Abstract
Introduction
Schistosomiasis (SCH) and soil transmitted helminthiases (STH) have been historically recognized as a major public health problem in Angola. However, lack of reliable, country wide prevalence data on these diseases has been a major hurdle to plan and implement programme actions to target these diseases. This study aimed to characterize SCH and STH prevalence and distribution in Angola.
Methods
A country wide mapping was conducted in October 2018 (1 province) and from July to December 2019 (14 provinces) in school aged (SAC) children in 15 (of 18) provinces in Angola, using WHO protocols and procedures. A total of 640 schools and an average of 50 students per school (N = 31,938 children) were sampled. Stool and urine samples were collected and processed using the Kato-Katz method and Urine Filtration. Prevalence estimates for SCH and STH infections were calculated for each province and district with 95% confidence intervals. Factors associated with SCH and STH infection, respectively, were explored using multivariable logistic regression accounting for clustering by school.
Results
Of the 131 districts surveyed, 112 (85.5%) are endemic for STH, 30 (22.9%) have a prevalence above 50%, 24 (18.3%) are at moderate risk (prevalence 20%-50%), and 58 (44.3%) are at low risk (<20% prevalence); similarly, 118 (90,1%) of surveyed districts are endemic for any SCH, 2 (1.5%) are at high risk (>50% prevalence), 59 (45.0%) are at moderate risk (10%-50% prevalence), and 57 (43.5%) are at low risk (<10% prevalence). There were higher STH infection rates in the northern provinces of Malanje and Lunda Norte, and higher SCH infection rates in the southern provinces of Benguela and Huila.
Conclusions
This mapping exercise provides essential information to Ministry of Health in Angola to accurately plan and implement SCH and STH control activities in the upcoming years. Data also provides a useful baseline contribution for Angola to track its progress towards the 2030 NTD roadmap targets set by WHO.
Author summary
Neglected Tropical Diseases (NTD) still affect nearly 1 billion people worldwide and are a major public health problem in Angola. Schistosomiasis (SCH) and soil transmitted Helminthiases (STH) affect disproportionally school aged children (SAC). In endemic areas, implementation of preventive chemoprevention through school-based Mass Drug Administration Campaigns is a key strategy used to reduce the burden of these infections. Mapping of schistosomiasis and soil transmitted helminthiases is essential to know where transmission occurs and is used to inform interventions planning. A country wide SCH and STH mapping was conducted across 15 of the 18 provinces of Angola. Parasitological analysis of nearly 32,000 children was conducted to detect SCH and STH infections and determine the prevalence of these diseases. Eighty Six percent of the mapped districts are endemic for STH and 22.9% have a prevalence above 50%. Similarly, 90% of surveyed districts are endemic for SCH. There were higher STH infection rates in the northern provinces of Malanje and Lunda Norte, and higher SCH infection rates in the southern provinces of Benguela and Huila. These results are of vital importance to map the prevalence of SCH and STH in Angola and to plan adequate interventions that support NTD control across the country.
Introduction
Neglected Tropical Diseases (NTD) are a group of poverty-related diseases, which are often chronic conditions impact nearly 1 billion an individual’s social and economic contributions worldwide [1]. Soil-transmitted helminthiases (STH) and schistosomiasis (SCH) can be controlled through interventions including preventive chemotherapy (often carried out as mass drug administration (MDA)) with impact and reinfection dependant on several factors such as the frequency, delivery and coverage of the campaigns, water and sanitation conditions, water contact patterns and sociodemographic factors [2–6].
Since the 2012 London Declaration on NTD, a global effort to eliminate NTDs was set and now reinforced with World Health Organization’s (WHO) road map for neglected tropical diseases 2021–2030 [1]. Regular treatment, adequate monitoring and evaluation are deemed as critical to achieve 2030 targets. In this context, mapping of SCH and STH is essential to know where transmission occurs and inform targeted interventions [7].
In Angola, STH and SCH are a recognized public health problem [8], with approximately 5–10% of the population in need of preventive chemotherapy for both diseases [1]. There is little published information on the distribution of these diseases and existing data are either outdated or covering limited geographical areas [9,10]. Passive data collection carried out in the mid 1900’s showed increasing Schistosoma haematobium cases reported in a number of provinces [11,12] and a survey in Bié confirmed that more than half of the population had urogenital SCH [13]. Subsequent surveys confirmed the existence of high prevalence rates of S. haematobium in both Malange [14] and in Huila provinces [15–17]. Additional mapping work carried out in Benguela, and Luanda found 93% of SAC to be infected with S. haematobium [18]. From 1980’s to 2000, the burden of SCH in Angola has been solely based on these estimations [19–21].
In contrast, for STH, surveys carried out in the 1950s in Zaire, Malange and Benguela estimated that up to 90% of the population was infected with Hookworm [11,14]. In Cuando Cubango, Huila and Kwanza Norte provinces, STH prevalence ranged from 65%-96% with Hookworm prevalence ranging from 40%-85% [15,22,23]. The first STH nationwide mapping conducted in 1964 registering prevalence around 75% in provinces like Huambo, Uige and Zaire [24]. Later surveys carried out in Bie found 86% of children had co-infections of 2 or more helminths [25]. While these referenced studies have shown a the presence of SCH and STH across Angola, their size and scope could not provide reliable prevalence estimates at district level, highlighting the need to conduct a country wide mapping.
In 2005, a STH and SCH mapping was carried out by the Angolan Ministry of Health (MoH) supported by the United Nations Children’s Fund (UNICEF) and WHO based on ecological regions. Results showed estimated nationwide urogenital SCH prevalence in Angola was 28%, with higher incidence in the southern (40,6%), central plateau (39,6%) and northern provinces [26]. STH was reported at 40% prevalence across the country with Ascaris (25,0%) and Hookworm (9,8%) reported as dominant [26]. Data from northern and central Angola confirms the pattern of predominance of Ascaris in SAC but also highlights the burden of Trichuris infection [9,27,28].
In 2014, a baseline mapping of SCH and STH infections was conducted through a collaboration between the Angolan Ministry of Health (MoH) The MENTOR Initiative (MENTOR) and the End Fund in the provinces of Uige, Zaire and Huambo. The mapping measures SCH and STH prevalence at district level as recommended by WHO confirming the high burden of STH, particularly in Uige but also moderate prevalence of SCH infections (from 10%-15%) with significant variations between districts within each province [29].
Since 2014, preventive chemotherapy interventions have been implemented firstly in Huambo, Uige and Zaire provinces followed by Cuanza Sul, Bié and Cuando Cubango in 2017, and Bengo in 2019. Recognizing the need to arrange intervention based on reliable mapping data, the MoH, supported by World Health Organization, led a country wide SCH and STH mapping exercise in October 2018 (1 province) and from July to December 2019 (14 provinces). The objective of this study was to quantify the prevalence and distribution of these diseases across fifteen provinces in Angola in order to be able to adequately plan preventive chemotherapy interventions. A secondary aim of this project was to explore whether the presence of a latrine or water source in the school reduced the risk of infection.
Methods
Ethics statement
This mapping was approved by the Angola Ministry of Health Ethics Committee (Approval number 27/2018) in June 2018. Informed Consent was sought from participants parents. The team liaised with School directors prior to the survey to ensure parents were aware of the benefits and harms of participating in the survey. Parents were provided with an explanatory information sheet and a consent form to take home to decide. Parents were asked to send their children to school with the signed form if they agreed.
All students participated in the mapping voluntarily. Children were briefed on the objectives of the mapping and only took part if they verbally assented to participate.
All data was kept anonymous. No personal information was collected as children were identified through a unique identifier number. All children enrolled were treated with a dose of Albendazole and Praziquantel according to their height.
Study design
A cross sectional survey using the standard WHO method for mapping [30] was implemented in October 2018 (Bengo province). Thereafter, from July to December 2019 in the remaining fourteen Angolan provinces not yet mapped for STH and SCH (Benguela, Bié, Cabinda, Cunene, Cuando Cubango, Cuanza Norte, Cuanza Sul, Lunda Norte, Lunda Sul, Malanje, Moxico, Huíla, Namibe and Luanda). A total of 131 districts were mapped. Parasitological examinations, knowledge attitudes and practices questionnaires were implemented in 640 public schools.
School selection procedures
An average of five schools per district were selected but the number of schools selected by district was determined according to population data and the geographical area to be covered. The total number of schools per district ranged from 1 (in unpopulated areas and/or in highly concentrated urban areas) to 13 (in large geographical areas).
School selection was done in two stages: in the first instance, simple random sampling was conducted to select a defined number of schools per district. Then, the list was assessed with direction from local authorities to verify the geographical spread of schools selected across the district. Schools selected close to one another were purposively replaced by schools in locations known to be in areas of increased risk for SCH transmission. The proximity to fresh water sources (river, lakes, lagoons or swamp areas) were considered for this exercise to ensure oversampling in these specific areas, as these provide the ideal ecological conditions for SCH transmission and are recommended as areas where mapping should be conducted30.
Study participants
Fifty children per school, 25 males and 25 females aged between 10–14 years old were invited to participate the day before the survey. School directors were asked to provide a list of all students to ensure systematic random selection.
For inclusion, only school-aged children resident in the study area for at least 2 years were considered to participate. Informed consent was requested of the child’s parents the day before and only those carrying signed informed consent were included in the study.
Children who had taken any antiparasitic drug in the previous 6 months (particularly Albendazole, Mebendazole, Praziquantel, Ivermectin) were not included in the survey.
Parasitological diagnosis
All children were provided with two plastic pots and requested to provide fresh stool and a urine sample. Kato Katz technique was used for analysing stool samples and urine filtration was used for the analysis of urine.
Kato Katz is a WHO reference technique for detecting and determining infection intensity for STH and Schistosoma mansoni allowing identification and quantification of these parasites [31] Microscopy using the Kato Katz technique requires fresh stool specimens, therefore analysis of specimens was conducted on site. The technique consisted of a microscopic examination of a sample of stool to examine the number of eggs in the faeces. All samples were collected, processed, and examined on the same day. All eggs were counted within one hour of preparing the slides. A single slide per student was prepared and reading was done once in the day of the collection as recommended for operational mapping30.
Urine filtration microscopy is the WHO standard technique for evaluating Schistosoma haematobium infection. A microscopic examination of a filter was used to collect the eggs of S. haematobium from 10 ml of urine. Macro-haematuria was visually inspected prior to the microscopic analysis of each sample.
Data collection and management (ESPEN Collect)
Data was collected though standard questionnaires using the ESPEN collect tool. The tool was developed by the Expanded Special Project for the Elimination of Neglected Tropical diseases (ESPEN) to allow collection of survey data and inform school and administrative level prevalence in real time. The tool was shaped to integrate key parameters under assessment and adjusted to Angola’s geographical regions by adding administrative boundaries to the mapping modules. ESPEN collect also allowed the generation of non-identifiable unique identifiers for every single child providing a useful resource to link parasitological data with school conditions and Knowledge, Attitudes and Practices (KAP) data.
The ESPEN Collect had four main questionnaires that were filled by a dedicated data manager in each team who was responsible to input all information of the survey.
Questionnaire 1. School Information sheet–This questionnaire collected information about school population (number of Students/teachers); Water source availability in the school and type of source to have water in the school; existence of freshwater bodies around the school; Presence and type of sanitation structure in the school; Presence and type of handwashing station in the school;
Questionnaire 2: Kato Katz sheet: This form provided collected individual data per student on the number of eggs counted of each species found in the slide (S. mansosi, Ascaris Lumbricoides, Trichuris Thricuria, Hookworm and others)
Questionnaire 3: Urine Filtration sheet: Also provided individual data per student about Macroscopic looking of the sample; Volume of Urine filtered and Number of S. haematobium eggs.
Questionnaire 4: Students’ hygiene and risk behaviours: This questionnaire was used to all enrolled children and collected information about children gender, age, and hygiene behaviours such as usual place of defecation and freshwater bodies regular contact.
School location details were recorded in all questionnaires alongside. A single student identification code was generated for each participant that was used in all forms. This unique identified was used to merge the four datasets generated and analyse data gathered.
Statistical analysis
Data were merged, cleaned, and analysed using Stata version 16 (College Station, TX: StataCorp LLC.). Prevalence (percentage and 95% confidence interval (CI)) and intensity of each infection (based on specified WHO thresholds30), any STH and any SCH infection were calculated based on the presence of eggs present in stool or urine samples, as appropriate, and presented by province and district (S1 and S2 Files).
STH and SCH risk for each district was determined using the calculated prevalence and based on the specified WHO thresholds [31]. The risk was mapped on a geographical map of Angola using ArcGIS version 10.3 (ESRI, Inc., Redlands, USA).
Finally, logistic regression using robust standard errors which accounted for clustering by school was used to explore whether the presence of a latrine or water source in the school was associated with STH or SCH infection, respectively. Models were adjusted for demographic factors including age, sex and province.
Results
Over the survey period, 31,938 children were sampled from 640 schools across 131 districts in Angola and covered 15 of the 18 provinces across the country. Children were aged between 10 and 14 with a median age of 12 (interquartile range: 11–13) (Table 1).
Table 1. Geographical Distribution of sampled schools and children and key characteristics of the sample.
| Province | Children | Schools | Male | Female |
|---|---|---|---|---|
| 31938 | 640 | 15966 | 15972 | |
| Bengo | 1549 (4.9%) | 31 (4.8%) | 780 (50.4%) | 769 (49.6%) |
| Benguela | 2150 (6.7%) | 43 (6.7%) | 1082 (50.3%) | 1068 (49.7%) |
| Bie | 2373 (7.4%) | 48 (7.5%) | 1192 (50.2%) | 1181 (49.8%) |
| Cabinda | 1150 (3.6%) | 23 (3.6%) | 575 (50.0%) | 575 (50.0%) |
| Cuando Cubango | 1850 (5.8%) | 37 (5.8%) | 925 (50.0%) | 925 (50.0%) |
| Cunene | 2100 (6.6%) | 42 (6.6%) | 1050 (50.0%) | 1050 (50.0%) |
| Huila | 3650 (11.4%) | 73 (11.4%) | 1826 (50.0%) | 1824 (50.0%) |
| Kwanza Norte | 2372 (7.4%) | 48 (7.5%) | 1195 (50.4%) | 1177 (49.6%) |
| Kwanza Sul | 2750 (8.6%) | 55 (8.6%) | 1377 (50.1%) | 1373 (49.9%) |
| Luanda | 1300 (4.1%) | 26 (4.1%) | 648 (49.8%) | 652 (50.2%) |
| Lunda Norte | 2450 (7.7%) | 49 (7.7%) | 1226 (50.0%) | 1224 (50.0%) |
| Lunda Sul | 1544 (4.8%) | 31 (4.8%) | 745 (48.3%) | 799 (51.7%) |
| Malanje | 2900 (9.1%) | 58 (9.1%) | 1449 (50.0%) | 1451 (50.0%) |
| Moxico | 2800 (8.8%) | 56 (8.8%) | 1396 (49.9%) | 1404 (50.1%) |
| Namibe | 1000 (3.1%) | 20 (3.1%) | 500 (50.0%) | 500 (50.0%) |
Prevalence of SCH infection
Overall, the sampled prevalence of any SCH infection amongst SAC children was 13.2% [95% CI: 12.8–13.5] with S. haematobium being the most prevalent species (12.6% [95% CI: 12.2–12.9]) compared to S. mansoni (0.9% [95% CI: 0.8–1.0]). The prevalence of any SCH infection was highest in Huila (32.3%), followed by Benguela (19.3%), Malanje (18.3%), and lowest in Lunda Sul (1.8%). Although S. haematobium was prevalent across all 15 sampled provinces, S. mansoni was only prevalent across 10 provinces. District specific prevalence with respective gender disaggregation can be found in S1 File.
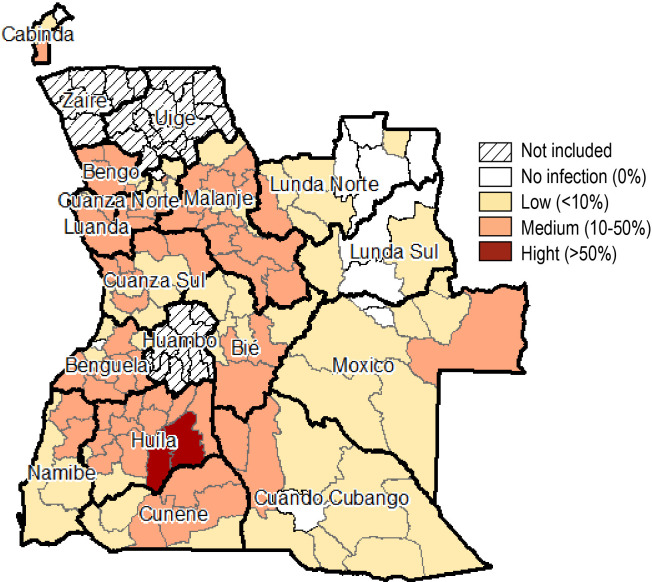
When calculating risk of SCH infection, only two districts had a high risk (>50% prevalence) of SCH infection, 59 with a medium risk of SCH infection (10–50% prevalence) and 57 with a low risk of SCH infection (<10% prevalence). No SCH infections were reported from nine districts (Fig 1).
Fig 1. Schistosomiasis risk (derived from Schistosomiasis prevalence) across 15 provinces of Angola (2018/2019).
(Source: Ministry of Health Angola).
Prevalence of STH infection
The prevalence of any STH infection was 24.1% [95% CI: 23.7–24.6] with A. lumbricoides being the most prevalent species (19.0% [95% CI: 18.6–19.5]) followed by Hookworms (5.8% [85% CI: 5.6–6.1]). T. trichiura (1.6% [95% CI: 1.4–1.7]) was the least prevalent species. The prevalence of any STH infection was highest in Kwanda Norte (69.7%), followed by Malanje (55.9%), Lunda Norte (50.5%), and lowest in Namibe (0.8%). Five provinces (Bengo, Kwanza Norte, Lunda Norte, Lunda Sul and Malanje) had a prevalence of >20% (moderate risk) of A. lumbricoides. Only two provinces (Malanje and Moxico) had a moderate risk of Hookworm infection. All provinces had a low risk (<20% prevalence) of T. trichiura. District specific prevalence with respective gender disaggregation can be found in S2 File.
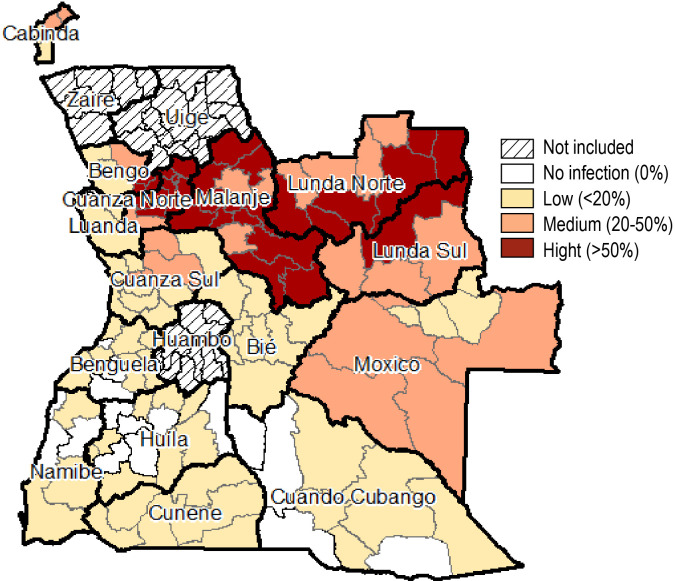
When calculating risk of STH infection by district, 30 districts had a high risk (>50% prevalence), 24 with a medium risk (20–50% prevalence) and 58 with a low risk (<20% prevalence) of STH infection. No STH infections were reported in 15 districts across the country (Fig 2).
Fig 2. Soil Transmitted Helminthiasis risk (derived from STH prevalence) across 15 provinces of Angola (2018/2019).
(Source: Ministry of Health Angola).
School data
School questionnaires were available for 639 schools (no data was recorded for one school in Namibe province), and therefore included in the following analyses. Only 30% (189/639) of schools reported having a water source in the school. For the majority of these schools, the water source was a protected fountain (26.5%; 50/189) or tap water (25.4%; 48/189) (S3 File).
Approximately 60% (385/639) of schools reported having latrine in the school grounds, with 37.7% (145/385) being a paved latrine, 26.8% (103/385) a non-paved latrine, 21.8% (84/385) a latrine with a flush and 12.7% (49/385) a ventilated improved pit (VIP) latrine. However, the majority (73.0%; 281/385) of schools with an available latrine reported never having water or toilet paper to use after using a latrine.
Student behaviour questionnaire
Analysis showed a large proportion of missing data, discrepancies and conflicting answers. For that reason, these results are presented in S4 File but were not considered robust enough to be included in further analysis.
Infection risk associated with latrine/water source in school
There was no association between the presence of a latrine (odds ratio (OR): 1.21 [95% confidence interval: 0.90, 1.62]) or water source (OR: 0.90 [0.67, 1.19]) in the school and SCH infection. This remained true in a multivariable logistic model (Table 2).
Table 2. Logistic regression assessing the association between presence of latrine or water source in the school and SCH and/or STH infection adjusted for age, sex and province.
| Univariable | Multivariable | ||||
|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| SCH infection | |||||
| Latrine in school | No | 1 | 0.21 | 1 | 0.72 |
| Yes | 1.21 (0.90, 1.62) | 1.04 (0.83, 1.31) | |||
| Water source in school | No | 1 | 0.45 | 1 | 0.33 |
| Yes | 0.90 (0.67, 1.19) | 0.88 (0.68, 1.14) | |||
| STH infection | |||||
| Latrine in school | No | 1 | 0.005 | 1 | 0.72 |
| Yes | 0.69 (0.54, 0.89) | 1.04 (0.83, 1.31) | |||
| Water source in school | No | 1 | 0.05 | 1 | 0.33 |
| Yes | 0.75 (0.57, 0.99) | 0.88 (0.68, 1.14) | |||
Although there seemed to be a reduced risk of STH infection with the presence of a latrine (OR: 0.69 [0.54, 0.89]) and water source (OR: 0.75 [0.57, 0.99]) in the school, these associations did not remain after adjustment for age, sex and province (Table 2).
Discussion
This SCH and STH mapping effort constitutes a landmark for NTD control in Angola. This has been the first country wide mapping exercise that sampled and collected SAC children data across several provinces to estimate the prevalence of these diseases. Despite the mapping exercises conducted in 2005 [26] and later in 2014 [29] and 2021 [9], this is the first mapping at country level that consistently follows WHO guidance for SCH and STH mapping in Africa [30].
Geographical distribution of SCH is consistent with historical data identifying high prevalence of this disease across Bié, Huila, Benguela, Bengo and Luanda provinces [9,10,13–18]. This is also in line with the 2005 mapping data that identified a higher prevalence of SCH across the central plateau [26]. S. haematobium prevalence is consistently higher than S. mansoni across these areas which is a consequence of the focal nature of SCH transmission, its association with human contact with infested water and the existence of a specific intermediate snail host [32].
Higher prevalence of STH was found in the northern provinces of Kwanza Norte, Malanje, Lunda Norte and Lunda Sul, with some schools mapped noting 100% of SAC infected with at least one STH. Ascaris has been the main infection found across these provinces, but Hookworm was frequently identified across Lunda Sul, Moxico and in Malanje, where there is a historical record of the disease [11,14]. These findings highlight the need to tailor communication interventions in these areas, particularly in impoverished rural areas where children tend to walk barefoot, a known risk factor for hookworm infection [33,34].
Less than a third of schools reported to have a water source in the school perimeter. Of these, only half had safe water source (protected fountain or tap water). Such results are in line with existing data about access to basic service water in Angola [35–37]. Similarly, sanitation information from schools mapped is aligned with existing information about sanitation access in schools in Angola [37]. These findings raise the need to improve water and sanitation conditions for SAC across the country. The low proportion of schools with sanitation equipment that had water or toilet paper to use after using a latrine suggests the need to invest in better sanitation equipment to ensure handwashing post defecation. Since poor WASH conditions are associated with increased risk of both SCH and STH transmission [38,39], it is essential to look at NTD control as an integrated approach that includes improvements of water and sanitation access and conditions in schools.
4041–44When controlling for age, sex and province, the presence of water and latrine in school was not associated with STH nor with SCH infections. This may be related to the absence of water and sanitation conditions in communities where children live. Children may have access to these conditions in schools but have limited or no access to adequate water and sanitation at home, a problem that has been previously identified [40]. Another major contributor may be the limited use of existing sanitation structures in schools [37]. Unfortunately, due to the high proportion of missing behavioural data, it was not possible to accurately assess these. But, when looking at the proportion of children reporting to urinate or defecate in school latrines, approximately only a fifth of children report to do so. WASH in school is essential to provide essential infrastructure, to foster its use and the adoption of healthy sanitation behaviours. This is not new as the requirement for investments in WASH in schools in Angola have been raised historically by UNICEF [37].
Results obtained by this mapping are essential to adequately plan MDA campaigns and ensure geographical areas are targeted based on the need. The Angola NTD Masterplan 2021–2025 [41] (final version awaiting ministerial approval) integrates this mapping data and projects its long-term results in line with WHO roadmap [1]. However, the main identified risk to attain those results is linked to the chronical lack of support to NTD activities in Angola. So far, financial support to conduct MDA has been provided by WHO and The MENTOR Initiative (through an End Fund Grant). MDA activities have been implemented across seven provinces with gradual appropriation of activities from several district and provincial health and education authorities over time. Nevertheless, mapping results “demand” the rapid scale up of MDA to several districts. For STH, 30 districts should be targeted bi-annually and 24 targeted annually corresponding to an estimated total of 2.650.000 SAC treated annually. For SCH, 2 districts should be targeted annually and 65 targeted at least once in 5 years, in an estimated total of 2.950.000 SAC children to be treated (Table 3). These treatment efforts need to be integrated with community level MDA, particularly where overlap of treatment with Albendazole is foreseen. However, as lymphatic filariasis mapping data is outdated, it is hard to accurately integrated these strategies.
Table 3. Number of districts in each province to be targeted for MDA according to mapped risk.
| Província | N | SCH | STH | ||||
|---|---|---|---|---|---|---|---|
| Low | Medium | High | Low | Medium | High | ||
| Bengo | 6 | 2 (33.3%) | 4 (66.7%) | - | 2 (33.3%) | 1 (16.7%) | 3 (50.0%) |
| Benguela | 10 | 3 (30.0%) | 6 (60.0%) | - | 7 (70.0%) | - | - |
| Bie | 9 | 6 (66.7%) | 3 (33.3%) | - | 9 (100.0%) | - | - |
| Cabinda | 4 | 2 (50.0%) | 1 (25.0%) | - | 2 (50.0%) | 2 (50.0%) | - |
| Cuando Cubango | 9 | 6 (66.7%) | 2 (22.2%) | - | 5 (55.6%) | - | - |
| Cunene | 6 | 3 (50.0%) | 3 (50.0%) | - | 6 (100.0%) | - | - |
| Huila | 14 | 2 (14.3%) | 10 (71.4%) | 2 (14.3%) | 8 (57.1%) | - | - |
| Kwanza Norte | 10 | 4 (40.0%) | 5 (50.0%) | - | - | 1 (10.0%) | 9 (90.0%) |
| Kwanza Sul | 12 | 5 (41.7%) | 7 (58.3%) | - | 8 (66.7%) | 4 (33.3%) | - |
| Luanda | 9 | 5 (55.6%) | 3 (33.3%) | - | 4 (44.4%) | 1 (11.1%) | - |
| Lunda Norte | 10 | 5 (50.0%) | 1 (10.0%) | - | - | 4 (40.0%) | 6 (60.0%) |
| Lunda Sul | 4 | 2 (50.0%) | - | - | - | 3 (75.0%) | 1 (25.0%) |
| Malanje | 14 | 3 (21.4%) | 11 (78.6%) | - | - | 3 (21.4%) | 11 (78.6%) |
| Moxico | 9 | 6 (66.7%) | 1 (11.1%) | - | 4 (44.4%) | 5 (55.6%) | - |
| Namibe | 5 | 3 (60.0%) | 2 (40.0%) | - | 3 (60.0%) | - | - |
One of the major limitations of this study is related to the sampling procedures and its impact on calculating SCH prevalence at sub district level. Sampling procedures were used to estimate prevalence at district level. Due to the focal nature of SCH, WHO recommends sub district estimates for SCH prevalence [42] and produced a tool to extrapolate prevalence at the sub-district level out of district level results. Such tool has also been adopted in Angola to refine mapping results. Another operational limitation of this was the non-inclusion of three provinces: Huambo, Uige and Zaire. These provinces have been mapped in 2014 and MDA have been implemented since. Considering that mapping should occur after 5 years of MDA, it was agreed that an impact assessment would take place in 2020 (delayed to 2021 due to the COVID-19 pandemic).
The use of single stool and urine samples for estimating the prevalence is also a major limitation to interpret the findings. The Kato Katz technique has recognized accuracy limitations particularly for low intensity infections. Therefore, it would have been preferable to collect several samples over time to improve accuracy [43]. However, considering the scope and size of the mapping needed to characterize STH and SCH prevalence in Angola and the available resources, such methods would have been unfeasible to implement across the country.
The inability to explore individual hygiene behaviours constitute a major challenge in order to fully understand STH and SCH risk behaviours of SAC in Angola. Nevertheless, this descriptive analysis, exploring the presence of a latrine or water source in the school provides important insights about key regions that have the highest risk of contracting SCH and STH. These should be targeted through information campaigns aiming to reduce well identified risk behaviours such as poor handwashing practices, open defecation, walking barefoot and bathing in rivers, dams, or lakes.
Since egg counting procedures and recording were not consistently done across all provinces, it was not possible to compute infection intensities. This poses a limitation to follow up the impact of NTD control interventions on infection intensity over time. In addition, it was not possible to map pre-SAC or other risk groups which constitutes a limitation to understand better transmission patterns across the whole Angolan population, including the role of adults in transmission of STH and ACH. Previous studies identified pregnant women to be at risk of infection in Angola [9]. Future research in Angola should consider the inclusion of adults that are not generally targeted in SCH and STH control interventions.
Conclusions
This first ever STH and SCH mapping in Angola achieved it main objective of quantifying the prevalence and distribution of these infections across the country. Results are of vital importance to map the prevalence and geographical distribution of these diseases and plan adequate interventions that support NTD control in Angola and contribute to WHO 2030 defined NTD control targets. Water and sanitation conditions in schools across Angola are still scarce and may be a significant factor contributing for the high endemicity of some NTD in Angola.
Supporting information
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
The authors would like to thank all lab workers and data managers who significantly contributed to ensure the implementation of field work. Likewise, to all logistic staff involved (drivers, mobilizers) who supported daily field activities. The authors also acknowledge all the Provincial and Municipal authorities support to the implementation of this mapping and, to Education and Health departments at provincial and district level who facilitated all contacts and, in the vast majority of the cases provided housing for field teams in remote settings. A major appreciation should also be given to all School Directors and teachers that contributed in a very positive way to make this mapping happen as they played a major role in mobilizing parents and children to enrol in the mapping. Finally, the authors would like to thank all parents and children engaged in the mapping for their vital contribution to better understand neglected tropical diseases in Angola.
Data Availability
Data cannot be shared publicly as it is MoH Angola data. Data are available from the MoH Institutional Data Access via geral@inis.ao or visit https://www.inis.ao/index.php/contactos.
Funding Statement
This mapping was funded by ESPEN/WHO (https://espen.afro.who.int/). The study was also partly funded by The END Fund under the current NTD country support program under implementation by The MENTOR Initiative. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization W. Ending the neglect to attain the Sustainable Development Goals: a road map for neglected tropical diseases 2021–2030. Geneva; 2020.
- 2.Kura K, Hardwick RJ, Truscott JE, Toor J, Hollingsworth TD, Anderson RM. The impact of mass drug administration on Schistosoma haematobium infection: what is required to achieve morbidity control and elimination? Parasites and Vectors. 2020;13: 554. doi: 10.1186/s13071-020-04409-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King CH, Kittur N, Binder S, Campbell CH, N’Goran EK, Meite A, et al. Impact of different mass drug administration strategies for gaining and sustaining control of Schistosoma mansoni and Schistosoma haematobium infection in Africa. American Journal of Tropical Medicine and Hygiene. 2020;103: 14–23. doi: 10.4269/ajtmh.19-0829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bronzan RN, Dorkenoo AM, Agbo YM, Halatoko W, Layibo Y, Adjeloh P, et al. Impact of community-based integrated mass drug administration on schistosomiasis and soil-transmitted helminth prevalence in Togo. Steinmann P, editor. PLOS Neglected Tropical Diseases. 2018;12: e0006551. doi: 10.1371/journal.pntd.0006551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunn JC, Bettis AA, Wyine NY, Lwin AMM, Tun A, Maung NS, et al. Soil-transmitted helminth reinfection four and six months after mass drug administration: Results from the delta region of Myanmar. PLoS Neglected Tropical Diseases. 2019;13. doi: 10.1371/journal.pntd.0006591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ortu G, Assoum M, Wittmann U, Knowles S, Clements M, Ndayishimiye O, et al. The impact of an 8-year mass drug administration programme on prevalence, intensity and co-infections of soil-transmitted helminthiases in Burundi. Parasites and Vectors. 2016;9: 1–17. doi: 10.1186/s13071-016-1794-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gabrielli AF, Montresor A, Chitsulo L, Engels D, Savioli L. Preventive chemotherapy in human helminthiasis: Theoretical and operational aspects. Trans R Soc Trop Med Hyg. 2011;105: 683–693. doi: 10.1016/j.trstmh.2011.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ministerio da Saude. Plano Nacional de Desenvolvimento Sanitário 2012–2025. Luanda; 2012.
- 9.Sousa-Figueiredo JC, Gamboa D, Pedro JM, Fançony C, Langa AJ, Soares Magalhães RJ, et al. Epidemiology of Malaria, Schistosomiasis, Geohelminths, Anemia and Malnutrition in the Context of a Demographic Surveillance System in Northern Angola. Noor AM, editor. PLoS ONE. 2012;7: e33189. doi: 10.1371/journal.pone.0033189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Figueiredo J. Contribuição para o estudo da epidemiologia e morbilidade da Schistosomose vesical na população adulta de Angola nas Províncias de Luanda, Bengo e Kwanza Sul. Available: https://run.unl.pt/bitstream/10362/52375/1/Tese_Mestrado_Parasitologia_Médica.pdf
- 11.Ferreira E. Distribuiçao e incidência de algumas endemias de Angola. An Inst Med Trop. 1953;10: 1739–1775. [Google Scholar]
- 12.Janz G, Morais de Carvalho A. Subsidios para o conhecimento das bilharzioses em Angola. An Inst Med Trop December de 1956, Vol 13, 4, pp 597–612. 1956;13: 597–612. [PubMed] [Google Scholar]
- 13.Sarmento A. Notas sobre um foco de bilharziose vesical em Angola. An Inst Med Trop. 1944;1: 375–380. [Google Scholar]
- 14.Ferreira EG, Gomes JA. Bilharziose (O Foco de Malanje). An Inst Med Trop. 1959;16: 407–432. [PubMed] [Google Scholar]
- 15.Cambournac JC, Casaca VR. Prospecção das endemias reinantes na área de Mulondo (Rio Cunene, Angola). An Inst Med Trop. 1956;13: 17–25. [PubMed] [Google Scholar]
- 16.Mesquita B. Considerações sobre a bilharziose vesical em Angola. An Inst Med Trop. 1952;9. [PubMed] [Google Scholar]
- 17.Carvalho RG. Bilharziose nos Ganguelas. An Inst Med Trop. 1959;16: 433–436. [PubMed] [Google Scholar]
- 18.Grácio MA. Contribuição para o conhecimento da incidencia da Bilharziose vesical no distrito de Benguela—I. An Inst Med Trop. 1977;5: 281–284. [PubMed] [Google Scholar]
- 19.Utroska JA, Chen MG, Dixon H, Yoon S-Y, Helling-Borda M, Hogerzeil H v, et al. An estimate of global needs for praziquantel within schistosomiasis control programmes / by Utroska J. A.… [et al.]. World Health Organization; 1990. p. PC. [Google Scholar]
- 20.Iarotski LS, Davis A. The schistosomiasis problem in the world: results of a WHO questionnaire survey. Bull World Health Organ. 1981;59: 115–127. [PMC free article] [PubMed] [Google Scholar]
- 21.Chitsulo L, Engels D, Montresor A, Savioli L. The global status of schistosomiasis and its control. Acta Tropica. 2000;77: 41–51. doi: 10.1016/s0001-706x(00)00122-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cambournac JC, Gândara AF, Casaca VR. Prospecção das endemias reinantes na área de Capelongo (Rio Cunene, Angola). An Inst Med Trop. 1956;13. [PubMed] [Google Scholar]
- 23.Cambournac FJ, Gândara AF, Pena AJ. Inquérito sobre bilharziose vesical e parasitoses intestinais nas áreas administrativas de Cuchi, Menongue e Longa (Angola). An Inst Med Trop. 1955;12: 549–574. [PubMed] [Google Scholar]
- 24.Janz GJ. Distribuição das parasitoses intestinais em Angola. An Inst Med Trop. 1964;21: 74–122. [PubMed] [Google Scholar]
- 25.Morais D de, Gouveia A, da Rosa J. Subsidios para o conhecimento medico e antropologico do povo undulo. An Inst Med Trop. 1975;3: 143–256. [PubMed] [Google Scholar]
- 26.Ministério da Saúde, UNICEF, WHO. Baseline survey for helmint control in school-aged children in Angola. Luanda; 2005.
- 27.Tomlinson M, Adams V, Chopra M, Jooste P, Strydom E, Dhansay A. Survey of iodine deficiency and intestinal parasitic infections in school-going children: Bie Province, Angola. Public Health Nutrition. Cambridge University Press; 2010. pp. 1314–1318. doi: 10.1017/S1368980010000510 [DOI] [PubMed] [Google Scholar]
- 28.Soares Magalhães RJ, Langa A, Pedro JM, Sousa-Figueiredo JC, Clements ACA, Vaz Nery S. Role of malnutrition and parasite infections in the spatial variation in children’s anaemia risk in northern Angola. Geospat Health. 2013;7: 341. doi: 10.4081/gh.2013.91 [DOI] [PubMed] [Google Scholar]
- 29.Sousa-Figueiredo J, The MENTOR Initiative, Ministry of Health Angola. Mapping of Schistosomiasis and Helminths Transmitted by Soil: Uige, Huambo and Zaire provinces. 2014.
- 30.World Health Organization Regional Office for Africa. Guide for Mapping Neglected Tropical Diseases Amenable to Preventive Chemotherapy in the African Region. 2018.
- 31.World Health Organization W. Deworming school-age children Helminth control in school-age children Second edition A guide for managers of control programmes. 2011. Available: http://www.who.int/neglected_diseases/en [Google Scholar]
- 32.CLENNON JA, MUNGAI PL, MUCHIRI EM, KING CH, KITRON U. SPATIAL AND TEMPORAL VARIATIONS IN LOCAL TRANSMISSION OF SCHISTOSOMA HAEMATOBIUM IN MSAMBWENI, KENYA. The American Journal of Tropical Medicine and Hygiene. 2006;75: 1034–1041. doi: 10.4269/AJTMH.2006.75.1034 [DOI] [PubMed] [Google Scholar]
- 33.Jiraanankul V, Aphijirawat W, Mungthin M, Khositnithikul R, Rangsin R, Traub RJ, et al. Incidence and Risk Factors of Hookworm Infection in a Rural Community of Central Thailand. The American Journal of Tropical Medicine and Hygiene. 2011;84: 594–598. doi: 10.4269/ajtmh.2011.10-0189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Errea RA, Vasquez-Rios G, Calderon ML, Siu D, Duque KR, Juarez LH, et al. Soil-Transmitted Helminthiasis in Children from a Rural Community Taking Part in a Periodic Deworming Program in the Peruvian Amazon. The American Journal of Tropical Medicine and Hygiene. 2019;101: 636–640. doi: 10.4269/ajtmh.18-1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Country (or area) | SDG 6 Data. [cited 16 Jul 2021]. Available: https://www.sdg6data.org/country-or-area/Angola
- 36.People using at least basic sanitation services, rural (% of rural population)—Angola | Data. [cited 16 Jul 2021]. Available: https://data.worldbank.org/indicator/SH.STA.BASS.RU.ZS?locations=AO
- 37.Angola U. WASH in Schools in Angola Diagnosis of water and sanitation conditions of 600 schools in 6 provinces of Angola. 2016. [cited 16 Jul 2021]. Available: www.unicef.org [Google Scholar]
- 38.Grimes JE, Croll D, Harrison WE, Utzinger J, Freeman MC, Templeton MR. The roles of water, sanitation and hygiene in reducing schistosomiasis: a review. Parasites & Vectors 2015 8:1. 2015;8: 1–16. doi: 10.1186/s13071-015-0766-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strunz EC, Addiss DG, Stocks ME, Ogden S, Utzinger J, Freeman MC. Water, Sanitation, Hygiene, and Soil-Transmitted Helminth Infection: A Systematic Review and Meta-Analysis. PLOS Medicine. 2014;11: e1001620. doi: 10.1371/journal.pmed.1001620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chard AN, Garn J v, Chang HH, Clasen T, Freeman MC. Impact of a school-based water, sanitation, and hygiene intervention on school absence, diarrhea, respiratory infection, and soil-transmitted helminths: results from the WASH HELPS cluster-randomized trial. Journal of Global Health. 2019;9. doi: 10.7189/JOGH.09.020402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ministério da Saúde. Plano Estratégico Nacional de Controlo de Doenças Tropicais Negligenciadas 2021–2025 (Rascunho). Luanda; 2021.
- 42.WHO. Report of the workshop on schistosomiasis sub-district level data review for shrinking the map; better utilization of available prevalenece data and sub-district level planning for selected anglophone countries. Brazzaville; 2019 Aug. Available: https://espen.afro.who.int/system/files/content/resources/Report%20of%20the%20Workshop%20on%20SCH%20sub-district%20level%20data%20review%20for%20shrinking%20the%20map.pdf
- 43.Lamberton PH, Kabatereine NB, Oguttu DW, Fenwick A, Webster JP. Sensitivity and specificity of multiple Kato-Katz thick smears and a circulating cathodic antigen test for Schistosoma mansoni diagnosis pre- and post-repeated-praziquantel treatment. PLoS Negl Trop Dis. 2014. Sep 11;8(9):e3139. doi: 10.1371/journal.pntd.0003139 ; PMCID: PMC4161328 [DOI] [PMC free article] [PubMed] [Google Scholar]