and of those that did, only one study (1.8%) used an independent reference that was not contingent upon a confirmatory test (ie, adrenal vein sampling to identify unilateral aldosterone excess, and where it was assumed that patients without lateralization were negative).30
Indirect and Direct Comparisons
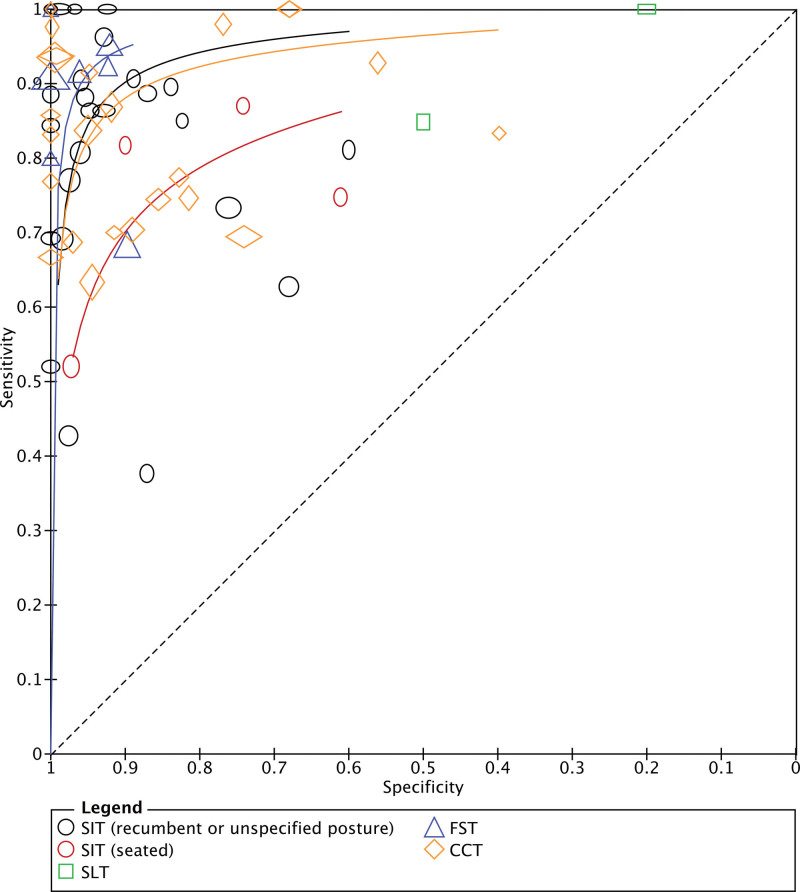
Sensitivities and specificities for each test were highly variable (Figure 1). There were visible differences in the ROC curves between tests (P=0.010 for global test; Figure 2). The FST appeared to have the best performance overall and its curve dominated across all specificities. There was considerable overlap between the curves corresponding to the recumbent SIT and CCT, suggesting comparable accuracies across testing thresholds. Few studies directly compared multiple confirmatory tests in the same patients against a common reference standard. When direct comparisons were made (Supplemental Material), the recumbent SIT was frequently more accurate than the CCT in the individual studies, but aggregate differences were not statistically significant (P=0.061).24,29,32,33,37 The seated SIT appeared to be more accurate than the recumbent SIT in 2 studies34,41; other head-to-head comparisons were limited by few studies.28,44
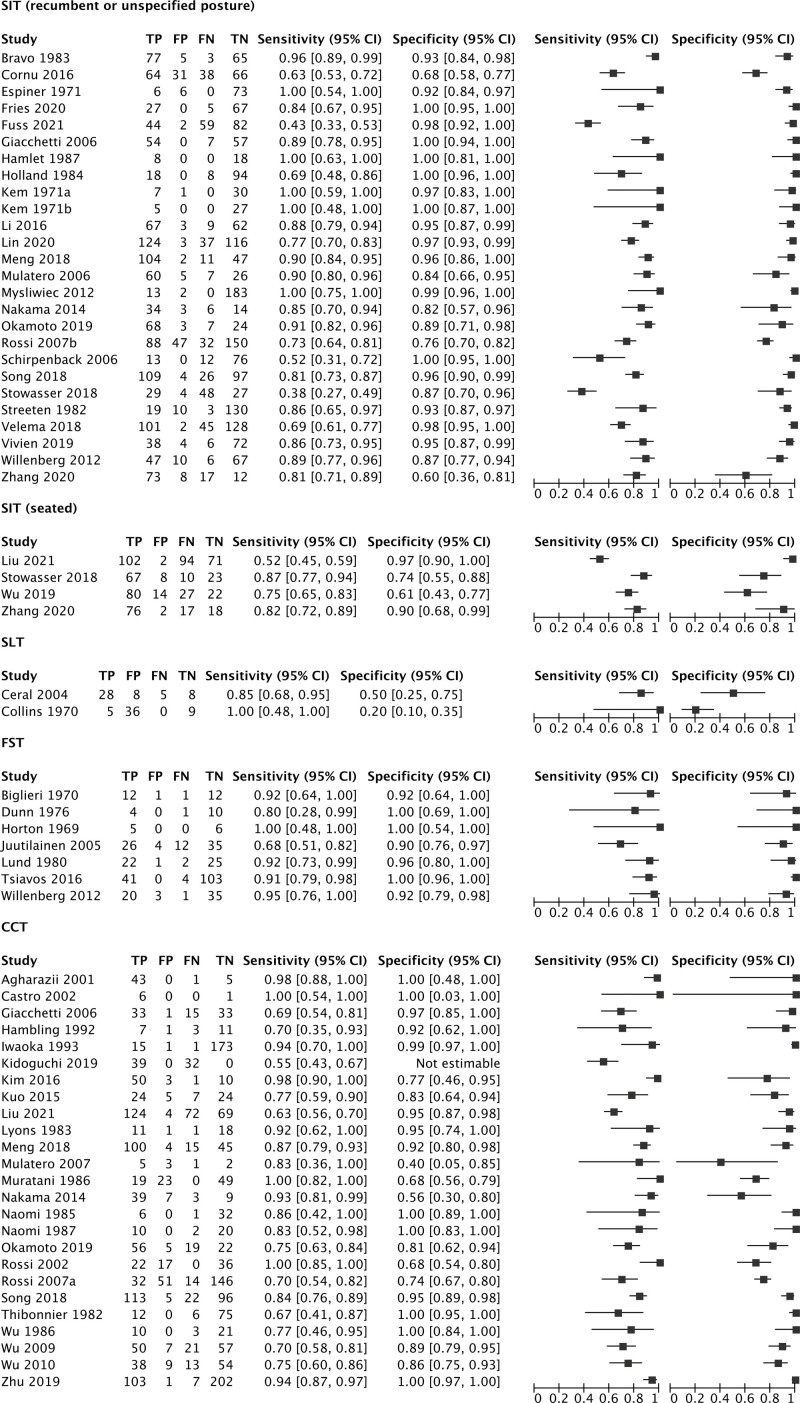
Figure 1.
Coupled forest plots. CCT indicates captopril challenge test; FN, false negative; FP, false positive; FST, fludrocortisone suppression test; SIT, intravenous saline infusion test; SLT, oral salt loading test; TN, true negative; and TP, true positive.
Figure 2.
Summary receiver operating characteristics curves. The clear markers correspond to individual studies. The size of each marker reflects study size (with height proportional to the number diseased and the width with the number nondiseased). A summary curve could not be provided for the oral salt loading test because there were only 2 studies. CCT indicates captopril challenge test; FST, fludrocortisone suppression test; SIT, intravenous saline infusion test; and SLT, oral salt loading test.
Intravenous SIT
The SIT was examined according to recumbent and seated postures using indirect comparisons. The corresponding summary ROC curves were approximately symmetrical (P=0.061) with comparable accuracy (P=0.058), implying similar performance irrespective of posture across the range of observed thresholds. While the vast majority of studies used a similar protocol (ie, 2 L of 0.9% NaCl infused over 4 hours) with few exceptions,18,19,21 there were large differences in diagnostic cut-offs (Supplemental Material). Comparisons were difficult as only 4 of 30 studies provided complete verification of all cases with a consistent reference standard,22,28,30,40 usually with another confirmatory test,22,28,40 and with the remaining studies applying partial or differential verification.
Oral SLT
Only 2 studies evaluated the SLT and these used highly different protocols.46,47 The SLT was considered diagnostic of PA when the urinary aldosterone was >5 µg/d (13.9 nmol/d) in one study and >13 µg/d (36.0 nmol/d) in the other.46,47 Using these cut-offs, the SLT had poor specificity (20% [95% CI, 10%–35%] to 50% [95% CI, 25%–75%]) and moderate to high sensitivity (85% [95% CI, 68%–95%] to 100% [95% CI, 48% to 100%]). The verification standard was inconsistent in the first study46 and based on the recumbent SIT in the second (using a low plasma aldosterone of >100 pmol/L [3.6 ng/dL] to define disease).47
Fludrocortisone Suppression Test
There were significant differences in the dosing of fludrocortisone, ranging from 0.4 28,49,50,53 to 1.2 mg/d48,51 for 3 to 4 days and with large variations in diagnostic cut-offs (ie, plasma aldosterone from 3.0 ng/dL [83 pmol/L] to 12.6 ng/dL [350 pmol/L]). Only one study described dexamethasone co-administration.53 In most studies, inconsistent reference standards were used48–51,53; in some, the FST was both the index test and part of its own reference standard.28,53 Complete verification with an independent reference was only provided in one of the 7 studies,52 which also reported the lowest sensitivity (68% [95% CI, 51%–82%]) and specificity (90% [95% CI, 76%–97%]).
Captopril Challenge Test
There were very large variations in captopril dosage, timing of blood collection, and diagnostic thresholds. In one study, all participants had PA, such that the false positive and true negative rates could not be determined.73 Complete verification of disease status with the same reference standard was only performed in 8 of the 25 studies, and in every case, another confirmatory test was the reference standard.57,58,63,65,66,69,70,72,73 The remaining 17 studies were limited by partial verification and the use of inconsistent standards, usually based on selective testing with another confirmatory test, but in some studies, the reference standard was based on the results of the CCT itself.29,37,67,74
Meta-regression Analysis
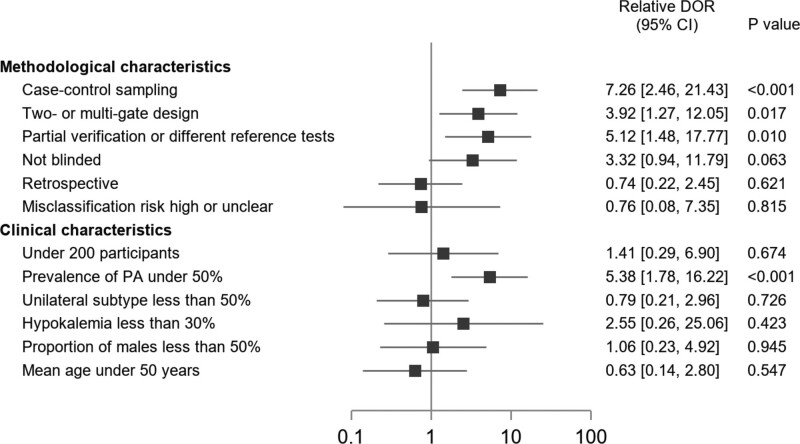
In addition to differences in testing protocols and thresholds, other major drivers of heterogeneity were related to study quality (Figure 3). Studies with case-control sampling overestimated test accuracy by 7-fold compared with those enrolling consecutive or randomly selected patients in whom there was diagnostic uncertainty (relative diagnostic odds ratio, 7.26 [95% CI, 2.46–21.43]). Similarly, the use of 2- or multi-gate designs (eg, inclusion of patients never suspected to have PA) was associated with a 4-fold overestimation of the diagnostic odds ratio (3.92 [95% CI, 1.27–12.05]), while partial verification or use of inconsistent reference standards resulted in a 5-fold overestimation (5.12 [95% CI, 1.48–17.77]). Post hoc interpretation of test results with knowledge of disease status potentially overestimated accuracy by 3-fold (3.32 [95% CI, 0.94–11.79]). Apart from disease prevalence, none of the clinical characteristics examined were associated with changes in test accuracy. For each test, the direction and magnitude of effects were consistent with the overall estimates, though meta-regression was sometimes underpowered owing to the small number of studies in some cases (Supplemental Material).
Figure 3.
Meta-regression analysis for diagnostic test accuracy variability. Relative diagnostic odds ratios (DOR) with 95% CI according to the main study characteristics. The reference category for all comparisons was the absence of the characteristic. PA indicates primary aldosteronism.
Publication Bias
Deeks’ funnel plot appeared asymmetrical for the recumbent SIT, FST, and CCT, suggesting publication bias, but statistical tests were nonsignificant (Supplemental Material).
Clinical Implications
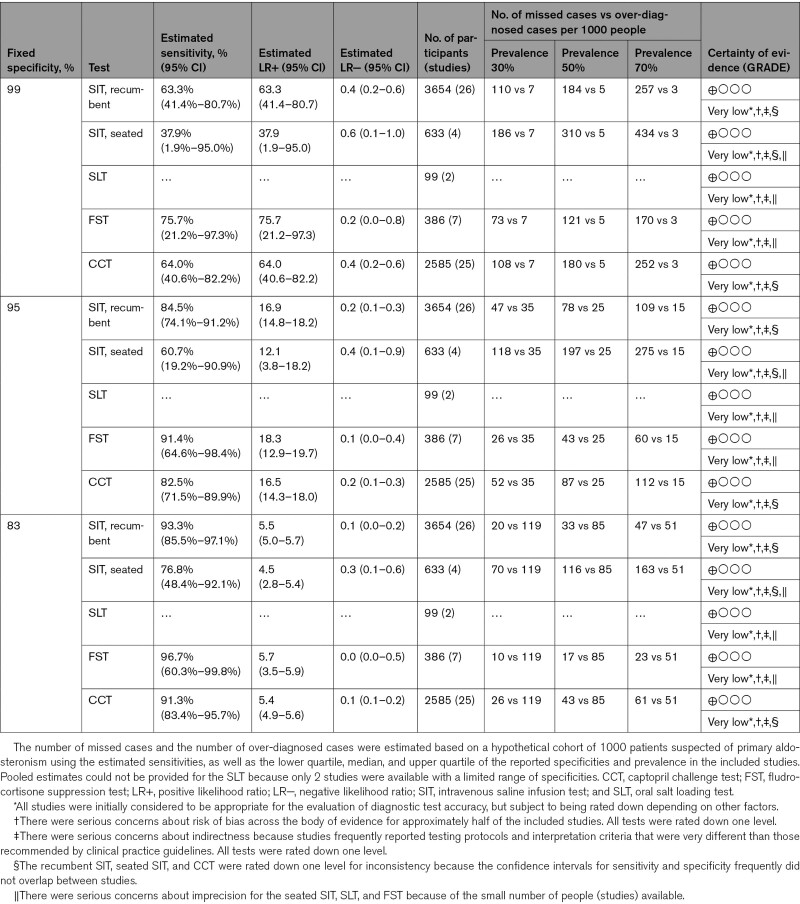
Each test was applied to a hypothetical cohort of 1000 patients to help contextualize the performance under different conditions (Table). With a fixed specificity of 95%, the number of missed cases (ie, patients with PA but normal results and therefore overlooked for opportunities for targeted treatment) exceeded the number over-diagnosed (ie, people without PA but who would potentially undergo unnecessary adrenal vein sampling) when disease prevalence ranged from 30% to 70% following positive ARR screening. This was true for every test, except the FST when disease prevalence was the lowest; in this latter scenario, the numbers of false negatives and false positives were approximately equal. Otherwise, under most scenarios, missed cases were often several fold higher than those over-diagnosed, except when test specificity was low. Finally, owing to serious concerns related to the risks of bias, indirectness, inconsistency, and imprecision across the body of evidence, the certainty of evidence for each confirmatory test was graded very low.
Table.
Summary of Findings
Discussion
We found large variations in how confirmatory tests for PA were conducted, interpreted, and verified, along with global concerns about study quality that limit their application in clinical practice. Spectrum bias (ie, generated by selection of cases and controls) and verification bias (ie, using different verification standards for positive and negative results) posed the greatest threats to study validity. There were almost no studies that completely verified disease status with a valid reference standard that was not itself a confirmatory test. It was impossible to distinguish a single best test or to produce meaningful summary sensitivities or specificities with any certainty. Therefore, the general reliance on historical studies to inform confirmatory testing is highly problematic.
Previous reviews did not consider the impact of study quality on test performance, but rather focused on clinical characteristics, and therefore, were unable to identify major sources of statistical heterogeneity.75,76 Moreover, these only included a subset of available studies and did not use recommended meta-analytic techniques to account for correlation between performance measures.11 In contrast, we found that diagnostic accuracy was highly dependent on study design. Indeed, study quality is well-recognized to impact estimates of association and failure to incorporate quality assessments in the analysis can dramatically distort the results of any review.7,77,78 Our results closely align with the work by Lijmer et al7 who also showed that case-control selection, use of different reference standards, and absence of blinding were the most important factors leading to exaggerated test accuracy. To frame the magnitude of overestimation in practical terms, in our review, an influential study that used different reference standards (ie, composite standards with different ways of ascertaining the presence of disease) would on average overstate the diagnostic odds ratio by 5-fold. Supposing a test had a fixed specificity of 90%, this would be equivalent to reporting a sensitivity of 90% when in reality the sensitivity should be 64%.
In light of this, perhaps it is time to reconsider the long tradition of confirmatory testing given the paucity of empirical evidence supporting its use. Contrary to classic dogma, many patients with PA can have suppressed aldosterone concentrations well below what is commonly believed to be possible with this condition (eg, under 5.0 ng/dL [140 pmol/L]), independent of medication effect, hypokalemia, circadian timing, or postural variation.30,79–82 Furthermore, testing can be dangerous (eg, customary drug washout can provoke symptomatic hypertension; volume expansion can lead to fluid overload or severe hypokalemia).83–85 As we have shown, the added value of confirmatory testing is minimal when there is a high pretest probability of PA, as positive tests only prove what was likely already known. Conversely, normal results modestly rule-out disease but with a high rate of false negative misclassification. Instead, relying on the basic ARR in combination with clinical characteristics (eg, multidrug hypertension, hypokalemia, and presence of adrenal nodule) appears to be sufficiently accurate for diagnosing PA in most instances,86,87 even without a subsequent confirmatory test. This approach has been safely adopted by a number of centers with high rates of treatment success without inordinate risks of performing unnecessary procedures.88,89
There were many strengths of our study (ie, inclusion of more than double the number of studies compared with previous reviews; robust analyses; identification of the major reasons for between-study differences; and standardized grading of the evidence), but there were some limitations. First, there was no universal definition of PA, so each study may have measured a slightly different construct. Part of the challenge lies in changing definitions of disease with increased recognition that PA is a continuous spectrum, such that any dichotomous classification is arbitrary.1 Second, it was impossible to include some studies because the 2×2 table could not be reconstructed (eg, AQUARR),90 but their inclusion would not likely have affected the overall findings, nor resolved the observed heterogeneity. Conversely, the inclusion of a large number of diverse studies was also the reason we observed large amounts of heterogeneity due to significant differences in testing protocols, interpretation criteria, and populations between studies, thus limiting the ability to pool and compare results. Third, PA was commonly defined by surrogate reference standards, and therefore, misclassification was not only possible, but expected.12 Patients with PA commonly have abnormal responses to one test, but not another.91 Admittedly, there is no perfect gold standard for diagnosing PA, and only one study had complete verification with an independent reference standard that did not include a confirmatory test itself.30 Addressing this, there is an ongoing trial assessing the SIT in consecutive patients suspected to have PA with complete verification using targeted treatment response as a reference standard (URL: https://www.clinicaltrials.gov; Unique identifier: NCT04422756).
Perspectives
Current recommendations for confirmatory testing in patients who have high probability features of PA are based on very low-quality evidence. The implication of our findings is that improvements in care may be realized by forgoing routine confirmatory tests if these add little to the diagnostic work-up, but present an unnecessary barrier in an already lengthy and complex diagnostic-care pathway. A potential future pathway may be to rely on the ARR together with clinical/biochemical characteristics to diagnose most cases of PA with early introduction of empirical mineralocorticoid receptor antagonist treatment in the majority, while reserving adrenal vein sampling for those who are most likely to benefit from potential surgery. Given that only a small fraction of patients with PA are ever diagnosed and treated,2 a paradigm shift is needed to meaningfully close care gaps and to improve clinical outcomes.
Article Information
Sources of Funding
This work was supported by the Canadian Institutes of Health Research (PJT-159533). A.A. Leung is supported by the Heart and Stroke Foundation of Canada’s National New Investigator Award. G.L. Hundemer is supported by the Kidney Research Scientist Core Education and National Training (KRESCENT) Program New Investigator Award (2019KP-NIA626990).
Disclosures
None.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- CCT
- captopril challenge test
- FST
- fludrocortisone suppression test
- PA
- primary aldosteronism
- ROC
- receiver operating characteristic
- SIT
- saline infusion test
- SLT
- salt loading test
Drs Leung and Symonds contributed equally.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.122.19377.
For Sources of Funding and Disclosures, see page 1842.
References
- 1.Brown JM, Siddiqui M, Calhoun DA, Carey RM, Hopkins PN, Williams GH, Vaidya A. The unrecognized prevalence of primary aldosteronism: a cross-sectional study. Ann Intern Med. 2020;173:10–20. doi: 10.7326/M20-0065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu YY, King J, Kline GA, Padwal RS, Pasieka JL, Chen G, So B, Harvey A, Chin A, Leung AA. Outcomes of a specialized clinic on rates of investigation and treatment of primary aldosteronism. JAMA Surg. 2021;156:541–549. doi: 10.1001/jamasurg.2021.0254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Incidence of atrial fibrillation and mineralocorticoid receptor activity in patients with medically and surgically treated primary aldosteronism. JAMA Cardiol. 2018;3:768–774. doi: 10.1001/jamacardio.2018.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Renal outcomes in medically and surgically treated primary aldosteronism. Hypertension. 2018;72:658–666. doi: 10.1161/HYPERTENSIONAHA.118.11568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6:51–59. doi: 10.1016/S2213-8587(17)30367-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, Stowasser M, Young WF, Jr. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:1889–1916. doi: 10.1210/jc.2015-4061 [DOI] [PubMed] [Google Scholar]
- 7.Lijmer JG, Mol BW, Heisterkamp S, Bonsel GJ, Prins MH, van der Meulen JH, Bossuyt PM. Empirical evidence of design-related bias in studies of diagnostic tests. JAMA. 1999;282:1061–1066. doi: 10.1001/jama.282.11.1061 [DOI] [PubMed] [Google Scholar]
- 8.Rutjes AW, Reitsma JB, Vandenbroucke JP, Glas AS, Bossuyt PM. Case-control and two-gate designs in diagnostic accuracy studies. Clin Chem. 2005;51:1335–1341. doi: 10.1373/clinchem.2005.048595 [DOI] [PubMed] [Google Scholar]
- 9.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM; QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 10.Rutter CM, Gatsonis CA. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat Med. 2001;20:2865–2884. doi: 10.1002/sim.942 [DOI] [PubMed] [Google Scholar]
- 11.Macaskill P, Takwoingi Y, Deeks JJ, Gatsonis C. Chapter 10: Understanding meta-analysis draft version (17 June 2021). Deeks JJ, Bossuyt PM, Leeflang MMG, Takwoingi Y, eds. In: Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 2. London: The Cochrane Collaboration, 2021. [Google Scholar]
- 12.Schünemann HJ, Mustafa RA, Brozek J, Steingart KR, Leeflang M, Murad MH, Bossuyt P, Glasziou P, Jaeschke R, Lange S, et al. ; GRADE Working Group. GRADE guidelines: 21 part 1. study design, risk of bias, and indirectness in rating the certainty across a body of evidence for test accuracy. J Clin Epidemiol. 2020;122:129–141. doi: 10.1016/j.jclinepi.2019.12.020 [DOI] [PubMed] [Google Scholar]
- 13.Schünemann HJ, Mustafa RA, Brozek J, Steingart KR, Leeflang M, Murad MH, Bossuyt P, Glasziou P, Jaeschke R, Lange S, et al. ; GRADE Working Group. GRADE guidelines: 21 part 2. test accuracy: inconsistency, imprecision, publication bias, and other domains for rating the certainty of evidence and presenting it in evidence profiles and summary of findings tables. J Clin Epidemiol. 2020;122:142–152. doi: 10.1016/j.jclinepi.2019.12.021 [DOI] [PubMed] [Google Scholar]
- 14.Kem DC, Weinberger MH, Mayes DM, Nugent CA. Saline suppression of plasma aldosterone in hypertension. Arch Intern Med. 1971;128:380–386. [PubMed] [Google Scholar]
- 15.Kem DC, Mayes D, Weinberger M, Nugent CA. Saline suppression of plasma aldosterone and plasma renin activity in hypertension. Ariz Med. 1971;28:264–266. [PubMed] [Google Scholar]
- 16.Espiner EA, Christlieb AR, Amsterdam EA, Jagger PI, Dobrzinsky SJ, Lauler DP, Hickler RB. The pattern of plasma renin activity and aldosterone secretion in normal and hypertensive subjects before and after saline infusions. Am J Cardiol. 1971;27:585–594. doi: 10.1016/0002-9149(71)90221-9 [DOI] [PubMed] [Google Scholar]
- 17.Streeten DH, Tomycz N, Anderson GH. Reliability of screening methods for the diagnosis of primary aldosteronism. Am J Med. 1979;67:403–413. doi: 10.1016/0002-9343(79)90786-1 [DOI] [PubMed] [Google Scholar]
- 18.Streeten DHP, Anderson GH, Jr, Springer JM. Outpatient screening procedures for primary aldosteronism. Clinical Science. 1982;63:125S–127S. doi: 10.1016/j.surg.2019.05.087 [Google Scholar]
- 19.Bravo EL, Tarazi RC, Dustan HP, Fouad FM, Textor SC, Gifford RW, Vidt DG. The changing clinical spectrum of primary aldosteronism. Am J Med. 1983;74:641–651. doi: 10.1016/0002-9343(83)91022-7 [DOI] [PubMed] [Google Scholar]
- 20.Holland OB, Brown H, Kuhnert L, Fairchild C, Risk M, Gomez-Sanchez CE. Further evaluation of saline infusion for the diagnosis of primary aldosteronism. Hypertension. 1984;6:717–723. doi: 10.1161/01.hyp.6.5.717 [DOI] [PubMed] [Google Scholar]
- 21.Hamlet SM, Tunny TJ, Klemm SA, Gordon RD. Aldosterone regulation during saline infusion: usefulness of aldosterone/cortisol ratio in the diagnosis of aldosterone-producing adenoma. Clin Exp Pharmacol Physiol. 1987;14:215–219. doi: 10.1111/j.1440-1681.1987.tb00378.x [DOI] [PubMed] [Google Scholar]
- 22.Mulatero P, Milan A, Fallo F, Regolisti G, Pizzolo F, Fardella C, Mosso L, Marafetti L, Veglio F, Maccario M. Comparison of confirmatory tests for the diagnosis of primary aldosteronism. J Clin Endocrinol Metab. 2006;91:2618–2623. doi: 10.1210/jc.2006-0078 [DOI] [PubMed] [Google Scholar]
- 23.Schirpenbach C, Seiler L, Maser-Gluth C, Rüdiger F, Nickel C, Beuschlein F, Reincke M. Confirmatory testing in normokalaemic primary aldosteronism: the value of the saline infusion test and urinary aldosterone metabolites. Eur J Endocrinol. 2006;154:865–873. doi: 10.1530/eje.1.02164 [DOI] [PubMed] [Google Scholar]
- 24.Giacchetti G, Ronconi V, Lucarelli G, Boscaro M, Mantero F. Analysis of screening and confirmatory tests in the diagnosis of primary aldosteronism: need for a standardized protocol. J Hypertens. 2006;24:737–745. doi: 10.1097/01.hjh.0000217857.20241.0f [DOI] [PubMed] [Google Scholar]
- 25.Rossi GP, Belfiore A, Bernini G, Desideri G, Fabris B, Ferri C, Giacchetti G, Letizia C, Maccario M, Mallamaci F, et al. ; PAPY Study Investigators. Prospective evaluation of the saline infusion test for excluding primary aldosteronism due to aldosterone-producing adenoma. J Hypertens. 2007;25:1433–1442. doi: 10.1097/HJH.0b013e328126856e [DOI] [PubMed] [Google Scholar]
- 26.Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, Ganzaroli C, Giacchetti G, Letizia C, Maccario M, et al. ; PAPY Study Investigators. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48:2293–2300. doi: 10.1016/j.jacc.2006.07.059 [DOI] [PubMed] [Google Scholar]
- 27.Myśliwiec J, Zukowski Ł, Grodzka A, Piłaszewicz A, Dragowski S, Górska M. Diagnostics of primary aldosteronism: is obligatory use of confirmatory tests justified? J Renin Angiotensin Aldosterone Syst. 2012;13:367–371. doi: 10.1177/1470320312438791 [DOI] [PubMed] [Google Scholar]
- 28.Willenberg HS, Vonend O, Schott M, Gao X, Blondin D, Saleh A, Rump LC, Scherbaum WA. Comparison of the saline infusion test and the fludrocortisone suppression test for the diagnosis of primary aldosteronism. Horm Metab Res. 2012;44:527–532. doi: 10.1055/s-0032-1314786 [DOI] [PubMed] [Google Scholar]
- 29.Nakama C, Kamide K, Kawai T, Hongyo K, Ito N, Onishi M, Takeya Y, Yamamoto K, Sugimoto K, Rakugi H. The influence of aging on the diagnosis of primary aldosteronism. Hypertens Res. 2014;37:1062–1067. doi: 10.1038/hr.2014.129 [DOI] [PubMed] [Google Scholar]
- 30.Cornu E, Steichen O, Nogueira-Silva L, Küpers E, Pagny JY, Grataloup C, Baron S, Zinzindohoue F, Plouin PF, Amar L. Suppression of aldosterone secretion after recumbent saline infusion does not exclude lateralized primary aldosteronism. Hypertension. 2016;68:989–994. doi: 10.1161/HYPERTENSIONAHA.116.07214 [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Liu Y, Li J, Wang X, Yu Y. Sodium infusion test for diagnosis of primary aldosteronism in chinese population. J Clin Endocrinol Metab. 2016;101:89–95. doi: 10.1210/jc.2015-2840 [DOI] [PubMed] [Google Scholar]
- 32.Song Y, Yang S, He W, Hu J, Cheng Q, Wang Y, Luo T, Ma L, Zhen Q, Zhang S, et al. ; Chongqing Primary Aldosteronism Study (CONPASS) Group†. Confirmatory tests for the diagnosis of primary aldosteronism: a prospective diagnostic accuracy study. Hypertension. 2018;71:118–124. doi: 10.1161/HYPERTENSIONAHA.117.10197 [DOI] [PubMed] [Google Scholar]
- 33.Meng X, Li Y, Wang X, Li J, Liu Y, Yu Y. Evaluation of the saline infusion test and the captopril challenge test in chinese patients with primary aldosteronism. J Clin Endocrinol Metab. 2018;103:853–860. doi: 10.1210/jc.2017-01530 [DOI] [PubMed] [Google Scholar]
- 34.Stowasser M, Ahmed AH, Cowley D, Wolley M, Guo Z, McWhinney BC, Ungerer JP, Gordon RD. Comparison of seated with recumbent saline suppression testing for the diagnosis of primary aldosteronism. J Clin Endocrinol Metab. 2018;103:4113–4124. doi: 10.1210/jc.2018-01394 [DOI] [PubMed] [Google Scholar]
- 35.Ahmed AH, Cowley D, Wolley M, Gordon RD, Xu S, Taylor PJ, Stowasser M. Seated saline suppression testing for the diagnosis of primary aldosteronism: a preliminary study. J Clin Endocrinol Metab. 2014;99:2745–2753. doi: 10.1210/jc.2014-1153 [DOI] [PubMed] [Google Scholar]
- 36.Velema MS, Linssen EJM, Hermus ARMM, Groenewoud HJMM, van der Wilt GJ, van Herwaarden AE, Lenders JWM, Timmers HJLM, Deinum J. A prediction model for primary aldosteronism when the salt loading test is inconclusive. Endocr Connect. 2018;7:1308–1314. doi: 10.1530/EC-18-0358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okamoto R, Taniguchi M, Onishi Y, Kumagai N, Uraki J, Fujimoto N, Fujii E, Yano Y, Ogura T, Ito M. Predictors of confirmatory test results for the diagnosis of primary hyperaldosteronism in hypertensive patients with an aldosterone-to-renin ratio greater than 20. The SHRIMP study. Hypertens Res. 2019;42:40–51. doi: 10.1038/s41440-018-0126-1 [DOI] [PubMed] [Google Scholar]
- 38.Vivien M, Deberles E, Morello R, Haddouche A, Guenet D, Reznik Y. Evaluation of biochemical conditions allowing bypass of confirmatory testing in the workup of primary aldosteronism: a retrospective study in a french hypertensive population. Horm Metab Res. 2019;51:172–177. doi: 10.1055/a-0857-1620 [DOI] [PubMed] [Google Scholar]
- 39.Fries CM, Bae YJ, Rayes N, Sandner B, Isermann B, Stumvoll M, Fagotto V, Reincke M, Bidlingmaier M, Mandy V, et al. Prospective evaluation of aldosterone LC-MS/MS-specific cutoffs for the saline infusion test. Eur J Endocrinol. 2020;183:191–201. doi: 10.1530/EJE-20-0030 [DOI] [PubMed] [Google Scholar]
- 40.Lin C, Yang J, Fuller PJ, Jing H, Song Y, He W, Du Z, Luo T, Cheng Q, Yang S, et al. ; Chongqing Primary Aldosteronism Study (CONPASS) Group. A combination of captopril challenge test after saline infusion test improves diagnostic accuracy for primary aldosteronism. Clin Endocrinol (Oxf). 2020;92:131–137. doi: 10.1111/cen.14134 [DOI] [PubMed] [Google Scholar]
- 41.Zhang D, Chen T, Tian H, Li Y, Mo D, Zhang T, Wang W, Zhang G, Liu Y, Tang L, et al. Exploration of the seated saline suppression test for the diagnosis of primary aldosteronism in the chinese population. Endocr Pract. 2020;26:891–899. doi: 10.4158/EP-2020-0064 [DOI] [PubMed] [Google Scholar]
- 42.Fuss CT, Brohm K, Kurlbaum M, Hannemann A, Kendl S, Fassnacht M, Deutschbein T, Hahner S, Kroiss M. Confirmatory testing of primary aldosteronism with saline infusion test and LC-MS/MS. Eur J Endocrinol. 2021;184:167–178. doi: 10.1530/EJE-20-0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu CH, Wu V, Yang YW, Lin YH, Yang SY, Lin PC, Chang CC, Tsai YC, Wang SM; TAIPAI group. Plasma aldosterone after seated saline infusion test outperforms captopril test at predicting clinical outcomes after adrenalectomy for primary aldosteronism. Am J Hypertens. 2019;32:1066–1074. doi: 10.1093/ajh/hpz098 [DOI] [PubMed] [Google Scholar]
- 44.Liu B, Hu J, Song Y, He W, Cheng Q, Wang Z, Feng Z, Du Z, Xu Z, Yang J, et al. ; Chongqing Primary Aldosteronism Study (CONPASS) Group. Seated saline suppression test is comparable with captopril challenge test for the diagnosis of primary aldosteronism: a prospective study. Endocr Pract. 2021;27:326–333. doi: 10.1016/j.eprac.2020.10.016 [DOI] [PubMed] [Google Scholar]
- 45.Thuzar M, Young K, Ahmed AH, Ward G, Wolley M, Guo Z, Gordon RD, McWhinney BC, Ungerer JP, Stowasser M. Diagnosis of primary aldosteronism by seated saline suppression test-variability between immunoassay and HPLC-MS/MS. J Clin Endocrinol Metab. 2020;105:dgz150. doi: 10.1210/clinem/dgz150 [DOI] [PubMed] [Google Scholar]
- 46.Collins RD, Weinberger MH, Dowdy AJ, Nokes GW, Gonzales CM, Luetscher JA. Abnormally sustained aldosterone secretion during salt loading in patients with various forms of benign hypertension; relation to plasma renin activity. J Clin Invest. 1970;49:1415–1426. doi: 10.1172/JCI106359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ceral J, Malirova E, Ballon M, Solar M. The role of urinary aldosterone for the diagnosis of primary aldosteronism. Horm Metab Res. 2014;46:663–667. doi: 10.1055/s-0034-1374638 [DOI] [PubMed] [Google Scholar]
- 48.Horton R. Stimulation and suppression of aldosterone in plasma of normal man and in primary aldosteronism. J Clin Invest. 1969;48:1230–1236. doi: 10.1172/JCI106087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Biglieri EG, Stockigt JR, Schambelan M. A preliminary evaluation for primary aldosteronism. Arch Intern Med. 1970;126:1004–1007. [PubMed] [Google Scholar]
- 50.Dunn PJ, Espiner EA. Outpatient screening tests for primary aldosteronism. Aust N Z J Med. 1976;6:131–135. doi: 10.1111/j.1445-5994.1976.tb03306.x [DOI] [PubMed] [Google Scholar]
- 51.Lund JO, Nielsen MD. Fludrocortisone suppression test in normal subjects, in patients with essential hypertension and in patients with various forms of aldosteronism. Acta Endocrinol (Copenh). 1980;93:100–107. doi: 10.1530/acta.0.0930100 [DOI] [PubMed] [Google Scholar]
- 52.Juutilainen AM, Voutilainen ET, Mykkänen L, Niskanen L. Plasma aldosterone to renin ratio predicts treatment response in primary aldosteronism: is volume loading needed? Blood Press. 2005;14:245–250. doi: 10.1080/08037050510034329 [DOI] [PubMed] [Google Scholar]
- 53.Tsiavos V, Markou A, Papanastasiou L, Kounadi T, Androulakis II, Voulgaris N, Zachaki A, Kassi E, Kaltsas G, Chrousos GP, Piaditis GP. A new highly sensitive and specific overnight combined screening and diagnostic test for primary aldosteronism. Eur J Endocrinol. 2016;175:21–28. doi: 10.1530/EJE-16-0003 [DOI] [PubMed] [Google Scholar]
- 54.Thibonnier M, Sassano P, Joseph A, Plouin PF, Corvol P, Menard J. Diagnostic value of a single dose of captopril in renin- and aldosterone-dependent, surgically curable hypertension. Cardiovasc Rev Rep. 1982;3:1659–1667. [Google Scholar]
- 55.Lyons DF, Kem DC, Brown RD, Hanson CS, Carollo ML. Single dose captopril as a diagnostic test for primary aldosteronism. J Clin Endocrinol Metab. 1983;57:892–896. doi: 10.1210/jcem-57-5-892 [DOI] [PubMed] [Google Scholar]
- 56.Naomi S, Iwaoka T, Umeda T, Inoue J, Hamasaki S, Miura F, Fujii Y, Sato T. Clinical evaluation of the captopril screening test for primary aldosteronism. Jpn Heart J. 1985;26:549–556. doi: 10.1536/ihj.26.549 [DOI] [PubMed] [Google Scholar]
- 57.Muratani H, Abe I, Tomita Y, Ueno M, Kawazoe N, Kimura Y, Tsuchihashi T, Takishita S, Uezono K, Kawasaki T. Is single oral administration of captopril beneficial in screening for primary aldosteronism? Am Heart J. 1986;112:361–367. doi: 10.1016/0002-8703(86)90276-0 [DOI] [PubMed] [Google Scholar]
- 58.Muratani H, Abe I, Tomita Y, Ueno M, Takishita S, Kawazoe N, Tsuchihashi T, Kawasaki T, Fujishima M. Single oral administration of captopril may not bring an improvement in screening of primary aldosteronism. Clin Exp Hypertens A. 1987;9:611–614. doi: 10.3109/10641968709164232 [DOI] [PubMed] [Google Scholar]
- 59.Wu KD, Hsieh BS, Chen WY, Yen TS, Kuo YM, Tsai TJ. Diagnostic value of captopril test in primary aldosteronism. Taiwan Yi Xue Hui Za Zhi. 1986;85:435–442. [PubMed] [Google Scholar]
- 60.Naomi S, Umeda T, Iwaoka T, Sato T. Effects of sodium intake on the captopril test for primary aldosteronism. Jpn Heart J. 1987;28:357–365. doi: 10.1536/ihj.28.357 [DOI] [PubMed] [Google Scholar]
- 61.Hambling C, Jung RT, Gunn A, Browning MC, Bartlett WA. Re-evaluation of the captopril test for the diagnosis of primary hyperaldosteronism. Clin Endocrinol (Oxf). 1992;36:499–503. doi: 10.1111/j.1365-2265.1992.tb02252.x [DOI] [PubMed] [Google Scholar]
- 62.Iwaoka T, Umeda T, Naomi S, Inoue J, Sasaki M, Yamauchi J, Sato T. The usefulness of the captopril test as a simultaneous screening for primary aldosteronism and renovascular hypertension. Am J Hypertens. 1993;6(11 Pt 1):899–906. doi: 10.1093/ajh/6.11.899 [DOI] [PubMed] [Google Scholar]
- 63.Agharazii M, Douville P, Grose JH, Lebel M. Captopril suppression versus salt loading in confirming primary aldosteronism. Hypertension. 2001;37:1440–1443. doi: 10.1161/01.hyp.37.6.1440 [DOI] [PubMed] [Google Scholar]
- 64.Castro OL, Yu X, Kem DC. Diagnostic value of the post-captopril test in primary aldosteronism. Hypertension. 2002;39:935–938. doi: 10.1161/01.hyp.0000014324.68506.ca [DOI] [PubMed] [Google Scholar]
- 65.Rossi E, Regolisti G, Negro A, Sani C, Davoli S, Perazzoli F. High prevalence of primary aldosteronism using postcaptopril plasma aldosterone to renin ratio as a screening test among Italian hypertensives. Am J Hypertens. 2002;15(10 Pt 1):896–902. doi: 10.1016/s0895-7061(02)02969-2 [DOI] [PubMed] [Google Scholar]
- 66.Mulatero P, Bertello C, Garrone C, Rossato D, Mengozzi G, Verhovez A, Fallo F, Veglio F. Captopril test can give misleading results in patients with suspect primary aldosteronism. Hypertension. 2007;50:e26–e27. doi: 10.1161/HYPERTENSIONAHA.107.093468 [DOI] [PubMed] [Google Scholar]
- 67.Rossi GP, Belfiore A, Bernini G, Desideri G, Fabris B, Ferri C, Giacchetti G, Letizia C, Maccario M, Mallamaci F, et al. ; Primary Aldosteronism Prevalence in Italy Study Investigators. Comparison of the captopril and the saline infusion test for excluding aldosterone-producing adenoma. Hypertension. 2007;50:424–431. doi: 10.1161/HYPERTENSIONAHA.107.091827 [DOI] [PubMed] [Google Scholar]
- 68.Rossi GP, Barisa M, Belfiore A, Desideri G, Ferri C, Letizia C, Maccario M, Morganti A, Palumbo G, Patalano A, et al. ; PAPY study Investigators. The aldosterone-renin ratio based on the plasma renin activity and the direct renin assay for diagnosing aldosterone-producing adenoma. J Hypertens. 2010;28:1892–1899. doi: 10.1097/HJH.0b013e32833d2192 [DOI] [PubMed] [Google Scholar]
- 69.Wu VC, Chang HW, Liu KL, Lin YH, Chueh SC, Lin WC, Ho YL, Huang JW, Chiang CK, Yang SY, et al. ; TAIPAI Study Group. Primary aldosteronism: diagnostic accuracy of the losartan and captopril tests. Am J Hypertens. 2009;22:821–827. doi: 10.1038/ajh.2009.89 [DOI] [PubMed] [Google Scholar]
- 70.Wu VC, Kuo CC, Chang HW, Tsai CT, Lin CY, Lin LY, Lin YH, Wang SM, Huang KH, Fang CC, et al. ; TAIPAI study group. Diagnosis of primary aldosteronism: comparison of post-captopril active renin concentration and plasma renin activity. Clin Chim Acta. 2010;411:657–663. doi: 10.1016/j.cca.2010.01.027 [DOI] [PubMed] [Google Scholar]
- 71.Kuo CC, Balakrishnan P, Hsein YC, Wu VC, Chueh SC, Chen YM, Wu KD, Wang MJ; TAIPAI group. The value of losartan suppression test in the confirmatory diagnosis of primary aldosteronism in patients over 50 years old. J Renin Angiotensin Aldosterone Syst. 2015;16:587–598. doi: 10.1177/1470320313498632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim JH, Park KS, Hong AR, Shin CS, Kim SY, Kim SW. Diagnostic role of captopril challenge test in korean subjects with high aldosterone-to-renin ratios. Endocrinol Metab (Seoul). 2016;31:277–283. doi: 10.3803/EnM.2016.31.2.277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kidoguchi S, Sugano N, Hayashi-Ishikawa N, Morisawa N, Tokudome G, Yokoo T. The characteristics of captopril challenge test-positive patients using various criteria. J Renin Angiotensin Aldosterone Syst. 2019;20:1470320319870891. doi: 10.1177/1470320319870891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu KY, Zhang Y, Zhang WJ, Li HY, Feng WH, Zhu DL, Li P. The captopril challenge test for diagnosing primary aldosteronism in a chinese population. BMC Endocr Disord. 2019;19:65. doi: 10.1186/s12902-019-0390-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu S, Yang J, Hu J, Song Y, He W, Yang S, Luo R, Li Q. Confirmatory tests for the diagnosis of primary aldosteronism: a systematic review and meta-analysis. Clin Endocrinol (Oxf). 2019;90:641–648. doi: 10.1111/cen.13943 [DOI] [PubMed] [Google Scholar]
- 76.Xiang Q, Wang W, Chen T, Yu K, Li Q, Zhang T, Tian H, Ren Y. The value of the post-captopril aldosterone/renin ratio for the diagnosis of primary aldosteronism and the influential factors: a meta-analysis. J Renin Angiotensin Aldosterone Syst. 2020;21:1470320320972032. doi: 10.1177/1470320320972032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jüni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ. 2001;323:42–46. doi: 10.1136/bmj.323.7303.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, Tugwell P, Klassen TP. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet. 1998;352:609–613. doi: 10.1016/S0140-6736(98)01085-X [DOI] [PubMed] [Google Scholar]
- 79.Carroll R, Gould A, Feltham J, Harper S. A case of confirmed primary hyperaldosteronism diagnosed despite normal screening investigations. N Z Med J. 2017;130:129–132. [PubMed] [Google Scholar]
- 80.Kline GA, Darras P, Leung AA, So B, Chin A, Holmes DT. Surprisingly low aldosterone levels in peripheral veins following intravenous sedation during adrenal vein sampling: implications for the concept of nonsuppressibility in primary aldosteronism. J Hypertens. 2019;37:596–602. doi: 10.1097/HJH.0000000000001905 [DOI] [PubMed] [Google Scholar]
- 81.Umakoshi H, Naruse M, Wada N, Ichijo T, Kamemura K, Matsuda Y, Fujii Y, Kai T, Fukuoka T, Sakamoto R, et al. ; WAVES-J Study Group. Adrenal venous sampling in patients with positive screening but negative confirmatory testing for primary aldosteronism. Hypertension. 2016;67:1014–1019. doi: 10.1161/HYPERTENSIONAHA.115.06607 [DOI] [PubMed] [Google Scholar]
- 82.Yozamp N, Hundemer GL, Moussa M, Underhill J, Fudim T, Sacks B, Vaidya A. Variability of aldosterone measurements during adrenal venous sampling for primary aldosteronism. Am J Hypertens. 2021;34:34–45. doi: 10.1093/ajh/hpaa151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fischer E, Beuschlein F, Bidlingmaier M, Reincke M. Commentary on the endocrine society practice guidelines: consequences of adjustment of antihypertensive medication in screening of primary aldosteronism. Rev Endocr Metab Disord. 2011;12:43–48. doi: 10.1007/s11154-011-9163-7 [DOI] [PubMed] [Google Scholar]
- 84.Heinrich DA, Adolf C, Quinkler M, Holler F, Lechner B, Nirschl N, Sturm L, Görge V, Beuschlein F, Reincke M. Safety of medical adjustment and confirmatory testing in the diagnostic work-up of primary aldosteronism. Eur J Endocrinol. 2019;181:421–428. doi: 10.1530/EJE-19-0138 [DOI] [PubMed] [Google Scholar]
- 85.Lee MH, Moxey JE, Derbyshire MM, Ward GM, MacIsaac RJ, Sachithanandan N. Decrease in serum potassium levels post saline suppression test in primary aldosteronism: an under-recognised phenomenon? J Hum Hypertens. 2016;30:664–665. doi: 10.1038/jhh.2016.7 [DOI] [PubMed] [Google Scholar]
- 86.Wang K, Hu J, Yang J, Song Y, Fuller PJ, Hashimura H, He W, Feng Z, Cheng Q, Du Z, et al. Development and validation of criteria for sparing confirmatory tests in diagnosing primary aldosteronism. J Clin Endocrinol Metab. 2020;105:dgaa282. doi: 10.1210/clinem/dgaa282 [DOI] [PubMed] [Google Scholar]
- 87.Burrello J, Amongero M, Buffolo F, Sconfienza E, Forestiero V, Burrello A, Adolf C, Handgriff L, Reincke M, Veglio F, et al. Development of a prediction score to avoid confirmatory testing in patients with suspected primary aldosteronism. J Clin Endocrinol Metab. 2021;106:e1708–e1716. doi: 10.1210/clinem/dgaa974 [DOI] [PubMed] [Google Scholar]
- 88.Violari EG, Arici M, Singh CK, Caetano CM, Georgiades CS, Grady J, Tendler BR, Shichman SJ, Malchoff CD. Adrenal vein sampling with and without cosyntropin stimulation for detection of surgically remediable aldosteronism. Endocrinol Diabetes Metab. 2019;2:e00066. doi: 10.1002/edm2.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kline GA, Pasieka JL, Harvey A, So B, Dias VC. High-probability features of primary aldosteronism may obviate the need for confirmatory testing without increasing false-positive diagnoses. J Clin Hypertens (Greenwich). 2014;16:488–496. doi: 10.1111/jch.12342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maiolino G, Mareso S, Bisogni V, Rossitto G, Azzolini M, Cesari M, Seccia TM, Calò L, Rossi GP. Assessment of the quantitative value usefulness of the aldosterone-renin ratio (ARR) for primary aldosteronism (AQUARR) study. High Blood Press Cardiovasc Prev. 2016;23:19–23. doi: 10.1007/s40292-015-0125-0 [DOI] [PubMed] [Google Scholar]
- 91.Saiki A, Tamada D, Hayashi R, Mukai K, Kitamura T, Takahara M, Otsuki M, Shimomura I. The number of positive confirmatory tests is associated with the clinical presentation and incidence of cardiovascular and cerebrovascular events in primary aldosteronism. Hypertens Res. 2019;42:1186–1191. doi: 10.1038/s41440-019-0247-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.