Abstract
Background:
The present study directly tested the crucial role of intestinal gastrin/CCKBR (cholecystokinin B receptor) in the treatment of salt-sensitive hypertension.
Methods:
Adult intestine-specific Cckbr-knockout mice (Cckbrfl/fl villin-Cre) and Dahl salt-sensitive rats were studied on the effect of high salt intake (8% NaCl, 6–7 weeks) on intestinal Na+/H+ exchanger 3 expression, urine sodium concentration, and blood pressure. High-salt diet increased urine sodium concentration and systolic blood pressure to a greater extent in Cckbrfl/fl villin-Cre mice and Dahl salt-sensitive rats than their respective controls, Cckbrfl/fl villin mice and SS13BN rats. We constructed gastrin-SiO2 microspheres to enable gastrin to stimulate specifically and selectively intestinal CCKBR without its absorption into the circulation.
Results:
Gastrin-SiO2 microspheres treatment prevented the high salt-induced hypertension and increase in urine Na concentration by inhibiting intestinal Na+/H+ exchanger 3 trafficking and activity, increasing stool sodium without inducing diarrhea. Gastrin-mediated inhibition of intestinal Na+/H+ exchanger 3 activity, related to a PKC (protein kinase C)-mediated activation of NHERF1 and NHERF2.
Conclusions:
These results support a crucial role of intestinal gastrin/CCKBR in decreasing intestinal sodium absorption and keeping the blood pressure in the normal range. The gastrointestinal administration of gastrin-SiO2 microspheres is a promising and safe strategy to treat salt-sensitive hypertension without side effects.
Keywords: blood pressure, cholecystokinin, gastrins, intestines, sodium
Novelty and Relevance.
What Is New?
Our study first showed that intestinal CCKBR (cholecystokinin B receptor) is important in the regulation of sodium balance and blood pressure.
Our study shows for the first time that gastrin-SiO2 microspheres, via intestinal CCKBR, inhibit Na+/H+ exchanger 3-mediated intestinal sodium absorption without causing diarrhea, and is therefore a potential clinical approach in the treatment of salt-sensitive hypertension.
What Is Relevant?
Intestinal CCKBR deficiency contributes to the pathogenesis and maintenance of salt-sensitive hypertension, but the cellular and molecular mechanisms remain to be determined.
Gastrin-SiO2 microspheres maintaining intestinal sodium metabolism by inhibiting Na+/H+ exchanger 3 activity via reducing cell surface Na+/H+ exchanger 3 protein through a NHERF1-NHERF2 and phospholipase C/PKC (protein kinase C) pathway.
Clinical/Pathophysiological Implications?
Gastrin-SiO2 microspheres, given by gavage, ameliorated salt-sensitive hypertension and organ damage by partial inhibition of Na+/H+ exchanger 3 activity to prevent inappropriate intestinal sodium absorption, without causing diarrhea and is a promising clinical approach in the treatment of salt-sensitive hypertension.
Gastrin-SiO2 microspheres stimulate CCKBR only in the intestines and decrease not only intestinal sodium absorption but also the risk of inflammation and cancer, enhancing their biocompatibility and safety, which might be promising for future clinical application.
Hypertension is a multifunctional disorder resulting from the interaction of the environment, genetics, epigenetics, and behavior, among which high salt intake is a common risk factor.1. Excessive sodium consumption (defined by the World Health Organization as >2 g sodium (5 g NaCl) per day2 increases blood pressure (BP) and causes hypertension, cardiovascular complications,1,3 and chronic kidney disease.4. However, several cohort studies4–6 and meta-analyses7,8 of such studies have shown that the relationship between sodium intake and BP or poor prognosis is not linear, but rather a J-shaped curve. These studies undermine the traditional view that the lower the sodium intake, the better is the overall health. The challenge stands on the way of dietary sodium restriction: the worldwide sodium intake ranges from 3.5 to 5.5 g/d (corresponding to 8.8–13.8 g of salt [NaCl] per day), which is much higher than the WHO recommendation. Therefore, interventions to inhibit the intestinal absorption of sodium to mitigate the deleterious consequences of inappropriate salt intake may be a novel therapy for hypertension.
In mammals, most of the orally ingested sodium is absorbed by the gut, primarily by the small intestines.9,10. Na+/H+ exchanger 3 (NHE3) at the intestinal brush border accounts for the majority of sodium absorbed by the intestines both in the basal state and in the late postprandial period.11. Angiotensin II–induced hypertension is attenuated in mice with global deletion of Nhe3 (Nhe3−/−)12 and mice with transgenic rescue of the Nhe3 gene (tgNhe3−/−) in the small intestines.13–16. Accordingly, tenapanor and SAR218034, inhibitors of NHE3 activity, were designed to reduce the high BP in rodents and humans. However, the clinical trials of tenapanor15 and SAR21803417 were discontinued, because of the heavy diarrhea. Rieg et al18 also reported that small intestine-specific Nhe3 knockout mice died within a few days after birth with no adult survival. Therefore, partially inhibiting NHE3 may be a way to decrease BP without or with minimal side effects.
Gastrin is a peptide hormone secreted by G cells in the stomach and duodenum, which induces acid secretion19–21. Gastrin can also regulate sodium balance and BP22,24 via its receptor, CCKBR (cholecystokinin B receptor) by inhibiting the activities of renal Na+/K+-ATPase24,25 and NHE3.25 We have reported that global knockout of gastrin (Gast)26 or Cckbr27 in mice causes salt-sensitive hypertension. Pharmacological inhibition of intestinal NHE3 decreases the BP of spontaneously hypertensive-obese rats.28 Therefore, we hypothesize that intestinal CCKBR, stimulated by gastrin, may play an important role in the intestinal sodium absorption and BP regulation by inhibiting intestinal NHE3 activity. However, increased circulating gastrin levels may promote cancer.29 We designed and constructed gastrin-SiO2 microspheres with the diameter of 70 μm to prevent its absorption in gastrointestinal tract.and determined if intestinal CCKBR, independent of renal CCKBR, can regulate BP.
Materials and Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. Details on the animal and synthesis and characterizations of SiO2 microspheres and molecular assays are in the Supplemental Material.
Results
CCKBR Protein Expression in the Intestine of Human and Mouse
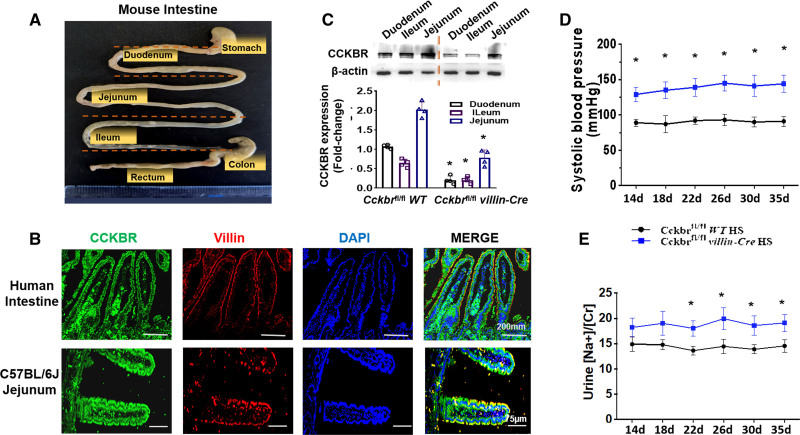
CCKBR is expressed in the renal proximal tubule and intestinal cells.30 Human protein atlas (https://www.proteinatlas.org) was used to retrieve the data on the expression of CCKBR in human tissues. Analyses of 3 databases, human protein atlas, genotype-tissue expression, and functional annotation of the mammalian genome 5, showed that the mRNA expression of Cckbr was highest in the duodenum, relative to other segments of the intestines (Figure S3). We, then, quantified the CCKBR protein expression in the different segments of mouse small intestine, divided into duodenum, jejunum, and ileum (Figure 1A). We found that CCKBR expression was highest in the jejunum, followed by the duodenum, and least in the ileum in wild-type mice (Figure 1B). Immunofluorescence study showed that CCKBR colocalized with villin and is mainly expressed at the microvillar brush border membrane of human intestine and mouse jejunum (Figure 1C).
Figure 1.
Intestinal CCKBR (cholecystokinin B receptor) is involved in the regulation of sodium balance and blood pressure (BP). A, Divisions of mouse gastrointestinal tract. B, CCKBR protein expression in 3 segments of the intestines in Cckbrfl/fl (wild type [WT]) mice and Cckbrfl/fl villin-Cre (knockout [KO]) mice. C, Immunofluorescence of Alexa Fluor 488-labeled CCKBR (green), Alexa Fluor 568-labeled villin (red) and DAPI(blue) in human intestine and C57BL/6J mouse jejunum. D, Systolic BP was measured by telemetry and the data were analyzed by Acqknowledge 5.0 software. E, Urine sodium concentration was measured by flame photometry (*P<0.05 vs Cckbrfl/fl WT+high-salt (HS), 1-way ANOVA, Tukey test).
Intestine-Specific Knockout of Cckbr Increases BP and Urine Na Concentration
Western blot showed that intestinal CCKBR expression was markedly lower in Cckbrfl/fl villin-Cre mice than Cckbrfl/fl WT mice (Figure 1B). However, there was no difference in renal CCKBR expression (P>0.05) in these 2 groups of mice (Figure S4), indicating intestinal-specific deletion of Cckbr. The BP from the carotid artery was measured continuously by radiotelemetry in conscious mice. Cckbrfl/fl villin-Cre mice had increased BP (P>0.05) but normal urine Na/Cr on normal-salt (0.49% NaCl) diet (Figure S5). Systolic BP in Cckbrfl/fl villin-Cre mice increased by the second week (129±10 mm Hg) and remained elevated until the fifth week (142±12 mm Hg) of high-salt (4% NaCl) diet. These BPs were significantly higher than those in WT mice (89±3–91±6 mm Hg; Figure 1D). As shown in Figure 1E, Cckbrfl/fl villin-Cre mice had higher urine Na concentration (18.22±1.85–19.10±1.67 Na+/Cr) than WT mice (14.90±1.41–14.56±1.26 Na+/Cr) from the second to the fifth week of high-salt diet.
Chronic high BP causes organ damage in the cardiovascular system.31 On normal-salt diet the expressions of the organ injury markers, MMP (matrix metalloproteinase)-9 and MMP-2, were higher in the kidneys of Cckbrfl/fl villin-Cre mice than WT mice (P<0.05; Figure S6A). The high-salt diet did not increase further the elevated renal MMP-9 and MMP-2 in Cckbrfl/fl villin-Cre mice but increased MMP-9 expression in WT mice to the same level as that observed in Cckbrfl/fl villin-Cre mice. No obvious pathological differences were observed in the sections of the kidney and heart between these 2 mice groups (Figure S6B). Serum biochemical parameters, including liver function (alanine aminotransferase [ALT] and AST [aspartate transaminase]) and renal function (urea nitrogen and uric acid) and cardiovascular-related tests (LDL-C [low-density lipoprotein cholesterol] and CK [creatine kinase]) were markedly increased in high salt-fed Cckbrfl/fl villin-Cre mice. These were minimally increased in WT mice (Figure S6C) except for serum ALT which was markedly increased by high-salt diet in WT mice.
Gastrin-SiO2 Microspheres Specifically Stimulate Small Intestinal CCKBR
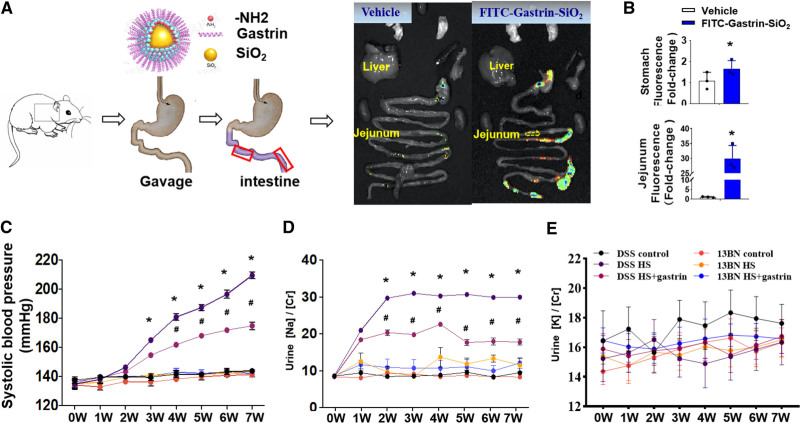
Gastrin could regulate sodium balance by inhibiting sodium transport in the gut and the kidney.22–25 Gastrin-SiO2 microspheres were designed to work in the intestine but not in the stomach (Figure 2A). The intestinal fluorescence was visually stronger in fluorescein isothiocyanate (FITC)-gastrin-SiO2-gavaged mice than control mice, especially in the jejunum and ileum, with less fluorescence in the latter that in the former (Figure 2A). The signals in the stomach which may be due to spontaneous luminescence of chyme. The quantified luminescence in the jejunum was much greater in gastrin-SiO2-FITC than gastrin-SiO2-vehicle treated mice (Figure 2B). We also constructed fluorescent dye-conjugated gastrin (not conjugated to SiO2). Figure S7A and S7B showed strong signals in the stomach in the FITC-gastrin group, with no signal in the intestines. By contrast, the signals in the FITC-gastrin-SiO2 microspheres group were concentrated in the jejunum (Figure S7C), which demonstrated that without microspheres, gastrin would bind to CCKBR in the stomach, such that there may not be enough gastrin to activate intestinal CCKBR. Time-dependent fluorescence of FITC-labeled gastrin-SiO2 microspheres was measured (Figure S8). There was strong signal in jejunum and other intestinal segments at 8-hour postgastrin-SiO2 microspheres administration, which was not significantly decreased until 24-hour postadministration. Gastrin was not detected in the serum in either the vehicle- or gastrin-SiO2 microspheres-treated group, indicating that gastrin in gastrin-SiO2 microspheres was not absorbed from the intestines and released into the circulation (Figure S1). Therefore, gastrin-SiO2 microspheres were used to stimulate specifically and selectively intestinal CCKBR in Dahl salt-sensitive rats and the daily gavage was sufficient for intestine CCKBR activation.
Figure 2.
Gastrin-SiO2 microspheres attenuates the sodium-induced increase in blood pressure (BP). A, Protocol for gastrin-SiO2 microspheres generation and intestinal location in vivo; gastrin-SiO2 microspheres location determined by fluorescence emission tomography. B, Immunofluorescence of stomach and jejunum in the 2 groups of mice (n=3/group, *P<0.05 vs control). C and D, Gastrin-SiO2 microspheres ameliorated the high salt (HS)-elevated systolic BP and urine Na concentration in Dahl salt-sensitive rats fed normal (N, 0.49% NaCl) or HS (8% NaCl) diets (*P<0.05 vs Dahl salt-sensitive control [normal-salt diet], # vs Dahl salt-sensitive + HS diet, 1-way ANOVA, Tukey test). E, Urine potassium excretion was not different among the groups. FITC indicates fluorescein isothiocyanate.
Gastrin-SiO2 Microspheres Treatment Mitigates Salt-Sensitive Hypertension
Conscious Dahl salt-sensitive rats had a progressive increase in systolic BP, measured by telemetry, after the second week of high-salt (8% NaCl) diet; the systolic BP of the control rats fed normal-salt (0.49% NaCl) diet was not altered during the 7-week period of observation (Figure 2C). Gastrin-SiO2 microspheres treatment attenuated the high salt-induced increase in systolic BP (164.8±4.2 versus 154.5±4.6 mm Hg) from the fourth week to the seventh week (209.3±5.8 versus 174.7±6.3 mm Hg; P<0.05). By contrast, there was no effect of the high-salt diet on systolic BP in SS13BN rats (Figure 2C). Urinary sodium (Figure 2D; P≤0.05) excretion, which was elevated by the high-salt diet in Dahl salt-sensitive rats, was decreased by gastrin-SiO2 microspheres treatment. Urinary potassium excretion was not different among the groups (Figure 2E).
Western blotting showed that the elevated MMP-9 and MMP-2 expressions in the kidney of Dahl salt-sensitive rats (high salt group) were downregulated by gastrin-SiO2 microsphere treatment (Figure S9A and S9B). The food intake (g/wk) and body weight (g) were similar among the 3 groups (Dahl salt-sensitive rats fed normal-salt (control) diet, high-salt, or high-salt and gastrin-SiO2 microspheres (Tables S1 and S2). Renal histopathologic staining and plasma biochemical parameters, except for serum LDL-C, indicated that gastrin-SiO2 microsphere treatment protected the organs (kidney, heart, and liver) from damage (Figures S9C and S10).
It is also critical to determine if there are unfavorable effects of gastrin-SiO2 microspheres treatment because gastrin stimulation may take part in colon or stomach carcinogenesis by increasing proliferation and information in tumor cells.30–33 As shown in Figure S11, the colon cancer promoting markers (cancerantigen 199, prostate-specific antigen, and carcino-embryonic antigen) were not affected by high-salt diet or gastrin-SiO2 microsphere treatment. The mRNA expressions of inflammatory factors (TNF [tumor necrosis factor]-α, MCP [monocytechemoattractantprotein]-1, MCP-2, IL [interleukin]-1β, IL-6, and NF-κB [nuclear factor-κB]) were increased in the high-salt group (P<0.05) but not aggravated by gastrin-SiO2 microspheres treatment. These results indicated that gastrin-SiO2 microspheres are biocompatible without obvious adverse side effects and could be used to treat salt-sensitive hypertension and its complications.
Gastrin/CCKBR Decreases NHE3 Expression and Activity in Intestinal Brush Border Membrane
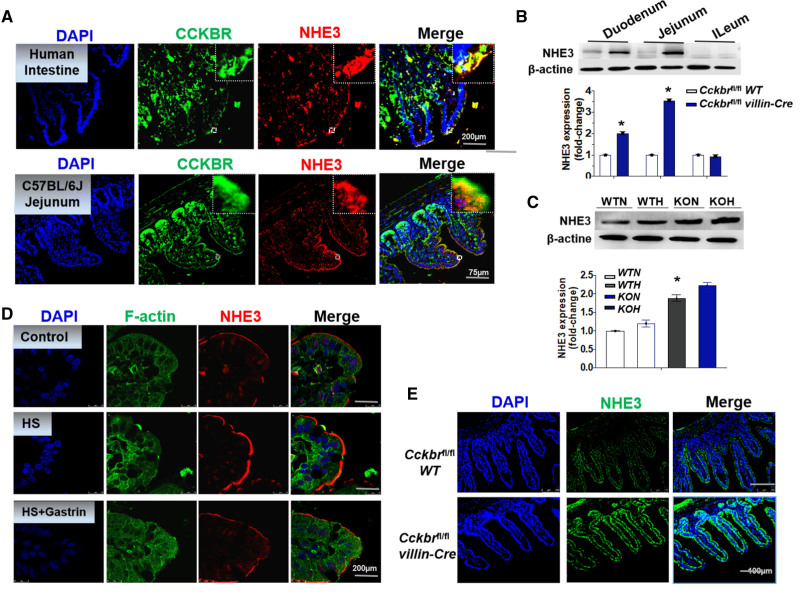
Intestinal NHE3-regulated sodium absorption may participate in the regulation of BP.14,15 Immunofluorescence studies showed that NHE3 was expressed in intestines and colocalized with CCKBR at the microvillar brush border membranes of human intestine and mouse jejunum (Figure 3A).
Figure 3.
Gastrin/CCKBR (cholecystokinin B receptor) decreases Na+/H+ exchanger 3 (NHE3) expression and activity in intestinal brush border membrane. A, Immunofluorescence of Alexa Fluor 488-labeled CCKBR (green), and Alexa Fluor 568-labeled NHE3 (red) in human intestine (scan bar is 200 μm) and mouse jejunum (scan bar is 75 μm). B and C, NHE3 expression in the 3 intestinal segments in knockout (KO) and wild-type (WT) mice fed normal (N) or high (H) salt diet (n=4/group, *P<0.01 vs Cckbrfl/fl WT, 1-way ANOVA, Holm-Sidak test). D, Immunofluorescence of Alexa Fluor 488-labeled F-actin (green) and Alexa Fluor 568-labeled NHE3 (red) in Dahl salt-sensitive rats (scan bar is 200 μm). E, Immunofluorescence of Alexa Fluor 488-labeled NHE3 (green) in WT and KO mice (scan bar is 100 μm).
NHE3 expression was increased in the duodenum and jejunum but not the ileum of Cckbrfl/fl villin-Cre mice compared with Cckbrfl/fl WT mice (Figure 3B). High-salt diet upregulated NHE3 expression in jejunum of Cckbrfl/fl WT that was further increased in Cckbrfl/fl villin-Cre mice (P<0.05; Figure 3C). Immunofluorescence microscopy also showed that NHE3 expression was greater in the brush border membrane of Cckbrfl/fl villin-Cre mice than Cckbrfl/fl WT mice (Figure 3D). Phospho-NHE3 in the jejunum was increased by high-salt diet, which was prevented by 2 different doses (0.02 and 0.1 mg/kg) of gastrin-SiO2 microspheres (Figure S12A). These results suggest a gastrin/CCKBR cross talk with NHE3 in the jejunum; gastrin-SiO2 microspheres treatment may mitigate high salt-induced hypertension by inhibiting NHE3 expression in the intestinal brush border membrane.
Trafficking of NHE3 in and out of the apical membrane is an important mechanism regulating its activity.34 Immunofluorescence study showed that NHE3 (red) is located on the surface of membrane fraction of the jejunum of the Dahl salt-sensitive rat, which was increased after a high-salt diet. Gastrin-SiO2 microspheres treatment markedly reduced the jejunal cell surface NHE3 expression (Figure 3D) and activity (Figure S12B) in these rats. Our study in Dahl salt-sensitive rats also showed that stool sodium (Figure S12C) in gastrin-SiO2 group was increased, which may the consequence of the decrease in intestinal sodium absorption. Global NHE3 knockout mice have diarrhea.35 There was no significant difference in stool sodium concentration of Cckbrfl/fl villin-Cre and Cckbrfl/fl WT mice fed high-salt diet (Figure S12D). The shapes and types of the feces were evaluated in gastrin-SiO2 microspheres group using the Bristol Stool Chart,36 which were normal (Bristol grade 3–4; Figure S13A). We also calculated the water in the feces (35±7%; formula in the Supplemental Material) and found no difference between Cckbrfl/fl villin-Cre and Cckbrfl/fl WT mice fed high-salt diet (Figure S13B). According to our design, gastrin-SiO2 microspheres do not have any ionic charge. Therefore, we measured the expression of 2 other proteins involved in intestinal ion transport. We found that high-salt diet minimally and nonsignificantly increased small intestinal Epithelial sodium channel(ENaC) expression, which was not decreased by gastrin treatment. High-salt diet increased the expression of Na+-K+-2Cl- cotransporter1(NKCC1) that was markedly decreased by gastrin-SiO2 microspheres treatment but not below control levels (Figure S13C through S13E). Therefore, gastrin-SiO2 microspheres inhibit intestinal NHE3 activity with no complicating diarrhea.
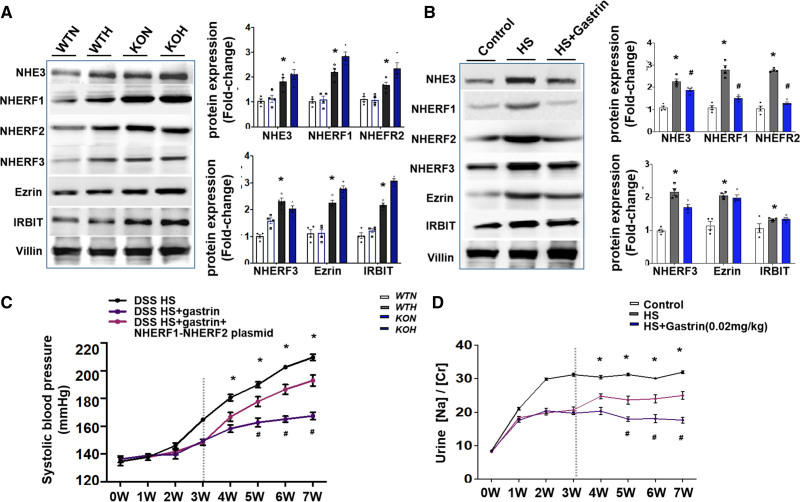
The Mechanism of the Gastrin/CCKBR-Mediated Suppression of Intestinal NHE3 Activity
Electroneutral NaCl transport requires the formation of macromolecular complexes, including that caused by NHE3, which is mediated by the NHE regulatory factor (NHERF) family of scaffold proteins.37,38 Ezrin and NHERFs interact with NHE3,39 and inositol-1,4,5-trisphosphate (IP3) receptor-binding protein (IRBIT) is an NHE3-binding partner, involving the phospholipase C (PLC)/PKC (protein kinase C) pathway.40 The expressions of NHE3, NHERF1, NHERF2, NHERF3, ezrin, and IRBIT were higher in Cckbrfl/fl villin-Cre mice than Cckbrfl/fl WT mice (Figure 4A), which were further increased by high-salt diet. Gastrin-SiO2 microspheres downregulated high salt elevated expressions of NHE3, NHERF1, and NHERF2 but not NHERF3, ezrin, or IRBIT in Dahl salt-sensitive rats (Figure 4B). The intravenous injection of the combination of Adeno-associated virus(AAV)-NHERF1 and AAV-NHERF2 plasmids partially counteracted the gastrin-SiO2 microspheres-induced amelioration of the high BP and urine Na concentration in Dahl salt-sensitive rats (Figure 4C and 4D).
Figure 4.
Gastrin inhibits Na+/H+ exchanger 3 (NHE3) expression via NHERF1 and NHERF2-mediated protein trafficking. A, Expressions of NHE3, NHERF1, NHERF2, NHERF3, ezrin, and IRBIT in intestinal brush border membranes of knockout (KO) mice and wild-type (WT) mice fed normal (N) or high (H) salt diet (n=4/group, *P<0.01 vs Cckbrfl/fl WT, 1-way ANOVA, Holm-Sidak test). B, Expressions of NHE3, NHERF1, NHERF2, NHERF3, ezrin, and IRBIT in the intestinal brush border membrane of Dahl salt-sensitive (DSS) rats. Villin was used as an internal marker of intestinal epithelial cells (*P<0.05 vs DSS control [0.49% NaCl], #P<0.05 vs DSS + high salt [HS, 8% NaCl], 1-way ANOVA, Tukey test). C and D, AAV-NHERF1 and AAV-NHERF2 plasmids impair the effectiveness of the gastrin-SiO2 microspheres in ameliorating the increase in systolic blood pressure (BP) and impairing the increase in urine Na concentration in DSS rats fed HS diet (*P<0.05 vs others, # vs DSS + others, 1-way ANOVA, Tukey test).
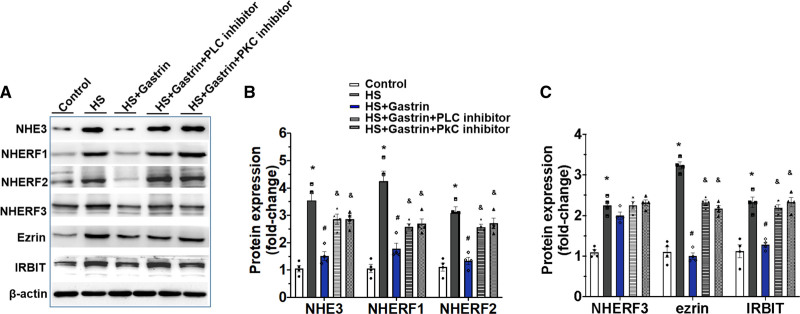
High-salt concentration increased NHE3, NHERF1, NHERF2, NHERF3, ezrin, and IRBIT expressions, which were decreased by gastrin in Caco-2 cells. A PLC or PKC inhibitor blocked the gastrin-mediated decrease in these protein (Figure 5A through 5C). These results demonstrated that the gastrin/CCKBR-mediated amelioration of salt-sensitive hypertension-related genes, and thus hypertension, is via a reduction of NHERF1-NHERF2-induced small intestinal brush border membrane NHE3 trafficking, through a PLC/PKC-dependent pathway (Figure 6).
Figure 5.
Gastrin-mediated Na+/H+ exchanger 3 (NHE3) inhibition via a phospholipase C (PLC)/PKC (protein kinase C)-dependent manner. A–C, Protein expressions of NHE3, NHERF1, NHERF2, NHERF3, Ezrin, and IRBIT on membranes of Caco-2 cells treated with 140 mmol/L Na+ (control), 240 mmol/L Na+ (high salt [HS]), HS incubated cells pretreated with gastrin (10−8 mol/L, HS + gastrin), U73122 (PLC inhibitor, 5×10−6 mol/L) + gastrin, or Go6983 (PKC inhibitor, 5×10−6 mol/L) + gastrin (*P<0.05 vs control, # vs HS, & vs HS + gastrin, 1-way ANOVA, Tukey test). KOH indicates knockout mice on high-salt diet; KON, knockout mice on normal-salt diet; WTH, WT mice on high-salt diet; and WTN, control group of WT mice on normal-salt diet.
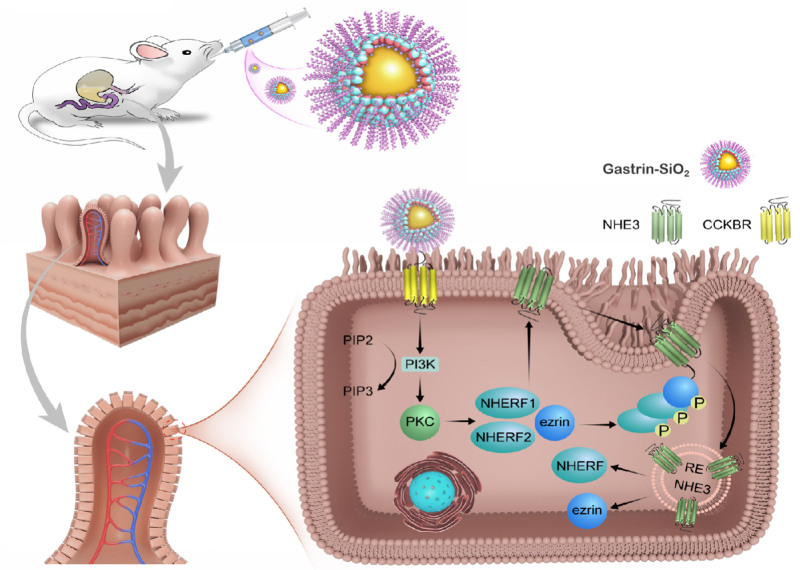
Figure 6.
Gastrin-SiO2 microspheres effectively inhibit Na+/H+ exchanger 3 (NHE3) activity caused by NHERF1 and NHERF2-inducedvsmall intestinal brush border membrane NHE3 trafficking, through a phospholipase C (PLC)/PKC (protein kinase C)-dependent pathway.
Discussion
Gastrin, by stimulating its receptor, CCKBR,26 exerts a natriuretic effect due to inhibition of NHE activity, such as NHE3.25,41 Gastrin can also induce the expression of its own receptor (CCKBR), which would further amplify cellular CCKBR signaling.42 Genome-wide association studies have shown that the chromosomal loci of CCKBR (11p15.5)43 and GAST (17q21)44 are linked to human essential hypertension.45 Germline deletion of Gast46 or Cckbr27 in mice decreases urine sodium concentration and increases BP. Pentagastrin can also decrease intestinal sodium absorption that is potentiated by stimulation of cholinergic or inhibition of sympathetic nerves.47 To determine if intestinal CCKBR, independent of renal CCKBR, can regulate BP by inhibiting intestinal NHE3, we generated mice lacking Cckbr only in the intestines, that is, Cckbrfl/fl villin-Cre mice. Cckbrfl/fl villin-Cre mice had increased BP and elevated duodenal and jejunal NHE3 expression. The expression of NHE3 is highest in the jejunum, followed by the duodenum, and lowest in the ileum48; NHE3 is also expressed in the colon.49 However, only about 4% of fluids and electrolytes are absorbed in the colon.50 Therefore, lack of CCKBR in the intestines can increase sodium balance by stimulating intestinal sodium absorption, via NHE3.
The gavage of gastrin-SiO2 microspheres mitigated the high-salt diet-induced hypertension in Dahl salt-sensitive rats with a decrease in intestinal NHE3 expression and activity, increase in stool sodium. Inhibition of NHE3 activity in the gut decreases BP but causes heavy diarrhea.15,17 In our study, the stool shapes and water content were normal, which effectively prevented the side effect of diarrhea. In addition to NHE3, ENaC-mediated electrogenic Na absorption is important for fluid and electrolyte absorption in the distal colon.51 Decreased ENaC activity is involved in the pathogenesis of diarrhea.52 In our study, ENaC expression was not affected by gastrin-SiO2 microspheres treatment. The rate of epithelial Cl secretion is determined, in large part by the activity of basolateral transporters, such as NKCC1.51 The intestinal expression of NKCC1 was increased by high-salt diet and markedly decreased by gastrin-SiO2 treatment but not below control levels. Therefore, normal expression of ENaC and NKCC1 after gastrin-SiO2 microspheres treatment may have compensated for the inhibition of NHE3, thus preventing a marked increase in stool sodium and, therefore, the development of diarrhea.
There are beneficial effects of inhibition of intestinal NHE3 in addition to BP regulation and end-organ protection.28,54 The long-term subcutaneous infusion of gastrin for 7 to 28 days protected against hypertensive nephropathy by normalizing BP, decreasing renal tubule cell apoptosis, and increasing macrophage efferocytosis55 Gastrin treatment for 28 days also exerted a protective effect on myocardial infarction.56 In our study, the high-salt diet-mediated increase in renal injury was mitigated by gastrin-SiO2 microspheres treatment for 7 weeks.
Gastrin acts as a growth factor for the gastric oxyntic mucosa and plays a role in carcinogenesis, colorectal neoplasia, in particular.57,58 The carcinogenic properties of gastrin has been mainly described with increased circulating gastrin.29 As aforementioned, ingestion of gastrin-SiO2 does not cause hypergastrinemia. We also found that carcinogenic and inflammatory factors in the small intestines were not increased by gastrin-SiO2 microspheres. The gut microbiome participate in the development of hypertension.59–61 Excessive salt intake leads to changes in intestinal microbiota and promotes the activation of innate and adaptive immune systems, resulting in salt-sensitive hypertension.62 However, only few studies have explored on the role of gastrin in the gut microbiome. One study showed that charred Crataegi fructus can promote gastrin secretion and restore the composition of disturbed intestinal microbiota to normal levels, including Bacteroides, Akkermansia, and Intestinimonas.63 Therefore, the beneficial effect of orally administered gastrin-SiO2 microspheres on gut microbiota could be the subject of future studies.
In a population study of working-class Brazilians, hypertension increased the risk nonalcoholic fatty liver disease. Hypertension is an independent of predictor of advanced liver; other predictors were high serum ALT and C-peptide levels.64 High-salt diet aggravated the BP of spontaneously hypertensive rats was associated with increased serum ALT levels.65 Dahl salt-sensitive rats fed a high-salt diet are hypertensive and hyperlipidemic.66 We also found that a high-salt diet increased serum ALT but not AST levels in Cckbrfl/fl WT mice. By contrast, high-salt diet increased both serum ALT and AST levels in Cckbrfl/fl villin-Cre mice and Dahl salt-sensitive rats that were normalized by gastrin-SiO2 microspheres treatment. But, the food intake (g/wk) and body weight (g) were similar among the these groups. Therefore, the elevated serum ALT and AST levels is not caused by excessive food intake.
We suggest a unifying mechanism that may account for the decrease in intestinal NHE3 expression induced by gastrin/CCKBR stimulation. This involves a decrease in NHERF1 and NHERF2 expression and stimulation of the PI3K (phosphatidylinositol 3-kinase)/PKC pathway. Among the NHERFs, NHERF1, NHERF2, and NHERF3, have the highest tendency to complex with and inhibit NHE3 activity.57,68 In NHERF1-deficient mice, the intestinal brush border expression of NHE3 is normal but total NHE3 expression is reduced.69 By contrast, in NHERF2-null mice, basal NHE3 activity is decreased, associated with decreased expression of NHE3 in the apical membrane, without a change in total NHE3 expression.70 We found that intestinal NHERF1 and NHERF2 overexpression minimized the gastrin-SiO2 microspheres-induced amelioration of BP and decrease in urinary sodium, reinforcing the importance of NHERF1 and NHERF2 in the stimulation of NHE3 activity. The activation of p38 MAP (mitogen-activated protein) kinase, followed by the activation of MAPK (MAP kinase activated kinase 2)/APK-2, PI3K, and Akt2, leads to brush border membrane NHE3 translocation and stimulation of NHE3 activity.71 Our in vitro study in Caco-2 cells indicated that NHERF1 and NHERF2, but not NHERF3, ezrin, and IRBIT participate in the gastrin-mediated NHE3 inhibition via a PLC/PKC-dependent manner. We suspect that there may be other signaling pathways involved in the regulation of NHE3 translocation, which need further exploration. We conclude that intestinal gastrin-SiO2 microspheres treatment inhibits NHE3 activity by reducing cell surface NHE3 protein through a NHERF1-NHERF2 and PLC/PKC pathway.
Perspectives
By using in vivo and in vitro experiments, we demonstrated that gastrin-SiO2 microspheres, via intestinal CCKBR, ameliorated salt-sensitive hypertension and organ damage by partial inhibition of NHE3 activity that is PLC/PKC dependent, without causing diarrhea. Gastrin-SiO2 microspheres, which cannot be absorbed into the circulation, reduce the risk of inflammation and cancer. These observations broaden our understanding of the function of intestinal gastrin/CCKBR and mechanisms of intestinal sodium transport. Our study indicates that targeting intestinal CCKBR is a prospective clinical therapeutic strategy for salt-sensitive hypertension, by inhibiting inappropriate intestinal sodium absorption.
Additional Information
Data on additional characterizations of the reagents, such as FITC fluorescent and FT-IR spectra of SiO2 and SiO2-NH2, analysis of gastrin-SiO2 microspheres and detection of serum gastrin by mass spectrometry, measurement of serum markers of colon cancer and intestinal inflammation, H&E staining and analysis of the biocompatibility of gastrin-SiO2 microspheres are shown in the Supplemental Material.
Article Information
Sources of Funding
These studies were supported, in part, by grants from the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (CIFMS, CAMS-I2M, 2021-I2M-1-072), the National Natural Science Foundation (China; 81970358, 81800402, and 82100902), Beijing Outstanding Young Scientist Program (grant no. BJJWZYJH 01201910010024), and the National Institutes of Health (United States; (R01DK039308, P01HL074940, R01DK119652, P.A. Jose).
Disclosures
None.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- ALT
- alanine aminotransferase
- AST
- aspartate transaminase
- BP
- blood pressure
- CCKBR
- cholecystokinin B receptor
- CK
- creatine kinase
- FITC
- fluorescein isothiocyanate
- IL-1β
- interleukin-1β
- IL-6
- interleukin 6
- LDL-C
- low-density lipoprotein cholesterol
- MAP
- mitogen-activated protein
- MAPK-2
- MAP kinase activated kinase 2
- MCP-1
- monocytechemoattractantprotein 1
- MCP-2
- monocytechemoattractantprotein 2
- MMP-2
- matrix metalloproteinase 2
- MMP-9
- matrix metalloproteinase 9
- NF-κB
- nuclear factor-κB
- NHE3
- Na+/H+ exchanger 3
- PI3K
- phosphatidylinositol 3-kinase
- PKC
- protein kinase C
- PLC
- phospholipase C
- TNF-α
- tumor necrosis factor α
X. Jiang, Y. Liu, and X.-Y. Zhang contributed equally.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.121.18791.
For Sources of Funding and Disclosures, see page 1677.
References
- 1.Strazzullo P, D’Elia L, Kandala NB, Cappuccio FP. Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies. BMJ. 2009;339:b4567. doi: 10.1136/bmj.b4567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guideline: Sodium Intake for Adults and Children. Geneva: World Health Organization. 2012. [PubMed] [Google Scholar]
- 3.Grillo A, Salvi L, Coruzzi P, Salvi P, Parati G. Sodium intake and hypertension. Nutrients. 2019;11:E1970. doi: 10.3390/nu11091970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas MC, Moran J, Forsblom C, Harjutsalo V, Thorn L, Ahola A, Wadén J, Tolonen N, Saraheimo M, Gordin D, et al. ; FinnDiane Study Group. The association between dietary sodium intake, ESRD, and all-cause mortality in patients with type 1 diabetes. Diabetes Care. 2011;34:861–866. doi: 10.2337/dc10-1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mozaffarian D, Singh GM, Powles J. Sodium and cardiovascular disease. N Engl J Med. 2014;371:2138–2139. doi: 10.1056/NEJMc1412113 [DOI] [PubMed] [Google Scholar]
- 6.O’Donnell M, Mente A, Rangarajan S, McQueen MJ, Wang X, Liu L, Yan H, Lee SF, Mony P, Devanath A, et al. ; PURE Investigators. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med. 2014;371:612–623. doi: 10.1056/NEJMoa1311889 [DOI] [PubMed] [Google Scholar]
- 7.Mente A, O’Donnell M, Rangarajan S, Dagenais G, Lear S, McQueen M, Diaz R, Avezum A, Lopez-Jaramillo P, Lanas F, et al. ; PURE, EPIDREAM and ONTARGET/TRANSCEND Investigators. Associations of urinary sodium excretion with cardiovascular events in individuals with and without hypertension: a pooled analysis of data from four studies. Lancet. 2016;388:465–475. doi: 10.1016/S0140-6736(16)30467-6 [DOI] [PubMed] [Google Scholar]
- 8.Graudal N, Jürgens G, Baslund B, Alderman MH. Compared with usual sodium intake, low- and excessive-sodium diets are associated with increased mortality: a meta-analysis. Am J Hypertens. 2014;27:1129–1137. doi: 10.1093/ajh/hpu028 [DOI] [PubMed] [Google Scholar]
- 9.Bookstein C, DePaoli AM, Xie Y, Niu P, Musch MW, Rao MC, Chang EB. Na+/H+ exchangers, NHE-1 and NHE-3, of rat intestine. Expression and localization. J Clin Invest. 1994;93:106–113. doi: 10.1172/JCI116933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fenton RA, Poulsen SB, de la Mora Chavez S, Soleimani M, Busslinger M, Dominguez Rieg JA, Rieg T. Caffeine-induced diuresis and natriuresis is independent of renal tubular NHE3. Am J Physiol Renal Physiol. 2015;308:F1409–F1420. doi: 10.1152/ajprenal.00129.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen T, Hubbard A, Murtazina R, Price J, Yang J, Cha B, Sarker R, Donowitz M. Myosin VI mediates the movement of NHE3 down the microvillus in intestinal epithelial cells. J Cell Sci. 2014;127(Pt 16):3535–3545. doi: 10.1242/jcs.149930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li XC, Shull GE, Miguel-Qin E, Zhuo JL. Role of the Na+/H+ exchanger 3 in angiotensin II-induced hypertension. Physiol Genomics. 2015;47:479–487. doi: 10.1152/physiolgenomics.00056.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li XC, Shull GE, Miguel-Qin E, Chen F, Zhuo JL. Role of the Na+/H+ exchanger 3 in angiotensin II-induced hypertension in NHE3-deficient mice with transgenic rescue of NHE3 in small intestines. Physiol Rep. 2015;3:e12605. doi: 10.14814/phy2.12605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linz B, Hohl M, Reil JC, Böhm M, Linz D. Inhibition of NHE3-mediated sodium absorption in the gut reduced cardiac end-organ damage without deteriorating renal function in obese spontaneously hypertensive rats. J Cardiovasc Pharmacol. 2016;67:225–231. doi: 10.1097/FJC.0000000000000336 [DOI] [PubMed] [Google Scholar]
- 15.Spencer AG, Labonte ED, Rosenbaum DP, Plato CF, Carreras CW, Leadbetter MR, Kozuka K, Kohler J, Koo-McCoy S, He L, et al. Intestinal inhibition of the Na+/H+ exchanger 3 prevents cardiorenal damage in rats and inhibits Na+ uptake in humans. Sci Transl Med. 2014;6:227ra36. doi: 10.1126/scitranslmed.3007790 [DOI] [PubMed] [Google Scholar]
- 16.Tanaka M, Itoh H. Hypertension as a metabolic disorder and the novel role of the gut. Curr Hypertens Rep. 2019;21:63. doi: 10.1007/s11906-019-0964-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linz D, Wirth K, Linz W, Heuer HO, Frick W, Hofmeister A, Heinelt U, Arndt P, Schwahn U, Böhm M, et al. Antihypertensive and laxative effects by pharmacological inhibition of sodium-proton-exchanger subtype 3-mediated sodium absorption in the gut. Hypertension. 2012;60:1560–1567. doi: 10.1161/HYPERTENSIONAHA.112.201590 [DOI] [PubMed] [Google Scholar]
- 18.Rieg JAD, Chavez SdelaM, Rieg T. Novel developments in differentiating the role of renal and intestinal sodium hydrogen exchanger 3. Am J Physiol Regul Integr Comp Physiol. 2016; 311: R1186–R1191. doi: 10.1152/ajpregu.00372.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ericsson P, Håkanson R, Rehfeld JF, Norlén P. Gastrin release: antrum microdialysis reveals a complex neural control. Regul Pept. 2010;161:22–32. doi: 10.1016/j.regpep.2010.01.004 [DOI] [PubMed] [Google Scholar]
- 20.Michell AR, Debnam ES, Unwin RJ. Regulation of renal function by the gastrointestinal tract: potential role of gut-derived peptides and hormones. Annu Rev Physiol. 2008;70:379–403. doi: 10.1146/annurev.physiol.69.040705.141330 [DOI] [PubMed] [Google Scholar]
- 21.Jose PA, Yang Z, Zeng C, Felder RA. The importance of the gastrorenal axis in the control of body sodium homeostasis. Exp Physiol. 2016;101:465–470. doi: 10.1113/EP085286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, Asico LD, Zheng S, Villar VA, He D, Zhou L, Zeng C, Jose PA. Gastrin and D1 dopamine receptor interact to induce natriuresis and diuresis. Hypertension. 2013;62:927–933. doi: 10.1161/HYPERTENSIONAHA.113.01094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Z, Zeng C, Villar VA, Chen SY, Konkalmatt P, Wang X, Asico LD, Jones JE, Yang Y, Sanada H, et al. Human GRK4γ142V variant promotes angiotensin II type I receptor-mediated hypertension via renal histone deacetylase type 1 inhibition. Hypertension. 2016;67:325–334. doi: 10.1161/HYPERTENSIONAHA.115.05962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu T, Konkalmatt PR, Yang Y, Jose PA. Gastrin decreases Na+,K+-ATPase activity via a PI 3-kinase- and PKC-dependent pathway in human renal proximal tubule cells. Am J Physiol Endocrinol Metab. 2016;310:E565–E571. doi: 10.1152/ajpendo.00360.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu T, Jose PA. Gastrin induces sodium-hydrogen exchanger 3 phosphorylation and mTOR activation via a phosphoinositide 3-kinase-/protein kinase C-dependent but AKT-independent pathway in renal proximal tubule cells derived from a normotensive male human. Endocrinology. 2013;154:865–875. doi: 10.1210/en.2012-1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pisegna JR, Tarasova NI, Kopp JA, Asico LD, Jose P, Farnsworth DW, Michejda CJ, Wank SA. Postprandial changes in renal function are mediated by elevated serum gastrin acting at cholecystokinin type B receptors (CCKBR) in the kidney. Gastroenterology. 1996;110:1106A. [Google Scholar]
- 27.Jiang X, Chen W, Liu X, Wang Z, Liu Y, Felder RA, Gildea JJ, Jose PA, Qin C, Yang Z. The synergistic roles of cholecystokinin B and dopamine D5 receptors on the regulation of renal sodium excretion. PLoS One. 2016;11:e0146641. doi: 10.1371/journal.pone.0146641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Linz B, Hohl M, Mishima R, Saljic A, Lau DH, Jespersen T, Schotten U, Sanders P, Linz D. Pharmacological inhibition of sodium-proton-exchanger subtype 3-mediated sodium absorption in the gut reduces atrial fibrillation susceptibility in obese spontaneously hypertensive rats. Int J Cardiol Heart Vasc. 2020;28:100534. doi: 10.1016/j.ijcha.2020.100534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rozengurt E, Walsh JH. Gastrin, CCK, signaling, and cancer. Annu Rev Physiol. 2001;63:49–76. doi: 10.1146/annurev.physiol.63.1.49 [DOI] [PubMed] [Google Scholar]
- 30.Koh TJ. Extragastric effects of gastrin gene knock-out mice. Pharmacol Toxicol. 2002;91:368–374. doi: 10.1034/j.1600-0773.2002.910615.x [DOI] [PubMed] [Google Scholar]
- 31.Muñoz-Durango N, Fuentes AC, Castillo AE, González-Gómez LM, Vecchiola A, Fardella CF, Kalergis AM. Role of the renin-angiotensin-aldosterone system beyond blood pressure regulation: molecular and cellular mechanisms involved in end-organ damage during arterial hypertension. Int J Mol Sci. 2016; 17: 797. doi: 10.3390/ijms17070797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kun Z, Hanqing G, Hailing T, Yuan Y, Jun Z, Lingxia Z, Kun H, Xin Z. Gastrin enhances autophagy and promotes gastric carcinoma proliferation via inducing AMPKα. Oncol Res. 2017;25:1399–1407. doi: 10.3727/096504016X14823648620870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao J, Yu JP, Liu CH, Zhou L, Yu HG. Effects of gastrin 17 on beta-catenin/Tcf-4 pathway in Colo320WT colon cancer cells. World J Gastroenterol. 2006;12:7482–7487. doi: 10.3748/wjg.v12.i46.7482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murtazina R, Kovbasnjuk O, Donowitz M, Li X. Na+/H+ exchanger NHE3 activity and trafficking are lipid Raft-dependent. J Biol Chem. 2006;281:17845–17855. doi: 10.1074/jbc.M601740200 [DOI] [PubMed] [Google Scholar]
- 35.Ledoussal C, Woo AL, Miller ML, Shull GE. Loss of the NHE2 Na(+)/H(+) exchanger has no apparent effect on diarrheal state of NHE3-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1385–G1396. doi: 10.1152/ajpgi.2001.281.6.G1385 [DOI] [PubMed] [Google Scholar]
- 36.Lemay DG, Baldiviez LM, Chin EL, Spearman SS, Cervantes E, Woodhouse LR, Keim NL, Stephensen CB, Laugero KD. Technician-scored stool consistency spans the full range of the bristol scale in a healthy US population and differs by diet and chronic stress load. J Nutr. 2021;151:1443–1452. doi: 10.1093/jn/nxab019 [DOI] [PubMed] [Google Scholar]
- 37.Zhu X, Cha B, Zachos NC, Sarker R, Chakraborty M, Chen TE, Kovbasnjuk O, Donowitz M. Elevated calcium acutely regulates dynamic interactions of NHERF2 and NHE3 proteins in opossum kidney (OK) cell microvilli. J Biol Chem. 2011;286:34486–34496. doi: 10.1074/jbc.M111.230219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang J, Singh V, Cha B, Chen TE, Sarker R, Murtazina R, Jin S, Zachos NC, Patterson GH, Tse CM, et al. NHERF2 protein mobility rate is determined by a unique C-terminal domain that is also necessary for its regulation of NHE3 protein in OK cells. J Biol Chem. 2013;288:16960–16974. doi: 10.1074/jbc.M113.470799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seidler U, Singh AK, Cinar A, Chen M, Hillesheim J, Hogema B, Riederer B. The role of the NHERF family of PDZ scaffolding proteins in the regulation of salt and water transport. Ann N Y Acad Sci. 2009;1165:249–260. doi: 10.1111/j.1749-6632.2009.04046.x [DOI] [PubMed] [Google Scholar]
- 40.He P, Zhang H, Yun CC. IRBIT, inositol 1,4,5-triphosphate (IP3) receptor-binding protein released with IP3, binds Na+/H+ exchanger NHE3 and activates NHE3 activity in response to calcium. J Biol Chem. 2008;283:33544–33553. doi: 10.1074/jbc.M805534200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Girardi AC, Di Sole F. Deciphering the mechanisms of the Na+/H+ exchanger-3 regulation in organ dysfunction. Am J Physiol Cell Physiol. 2012;302:C1569–C1587. doi: 10.1152/ajpcell.00017.2012 [DOI] [PubMed] [Google Scholar]
- 42.Ashurst HL, Varro A, Dimaline R. Regulation of mammalian gastrin/CCK receptor (CCK2R) expression in vitro and in vivo. Exp Physiol. 2008;93:223–236. doi: 10.1113/expphysiol.2007.040683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rankinen T, An P, Rice T, Sun G, Chagnon YC, Gagnon J, Leon AS, Skinner JS, Wilmore JH, Rao DC, et al. Genomic scan for exercise blood pressure in the Health, Risk Factors, Exercise Training and Genetics (HERITAGE) Family Study. Hypertension. 2001;38:30–37. doi: 10.1161/01.hyp.38.1.30 [DOI] [PubMed] [Google Scholar]
- 44.Cui J, Zhou X, Chazaro I, DeStefano AL, Manolis AJ, Baldwin CT, Gavras H. Association of polymorphisms in the promoter region of the PNMT gene with essential hypertension in African Americans but not in whites. Am J Hypertens. 2003;16:859–863. doi: 10.1016/s0895-7061(03)01026-4 [DOI] [PubMed] [Google Scholar]
- 45.Asico LD, et al. Minocycline normalizes the hypertension in gastrin-/- mice. FASEB J. 33; S1:724.6, 2019. [Google Scholar]
- 46.Mailman D. Effects of pentagastrin on intestinal absorption and blood flow in the anaesthetized dog. J Physiol. 1980;307:429–442. doi: 10.1113/jphysiol.1980.sp013444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang JL, Zhao L, Zhu J, Wang DK, Ren MJ, Wang M, Liu Y, Boron WF, Chen LM. Expression, localization, and effect of high salt intake on electroneutral Na+/HCO3 - Cotransporter NBCn2 in Rat small intestine: implication in intestinal NaCl absorption. Front Physiol. 2019;10:1334. doi: 10.3389/fphys.2019.01334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Melvin JE, Park K, Richardson L, Schultheis PJ, Shull GE. Mouse down-regulated in adenoma (DRA) is an intestinal Cl(-)/HCO(3)(-) exchanger and is up-regulated in colon of mice lacking the NHE3 Na(+)/H(+) exchanger. J Biol Chem. 1999;274:22855–22861. doi: 10.1074/jbc.274.32.22855 [DOI] [PubMed] [Google Scholar]
- 49.Yang J, Jose PA, Zeng C. Gastrointestinal-renal axis: role in the regulation of blood pressure. J Am Heart Assoc. 2017;6:e005536. doi: 10.1161/JAHA.117.005536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anbazhagan AN, Priyamvada S, Alrefai WA, Dudeja PK. Pathophysiology of IBD associated diarrhea. Tissue Barriers. 2018;6:e1463897. doi: 10.1080/21688370.2018.1463897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martínez-Augustin O, Romero-Calvo I, Suárez MD, Zarzuelo A, de Medina FS. Molecular bases of impaired water and ion movements in inflammatory bowel diseases. Inflamm Bowel Dis. 2009;15:114–127. doi: 10.1002/ibd.20579 [DOI] [PubMed] [Google Scholar]
- 52.Matthews JB. Molecular regulation of Na+-K+-2Cl- cotransporter (NKCC1) and epithelial chloride secretion. World J Surg. 2002;26:826–830. doi: 10.1007/s00268-002-4059-z [DOI] [PubMed] [Google Scholar]
- 53.King AJ, Siegel M, He Y, Nie B, Wang J, Koo-McCoy S, Minassian NA, Jafri Q, Pan D, Kohler J, et al. Inhibition of sodium/hydrogen exchanger 3 in the gastrointestinal tract by tenapanor reduces paracellular phosphate permeability. Sci Transl Med. 2018;10:eaam6474. doi: 10.1126/scitranslmed.aam6474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gu D, Fang D, Zhang M, Guo J, Ren H, Li X, Zhang Z, Yang D, Zou X, Liu Y, et al. Gastrin, via activation of PPARα, protects the kidney against hypertensive injury. Clin Sci (Lond). 2021;135:409–427. doi: 10.1042/CS20201340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fu J, Tang Y, Zhang Z, Tong L, Yue R, Cai L. Gastrin exerts a protective effect against myocardial infarction via promoting angiogenesis. Mol Med. 2021;27:90. doi: 10.1186/s10020-021-00352-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jin G, Westphalen CB, Hayakawa Y, Worthley DL, Asfaha S, Yang X, Chen X, Si Y, Wang H, Tailor Y, et al. Progastrin stimulates colonic cell proliferation via CCK2R- and β-arrestin-dependent suppression of BMP2. Gastroenterology. 2013;145:820–30.e10. doi: 10.1053/j.gastro.2013.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jin G, Ramanathan V, Quante M, Baik GH, Yang X, Wang SS, Tu S, Gordon SA, Pritchard DM, Varro A, et al. Inactivating cholecystokinin-2 receptor inhibits progastrin-dependent colonic crypt fission, proliferation, and colorectal cancer in mice. J Clin Invest. 2009;119:2691–2701. doi: 10.1172/JCI38918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Richards EM, Li J, Stevens BR, Pepine CJ, Raizada MK. Gut microbiome and neuroinflammation in hypertension. Circ Res. 2022;130:401–417. doi: 10.1161/CIRCRESAHA.121.319816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tang WH, Kitai T, Hazen SL. Gut microbiota in cardiovascular health and disease. Circ Res. 2017;120:1183–1196. doi: 10.1161/CIRCRESAHA.117.309715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, Zadeh M, Gong M, Qi Y, Zubcevic J, et al. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65:1331–1340. doi: 10.1161/HYPERTENSIONAHA.115.05315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferguson JF, Aden LA, Barbaro NR, Van Beusecum JP, Xiao L, Simmons AJ, Warden C, Pasic L, Himmel LE, Washington MK, et al. High dietary salt-induced dendritic cell activation underlies microbial dysbiosis-associated hypertension. JCI Insight. 2019;5:126241. doi: 10.1172/jci.insight.126241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu C, He Q, Zeng L, Shen L, Luo Q, Zhang W, Zhou X, Wan J. Digestion-promoting effects and mechanisms of dashanzha pill based on raw and charred crataegi fructus. Chem Biodivers. 2021;18:e2100705. doi: 10.1002/cbdv.202100705 [DOI] [PubMed] [Google Scholar]
- 63.Dixon JB, Bhathal PS, O’Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91–100. doi: 10.1053/gast.2001.25540 [DOI] [PubMed] [Google Scholar]
- 64.Arima S, Uto H, Ibusuki R, Kumamoto R, Tanoue S, Mawatari S, Oda K, Numata M, Fujita H, Oketani M, et al. Hypertension exacerbates liver injury and hepatic fibrosis induced by a choline-deficient L-amino acid-defined diet in rats. Int J Mol Med. 2014;33:68–76. doi: 10.3892/ijmm.2013.1544 [DOI] [PubMed] [Google Scholar]
- 65.Atarashi K, Ishiyama A, Takagi M, Minami M, Kimura K, Goto A, Omata M. Vitamin E ameliorates the renal injury of Dahl salt-sensitive rats. Am J Hypertens. 1997;10(5 Pt 2):116S–119S. [PubMed] [Google Scholar]
- 66.Yang J, Singh V, Chen TE, Sarker R, Xiong L, Cha B, Jin S, Li X, Tse CM, Zachos NC, et al. NHERF2/NHERF3 protein heterodimerization and macrocomplex formation are required for the inhibition of NHE3 activity by carbachol. J Biol Chem. 2014;289:20039–20053. doi: 10.1074/jbc.M114.562413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Avula LR, Chen T, Kovbasnjuk O, Donowitz M. Both NHERF3 and NHERF2 are necessary for multiple aspects of acute regulation of NHE3 by elevated Ca2+, cGMP, and lysophosphatidic acid. Am J Physiol Gastrointest Liver Physiol. 2018;314:G81–G90. doi: 10.1152/ajpgi.00140.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Broere N, Chen M, Cinar A, Singh AK, Hillesheim J, Riederer B, Lünnemann M, Rottinghaus I, Krabbenhöft A, Engelhardt R, et al. Defective jejunal and colonic salt absorption and alteredNa(+)/H (+) exchanger 3 (NHE3) activity in NHE regulatory factor 1 (NHERF1) adaptor protein-deficient mice. Pflugers Arch. 2009;457:1079–1091. doi: 10.1007/s00424-008-0579-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murtazina R, Kovbasnjuk O, Chen TE, Zachos NC, Chen Y, Kocinsky HS, Hogema BM, Seidler U, de Jonge HR, Donowitz M. NHERF2 is necessary for basal activity, second messenger inhibition, and LPA stimulation of NHE3 in mouse distal ileum. Am J Physiol Cell Physiol. 2011;301:C126–C136. doi: 10.1152/ajpcell.00311.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cha B, Donowitz M. The epithelial brush border Na+/H+ exchanger NHE3 associates with the actin cytoskeleton by binding to ezrin directly and via PDZ domain-containing Na+/H+ exchanger regulatory factor (NHERF) proteins. Clin Exp Pharmacol Physiol. 2008;35:863–871. doi: 10.1111/j.1440-1681.2008.04931.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.