Abstract
T cell co-stimulation is important for the maintenance of immunologic tolerance. Co-inhibitory receptors including programmed cell death-1 (PD-1) confer peripheral tolerance to prevent autoimmunity. SAP (SH2D1A) is an adaptor molecule that is important in T cell signaling and has been shown to interact with signaling lymphocytic activation molecule (SLAM) family receptors also in the context of self-tolerance. We recently reported that SAP interferes with PD-1 function. In the current study, we investigated the levels of SAP and PD-1 in patients with rheumatoid arthritis (RA) to further understand what role they play in disease activity. We observed increased SAP levels in lymphocytes of RA patients and found that PD-1 levels correlated positively with RA disease activity. Additionally, we found that SAP interacts with CD28 to inhibit T cell signaling in vitro. This work demonstrates a putative molecular mechanism for SAP mediated PD-1 inhibition.
Keywords: SAP, PD-1, rheumatoid arthritis, CD28, DAS28
1. Introduction:
Rheumatoid arthritis (RA) affects approximately 1% of the world’s population (1). While the etiology is complex, T cells have been shown to play a significant role in pathogenesis (2, 3). During the development of a normal immune response, T cell tolerance is maintained by dampening positive signaling in the periphery. To accomplish this, co-inhibitory receptors diminish T cell activation and cytokine secretion. Programmed cell death-1 (PD-1) is an important co-inhibitory receptor critical to maintain peripheral tolerance. PD-1 inhibition has led to a revolution in the treatment of certain malignancies, including melanoma, pulmonary malignancies and genitourinary malignancies, among others, with durable responses in many patients (4). Conversely, therapeutic strategies for autoimmunity involving co-receptors have also proven productive; a soluble CTLA-4-Ig fusion engages CD80/86 and blocks CD28 signaling. This strategy has been shown to lead to clinical responses in the treatment of inflammatory arthritides (5).
Co-inhibitory receptor signaling is an area of intense investigation in immunology and oncology. However, the signaling pathways downstream of PD-1 that result in the inhibition of T cells remain poorly understood. By affinity purifying PD-1 from human T cells we previously identified signaling lymphocytic activation molecule (SLAM)-associated protein (SAP) as a negative regulator of PD-1 signaling (6) and that SAP expression was inversely correlated with PD-1 function (4). SAP, encoded by the SH2D1A gene is a 14 kDa adaptor protein and consists mostly of one SH2 domain that canonically binds to SLAM receptors via conserved tyrosine interactions (7). In the present study we confirm that PD-1 levels are elevated in synovial fluid and peripheral blood T cells of patients with RA. In addition, we found elevated SAP levels that could render the rheumatoid PD-1 ineffective. Further, we show that SAP exerts it inhibitory effect by binding to phosphorylated tyrosine 173 of CD28 and thereby blocking dephosphorylation by SH2 domain containing protein tyrosine phosphatase (SHP2).
2. Materials and Methods:
2.1. Patients and Controls.
Research involving human subjects was performed according to the Institutional Review Boards at NYU Langone Health through an approved protocol with appropriate informed consent as required. Patients with RA fulfilled the ACR 2010 RA classification criteria. Rheumatoid factor and anti-CCP antibody status, C-Reactive protein level, and medications usage were obtained by review of electronic medical records. Biologic therapy was defined as use of anti-TNFα, Abatacept, Rituximab, Tocilizumab, or Tofacitinib. Synovial fluid samples were obtained from patients undergoing diagnostic or therapeutic arthrocentesis of an inflammatory knee effusion as directed by the treating rheumatologist. The same consent was signed for these samples as well as the peripheral blood. These samples were de-identified and therefore additional clinical information was not available, except for the patients from whom paired synovial fluid and blood were obtained. Blood samples for clinical phenotyping were obtained from patients seen at NYU Bellevue Hospital. For blood cell analyses in the cross-sectional cohort, DAS was measured by the treating clinician. Anti-CCP titers were measured at the Bellevue Hospital clinical laboratory, with a positive result defined as concentration >25 units per ml. Blood samples were acquired before initiation of a new biologic therapy or within 1 week of starting methotrexate. Concurrent prednisone use was permitted. All synovial fluid and blood samples were subjected to density centrifugation using Ficoll-Paque to isolate mononuclear cells, which were cryopreserved for batched analyses. Phenotypic analyses of blood T cells were performed on samples from both RA patients and healthy subjects, with similar results unless specifically indicated.
2.2. General Reagents.
RPMI medium 1640, DMEM, Dulbecco’s phosphate-buffered saline, and FBS were purchased from Life Technologies. Opti-MEM-I was purchased from Invitrogen. Ficoll-Paque was purchased from GE. The bicinchoninic acid (BCA) assay was purchased from Pierce Biotechnology. Poly-L-lysine, fibronectin, and orthovanadate were purchased from Sigma. Pervanadate was prepared by mixing orthovanadate and H2O2 at a 1:1 ratio and was used at a final concentration of 50 μM.
2.3. Cell Culture, Transfection, and Stimulation.
Jurkat T cells were obtained from the ATCC and maintained in RPMI medium supplemented with 10% FBS and 1% penicillin/streptomycin. CD28 constructs were introduced into the cells by nucleofection (Lonza) with an efficiency of 50–70%. For co-localization experiments, cells were stimulated with soluble anti-CD3 and anti-CD28 antibodies.
2.4. DNA Constructs.
Mutations in the tyrosine and serine residues of the intracellular CD28 domain (Y173F, Y190F, and S171A) were generated using the QuikChange Site-Directed Mutagenesis Kit (Agilent). mCherry-SAP and CD28-GFP were generated by cloning the SH2D1A gene from pDORN201-SH2D1A into mCherry-hLC3B-pcDNA3.1 and CD28 gene from DNASU Clone ID 641402 (DNASU) into the pGFP-N1 (Invitrogen). mCherry-hLC3B-pcDNA3.1 (Addgene 40827) was a gift from David Rubinsztein, Cambridge Institute of Medical Research, Cambridge, UK. SAP was generated from pLX304-SH2D1A (DNASU).
2.5. Flow Cytometry.
CD3 T cells were stained for 30 minutes at 4°C with the following anti-human antibodies (all from BioLegend): APC/Cy7-CD3 (clone HIT3a), Alexa Fluor-CD4 (clone RPA-T4), Pacific Blue-CD8 (clone RPA-T8), and Brilliant Violet 711-PD-1 (clone EH12.2H7. The cells were washed twice with cold PBS containing 2% FBS and were fixed in 2% paraformaldehyde. Fixed cells were washed twice with Intracellular Staining Permeabilization Wash Buffer (BioLegend) and stained with anti-human SAP antibody (clone XLP-1D12; eBioscience) for 30 minutes at room temperature. The cells were washed after intracellular staining and data were acquired on a BD LSR flow cytometer and were analyzed using FlowJo software.
2.6. Generating Stable Knockdown Jurkat T Cells.
CD28 was stably knocked down in Jurkat T cells by shRNA using Mission shRNA library (Sigma). Lentiviral particles were generated by transfecting HEK293T cells with pMD2G, psPAX2, and the shRNA plasmid using SuperFect (Qiagen). T cells were transduced by centrifugation and selected with puromycin. Knockdown was validated by flow cytometry (Supplemental Figure 3).
2.7. Immunoprecipitation and Affinity Enrichment.
Cell lysates were mixed with anti-GFP monoclonal antibody coupled to agarose beads (MBL) to enrich for GFP-tagged CD28 according to the manufacturer’s protocols. Pull-down lysates were separated by Tris-glycine SDS-PAGE, transferred to nitrocellulose filters, and visualized as described (3). CD28-GFP constructs were overexpressed in Jurkat T cells, validated by flow cytometry and SAP was overexpressed in HEK293T cells.
2.8. Cytokine Analysis.
To determine the concentration of secreted IL-2 following stimulation, the human IL-2 ELISA kit (BioLegend) was used according to the manufacturer’s protocols. Cells were stimulated with antibody-coated plates for 24 hours following supernatant collection and analysis, as described above.
2.9. Statistics.
Values are reported as mean ± SEM. Statistical analyses were performed using Student’s paired t test, Mann-Whitney test and 2 way ANOVA analysis. All statistical analyses were performed using GraphPad Prism (version 6.0).
3. Results:
3.1. RA patient T cells express high levels of PD-1 that correlate with disease activity.
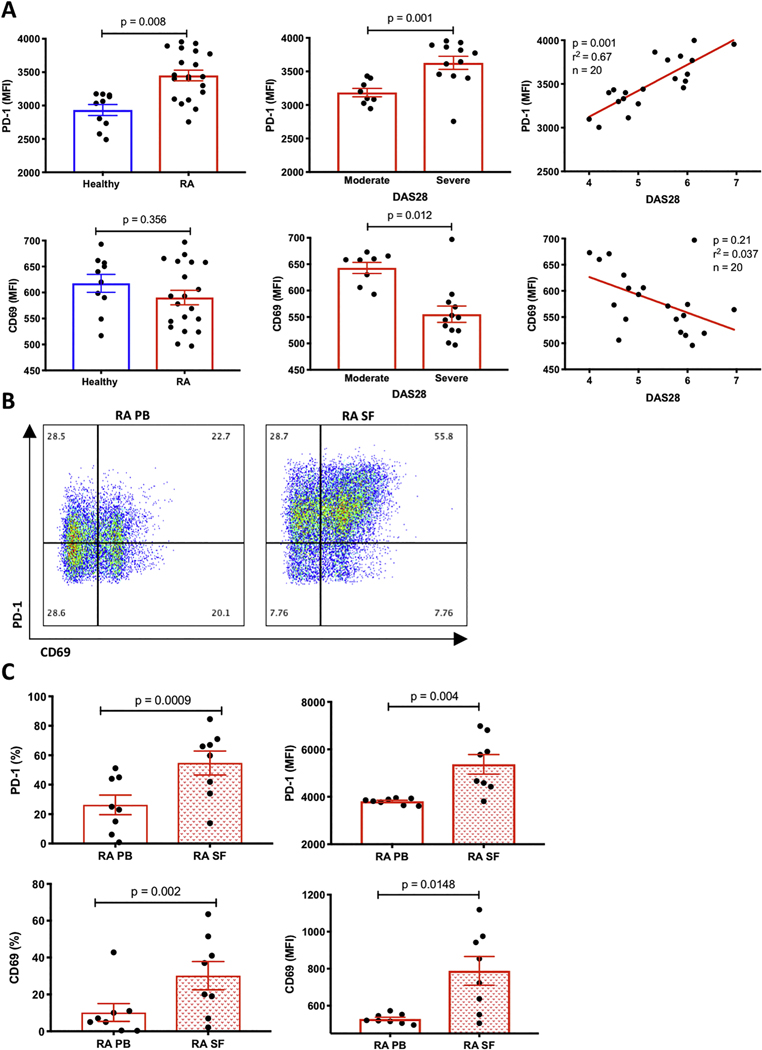
To document T cell changes associated with RA we collected blood and synovial fluid from seropositive (defined as positive for rheumatoid factor (RF) and/or cyclic citrullinated peptide (CCP)) RA patients meeting ACR criteria with moderate to high DAS28 scores (DAS28>3.2), as well as blood from healthy controls. Using flow cytometry (gating strategy Supplemental Fig. 2), we analyzed CD3 T cells from the peripheral blood and synovial fluid. In peripheral blood, we found that PD-1 levels were elevated on CD3 T cells from patients with RA compared to healthy controls (Fig. 1A). Furthermore, PD-1 MFI was higher in patients with high disease activity and correlated with DAS28 scores (Fig. 1A). Correlation studies revealed linear association between DAS28 and PD-1 levels (Fig. 1A). When we compared surface expression of CD69, a marker of T cell activation, on T cells isolated from healthy donors compared to RA T cells, we detected no difference. In contrast, CD69 expression was higher in patients with moderate RA disease activity than those with high disease activity, suggesting that the elevated PD-1 levels did not simply reflect non-specific T cell activation. In synovial fluid, we observed that RA T cells expressed significantly higher levels of PD-1 as compared to peripheral blood T cells from the same patients (Fig. 1B and 1C). Interestingly, we found that both PD-1 and CD69 levels were higher in synovial fluid compared to peripheral blood, which has been shown in other inflammatory arthritides (8). We also looked at other clinical markers of inflammation such as ESR and CRP in correlation with PD-1 levels, but they were not significant (Supplemental Fig. 1). In summary, these data suggest that while PD-1 is elevated, it is likely not just a marker of activation since patients with severe disease do not possess high levels of CD69.
Figure 1. Patients with RA exhibit increased PD-1 levels in T cells from different compartments and correlate with disease activity.
(A) PBMC from healthy donors or patients with RA were analyzed by flow cytometry for the expression of PD-1 and CD69 levels. DAS28 scores were calculated based on data from clinical charts, divided into moderate (DAS>3.2–5.1) and severe (DAS>5.2) and correlated with the expression levels of both PD-1 and CD69. Healthy controls: n=10, RA patients: n= 20, p values are as indicated. (B) A representative two-dimensional flow cytometry plot of PD-1 and CD69 from RA patient peripheral blood (PB) and synovial fluid (SF). Percentages in each quadrant are shown. (C) Quantification of PD-1 and CD69 expression levels, as measured by flow cytometry, from matched T cells isolated from peripheral blood (PB) and matched synovial fluid (SF) of 8 RA patients. Data is shown as percentage (%) and as MFI (mean fluorescence intensity), p values are as indicated in the figure based on calculated t-test.
3.2. SAP levels are elevated in T cells of patients with rheumatoid arthritis.
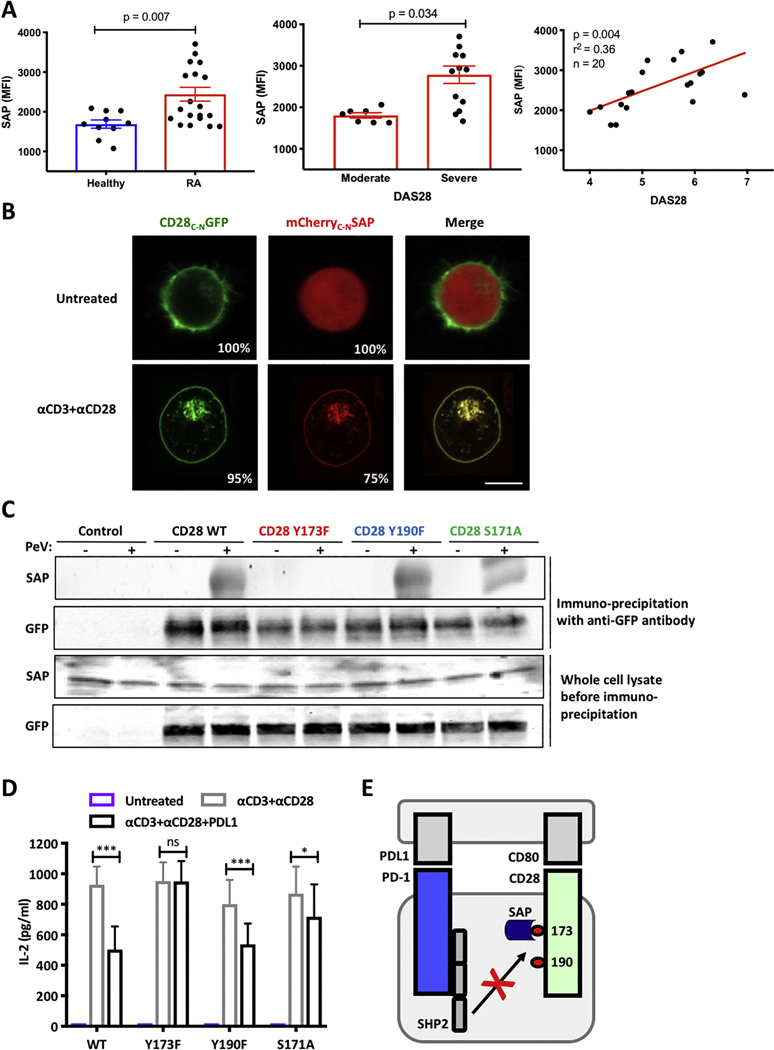
Since our prior work demonstrated that SAP inhibits the function of the PD-1 pathway, we were encouraged to study the relationship of SAP in T cells from patients with RA, which has not been extensively studied. The immunopathology of RA is T cell driven and PD-1 is known to be a negative regulator of T cell function. However, elevated PD-1 levels on the surface of RA T cells are surprising, suggesting that these cells are activated, but the PD-1 pathway is not active. Thus, we considered SAP as an inhibitor of PD-1 function and investigated whether SAP levels are altered in RA patients. Accordingly, we measured SAP levels in permeabilized CD3 T cells by flow cytometry and observed that T cells from patients with RA had elevated SAP levels compared to healthy controls (Fig. 2A). Importantly, these levels were higher in patients with active disease mirroring the PD-1 phenotype in RA T cells (Fig. 2A). Correlation studies revealed that SAP is elevated in patients with more severe disease activity suggesting that perhaps it is involved in inhibiting PD-1 signaling in RA patients (Fig. 2A). We next sought a mechanism to determine how SAP can inhibit PD-1 signaling in vitro.
Figure 2. SAP binds CD28 in Y173 dependent fashion.
(A) PBMC were isolated from 10 healthy controls and 20 patients with active RA and the levels of SAP expression were evaluated by flow cytometry. DAS were calculated as described in the method section. Moderate disease is defined as DAS 3.2–5.1 and severe disease is defined as DAS >5.2. Correlation between DAS scoring and SAP levels were calculated and Pearson coefficient (r2) is shown. (B) mCherry-SAP and CD28-GFP were over expressed in Jurkat T cells prior to stimulation as indicated. Images were acquired on live cells; representative cells are shown. 20 cells were counted in three independent experiments, average percentage of cells showing the dominant phenotype are shown. (C) Jurkat T cells overexpressing different versions of CD28-GFP were treated with pervanadate (at concertation of 100 uM) for 5 minutes and lysed. CD28 were pulled downs using anti-GFP antibodies. Whole cell lysate and the immune precipitate were blotted for SAP and GFP. A representative western blot is shown, n=3. (D) Jurkat T cells expressing different versions of CD28 were stimulated as indicated. IL-2 levels in the supernatant were measure by ELISA after 24 hours, n=3, ± SEM, ns not significant, * p<0.05, ** p<0.001. (E) A diagram showing the role of SAP blocking CD28 tyrosine 173 dephosphorylation by SHP2.
3.3. SAP binds to CD28 through tyrosine 173.
It has been reported that CD28 is dephosphorylated by SHP2 downstream of PD-1 signaling (9, 10). Interestingly, others groups have not found CD28 to be directly involved in the PD-1 pathway (11) or have shown dephosphorylation of CD28 by SHP1 in the absence of SHP2 (12). We evaluated the interaction of SAP and CD28 as a possible mechanism for SAP mediated PD-1 inhibition. To assess cellular proximity of SAP and CD28, we tagged the two proteins with monomeric Cherry (mCherry) and GFP, respectively, and localized them in Jurkat T cells using live-cell confocal imaging. As expected, CD28-GFP was expressed predominantly on the plasma membrane (PM) and this distribution was not altered by cellular activation (Fig. 2B). In resting cells mCherry-SAP was distributed in a cytosolic pattern with negatively imaged organelles (Fig. 2B). Upon activation of the Jurkat T cells with anti-CD3 and anti-CD28 antibodies, mCherry-SAP translocated to the PM and colocalized with CD28-GFP (Fig. 2B). This was also observed when transfected cells were treated with pervanadate (Supplemental Fig. 3). Thus, CD28 and SAP colocalize in T cells in an activation-dependent manner at the PM compartment.
To determine if this co-localization resulted from a protein-protein interaction between CD28 and SAP we performed an immuno-precipitation experiment with different versions of the CD28 tagged with GFP immobilized on agarose beads coated with anti-GFP antibodies. We expressed wild-type (WT) or mutant CD28-GFP in Jurkat T cells, treated these cells with pervanadate or vehicle control. To the washed agarose beads we applied lysate of Jurkat T cells as a source of endogenous SAP. In an unmutated CD28 protein, we observed that SAP was pulled down when the Jurkat T cells were treated with pervanadate, suggesting phosphotyrosine-dependent binding as indicated by the band on the western blot. Tyrosine 173 within the cytoplasmic domain of CD28, has been found to associate with p85 of PI3K leading to cell activation and is a known SH2 binding domain. The second tyrosine at position 190 of CD28 is an SH3 binding domain and has been shown to interact with Grb2 (an SH2 binding adaptor protein) (13)). Thus, to understand the site of binding, we generated phospho-deficient versions of CD28 that cannot bind to SH2-domain carrying proteins, such as SAP. For example, if tyrosine 173 was mutated (Y173F; meaning we replaced tyrosine (Y) in position 173 with phenylalanine (F)) and SAP was not pulled down, then the conclusion is that SAP does interact with this tyrosine. And indeed, when tyrosine 173 was mutated SAP was not pulled down (Fig. 2C). Next, we mutated tyrosine in the 190 position, in the same manner as described above. Given that removal of the tyrosine at position 190, still pulled down SAP, suggested that tyrosine 190 was not where SAP binds to CD28.
The best-established binding partners of SAP are phosphorylated tyrosine motifs on members of the SLAM receptor family (14). SLAM-SAP binding has been shown to be facilitated by a proximal serine residue (14, 15). Since serine residues are often seen as a residue to stabilize protein-protein interactions. We asked if the serine at position 171 was contributing to the binding at the Y173 position. Indeed, we found that when the serine residue was mutated to alanine, less SAP was bound to CD28, suggesting that for optimal SAP binding to CD28, a serine residue at position 171 is necessary, suggesting that this residue may stabilize the interaction (Fig. 2C).
To evaluate the functional significance of CD28 phosphorylation on tyrosine 173 we used CD28 deficient T cells and rescued CD28 function by transfection with WT or mutant versions of CD28 (as described above). When CD28 deficient Jurkat T cells were reconstituted with WT CD28 and stimulated by cross-linking CD3 and CD28 with antibodies to the two receptors, the cells secreted IL-2 (Fig. 2D). When PD-L1 was added to the cell stimulation, IL-2 production was diminished by 50% demonstrating productive engagement of PD-1 signaling. Whereas cells transduced with CD28 with a Y190F substitution showed the same degree of PD-L1-mediated inhibition as those transduced with WT CD28, stimulation of cells transduced with a Y173F mutation was unaffected by PD-L1. Cells rescued with CD28 with an S171A substitution showed an intermediate response. Thus, CD28 that cannot bind to SAP does not support PD-1 mediated inhibition of T cells (Fig. 2E).
4. Discussion:
The expression of co-inhibitory receptors on RA T cells has been described in the literature (16–19). In the present study, we evaluated the relationship between the T cell surface expression of PD-1 and RA clinical disease activity. In peripheral blood, PD-1 levels correlated with RA disease activity. In addition, we found that the PD-1 pathway is unlikely to inhibit T cells in synovial fluid as the cells have an elevated CD69 and PD-1 suggestive of an activated phenotype. Therefore, we wanted to understand this discrepancy and considered whether there may be a molecule downstream of PD-1 that may interfere with its function. In our previous work, we reported that SAP inhibits PD-1 activity in vitro (6). We found that SAP levels are also elevated in RA patient T cells. This is particularly interesting since both PD-1 and SAP are found in T follicular helper cells. In fact, an elegant study performed by Rao et al., (16) revealed the difference between PD-1+CXRX5+ and PD-1+CXCR5-T cells in patients with RA, looking at the synovium and peripheral blood. In their work, they characterized a new T cell subset, termed T peripheral helper cells, which are PD-1+ and CXCR5-. These cells were described as helpers to promote plasma cell differentiation through IL-21 and SLAMF5 interaction.
There are a few RA databases, which have identified associations mostly with increased susceptibility to RA with particular SNPs in PD-1 (20–22). More specifically, PD-1 was found to be associated with RA haplotype in RA patients from Hong Kong, but not with Japanese RA individuals (23). A tissue-specific susceptibility locus for inherited inflammatory disorders was found to be associated with the PD-1 gene. Meta-analysis of genetic polymorphisms in PD-1 reported associations with RA, ankylosing spondylitis, and type 1 diabetes (24, 25). Similar associations were not reported for SAP. One manuscript evaluated the levels of SAP transcript expression in RA patients and found that SAP levels were lower in a cohort of 21 Japanese RA patients compared to inactive SLE controls (26). In the manuscript by Rao et al., SAP was found to be elevated in PD-1 high, MHCII positive synovial fluid cells. In addition, the same authors showed PD-1 high CD4+ T cells from peripheral blood also expressed high levels of SAP (16).
Our studies in Jurkat T cells revealed a mechanism whereby SAP may limit PD-1 signaling by binding to phosphor-tyrosine 173 of CD28 thereby blocking dephosphorylation by SHP2. Our data suggest that T cells in the rheumatoid synovium escape PD-1 mediated exhaustion by upregulating protein expression or inhibiting the degradation of SAP. Interestingly, GWAS have identified point mutations in the CD28 gene, which increase susceptibility to RA (27, 28) as well as other autoimmune diseases such as systemic sclerosis and sclerosing cholangitis (29). These mutations occur mostly in non-coding regions. SLAMF6 is elevated on the surface of T and B cells in patients with RA compared to controls (30). In addition, SLAMF6 expression was found to be decreased in RA patients who were being treated with methotrexate (31) suggesting the potential that SLAM proteins are important in modulating RA disease activity. However, to our knowledge, studies have not linked SLAM-SAP signaling to PD-1-CD28 interactions.
The role of PD-1 positive cells in the SF of RA patients is unclear. We hypothesized that since PD-1 can be an early activation marker and these cells are also CD69 positive, these cells are in fact activated. In these cells PD-1 doesn’t function as an inhibitory receptor. Other manuscripts may support these findings. In a 2018 paper by Luo et al., the authors also observed increased PD-1 levels in active disease RA T cells especially in the synovial fluid and in the peripheral blood. This was observed more frequently on CD4+ cells, but also on CD8+ cells (17). In addition, it was observed by Bommarito et al., that PD-1+ T cells from RA patients were resistant to inhibition with a PD-1 ligand (19). Another interesting manuscript, highlighted a novel regulatory T cell subset that was deficient in inhibitory function, and these cells were PD-1+, CTLA-4+ and CD28 + (18). Other co-inhibitory receptors have been reported on synovial T cells, such as PD-1 TIM3 and TIGIT. Interestingly, the same authors also reported that PD-1 may be transferred to other cells via extracellular vesicles (32). Others have shown that PD-1 levels are upregulated in response to chronic antigen presentation in situations of autoimmunity (8, 17). Here we add elevated SAP levels as a potential mechanism to block PD-1 function. CD28 has been recently described as a signaling partner of PD-1 and we report that the function of PD-1 is interrupted by SAP binding to the proximal tyrosine residue on CD28, making it unavailable for downstream PD-1 mediating phosphatases.
Supplementary Material
Supplemental Figure 1. PBMC from healthy donors or patients with RA were analyzed by flow cytometry for the expression of PD-1 levels. Clinical markers of inflammation (CRP and ESR) were correlated with the expression level of PD-1 using simple linear regression analysis.
Supplemental Figure 2. Representative flow cytometric scatter plots show gating strategy used to identify different T cells in blood and synovial fluid. Viable cells were gated using a viability dye, first. Lymphocytes were gated on side scatter and forward scatter parameters. Doublets were excluded. CD3 T cells were gated from single cells.
Supplemental Figure 3. (A) mCherry-SAP and CD28-GFP were over expressed in Jurkat T cells prior to stimulation with pervanadate as indicated. Images were acquired on live cells; representative cells are shown. 20 cells were counted in three independent experiments, average percentage of cells showing the dominant phenotype are shown. (B) Efficient CD28 knockdown in Jurkat T cells. Flow cytometry analysis was performed on Jurkat T cells transfected with CD28 shRNA using anti-CD28 FITC antibody, red and green curves represent negative controls, orange is C28KD and blue is CD28WT.
Table 1.
Patient characteristics.
| Patient | Age | Sex | Disease Duration | Medications | DAS28 | Seropositive (RF and CCP) | Erosive |
|---|---|---|---|---|---|---|---|
| 1 | 62 | F | 12 y | Prednisone 5 mg daily | 5.92 | Both | Yes |
| 2 | 36 | M | 5 y | none | 5.86 | Both | N/A |
| 3 | 49 | F | 26 y | none | 6.34 | Both | Yes |
| 4 | 57 | F | 6 y | HCQ 400 mg daily | 4.74 | Both | Yes |
| 5 | 26 | F | New dx | none | 4.6 | Both | No |
| 6 | 44 | M | 9 y | Prednisone 15 mg daily | 6.14 | Both | Yes |
| 7 | 29 | F | 9 y | Adalimumab 40 mg biweekly | 6.1 | Both | Yes |
| 8 | 48 | F | New dx | none | 4.5 | Both | No |
| 9 | 49 | F | New dx | none | 5.96 | Both | No |
| 10 | 66 | M | 2 y | none | 6.95 | Both | Yes |
| 11 | 56 | F | 5 y | RTX, MTX 10 mg weekly | 4.8 | Both | Yes |
| 12 | 36 | F | 4 y | MTX 15mg weekly, Prednisone 15 mg daily | 5.77 | Both | Yes |
| 13 | 34 | F | N/A | N/A | 5.2 | Both | N/A |
| 14 | 53 | F | N/A | N/A | 6.1 | Both | N/A |
| 15 | 55 | F | N/A | N/A | 6.9 | Both | N/A |
| 16 | 22 | F | N/A | N/A | 4.2 | Both | N/A |
| 17 | 50 | F | N/A | N/A | 4.1 | Both | N/A |
| 18 | 32 | F | N/A | N/A | 6.3 | Both | N/A |
| 19 | 49 | M | N/A | N/A | 6.6 | Both | N/A |
| 20 | 28 | F | N/A | N/A | 7.0 | Both | N/A |
DAS28 disease activity in 28 joints; HCQ hydroxychloroquine; SC subcutaneous; RTX rituximab, MTX methotrexate, RF rheumatoid factor; CCP cyclic citrullinated protein.
RA patients with active disease have high levels of PD-1 and CD69 on synovial T lymphocytes.
Peripheral T lymphocytes from RA patients have high levels of PD-1 and SAP.
SAP binds to the proximal tyrosine residue on CD28, thereby abolishing PD-1 effect.
Mutation of the proximal tyrosine on the CD28 tail abolishes PD-1 function.
Acknowledgments
Funding:
This work was supported by grants from the NIH (AI25640 and CA231277) and from the Cancer Research Institute.
Footnotes
Declaration of Interest:
The authors declare that there are no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Spector TD. Rheumatoid arthritis. Rheum Dis Clin North Am. 1990;16(3):513–37. [PubMed] [Google Scholar]
- 2.Mellado M, Martinez-Munoz L, Cascio G, Lucas P, Pablos JL, Rodriguez-Frade JM. T Cell Migration in Rheumatoid Arthritis. Front Immunol. 2015;6:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cope AP, Schulze-Koops H, Aringer M. The central role of T cells in rheumatoid arthritis. Clin Exp Rheumatol. 2007;25(5 Suppl 46):S4–11. [PubMed] [Google Scholar]
- 4.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kremer JM, Westhovens R, Leon M, Di Giorgio E, Alten R, Steinfeld S, et al. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med. 2003;349(20):1907–15. [DOI] [PubMed] [Google Scholar]
- 6.Peled M, Tocheva AS, Sandigursky S, Nayak S, Philips EA, Nichols KE, et al. Affinity purification mass spectrometry analysis of PD-1 uncovers SAP as a new checkpoint inhibitor. Proc Natl Acad Sci U S A. 2018;115(3):E468–E77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartzberg PL, Mueller KL, Qi H, Cannons JL. SLAM receptors and SAP influence lymphocyte interactions, development and function. Nat Rev Immunol. 2009;9(1):39–46. [DOI] [PubMed] [Google Scholar]
- 8.Petrelli A, Mijnheer G, Hoytema van Konijnenburg DP, van der Wal MM, Giovannone B, Mocholi E, et al. PD-1+CD8+ T cells are clonally expanding effectors in human chronic inflammation. J Clin Invest. 2018;128(10):4669–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hui E, Cheung J, Zhu J, Su X, Taylor MJ, Wallweber HA, et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science. 2017;355(6332):1428–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamphorst AO, Wieland A, Nasti T, Yang S, Zhang R, Barber DL, et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science. 2017;355(6332):1423–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao Y, Harrison DL, Song Y, Ji J, Huang J, Hui E. Antigen-Presenting Cell-Intrinsic PD-1 Neutralizes PD-L1 in cis to Attenuate PD-1 Signaling in T Cells. Cell Rep. 2018;24(2):379–90 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Celis-Gutierrez J, Blattmann P, Zhai Y, Jarmuzynski N, Ruminski K, Gregoire C, et al. Quantitative Interactomics in Primary T Cells Provides a Rationale for Concomitant PD-1 and BTLA Coinhibitor Blockade in Cancer Immunotherapy. Cell Rep. 2019;27(11):3315–30 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Truitt KE, Hicks CM, Imboden JB. Stimulation of CD28 triggers an association between CD28 and phosphatidylinositol 3-kinase in Jurkat T cells. J Exp Med. 1994;179(3):1071–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shlapatska LM, Mikhalap SV, Berdova AG, Zelensky OM, Yun TJ, Nichols KE, et al. CD150 association with either the SH2-containing inositol phosphatase or the SH2-containing protein tyrosine phosphatase is regulated by the adaptor protein SH2D1A. J Immunol. 2001;166(9):5480–7. [DOI] [PubMed] [Google Scholar]
- 15.Hwang PM, Li C, Morra M, Lillywhite J, Muhandiram DR, Gertler F, et al. A “three-pronged” binding mechanism for the SAP/SH2D1A SH2 domain: structural basis and relevance to the XLP syndrome. EMBO J. 2002;21(3):314–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rao DA, Gurish MF, Marshall JL, Slowikowski K, Fonseka CY, Liu Y, et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature. 2017;542(7639):110–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo Q, Ye J, Zeng L, Luo Z, Deng Z, Li X, et al. Elevated expression of PD1 on T cells correlates with disease activity in rheumatoid arthritis. Mol Med Rep. 2018;17(2):3297–305. [DOI] [PubMed] [Google Scholar]
- 18.Fessler J, Raicht A, Husic R, Ficjan A, Schwarz C, Duftner C, et al. Novel Senescent Regulatory T-Cell Subset with Impaired Suppressive Function in Rheumatoid Arthritis. Front Immunol. 2017;8:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bommarito D, Hall C, Taams LS, Corrigall VM. Inflammatory cytokines compromise programmed cell death-1 (PD-1)-mediated T cell suppression in inflammatory arthritis through up-regulation of soluble PD-1. Clin Exp Immunol. 2017;188(3):455–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kong EK, Prokunina-Olsson L, Wong WH, Lau CS, Chan TM, Alarcon-Riquelme M, et al. A new haplotype of PDCD1 is associated with rheumatoid arthritis in Hong Kong Chinese. Arthritis Rheum. 2005;52(4):1058–62. [DOI] [PubMed] [Google Scholar]
- 21.James ES, Harney S, Wordsworth BP, Cookson WO, Davis SJ, Moffatt MF. PDCD1: a tissue-specific susceptibility locus for inherited inflammatory disorders. Genes Immun. 2005;6(5):430–7. [DOI] [PubMed] [Google Scholar]
- 22.Lee YH, Bae SC, Kim JH, Song GG. Meta-analysis of genetic polymorphisms in programmed cell death 1. Associations with rheumatoid arthritis, ankylosing spondylitis, and type 1 diabetes susceptibility. Z Rheumatol. 2015;74(3):230–9. [DOI] [PubMed] [Google Scholar]
- 23.Iwamoto T, Ikari K, Inoue E, Toyama Y, Hara M, Yamanaka H, et al. Failure to confirm association between PDCD1 polymorphisms and rheumatoid arthritis in a Japanese population. J Hum Genet. 2007;52(6):557–60. [DOI] [PubMed] [Google Scholar]
- 24.Gene and Autoimmune Disease Association Database [Available from: aarda.org.
- 25.Transcriptional data of inflammatory arthritis T cells [cited 2019. Available from: https://www.ncbi.nlm.nih.gov/bioproject/595368.
- 26.Takei M, Ishiwata T, Mitamura K, Fujiwara S, Sasaki K, Nishi T, et al. Decreased expression of signaling lymphocytic-activation molecule-associated protein (SAP) transcripts in T cells from patients with rheumatoid arthritis. Int Immunol. 2001;13(4):559–65. [DOI] [PubMed] [Google Scholar]
- 27.Raychaudhuri S, Thomson BP, Remmers EF, Eyre S, Hinks A, Guiducci C, et al. Genetic variants at CD28, PRDM1 and CD2/CD58 are associated with rheumatoid arthritis risk. Nat Genet. 2009;41(12):1313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marquez A, Kerick M, Zhernakova A, Gutierrez-Achury J, Chen WM, Onengut-Gumuscu S, et al. Meta-analysis of Immunochip data of four autoimmune diseases reveals novel single-disease and cross-phenotype associations. Genome Med. 2018;10(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu JZ, Hov JR, Folseraas T, Ellinghaus E, Rushbrook SM, Doncheva NT, et al. Dense genotyping of immune-related disease regions identifies nine new risk loci for primary sclerosing cholangitis. Nat Genet. 2013;45(6):670–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isomaki P, Aversa G, Cocks BG, Luukkainen R, Saario R, Toivanen P, et al. Increased expression of signaling lymphocytic activation molecule in patients with rheumatoid arthritis and its role in the regulation of cytokine production in rheumatoid synovium. J Immunol. 1997;159(6):2986–93. [PubMed] [Google Scholar]
- 31.Morita Y, Fukazawa T, Hirashima M, Kaga K, Kusaoi M, Morita T, et al. The effect of methotrexate (MTX) on expression of signalling lymphocytic activation molecule (SLAM) in patients with rheumatoid arthritis (RA) and its role in the regulation of cytokine production. Scand J Rheumatol. 2006;35(4):268–72. [DOI] [PubMed] [Google Scholar]
- 32.Greisen SR, Yan Y, Hansen AS, Veno MT, Nyengaard JR, Moestrup SK, et al. Extracellular Vesicles Transfer the Receptor Programmed Death-1 in Rheumatoid Arthritis. Front Immunol. 2017;8:851. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. PBMC from healthy donors or patients with RA were analyzed by flow cytometry for the expression of PD-1 levels. Clinical markers of inflammation (CRP and ESR) were correlated with the expression level of PD-1 using simple linear regression analysis.
Supplemental Figure 2. Representative flow cytometric scatter plots show gating strategy used to identify different T cells in blood and synovial fluid. Viable cells were gated using a viability dye, first. Lymphocytes were gated on side scatter and forward scatter parameters. Doublets were excluded. CD3 T cells were gated from single cells.
Supplemental Figure 3. (A) mCherry-SAP and CD28-GFP were over expressed in Jurkat T cells prior to stimulation with pervanadate as indicated. Images were acquired on live cells; representative cells are shown. 20 cells were counted in three independent experiments, average percentage of cells showing the dominant phenotype are shown. (B) Efficient CD28 knockdown in Jurkat T cells. Flow cytometry analysis was performed on Jurkat T cells transfected with CD28 shRNA using anti-CD28 FITC antibody, red and green curves represent negative controls, orange is C28KD and blue is CD28WT.