Abstract
The pharmacokinetic (PK) profile of budesonide oral suspension (BOS) was evaluated during a phase 2, randomized, double-blind, placebo-controlled, dose-ranging study in pediatric patients with eosinophilic esophagitis (EoE) (MPI 101-01/NCT00762073).
Non-compartmental methods were used to calculate PK parameters in 37 patients after receiving morning doses of BOS, with volume and dose adjusted for age (low dose: 0.35 or 0.5 mg; high dose: 1.4 or 2.0 mg [2–9 or 10–18 years old, respectively]). Relationships between apparent oral clearance and volume of distribution, and bodyweight and body mass index were also evaluated.
Budesonide systemic exposure increased with BOS dose. After oral administration, time to maximum plasma budesonide concentration occurred ~1 hour post dose and the half-life of budesonide was 3.3–3.5 hours. PK parameters were similar between age groups for low- and high-dose BOS, indicating that volume and dose adjustments for age were appropriate for pediatric patients with EoE. BOS was well tolerated.
Keywords: budesonide, pediatric study, systemic pharmacokinetics, topical swallowed corticosteroid
What Is Known
The efficacy and safety of budesonide oral suspension (BOS) for the treatment of eosinophilic esophagitis (EoE) in adult, adolescent, and pediatric patients have been described in 2 phase 2 and 2 phase 3 multicenter, randomized, placebo-controlled trials.
What Is New
Budesonide systemic exposure was similar between age groups when BOS volume and dose adjustments were made for younger (2–9 years old) and older (10–18 years old) children with EoE.
Volume and dose adjustments to account for age and a shorter esophageal length in younger children (less than 10 years old) are thus appropriate for the treatment of pediatric patients with EoE.
Eosinophilic esophagitis (EoE) is a chronic immune-/allergen–mediated disease characterized by eosinophilic infiltration of the esophageal mucosa (≥15 eosinophils per high-power field [eos/hpf]) and esophageal dysfunction (1,2).
Common signs and symptoms in pediatric patients include vomiting, abdominal pain, reflux-like symptoms, food refusal, and failure to thrive (2). There is currently no US Food and Drug Administration-approved swallowed topical corticosteroid for EoE (3). Clinical guidelines recommend topical corticosteroids and offer conditional recommendations for proton-pump inhibitors (PPIs) and/or dietary modification over no treatment (4). For pediatric patients, first-line pharmacologic options include PPIs and topical corticosteroids (5).
Budesonide oral suspension (BOS) is a swallowed, viscous, immediate-release topical corticosteroid developed for EoE and optimized to maximize mucosal contact at the esophageal surface (6,7). The efficacy and safety of BOS in EoE have been described in two phase 2 and two phase 3, randomized, placebo-controlled trials (6–9). To date, pharmacokinetic (PK) data for BOS have only been reported in healthy adults and in a population PK analysis in children and adults with EoE and healthy adult volunteers (10,11).
MPI 101-01/NCT00762073 was a phase 2 trial in pediatric patients with EoE; patients received low, medium or high doses of BOS, or placebo (6). Significantly more patients treated with medium or high doses of BOS than placebo experienced improvements in histologic and combined (histologic and symptom) outcomes (6). However, changes in EoE clinical symptom scores (CSS) were similar for BOS- and placebo-treated patients (6). We report the systemic PK profile of BOS from MPI 101-01.
METHODS
Study Design and Population
This phase 2, randomized, double-blind, placebo-controlled, dose-ranging study was conducted in patients 2–18 years old with EoE across 16 sites in the United States of America from January 2009 to April 2010 (MPI 101-01/NCT00762073) (6). This study was approved by the Institutional Review Board at each center and conducted in accordance with the International Council for Harmonisation of Good Clinical Practice guidelines and the principles of the Declaration of Helsinki.
Patients with symptoms of esophageal dysfunction (EoE CSS) and histologic evidence of EoE (≥ 20 eos/hpf) were eligible (Supplemental Digital Content 1 details further eligibility criteria, http://links.lww.com/MPG/C840) (6). Additional inclusion/exclusion criteria are presented elsewhere (6).
After a 4-week screening period, 81 eligible patients were randomized (1:1:1:1) to low-, medium- or high-dose BOS, or placebo. Patients in the low- and medium-dose BOS groups received placebo in the morning and BOS in the evening [low-dose: 0.35 mg or 0.5 mg; medium-dose: 1.4 mg or 2.0 mg (2–9 or 10–18 years old, respectively)]; patients in the high-dose BOS group received BOS twice-daily [1.4 mg or 2.0 mg (2–9 or 10–18 years old, respectively)]; placebo-treated patients received placebo twice-daily (Supplemental Digital Content 2, http://links.lww.com/MPG/C840) (6). Adjustments in volume [7 mL (2–9 years old); 10 mL (10–18 years old)] and dose were made to account for age and shorter esophageal lengths in younger versus older children (Supplemental Digital Content 2, http://links.lww.com/MPG/C840). After 12 weeks, patients began a 3-week taper period; patients received treatment once-daily (morning) during week 1 and doses were reduced by 50% during weeks 2 and 3 (6).
PK Sample Collection and Data Analyses
PK analyses were undertaken for BOS-treated patients who had sufficient blood samples to calculate PK parameters. Patients fasted overnight and delayed their morning dose until instructed to take it at the study site. Patients in the low- and medium-dose groups reversed their usual regimen to receive BOS in the morning and placebo in the evening to enable PK sampling at either the second, fourth, eighth, or twelfth week of therapy. Serial blood samples were taken pre-dose and at 0.5, 1, 2, 3, 4, 6, and 8 hours post-dose. Blood samples were combined with heparin, processed by centrifugation (1500 g for 10 minutes) to collect the plasma, and frozen at −80°C. Plasma samples were processed using solid-phase extraction and analyzed using liquid chromatography-mass spectrometry. Budesonide plasma concentration was assessed using a validated bioanalytical method. The lower limit of quantification for detecting blood plasma levels of budesonide was ~20 pg/mL (0.2 mL sample).
PK parameters were calculated using non-compartmental methods, based on actual sample collection times. Prespecified PK parameters included area under the plasma concentration-time curve from time zero to the last quantifiable concentration (AUC0–last); maximum observed plasma concentration (Cmax); time to Cmax (Tmax); and terminal elimination half-life (T1/2). Post hoc PK parameters included area under the plasma concentration-time curve during a dosing interval, where tau is 12 or 24 hours for twice-daily or once-daily BOS dosing, respectively (AUC0–tau); apparent oral clearance (CL/F); apparent volume of distribution associated with the terminal slope (VZ/F); geometric least-squares means [95% confidence interval (CI)] for AUC0–last and Cmax; and ratios of geometric least-squares means (90% CI) for high- versus low-dose BOS groups. Relationships between CL/F and VZ/F versus bodyweight and body mass index (BMI) were assessed.
PK assessments were measured for morning doses; therefore, patients in the medium- and high-dose groups received the same dose (Supplemental Digital Content 2, http://links.lww.com/MPG/C840); PK data for these groups were combined and are referred to as the high-dose group.
Safety Assessments
Safety outcomes have been previously reported (6) and included adverse events (AE); physical examinations; electrocardiograms; vital signs; height and bodyweight measurements; and clinical laboratory tests (chemistry, hematology, urinalysis, serum pregnancy, and morning serum cortisol levels) at prespecified visits throughout the study. Safety assessments for the PK analysis set were determined post hoc.
Supplemental Digital Content 1, http://links.lww.com/MPG/C840 details the statistical analyses.
RESULTS
Baseline Demographics
Thirty-seven BOS-treated patients had sufficient serum samples for PK analysis (Supplemental Digital Content 3, http://links.lww.com/MPG/C840). The mean (standard deviation) age was 9.6 (4.9) years; most patients were male (83.8%) and white (100%). There were 19 patients 2–9 years old and 18 patients 10–18 years old. Baseline demographics were similar between the low- (n = 9), medium- (n = 15), and high-dose (n = 13) BOS groups (Supplemental Digital Content 3, http://links.lww.com/MPG/C840).
PK Analyses
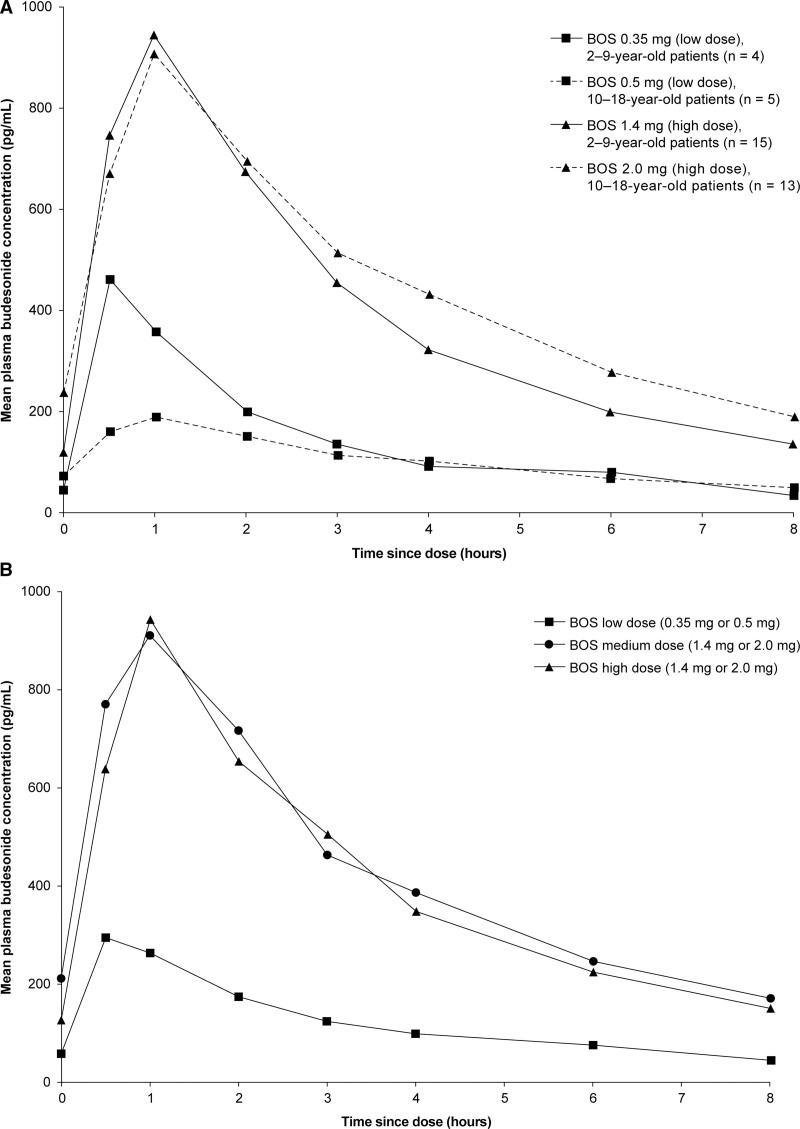
Mean budesonide plasma concentrations over time are shown in Figure 1; data were similar for patients in the medium- and high-dose groups, who received the same morning dose of BOS [1.4 mg or 2.0 mg (2–9 or 10–18 years old, respectively)], supporting the combination of these groups for the PK analyses. Drug exposure was consistent between age groups (Fig. 1 and Table 1) and Tmax was ~1 hour after dosing (Table 1). Mean AUC0–last, AUC0–tau, and Cmax increased from the low- to the high-dose group (Table 1). Mean Tmax, CL/F, and VZ/F were similar between patients treated with low and high doses of BOS and between age groups (Table 1); minimal variation was observed between treatment groups in T1/2, which ranged from 3.3 to 3.5 hours. There was no statistically significant correlation between CL/F and VZ/F, and bodyweight or BMI (Supplemental Digital Content 4, http://links.lww.com/MPG/C840 and Supplemental Digital Content 5, http://links.lww.com/MPG/C840). Systemic PK profiles were similar across age strata for each dose; however, for patients 2–9 years old, PK data were only available for four patients treated with low-dose BOS (0.35 mg) (Fig. 1 and Table 1). In this group, one patient (3 years old) had a Cmax (1060 pg/mL) 2–4 times greater than the other patients, leading to a disparity between the 2 low-dose BOS age groups (2–9 and 10–18 years old).
FIGURE 1.
Mean plasma concentrations of budesonide over 8 hours in patients 2–9 and 10–18 years old after an oral dose of BOS (PK analysis set) stratified by (A) age and low- or high-dose and (B) by low-, medium-, or high-dose. BOS = budesonide oral suspension; PK = pharmacokinetic.
TABLE 1.
Summary of prespecified and post hoc budesonide PK parameters (PK analysis set) after an oral dose of BOS (low or high dose)
| Parameter | Low-dose BOS | High-dose BOS | Ratio of geometric LS means (90% CI) for high- vs low-dose BOS | |||
|---|---|---|---|---|---|---|
| 0.35 mg | 0.5 mg | 1.4 mg | 2.0 mg | 2–9 | 10–18 | |
| (2–9 years old) | (10–18 years old) | (2–9 years old) | (10–18 years old) | years old | years old | |
| (n = 4) | (n = 5) | (n = 15) | (n = 13) | |||
| AUC0–last, hour×pg/mL | ||||||
| Mean | 1139.5 | 743.8 | 3259.3 | 3636.9 | ||
| SD | 800.8 | 425.3 | 2109.4 | 1769.9 | ||
| Geometric LS mean | 903.9 | 654.5 | 2636.4 | 3282.7 | 2.92 (1.4–6.2) | 5.02 (3.2–7.9) |
| 95% CI | 401.8–2033.4 | 410.9–1042.6 | 1734.6–4007.1 | 2459.5–4381.5 | ||
| Median | 1026.0 | 551.0 | 2580.0 | 2700.0 | ||
| Min, max | 286, 2220 | 328, 1390 | 320, 8270 | 1730, 7240 | ||
| AUC0–tau, hour×pg/mL* | ||||||
| Mean | 1710 | 1270 | 4260 | 4840 | ||
| SD | 846 | 713 | 2380 | 2580 | ||
| Median | 1780 | 1050 | 3150 | 3640 | ||
| Min, max | 613, 2670 | 726, 2230 | 1750, 10,100 | 2460, 10,100 | ||
| Cmax, pg/mL | ||||||
| Mean | 492.0 | 195.0 | 1019.5 | 958.4 | ||
| SD | 417.8 | 64.4 | 670.2 | 527.6 | ||
| Geometric LS mean | 355.2 | 187.2 | 805.2 | 841.1 | 2.27 (1.0–5.2) | 4.49 (2.9–7.0) |
| 95% CI | 143.9–877.0 | 118.4–296.1 | 504.9–1284.0 | 633.0–1117.7 | ||
| Median | 402.0 | 191.0 | 812.0 | 708.0 | ||
| Min, max | 104, 1060 | 139, 296 | 70, 2550 | 359, 2210 | ||
| Tmax, hour | ||||||
| Mean | 0.7 | 1.2 | 0.9 | 1.1 | ||
| SD | 0.4 | 0.4 | 0.4 | 0.5 | ||
| Median | 0.5 | 1.0 | 1.0 | 1.0 | ||
| Min, max | 0.5, 1.2 | 1, 2 | 0.5, 2 | 0.5, 2 | ||
| T1/2, hour | ||||||
| Mean | 3.3 | 3.4 | 3.5 | 3.5 | ||
| SD | 0.8 | 0.8 | 2.7 | 1.0 | ||
| Median | 3.2 | 3.5 | 2.7 | 3.6 | ||
| Min, max | 2.4, 4.4 | 2.3, 4.2 | 1.9, 12.7 | 2.1, 5.1 | ||
| CL/F, L/hour* | ||||||
| Mean | 274 | 491 | 417 | 516 | ||
| SD | 200 | 235 | 192 | 219 | ||
| Median | 198 | 525 | 446 | 550 | ||
| Min, max | 131, 571 | 224, 689 | 139, 800 | 199, 814 | ||
| VZ/F, L* | ||||||
| Mean | 1300 | 2240 | 1860 | 2570 | ||
| SD | 888 | 856 | 1220 | 1200 | ||
| Median | 1110 | 2110 | 1760 | 2660 | ||
| Min, max | 447, 2520 | 1360, 3380 | 496, 5490 | 1030, 4460 | ||
AUC0–last = area under the plasma concentration-time curve from time zero (T0) to the last quantifiable concentration; AUC0–tau = area under the plasma concentration-time curve during a dosing interval, where tau is 12 hours for twice-daily BOS dosing and 24 hours for once-daily BOS dosing; BOS = budesonide oral suspension; CI = confidence interval; CL/F = apparent oral clearance; Cmax = maximum observed plasma concentration; LS = least-squares; max = maximum; min = minimum; PK = pharmacokinetic; SD = standard deviation; T1/2 = terminal elimination half-life; Tmax = time to Cmax; VZ/F = apparent volume of distribution associated with the terminal slope.
0.5 mg, n = 4; 1.4 mg, n = 14.
Safety Assessments
Safety data from this study (N = 81) have been published (6) and demonstrated that all BOS doses were well tolerated. As determined post hoc, 75.7% (28/37) of patients from the PK analysis set reported at least one treatment-emergent adverse event (TEAE) over 12 weeks, the proportion of which was higher in patients who received medium- and high-dose BOS (80.0% and 84.6%, respectively) than low-dose BOS (55.6%). However, most TEAEs (83.8%) reported in patients from the PK analysis set were considered by the investigator to be unrelated to study drug; no severe TEAEs or serious AEs were reported, and no TEAEs resulted in study discontinuation. There were no clinically meaningful or dose-related changes in morning cortisol levels, or clinically important changes in other laboratory parameters.
DISCUSSION
This study determined the systemic PK profile of budesonide for different doses of BOS in patients 2–18 years old with EoE. Systemic exposure for low- and high-dose BOS was similar between age groups. Mean AUC0–last, AUC0–tau, and Cmax increased with dose and were consistent between age groups for low- and high-dose BOS. Mean Tmax and T1/2 were similar across age groups and doses. No correlation was observed between CL/F and VZ/F, and bodyweight or BMI. The lack of apparent association between these parameters suggests that bodyweight and BMI do not affect the PK profile of BOS. The previously reported efficacy and safety outcomes from MPI 101-01 informed other clinical trial protocols and led to the selection of BOS 2.0 mg twice-daily for further investigation in subsequent trials in adults and adolescents with EoE (6–9).
During our study, BOS was well tolerated and no clinically meaningful changes in morning cortisol levels were recorded (6). More patients who received medium- and high-dose BOS experienced TEAEs than those receiving low-dose BOS; however, most TEAEs were considered unrelated to study drug. A phase 3 study of BOS 2.0 mg twice-daily showed that adrenal effects were infrequently reported with long-term treatment (9).
The mean T1/2 of BOS (3.3 to 3.5 hours) indicates that accumulation is not expected with once-daily or twice-daily dosing. The short half-life of BOS is consistent with data on BOS 2.0 mg in healthy adults (10).
The results of one outlier in this study caused disparity between the two age groups; despite this, systemic drug exposure was generally consistent across age strata. Thus, the volume adjustments in this study were a satisfactory means of altering dosing to account for age and esophageal length. It has been reported that esophageal length is correlated with height (12); therefore, the age of 10 years was determined as the cutoff for age stratification, based on the association between puberty onset (typically at 10 years old) and an increase in height (12–14).
The systemic PK of BOS 2.0 mg has been evaluated in 47 adults with EoE (7,15). The geometric means for AUC0–tau [5071 hour×pg/mL (n = 24)] and Cmax (914.8 pg/mL) in these patients were similar to the mean AUC0–tau (4840 hour×pg/mL) and Cmax (958.4 pg/mL) in patients 10–18 years old in our study who received BOS 2.0 mg, indicating that volume and dose adjustments used in our study to account for age and esophageal length were appropriate. However, Tmax was slightly later (~2 hours) than in our pediatric population (~1 hour) (15).
Entocort [Enteric Coated (EC), 9.0 mg budesonide once-daily oral administration] has been evaluated in patients 9–14 years old with Crohn’s disease (16). Systemic exposure to budesonide was higher with Entocort EC than for our patients 10–18 years old who received high-dose BOS (Entocort EC vs BOS 2.0 mg; AUC from time 0 to 24 hours, 17.78 vs 9.68 hour×ng/mL; Cmax, 2.58 vs 0.96 ng/mL), suggesting that BOS 2.0 mg twice-daily may have an improved safety profile over Entocort EC 9.0 mg once-daily (16).
This study was limited by the small population size; one outlier in the low-dose 2–9-year-old BOS group inflated the mean Cmax for this group. Moreover, only the linear association between PK parameters and bodyweight or BMI was examined. Future dose-ranging studies of swallowed topical corticosteroids could consider alternative methods for volume and dose adjustments, such as using body surface area.
Conclusions
Overall, PK parameters were similar across age strata, suggesting that volume and dose adjustments were a satisfactory means of adjusting the BOS dose to account for age and esophageal length in pediatric patients. Additionally, the mean half-life of BOS indicated that accumulation is not expected with the dosing regimens utilized in this study.
These findings support an ongoing robust clinical development program of this topical corticosteroid optimized for esophageal delivery and will inform dose adjustments for patients younger than 11 years old for future studies of BOS.
Acknowledgments:
The authors would like to thank Elaine Phillips, PhD, for her contribution to this study. The authors also gratefully acknowledge the investigators, study coordinators, and patients and their families for their participation in this study.
Supplementary Material
Footnotes
https://clinicaltrials.gov registration number: NCT00762073.
The data sets, including the redacted study protocol, redacted statistical analysis plan, and individual participants’ data supporting the results reported in this article, will be made available within 3 months from initial request to researchers who provide a methodologically sound proposal. The data will be provided after its de-identification, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization.
Sources of Funding: This study was funded by Meritage Pharma, Inc., now part of Shire, a member of the Takeda group of companies. Medical writing support was provided by Joanna L Donnelly, PhD, of PharmaGenesis London, London, UK, and was funded by Takeda Pharmaceuticals USA, Inc.
This manuscript discusses the use of budesonide oral suspension, which, is not currently approved by the FDA for the use under discussion.
S.K.G. was a principal investigator for this study and is a consultant for Abbott, Adare Pharmaceuticals, Allakos, DBV Technologies, Gossamer Bio, Medscape, QOL Medical, Receptos/Celgene, and UpToDate and has received research support from Shire, a Takeda company.
M.H., J.M.V., and R.H.F. were employees of Meritage Pharma, Inc., now part of Shire, a Takeda company, and stockholders of Meritage Pharma, Inc., at the time this study was conducted.
N.K.D., J.W., and I.H.S. are employees of Takeda Development Center Americas, Inc., and stockholders of Takeda Pharmaceutical Company Limited.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jpgn.org).
REFERENCES
- 1.Dellon ES, Liacouras CA, Molina-Infante J, et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: proceedings of the AGREE conference. Gastroenterology. 2018;155:1022–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lucendo AJ, Molina-Infante J, Arias A, et al. Guidelines on eosinophilic esophagitis: evidence-based statements and recommendations for diagnosis and management in children and adults. United European Gastroenterol J. 2017;5:335–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.FDA News Release. FDA approves first treatment for eosinophilic esophagitis, a chronic immune disorder. Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-eosinophilic-esophagitis-chronic-immune-disorder#:~:text=Today%2C%20the%20U.S.%20Food%20and,of%20a%20treatment%20for%20EoE. Accessed June 10, 2022.
- 4.Hirano I, Chan ES, Rank MA, et al. AGA Institute and the Joint Task Force on Allergy-Immunology Practice Parameters clinical guidelines for the management of eosinophilic esophagitis. Gastroenterology. 2020;158:1776–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rossetti D, Isoldi S, Oliva S. Eosinophilic esophagitis: update on diagnosis and treatment in pediatric patients. Paediatr Drugs. 2020;22:343–56. [DOI] [PubMed] [Google Scholar]
- 6.Gupta SK, Vitanza JM, Collins MH. Efficacy and safety of oral budesonide suspension in pediatric patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2015;13:66–76. [DOI] [PubMed] [Google Scholar]
- 7.Hirano I, Collins MH, Katzka DA, et al. Budesonide oral suspension improves outcomes in patients with eosinophilic esophagitis: results from a phase 3 trial. Clin Gastroenterol Hepatol. 2022;20:525–34.e10. [DOI] [PubMed] [Google Scholar]
- 8.Dellon ES, Katzka DA, Collins MH, et al. Budesonide oral suspension improves symptomatic, endoscopic, and histologic parameters compared with placebo in patients with eosinophilic esophagitis. Gastroenterology. 2017;152:776–86. [DOI] [PubMed] [Google Scholar]
- 9.Dellon ES, Collins MH, Katzka DA, et al. Long-term treatment of eosinophilic esophagitis with budesonide oral suspension. Clin Gastroenterol Hepatol. 2021. [DOI] [PubMed] [Google Scholar]
- 10.Song I, Finkelman RD, Lan L. A pharmacokinetic bridging study to compare the bioavailability of budesonide between budesonide oral suspension and ENTOCORT EC in healthy subjects. Drugs R D. 2020;20:359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song IH, Rodgers T, Hayes S, et al. A population pharmacokinetic analysis of budesonide oral suspension in children and adults with eosinophilic esophagitis and healthy adult volunteers. Am J Gastroenterol. 2020;115:S220. [Google Scholar]
- 12.Yang GS, Bishop WP, Smith BJ, et al. Radiographic and endoscopic measurements of esophageal length in pediatric patients. Ann Otol Rhinol Laryngol. 2005;114:587–92. [DOI] [PubMed] [Google Scholar]
- 13.Limony Y, Koziel S, Friger M. Age of onset of a normally timed pubertal growth spurt affects the final height of children. Pediatr Res. 2015;78:351–5. [DOI] [PubMed] [Google Scholar]
- 14.Song TJ, Kim YH, Ryu HS, et al. Correlation of esophageal lengths with measurable external parameters. Korean J Intern Med. 1991;6:16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.A study in adolescents and adults with eosinophilic esophagitis (EoE) measuring histologic response and determine if reduction in dysphagia is achieved. 2021. ClinicalTrials.gov Website. Available at: https://clinicaltrials.gov/ct2/show/NCT02605837. Accessed June 9, 2022.
- 16.ENTOCORT EC (budesonide) extended-release capsules, for oral use. 2019. FDA Web site. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/021324s018lbl.pdf. Accessed June 9, 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.