Abstract
Social anxiety disorder (SAD) is characterized by aberrant prefrontal activity during reappraisal, an adaptive cognitive approach aimed at downregulating the automatic response evoked by a negative event. Cognitive behavioral therapy (CBT) is first-line psychotherapy for SAD, however, many remain symptomatic after treatment indicating baseline individual differences in neurofunctional activity may factor into CBT outcome. An emotion regulation strategy practiced in CBT is cognitive restructuring, a proxy for reappraisal. Therefore, neural response during reappraisal may serve as a brain-based predictor of CBT success. Prior to 12 weeks of individual CBT, 34 patients with SAD completed a validated emotion regulation task during fMRI. Task instructions included ‘Reappraise,’ that is, use a cognitive approach to reduce affective state to a negative image, which was contrasted with looking at a negative image (‘Look’). Regression results for Reappraise (vs. Look) revealed greater reduction in symptom severity was predicted by less pre-CBT activation in the dorsolateral prefrontal cortex (DLPFC). Regarding predictive validity, DLPFC significantly classified responder status. Post-hoc analysis revealed DLPFC activity, but not demographic data, baseline clinical measures, or reappraisal-related affective state during fMRI, significantly accounted for the variance in symptom reduction. Findings indicate patients with SAD are more likely to benefit from CBT if there is less pre-treatment DLPFC recruitment, a region strongly implicated in emotion regulation. Patients with reduced baseline frontal activation when reappraising negative stimuli may be especially helped by explicit cognitive interventions. Further research is necessary to establish DLPFC as a stable brain-based marker of treatment outcome.
1. Introduction
Social anxiety disorder (SAD) is a common psychiatric illness characterized by pervasive fears of potential scrutiny by others (Kessler et al., 2005). Inappropriate, excessive fears in SAD are due in part to emotion dysregulation as indicated by an overreliance on expressive suppression, a generally maladaptive avoidance regulation strategy to cope with emotion (Heimberg et al., 2014; Spokas et al., 2009). Suppression in SAD is mediated by social concerns, for example, the belief that inhibiting expressions reduces the likelihood of negative evaluation (Spokas et al., 2009). In addition to suppression, socially anxious individuals report low levels of self-efficacy when implementing emotion regulation strategies including cognitive reappraisal (Werner et al., 2011). Reappraisal is an antecedent form of regulation directed at cognitively changing a situation to alter its emotional significance; it is considered an adaptive, explicit regulation approach known to engender social support and psychological well-being (Gross and John, 2003).
Cognitive behavioral therapy (CBT) is a first-line psychotherapy for SAD and other anxiety disorders (Hofmann and Smits, 2008) that aims to reduce symptoms by increasing adaptive emotion regulation (O’Toole et al., 2015), and indeed, reappraisal is utilized more frequently in SAD patients who participated in CBT (Moscovitch et al., 2012). Although CBT is the “gold standard” psychotherapy for SAD, response rate varies considerably ranging from 4% to 80% (Loerinc et al., 2015). Thus, many patients remain symptomatic after completing treatment. Increasingly, the utility of brain-based predictors is being realized as they are frequently superior in foretelling who is likely to achieve clinically-meaningful response to CBT relative to demographic and/or baseline clinical information alone (Ball et al., 2014; Doehrmann et al., 2013; Thompson et al., 2015). Evidence brain-based markers may be more sensitive in predicting treatment outcome than non-imaging measures are in line with the conceptualization psychiatric illnesses are brain illnesses (Downar et al., 2016). In other words, it stands to reason that individual differences in brain pathophysiology will interact with mechanisms of treatment.
In neuroimaging studies, reappraisal includes interpreting (e.g., “reframing”) an aversive stimulus to decrease the negative affect evoked by it (Webb et al., 2012), an approach that generally echoes cognitive strategies employed in CBT, for example, generating alternative appraisals to negate negative beliefs (Hofmann, 2004; Hope et al., 2006). Cognitive reappraisal has been widely studied with functional magnetic resonance imaging (fMRI) and converging data in healthy individuals show reappraisal engages regions central in higher-order processes (e.g., attention, inhibition, working memory) such as dorsolateral prefrontal cortex (DLPFC), medial prefrontal cortex (MPFC), anterior cingulate cortex (ACC), inferior frontal gyrus (IFG), as well as ventromedial prefrontal cortex (VMPFC) and ventrolateral prefrontal cortex (VLPFC), though ventral areas have been less consistently observed (Buhle et al., 2014; Messina et al., 2015). Together, these regions form a cognitive control network which underlies the ability to deliberately modulate reactions to emotional stimuli.
Consistent with behavioral observations of emotion dysregulation in SAD, Goldin et al. (2009a) observed less DLPFC and less dorsal ACC (dACC) recruitment in SAD when reappraising negative faces compared to healthy controls. Moreover, SAD participants failed to exhibit more reappraise-related activity in any regions relative to controls. In a separate study, there was evidence of delayed engagement of DLPFC, dACC, VLPFC, MPFC, and dorsomedial PFC (DMPFC) when reappraising negative beliefs compared to controls (Goldin et al., 2009b). Findings suggest patients with SAD were able to eventually recruit regions that support regulation but may have required additional time to override the initial emotional reactivity to negative stimuli (Goldin et al., 2009b). Patterns of prefrontal hypo-activity or delayed reappraise-related recruitment in SAD relative to healthy controls has also been shown in other tasks involving negative socio-emotional stimuli (Ziv et al., 2013). Altogether, these data are consonant with reports of reduced activation in dorsal and ventral lateral regions during reappraisal in other anxiety disorders (e.g., panic disorder, generalized anxiety disorder) and major depressive disorder suggesting impairment in cognitive reappraisal capacity is a transdiagnostic factor in internalizing psychopathologies (Zilverstand et al., 2016).
The clinical implication is that pre-CBT variation in frontal activity during reappraise may interact with CBT outcome as this cognitive approach is a proxy to the interventions practiced in CBT (e.g., cognitive restructuring) (Arch and Craske, 2009). While this has yet to be examined in SAD, less baseline DLPFC activation in reappraise, relative to looking at aversive images, has been shown to predict CBT success in panic disorder (Reinecke et al., 2014), which has shared neurobiological features with SAD (Demenescu et al., 2013). These data suggest CBT may be most beneficial to those who have greater regulation deficiency when using a cognitive approach to downregulate emotional reactivity.
Results also indicate the DLPFC or other areas involved in emotion regulation may be a mechanistic target of CBT. In support, SAD patients who completed CBT relative to a waitlist control group exhibited increased DLPFC and DMPFC activity when reappraising negative social situations suggesting a generalization of the techniques learned in CBT (Goldin et al., 2013). There is also evidence of greater pre-to-post CBT reappraisal-related activation (compared to waitlist control) in the superior/middle frontal gyrus and middle occipital gyrus as well as less activity in posterior superior temporal gyrus, which collectively predicted 24% of the variance in symptom severity reduction. Yet, neural changes in other conditions (i.e., react praise, react criticism) did not significantly account for the decrease in social anxiety symptoms nor did self-reported pre-to-post-CBT decreases in negative emotion. Findings signify neural correlates of emotion regulation were a target of CBT. These data indicate reappraise-related disturbances in SAD interact with CBT and that alterations in brain response during reappraise contribute to a reduction in symptoms. However, it is not clear if frontal regions key to reappraisal predict CBT outcome in SAD.
The goal of the present study was to test whether pre-CBT brain activity using cognitive reappraisal to reduce negative affective state evoked by aversive images predicted clinical improvement in patients with SAD. To this end we employed an emotion regulation task (ERT) based on paradigms previously used to examine cognitive reappraisal mechanisms in other labs (Ochsner et al., 2002) and in our lab in healthy participants (Phan et al., 2005) and patient cohorts (MacNamara et al., 2016; Rabinak et al., 2014). We hypothesized larger decreases in symptom severity would be predicted by less pre-CBT activation in cognitive control areas when reappraising negative images compared to naturally reacting to negative images (i.e., “Look Negative”). We also hypothesized brain activity, but not demographic data, clinical measures, or on-line reappraisal ability, would significantly account for the pre-to-post change in symptom severity after completing CBT. Lastly, we explored whether baseline symptom severity correlated with less neurofunctional activity in reappraise relative to “Look Negative.”
2. Methods
2.1. Participants
All participants provided written informed consent as approved by the local Institutional Review Board at the University of Illinois at Chicago (UIC) and all procedures complied with the Helsinki Declaration. Diagnosis was based on the Structured Clinical Interview for DSM-IV (“SCID-IV”; First et al., 1995) and the clinician-administered Liebowitz Social Anxiety Scale total score (“LSAS”; Liebowitz, 1987) determined symptom severity. Depression level was evaluated with the clinician-administered Hamilton Depression Rating Scale (“HAM-D”; Hamilton, 1960). Regarding self-reported symptoms, the Beck Depression Inventory (BDI; Beck et al., 1996) and the Spielberger State-Trait Anxiety Inventory (Spielberger, 1983) assessed depression and general trait anxiety level, respectively. A master’s-level clinician trained in the SCID-IV and clinician-administered measures performed assessments. The Emotion Regulation Questionnaire (“ERQ”; Gross and John, 2003) was used to evaluate subjective habitual use of reappraisal and expressive suppression. Participants were compensated for their time.
Participants were between 18 and 55 years of age, free of major medical or neurologic illness as confirmed by a Board Certified physician. SAD was required to be the primary diagnosis and participants with comorbidity were not excluded. All participants were free of psychotropic medications and none were receiving concurrent psychotherapy. None of the participants tested positive for drugs.
Exclusion criteria included contraindications to magnetic resonance imaging (e.g., pregnancy, claustrophobia, non-removable ferrous objects), current substance abuse or dependence (within 6 months of study), or history of major psychiatric illness (e.g., bipolar disorder, psychotic disorder, pervasive developmental disorder).
2.2. Treatment
Within a week of the fMRI scan, patients began once-weekly sessions of manualized individual CBT for 12 weeks, which comprised psycho-education, cognitive restructuring, in vivo exposures to fears, and relapse prevention (Hope et al., 2006). A CBT-trained licensed clinical psychologist or post-doctoral clinical psychologist conducted treatment. The clinicians were supervised by a licensed clinical psychologist with expertise in CBT and clinical trials. Response to CBT was defined as a 50% (or more) reduction in symptom severity as indexed with the LSAS total score (Jakubovski and Bloch, 2014; Roy-Byrne et al., 2010; van Vliet et al., 1994). Symptom severity was assessed by a non-treating clinician.
2.3. fMRI task
During the ERT participants were instructed to: 1) use a cognitive-linguistic strategy to reduce negative affect evoked by an aversive image (“Reappraise”); 2) attend to, be aware of, and “feel what you naturally feel” when looking at an aversive image (“Look Negative”); or 3) view neutral images (“Look Neutral”). Prior to the scan, participants were instructed on the strategy of reappraisal (Ochsner et al., 2002; Phan et al., 2005) and all conditions were practiced with images not used in the experiment to confirm understanding of task instructions.
The task comprised 64 unpleasant and 32 neutral International Affective Picture System images (Lang et al., 2008). There were eight 20-s blocks of each condition (four images presented for 5 s each), which were interspersed with 20-s baseline blocks (comprising a white fixation cross presented on a black background). At the beginning of each block, the instruction to ‘Reappraise,’ ‘Look Negative,’ or ‘Look Neutral’, appeared in white text on a black screen for 5 s. Immediately following each task block, participants were asked to rate “How negative do you feel?” on a 5-point scale (1 = not at all, 5 = extremely) via button response. The order of blocks was pseudo-randomized over 4 separate runs of 5 min each.
2.4. fMRI data acquisition and preprocessing
Scanning was conducted on a 3 Tesla GE Signa System (General Electric; Milwaukee, WI) and performed with blood oxygen-level dependent (BOLD)-sensitive whole-brain fMRI using GE software (LX 8.3, Neuro-optimized gradients). Images were acquired using a gradient-echo echo-planar imaging sequence with the following parameters: TR = 2 s, TE = 25 ms, flip angle = 90°, field of view = 22 × 22 cm2, acquisition matrix 64 × 64; 44 axial, 3-mm-thick slices with no gap. For anatomical localization, a high-resolution, T1-weighted volumetric anatomical scan was acquired.
Data from all participants met criteria for quality with minimal motion correction (movements were <3 mm and <3° rotation in any one direction) and the first 4 volumes from each run were discarded to allow for T1 equilibration effects. Conventional preprocessing steps were used in the Statistical Parametric Mapping (SPM8) software package (Wellcome Trust Centre for Neuroimaging, London; www.fil.ion.ucl.ac.uk/spm). Briefly, images were temporally corrected to account for differences in slice time collection, spatially realigned to the first image of the first run, normalized to a Montreal Neurological Institute (MNI) template, resampled to 2 × 2 × 2 mm3 voxels, and smoothed with an 8 mm isotropic Gaussian kernel.
2.5. fMRI analysis
A general linear model was applied to the time series, convolved with the canonical hemodynamic response function and with a 128 s high-pass filter. Nuisance regressors comprising 6 motion parameters were included to correct for motion artifacts. Blocks of Reappraise, Look Negative, and Look Neutral trials, were modeled separately, the effects of which were estimated for each voxel for each participant and taken to the second level for random effects analysis.
To assess predictors of CBT response, contrasts of interest from SAD pre-treatment scans were entered into a whole-brain analysis of covariance, regressing LSAS change (ΔPreTx − PostTx) while initial severity (LSASPreTx) was controlled for as a regressor of no interest. To correct for multiple comparisons, simulation was performed using the 3dClustSim utility (10,000 iterations; updated and ‘bug-free’ on December 2015; [https://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html]; Cox, 1996) where residuals for each participant related to the contrast of interest was used to estimate smoothness and applied to second-level statistical results. Significance at α < 0.05 with a height threshold of p < 0.005 across the entire brain (i.e., a whole-brain gray matter mask [volume = 1,459,304 mm3]) revealed a minimum cluster size of 141 voxels (volume = 1128 mm3) for the regression analysis.
To illustrate the magnitude and direction of activation for effects, parameter estimates of peak activation for significant a priori findings (β weights, arbitrary units [a.u.]) were extracted from spherical (10-mm diameter) regions of interest (ROI) from each participant and submitted to Pearson correlations and scatter plots in the Statistical Package for the Social Sciences (SPSS) (Chicago, IL version 22). Importantly, statistical analyses were not performed on these Reappraise vs. Look Negative data as significance at the whole-brain level was already established. Additional analyses were performed in SPSS to explore the extent to which activation in an a priori ROI was driven by Reappraise or Look Negative. Accordingly, spherical ROIs generated for the Reappraise vs. Look Negative findings were subsequently applied to a non-emotional baseline: Reappraise vs. Look Neutral and Look Negative vs. Look Neutral. Beta values for these contrasts were then submitted to two-tailed Pearson correlations.
To explore the validity of significant clusters in predicting CBT response, ROC curves in SPSS evaluated whether activity in a priori whole-brain findings classified responder status. These same regions were submitted to cross-validation and split-sample validation analysis in SPSS to examine reproducibility. Lastly, post-hoc multiple regression analyses were performed in SPSS to test whether activation, in the context of demographic and clinical measures, significantly explained variance in pre-to-post CBT change in symptom severity. All analyses were two-tailed with alpha level set at 0.05.
3. Results
3.1. Participant characteristics
Thirty-four patients with SAD (65% female) had a mean age of 25.0 ± 4.7 years and an average symptom severity (LSAS total score) of 77.7 ± 14.0 meeting the standard cutoff of 60 for the generalized SAD subtype (Mennin et al., 2002). Depression levels ranged from mild to moderate as the clinician-administered HAM-D indicated patients were mildly symptomatic (average 8.3 ± 5.3) (Zimmerman et al., 2013) and self-report was suggestive of moderate depression symptoms (average 20.8 ± 9.5) (Beck et al., 1996). Regarding concurrent disorders, they were generalized anxiety disorder (n = 11), panic disorder (n = 4), specific phobia (n = 3), adjustment disorder (n = 1), posttraumatic stress disorder (n = 1), and an eating disorder (n = 1). Eleven SAD patients had another anxiety disorder, 8 had major depressive disorder, and 5 had comorbid dysthymia. Altogether, 11 patients had more than one current comorbid disorder. Concerning race/ethnicity, 64.7% self-identified as Caucasian, 17.6% as Asian, and 2.9% as African American. About 29% self-identified as Hispanic and 14.7% as unknown or other than Caucasian, Asian, African American, or Hispanic.
3.2. Treatment effects
After completing 12 weeks of CBT, symptom severity (i.e., LSAS) significantly decreased (pre = 77.7 ± 14.0; post = 47.3 ± 23.3) (p < 0.001) as did depression level as assessed with the HAM-D (pre = 8.3 ± 5.3; post = 3.2 ± 3.3) (p < 0.001) and the BDI (pre = 20.8 ± 9.5; post = 4.9 ± 7.2) (p b 0.001). Self-reported emotion regulation tendencies determined with the ERQ revealed an increase in the use of reappraisal (pre = 23.1 ± 5.9; post = 30.0 ± 5.3) (p < 0.001) and a decrease in the use of expressive suppression (pre = 17.9 ± 5.1; post = 15.2 ± 6.0) (p < 0.007). Approximately 32% (11/34) were ‘responders’ as they demonstrated at least a 50% reduction in symptom severity based on the LSAS total score and 68% (23/34) were ‘non-responders’. These groups did not differ in baseline LSAS [t(32) = 0.57, p = 0.58], HAM-D [t(32) = 0.83, p = 0.41], BDI [t(32) = 1.77, p = 0.09], trait anxiety level [t(32) = 0.45, p = 0.66], self-reported reappraisal [t(32) = 0.09, p = 0.93] or suppression [t(32) = 0.91, p = 0.37], age, [t(32) = 0.13, p = 0.90], educational level [t(32) = 0.88, p = 0.38], gender [χ2(1) = 0.46, p = 0.50], or race/ethnicity [χ2(3) = 1.45, p = 0.69].
3.3. Subjective ratings during fMRI
Planned comparisons with two-tailed t-tests revealed more negative affect after attending to negative images (3.06 ± 0.65) relative to reappraise (2.48 ± 0.73) [t(33) = 5.71, p < 0.001] and looking at neutral pictures (1.30 ± 0.47) [t(33) = 17.16, p < 0.001]. Negative affect was also reported as greater after reappraising negative images relative to looking at neutral images [t(33) = 11.46, p < 0.001].
3.4. fMRI results
Whole-brain regression findings for Reappraise vs. Look Negative, corrected for multiple comparisons at alpha level p < 0.05, revealed greater reduction in symptom severity after CBT was predicted by less baseline activity in the left DLPFC [(− 44, 18, 48) z = 3.34, k = 162, volume = 1296 mm3] (Fig. 1A). As expected, a negative relationship between DLPFC activity and symptom change (r = − 0.54) was illustrated in SPSS (Fig. 1B). Post-hoc ROC analysis in SPSS showed DLPFC activity accurately discriminated between responders and non-responders [(AUC) = 0.79; p < 0.007] (Fig. 2).
Fig. 1.

A) Regressing LSAS change (ΔPreTx − PostTx) while initial severity (LSASPreTx) is controlled for as a regressor of no interest, brain map depicts whole-brain analysis of covariance showing dorsolateral prefrontal cortex in SAD as denoted by negative parameter estimates of activation based on Reappraise vs. Look Negative displayed on statistical t-map at p < 0.005. B) Scatter plot of the regression analyses depicting extracted parameter estimates of activation from dorsolateral prefrontal cortex showing greater response to CBT in SAD was predicted by less dorsolateral prefrontal cortex activity during Reappraise vs. Look Negative.
Fig. 2.
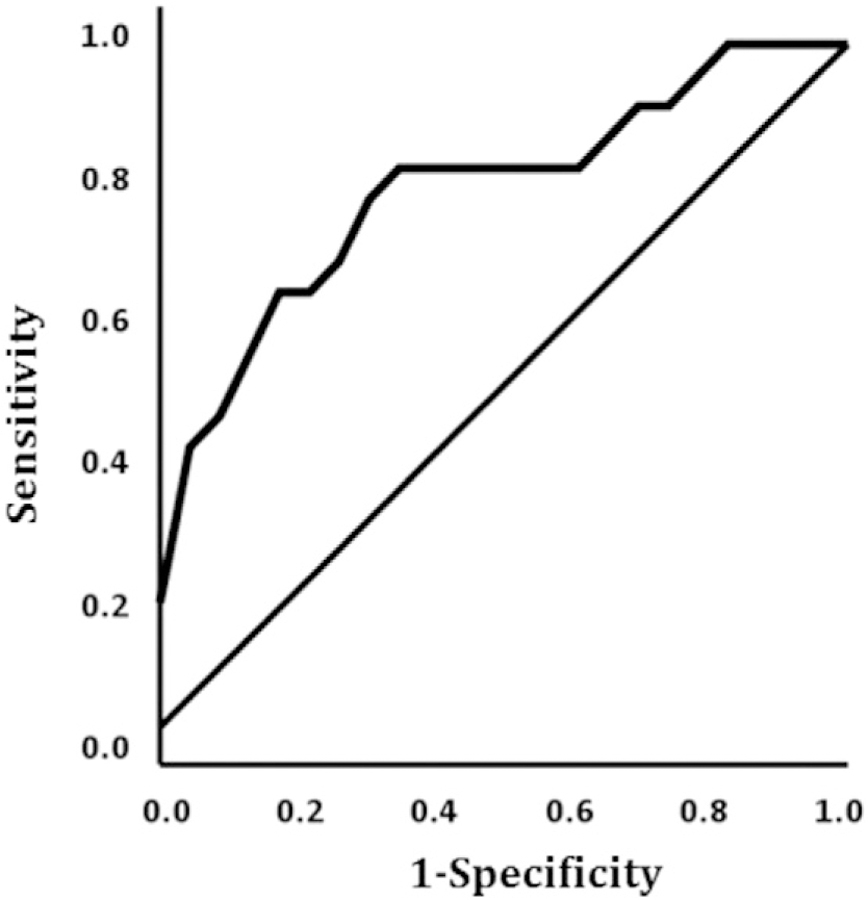
ROC curve illustrating classification of responder status based on pre-CBT dorsolateral prefrontal cortex activation.
When applying the DLPFC ROI generated for Reappraise vs. Look Negative to a non-emotional baseline, there was no correlation between Reappraise vs. Look Neutral and change in symptom severity (r = −0.28, p = 0.11) or Look Negative vs. Look Neutral and change in symptom severity (r = 0.10, p = 0.56). However, within group analyses demonstrated Reappraise vs. Look Neutral was negatively linked with symptom severity in responders (r = − 0.69, p < 0.02) but not non-responders (r = −0.01, p = 0.98). No associations were observed between change in symptom severity and Look Negative vs. Look Neutral in responders (r = −0.47, p = 0.15) or non-responders (r = 0.14, p = 0.53). When exploring links between baseline symptom severity and baseline Reappraise vs. Look Negative, only negative relationships emerged; for example, higher LSAS total scores were associated with less DMPFC [(−8, 48, 16) z = 2.84, k = 39, volume = 312 mm3] activity. However, this whole-brain finding and other clusters did not survive adjustment for multiple comparisons (all clusters < 141 contiguous voxels).
3.5. Post-hoc analysis
A two-step hierarchical multiple regression analysis (forced entry) showed that in Step 1, change in symptom severity was not predicted by baseline clinical measures (LSAS, HAM-D, trait anxiety, Reappraisal, Suppression), demographic data (i.e., age, education level), or affective state related to Reappraisal during the fMRI task [R2 = 0.19, F(7,26) = 0.86, p = 0.55]. However, when DLPFC activation for Reappraise vs. Look Negative was entered in Step 2, it accounted for significant variance in symptom severity reduction (standardized β = − 0.66; p < 0.003) (zero-order correlation = − 0.540, partial correlation = −0.55) resulting in significance in the full model [R2 = 0.44, F(8,25) = 2.42, p < 0.04]. To evaluate predictive reproducibility, DLPFC activation was submitted to a 10-fold cross-validation analysis in SPSS, which revealed overall performance was 79.4% (risk estimate 0.35; SEM 0.08). Split-sample validation using random assignment of 2 separate cohorts (training = 60% of cases, test = 40% of cases) showed 68.8% of responders were classified correctly and 66.7% of test cases were classified correctly (risk estimate = 0.31 and 0.33, respectively).
4. Discussion
The primary objective of the current study was to test whether response in CBT was predicted by reappraisal-related neural activity in patients with SAD. Explicit emotion regulation with a cognitive approach such as reappraisal is a proxy to the cognitive interventions practiced in CBT (e.g., cognitive restructuring). Therefore, neural activity in structures that support regulation before treatment may interact with CBT. We hypothesized less baseline frontal activity during reappraisal in the context of negative images would predict reduction in symptom severity. In support of our hypothesis, treatment outcome was significantly foretold by pre-CBT activation in the dorsolateral prefrontal cortex (DLPFC) during emotion regulation. However, despite evidence of a brain-based predictor, there was no significant relationship between initial symptom severity and regulation-related neural activity.
Specifically, patients with SAD exhibited a significant decrease in social anxiety symptoms (i.e., LSAS change (ΔPreTx − PostTx)) after 12 weeks of individual CBT, which was predicted by less pre-treatment activation in DLPFC when interpreting (e.g., reframing) images of general negative content (“Reappraise”) relative to being aware of, and experiencing naturally, the emotions brought about by aversive images (“Look Negative”). To evaluate whether baseline DLPFC activation was primarily driven by regulation or reactivity to negative images, post-hoc analyses were conducted contrasting Reappraise and Look Negative separately with a non-emotional baseline. Results showed deficient DLPFC recruitment was primarily driven by Reappraise in treatment responders (i.e., ≥ 50% reduction in social anxiety symptoms) though findings need to be interpreted with caution given the exploratory nature of correlations and small number of patients who attained responder status. Furthermore, findings did not extend to pre-treatment analysis. That is, baseline symptom severity alone did not correlate with neurofunctional activity during emotion regulation. The lack of significant results may have been due to a restriction of range in symptomatology and without a healthy control comparison group, we cannot determine the extent to which pre-CBT activity was aberrant.
With regard to predictive validity, DLPFC significantly classified responder status and cross-validation and split-sample validation approaches suggest DLPFC’s predictive ability was stable. The DLPFC finding is important as its recruitment has consistently been observed in studies of cognitive reappraisal (Buhle et al., 2014; Messina et al., 2015). It is a domain-general cognitive control area well-positioned to integrate sensory information and behavioral control given its interconnections with other prefrontal areas (Miller and Cohen, 2001), motor regions (e.g., supplementary motor area) (Petrides and Pandya, 1999), and input from parietal areas (Petrides and Pandya, 1999). Most relevant to the process of reappraisal, the DLPFC is engaged in volitional attention and working memory, functions integral to the monitoring and manipulation of information (Ochsner and Gross, 2005). Our data indicate individual differences in DLPFC activity during regulation interacts with treatment though further study is needed to determine whether its predictive ability is unique to CBT or generalizes to other interventions (e.g., pharmacotherapy).
Behaviorally, patients reported a reduction in negative affective state in the reappraisal condition suggesting a capacity to downregulate reactivity to negative information when instructed. It is also notable that after completing CBT, patients reported an increase in habitual reappraisal tendencies and a decrease in suppression indicating a shift to more adaptive means of regulation after treatment. Yet, individual differences in baseline ‘on-line’ regulation ability and pre-CBT self-reported regulation propensities did not significantly correspond with CBT outcome.
Additionally, post-hoc multiple regression results revealed DLPFC activation significantly accounted for decreases in symptoms (ΔPreTx − PostTx) whereas demographic data, clinical measures, and baseline affective state related to the reappraisal task were non-significant. These data add to accruing evidence regarding the advantage of neural biomarkers to predict which patient is likely to improve in CBT (Ball et al., 2014; Doehrmann et al., 2013; Thompson et al., 2015). That said, we recognize baseline regulation-related DLPFC activity tracked change in symptom severity as indexed with LSAS. Further work in neuroscience-based taxonomy is needed to identify and advance neural predictors that do not solely rely on symptom measures, which are prone to bias and demand characteristics.
Our observation that CBT may be especially beneficial to patients with SAD who have less neurofunctional regulation capacity expands on previous studies of neural predictors in SAD. For example, less baseline dACC reactivity to social criticism was associated with CBT success a year after completing treatment in SAD patients (Månsson et al., 2015) and when demands on attentional resources are high (e.g., ‘ignore’ threat distractors under high perceptual load), greater reduction in symptom severity was predicted by more baseline disruption in frontal regions (less dACC-DLPFC connectivity) (Klumpp et al., 2016). In contrast, studies of emotion processing have shown pronounced symptom improvement is associated with more pre-CBT activation in frontal regions (e.g., medial orbitofrontal, dorsomedial frontal gyrus) and secondary visual areas (e.g., occipitotemporal cortex) when attending to threat faces (Doehrmann et al., 2013; Klumpp et al., 2013). Also, when attention is simply directed away from emotional stimuli, via attentional deployment to non-emotional stimuli, greater baseline activation in frontal areas predicted greater change in symptom severity (e.g., dACC; Klumpp et al., 2014). Considered together, the direction of activation in predicting CBT response in SAD appears to be sensitive to the circuitry probed by a paradigm. Further study is needed to delineate what pre-treatment neural properties CBT improves on or what baseline strengths CBT capitalizes on.
In summary, greater reduction in symptom severity following CBT was predicted by less baseline activity in the DLPFC, a region strongly implicated in cognitive reappraisal (Buhle et al., 2014; Messina et al., 2015). Potentially, patients with reduced frontal recruitment when downregulating emotions elicited by negative cues are more likely to improve with CBT techniques that heavily rely on higher-order functions such as cognitive restructuring, a multi-step process that begins with the monitoring of maladaptive thoughts and its relationship to emotions. Approaches used by therapists (e.g., Socratic method, logical empiricism, behavioral experimentation) aid in the identification of negative thoughts and faulty beliefs that are subsequently challenged with the adoption of a rational, objective perspective (Arch and Craske, 2009). Therefore, the process of restructuring would appear to involve executive functions important for volitional regulation such as cognitive flexibility, inhibition (e.g., ability to ignore internal or external distractors), attention (capacity to sustain focus), and working memory.
While our data indicate CBT capitalizes on less baseline frontal recruitment when modulating emotional reactivity, other studies report a different pattern in tasks that entail executive functions. For example, in posttraumatic stress disorder (PTSD) decreases in symptom severity is predicted by more efficient inhibitory control (e.g., greater pre-CBT activation in frontal network) (Falconer et al., 2013) and in SAD more and less pre-to-post CBT activation when reappraising criticism significantly accounts for clinical improvement (Goldin et al., 2014). Possible reasons for mixed results include differences in the neurobiology between SAD and PTSD (e.g., medial PFC hypo-activity in PTSD; Etkin and Wager, 2007) and the method used to identify neural predictors in SAD (Goldin et al., 2014). Namely, regions linked to pre-to-post treatment changes in activation may not necessarily serve as brain-based predictors of CBT response (Fu et al., 2008; Klumpp et al., 2013; Yang et al., 2014). Here, we used a whole-brain approach focused on pre-CBT activation; still, we cannot rule out inconsistent findings may be due to other methodological differences between studies (e.g., type of paradigm, neuroimaging acquisition parameters, patient sample).
Results need to be considered in the context of important limitations. First and foremost, there was no treatment control or waitlist control group, therefore, neural and clinical findings cannot be causally attributed to CBT and could be due to factors not related to treatment such as differential regression to the mean in patients. Second, our sample size was relatively modest and is not fully representative of typical patients as none were taking a psychotropic medication. Third, findings are based at the group, as opposed to single-subject, level of analysis thus reducing the clinical utility of using baseline fMRI data to predict which patient has a high probability of responding to CBT. Fourth, treatment responders were required to have at least a 50% reduction in social anxiety symptoms, which is consistent with other studies (Jakubovski and Bloch, 2014; Roy-Byrne et al., 2010; van Vliet et al., 1994). Nevertheless, it has been argued that this cutoff may be too conservative (Bandelow et al., 2006) and may explain why relatively few ‘responded’ to CBT. Other factors that potentially contributed to the low response rate include depression symptoms and relatively high comorbidity, which have been associated with poorer outcome (Chambless et al., 1997; Hoyer et al., 2016; Ledley et al., 2005; Mululo et al., 2012). Fifth, emotion regulation results involved general negative images and may not generalize to other types of negative content (e.g., ideographic stimuli). Lastly, CBT encompasses an amalgam of techniques; therefore, we cannot conclude neural predictors are limited to one element of CBT (e.g., cognitive restructuring).
5. Conclusions
Our study indicates patients with SAD are more likely to benefit after completing CBT if they have less baseline recruitment of a regulatory structure when using a cognitive approach to reduce negative affective state. Specifically, greater reduction in symptom severity was predicted by less pre-CBT activity in the DLPFC when using reappraisal to downregulate emotions elicited by a negative stimulus. Reappraisal is a proxy for the cognitive strategies practiced in CBT aimed at altering the dysfunctional thinking associated with SAD. Accordingly, findings suggest patients with greater pre-CBT impairment in explicit regulation may be especially helped by explicit cognitive interventions practiced in CBT. Further research is necessary to determine whether DLPFC is a stable predictor of CBT outcome or other standard interventions for SAD.
Acknowledgements
Funding: This work was supported by NIMH K23MH093679 and Brain & Behavior Research Foundation (formerly NARSAD) Award to H.K. and in part by NIMH R01MH101497 to K.L.P. and the Center for Clinical and Translational Research (CCTS) UL1RR029879. The funding sources did not participate in the study design, collection, analysis and interpretation of data, writing the report, or in the decision to submit the article for publication.
Footnotes
Conflict of interest
None.
References
- Arch JJ, Craske MG, 2009. First-line treatment: a critical appraisal of cognitive behavioral therapy developments and alternatives. Psychiatr. Clin. North Am 32, 525–547. [DOI] [PubMed] [Google Scholar]
- Ball TM, Stein MB, Ramsawh HJ, Campbell-Sills L, Paulus MP, 2014. Single-subject anxiety treatment outcome prediction using functional neuroimaging. Neuropsychopharmacology 39, 1254–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandelow B, Baldwin DS, Dolberg OT, Andersen HF, Stein DJ, 2006. What is the threshold for symptomatic response and remission for major depressive disorder, panic disorder, social anxiety disorder, and generalized anxiety disorder? J. Clin. Psychiatry 67, 1428–1434. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK, 1996. Manual for the Beck Depression Inventory-II. Psychological Corporation, San Antonio, TX. [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Weber J, Ochsner KN, 2014. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb. Cortex 24, 2981–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambless DL, Tran GQ, Glass CR, 1997. Predictors of response to cognitive-behavioral group therapy for social phobia. J. Anxiety Disord 11, 221–240. [DOI] [PubMed] [Google Scholar]
- Cox RW, 1996. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res 29, 162–173. [DOI] [PubMed] [Google Scholar]
- Demenescu LR, Kortekaas R, Cremers HR, Renken RJ, van Tol M-J, van der Wee NJA, Veltman DJ, den Boer JA, Roelofs K, Aleman A, 2013. Amygdala activation and its functional connectivity during perception of emotional faces in social phobia and panic disorder. J. Psychiatr. Res 47, 1024–1031. [DOI] [PubMed] [Google Scholar]
- Doehrmann O, Ghosh SS, Polli FE, Reynolds GO, Horn F, Keshavan A, Triantafyllou C, Saygin ZM, Whitfield-Gabrieli S, Hofmann SG, Pollack M, Gabrieli JD, 2013. Predicting treatment response in social anxiety disorder from functional magnetic resonance imaging. JAMA Psychiatry 70, 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downar J, Blumberger DM, Daskalakis ZJ, 2016. The neural crossroads of psychiatric illness: an emerging target for brain stimulation. Trends Cogn. Sci 20, 107–120. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD, 2007. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry 164, 1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer E, Allen A, Felmingham KL, Williams LM, Bryant RA, 2013. Inhibitory neural activity predicts response to cognitive-behavioral therapy for posttraumatic stress disorder. J. Clin. Psychiatry 74, 895–901. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J, 1995. Structured Clinical Interview for DSM-IV Axis I Disorders. Patient Edition (SCID-P), Version 2. Biometrics Research, New York, NY. [Google Scholar]
- Fu CHY, Williams SCR, Cleare AJ, Scott J, Mitterschiffthaler MT, Walsh ND, Donaldson C, Suckling J, Andrew C, Steiner H, Murray RM, 2008. Neural responses to sad facial expressions in major depression following cognitive behavioral therapy. Biol. Psychiatry 64, 505–512. [DOI] [PubMed] [Google Scholar]
- Goldin PR, Manber-Ball T, Werner K, Heimberg R, Gross JJ, 2009a. Neural mechanisms of cognitive reappraisal of negative self-beliefs in social anxiety disorder. Biol. Psychiatry 66, 1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin P, Manber T, Hakimi S, Canli T, Gross JJ, 2009b. Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Arch. Gen. Psychiatry 66, 170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Ziv M, Jazaieri H, Hahn K, Heimberg R, Gross JJ, 2013. Impact of cognitive behavioral therapy for social anxiety disorder on the neural dynamics of cognitive reappraisal of negative self-beliefs: randomized clinical trial. JAMA Psychiatry 70, 1048–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Ziv M, Jazaieri H, Weeks J, Heimberg RG, Gross JJ, 2014. Impact of cognitive-behavioral therapy for social anxiety disorder on the neural bases of emotional reactivity to and regulation of social evaluation. Behav. Res. Ther 62, 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ, John OP, 2003. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. J. Pers. Soc. Psychol 85, 348–362. [DOI] [PubMed] [Google Scholar]
- Hamilton M, 1960. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimberg RG, Hofmann SG, Liebowitz MR, Schneier FR, Smits JAJ, Stein MB, Hinton DE, Craske MG, 2014. Social anxiety disorder in DSM-5. Depress. Anxiety 31, 472–479. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, 2004. Cognitive mediation of treatment change in social phobia. J. Consult. Clin. Psychol 72, 393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Smits JAJ, 2008. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J. Clin. Psychiatry 69, 621–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope DA, Heimberg RG, Turk CL, 2006. Managing Social Anxiety: A Cognitive-Behavioral Therapy Approach first ed. Oxford University Press, New York, USA. [Google Scholar]
- Hoyer J, Wiltink J, Hiller W, Miller R, Salzer S, Sarnowsky S, Stangier U, Strauss B, Willutzki U, Leibing E, 2016. Baseline patient characteristics predicting outcome and attrition in cognitive therapy for social phobia: results from a large multicentre trial: predictors of outcome in social phobia. Clin. Psychol. Psychother 23, 35–46. [DOI] [PubMed] [Google Scholar]
- Jakubovski E, Bloch MH, 2014. Prognostic subgroups for citalopram response in the STAR*D trial. J. Clin. Psychiatry 75, 738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE, 2005. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62, 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H, Fitzgerald DA, Phan KL, 2013. Neural predictors and mechanisms of cognitive behavioral therapy on threat processing in social anxiety disorder. Prog. NeuroPsychopharmacol. Biol. Psychiatry 45, 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H, Fitzgerald DA, Angstadt M, Post D, Phan KL, 2014. Neural response during attentional control and emotion processing predicts improvement after cognitive behavioral therapy in generalized social anxiety disorder. Psychol. Med 44, 3109–3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H, Fitzgerald DA, Piejko K, Roberts J, Kennedy AE, Phan KL, 2016. Prefrontal control and predictors of cognitive behavioral therapy response in social anxiety disorder. Soc. Cogn. Affect. Neurosci 11, 630–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN, 2008. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual (Technical Report A-8). University of Florida, Gainesville, FL. [Google Scholar]
- Ledley DR, Huppert JD, Foa EB, Davidson JRT, Keefe FJ, Potts NLS, 2005. Impact of depressive symptoms on the treatment of generalized social anxiety disorder. Depress. Anxiety 22, 161–167. [DOI] [PubMed] [Google Scholar]
- Liebowitz MR, 1987. Social phobia. Mod. Probl. Pharmacopsychiatry 22, 141–173. [DOI] [PubMed] [Google Scholar]
- Loerinc AG, Meuret AE, Twohig MP, Rosenfield D, Bluett EJ, Craske MG, 2015. Response rates for CBT for anxiety disorders: need for standardized criteria. Clin. Psychol. Rev 42, 72–82. [DOI] [PubMed] [Google Scholar]
- MacNamara A, Rabinak CA, Kennedy AE, Fitzgerald DA, Liberzon I, Stein MB, Phan KL, 2016. Emotion regulatory brain function and SSRI treatment in PTSD: neural correlates and predictors of change. Neuropsychopharmacology 41, 611–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Månsson KNT, Frick A, Boraxbekk C-J, Marquand AF, Williams SCR, Carlbring P, Andersson G, Furmark T, 2015. Predicting long-term outcome of internet-delivered cognitive behavior therapy for social anxiety disorder using fMRI and support vector machine learning. Transl. Psychiatry 5, e530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennin DS, Fresco DM, Heimberg RG, Schneier FR, Davies SO, Liebowitz MR, 2002. Screening for social anxiety disorder in the clinical setting: using the Liebowitz Social Anxiety Scale. J. Anxiety Disord 16, 661–673. [DOI] [PubMed] [Google Scholar]
- Messina I, Bianco S, Sambin M, Viviani R, 2015. Executive and semantic processes in reappraisal of negative stimuli: insights from a meta-analysis of neuroimaging studies. Front. Psychol 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD, 2001. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci 24, 167–202. [DOI] [PubMed] [Google Scholar]
- Moscovitch DA, Gavric DL, Senn JM, Santesso DL, Miskovic V, Schmidt LA, McCabe RE, Antony MM, 2012. Changes in judgment biases and use of emotion regulation strategies during cognitive-behavioral therapy for social anxiety disorder: distinguishing treatment responders from nonresponders. Cogn. Ther. Res 36, 261–271. [Google Scholar]
- Mululo SCC, de Menezes GB, Vigne P, Fontenelle LF, 2012. A review on predictors of treatment outcome in social anxiety disorder. Rev. Bras. Psiquiatr 34, 92–100 Sao Paulo Brazil. 1999. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ, 2005. The cognitive control of emotion. Trends Cogn. Sci 9, 242–249. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE, 2002. Rethinking feelings: an fMRI study of the cognitive regulation of emotion. J. Cogn. Neurosci 14, 1215–1229. [DOI] [PubMed] [Google Scholar]
- O’Toole MS, Mennin DS, Hougaard E, Zachariae R, Rosenberg NK, 2015. Cognitive and emotion regulation change processes in cognitive behavioural therapy for social anxiety disorder. Clin. Psychol. Psychother 22, 667–676. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN, 1999. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur. J. Neurosci 11, 1011–1036. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME, 2005. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol. Psychiatry 57, 210–219. [DOI] [PubMed] [Google Scholar]
- Rabinak CA, MacNamara A, Kennedy AE, Angstadt M, Stein MB, Liberzon I, Phan KL, 2014. Focal and aberrant prefrontal engagement during emotion regulation in veterans with posttraumatic stress disorder. Depress. Anxiety 31, 851–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinecke A, Thilo K, Filippini N, Croft A, Harmer CJ, 2014. Predicting rapid response to cognitive-behavioural treatment for panic disorder: the role of hippocampus, insula, and dorsolateral prefrontal cortex. Behav. Res. Ther 62, 120–128. [DOI] [PubMed] [Google Scholar]
- Roy-Byrne P, Craske MG, Sullivan G, Rose RD, Edlund MJ, Lang AJ, Bystritsky A, Welch SS, Chavira DA, Golinelli D, Campbell-Sills L, Sherbourne CD, Stein MB, 2010. Delivery of evidence-based treatment for multiple anxiety disorders in primary care: a randomized controlled trial. JAMA 303, 1921–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, 1983. Manual for the State-Trait Anxiety Inventory (Form Y). Consulting Psychologists Press. [Google Scholar]
- Spokas M, Luterek JA, Heimberg RG, 2009. Social anxiety and emotional suppression: the mediating role of beliefs. J. Behav. Ther. Exp. Psychiatry 40, 283–291. [DOI] [PubMed] [Google Scholar]
- Thompson DG, Kesler SR, Sudheimer K, Mehta KM, Thompson LW, Marquett RM, Holland JM, Reiser R, Rasgon N, Schatzberg A, O’Hara RM, 2015. FMRI activation during executive function predicts response to cognitive behavioral therapy in older, depressed adults. Am. J. Geriatr. Psychiatry 23, 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vliet IM, den Boer JA, Westenberg HG, 1994. Psychopharmacological treatment of social phobia; a double blind placebo controlled study with fluvoxamine. Psychopharmacology 115, 128–134. [DOI] [PubMed] [Google Scholar]
- Webb TL, Miles E, Sheeran P, 2012. Dealing with feeling: a meta-analysis of the effectiveness of strategies derived from the process model of emotion regulation. Psychol. Bull 138, 775–808. [DOI] [PubMed] [Google Scholar]
- Werner KH, Goldin PR, Ball TM, Heimberg RG, Gross JJ, 2011. Assessing emotion regulation in social anxiety disorder: the emotion regulation interview. J. Psychopathol. Behav. Assess 33, 346–354. [Google Scholar]
- Yang Y, Kircher T, Straube B, 2014. The neural correlates of cognitive behavioral therapy: recent progress in the investigation of patients with panic disorder. Behav. Res. Ther 62, 88–96. [DOI] [PubMed] [Google Scholar]
- Zilverstand A, Parvaz MA, Goldstein RZ, 2016. Neuroimaging cognitive reappraisal in clinical populations to define neural targets for enhancing emotion regulation. A Systematic Review. NeuroImage 10.1016/j.neuroimage.2016.06.009 (Epub ahead of print). [DOI] [PMC free article] [PubMed]
- Zimmerman M, Martinez JH, Young D, Chelminski I, Dalrymple K, 2013. Severity classification on the Hamilton depression rating scale. J. Affect. Disord 150, 384–388. [DOI] [PubMed] [Google Scholar]
- Ziv M, Goldin P, Jazaieri H, Hahn KS, Gross JJ, 2013. Is there less to social anxiety than meets the eye? Behavioral and neural responses to three socio-emotional tasks. Biol. Mood Anxiety Disord 3, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]


