Abstract
Aedes-borne viruses (ABVs) such as dengue (DENV), chikungunya (CHIKV), and Zika (ZIKV) contribute significantly to the global burden of infectious diseases, disproportionately affecting disadvantaged populations from tropical and subtropical urban areas. ABVs can be transmitted from female mosquitoes to their progeny by vertical transmission via transovarial and/or trans-egg vertical transmission and contribute to the maintenance of infected-mosquito populations year-round in endemic regions. This study describes the natural infection rate of DENV, CHIKV, and ZIKV in field-caught male Aedes (Sergentomyia) aegypti (Linnaeus) mosquitoes from Mérida, Yucatán, México, as a proxy for the occurrence of vertical virus transmission. We used indoor sequential sampling with Prokopack aspirators to collect all mosquitoes inside houses from ABV hotspots areas. Collections were performed in a DENV and CHIKV post-epidemic phase and during a period of active ZIKV transmission. We individually RT-qPCR tested all indoor collected Ae. aegypti males (1,278) followed by Sanger sequencing analysis for final confirmation. A total of 6.7% male mosquitoes were positive for ABV (CHIKV = 5.7%; DENV = 0.9%; ZIKV = 0.1%) and came from 21.0% (30/143) houses infested with males. Most ABV-positive male mosquitoes were positive for CHIKV (84.8%). The distribution of ABV-positive Ae. aegypti males was aggregated in a few households, with two houses having 11 ABV-positive males each. We found a positive association between ABV-positive males and females per house. These findings suggested the occurrence of vertical arbovirus transmission within the mosquito populations in an ABV-endemic area and, a mechanism contributing to viral maintenance and virus re-emergence among humans in post-epidemic periods.
Keywords: Aedes, male, density, transmission, infection
Graphical Abstract
Graphical Abstract.
Aedes-borne viruses (ABV) such as dengue (DENV), Zika (ZIKV) (family Flaviviridae, genus Flavivirus), and chikungunya (CHIKV) (family Togaviridae, genus Alphavirus) are transmitted by the bites of infected female Aedes (Sergentoyia) aegypti (Linnaeus), considered the primary vector in urban and peri-urban areas (Gubler 2006, Wilder-Smith et al. 2020). Ae. aegypti, generally described as a highly anthropophilic and endophilic mosquito, is considered a very efficient ABV vector even at low population densities (Kuno 1997, Braks et al. 2006, Barrera et al. 2019, Devine et al. 2021). The widespread distribution of ZIKV and CHIKV in areas co-circulating with the four DENV serotypes is a significant public health concern, challenging disease surveillance, clinical diagnosis, and case management, especially in endemic areas (Pistone et al. 2009, Dash et al. 2013, Dzul-Manzanilla et al. 2016, Pavía-Ruz et al. 2018a, Aragao et al. 2019, Dos Reis et al. 2019, Dzul-Manzanilla et al. 2021). With no effective antiviral therapy or commercially available vaccine, surveillance and vector control are the primary approaches to detect and prevent ABVs transmissions in human populations. The implementation of highly effective vector control strategies has been challenging to sustain (Vazquez-Prokopec et al. 2010, Bowman et al. 2016, Manrique-Saide et al. 2020). Early detection of ABV transmission, including asymptomatic carriers, and a more accurate and reliable methodology to quantify ABV natural infection in mosquitoes are key elements for improving ABVs control.
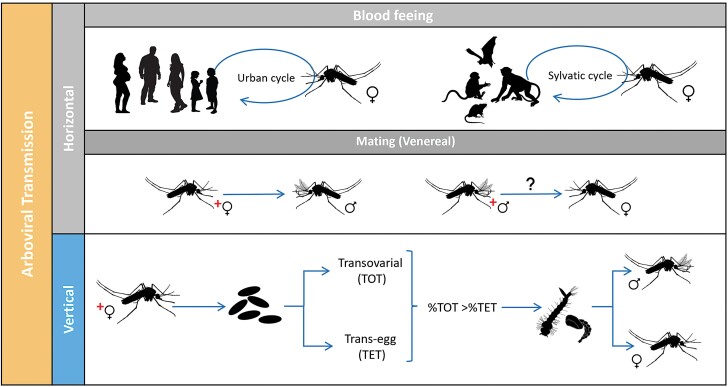
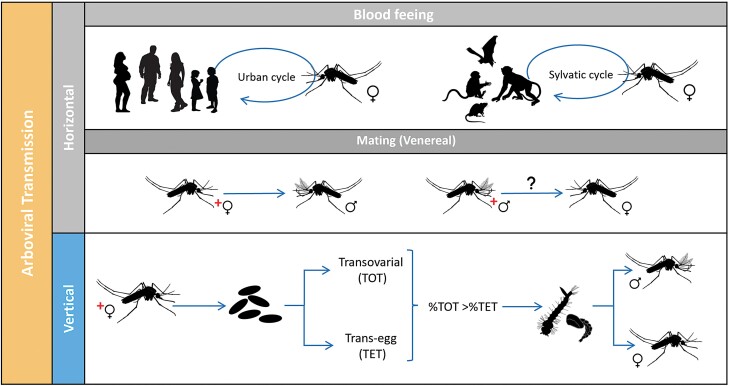
DENV, ZIKV, and CHIKV, as other arboviruses, are horizontally transmitted (HT) between a vertebrate and Ae. aegypti mosquitoes and maintained due to significant heterogeneities at the mosquito – virus – human – environment interface (Lequime et al. 2016, Vazquez-Prokopec et al. 2016). In the Americas, the expected pattern of ABVs transmission is characterized by inter-epidemic periods replaced by aggressive breakouts (Rojas et al. 2018, Segura et al. 2021). What is often challenging to understand is how ABVs are maintained in the population even in the absence of reported illnesses in the passive surveillance system and during unfavorable conditions for vector activity. Mosquito populations fluctuate, impacting human-mosquito contacts and horizontal virus transmission (Fig. 1), due to micro and macro environmental variability such as weather or vector control campaigns that can reduce vector density and thus opportunities for HT (Conway et al. 2014, Erguler et al. 2017, Gould et al. 2017). Additionally, reductions in human susceptibility to ABVs can prevent HT during subsequent epidemic periods and could lead to interannual variability if no new viruses (e.g., DENV serotypes) are introduced into a given area.
Fig. 1.
Potential transmission routes for Aedes-borne viruses: Horizontal (HT) when a female mosquito vector acquires the virus through blood-feeding on an infected vertebrate host (vector-borne transmission) is the most common and efficient route. Another type of HT includes the passage of the virus during mating (venereal transmission, HVT). Vertical transmission (TV), also called hereditary, occurs when an infected female mosquito transmits the virus to its progeny. VT can occur when germinal tissues of the female mosquitoes are infected (transovarial transmission, TOT) or during oviposition (trans-egg transmission, TET).
There are several possibilities for how ABVs can be maintained year-round in a population, the most likely route is introducing or re-introducing ABVs across multiple years from other regions (Lequime et al. 2016), and/or mechanisms where viruses are transmitted between mosquitoes and then to vertebrate host populations, known as mix-mode transmission (Mavale et al. 2010, Lequime et al. 2016). Another possibility is alternative host species or asymptomatic human carriers that may act as a target amplifier host of ABVs (Chevillon et al. 2008, Moghadas et al. 2017, Munoz 2018).
ABVs can be transmitted from female mosquitoes to their progeny by vertical transmission (VT, Fig. 1) before eggs are fertilized (transovarial transmission, TOT) or during fertilization immediately followed by oviposition (trans-egg vertical transmission, TET). While TOT leads to a much higher percentage of progeny that carries the viruses, both ways may contribute to the mix-mode transmission model to maintaining an ABV infected population, at a certain level, to explain year-to-year transmission (Adams and Boots 2010, Lequime and Lambrechts 2014, Lequime et al. 2016, Agboli et al. 2019). Additionally, ABVs may persist during unfavorable periods through the infection of eggs, larvae, or adults (including diapausing individuals) without the need for HT (Lequime et al. 2016). Another route of ABVs transmission at a lower rate could be during mating (horizontal venereal transmission, HVT, Fig. 1). A better understanding of the nature and importance of these alternative routes of viral persistence is fundamental for understanding ABV epidemiology in inter-epidemic periods.
In the published literature, several studies have provided field and laboratory evidence demonstrating the existence of alternative transmission modes of DENV, ZIKV, and CHIKV. Laboratory trials include experimental feeding of field-collected and/or lab-reared mosquitoes with artificially infected blood (Chompoosri et al. 2016, Ciota et al. 2017, Honório et al. 2019, Manuel et al. 2020) or infected blood from positive patients (Goncalves et al. 2020) or in vivo intra-thoracic inoculation of parental mosquitoes (Freier and Rosen 1987, Bosio et al. 1992, Joshi et al. 2002). Despite positive results and persistence of infection over consecutive generations in Ae. aegypti, infections are ephemeral, and the significance of maintenance of mosquito ABV-infections in nature is not yet elucidated.
Evidence from field observations includes detecting ABV-infections by virus isolation and/ or PCR-based molecular assays on immature and adult stages from field-collected Ae. aegypti and Aedes (Sergentoyia) albopictus (Skuse). In Morelos, México, ZIKV has been detected and successfully isolated from larvae hatched from field-collected eggs of Ae. aegypti mosquitoes (Izquierdo-Suzan et al. 2019). Studies carried out in Oaxaca and Acapulco, México, reported similar results for DENV (Günther et al. 2007, Martínez et al. 2014). Additionally, results from entomological collections performed in urban environments in Culiacan and Navolato, Sinaloa, México, revealed infected larvae of Ae. aegypti DENV-2 and DENV-4 (Apodaca-Medina et al. 2018, Torres-Avendano et al. 2021). Previous studies in the Brazilian Amazon have shown similar results (da Costa et al. 2018). In Kenya, sample pools of field-caught adult males, females, and immature stages of Aedes mosquitoes were PCR-positive for DENV and CHIKV (Heath et al. 2020).
Natural ABV-infected male mosquitoes could serve as a proxy for quantifying the rate of alternative ways of viral transmission. In a study carried out in Chennai, Tamil Nadu, India, DENV-2 and DENV-3 positive male adult Ae. aegypti were detected using immunological assays (Arunachalam et al. 2008). Similar findings were reported in Singapore, reporting that male Ae. aegypti and Ae. albopictus were positive for four DENV serotypes (Kow et al. 2001). Most of those studies relied on testing pooled males and originated from sampling the adult population with methods that could not help inferring the natural vertical AVB infection rates and its frequency in nature.
In the current study, we report ABV infections in individually-tested male Ae. aegypti mosquitoes collected by complete removal of vectors from houses using Prokopack aspirators in a previously identified ABV hotspot located in the city of Mérida, Yucatán, México (Vazquez-Prokopec et al. 2009, Bisanzio et al. 2018), and during a DENV and CHIKV postepidemic period and as ZIKV was actively transmitted in the area. We describe the distribution of arbovirus infection in male Ae. aegypti across houses and the association between ABV infections in male Ae. aegypti and the presence of infected female Ae. aegypti, for which infection rates and distributions were previously published (Kirstein et al. 2021).
Materials and Methods
Study Design
Samples were collected from 200 houses located in ABV transmission hotspots in the city of Mérida, Yucatán, México (Bisanzio et al. 2018, Dzul-Manzanilla et al. 2021). Hotspot areas are the results of a risk stratification study using historical human ABV infection data (2008–2022) to identify census tracts within the city where ABV transmission intensity is consistently and significantly higher than expected by chance. The analysis is based on the Getis Gi*(d) statistic (Bisanzio et al. 2018, Dzul-Manzanilla et al. 2021). DENV hotspots are located in the central-southern area of Mérida and were also found to be associated with CHIKV and ZIKV hotspots (Bisanzio et al. 2018, Dzul-Manzanilla et al. 2021). ABV transmission in Mérida peaks during the rainy season (July–November). Collections occurred in 2016 (N = 83 houses) and 2017 (N = 117 houses) between June and December. Houses were located within city blocks where recent (one to two months) ABV human cases were reported by the passive surveillance data collected by the Yucatán Ministry of Health (MOH). Supp Fig. 1 (online only) shows the yearly trend in cases reported to Yucatan’s MOH in relation to our field collections. DENV transmission (human cases) was reported by the MOH during 2016–2017 (SINAVE 2017), while CHIKV was first reported and caused an outbreak during 2015 and sharply decreased with very few cases reported during 2016. As for ZIKV, human cases were first reported in 2016 and continued with an outbreak during 2017 (Bisanzio et al. 2018, Dzul-Manzanilla et al. 2021).
As previously described (Koyoc-Cardeña et al. 2019), the study design originally involved selecting 200 houses where recent (within one month) CHIKV, ZIKV, or DENV occurred. The study team was provided a list of blocks that the MOH identified as having had a human ABV infection within the previous 1–2 mo. No information of the number of cases or how many individuals were infected (or when onset of symptoms occurred) was provided due to patient confidentiality restrictions. The entomological team only had a list of houses to visit, and they were blind to any information about arbovirus infection status. Field technicians from the Collaborative Unit for Entomological Bioassays of the Autonomous University of Yucatán (UCBE-UADY) captured adult indoor resting Ae. aegypti using Prokopack aspirators by active removal sampling over three hours at predetermined intervals of 10 minutes or if no mosquitoes were aspirated in two consecutive rounds. Collectors and field staff wore traditional field clothing, including closed-toe shoes, socks, pants, a long-sleeved shirt, and no personal insecticide or repellent was applied.
Collected mosquitoes were transferred alive to the entomology lab (UCBE-UADY) for sexing and identification. Male and female Ae. aegypti were individually dissected, their heads and bodies were separated and preserved in 1.5 ml vials containing 1ml RNALater (Thermo Fisher Scientific, Waltham, MA) and 1 μl of Tween 20 (Sigma-Aldrich Co. St. Louis, MO) to increase cuticle penetrance and break the superficial tension of the solution. Samples were then stored at −20°C for future virus detection.
RNA Extraction and Molecular Detection of Arboviral Infections
The nucleic acids isolation and detection of DENV, ZIKV, and CHIKV genomes in individually processed adult male and female Ae. aegypti was conducted at the Laboratorio de Virología, Centro de Investigaciones Regionales ‘Dr. Hideyo Noguchi’, Universidad Autónoma de Yucatán. The molecular processing of the samples was performed following previously described procedures (Kirstein et al. 2021). Briefly, RNA was extracted from 3,439 male and female Ae. aegypti (including thorax, abdomen, and extremities) using QIAamp Viral RNA Mini Kit (QIAGEN). Viral RNA was detected by reverse transcription quantitative real-time PCR (RT-qPCR) using a QuantiFast Probe RT-PCR Kit (QIAGEN). RT-qPCR reactions were performed following standard protocols in a Step One Plus Real-Time PCR System (Applied Biosystems). Reactions (samples) were considered positive when a sigmoidal curve was detected at a Ct value of ≤38 cycles of amplification. Supp Table 1 (online only) shows the Primers and probes used to target CHIKV, ZIKV (Lanciotti et al. 2007, 2008), and DENV (personal communication from Davis Arbovirus Research & Training). Positive samples for CHIKV and ZIKV were confirmed by end-point RT-PCR using a high-fidelity polymerase, SuperScript III One-Step RT-qPCR System with Platinum Taq DNA polymerase (Thermo Fisher Scientific) as previously described (Kirstein et al. 2021). Detection of ABVs by RT-qPCR was confirmed by Sanger sequencing of positive amplicons (Macrogen corp., MD, USA).
Sequence Analysis
Raw sequencing data obtained using single forward and reverse primers were filtered and trimmed based on quality scores. BLASTN analysis against the NCBI nucleotides database (https://blast.ncbi.nlm.nih.gov/Blast.cgi) (Acland et al. 2013, Boratyn et al. 2013, Thannesberger et al. 2021) with a minimum E-value threshold of 1E−05, and retention of at least the first 10 hits, was used to assign sequences identity. BLASTN hits that did not fulfill these conditions were considered potential chimeric sequences and discarded. To confirm BLASTN results, high-quality sequences were mapped to the virus reference genome in the software Geneious Prime 2020 using the highest sensitivity and up to 5-times iterations. Forward and reverse sequences were also merged using De Novo Assemble (Geneious Assembler) with high sensitivity, and these consensus sequences were also compared to the GenBank database using NCBI BLASTN and the parameters as mentioned above.
Visualization of electropherograms, nucleotide sequences alignment, and analysis was performed using the software Geneious Prime 2020. Reference genomes were downloaded from RefSeq NCBI (Reference Sequence Database, https://www.ncbi.nlm.nih.gov/refseq/), and the accession numbers of each reference genomes used are: MG967666.1 for CIHKV, NC_012532.1 for ZIKV, and NC_002640.1 for DENV. BLASTN Hit Table shows only the first hit out of 10 retained.
Data Analysis
Absolute Ae. aegypti density per house (termed ‘total catch’) was calculated as the sum of adult males collected across all sampling rounds. For analyses, houses were categorized based on the total catch of male Ae. aegypti as high density (≥10 collected) or low density (<10 collected); houses without mosquitoes were excluded from the analysis (Koyoc-Cardeña et al. 2019). The absolute natural infection rate was calculated as the total number of infected males divided by the total catch per house. A chi-squared test was used to compare infection rates by houses, based on their density category (low vs. high). A general linear model (GLM) was used to quantify the relationship between the ABV infections in males at house level (as response binary variable: infected = 1, not infected = 0) and infected female adult Ae. aegypti density (as an explanatory variable), assuming binomial distribution and logit link function. Additionally, a negative binomial regression model (NegGLM) quantified the association between total ABV-infected males per house (as an explanatory variable) and total ABV-infected females (as response variable) per infested houses. All analyses were performed within the R programming environment (https://www.r-project.org/) using the packages ‘stats’, ‘lme4’, and ‘ggplot2’.
Data Availability
Original data of this study including mosquito collections raw data, DNA raw sequences and sequences analysis, are available online via Mendeley at: https://data.mendeley.com/datasets/whr6x8std7/draft?a=7f8057ae-9ff7-4ca3-821b-7cc63a622302.
Ethics Statement
Protocols for this study were approved by Emory University’s ethics committee under the protocol ID: IRB00082848 and the Ethics and Research Committee from the O’Horan General Hospital from the Ministry of Health of Yucatan, Register No. CEI-0-34-1-14. Written informed consent was obtained from the head of the household prior to mosquito collections.
Results
A total of 3,439 Ae. aegypti were collected: 1,278 (37.2%) were males and 2,161 (62.8%) were females (sex ratio: 1.7:1). The majority of male specimens were collected in 2017, but the average number of male Ae. aegypti per house and their sex ratio were similar between 2016 and 2017 (8.7 and 9.0, respectively) (Table 1). At the house level, 179 out of 200 premises were infested with Ae. aegypti. At least one male Ae. aegypti was found in 79.9% (143 houses infested with Ae. aegypti males/ 179 houses infested with male and female Ae. aegypti) of the houses. The Ae. aegypti male infestation prevalence by premises was similar between 2016 and 2017 (77.8% and 84.5%, respectively). Finally, the distribution of male Ae. aegypti per house was aggregated in a few premises [80.0% (1,023/1,278) of males were collected in 41.2% (59/143) houses infested with male Ae. Aegypti], with five houses harboring at least seven males each and one house having up to 69 males. This pattern was also consistent during both years.
Table 1.
Descriptive measures and infection rates in Aedes aegypti males collected indoors in houses of suspected ABV-infected cases from Yucatán, Mexico, 2016–2017
| Entomologic measure | Collection year | Total | |
|---|---|---|---|
| 2016 | 2017 | ||
| # of houses screened | 83 | 117 | 200 |
| # of infested houses with Ae. aegypti (% of infested houses) | 72 (86.7%) | 107(91.4%) | 179 (89.5%) |
| # of infested houses with Ae. aegypti females (% of positive houses) | 66 (91.7%) | 103 (96.3%) | 169 (94.4%) |
| # of infested houses with Ae. aegypti males (% of positive houses) | 56 (77.8%) | 87 (84.5%) | 143 (79.9%) |
| Total # of Ae. aegypti | 1,341 | 2,098 | 3,439 |
| # of Ae. aegypti females (% of females) | 851 (63.5%) | 1,310 (62.4%) | 2,161 (62.8%) |
| # of Ae. aegypti males (% of males) | 490 (36.5%) | 788 (37.5%) | 1,278 (37.2%) |
| Sex ratio F:M | 1.7:1 | 1.7:1 | 1.7:1 |
| Ae. aegypti males | |||
| # of positive Ae. aegypti males for any virus (% males tested) | 75 (15.3%) | 11 (1.4%) | 86 (6.7%) |
| # of positive Ae. aegypti males for CHIKV (% males tested) | 73 (14.9%) | 0 (0%) | 73 (5.7%) |
| # of positive Ae. aegypti males for DENV (% males tested) | 2 (0.4%) | 10 (1.3%) | 12 (0.9%) |
| # of positive Ae. aegypti males for ZIKV (% males tested) | 0 (0%) | 1 (0.1%) | 1 (0.1%) |
| Fraction of each virus to all ABV positive male mosquitoes (CHIKV, DENV, ZIKV) | (98.6%, 2.6%, 0%) | (0%, 90.9%, 9.9%) | (84.8%, 13.9%, 1.1%) |
| # of houses with positive Ae. aegypti (+) for any virus (% of tested mosquitoes/infested houses) | 30 (41.6%) | 22 (20.5%) | 52 (29.5%) |
| # of houses with positive Ae. aegypti males (+) for any virus (% of males tested/infested houses with males) | 23 (41.1%) | 7 (8.0%) | 30 (21.0%) |
| # of houses (+) CHIKV (% of males tested/infested houses with males) | 21 (37.5%) | 0 (0%) | 21 (14.7%) |
| # of houses (+) DENV (% of males tested/infested houses with males) | 1 (1.8%) | 6 (6.9%) | 7 (4.9%) |
| # of houses (+) ZIKV (% of males tested/ infested houses with males) | – | – | – |
| # of houses (+) ZIKV + DENV (% of males tested/ infested houses with males) | 0 (0%) | 1 (1.1%) | 1 (0.7%) |
| # of houses (+) CHIKV + DENV (% of males tested/ infested houses with males) | 1 (1.8%) | 0 (0%) | 1 (0.7%) |
Natural ABV Infection Rate of Male Ae. aegypti
From the total of 1,278 males collected, 86 (6.7%) were positive for arbovirus infection (Table 1). The majority of ABV-positive male mosquitoes were positive for CHIKV (5.7%), followed by DENV (0.9%) and ZIKV (0.1%). Overall, the fraction of each virus to all ABV-positive male mosquitoes was 84.8% for CHIKV, 13.9% for DENV, and 1.1% for ZIKV. Although the average number of male Ae. aegypti per house was similar between 2016 and 2017 (8.7 and 9.0, respectively), the rate of ABV-positive male Ae. aegypti did differ significantly between years (15.3% in 2016 and 1.4% in 2017; X2 = 93.1, df = 1; P <0.001; Table 1). CHIKV positive males were only prevalent in 2016 (14.9%), while DENV positive males were slightly but not significantly less prevalent in 2016 than in 2017 (0.4–1.3%, respectively). Male mosquitoes positive with ZIKV were detected only in 2017 (0% in 2016 and 0.1% in 2017) (Table 1).
ABV-positive Ae. aegypti males were collected in 30 (21.0%) houses out of 143 infested with male mosquitoes (Table 1). CHIKV-positive male mosquitoes were found in 14.7% of the houses, only during 2016, while 4.9% of the houses were infested with DENV-positive Ae. aegypti males. The only ZIKV-positive male Ae. aegypti was concurrent with DENV-positive males in one house (0.7%) during a collection performed in 2017. Additionally, CHIKV- and DENV-positive male Ae. aegypti were also concurrent in one house (0.7%) (Table 1).
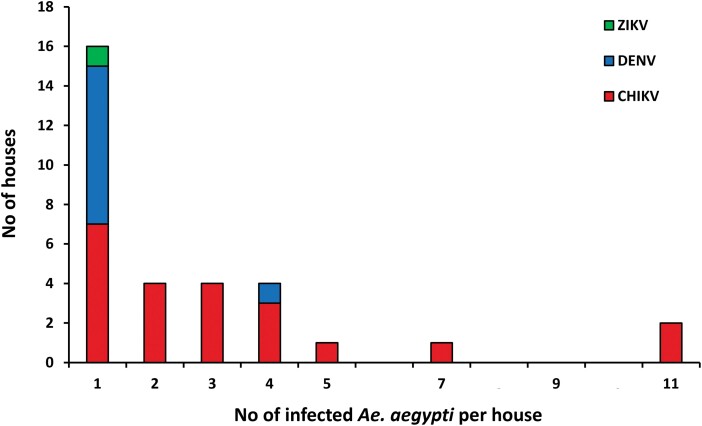
The median number of positive male mosquitoes collected per ABV-positive house was 1 (interquartile range [IQR] = 0–2.3). The ABV-positive male distribution per house was highly heterogeneous, with a maximum of 11 CHIKV-positive males detected in two houses (Fig. 2). Male infection was highly aggregated in a few houses, with 81.3% (70/86) infected mosquitoes collected in 46.6% (14/30) houses infested with male Ae. aegypti (Fig. 2).
Fig. 2.
Distribution of the number of male Ae. aegypti positive for CHIKV, DENV, and ZIKV detected per house in Mérida, Yucatán, México during 2016–2017.
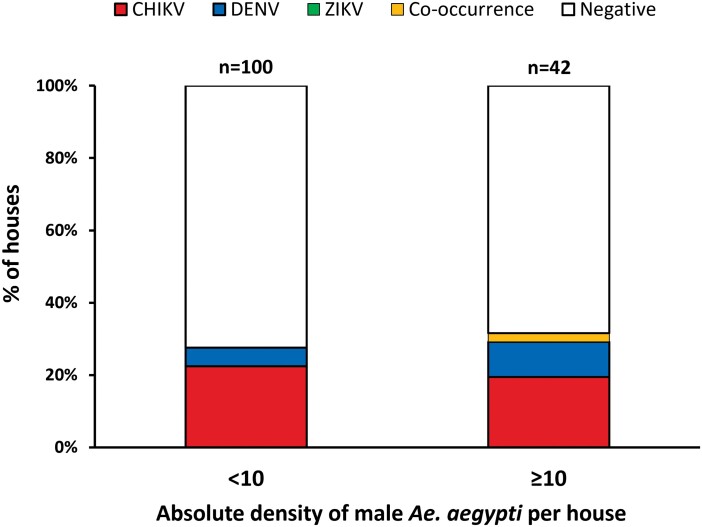
Overall, ABV-positive males were significantly more abundant in houses with a high density of males collected [33.3% (14 houses with positive Ae. aegypti for any virus/42 house infested with 10 or more male Ae. aegypti)] compared to houses with a low density of males [15.8% (16 houses with positive Ae. aegypti for any virus/101 houses infested with less than 10 Ae. aegypti males)] (X2 = 4.3, df = 1; P < 0.05) (Fig. 3). A larger proportion of houses harbored CHIKV-positive males in both low- and high-density houses (13.0 and 19.0%, respectively) compared to DENV-positive mosquitoes (3.0 and 10.0%, respectively); such difference was not significantly significant (X2 = 1.5, df = 1; P > 0.05). Co-occurrence of CHIKV- and DENV-positive males was observed in one high-density house [2.4% (1/42 house infested with 10 or more male Ae. aegypti)]. Additionally, only one ZIKV-positive male Ae. aegypti was detected in co-occurrence with DENV-positive male mosquitoes in a high-density house (2.4%) (Fig. 3).
Fig. 3.
Percentage of houses infested with CHIKV, DENV, and/or ZIKV-positive male Ae. aegypti from low-density (<10 total male mosquitos collected per house, N = 101) and high-density (>10 total male mosquitoes collected per house, N = 42) premises in Yucatán, México. The variable co-occurrence contains percentages of houses where mosquitoes were positive for two viruses within the same house.
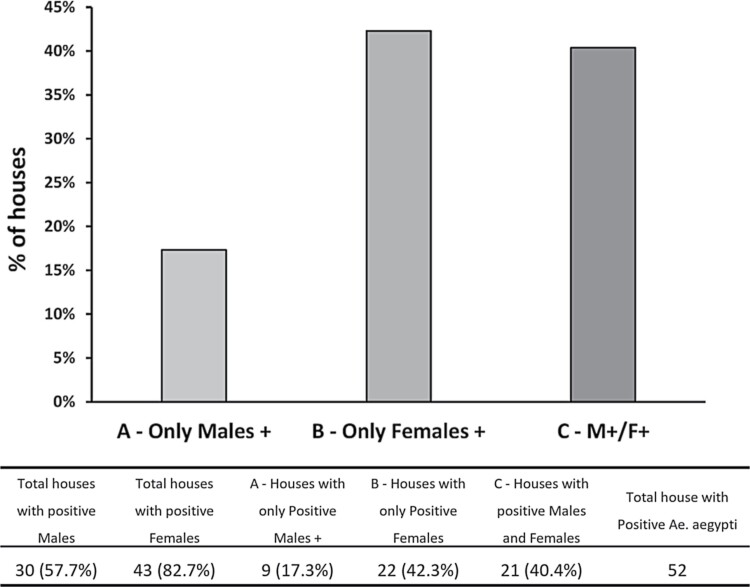
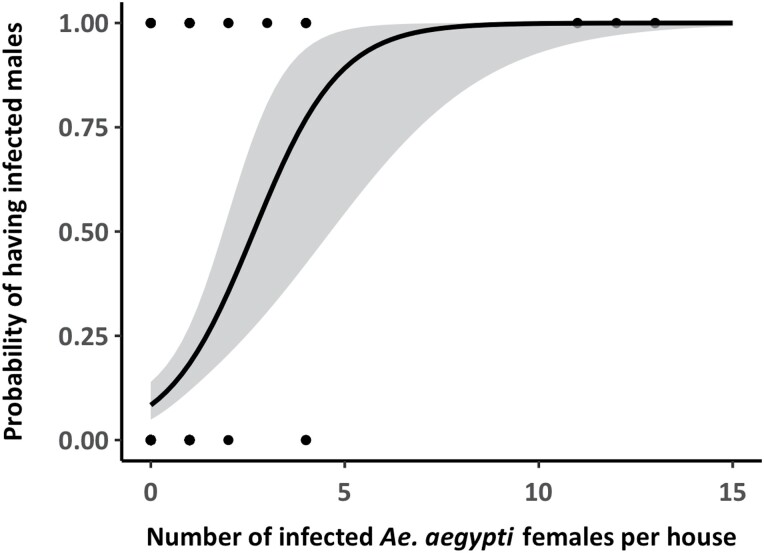
ABV-positive males were found in 16.7% (30 houses with positive Ae. aegypti males for any virus/179 houses infested with male and female Ae. aegypti) of the houses infested with mosquitoes (males and females), while ABV-positive males and females co-occurred in 40.4% (21/52 houses with positive Ae. aegypti for any virus) of houses infested with infected male and female Ae. aegypti mosquitoes (Fig. 4). The number of ABV-positive males was significantly and positively associated with the number of positive females (NegGLM, IRR = 1.3, 65% CI = 1.2–1.5, P < 0.001). The probability of finding ABV-positive males was significantly and positively associated with the presence of infected Ae. aegypti females in the same house (Binomial GLM, OD = 2.4, 95% CI = 1.6–3.9, P < 0.001), with houses having more than four infected females having a probability of observing positive males above 87.3% (Fig. 5).
Fig. 4.
Relationship between infected female Ae. aegypti and positive males at house level.
Fig. 5.
Predicted probability of ABV infection in male Ae. aegypti as a function of the number of infected females detected in the same house. The solid line represents the mean prediction from a binomial generalized linear model (GLM) and gray band the 95% CI of the prediction. The dots indicate the binomial data, with dark dots showing multiple (overlapping) observations.
Nucleotide Sequence Analysis
ABVs detected by RT-qPCR were identified with high fidelity by Sanger sequencing (Table 2). High-quality reads matched DENV and CHIKV genomes published in NCBI GenBank (BLASTN search hit >90 percent identity). DENV-4 was confirmed as the circulating serotype (Table 2). The two sequences that were DENV-4 were also found in houses along with DENV-4 infected female Ae. aegypti. One sample showed a positive peak for RT-qPCR using ZIKV specific primers, but no sequence was obtained. However, we decided to consider this sample ZIKV-positive. Supplementary information (online only) spreadsheets show sequences of quality information and BLASTN results for each sequenced sample; only the first most relevant hits are shown. Nucleotide raw data and BLASTN hits are available at Mendley data (D2, D3).
Table 2.
Summary of sequence analysis of all ABV-positive male Ae. aegypti considered in this study
| Sex | Virus | Positive samples | # of sequences from samples | Blast samples |
|---|---|---|---|---|
| Males | CHIKV | 73 | 58 | 51 |
| DENVa | 12 | 10 | 5 | |
| ZIKV | 1 | No sample sequence | ||
The number is based in samples, for each sample forward and reverse sequences were obtained and analyzed
DENV serotype corresponded to DENV-4.
Discussion
Here, we report the natural infection rates of CHIKV, DENV, and ZIKV in field-caught male Aedes aegypti mosquitoes collected in houses located within identified ABV-disease transmission hotspots in the city of Mérida, Yucatán, México (Dzul-Manzanilla et al. 2021). We found that 6.7% of the total male mosquitoes collected were RT-PCR-positive for an ABV, and up to 20% of houses had evidence of at least one positive male Ae. aegypti.
Multiple factors may be responsible for maintaining arboviral infections in non-epidemic periods, ranging from infrequent virus re-introductions, low-level horizontal transmission, and possible vertical transmission between mosquitoes. Horizontal transmission of ABVs between a vertebrate host and the mosquito vector is considered the most efficient transmission route (Lequime and Lambrechts 2014, Lequime et al. 2016, Agboli et al. 2019), particularly during epidemics periods. Evidence of alternative routes of ABV transmission such as TOT or TET as well as VT between male and female mosquitoes (Kow et al. 2001; Dzul-Manzanilla et al. 2015, 2016; Dos Reis et al. 2019; Heath et al. 2020; Manuel et al. 2020) have been considered infrequent or alternative routes of ABV transmission between mosquitoes (Lequime and Lambrechts 2014, Goncalves et al. 2020). These strategies could play an important role in maintaining the circulation of the virus during inter or post-epidemic periods and unfavorable climate conditions when horizontal transmission becomes difficult (Freier and Rosen 1987, Kuno 1997, Goncalves et al. 2020). However, the epidemiological role of such transmissions is still unclear (Adams and Boots 2010, Lequime and Lambrechts 2014, Grunnill and Boots 2016, Lequime et al. 2016).
The idea of alternative ways of viral transmission in mosquitoes has rarely taken center stage in arbovirology and has remained a debate topic since it was initially discovered more than a century ago (Lequime and Lambrechts 2014, Grunnill and Boots 2016). Studies from laboratory experiments have reported the existence of VT of ABVs such as CHIKV, DENV, and ZIVK in Ae. aegypti and Ae. albopictus with a certain level of success (Arunachalam et al. 2008, Grunnill and Boots 2016, Apodaca-Medina et al. 2018, Aragao et al. 2019). A review of the literature by Adams and Boots (2010) described that vertical infection efficiencies for DENV are 1–4% (Adams and Boots 2010). Another comprehensive review by Grunnill and Boots (2016) reported the calculation of three measures to compute VT of DENV in laboratory experiments [the vertical transmission rate (VTR), the filial infection rate (FIR), and the vertical infection rate (VIR)] demonstrating meager rates of transmission, typically <10/1,000 MIR or MLE. This report points out the existing bias when infections rates are calculated based on pools of samples, inappropriate collection methodologies and sample size, lack of statistical analysis, and rigorous tests of the sensitivity of the different molecular and immunological screening methods in detecting DENVs (and other ABVs) in larvae and other life stages (Grunnill and Boots 2016).
Evidence of ABV VT, detected from field-collected immature stages, and males in México led to estimates of natural VT prevalence <0.1% (Günther et al. 2007, Dzul-Manzanilla et al. 2015, Apodaca-Medina et al. 2018, Torres-Avendano et al. 2021). Our study shows that male Ae. aegypti can have a higher prevalence of ABV infection when the natural infection rate is calculated. Most males were positive for CHIKV (5.7%) and only found during 2016, followed by DENV and ZIKV. These findings are consistent with our previously published data on ABV infections in Ae. aegypti females from the same population, showing a relatively high natural infection rate for CHIKV (6.0%, only in 2016) compared to other virus (Kirstein et al. 2021).
Our first collections started a year after the first case of CHIKV was reported in Mérida and months after a CHIKV outbreak occurred in Mérida (with 1,531 cases reported) during the year 2015. During the study (2016–2017), CHIKV transmission (human cases reported by the MOH) decreased from 11 cases in 2016 to 0 cases in 2017. ZIKV transmission was initially detected in May 2016, with 2,199 cases reported, but transmission decreased to 24 cases in 2017 (Bisanzio et al. 2018, Dzul-Manzanilla et al. 2021). Reports of DENV human cases were low during this period in Mérida (158 cases in 2016 and 30 cases in 2017) (SINAVE 2020). Thus, a collection of more male Ae. aegypti mosquitoes with CHIKV during a post-epidemic year suggest the existence of alternative routes for virus maintenance like VT. To better support our results, we sequenced single positive male samples to confirm the RT-PCR results. Our sequence readings backed up the RT-PCR results. We also found a correlation between the DENV serotype in females and males from the same house. We identified the DENV serotype as DENV-4. This finding is consistent with prior findings from other studies in the area, which identified DENV-4 being the most common serotype in most years, followed by DENV-1 and DENV-3 (Bisanzio et al. 2018, Pavía-Ruz et al. 2018b).
Our results show few male Ae. aegypti mosquitoes positive for DENV lines up with the infection rates found in females (Kirstein et al. 2021) and a low number of reported symptomatic DENV human cases captured by the MOH during 2016–2017. Nevertheless, we expected to find more male and female Ae. aegypti mosquitoes positive for ZIKV since it was first reported in Mérida in 2016 and continued to spread throughout 2017. Furthermore, such findings were confirmed by the Yucatán MOH and by the longitudinal family cohort study ongoing in Mérida at the time of the entomological collections (Pavía-Ruz et al. 2018b). These data sets did not register any specific ZIKV human case within the area or houses where mosquito collections were conducted weeks or months before collection.
We are aware of several limitations of our study. First, because we can only use males as proxies to assume a VT route, we assumed that male infections might follow infections in females (i.e., male VT prevalence would translate to similar VT prevalence in females). Furthermore, since we conducted an observational study, we could not identify females that were infected by VT versus horizontal infection. Similarly, Sanger sequencing was used as confirmation of viral infection, but more in-depth sequencing would have provided information about the genomic match between viruses in males and females, likely increasing the resolution of our data to ascertain the rate of VT in females. Finally, we conducted this evaluation as a cross-sectional study during a period following active ABV infection. While few cases were detected during the study, all selected houses were located within city blocks where recent AVB (within 1–2 mo) infections in humans occurred (Koyoc-Cardeña et al. 2019, Kirstein et al. 2021). Conducting a longitudinal study throughout the low transmission season would have expanded our findings to determine whether VT could be a mechanism for the persistence of ABVs during periods of low transmission.
The distribution of ABV-positive males Ae. aegypti within houses was strongly aggregated, with four houses harboring more than five ABV-positive males each and two houses with up to 11 CHIKV-positive males. Such evidence of aggregation (rarely observed, as mosquitoes are generally pooled per house) supports the hypothesis of an alternative route or transmission, such as VT, as it is very plausible that those males collected at the same house and time were all siblings. Aggregated pockets of infection at the household level were previously reported for the identical houses when female Ae. aegypti were analyzed for ABV infections (Koyoc-Cardeña et al. 2019, Kirstein et al. 2021). Not surprisingly, the probability of finding infected males was associated with the number of ABV-infected females in the house; residences with four or more infected females had a probability of having positive males of more than 80%. It is likely, then, that some of those ABV-infected females (especially in houses with high male infection rates) may have been infected by VT instead of horizontal transmission. Infections in females were quantified from their heads (Koyoc-Cardeña et al. 2019, Kirstein et al. 2021), leading us to assume that if VT occurred, it likely led to potentially infectious females.
In conclusion, we found a large number of male Ae. aegypti positive for DENV, CHIKV, and ZIKV, circulation inside houses in the city of Mérida Yucatán, México, and the number of ABV-positive males was significantly and positively associated with the number of infected females within the same premises. VT of ABVs from female mosquitoes to their progeny may be a critical natural phenomenon that contributes to maintaining an ABV infected population of mosquitoes in endemic areas and may lead to enhance sporadic outbreaks affecting human populations yearly. A better understanding of the nature and importance of these alternative routes of viral persistence is fundamental for understanding ABV epidemiology in inter or post-epidemic periods and facilitating the establishment of active surveillance and vector control strategies to prematurely detect and prevent ABVs transmissions.
Supplementary Material
Acknowledgments
We thank the residents of Mérida, Yucatán, for kindly allowing us to conduct this vital research. Research funding was provided by an Interagency Agreement between USAID and the US Centers for Disease Control and Prevention (CDC: OADS BAA 2016-N-17844; PI, Vazquez-Prokopec G.M.), by the Canadian Institutes of Health Research (CIHR) and IDRC (Preventing Zika disease with novel vector control approaches, Project 108412) by Fondo Mixto CONACyT (México)-Gobierno del Estado de Yucatán (Project YUC-2017-03-01-556), the National Institutes of Health (NIH/NIAID: U01AI148069) and Emory University via the MP3 initiative (Vazquez-Prokopec, PI). The opinions expressed herein are those of the author(s) and do not necessarily reflect the views of Emory University or the U.S. Agency for International Development. The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Contributor Information
Oscar D Kirstein, Department of Environmental Sciences. Emory University, Atlanta, GA, USA.
Guadalupe Ayora Talavera, Laboratorio de Virología, Centro de Investigaciones Regionales “Dr. Hideyo Noguchi”, Universidad Autónoma de Yucatán, Mérida, Yucatán, México.
Zhuoran Wei, Department of Environmental Sciences. Emory University, Atlanta, GA, USA.
Karina J Ciau-Carrilo, Laboratorio de Virología, Centro de Investigaciones Regionales “Dr. Hideyo Noguchi”, Universidad Autónoma de Yucatán, Mérida, Yucatán, México.
Edgar Koyoc-Cardeña, Unidad Colaborativa para Bioensayos Entomológicos, Universidad Autónoma de Yucatán, Mérida, Yucatán, México.
Henry Puerta-Guardo, Laboratorio de Virología, Centro de Investigaciones Regionales “Dr. Hideyo Noguchi”, Universidad Autónoma de Yucatán, Mérida, Yucatán, México; Unidad Colaborativa para Bioensayos Entomológicos, Universidad Autónoma de Yucatán, Mérida, Yucatán, México.
Ester Rodríguez-Martín, Laboratorio de Virología, Centro de Investigaciones Regionales “Dr. Hideyo Noguchi”, Universidad Autónoma de Yucatán, Mérida, Yucatán, México.
Anuar Medina-Barreiro, Unidad Colaborativa para Bioensayos Entomológicos, Universidad Autónoma de Yucatán, Mérida, Yucatán, México.
Azael Che Mendoza, Unidad Colaborativa para Bioensayos Entomológicos, Universidad Autónoma de Yucatán, Mérida, Yucatán, México.
Anne L Piantadosi, Department of Pathology and Laboratory Medicine, Emory University School of Medicine, Atlanta, GA, USA.
Pablo Manrique-Saide, Unidad Colaborativa para Bioensayos Entomológicos, Universidad Autónoma de Yucatán, Mérida, Yucatán, México.
Gonzalo M Vazquez-Prokopec, Department of Environmental Sciences. Emory University, Atlanta, GA, USA.
References Cited
- Acland, A., Agarwala R., Barrett T., Beck J., Benson D. A., Bollin C., Bolton E., Bryant S. H., Canese K., and Church D. M.. 2013. Database resources of the national center for biotechnology information. Nucleic Acids Res. 41: D8–D20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams, B., and Boots M.. 2010. How important is vertical transmission in mosquitoes for the persistence of dengue? Insights from a mathematical model. Epidemics. 2: 1–10. [DOI] [PubMed] [Google Scholar]
- Agboli, E., Leggewie M., Altinli M., and Schnettler E.. 2019. Mosquito-specific viruses-transmission and interaction. Viruses. 11(9): 873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apodaca-Medina, A. I., Torres-Avendaño J. I., Rendón-Maldonado J. G., Torres-Montoya E. H., Flores-López B. A., Del Angel R. M., Velarde-Félix J. S., Salomón-Soto V. M., and Castillo-Ureta H.. 2018. First evidence of vertical infection of Dengue virus 2 in Aedes aegypti mosquitoes from Sinaloa, Mexico. Vector Borne Zoonotic Dis. 18: 231–233. [DOI] [PubMed] [Google Scholar]
- Aragão, C. F., Pinheiro V. C. S., Nunes Neto J. P., Silva E. V. P. D., Pereira G. J. G., Nascimento B. L. S. D., Castro K. D. S., Maia A. M., Catete C. P., and Martins L. C., . et al. 2019. Natural infection of Aedes aegypti by Chikungunya and Dengue type 2 virus in a transition area of North-Northeast Brazil. Viruses. 11(12): 1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunachalam, N., Tewari S., Thenmozhi V., Rajendran R., Paramasivan R., Manavalan R., Ayanar K., and Tyagi B.. 2008. Natural vertical transmission of dengue viruses by Aedes aegypti in Chennai, Tamil Nadu, India. Indian J. Med. Res. 127: 395. [PubMed] [Google Scholar]
- Barrera, R., Amador M., Acevedo V., Beltran M., and Munoz J.. 2019. A comparison of mosquito densities, weather and infection rates of Aedes aegypti during the first epidemics of Chikungunya (2014) and Zika (2016) in areas with and without vector control in Puerto Rico. Med. Vet. Entomol. 33: 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisanzio, D., Dzul-Manzanilla F., Gomez-Dantés H., Pavia-Ruz N., Hladish T. J., Lenhart A., Palacio-Vargas J., González Roldan J. F., Correa-Morales F., and Sánchez-Tejeda G.. 2018. Spatio-temporal coherence of dengue, chikungunya and Zika outbreaks in Merida, Mexico. PLoS Negl. Trop. Dis. 12: e0006298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boratyn, G. M., Camacho C., Cooper P. S., Coulouris G., Fong A., Ma N., Madden T. L., Matten W. T., McGinnis S. D., and Merezhuk Y.. 2013. BLAST: a more efficient report with usability improvements. Nucleic Acids Res. 41: W29–W33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosio, C. F., Thomas R. E., Grimstad P. R., and Rai K. S.. 1992. Variation in the efficiency of vertical transmission of dengue-1 virus by strains of Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 29: 985–989. [DOI] [PubMed] [Google Scholar]
- Bowman, L. R., Donegan S., and McCall P. J.. 2016. Is dengue vector control deficient in effectiveness or evidence?: Systematic review and meta-analysis. PLoS Negl. Trop. Dis. 10: e0004551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braks, M., Juliano S., and Lounibos L.. 2006. Superior reproductive success on human blood without sugar is not limited to highly anthropophilic mosquito species. Med. Vet. Entomol. 20: 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevillon, C., Briant L., Renaud F., and Devaux C.. 2008. The Chikungunya threat: an ecological and evolutionary perspective. Trends Microbiol. 16: 80–88. [DOI] [PubMed] [Google Scholar]
- Chompoosri, J., Thavara U., Tawatsin A., Boonserm R., Phumee A., Sangkitporn S., and Siriyasatien P.. 2016. Vertical transmission of Indian Ocean Lineage of chikungunya virus in Aedes aegypti and Aedes albopictus mosquitoes. Parasites Vectors. 9: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciota, A. T., Bialosuknia S. M., Ehrbar D. J., and Kramer L. D.. 2017. Vertical transmission of Zika virus by Aedes aegypti and Ae. albopictus mosquitoes. Emerg. Infect. Dis. 23: 880–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway, M. J., Colpitts T. M., and Fikrig E.. 2014. Role of the vector in arbovirus transmission. Ann. Rev. Virol. 1: 71–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa, C. F., da Silva A. V., do Nascimento V. A., de Souza V. C., Monteiro D., Terrazas W. C. M., Dos Passos R. A., Nascimento S., Lima J. B. P., and Naveca F. G.. 2018. Evidence of vertical transmission of Zika virus in field-collected eggs of Aedes aegypti in the Brazilian Amazon. PLoS Negl. Trop. Dis. 12: e0006594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash, A., Bhatia R., Sunyoto T., and Mourya D.. 2013. Emerging and re-emerging arboviral diseases in Southeast Asia. J. Vector Borne Dis. 50: 77. [PubMed] [Google Scholar]
- Devine, G. J., Vazquez-Prokopec G. M., Bibiano-Marín W., Pavia-Ruz N., Che-Mendoza A., Medina-Barreiro A., Villegas J., Gonzalez-Olvera G., Dunbar M. W., and Ong O.. 2021. The entomological impact of passive metofluthrin emanators against indoor Aedes aegypti: a randomized field trial. PLoS Negl. Trop. Dis. 15: e0009036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Reis, I. C., Gibson G., Ayllon T., de Medeiros Tavares A., de Araujo J. M. G., da Silva Monteiro E., Rodrigues Aguiar A., de Oliveira J. V., de Paiva A. A. P., and Wana Bezerra Pereira H., . et al. 2019. Entomo-virological surveillance strategy for dengue, Zika and chikungunya arboviruses in field-caught Aedes mosquitoes in an endemic urban area of the Northeast of Brazil. Acta Trop. 197: 105061. [DOI] [PubMed] [Google Scholar]
- Dzul-Manzanilla, F., Martínez N. E., Cruz-Nolasco M., Gutiérrez-Castro C., López-Damián L., Ibarra-López J., Martini A., Torres-Leyva J., Bibiano-Marín W., and Tornez-Benitez C.. 2015. Arbovirus surveillance and first report of chikungunya virus in wild populations of Aedes aegypti from Guerrero, Mexico. J. Am. Mosq. Control Assoc. 31: 275–277. [DOI] [PubMed] [Google Scholar]
- Dzul-Manzanilla, F., Correa-Morales F., Che-Mendoza A., Palacio-Vargas J., Sánchez-Tejeda G., González-Roldan J. F., López-Gatell H., Flores-Suárez A. E., Gómez-Dantes H., and Coelho G. E.. 2021. Identifying urban hotspots of dengue, chikungunya, and Zika transmission in Mexico to support risk stratification efforts: a spatial analysis. Lancet Planet. Health. 5: e277–e285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzul-Manzanilla, F., Martinez N. E., Cruz-Nolasco M., Gutierrez-Castro C., Lopez-Damian L., Ibarra-Lopez J., Martini-Jaimes A., Bibiano-Marin W., Tornez-Benitez C., and Vazquez-Prokopec G. M., . et al. 2016. Evidence of vertical transmission and co-circulation of chikungunya and dengue viruses in field populations of Aedes aegypti (L.) from Guerrero, Mexico. Trans. R Soc. Trop. Med. Hyg. 110: 141–144. [DOI] [PubMed] [Google Scholar]
- Erguler, K., Chandra N. L., Proestos Y., Lelieveld J., Christophides G. K., and Parham P. E.. 2017. A large-scale stochastic spatiotemporal model for Aedes albopictus-borne chikungunya epidemiology. PLoS One. 12: e0174293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freier, J. E., and Rosen L.. 1987. Vertical transmission of dengue viruses by mosquitoes of the Aedes scutellaris group. Am. J. Trop. Med. Hyg. 37: 640–647. [DOI] [PubMed] [Google Scholar]
- Goncalves, D. D. S., Hue K. D. T., Thuy V. T., Tuyet N. V., Thi G. N., Thi Thuy V. H., Xuan T. H. T., Thi D. L., Vo L. T., and Le Anh Huy H., . et al. 2020. Assessing the vertical transmission potential of dengue virus in field-reared Aedes aegypti using patient-derived blood meals in Ho Chi Minh City, Vietnam. Parasites Vectors. 13: 468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould, E., Pettersson J., Higgs S., Charrel R., and De Lamballerie X.. 2017. Emerging arboviruses: why today? One Health. 4: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunnill, M., and Boots M.. 2016. How important is vertical transmission of dengue viruses by mosquitoes (Diptera: Culicidae)? J. Med. Entomol. 53: 1–19. [DOI] [PubMed] [Google Scholar]
- Gubler, D. J. 2006. Dengue/dengue haemorrhagic fever: history and current status. InNovartis foundation symposium. John Wiley,Chichester, New York. [DOI] [PubMed] [Google Scholar]
- Günther, J., Martínez-Muñoz J. P., Pérez-Ishiwara D. G., and Salas-Benito J.. 2007. Evidence of vertical transmission of dengue virus in two endemic localities in the state of Oaxaca, Mexico. Intervirology. 50: 347–352. [DOI] [PubMed] [Google Scholar]
- Heath, C. J., Grossi-Soyster E. N., Ndenga B. A., Mutuku F. M., Sahoo M. K., Ngugi H. N., Mbakaya J. O., Siema P., Kitron U., and Zahiri N.. 2020. Evidence of transovarial transmission of Chikungunya and Dengue viruses in field-caught mosquitoes in Kenya. PLoS Negl. Trop. Dis. 14: e0008362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honório, N. A., Wiggins K., Eastmond B., Câmara D. C. P., and Alto B. W.. 2019. Experimental vertical transmission of chikungunya virus by Brazilian and Florida aedes albopictus populations. Viruses. 11: 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo-Suzan, M., Zarate S., Torres-Flores J., Correa-Morales F., Gonzalez-Acosta C., Sevilla-Reyes E. E., Lira R., Alcaraz-Estrada S. L., and Yocupicio-Monroy M.. 2019. Natural vertical transmission of Zika virus in larval Aedes aegypti populations, Morelos, Mexico. Emerg. Infect. Dis. 25: 1477–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi, V., Mourya D., and Sharma R.. 2002. Persistence of dengue-3 virus through transovarial transmission passage in successive generations of Aedes aegypti mosquitoes. Am. J. Trop. Med. Hyg. 67: 158–161. [DOI] [PubMed] [Google Scholar]
- Kirstein, O. D., Ayora-Talavera G., Koyoc-Cardeña E., Chan Espinoza D., Che-Mendoza A., Cohuo-Rodriguez A., Granja-Pérez P., Puerta-Guardo H., Pavia-Ruz N., and Dunbar M. W.. 2021. Natural arbovirus infection rate and detectability of indoor female Aedes aegypti from Mérida, Yucatán, Mexico. PLoS Negl. Trop. Dis. 15: e0008972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kow, C. Y., Koon L. L., and Yin P. F.. 2001. Detection of dengue viruses in field caught male Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in Singapore by type-specific PCR. J. Med. Entomol. 38: 475–479. [DOI] [PubMed] [Google Scholar]
- Koyoc-Cardeña, E., Medina-Barreiro A., Cohuo-Rodríguez A., Pavía-Ruz N., Lenhart A., Ayora-Talavera G., Dunbar M., Manrique-Saide P., and Vazquez-Prokopec G.. 2019. Estimating absolute indoor density of Aedes aegypti using removal sampling. Parasites Vectors. 12: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno, G. 1997. Factors influencing the transmission of dengue viruses. Dengue Dengue Hemorrhagic Fever. 1: 23–39. [Google Scholar]
- Lanciotti, R. S., Kosoy O. L., Laven J. J., Panella A. J., Velez J. O., Lambert A. J., and Campbell G. L.. 2007. Chikungunya virus in US travelers returning from India, 2006. Emerg. Infect. Dis. 13: 764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti, R. S., Kosoy O. L., Laven J. J., Velez J. O., Lambert A. J., Johnson A. J., Stanfield S. M., and Duffy M. R.. 2008. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 14: 1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lequime, S., and Lambrechts L.. 2014. Vertical transmission of arboviruses in mosquitoes: a historical perspective. Infect. Genet. Evol. 28: 681–690. [DOI] [PubMed] [Google Scholar]
- Lequime, S., Paul R. E., and Lambrechts L.. 2016. Determinants of arbovirus vertical transmission in mosquitoes. PLoS Pathog. 12: e1005548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manrique-Saide, P., Dean N. E., Halloran M. E., Longini I. M., Collins M. H., Waller L. A., Gomez-Dantes H., Lenhart A., Hladish T. J., and Che-Mendoza A.. 2020. The TIRS trial: protocol for a cluster randomized controlled trial assessing the efficacy of preventive targeted indoor residual spraying to reduce Aedes-borne viral illnesses in Mérida, Mexico. Trialsm. 21: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuel, M., Missé D., and Pompon J.. 2020. Highly efficient vertical transmission for Zika virus in Aedes aegypti after long extrinsic incubation time. Pathogens. 9(5): 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez, N. E., Dzul-Manzanilla F., Gutiérrez-Castro C., Ibarra-López J., Bibiano-Marín W., López-Damián L., Martini-Jaimes A., Huerta H., Che-Mendoza A., and Ayora-Talavera G.. 2014. Natural vertical transmission of dengue-1 virus in Aedes aegypti populations in Acapulco, Mexico. J. Am. Mosq. Control Assoc. 30: 143–146. [DOI] [PubMed] [Google Scholar]
- Mavale, M., Parashar D., Sudeep A., Gokhale M., Ghodke Y., Geevarghese G., Arankalle V., and Mishra A. C.. 2010. Venereal transmission of chikungunya virus by Aedes aegypti mosquitoes (Diptera: Culicidae). Am. J. Trop. Med. Hyg. 83: 1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghadas, S. M., Shoukat A., Espindola A. L., Pereira R. S., Abdirizak F., Laskowski M., Viboud C., and Chowell G.. 2017. Asymptomatic transmission and the dynamics of Zika infection. Sci. Rep. 7: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz, A. 2018. How relevant is the asymptomatic population in dengue transmission? Appl. Math. Sci. 12: 1699–1708. [Google Scholar]
- Pavía-Ruz, N., Contreras-Capetillo S., Valadéz-González N., Villegas-Chim J., Carcaño-Castillo R., Valencia-Pacheco G., Vera-Gamboa L., Correa-Morales F., Herrera-Bojórquez J., and Delfín-González H.. 2018a. An integrated intervention model for the prevention of Zika and other Aedes-borne diseases in women and their families in Mexico. Current Topics in Zika, p. 49. [Google Scholar]
- Pavía-Ruz, N., Barrera-Fuentes G. A., Villanueva-Jorge S., Che-Mendoza A., Campuzano-Rincón J. C., Manrique-Saide P., Rojas D. P., Vazquez-Prokopec G. M., Halloran M. E., and Longini I. M.. 2018b. Dengue seroprevalence in a cohort of schoolchildren and their siblings in Yucatan, Mexico (2015-2016). PLoS Negl. Trop. Dis. 12: e000674m8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistone, T., Ezzedine K., Schuffenecker I., Receveur M. -C., and Malvy D.. 2009. An imported case of Chikungunya fever from Madagascar: use of the sentinel traveller for detecting emerging arboviral infections in tropical and European countries. Travel Med. Infect. Dis. 7: 52–54. [DOI] [PubMed] [Google Scholar]
- Rojas, D. P., Barrera-Fuentes G. A., Pavia-Ruz N., Salgado-Rodriguez M., Che-Mendoza A., Manrique-Saide P., Vazquez-Prokopec G. M., Halloran M. E., Longini I. M., and Gomez-Dantes H.. 2018. Epidemiology of dengue and other arboviruses in a cohort of school children and their families in Yucatan, Mexico: Baseline and first year follow-up. PLoS Negl. Trop. Dis. 12: e0006847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura, N. A., Muñoz A. L., Losada-Barragán M., Torres O., Rodríguez A. K., Rangel H., and Bello F.. 2021. Minireview: epidemiological impact of arboviral diseases in Latin American countries, arbovirus-vector interactions and control strategies. Pathogens Dis. 79: ftab043. [DOI] [PubMed] [Google Scholar]
- Sistema Nacional de Vigilancia Epidemiologica (SINAVE). 2017. Panorama Epidemiológico de Dengue 2017. Dirección General de Epidemiología, México. https://www.gob.mx/. [Google Scholar]
- Sistema Nacional de Vigilancia Epidemiologica (SINAVE). 2020. Panorama Epidemiológico de Dengue 2020. Dirección General de Epidemiología, México. https://www.gob.mx/. [Google Scholar]
- Thannesberger, J., Rascovan N., Eisenmann A., Klymiuk I., Zittra C., Fuehrer H., Scantlebury-Manning T., Hilaire M. G., Austin S., and Landis R.. 2021. Viral metagenomics reveals the presence of novel Zika virus variants in Aedes mosquitoes from Barbados. Parasites Vectors. 14: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Avendaño, J. I., Apodaca-Medina A. I., Castillo-Ureta H., Rendón-Maldonado J. G., Torres-Montoya E. H., Cota-Medina A., Ríos-Tostado J. J., and Zazueta-Moreno J. M.. 2021. Natural vertical transmission of dengue virus serotype 4 in Aedes aegypti larvae from urban areas in Sinaloa, Mexico. Vector-Borne Zoonotic Dis. 21(6): 478–481. [DOI] [PubMed] [Google Scholar]
- Vazquez-Prokopec, G. M., Galvin W. A., Kelly R., and Kitron U.. 2009. A new, cost-effective, battery-powered aspirator for adult mosquito collections. J. Med. Entomol. 46: 1256–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Prokopec, G. M., Chaves L. F., Ritchie S. A., Davis J., and Kitron U.. 2010. Unforeseen costs of cutting mosquito surveillance budgets. PLoS Negl. Trop. Dis. 4: e858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Prokopec, G. M., Perkins T. A., Waller L. A., Lloyd A. L., R. C.Reiner, Jr, Scott T. W., and Kitron U.. 2016. Coupled heterogeneities and their impact on parasite transmission and control. Trends Parasitol. 32: 356–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder-Smith, A., Lindsay S. W., Scott T. W., Ooi E. E., Gubler D. J., and Das P.. 2020. The Lancet Commission on dengue and other Aedes-transmitted viral diseases. Lancet (Lond. Engl.). 395: 1890–1891. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Original data of this study including mosquito collections raw data, DNA raw sequences and sequences analysis, are available online via Mendeley at: https://data.mendeley.com/datasets/whr6x8std7/draft?a=7f8057ae-9ff7-4ca3-821b-7cc63a622302.