Abstract
The objective of this experiment was to evaluate the effects of a multi-strain Bacillus subtilis-based direct-fed microbial (DFM) on nursery pig health as indicated by intestinal mucosal and blood plasma immunological markers and intestinal morphology. Eighty pigs, of equal number of barrows and gilts (initial BW: 7.0 ± 0.60 kg), weaned at 21 ± 1 d of age were randomly allotted to sixteen pens, with five pigs per pen. Two dietary treatments were implemented, a basal control (CON) and a basal control plus DFM (CDFM). Both diets were corn, soybean meal, and distillers dried grains based and were formulated to meet or exceed all nutritional requirements (NRC, 2012) and manufactured on site. Diets were fed for 42 d. On d 21 and 42 of the experiment, one pig per pen was randomly selected and euthanized, with equal number of males and females represented. Blood samples were collected prior to euthanasia for assessment of plasma concentrations of immunoglobulin A (IgA) and intestinal fatty acid binding protein. Segments of the gastrointestinal tract including duodenum, jejunum, ileum, ascending and distal colon were removed for analysis of intestinal morphology, and levels of interleukin 6, interleukin 10 (IL-10), and tumor necrosis factor alpha. Jejunal villus height was greater in the CDFM pigs as compared with CON pigs (P = 0.02) and ascending colon crypt depth tended to be greater on d 21 (P = 0.10). Compared to CON, CDFM significantly increased overall plasma IgA (P = 0.03) (0.58 vs. 0.73 0.05 mg/mL, respectively), while it tended to increase plasma IgA (P = 0.06) on d 21 (0.34 vs. 0.54 ± 0.07 mg/mL, respectively) and tended to increase overall IL-10 (P = 0.10) in the jejunum (113 vs. 195 ± 35 pg/mL, respectively). Addition of a multi-strain Bacillus subtilis-based DFM may have an early benefit to nursery pig health status, observed through specific changes in morphology and both systemic and localized immunological markers.
Keywords: direct-fed microbial, immunity, morphology, weanling pig
INTRODUCTION
The nursery phase of production is arguably the most stressful time of the market pig’s life, greatly impacting the pig’s gut health, immune status, and growth rate, all of which has been previously reviewed (Moeser et al., 2017 ; Campbell et al., 2013; Pluske, 2013). As the industry moves away from the use of antibiotic growth promoters, finding new ways to minimize the negative impact of weaning has become of great importance. Among the many researched antibiotic alternatives, direct-fed microbials (DFM) are one of great promise. Studies utilizing a DFM composed of Bacillus subtilis suggest an impact on health status through specific changes in immunological markers, intestinal morphology, or microbiota populations (Bhandari et al., 2008; Lee et al., 2014; Zhang et al., 2017). However, there is considerable genomic diversity and strain-specific capabilities within the B. subtilis species, making each single or multi-strain combination unique (Earl et al., 2008 ). A two-strain B. subtilis combinations comprised of strains isolated from intestinal epithelial scrapings of high-performing pigs showed promising performance benefits for nursery pigs, increasing gain by 5–10% and lowering feed per gain ratio up to 5% (Augspurger et al., 2016). However, the mechanisms for improved growth were not elucidated. Given the gastrointestinal origin and findings of the research mentioned above, it was hypothesized that the multi-strain combination may provide benefit through changes to specific gut-related health indicators. Therefore, the specific objective of this study was to evaluate the effect of a multi-strain B. subtilis-based DFM on immunological markers and intestinal morphology as indicators of health status of the 21-d-old weanling pig. The hypothesis was that pigs supplemented with B. subtilis would have improved health status indicated by positive changes in both plasma and mucosal immunological markers, and greater morphological development of individual segments of the gastrointestinal tract (GIT).
MATERIALS AND METHODS
Animals, Housing, and Experimental Design
The Institutional Animal Care and Use Committee at Michigan State University reviewed and approved the protocol (PROTO201900154) for this experiment. The animal study was structured as a completely randomized design and conducted between the months of August and September 2019 at the Michigan State University Swine Teaching and Research Center. Eighty crossbred pigs (PIC 359 × Yorkshire) from the MSU Swine Farm were weaned at 21 ± 1 d (7.0 ± 0.60 kg, initial BW) and randomly allotted into 16 cohorts with five pigs per cohort. Cohorts were then randomly allotted to one of 16 pens (1.22 × 1.83 m) located in one of four mechanically-ventilated nursery rooms at the Michigan State University Swine Teaching and Research Center. Cohort allotment was based on litter (dam), weight, and sex, and maintaining a similar average weight in each pen. Treatments were randomly assigned to each pen. Each pen held five pigs with four of the pens within each treatment containing three gilts and two barrows and the remaining four pens containing three barrows and two gilts. Pens were equipped with round-rod steel flooring, vertical-rod, fiberglass fencing and gates, single-sided two-hole feeders, and one nipple drinker. Pen to pen cross-contamination between the two treatments was considered minimal as pens and alleyways were cleaned on a regular basis. The same eighty pigs were utilized in the first portion of this study previously published (Lewton et al., 2021). As such, all housing, vaccinating, and processing of piglets and dams was conducted as explained by Lewton et al. (2021).
Diets and Feeding
Two dietary treatments were used: a control diet with no DFM supplementation (CON) and diet with supplementation of a multi-strain B. subtilis-based DFM (CDFM) (United Animal Health, Sheridan, IN) comprised of a dried spore preparation having a guaranteed count of 1.48 × 108 CFU/g and included at a rate of 0.5 g/kg of feed to provide a final count of at least 7.35 × 104 CFU/g of complete feed. The nursery study was split up into three dietary phases (d 0–14, d 14–28, and d 28–42), with d 0 representing the day of weaning (21 ± 1 d of age). All diets were fed as a mash. Following the procedures of Lewton et al. (2021), all diets were based on requirements published by the NRC (2012) and formulated according to example diets made available online by Kansas State University (Menegat et al. 2019, https://www.asi.k-state.edu/research-and-extension/swine/premix-and-diet-recommendations.html, accessed: August 25, 2019) (Tables 1 and 2). Dietary Cu and Zn were maintained at requirement (NRC, 2012). Diets were mixed at the Michigan State University swine farm using a 113-kg paddle ribbon mixer. The mixer was emptied and wiped clean between each batch to minimize cross-contamination. Analyzed feed values were obtained for each dietary phase, from composite samples of individual feeders from the same treatment (Table 3).
Table 1.
Composition of diets, control, and diet containing multi-strain Bacillus subtilis-based direct-fed microbial (DFM), across all three dietary phases, as-fed basis1,2
| Ingredient % | Phase 1, d 0–14 | Phase 2, d 14–28 | Phase 3, d 28–42 |
|---|---|---|---|
| Corn | 40.18 | 48.25 | 50.89 |
| Soybean meal, 47.5% CP | 17.30 | 21.15 | 22.65 |
| Corn DDGS, 7.5% oil | 5.00 | 10.00 | 20.00 |
| Dried whey, 72% lactose | 25.00 | 10.00 | – |
| Fish meal | 3.00 | 4.50 | – |
| Spray-dried bovine plasma | 4.00 | – | – |
| Corn oil | 3.00 | 3.00 | 3.00 |
| Calcium carbonate, 38.5% Ca | 0.65 | 0.60 | 0.85 |
| Monocalcium phosphate, 21.5% P | 0.55 | 0.55 | 0.45 |
| Salt | 0.30 | 0.55 | 0.60 |
| l-Lysine HCl | 0.35 | 0.50 | 0.65 |
| dl-Methionine | 0.15 | 0.15 | 0.14 |
| l-Threonine | 0.13 | 0.19 | 0.21 |
| l-Tryptophan | 0.03 | 0.06 | 0.06 |
| l-Valine | 0.07 | 0.10 | 0.10 |
| VTM premix3 | 0.25 | 0.25 | 0.25 |
| Phytase | 0.05 | 0.05 | 0.05 |
| Total, 100% | 100 | 100 | 100 |
CON and DFM diets differed only by the inclusion of DFM at 0.05 g/kg or 1.48 × 108 CFU/g of complete feed.
Direct-fed microbial (United Animal Health, Sheridan, IN) had a guaranteed count of 1.48 × 108 CFU/g and was included at a rate of 0.5 g/kg of feed to provide a final count of at least 7.35 × 104 CFU/g of complete feed.
VTM premix provided the following vitamin and micromineral concentrations per kilogram of premix: zinc 83.4 g, iron 66.7 g, manganese 33.4 g, copper 10 g, iodine 0.3 g, selenium 0.2 g, vitamin A 7,363 KIU, vitamin D 1,177 KIU, vitamin E 44,112 IU, menadione 1.5 g, vitamin B12 0.02 g, riboflavin 4.7 g, pantothenic acid 14.7 g, niacin 29.4 g, thiamine 0.7 g, pyridoxine 2.9 g, folic acid 1.1 g, and biotin 0.1 g.
Table 2.
Calculated analysis of diets, control, and diet containing multi-strain B. subtilis-based direct-fed microbial (DFM), across all three dietary phases, as-fed basis1,2
| Item | Phase 1, d 0–14 | Phase 2, d 14–28 | Phase 3, d 28–42 |
|---|---|---|---|
| ME, kcal/kg | 3,477 | 3,439 | 3,417 |
| CP, % | 21.40 | 21.70 | 21.60 |
| Lys SID, % | 1.40 | 1.35 | 1.30 |
| His SID, % | 0.48 | 0.47 | 0.48 |
| Ile SID, % | 0.77 | 0.76 | 0.70 |
| Leu SID, % | 1.65 | 1.62 | 1.69 |
| Met + Cys SID, % | 0.78 | 0.76 | 0.73 |
| Thr, SID, % | 0.88 | 0.85 | 0.82 |
| Trp SID, % | 0.27 | 0.26 | 0.25 |
| Val SID, % | 0.97 | 0.93 | 0.90 |
| Ca, % | 0.78 | 0.74 | 0.59 |
| STTD P, % | 0.63 | 0.56 | 0.43 |
| Ca:P, ratio | 1.12 | 1.11 | 1.09 |
| Phytase, FTU/kg | 257.5 | 257.5 | 257.5 |
| Na, % | 0.51 | 0.38 | 0.31 |
| Cl, % | 0.69 | 0.62 | 0.51 |
CON and DFM diets differed only by the inclusion of DFM at 0.05 g/kg or 1.48 × 108 CFU/g of complete feed.
Direct-fed microbial (United Animal Health, Sheridan, IN) had a guaranteed count of 1.48 × 108 CFU/g and was included at a rate of 0.5 g/kg of feed to provide a final count of at least 7.35 × 104 CFU/g of complete feed.
Table 3.
Analyzed composition of control diet and diet containing multi-strain B. subtilis-based direct-fed microbial (DFM)1, across all three dietary phases
| Item | Phase 1, d 0–14 | Phase 2, d 14–28 | Phase 3, d 28–42 | |||
|---|---|---|---|---|---|---|
| Control | DFM | Control | DFM | Control | DFM | |
| DM, % | 91.45 | 91.40 | 90.31 | 90.34 | 90.61 | 90.91 |
| GE, kcal/kg | 4,608 | 4,611 | 4,678 | 4,597 | 4,762 | 4,736 |
| CP, % | 21.70 | 21.40 | 21.70 | 22.00 | 21.90 | 22.60 |
| Crude Fat, % | 4.50 | 4.30 | 5.20 | 5.10 | 6.60 | 6.70 |
| Crude Fiber, % | 1.60 | 1.70 | 2.10 | 2.20 | 3.30 | 3.00 |
| NDF, % | 6.20 | 6.20 | 8.30 | 8.10 | 11.10 | 12.00 |
| ADF, % | 2.90 | 2.50 | 4.00 | 4.00 | 5.50 | 5.80 |
| Indispensable AA, % | ||||||
| Arg | 1.10 | 1.10 | 1.10 | 1.10 | 1.10 | 1.10 |
| His | 0.51 | 0.51 | 0.50 | 0.50 | 0.52 | 0.53 |
| Ile | 0.91 | 0.92 | 0.90 | 0.91 | 0.87 | 0.88 |
| Leu | 1.85 | 1.89 | 1.83 | 1.84 | 1.95 | 2.02 |
| Lys | 1.58 | 1.53 | 1.56 | 1.46 | 1.49 | 1.56 |
| Met | 0.50 | 0.42 | 0.45 | 0.48 | 0.42 | 0.46 |
| Met + Cys | 0.91 | 0.81 | 0.76 | 0.81 | 0.76 | 0.79 |
| Phe | 0.98 | 0.97 | 0.98 | 0.99 | 1.05 | 1.07 |
| Thr | 1.00 | 1.03 | 0.97 | 0.96 | 0.91 | 0.94 |
| Trp | 0.30 | 0.30 | 0.28 | 0.34 | 0.26 | 0.28 |
| Val | 1.13 | 1.15 | 1.08 | 1.10 | 1.05 | 1.07 |
| Dispensable AA, % | ||||||
| Ala | 1.06 | 1.07 | 1.11 | 1.09 | 1.13 | 1.16 |
| Asp | 1.94 | 1.93 | 1.86 | 1.87 | 1.77 | 1.73 |
| Cys | 0.41 | 0.39 | 0.31 | 0.33 | 0.34 | 0.33 |
| Glu | 3.28 | 3.27 | 3.32 | 3.33 | 3.37 | 3.32 |
| Gly | 0.80 | 0.80 | 0.88 | 0.86 | 0.79 | 0.79 |
| Pro | 1.17 | 1.19 | 1.23 | 1.20 | 1.29 | 1.32 |
| Ser | 0.84 | 0.84 | 0.80 | 0.80 | 0.82 | 0.82 |
| Tyr | 0.66 | 0.68 | 0.65 | 0.63 | 0.68 | 0.68 |
Direct-fed microbial (United Animal Health, Sheridan, IN) had a guaranteed count of 1.48 × 108 CFU/g and was included at a rate of 0.5 g/kg of feed to provide a final count of at least 7.35 × 104 CFU/g of complete feed.
Data Recording and Sample Collection
At the end of wk 3 and 6, one pig per pen, was euthanized for analysis of systemic and localized immunity and intestinal morphology. Following the procedure of Lewton et al. (2021), an equal number of males and females were euthanized from both treatments to leave 4 pigs per pen at the conclusion of wk 3, and to maintain 2 of each sex per pen for the remainder of the study. All euthanized pigs were randomly selected based on weight to achieve a similar weight distribution between the two treatments. One pig was euthanized at a time alternating between CON and CDFM. Pigs were sedated using a combination of Telazol (2.5 mg/kg), Ketamine (1.25 mg/kg), and Xylazine (1.25 mg/kg) in a single intramuscular injection with a 22 G needle. Pigs were then euthanized using sodium pentobarbital (1 mL/4.5 kg) in a single intra-cardiac injection with an 18-G needle.
Post sedation and prior to euthanasia, a single blood sample for plasma analysis was collected from each pig using a 10 mL BD Vacutainer via jugular venipuncture. Blood tubes were temporarily placed on ice until aliquoted.
After confirmation of death, carcasses were opened lengthwise and the cecum was located. Collection of the duodenum, jejunum, ileum, ascending colon, and distal colon was conducted according to the procedures detailed by Lewton et al. (2021). Upon removal, sections of intestine were placed on a metal pan and a portion of tissue was collected for histology, opened lengthwise and immediately placed in chilled 1× phosphate-buffered solution (PBS). For each tissue section collected, the most distal end was taken for histological assessment in order to maintain consistency. Once cleaned, these tissues were flattened and stapled, mucosa side up, to appropriately labeled cardboard paper and stored in Carnoy’s 2000 (MasterTech Scientific, Inc.) for 24 hr to prep for histological assessment.
After removal of digesta a portion of each section, excluding the distal colon (ileum, jejunum, duodenum, and ascending colon) was placed in 1× PBS until clean, and laid flat on a metal pan to collect the mucosal layer. Mucosal scrapes were collected using two glass slides to gently remove the mucosa from the muscular layer of the tissue. Scrapings were then flash frozen, wrapped in whirl pack bags, rolled up, and placed in liquid nitrogen. These were then transferred to a ‐80 °C for storage until analysis of localized immunity markers.
Chemical Analysis
Blood tubes were placed directly on ice and then centrifuged for 30 min at 2,300 rpm and 4 °C. Plasma was then aliquoted into labeled sample tubes in a covered sample container and stored at ‐80 °C until analysis of specific cytokines and immunoglobulins. Plasma samples were analyzed for intestinal fatty acid binding protein (iFABP) and immunoglobulin A (IgA). Fatty acid binding protein was analyzed using a human FABP2/I-FABP immunoassay from R&D Systems, Inc (Minneapolis, MN). Samples and all materials were allowed 2 hr to thaw, prior to dilution of samples (25 µL sample per 300 µL calibrator diluent), and preparation of eight standards (0–1,000 pg/mL). Upon completion of the immunoassay, optical density of each well was determined using a microplate reader at 450 nm within 30 min of adding stop solution. Assay sensitivity was 3.63 pg/mL with intra- and inter-assay coefficients of variations of 3.50% and 8.40%, respectively. Immunoglobulin A was analyzed in a similar manner using a pig IgA ELISA kit from Bethyl Laboratories, Inc. (Montgomery, TX). Samples were thawed for 2 hr prior to diluting with a 1:15,000 dilution scheme of sample to diluent. A standard curve was established using a series of eight dilutions (0–1,000 ng/mL). Plates were measured for optical density at an absorbance of 450 nm within 30 min of adding stop solution. Assay sensitivity was 1.37 ng/mL with intra- and inter-assay coefficients of variations of 2.79% and 5.79%, respectively.
Interleukin 6 (IL-6), interleukin 10 (IL-10), and tumor necrosis factor alpha (TNFα) were measured as general markers of immune system activity. Markers were analyzed using ELISA kits produced by RayBiotech, Inc. (Norcross, GA) for IL-6, and R&D Systems, Inc. (Minneapolis, MN) for IL-10 and TNFα. All ELISAs followed the same principles as discussed above for analysis of systemic immunity markers. Protein concentration was diluted two-fold prior to running ELISAs for IL-6 and IL-10. Standard curves were prepared for IL-6 (0–10,000 pg/mL), IL-10 (0–2,000 pg/mL) and TNFα (0–1,500 pg/mL) using a series of eight dilutions. Upon completion of assay and addition of stop solution, optical density was measured at an absorbance of 450 nm. Assay sensitivity for these three markers were IL-6 (45.0 pg/mL), IL-10 (3.5 pg/mL), and TNF (3.7 pg/mL). Intra- and inter-assay coefficients of variation for each of these assays were IL-6 (9.94% and 11.49%), IL-10 (3.43% and 6.17%), and TNF (4.87% and 8.90%), respectively.
Twenty-four hour post collection, tissues for analysis of intestinal morphology were moved from Carnoy’s 2000 (MasterTech Scientific, Inc.) to a new container with 1× PBS + 0.1% sodium azide (1.50 L 1× PBS + 1.5 g sodium azide), where they remained for an additional 24 hr. Following this time period, sections of each tissue were cut and moved into a separate plastic cassette, placed in 70% ethanol, and delivered to the Michigan State University Investigative Histopathology Laboratory (East Lansing, MI) for slide preparation and staining. According to their procedures previously fixed tissues were processed and vacuum infiltrated with paraffin on the Sakura VIP 2000 tissue processor, followed by embedding with the ThermoFisher HistoCentre III embedding station. Once blocks were cooled, excess paraffin was removed from the edges; placed on a Reichert Jung 2030 rotary microtome and faced to expose tissue samples. Once the block was faced it was cooled and finely sectioned at 4–5 µm. Sections were dried at 56 °C slide incubator to ensure adherence to the slides for 2–24 hr not exceeding this temperature which would potentially destroy antigenic components. Slides were removed from the incubator and stained with a routine hematoxylin and eosin method, followed by cover-slipping with synthetic mounting media for permanent retention and visualization with light microscopy.
Slides were then viewed microscopically using a Leica DM750 brightfield microscope fit with Leica LAS EZ software (Buffalo Grove, IL). Within the ascending colon, crypt depth, and muscle thickness were measured using a minimum of 20 representative crypts and measurements of muscle thickness. Within the duodenum, jejunum, and ileum, villi height, and crypt depth were evaluated by selecting a minimum of 20 representative villi and crypt. Villi to crypt ratio was calculated using a minimum of eight adjacent villi and crypt. Photos taken and used for analysis of villi and crypt were also used to evaluate goblet cell concentrations within the duodenum and ascending colon. Using 8–15 representative crypt, total goblet cells per crypt and goblet cells per µm of crypt depth were counted. The goblet cell concentrations in the jejunum and ileum were not evaluated, because of poor quality photos of those two segments.
Statistical Analysis
All data were analyzed using the PROC GLIMMIX procedure in SAS 9.4 (SAS Institute, Inc., Cary, NC) specifying pen as the experimental unit. The model included the fixed effects of treatment and their interactions with week and intestinal segment. Pen nested within treatments were specified to be random effects in order to define pen as the experimental unit for treatment. Individual treatment means were separate using the Tukey-Kramer multiple comparison test. Differences were considered significant at P < 0.05 and tendencies at 0.05 < P < 0.10.
RESULTS AND DISCUSSION
Growth Performance, Morbidity and Mortality
Growth performance of pigs in this experiment was reported previously by Lewton et al. (2021). The overall health of pigs was excellent with no mortalities and only two pigs being treated due to lameness and/or weight loss, for a total morbidity of 2.5%, as mentioned by Lewton et al. (2021). This study involved pigs originating from a high health herd aside from a few common underlying health challenges including rotavirus, which was tested positive for in the pigs used in this experiment. As discussed by Lewton et al. (2021), in the current study, with pen being defined as the experimental unit, eight pens were assigned to each treatment, as this was considered enough statistical power to detect differences in immunological markers, intestinal morphology, or microbiota populations (Bhandari et al., 2008; Lee et al., 2014; Zhang et al., 2017), however, this was not expected to provide enough power to mimic the differences in growth performance obtained by Augspurger et al. (2016), nor detect statistical differences in morbidity or mortality. Differences in performance observed between the current group of pigs and those of Augspurger et al. (2016), are reported and discussed by Lewton et al. (2021) and may be related to differences in overall herd health status. Furthermore, pen density, with five pigs per pen, favored maximum health and performance.
Intestinal Morphology
In the current study, differences were observed between treatments in intestinal morphology. Difference observed appeared to be correlated with our results available elsewhere (Lewton et al., 2021). The DFM had the greatest impact on both amino acid digestibility and morphology within the jejunum. Relative to CON, CDFM improved overall jejunal villus height by 9% (P = 0.02; 385 vs. 422 ± 10 µm, respectively; Table 4). While not statistically significant similar numerical treatment differences were observed between CON and CDFM jejunal villus height on both d 21 (P = 0.24; 370 vs. 406 ± 21 µm) and 42 (P = 0.20; 399 vs. 439 ± 21 µm) suggesting an early, yet lasting impact of the DFM (Table 4). However, there did not appear to be any impact on intestinal morphology within the duodenum or ileum. Other groups have evaluated B. subtilis-based products for morphological changes, with varied results. Similar to the current study, Walsh et al. (2007) fed weanling pigs a mixed Bacillus-based DFM and observed an increase in ileal villus height on d 34 of the trial. These improvements were limited to pigs given a single bolus dose in addition to the DFM being supplied in the feed. Lee et al. (2014) observed greater differences when feeding a B. subtilis fermented biomass to 21-d-old weanling pigs. A linear improvement was observed on d 28 of the study, with the highest inclusion of the biomass (4.5 g/kg) leading to increased villus height and villus height to crypt depth ratio in the duodenum, jejunum, and ileum. Still other research has shown improvements in ileal villus height of 7-d-old piglets receiving formula milk supplemented with B. subtilis PB6, appearing to have the greatest impact on pigs suffering from intrauterine growth restriction (Hu et al., 2016). The same variation in response occurs across species, with poultry studies observing variable responses depending on the specific product (Samanya and Yamauchi, 2002; Sikander et al., 2017). In agreeance with the current study, these studies all appear to suggest that B. subtilis has a greater impact on villus height than crypt depth within the small intestine.
Table 4.
Intestinal morphology across gastrointestinal segments, measured at two time points for control diet and multi-strain B. subtilis-based direct-fed microbial (DFM)1
| Day | Segment | Control | DFM | SEM | P-value |
|---|---|---|---|---|---|
| Villi height, µm | |||||
| 21 | Duodenum | 432 | 451 | 22 | 0.55 |
| Jejunum | 370 | 406 | 21 | 0.24 | |
| Ileum | 370 | 381 | 12 | 0.51 | |
| 42 | Duodenum | 512 | 486 | 20 | 0.38 |
| Jejunum | 399 | 439 | 21 | 0.20 | |
| Ileum | 407 | 404 | 12 | 0.89 | |
| Overall2 | Duodenum | 472 | 468 | 17 | 0.87 |
| Jejunum | 385b | 422a | 10 | 0.02 | |
| Ileum | 388 | 393 | 8 | 0.67 | |
| Crypt depth, µm | |||||
| 21 | Duodenum | 340 | 359 | 11 | 0.25 |
| Jejunum | 297 | 319 | 13 | 0.24 | |
| Ileum | 238 | 233 | 7 | 0.67 | |
| Ascending colon | 337y | 373x | 14 | 0.10 | |
| 42 | Duodenum | 354 | 359 | 11 | 0.72 |
| Jejunum | 316 | 321 | 13 | 0.81 | |
| Ileum | 252 | 248 | 7 | 0.73 | |
| Ascending colon | 410 | 424 | 14 | 0.52 | |
| Overall | Duodenum | 347 | 359 | 8 | 0.29 |
| Jejunum | 307 | 320 | 8 | 0.24 | |
| Ileum | 245 | 241 | 5 | 0.60 | |
| Ascending colon | 374 | 398 | 11 | 0.15 | |
Direct-fed microbial (United Animal Health, Sheridan, IN), n = 6–8 observations per treatment, 1–2 missing values in duodenum and ileum due to damaged tissue upon collection.
Overall values are based on average of d 21 and 42 LSmeans.
Values in a common row lacking a common superscript differ (P ≤ 0.05).
Values in a common row lacking a common superscript tend to differ (P ≤ 0.10).
In the current study, overall crypt depth within the ascending colon did not differ between treatments (P = 0.15). However, crypt depth on d 21 tended to increase (P = 0.10) with addition of CDFM relative to CON (337 vs. 373 ± 14 µm, respectively) (Table 4). Jin et al. (1994) have shown high fiber diets increase colon crypt depth of 13 kg pigs, being significantly altered within a 14-d time span. More recent studies have confirmed this, observing increased colon crypt depth when feeding high fiber or resistant starch diets to nursery pigs (Hedemann et al., 2006; Hedemann and Knudsen, 2007). A study evaluating a different Bacillus species found no differences in crypt depth between treatments in either the small intestine or colon (Reiter et al., 2006). However, limited other research has evaluated the effects of a DFM on colonic morphology. Deeper crypts in the small intestine have been associated with greater cell production and turnover and are often associated with reduced growth performance (Hedemann et al., 2003; Awad et al., 2009). Additionally, a study in mice suggested that the crypt may also serve to protect stem cells from harmful metabolites generated by the microbiota primarily located within the colon (Kaiko et al., 2016). An observed increase in colon crypt depth may indicate the cells responding to greater concentrations of metabolites produced in the colon. As some of these metabolites, including butyrate and other volatile fatty acids, are beneficial to the host, serving as secondary energy sources, increased colonic crypt depth with the addition of B. subtilis may be related to positive changes in the microbiota, increasing metabolite production (Wong et al., 2006; Kaiko et al., 2016). Still other researchers have demonstrated the importance of increased crypt size for the initiation of crypt fission, a necessary part of intestinal growth and regeneration (Park et al., 1997).
Goblet Cell Concentrations
In the current study, no treatment differences were observed in goblet cell concentration within the duodenum or ascending colon, when expressed either as goblet cells per crypt or goblet cells per µm of crypt depth. Concentrations of goblet cells per crypt d 21 were 14.1 vs. 15.3 ± 1.6 cells (P = 0.59) in the duodenum, and 20.3 vs. 20.6 ± 2.6 cells (P = 0.93) in the ascending colon for CON and CDFM, respectively. On d 42, goblet cell concentrations were 21.3 vs. 19.2 ± 1.5 cells (P = 0.35) in the duodenum, and 26.7 vs. 28.7 ± 2.6 cells (P = 0.60) in the ascending colon for CON and CDFM, respectively. When expressed as goblet cells per µm of crypt depth, overall concentrations for CON and CDFM were 0.049 vs. 0.047 ± 0.002 cells/µm (P = 0.66) in the duodenum, and 0.083 vs. 0.081 ± 0.007 cells/µm (P = 0.84) in the ascending colon, respectively. While there are multiple classes of goblet cells, being neutral or acidic, and within the acidic class existing as either sulfated or non-sulfated (Croix et al., 2011), they were not distinguished in the current study. Davis et al. (2007) found that administering Lactobacillus brevis to piglets throughout lactation and the nursery phase led to greater production of non-sulfated goblet cells in the duodenum, while decreasing the number of sulfated goblet cells in the duodenum and jejunum. Similar decreases in the non-sulfated goblet cells were observed in the pigs supplemented L. brevis during lactation and followed with either an antibiotic or Bacillus DFM in the nursery (Davis et al., 2007). Brown et al. (2006) found no differences in goblet cells when comparing two different management strategies, conventional and segregated early weaning, observing no effects of management on goblet cell concentration. However, neutral goblet cells significantly decreased upon weaning, returning to above pre-weaning concentrations by 25 d post-weaning (Brown et al., 2006). Another study agreed with these results, observing effects of weaning on goblet cell concentration, with pigs evaluated at weaning having greater goblet cell concentration than those evaluated 5 d post-weaning (Bruininx et al., 2002). However, in the current study pigs were not evaluated until wk 3 and 6 post-weaning, likely beyond the minimal time required for full recovery of the effects of weaning on goblet cell concentrations. Goblet cells primarily function as protective cells, secreting mucins that form a layer of protection across the lumen of the small and large intestine (Specian and Oliver, 1991). A greater concentration of goblet cells in a specific segment of the GIT may be an indication of greater protection of the mucosal layer. Additionally, goblet cell mucin production has been shown to increase in response to microbes, as colonization of mucus provides a great platform for bacterial growth (Deplancke and Gaskins, 2001). However, that did not appear to be the case in the current study, as goblet cell concentration was not increased to coincide with the tendency of deeper crypts within the ascending colon in pigs fed CDFM (Table 4).
Immunological Markers
In the current study, CDFM led to an increase in overall plasma levels of IgA (P = 0.03) by more than 20% relative to CON (0.73 vs 0.58 ± 0.05 mg/mL, respectively). Relative to CON, CDFM also tended to increase d 21 plasma IgA concentrations (P = 0.06) (0.34 vs. 0.54 ± 0.07 mg/mL, respectively), suggesting an early impact of the DFM on mucosal immunity (Table 5). This antibody plays an important role in clearing antigens present in the gut lumen, preventing them from entering the epithelium (Corthësy, 2009). The current results are in agreeance with other B. subtilis studies involving nursery pigs. Lee et al. (2014) observed an increase in plasma IgA, with a linear increase as more B. subtilis was added to the diet, leading up to a 13% increase relative to pigs on the control diet. Dong et al. (2014) observed a similar increase in plasma IgA in 70-d-old pigs, when supplemented with B. subtilis alone, but observed no differences when B. subtilis was combined with Lactobacillus plantarum. Another study supplementing B. subtilis as part of a mixed cocktail DFM did not observe any differences in plasma IgA concentrations (Kim et al., 2014). These observed differences may be due to differences in the mechanism between mixed DFM and those composed of B. subtilis alone. Poultry studies also appear to indicate an immune enhancement by B. subtilis, observed through significant increases in plasma or mucosal IgA concentrations (Amerah et al., 2013; Rajput et al., 2013; Bai et al., 2018). Bacillus subtilis appears to play an important role in stimulating a systemic immune response, inducting IgA production.
Table 5.
Immunological markers in both plasma and intestinal mucosa, measured at two time points for control diet and multi-strain B. subtilis-based direct-fed microbial (DFM)1
| Location/item | Day | Control | DFM | SEM | P-value |
|---|---|---|---|---|---|
| Plasma | |||||
| IgA, mg/mL | 21 | 0.34y | 0.54x | 0.07 | 0.06 |
| 42 | 0.82 | 0.92 | 0.07 | 0.29 | |
| Overall2 | 0.58b | 0.73a | 0.05 | 0.03 | |
| iFABP, pg/mL | 21 | 467 | 410 | 83 | 0.63 |
| 42 | 496 | 347 | 88 | 0.24 | |
| Overall | 481 | 379 | 61 | 0.25 | |
| Ileum | |||||
| IL-6, pg/mL | 21 | 363 | 331 | 41 | 0.57 |
| 42 | 437 | 480 | 39 | 0.44 | |
| Overall | 400 | 405 | 29 | 0.90 | |
| IL-10, pg/mL | 21 | 147 | 168 | 48 | 0.77 |
| 42 | 225 | 258 | 56 | 0.69 | |
| Overall | 186 | 213 | 36 | 0.61 | |
| TNF-α, pg/mL | 21 | 110 | 85 | 16 | 0.27 |
| 42 | 110 | 101 | 15 | 0.68 | |
| Overall | 110 | 93 | 11 | 0.30 | |
| Jejunum | |||||
| IL-6, pg/mL | 21 | 328 | 382 | 38 | 0.33 |
| 42 | 403 | 384 | 40 | 0.72 | |
| Overall | 366 | 382 | 30 | 0.69 | |
| IL-10, pg/mL | 21 | 93 | 152 | 35 | 0.26 |
| 42 | 133 | 237 | 49 | 0.13 | |
| Overall | 113y | 195x | 35 | 0.10 | |
| TNF-α, pg/mL | 21 | 79 | 76 | 15 | 0.89 |
| 42 | 78 | 62 | 15 | 0.46 | |
| Overall | 78 | 69 | 11 | 0.54 | |
Direct-fed microbial (United Animal Health, Sheridan, IN), n = 6–8 observations per treatment.
Overall values are based on average of d 21 and 42 LSmeans.
Values in a common row lacking a common superscript differ (P ≤ 0.05).
Values in a common row lacking a common superscript tend to differ (P ≤ 0.10).
Intestinal fatty acid binding protein has been recognized as a biomarker of mucosal damage, as the two are highly correlated (Lau et al., 2016; Pietro et al., 2019). In the current study, no treatment differences were observed in plasma iFABP (Table 5). We are aware of no other studies evaluating Bacillus-based products on iFABP concentrations as a marker for intestinal tissue injury. Research comparing plasma iFABP in weaned or nursing pigs indicated that differences in plasma iFABP may involve factors beyond those associated with weaning, observing a greater within-litter correlation to iFABP levels of their mother (Berkeveld et al., 2008). A study using a rodent model evaluated the ability of B. subtilis to minimize the adverse effects associated with excessive exercise. Supplemented rats were able to maintain a similar concentration of plasma iFABP to that of sedentary rats, while control exercised rats had significantly greater concentrations, indicating a protective role of B. subtilis to environmental stressors (Ducray et al., 2019). The pigs used in the current study originated from a high health herd and had minimal enteric challenges other than that normally observed post-weaning in a nursery setting. This likely decreased overall damage to the intestinal epithelium, which may explain why no differences in iFABP concentrations were observed in this case.
Gastrointestinal functionality biomarkers have been evaluated in many studies involving a B. subtilis-based DFM, however there does not appear to be a single, representative biomarker for capturing its local effects within the GIT, but rather requires analysis of several markers for an accurate interpretation (Pietro et al., 2019). In the current study, relative to CON, CDFM tended to increase overall jejunal IL-10 concentrations (113 vs. 195 ± 35 pg/mL; P = 0.10) but negating other results of this study, had no specific impact on d 21 (P = 0.26). No other differences were observed in IL-6, IL-10, or TNFα concentrations (Table 5). This increase in anti-inflammatory cytokine IL-10 has been previously observed. Zhang et al. (2017) found when feeding B. subtilis fermented soybean meal, relative to a control diet, fermented soybean meal led to decreases in IL-6 and increases in IL-10 in both the jejunum and ileum. In contrast, Walsh et al. (2012) found that Salmonella typhimurium challenged pigs supplemented with B. subtilis did not have greater mucosal TNFα concentrations, relative to a negative control group. Others observed no changes in plasma TNFα with B. subtilis and two different concentrations of fiber (Jaworski et al., 2017). However, other researchers feeding B. subtilis to pre-weaned dairy calves found no changes in either IL-6 or IL-10 (Sun et al., 2010). Variations in these results may involve several factors such as dietary composition and the health status of the animals, in addition to differences in the bacterial strains used in the study. Rodent macrophages cultured with B. subtilis displayed a stimulated immune response with greater concentrations of nitric oxide and various cytokines, including IL-10 and IL-6 (Xu et al., 2012). These results appear to indicate that B. subtilis may support the simulation of a local intestinal immune response by altering concentrations of specific cytokines and other immunity markers.
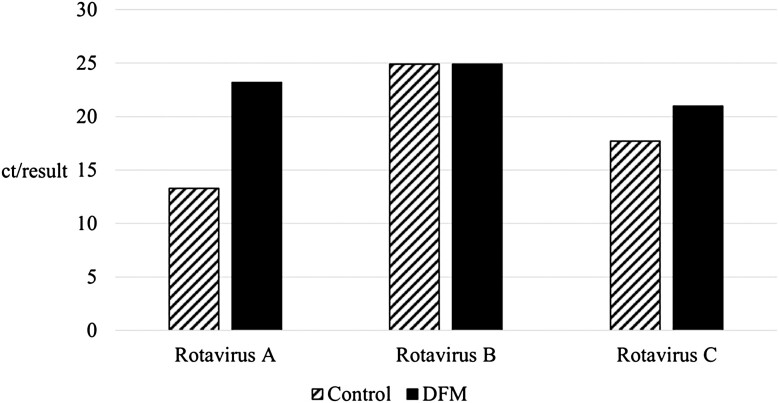
Rotavirus
Within the first week of the study, mild diarrhea cases occurred throughout the nursery. Pooled fecal samples collected on d 9 of the study were sent to Iowa State University Veterinary Diagnostic Lab (Ames, IA) for analysis. Laboratory analysis of these samples indicated the presence of rotavirus in the feces of sampled pigs, from both treatments. However, PCR results indicated a numerically lower concentration of rotavirus A and C in pigs supplemented with CDFM (Figure 1). Statistical analysis of these samples was not conducted as it was not a planned comparison and analysis was limited to two pooled samples per treatment. These differences may help explain other observed results, as an indicator of the B. subtilis competitively excluding specific pathogens present in the GIT, helping to restore the balance of the microbiome (Zimmerman et al., 2001). The role of B. subtilis may be two part, directly decreasing the presence of pathogens, and increasing the production of IgA. In monogastric, including pigs, secretory IgA is the primary immunoglobulin present in milk (Saif and Fernandez, 1996). While in lactation, piglets receive needed immunoglobulins in the form of milk, however the transition to grain-based diets upon weaning eliminates the passive immunity received from the mother. In the current study, B. subtilis led to a significant increase in plasma IgA (P = 0.03), an increase that tended to appear within the first 21 d post-weaning (P = 0.06) (Table 5). This increase in IgA, an antibody important for clearing antigens to protect the epithelial layer (Corthësy, 2009), possibly decreased the presence of specific pathogens, including rotavirus A and C, in the GIT of DFM supplemented pigs.
Figure 1.
PCR of pooled fecal sample analysis, a total of two representative pooled samples from a total of four pigs per treatment, taken on d 9 of the trial, represented as cycle threshold per test (ct/test), for control diet and multi-strain Bacillus subtilis-based direct-fed microbial (DFM).
In conclusion, addition of a multi-strain B. subtilis-based DFM in nursery diets appeared to lead to beneficial impacts on intestinal morphology, observed through greater villus height in the jejunum, and deeper crypts in the ascending colon. In agreeance with other results of our research (Lewton et al., 2021), a multi-strain B. subtilis-based DFM appears to provide benefits specific to the mid-jejunum. These changes may appear as soon as 3 wks into supplementation. Bacillus subtilis appears to improve the immune function of nursery pigs, observed in 20% higher plasma IgA concentrations and increased expression of jejunal anti-inflammatory cytokine IL-10. The current study was limited by the overall superior health of the pigs involved. Compared to other similar studies, this group of pigs performed exceptionally well (Lewton et al., 2021), which may have limited the effects of DFM on intestinal morphology and immunological markers.
ACKNOWLEDGMENTS
The authors acknowledge United Animal Health (Sheridan, IN) for providing the direct-fed microbial product and for giving to the Michigan State University Swine Nutrition and Production Management Master’s Degree Fund which supported J. R. Lewton.
Glossary
Abbreviations
- AA
amino acid
- CDFM
control plus direct-fed microbial
- CON
control
- DFM
direct-fed microbial
- GIT
gastrointestinal tract
- iFABP
intestinal fatty acid binding protein
- IgA
immunoglobulin A
- IL-6
Interleukin 6
- IL-10
Interleukin 10
- PBS
phosphate-buffered solution
- TNFα
tumor necrosis factor alpha
Contributor Information
Jaron R Lewton, Department of Animal Science, Michigan State University, East Lansing, MI 48824, USA.
Adrienne D Woodward, United Animal Health, Sheridan, IN 46069, USA.
Ronny L Moser, United Animal Health, Sheridan, IN 46069, USA.
Kyan M Thelen, Department of Large Animal Clinical Sciences, College of Veterinary Medicine, Michigan State University, East Lansing, MI 48824, USA.
Adam J Moeser, Department of Large Animal Clinical Sciences, College of Veterinary Medicine, Michigan State University, East Lansing, MI 48824, USA.
Nathalie L Trottier, Department of Animal Science, Cornell University, Ithaca, NY 14853, Greece.
Robert J Tempelman, Department of Animal Science, Michigan State University, East Lansing, MI 48824, USA.
Dale W Rozeboom, Department of Animal Science, Michigan State University, East Lansing, MI 48824, USA.
DISCLOSURES
Michigan State University has no financial interests in the product evaluated or in the company providing that product. Because of the perception of a conflict of interest and in the interest of full transparency, we are disclosing 1) that employees of the company providing said product were co-investigators or researchers, and are co-authors of this manuscript, 2) the company provided the product gratis, and 3) the company had previously donated to the Michigan State University Swine Nutrition and Production Management Master’s Degree program which supported J. R. Lewton.
LITERATURE CITED
- Amerah, A. M., Quiles A., Medel P., Sanchez J., Lehtinen M. J., and Gracia M. I.. . 2013. Effect of pelleting temperature and probiotic supplementation on growth performance and immune function of broilers fed maize/soy-based diets. Anim. Feed Sci. Technol. 180:55–63. doi: 10.1016/j.anifeedsci.2013.01.002. [DOI] [Google Scholar]
- Augspurger, N. R., Spencer J. D., Son S., Ley J. A., and King M. R.. . 2016. Improved growth performance of nursery pigs fed diets supplemented with a Bacillus subtilis-based direct-fed microbial feed additive. J. Anim. Sci. 94(Suppl. 2):76. doi: 10.2527/msasas2016-162. [DOI] [Google Scholar]
- Awad, W., Abdel-Raheem S. M., Ghareeb K., and Bohm J.. . 2009. Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poult. Sci. 88:49–55. doi: 10.3382/ps.2008-00244. [DOI] [PubMed] [Google Scholar]
- Bai, K., Feng C., Jiang L., Zhang L., Zhang J., Zhang L., and Wang T.. . 2018. Dietary effects of Bacillus subtilis fmbj on growth performance, small intestinal morphology, and its antioxidant capacity of broilers. Poult. Sci. 97:2312–2321. doi: 10.3382/ps/pey116. [DOI] [PubMed] [Google Scholar]
- Berkeveld, M., Langendijk P., Verheijden J. H. M., Taverne M. A. M., van Nes A., van Haard P., and Koets A. P.. . 2008. Citrulline and intestinal fatty acid-binding protein: longitudinal markers of postweaning small intestinal function in pigs. J. Anim. Sci. 86:3440–3449. doi: 10.2527/jas.2008-1167. [DOI] [PubMed] [Google Scholar]
- Bhandari, S. K., Xu B., Nyachoti C. M., Giesting D. W., and Krause D. O.. . 2008. Evaluation of alternatives to antibiotics using an Escherichia coli K88+ model of piglet diarrhea: effects on gut microbial ecology. J. Anim. Sci. 85:3256–3266. doi: 10.2527/jas.2007-0320. [DOI] [PubMed] [Google Scholar]
- Brown, D. C., Maxwell C. V., Erf G. F., Davis M. E., Singh S., and Johnson Z. B.. . 2006. The influence of different management systems and age on intestinal morphology, immune cell numbers and mucin production from goblet cells in post-weaning pigs. Vet. Immunol. Immunopathol. 111:187–198. doi: 10.1016/j.vetimm.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Bruininx, E. M. A. M., Schellingerhout A. B., Lensen E. G. C., van der Peet-Schwering C. M. C., Schrama J. W., Everts H., den Hartog L. A., and Beynen A. C.. . 2002. Associations between individual food intake characteristics and indicators of gut physiology of group-housed weanling pigs differing in genotype. Anim. Sci. 75:103–113. doi: 10.1017/S1357729800052887. [DOI] [Google Scholar]
- Campbell, J. M., Crenshaw J. D., and Polo J.. . 2013. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 4:19. doi: 10.1186/2049-1891-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corthësy, B. 2009. Secretory immunoglobulin A: well beyond immune exclusion at mucosal surfaces. Immunopharm. Immunotoxicol. 31:174–179. doi: 10.1080/08923970802438441. [DOI] [PubMed] [Google Scholar]
- Croix, J. A., Carbonero F., Nava G. M., Russell M., Greenberg E., and Gaskins H. R.. . 2011. On the relationship between sialmucin and sulfomucin expression and hydrogenotrophic microbes in the human colonic mucosa. PLoS One 6:e24447. doi: 10.1371/journal.pone.0024447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, M. E., Brown D. C., Baker A., Bos K., Dirain M. S., Halbrook E., Johnson Z. B., Maxwell C., and Rehberger T.. . 2007. Effect of direct-fed microbial and antibiotic supplementation on gastrointestinal microflora, mucin histochemical characterization, and immune populations of weanling pigs. Livest. Sci. 108:249–253. doi: 10.1016/j.livsci.2007.01.063. [DOI] [Google Scholar]
- Deplancke, B., and Gaskins H. R.. . 2001. Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am. J. Clin. Nutr. 73:1131S–1141S. doi: 10.1093/ajcn/73.6.1131S. [DOI] [PubMed] [Google Scholar]
- Dong, X., Zhang N., Zhou M., Tu Y., Deng K., and Diao Q.. . 2014. Effects of dietary probiotics on growth performance, faecal microbiota and serum profiles in weaned piglets. Anim. Prod. Sci. 54:616–621. doi: 10.1071/an12372. [DOI] [Google Scholar]
- Ducray, H. A. G., Globa L., Pustovyy O., Roberts M. D., Rudisill M., Vodyanoy V., and Sorokulova I.. . 2019. Prevention of excessive exercise-induced adverse effects in rats with Bacillus subtilis BSB3. J. Appl. Microb. 128:1163–1178. doi: 10.1111/jam.14544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl, A. M., Losick, R., and Kolter R.. . 2008. Ecology and genomics of Bacillus subtilis. Trends Microbiol. 16:269. doi: 10.1016/j.tim.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedemann, M. S., Eskildsen M., Lærke H. N., Pedersen C., Lindberg J. E., Laurinen P., Bach K., and Knudsen K. E.. . 2006. Intestinal morphology and enzymatic activity in newly weaned pigs fed contrasting fiber concentrations and fiber properties. J. Anim. Sci. 84:1375–1386. doi: 10.2527/2006.8461375x. [DOI] [PubMed] [Google Scholar]
- Hedemann, M. S., Hojsgaard S., and Jensen B. B.. . 2003. Small intestinal morphology and activity of intestinal peptidases in piglets around weaning. J. Anim. Phys. Anim. Nutr 87:32–41. doi: 10.1046/j.1439-0396.2003.00405.x. [DOI] [PubMed] [Google Scholar]
- Hedemann, M. S., and Knudsen K. E. B.. . 2007. Resistant starch for weaning pigs-effect on concentration of short chain fatty acids in digesta and intestinal morphology. Livest. Sci. 108:175–177. doi: 10.1016/j.livsci.2007.01.045. [DOI] [Google Scholar]
- Hu, L., Che L., Peng X., Xu Q., Fang Z., Xu S., Lin Y., and Wu D.. . 2016. Probiotic treatment using Bacillus subtilis PB6 improves the growth performance, intestinal morphology, enzyme activities and barrier function in low birth weight piglets. J. Anim. Sci. 94:829. doi: 10.2527/jam2016-1731. [DOI] [Google Scholar]
- Jaworski, N. W., Owusu-Asiedu A., Walsh M. C., McCann J. C., Loor J. J., and Stein H. H.. . 2017. Effects of a 3 strain Bacillus-based direct-fed microbial and dietary fiber concentration on growth performance and expression of genes related to absorption and metabolism of volatile fatty acids in weanling pigs. J. Anim. Sci. 95:308–319. doi: 10.2527/jas.2016.0557. [DOI] [PubMed] [Google Scholar]
- Jin, L., Reynolds L. P., Redmer D. A., Caton J. S., and Crenshaw J. D.. . 1994. Effects of dietary fiber on intestinal growth, cell proliferation, and morphology in growing pigs. J. Anim. Sci. 72:2270–2278. doi: 10.2527/1994.7292270x. [DOI] [PubMed] [Google Scholar]
- Kaiko, G., Ryu S. H., Koues O. I., Collins P. L., Solnica-Krezel L., Pearce E. J., Pearce E. L., Oltz E. M., and Stappenbeck T. S.. . 2016. The colonic crypt protects stem cells from microbiota-derived metabolites. Cell 165:1708–1720. doi: 10.1016/j.cell.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, K. H., Ingale S. L., Kim J. S., Lee S. H., Lee J. H., Kwon I. K., and Chae B. J.. . 2014. Bacteriophage and probiotics both enhance the performance of growing pigs, but bacteriophage is more effective. Anim. Feed Sci. Technol. 196:88–95. doi: 10.1016/j.anifeedsci.2014.06.012. [DOI] [Google Scholar]
- Lau, E., Marques C., Pestana D., Santoalha M., Carvalho D., Freitas P., and Calhau C.. . 2016. The role of I-FABP as a biomarker of intestinal barrier dysfunction driven by gut microbiota changes in obesity. Nutr. Metab. 13. doi: 10.1186/s12986-016-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. H., Ingale S. L., Kim J. S., Kim K. H., Lokhande A., Kim E. K., and Kwon I. K.. . 2014. Effects of dietary supplementation with Bacillus subtilis LS 1–2 fermentation biomass on growth performance, nutrient digestibility, cecal microbiota and intestinal morphology of weanling pig. Anim. Feed Sci. Technol. 188:102–110. doi: 10.1016/j.anifeedsci.2013.12.001. [DOI] [Google Scholar]
- Lewton, J. R., Woodward A. D., Moser R. L., Thelen K. M., Moeser A. J., Trottier N. L., Tempelman R. J., and Rozeboom D. W.. . 2021. Effects of a multi-strain Bacillus subtilis-based direct-fed microbial on weanling pig growth performance and nutrient digestibility. Transl. Anim. Sci. 5:1–12. doi: 10.1093/tas/txab058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeser, A. J., Pohl C. S., and Rajput M.. . 2017. Weaning stress and gastrointestinal barrier development: implications for lifelong gut health in pigs. Anim. Nutr. 3:313–321. doi: 10.1016/j.aninu.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegat, M. B., Goodband R. D., DeRouchey J. M., Tokach M. D., Woodworth J. C., and Dritz S. S.. . 2019. Example of swine nursery diets. Kansas State University Swine Extension. Accessed July 10, 2019. www.asi.k-state.edu. [Google Scholar]
- NRC. 2012. Nutrient requirements of swine. 11th rev. ed. National Academic Press, Washington, DC. [Google Scholar]
- Park, H. S., Goodlad R. A., Ahnen D. J., Winnett A., Sasieni P., Lee C. Y., and Wright N. A.. . 1997. Effects of epidermal growth factor and dimethylhydrazine on crypt size, cell proliferation, and crypt fission in the rat colon. Am. J. Pathol. 151:843–852. https://pubmed.ncbi.nlm.nih.gov/9284833/ [PMC free article] [PubMed] [Google Scholar]
- Pietro, C., Viviane V., Estefania P. C., Jerome S., and Anna-Maria K.. . 2019. Biomarkers of gastrointestinal functionality in animal nutrition and health. Anim. Feed Sci. Technol. 250:9–31. doi: 10.1016/j.anifeedsci.2018.07.012. [DOI] [Google Scholar]
- Pluske, J. R. 2013. Feed and feed additives-related aspects of gut health and development in weanling pigs. J. Anim. Sci. Biotechnol. 4:1. doi: 10.1186/2049-1891-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajput, I. R., Li L. Y., Xin X., Wu B. B., Juan Z. W., Cui Z. W., Yu D. Y., and Li W. F.. . 2013. Effect of Saccharomyces boulardii and Bacillus subtilis B10 on intestinal ultrastructure modulation and mucosal immunity development mechanism in broiler chickens. Poult. Sci. 92:956–965. doi: 10.3382/ps.2012-02845. [DOI] [PubMed] [Google Scholar]
- Reiter, K., Eggebrecht S., Drewes B., Riess M., and Weyrauch K. D.. . 2006. Effects of Enterococcus faecium and Bacillus cereus var. toyoi on the morphology of the intestinal mucous membrane in piglets. Biol. Brat. 61:803–809. doi: 10.2478/s11756-006-0161-2. [DOI] [Google Scholar]
- Saif, L. J., and Fernandez F. M.. . 1996. Group A rotavirus veterinary vaccines. J. Infect. Dis. 174:S98–106. doi: 10.1093/infdis/174.supplement_1.s98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanya, M., and Yamauchi K.. . 2002. Histological alterations of intestinal villi in chickens fed dried Bacillus subtilis var. natto. Comp. Biochem. Phys. 133:95–104. doi: 10.1016/S1095-6433(02)00121-6. [DOI] [PubMed] [Google Scholar]
- Sikandar, A., Zaneb H., Younus M., Masood S., Aslam A., Shah M., and Rehman H.. . 2017. Growth performance, immune status and organ morphometry in broilers fed Bacillus subtilis-supplemented diet. S Afr. J. Anim. Sci. 47:3. doi: 10.4314/sajas.v47i3.14. [DOI] [Google Scholar]
- Specian, R. D., and Oliver M. G.. . 1991. Functional biology of intestinal goblet cells. Am. J. Physiol. 260:C183–C193. doi: 10.1152/ajpcell.1991.260.2.C183. [DOI] [PubMed] [Google Scholar]
- Sun, P., Wang J. Q., and Zhang H. T.. . 2010. Effects of Bacillus subtilis natto on performance and immune function of preweaning calves. J. Dairy Sci. 93:5851–5855. doi: 10.3168/jds.2010-3263. [DOI] [PubMed] [Google Scholar]
- Walsh, M. C., Rostagno M. H., Gardiner G. E., Sutton A. L., Richert B. T., and Radcliffe J. S.. . 2012. Controlling Salmonella infection in weanling pigs through water delivery of direct-fed microbials or organic acids. Part I: effects on growth performance, microbial populations, and immune status. J. Anim. Sci. 90:261–271. doi: 10.2527/jas.2010-3598. [DOI] [PubMed] [Google Scholar]
- Walsh, M. C., Saddoris K. L., Sholly D. M., Hinson R. B., Sutton A. L., Applegate T. J., Richert B. T., and Radcliffe J. S.. . 2007. The effects of direct fed microbials delivered through the feed and/or in a bolus at weaning on growth performance and gut health. Livest. Sci. 108:254–257. doi: 10.1016/j.livsci.2007.01.051. [DOI] [Google Scholar]
- Wong, J. M. W., de Souza R., Cyril K. W. C., Azadeh E., and Jenkins D. J. A.. . 2006. Colonic health: fermentation and short chain fatty acids. J. Clin. Gastroenterol. 40:235–243. doi: 10.1097/00004836-200603000-00015 [DOI] [PubMed] [Google Scholar]
- Xu, X., Huang Q., Mao Y., Cui Z., Li Y., Huang Y., Rajput I. R., Yu D., and Li W.. . 2012. Immunomodulatory effects of Bacillus subtilis (natto) B4 spores on murine macrophages. Microbiol. Immunol. 56:817–824. doi: 10.1111/j.1348-0421.2012.00508.x. [DOI] [PubMed] [Google Scholar]
- Zhang, Y., Shi C., Wang C., Lu Z., Wang F., Feng J., and Wang Y.. . 2017. Effect of soybean meal fermented with Bacillus subtilis BS12 on growth performance and small intestinal immune status of piglets. Food Agric. Immunol. 29:133–146. doi: 10.1080/09540105.2017.1360258. [DOI] [Google Scholar]
- Zimmermann, B., Bauer E., and Mosenthin R.. . 2001. Pro- and prebiotics in pig nutrition—potential modulators of gut health. J. Anim. Feed Sci. 10:47–56. doi: 10.22358/jafs/67940/2001. [DOI] [Google Scholar]