Abstract
Introduction
Co-occurrence of e-cigarette use and alcohol consumption during adolescence is frequent. Here, we examined whether adolescent co-exposure to alcohol drinking and vaporized nicotine would impact reward- and cognition-related behaviors in adult male and female rats during adulthood.
Aims and Methods
Four groups of male and female Sprague Dawley rats (n = 8–11/group/sex) received either nicotine (JUUL 5% nicotine pods) or vehicle vapor for 10 minutes daily between postnatal days 30–46, while having continuous voluntary access to ethanol and water during this time in a two-bottle preference design. Upon reaching adulthood, all rats underwent behavioral testing (ie, Pavlovian conditioned approach testing, fear conditioning and a two-bottle alcohol preference).
Results
A sex-dependent effect, not related to adolescent nicotine or alcohol exposure, on alcohol drinking in adulthood was found, such that females had a higher intake and preference for alcohol compared to males; both male and female adult rats also had greater alcohol preference compared to their alcohol preference as adolescents. Male rats exposed to vaporized nicotine with or without alcohol drinking during adolescence exhibited altered reward-related learning in adulthood, evidenced by enhanced levels of sign-tracking behavior. Male rats that drank alcohol with or without nicotine vapor in adolescence showed deficits in associative fear learning and memory as adults. In contrast, these effects were not seen in female rats exposed to alcohol and nicotine vapor during adolescence.
Conclusions
The present study provides evidence that co-exposure to alcohol and vaporized nicotine during adolescence in male, but not female, rats produces long-term changes in reward- and cognition-related behaviors.
Implications
These findings enhance our understanding of the effects of alcohol drinking and nicotine vapor exposure in adolescence. Moreover, they highlight potential sex differences that exist in the response to alcohol and nicotine vapor, underscoring the need for follow-up studies elucidating the neurobiological mechanisms that drive these sex differences, as well as the long-term effects of alcohol and nicotine vapor use.
Introduction
E-cigarettes have become increasingly popular among adolescents1; in 2020, 30.7% of 10th graders and 34.5% of 12th graders reported using e-cigarettes, following a twofold increase over the past 2 years.1 An equally important issue is the consumption of alcohol by adolescents, whereby 7.10 million have reported consuming alcohol in the last month, and an estimated 414 000 adolescents meet the criteria for DSM-IV lifetime alcohol abuse.2 Alcohol and nicotine are frequently used sequentially and simultaneously by adolescents3 with a high prevalence of concurrent e-cigarette vaping and alcohol consumption.4 Vaping high school students also showed greater alcohol drinking compared to nonvapers,5 and alcohol and e-cigarette use is the most common type of co-use in this population.6 The long-term effects of vaping are currently unknown, as are the consequences of e-cigarette/alcohol co-use.6 In contrast, the additive effects of combustible tobacco and alcohol co-use are well established. For example, cigarette smoking amplifies cognitive deficits in adults who excessively drink alcohol, and alcohol-dependent adults who smoke cigarettes show pronounced neuropsychological damage compared to alcohol-dependent nonsmokers.7,8 Unfortunately, the consequences of nicotine and alcohol co-use in adolescence on subsequent behavior in adulthood are limited.
The adolescent brain undergoes critical neuronal and structural development, making adolescence a period of vulnerability to the effects of drugs,9 with brain imaging studies suggesting altered brain structure and function in adolescent users compared to nonusers.9 However, the causal consequences of adolescent co-use cannot be systematically examined in humans and thus, animal models are required to explore the cause–effect relationship. Preclinical studies suggest that adolescent alcohol and nicotine co-use may produce additive effects on behavioral outcomes. In adolescent male rats, concurrent intravenous self-administration of nicotine and alcohol was more reinforcing than either drug alone—an effect not observed in adults.10 A combination of the two substances also increases ambulatory activity, and decreases anxiety-like behaviors in adolescent, but not adult, males.11
Despite the paucity of preclinical research that explores the long-term ramifications of adolescent nicotine and alcohol co-use, both drugs have been examined in isolation. Nicotine or alcohol exposure in adolescence increases the risk of substance use later in life, where alcohol exposure in adolescent rats increased voluntary ethanol drinking and preference in adulthood,12 and adolescent nicotine-exposed rodents showed increased vulnerability to nicotine’s reinforcing effects and enhanced reward responses to other drugs as adults.13 These drug-induced neuroadaptations may result in the sensitization of reward-incentive processes such that reward-related stimuli acquire enhanced salience.14 Repeated exposure to alcohol or nicotine in adolescent rats increased conditioned approach toward reward-associated cues in adulthood.15–17 Moreover, adult rodents exhibit long-term impairments across several cognitive domains as a consequence of alcohol exposure in adolescence, including spatial working memory18 and fear retention.19 Similarly, rats treated with nicotine during adolescence display long-lasting dysfunctions in attention, impulsive behavior20 and serial pattern learning.21
To date, no preclinical studies have directly tested the effects of adolescent alcohol drinking and nicotine vapor co-exposure on reward- and cognitive-related behaviors in adulthood. Given the known sex differences in the response to alcohol and nicotine,22 as well as sex differences in brain development,23 it is imperative to study these effects in both male and female rodents. We hypothesized that the co-exposure to nicotine vapor and alcohol in adolescence would produce pronounced changes in reward- and cognitive-associated behavior when compared with exposure to either drug alone. Specifically, we predicted that the co-exposure to alcohol and vaporized nicotine would increase alcohol intake and preference in adulthood, enhance the incentive salience of reward-predictive stimuli and impair fear associative learning and memory.
Methods
All procedures were approved by the Animal Care Committee at the University of Guelph under Canadian Council on Animal Care Guidelines. Male (N = 39) and female (N = 35) Sprague-Dawley rats aged postnatal day (PND) 21 upon arrival were obtained from Charles River with lactating dams (Montreal, Canada), weaned on PND25–26, and were given nine days of acclimatization to the facility before any handling or experimental procedures began. The animals were maintained on a 12-hour light–dark cycle (7:00 am–7:00 pm). Upon weaning, same-sex littermates were housed two per cage. On PND28, pair-housed rats were separated by a mesh divider to measure individual food, water and alcohol intake while still having social contact. All animals had access to standard chow and water ad libitum until behavioral testing commenced. The rats were randomly assigned to one of four exposure groups: (1) Vehicle Vapor (control for Nicotine)/Water(control for Alcohol) (Control [CO]) (N = 8/sex); (2) Vehicle Vapor/Alcohol (AO) (N = 11/9, male/female); (3) Nicotine Vapor/Water (NO) (N = 8/sex); or (4) Nicotine Vapor/Alcohol (AN) (N = 11/9, male, female). (Supplementary Figure S1 schematically illustrates the experimental protocol).
Drug Preparation
Nicotine
JUUL mint flavored 5% nicotine e-liquid pods (59 mg/mL) (JUUL Labs, Toronto, Canada) (one of the most popular JUUL flavors among adolescents24) or vehicle e-liquid without flavoring was administered. The vehicle e-liquid was a mixture of 30:70 propylene glycol (PG) to vegetable glycerine (VG), representative of commercially available JUUL pods.25
Alcohol
Ethanol (Commercial Alcohols, Brampton, Canada) was diluted to a final concentration of 10% vol/vol in tap water.12
Vapor Administration Procedure
Beginning at PND30, passive nicotine vapor exposure was conducted using the OpenVape apparatus.26 Animals in the nicotine vapor only (NO) and concurrent alcohol drinking and nicotine vapor (AN) exposure groups received nicotine vapor. Animals in the control (CO) and alcohol only (AO) exposure groups received vehicle vapor. All animals received 10 minutes of vapor exposure a day (beginning at 7:00 am) for 16 days, with each epoch of pump activation resulting in 10, 2-second puffs/min.26 The nicotine exposure duration was chosen based on previous findings from Frie et al.26 demonstrating behavioral and pharmacological relevant results following nicotine vapor exposure using the same vapor apparatus in adult and adolescent male rats. Animals from each exposure group and sex were placed in the exposure chamber together.
Adolescent Alcohol Exposure
During the duration of vapor exposure (PND30–46), rats assigned to AO and AN exposure groups were given continuous access (except during vapor exposure) to 10% ethanol and tap water in a two-bottle preference design (described in detail below). Rats assigned to the CO and NO exposure groups received continuous access (except during vapor exposure) to two bottles of tap water.
Two-Bottle Preference Test
The effects of adolescent voluntary alcohol drinking (with or without nicotine vapor inhalation) on adulthood alcohol consumption and preference were quantified using a two-bottle alcohol preference test.27 Beginning at PND110, all rats were given 24-hour access to 10% ethanol and tap water for 4 days. To prevent location preference development, the position of the bottles was switched daily. Bottle weights were recorded daily. The main outcome measurements were alcohol consumption (in g/kg) and alcohol preference (%). Alcohol preference was calculated as:
Effects of Adolescent Nicotine Vapor and Alcohol Co-exposure on Reward-Related Behavior in Adulthood
The incentive salience of rewarding cues can be inferred utilizing Pavlovian conditioned approach (PCA) tasks, wherein a previously neutral stimulus such as a lever, becomes a conditioned stimulus (CS) when it is repeatedly paired with the delivery of an unconditioned stimulus (US) such as a food reward.28 Animals that develop this conditioned response (CR) to approach the CS are termed sign-trackers, whereas others will preferentially approach the reward delivery location when the cue is presented; termed goal-trackers. Though both behaviors require associative learning, the degree of sign-tracking represents the incentive salience that is attributed to the cue.
Pavlovian Conditioned Approach Apparatus
Behavioral procedures were carried out in standard operant chambers (Coulbourn Instruments, Allentown, PA) enclosed in sound-attenuating cubicles (76.2 × 46.99 × 44.96 cm). The chambers were outfitted with a food cup located in the center of the right sidewall and retractable levers were positioned to the left and right of the food cup. The cubicles also contained surveillance cameras to monitor behaviors during testing. Data collection from Graphic State software (Coulbourn Instruments, Allentown, PA) was transformed using a customized Microsoft Excel macro.
Behavioral Procedure
Beginning on PND72, body weights were reduced to 85% of baseline body weight. All rats remained food restricted until the completion of the experiment and were fed after each testing session. All rats were given 45 mg Dustless Precision sucrose banana flavored pellets (Bio-Serv, Flemington, NJ, product #F0024) in their home cages for 1 day to reduce potential neophobia. On the first day of the experiment (PND85), each rat was assigned to a chamber and received one 30-min magazine training session, during which a single banana pellet was freely delivered on a random time 30-second schedule. PCA testing began 24 hours following magazine training and lasted 12 days. Each daily testing session was 60 minutes in duration and consisted of 25 CS+ and 25 CS− trials with an average inter-trial interval (ITI) of 60 seconds. CS+ trials consisted of a 10-second extension of a lever followed by the delivery of two banana pellets upon lever retraction. During CS− trials, a 10-second extension of the other lever occurred, but no reinforcer was delivered upon retraction. The presentation of the CS was pseudorandom such that no more than two of the same CS presentations could occur consecutively. The assignment of the left and right levers as either CS+ or CS− was counterbalanced across animals and within exposure groups.
Effects of Adolescent Vaporized Nicotine and Alcohol Co-exposure on Associative Memory in Adulthood
The fear conditioning paradigm is used to assess associative learning and memory. Fear conditioning is induced when an innocuous CS is contingently paired with an aversive US that reflexively creates an unconditioned response. Through the formation of a conditioned and unconditioned association, the CS will come to elicit a CR, similar to that of innate fear responses.29
Fear Conditioning Apparatus
Experiments were conducted in four standard fear-conditioning chambers (Med Associates Inc., St. Albans, VT; 29.53 × 23.5 × 20.96 cm) enclosed in sound-attenuating cubicles (63.5 × 36.83 × 74.93 cm). A tone generator presented an auditory cue (90 dB, 2-kHz) as the CS. The steel bars were wired to a shock generator to deliver an electric foot shock (1.0 mA) as the US. Chambers were outfitted with either a lemon or vanilla scent and flat or zig-zag grid floors as contextual cues, with use of these cues counterbalanced across animals within each exposure group. Freezing behavior, defined as total motor immobility except for movement necessitated by respiration,30 was recorded and analyzed using Video Freeze Software. On the cue testing day, animals were introduced to a novel context arrangement (eg, zig-zag grid floor substituted for flat grid floors, lemon substituted for vanilla scent) and a plastic sheet that rounded the chamber walls was inserted to provide a new context.
Behavioral Procedure
As previously described,31 the training session consisted of five 10-second tone presentations followed by foot-shock delivery during the last 2 seconds of the tone with an ITI of 64 seconds. Fear conditioning began on PND103 for all animals, with the first trial starting 3 minutes after the rat is placed in the chamber. After a 24-hour period, freezing to the context was assessed by returning the rats to their original chamber for 8 minutes, with no tone or shock presented. One day later, fear conditioning to the tone was assessed by placing rats in a novel context. Following an initial 30-second delay, the tone was presented 20 times for 10 seconds each with a 30-second ITI between tone presentations. No shock was delivered to the animals. Incidences of freezing were recorded during the ITIs on training and tone test sessions, and in 64-second bins during the context test session.31
Statistical Analyses
Data analysis was performed using IBM SPSS Statistics 27 (Armonk, NY) software. All data will be available upon publication at https://www.khokharlab.com/.
Two-Bottle Preference Test Data Analysis
Average alcohol intake and preference in adolescence and adulthood were compared both within- and between- ages using a two-way between-subject ANOVA with adolescent nicotine exposure and age as between-subject factors, for males and females in groups exposed to alcohol in adolescence. Average alcohol intake and preference data from adulthood only was analyzed by a two-way between-subject ANOVA with adolescent exposure and sex as between-subject factors.
PCA Behavioral Data Analysis
Behavioral dependent measures included: (1) number of lever presses and food cup entries per session, (2) probability of pressing the lever and entering the food cup during a trial, and (3) the latency to press the lever and enter the food cup during the 10-second CS presentation. Given previously observed sex differences in the impact of adolescent nicotine and alcohol exposure on behavior,21,22 males and females were analyzed separately for each behavioral measure using a two-way-repeated measures factorial ANOVA with exposure (nicotine vapor and alcohol) as a between-subject factor and day as a within-subject factor. Average PCA index scores (described below) for males and females were analyzed with a two-way ANOVA with exposure (nicotine vapor and alcohol) as the between-subject factor. Cohen’s d was also calculated as an estimation of effect size. The interpretation criteria for effect sizes were considered small, medium, or large if they corresponded to partial η 2 and Cohen’s d of at least 0.0099 (d = 0.29–0.49), 0.0588 (d = 0.50–0.79) and 0.13790 (d = ≥ 0.80), respectively.
Quantification of Sign- and Goal-Tracking Behavior
A PCA index score was calculated to classify animals as goal-trackers, sign-trackers, or intermediates based on average performance during all 12 sessions.28 The PCA Index score was calculated as the sum of (1) Response Bias (contacting the CS+ lever or food cup entries in relation to total number of CS+ or food cup responses), (2) Probability Difference (difference between the probability of pressing the CS+ lever and probability entering the food cup), and (3) Latency Score (the difference between latency to contact the CS+ lever and latency to enter the food cup), which was then divided by 3. Animals with scores of −1.0 to −0.3 were classified as goal-trackers and animals with scores of +0.3 to +1.0 were classified as sign-trackers. Animals that were within the range of −0.29 to +0.29 were defined as intermediates.
Fear Conditioning Data Analysis
Separate two-way analyses were conducted for males and females given that they display distinct patterns of fear expression.32 Freezing behavior was analyzed using repeated measures ANOVA on each of the 3 days with adolescent exposure (nicotine vapor and alcohol) as the between-subject factor and trial or block (64-second epoch) as the within subjects factor. One male animal in the CO and two male animals in the AN group were excluded due to their freezing being more than 2 standard deviations outside the mean.
Results
Two-Bottle Preference Test
Female Rats Consumed More Alcohol than Males in Adulthood, with Both Sexes Showing Greater Alcohol Preference in Adulthood Compared to Adolescence
No main effect of exposure or an exposure by sex interaction was revealed for alcohol intake or preference in adolescence or adulthood. A two-way ANOVA comparing alcohol preference from adolescence and adulthood revealed a main effect of age for males [F(1,40) = 8.3; p = .006, η p2 = 0.173] and females [F(1,32) = 30.8; p < .05, η p2 = 0.491] where preference for alcohol was higher in adulthood relative to adolescence (Supplementary Figure S2, A and B). A significant main effect of age for average alcohol intake (g/kg) was observed in females [F(1,32) = 9.3; p = .021, η p2 = 0.156] but not in males, indicating alcohol intake was higher in adulthood relative to intake in adolescence for female rats (Supplementary Figure S2, C and D). An overall increase in alcohol intake [F(1,66) = 19.8; p < .05, η p2 = 0.231] and preference [F(1,66) = 21.4; p < .05, η p2 = 0.245] in adulthood was detected in female rats compared to male rats across all exposure groups (Supplementary Figure S2, E and F).
Pavlovian Conditioned Approach
Male Adolescent Nicotine Vapor Exposure Altered Reward-Associated Learning in Adulthood
Sign-tracking was measured as lever pressing during CS+ presentation over the 12 testing sessions. Analysis of the number of lever presses revealed a main effect of session for males [F(3.3,116.9) = 11.4; p < .05, η p2 = 0.246] and females [F(3.0,94.1) = 9.3; p < .05, η p2 = 0.231] with lever pressing increasing across sessions. A main effect of nicotine vapor was reported for males [F(1,35) = 4.9; p = .03, η p2 = 0.123] in the between-subject analysis, with both adolescent nicotine vapor-exposed male groups exhibiting significantly higher lever pressing compared to CO males (d = 1.0) (Supplementary Figure S3, A). No effect of nicotine vapor or alcohol drinking was observed in females (Supplementary Figure S3, B). Analysis of the probability to lever press revealed a main effect of session in males [F(3.5,122.9) = 25.6; p < .05, η p2 = 0.422] and females [F(4.0,124.1) = 24.3; p < .05, η p2 = 0.440] with the probability to press the lever increasing across sessions. A main effect of nicotine vapor exposure was revealed in males [F(1,35) = 9.5; p = .004, η p2 = 0.214] where both nicotine vapor exposed groups displayed a higher probability to press the lever compared to CO (d = 1.2) and AO (d = 0.8) exposure groups (Supplementary Figure S3, C). This effect was not observed in females (Supplementary Figure S3, D). Analysis of latency to lever press revealed a main effect of session in males [F(3.4,117.8) = 24.9; p < .05, η p2 = 0.416] and females [F(4.0,123.4) = 24.7; p < .05, η p2 = 0.443] with latency to press the lever decreasing across sessions. Male rats from both groups exposed to vaporized nicotine in adolescence demonstrated a shorter latency to press the lever [F(1,35) = 9.1; p = .005, η p2 = 0.206] relative to CO (d = 1.2) and AO (d = 0.8) exposure groups (Supplementary Figure S3, E). No differences were present amongst exposure groups in female rats (Supplementary Figure S3, F).
Goal-tracking was measured as food cup entries during CS+ presentation over the 12 testing sessions. Analysis of the number of food cup entries revealed a main effect of session for females [F(3.5,108.9) = 6.7; p < .05, η p2 = 0.177] but not males. There was no effect of nicotine vapor or alcohol exposure in either males or females (Supplementary Figure S4, A and B). Analysis revealed a main effect of session for the probability and latency to enter the food cup in males [F(2.4,85.6) = 10.9; p < .05, η p2 = 0.237], [F(2.6,91.0) = 24.2; p < .05, η p2 = 0.408] and females [F(2.4,74.9) = 10.6; p < .05, η p2 = 0.256], [F(3.1,96.2) = 25; p < .05, η p2 = 0.446]. An effect of nicotine vapor for males in the probability and latency to enter the food cup was observed [F(1,35) = 5.1; p = .03, η p2 = 0.128], [F(1,35) = 6.6; p = .02, η p2 = 0.158], with AN exposed males demonstrating a lower probability and longer latency to enter the food cup compared to CO (d = 1.1) and AO (d = 1.0) exposed males (Supplementary Figure S4, C and E). In contrast, nicotine vapor exposure had no effect in females for either the probability or latency to enter the food cup (Supplementary Figure S4, D and F). A repeated measures ANOVA for CS− approach revealed no significant effects of session and exposure across all behavioral metrics for males and females (data not shown).
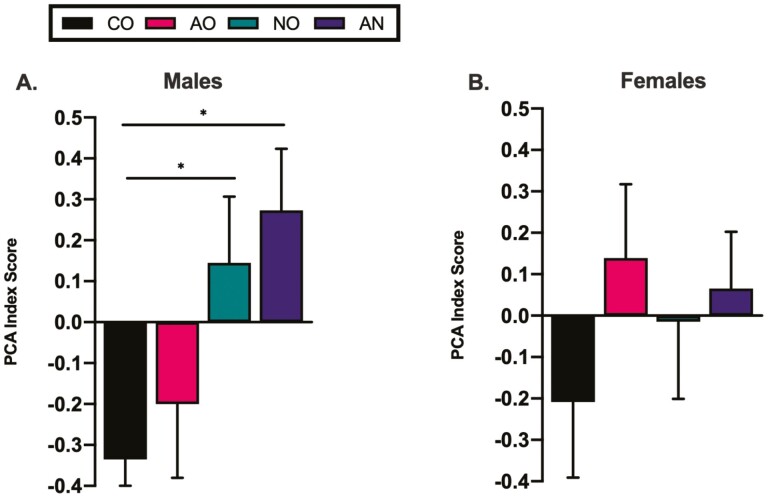
Analysis of the average PCA index score revealed a main effect of nicotine vapor [F(1,35) = 8.4; p = .006, η p2 = 0.194] in males exposed to vaporized nicotine. According to Cohen’s criteria, a large effect was observed in the comparison between AN and CO (d = 1.2) exposed males, AN and AO (d = 0.9) exposed males, and between NO and CO (d = 0.9) exposed males, where male groups exposed to vaporized nicotine had a higher PCA index score (Figure 1A). No such effect was observed in females (Figure 1B). Co-exposure of alcohol and nicotine vapor increased the number of male sign-trackers to 63.6% relative to male controls of 0%. Conversely, co-exposure of alcohol and nicotine vapor had no effect on the number of female sign-trackers relative to female controls with 22.2% of co-exposed females presenting with a sign-tracking phenotype compared to 25% of female controls (Supplementary Table 1).
Figure 1.
Lever press and food cup entry number, probability and latency were combined into a PCA index score for each session and averaged over the 12 PCA sessions. PCA index scores are used to classify rats as sign-trackers (STs) (score +0.3 to +1.0), intermediates (Int.) (score −0.29 to +0.29) and goal-trackers (GTs) (score −1.0 to −0.3). (A) Male groups exposed to vaporized nicotine in adolescence had a higher PCA index score compared to CO males. (B) No significant differences for PCA index scores were detected in females. The data are presented as mean ± SEM. *p ≤ .05 significant difference between males that received vaporized nicotine and males that did not receive vaporized nicotine in adolescence.
Fear Conditioning
Adolescent Alcohol Exposure in Male Rats Impaired Learning and Contextual Fear Memory
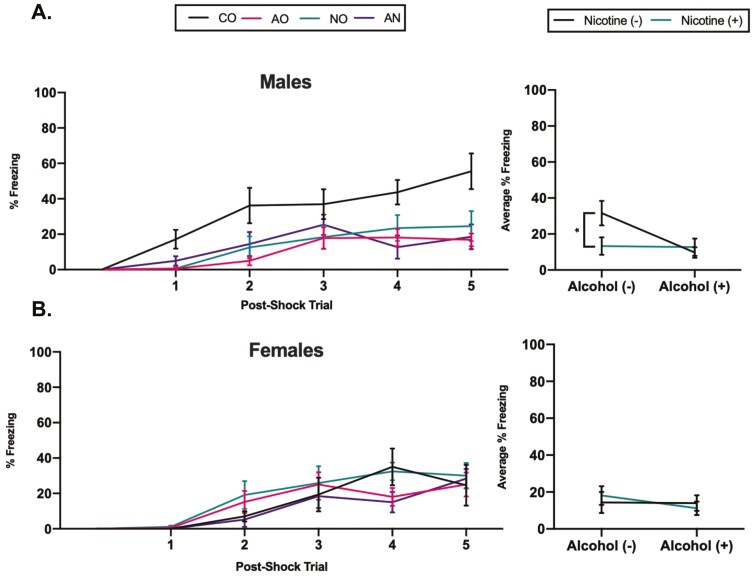
A main effect of post-shock trial for males [F(3.6,116.4) = 26.8; p < .05, η p2 = 0.456] and females [F(3.1,93.4) = 22.5; p < .05, η p2 = 0.428] was observed on the conditioning day, indicating a progressive increase in freezing behavior as trials proceeded. During the conditioning session, a main effect of alcohol [F(1,32) = 9.2; p = .005, η p2 = 0.224], nicotine vapor [F(1,32) = 4.3; p = .46, η p2 = 0.119] and an alcohol-by-nicotine vapor interaction [F(1,32) = 8.3; p = .007, η p2 = 0.206] was detected in males (Figure 2A), where male adolescent drug exposure resulted in significant deficits in fear acquisition relative to CO males. The level of postshock freezing in females was comparable in all exposure groups during training (Figure 2B). Male and female rats in each group exhibited freezing during the context test, which diminished over the course of the session. This was confirmed by a repeated measures ANOVA in which there was a main effect of epoch in males [F(4.3,138.9) = 7.1; p < .05, η p2 = 0.181] and females [F(3.6,106.8) = 5.7; p < .05, η p2 = 0.160]. Analysis revealed a main effect of alcohol in males [F(1,32) = 5.6; p = .02, η p2 = 0.150] where AO and AN exposed males showed less freezing behavior and demonstrated deficits in context-related memory (Supplementary Figure S5, A). There was no main effect of exposure on context-related memory for female rats (Supplementary Figure S5, B). A main effect of trial on cue testing day was revealed for males [F(10.8,345.6) = 8.3; p < .05, η p2 = 0.207] and females [F(8.0,240.1) = 7.2; p < .05, η p2 = 0.194], indicating both sexes demonstrated extinction of the fear response over repeated tone administration. No effect of exposure on cue-related memory was detected for either male or female rats (Supplementary Figure S5, C and D).
Figure 2.
Effect of adolescent drug exposure on conditioned freezing behavior in male and female rats. Adolescent drug exposure significantly impaired fear acquisition in (A) males but not in (B) females. Data are presented as mean ± SEM. *p ≤ .05 significant difference between male exposure groups and CO males.
Discussion
Despite the epidemiological findings indicating frequent associations between e-cigarette use and alcohol consumption,4,5 there is a scarcity of information regarding the long-term behavioral consequences of their co-use during adolescence. Using a preclinical model of moderate voluntary adolescent alcohol intake and passive nicotine vapor administration, the current study found that exposure to alcohol and vaporized nicotine during this critical developmental period promotes unique sex-specific effects on incentive and associative learning processes.
Adult male, but not female, rats exposed to vaporized nicotine in adolescence attributed greater incentive value to reward-predicting cues, evidenced by increased approach toward the cue (ie, CS+), and were more likely to express a sign-tracking phenotype. The current findings confirm previous observations showing that subcutaneous nicotine injections during adolescence produce long-lasting alterations in reward-associated learning in adult male rats.17 Several studies have shown that exposure to substances of abuse promote sign-tracking behaviors,15,16 which may contribute to reduced impulse control, psychomotor sensitization, and addiction vulnerability.33 Impulsivity is especially of interest, as impulsive-like and sign-tracking behaviors have been reported to be correlated34; the increased impulsivity observed in adult rats after adolescent nicotine exposure may provide a potential mechanism underlying the increased sign-tracking behavior that might also contribute to future substance-use vulnerability.20 Our study is also supported by clinical findings suggesting that nicotine use increases attentional bias to drug-associated cues in smokers.35 The ability of nicotine vapor to enhance the incentive value of reward-predicting cues may be relevant to the initiation of future smoking and other drug use. A meta-analysis revealed adolescent e-cigarette users had more than three times the odds of subsequent cigarette use and four times the odds of past 30-day smoking than nonusers.36 Furthermore, in a human laboratory paradigm, the exposure to passive e-cigarette use increased adolescents’ urge to smoke a regular cigarette.37 Based on our observations of increased sign-tracking, and the epidemiological evidence, e-cigarette use in adolescence might pose as a risk factor for future tobacco or other drug use and dependence through increasing the incentive salience associated with these substances.
Adult female rats exposed to nicotine in adolescence appeared to be resistant to the long-lasting incentive-salience related effects observed in male rats. This contrasts prior findings that nicotine exposure in female rodents enhances the expression of sign-tracking behaviors.38 However, these studies were performed in females injected with nicotine in adulthood, whereas repeated injection with nicotine in adolescence reduced approach behaviors in adult female rats.17 These sex differences highlight the potential impact of route of administration, as well as the age of exposure on the long-term effects of adolescent nicotine vapor exposure on reward-related behaviors. Additionally, we observed pronounced sign-tracking behavior in female controls compared to male controls. These findings are consistent with previous reports suggesting sex alone (independent of drug exposure) impacts conditioned approach16,38 where females may be more likely to attribute greater incentive value to reward-related cues. Though, several other factors could account for this sex difference in performance such as differences in exploration,39 learning,40 and impulsivity.41 Follow-up studies are needed to determine the mechanistic differences that underlie these sex-dependent behavioral effects.
Conversely, adolescent exposure to alcohol had no effect on sign-tracking behaviors in adulthood. This adds to the mixed literature suggesting that prior alcohol exposure can lead to either an enhanced15,16,42 or absent16 sign-tracking response. One factor contributing to this discrepancy might be differences in task design; previously, a divergence in behavioral responses between adolescent alcohol-exposed and control animals was detected by the 14th day of testing,42 requiring more training days than our study included. Interestingly, nicotine vapor and alcohol co-exposure led to similar behavioral profiles to nicotine vapor alone in the majority of the PCA measures, suggesting that a synergistic or additive effect of nicotine and alcohol was not present for these behaviors and their combined effect was primarily driven by the actions of nicotine vapor. However, with respect to goal-tracking, males co-exposed to nicotine vapor and alcohol during adolescence, but not nicotine vapor alone, showed decreased goal-tracking behaviors relative to males exposed to alcohol alone, suggesting a potentiation of nicotine vapor’s effects by alcohol. Consistently, co-administration of alcohol and nicotine produces an additive release of dopamine in the nucleus accumbens core compared to each drug in isolation,43 as well as behavioral disruptions not observed following alcohol administration exclusively.44
Male, but not female, rats exposed to alcohol, nicotine or the combination during adolescence exhibited a deficit in fear acquisition compared to controls as denoted by a reduction in the percentage of freezing time across the postshock trials. These results are consistent with previous work suggesting that sex moderates the effects of adolescent drug exposure on cognitive function.45 The reduced fear response in adolescent drug-exposed males may hinge on impairments in underlying learning mechanisms. Our results are also in agreement with others that found no differences in tone freezing responses in adult rats exposed to alcohol or nicotine during adolescence.19,46 Moreover, adolescent alcohol-exposed male rats showed contextual memory deficits in adulthood—a cognitive domain dependent on the hippocampus.47 However, given that alcohol exposure compromised fear acquisition, the context memory deficits may not be due to memory retention, but a result of learning impairments. Nonetheless, these effects corroborate previous literature that have reported alcohol-induced disruptions of contextual fear conditioning during adolescence.19
In contrast to earlier findings with nicotine,46,48 we found no effect of adolescent nicotine vapor exposure during contextual fear conditioning in either male or female rats. However, these inconsistencies may be a result of differing study parameters such as the strain of animal, dose and route of administration, and dependent measure to assess fear conditioning. In fact, studies utilizing freezing as the dependent measure produced a similar lack of effects,49 whereas lick suppression protocols showed adolescent nicotine-induced deficits.48 As mentioned, male rats expressed enhanced freezing behaviors compared to female rats. However, these discrepancies may not reflect genuine learning and memory deficits in females, but rather be a product of our fear assessment method. Male rats are more likely to perform inactive responses such as freezing, while females engage in active responses such as darting representing escape-like behavior, therefore exhibiting lower levels of freezing.32 For that reason, future studies should utilize multiple indices of fear behaviors, particularly when comparing sexes. It is also possible that the lower expression of fear precluded our ability to detect impairments in fear learning in the female rats. While the exact neurobiological basis that underlies this sexual dimorphism remains unknown, these findings add to our current understanding regarding the role of sex and drug use in learning and memory.
Contrary to our predictions, we found that the exposure to nicotine vapor, alcohol or its combination in adolescence had no effect on alcohol intake or preference in adulthood. These results are consistent with previous findings on adolescent nicotine and alcohol exposure that enforced voluntary access to alcohol in adulthood.50,51 However, there are inconsistencies throughout the literature, where previous studies have shown early exposure to alcohol either increased12,52 or decreased27 subsequent alcohol intake. Additionally, both the exposure to nicotine and the co-exposure of nicotine and alcohol in adolescence has been reported to enhance alcohol drinking later in life.10 These conflicting patterns of responses may be dependent on methodological distinctions such as regimen of administration during adolescence and choice of alcohol consumption paradigms used in adulthood. For instance, forced alcohol exposure has been shown to induce stress as opposed to voluntary access, and as a result increases alcohol preference.51 A pharmacokinetic interaction (competitive inhibition) may also be present between alcohol and PG, since both are metabolized by alcohol dehydrogenase in the liver.6,53 This possibility may have confounded our ability to detect the effects of adolescent alcohol and nicotine vapor exposure on alcohol drinking in adulthood, as all animals were exposed to the PG/VG vehicle.
Consistent with previous research,52 we found that female rats consumed significantly more alcohol than male rats. A potential explanation for this sex-specific pattern may be the fact that female rats are less sensitive to the hypnotic effects of alcohol relative to males.54 This insensitivity would serve as a permissive feature, leading females to consume more alcohol before experiencing pharmacological feedback that would moderate their intake. Although animals exposed to alcohol in adolescence did not differ from controls in alcohol consumption during adulthood, within-animal differences in the consumption of alcohol during adolescence compared to later drinking demonstrated increases in preference for both male and female rats. Taken together with the literature, our findings indicate that the effect of adolescent exposure to nicotine vapor, alcohol or the combination in regulating alcohol consumption in adulthood heavily depends on multiple variables, and the complexity of these findings highlight the significance in considering intra-individual differences as opposed to solely group differences in such analyses.
The present study is the first to investigate the long-term sex-specific effects of adolescent concurrent nicotine vapor and alcohol exposure on subsequent behaviors, showing that adolescent alcohol drinking, vaporized nicotine and their combined exposure impact reward-driven and cognitive-associated behaviors later in life. We did not find additive effects of the combination of alcohol drinking and nicotine vapor; it is likely that alternate dose combinations may be needed to observe a drug interaction. Furthermore, we did not assess the impact of co-exposure on the pharmacokinetics (ie, blood nicotine or alcohol levels) and pharmacodynamics of individual drugs, and how they may be influenced by age or sex. This is of critical importance considering that the majority of our findings were sex-specific. Future studies incorporating assessments of plasma drug levels and receptor expression (eg, nicotinic acetylcholine receptors) will be conducted to establish the potential contribution of such pharmacokinetic and pharmacodynamic changes in order to provide better insight into these observed behavioral results. Moreover, there may be additional differences between males and females (eg, respiration rates) that might alter the pharmacokinetic profiles of nicotine vapor exposure, thereby warranting careful study of these parameters. Nevertheless, our results add to the growing list of findings that highlight the sex-related distinctions that emerge and call attention to the importance of utilizing both sexes when measuring behavioral and cognitive outcomes. With the recent escalation of e-cigarette use among teens and its association with the consumption of alcohol, these findings underscore the importance of studying the causal consequences of e-cigarette and alcohol co-use during adolescence, and ultimately elucidate the neurobiological underpinnings that drive the effects of these drugs.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://academic.oup.com/ntr.
Contributor Information
Jessica Ruffolo, Department of Biomedical Sciences, University of Guelph, Guelph, ON, Canada; Collaborative Neurosciences Graduate Program, University of Guelph, Guelph, ON, Canada.
Jude A Frie, Department of Biomedical Sciences, University of Guelph, Guelph, ON, Canada; Collaborative Neurosciences Graduate Program, University of Guelph, Guelph, ON, Canada.
Hayley H A Thorpe, Department of Biomedical Sciences, University of Guelph, Guelph, ON, Canada; Collaborative Neurosciences Graduate Program, University of Guelph, Guelph, ON, Canada.
Malik Asfandyaar Talhat, Department of Biomedical Sciences, University of Guelph, Guelph, ON, Canada.
Jibran Y Khokhar, Department of Biomedical Sciences, University of Guelph, Guelph, ON, Canada; Collaborative Neurosciences Graduate Program, University of Guelph, Guelph, ON, Canada.
Funding
This work was supported by the Canadian Institutes of Health Research (CIHR) Catalyst (Grant Number 442011) awarded to JYK and supported by CIHR Vanier Graduate Scholarship awarded to JAF.
Declaration of Interests
None declared.
Authors’ Contribution
JR, JAF, HHAT, and JYK conceptualized the paper. JR, JAF, HHAT, and MAT performed experiments. JR and JAF performed analyses. JR wrote the manuscript and JYK, JAF, and HHAT provided manuscript revisions and finalized the manuscript for submission; all authors have given feedback on the final manuscript and approved its submission. JR and JAF equally contributed to this study.
References
- 1. Miech R, Leventhal A, Johnston L, O’Malley PM, Patrick ME, Barrington-Trimis J. Trends in use and perceptions of nicotine vaping among US youth from 2017 to 2020. JAMA Pediatr. 2021;175(2):185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Swendsen J, Burstein M, Case B, et al. Use and abuse of alcohol and illicit drugs in US adolescents: results of the National Comorbidity Survey-Adolescent Supplement. Arch Gen Psychiatry. 2012;69(4):390–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Crummy EA, O’Neal TJ, Baskin BM, Ferguson SM. One is not enough: understanding and modeling polysubstance use. Front Neurosci. 2020;14:569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zuckermann AME, Williams G, Battista K, de Groh M, Jiang Y, Leatherdale ST. Trends of poly-substance use among Canadian youth. Addict Behav Rep. 2019;10:100189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rothrock AN, Andris H, Swetland SB, et al. Association of E-cigarettes with adolescent alcohol use and binge drinking-drunkenness: a systematic review and meta-analysis. Am J Drug Alcohol Abuse. 2020;46(6):684–698. [DOI] [PubMed] [Google Scholar]
- 6. Frie JA, Nolan CJ, Murray JE, Khokhar JY.. Addiction-related outcomes of nicotine and alcohol co-use: new insights following the rise in vaping. Nicotine Tobacco Res. 2022;24(8):1141–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marshall AM, Heffernan T, Hamilton C. The synergistic impact of excessive alcohol drinking and cigarette smoking upon prospective memory. Front Psychiatry. 2016;7:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Durazzo TC, Mon A, Gazdzinski S, Meyerhoff DJ. Chronic cigarette smoking in alcohol dependence: associations with cortical thickness and N-acetylaspartate levels in the extended brain reward system. Addict Biol. 2013;18(2):379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hamidullah S, Thorpe HHA, Frie JA, Mccurdy RD, Khokhar JY. Adolescent substance use and the brain: behavioral, cognitive and neuroimaging correlates. Front Hum Neurosci. 2020;14:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lárraga A, Belluzzi JD, Leslie FM. Nicotine increases alcohol intake in adolescent male rats. Front Behav Neurosci. 2017;11:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cross SJ, Leslie FM. Combined nicotine and ethanol age-dependently alter neural and behavioral responses in male rats. Behav Pharmacol. 2021;32(4):321–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alaux-Cantin S, Warnault V, Legastelois R, et al. Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology. 2013;67:521–531. [DOI] [PubMed] [Google Scholar]
- 13. Alajaji M, Lazenka MF, Kota D, et al. Early adolescent nicotine exposure affects later-life cocaine reward in mice. Neuropharmacology. 2016;105:308–317. [DOI] [PubMed] [Google Scholar]
- 14. Berridge KC, Robinson TE. Liking, wanting, and the incentive-sensitization theory of addiction. Am Psychol. 2016;71(8):670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McClory AJ, Spear LP. Effects of ethanol exposure during adolescence or in adulthood on Pavlovian conditioned approach in Sprague-Dawley rats. Alcohol. 2014;48(8):755–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Madayag AC, Stringfield SJ, Reissner KJ, Boettiger CA, Robinson DL. Sex and adolescent ethanol exposure influence Pavlovian conditioned approach. Alcohol Clin Exp Res. 2017;41(4):846–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Quick SL, Olausson P, Addy NA, Taylor JR. Repeated nicotine exposure during adolescence alters reward-related learning in male and female rats. Behav Brain Res. 2014;261:171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. White AM, Ghia AJ, Levin ED, Swartzwelder HS. Binge pattern ethanol exposure in adolescent and adult rats: differential impact on subsequent responsiveness to ethanol. Alcohol Clin Exp Res. 2000;24(8):1251–1256. [PubMed] [Google Scholar]
- 19. Broadwater M, Spear LP. Consequences of adolescent or adult ethanol exposure on tone and context fear retention: effects of an acute ethanol challenge during conditioning. Alcohol Clin Exp Res. 2014;38(5):1454–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Counotte DS, Spijker S, Van de Burgwal LH, et al. Long-lasting cognitive deficits resulting from adolescent nicotine exposure in rats. Neuropsychopharmacology. 2009;34(2):299–306. [DOI] [PubMed] [Google Scholar]
- 21. Fountain SB, Rowan JD, Kelley BM, Willey AR, Nolley EP. Adolescent exposure to nicotine impairs adult serial pattern learning in rats. Exp Brain Res. 2008;187(4):651–656. [DOI] [PubMed] [Google Scholar]
- 22. Kasten CR, Carzoli KL, Sharfman NM, et al. Adolescent alcohol exposure produces sex differences in negative affect-like behavior and group I mGluR BNST plasticity. Neuropsychopharmacology. 2020;45(8):1306–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kaczkurkin AN, Raznahan A, Satterthwaite TD. Sex differences in the developing brain: insights from multimodal neuroimaging. Neuropsychopharmacology. 2019;44(1):71–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Davis DR, Morean ME, Bold KW, et al. Cooling e-cigarette flavors and the association with e-cigarette use among a sample of high school students. PLoS One. 2021;16(9):e0256844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Talih S, Salman R, El-Hage R, et al. Characteristics and toxicant emissions of JUUL electronic cigarettes. Tob Control. 2019;28(6):678–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Frie JA, Underhill J, Zhao B, de Guglielmo G, Tyndale RF, Khokhar JY. OpenVape: an open-source e-cigarette vapor exposure device for rodents. eNeuro. 2020;7(5):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hamidullah S, Lutelmowski CD, Creighton SD, et al. Effects of vapourized THC and voluntary alcohol drinking during adolescence on cognition, reward, and anxiety-like behaviours in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2021;106:110141. [DOI] [PubMed] [Google Scholar]
- 28. Meyer PJ, Lovic V, Saunders BT, et al. Quantifying individual variation in the propensity to attribute incentive salience to reward cues. PLoS One. 2012;7(6):e38987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Curzon P, Rustay NR, Browman KE. Frontiers in neuroscience cued and contextual fear conditioning for rodents. In: Buccafusco JJ, ed. Methods of Behavior Analysis in Neuroscience. Boca Raton, FL: CRC Press/Taylor & Francis; 2009. [PubMed] [Google Scholar]
- 30. Fanselow MS. Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci. 1980;15(4):177–182. [DOI] [PubMed] [Google Scholar]
- 31. Keene CS, Bucci DJ. Neurotoxic lesions of retrosplenial cortex disrupt signaled and unsignaled contextual fear conditioning. Behav Neurosci. 2008;122(5):1070–1077. [DOI] [PubMed] [Google Scholar]
- 32. Gruene TM, Flick K, Stefano A, Shea SD, Shansky RM. Sexually divergent expression of active and passive conditioned fear responses in rats. Elife. 2015;4:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tomie A, Grimes KL, Pohorecky LA. Behavioral characteristics and neurobiological substrates shared by Pavlovian sign-tracking and drug abuse. Brain Res Rev. 2008;58(1):121–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tomie A, Aguado AS, Pohorecky LA, Benjamin D. Ethanol induces impulsive-like responding in a delay-of-reward operant choice procedure: impulsivity predicts autoshaping. Psychopharmacology (Berl). 1998;139(4):376–382. [DOI] [PubMed] [Google Scholar]
- 35. Chanon VW, Sours CR, Boettiger CA. Attentional bias toward cigarette cues in active smokers. Psychopharmacology (Berl). 2010;212(3):309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Soneji S, Barrington-Trimis JL, Wills TA, et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults: a systematic review and meta-analysis. JAMA Pediatr. 2017;171(8):788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. King AC, Smith LJ, McNamara PJ, Matthews AK, Fridberg DJ. Passive exposure to electronic cigarette (e-cigarette) use increases desire for combustible and e-cigarettes in young adult smokers. Tob Control. 2015;24(5):501–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stringfield SJ, Madayag AC, Boettiger CA, Robinson DL. Sex differences in nicotine-enhanced Pavlovian conditioned approach in rats. Biol Sex Differ. 2019;10(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Alstott J, Timberlake W. Effects of rat sex differences and lighting on locomotor exploration of a circular open field with free-standing central corners and without peripheral walls. Behav Brain Res. 2009;196(2):214–219. [DOI] [PubMed] [Google Scholar]
- 40. Dalla C, Shors TJ. Sex differences in learning processes of classical and operant conditioning. Physiol Behav. 2009;97(2):229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lovic V, Saunders BT, Yager LM, Robinson TE. Rats prone to attribute incentive salience to reward cues are also prone to impulsive action. Behav Brain Res. 2011;223(2):255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Spoelder M, Tsutsui KT, Lesscher HM, Vanderschuren LJ, Clark JJ. Adolescent alcohol exposure amplifies the incentive value of reward-predictive cues through potentiation of phasic dopamine signaling. Neuropsychopharmacology. 2015;40(13):2873–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tizabi Y, Bai L, Copeland RL Jr, Taylor RE. Combined effects of systemic alcohol and nicotine on dopamine release in the nucleus accumbens shell. Alcohol Alcohol. 2007;42(5):413–416. [DOI] [PubMed] [Google Scholar]
- 44. Rezvani AH, Levin ED. Nicotine-alcohol interactions and cognitive function in rats. Pharmacol Biochem Behav. 2002;72(4):865–872. [DOI] [PubMed] [Google Scholar]
- 45. Noorbakhsh S, Afzali MH, Boers E, Conrod PJ. Cognitive function impairments linked to alcohol and cannabis use during adolescence: a study of gender differences. Front Hum Neurosci. 2020;14:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Portugal GS, Wilkinson DS, Turner JR, Blendy JA, Gould TJ. Developmental effects of acute, chronic, and withdrawal from chronic nicotine on fear conditioning. Neurobiol Learn Mem. 2012;97(4):482–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sanders MJ, Wiltgen BJ, Fanselow MS. The place of the hippocampus in fear conditioning. Eur J Pharmacol. 2003;463(1-3):217–223. [DOI] [PubMed] [Google Scholar]
- 48. Spaeth AM, Barnet RC, Hunt PS, Burk JA. Adolescent nicotine exposure disrupts context conditioning in adulthood in rats. Pharmacol Biochem Behav. 2010;96(4):501–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Smith LN, McDonald CG, Bergstrom HC, et al. Long-term changes in fear conditioning and anxiety-like behavior following nicotine exposure in adult versus adolescent rats. Pharmacol Biochem Behav. 2006;85(1):91–97. [DOI] [PubMed] [Google Scholar]
- 50. Smith AM, Kelly RB, Chen WJ. Chronic continuous nicotine exposure during periadolescence does not increase ethanol intake during adulthood in rats. Alcohol Clin Exp Res. 2002;26(7):976–979. [DOI] [PubMed] [Google Scholar]
- 51. Siegmund S, Vengeliene V, Singer MV, Spanagel R. Influence of age at drinking onset on long-term ethanol self-administration with deprivation and stress phases. Alcohol Clin Exp Res. 2005;29(7):1139–1145. [DOI] [PubMed] [Google Scholar]
- 52. Amodeo LR, Kneiber D, Wills DN, Ehlers CL. Alcohol drinking during adolescence increases consumptive responses to alcohol in adulthood in Wistar rats. Alcohol. 2017;59:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cunningham CA, Ku K, Sue GR. Propylene glycol poisoning from excess whiskey ingestion: a case of high osmolal gap metabolic acidosis. J Investig Med High Impact Case Rep. 2015;3(3):2324709615603722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cha YM, Li Q, Wilson WA, Swartzwelder HS. Sedative and GABAergic effects of ethanol on male and female rats. Alcohol Clin Exp Res. 2006;30(1):113–118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.