Abstract
More than 60% of species examined from a total of 421 strains of heterotrophic marine bacteria which were isolated from marine sponges and seawater were observed to have no detectable siderophore production even when Fe(III) was present in the culture medium at a concentration of 1.0 pM. The growth of one such non-siderophore-producing strain, alpha proteobacterium V0210, was stimulated under iron-limited conditions with the addition of an isolated exogenous siderophore, N,N′-bis (2,3-dihydroxybenzoyl)-O-serylserine from a Vibrio sp. Growth was also stimulated by the addition of three exogenous siderophore extracts from siderophore-producing bacteria. Radioisotope studies using 59Fe showed that the iron uptake ability of V0210 increased only with the addition of exogenous siderophores. Biosynthesis of a hydroxamate siderophore by V0210 was shown by paper electrophoresis and chemical assays for the detection of hydroxamates and catechols. An 85-kDa iron-regulated outer membrane protein was induced only under iron-limited conditions in the presence of exogenous siderophores. This is the first report of bacterial iron uptake through an induced siderophore in response to exogenous siderophores. Our results suggest that siderophores are necessary signaling compounds for growth and for iron uptake by some non-siderophore-producing marine bacteria under iron-limited conditions.
Iron is an essential element for most microorganisms owing to its importance in a variety of biochemical reactions, including respiration, photosynthetic transport, nitrate reduction, chlorophyll synthesis, nitrogen fixation, and detoxification of oxygen radicals. In spite of its high abundance in the earth's crust, dissolved iron concentrations are particularly low (<0.4 μM) in the surface waters of the open ocean (25). Under iron-limited conditions, most prokaryotic cells and certain fungi and plants secrete siderophores, which are compounds with small molecular masses which bind ferric ions with high affinity (23). Siderophores usually fall into two groups, the hydroxamates and the catecholates, based on their structural features (23, 38, 39). Organisms which are capable of siderophore production have molecular systems which transport iron by siderophore-iron complexes into cells through iron-regulated outer membrane proteins (IROMP) (4). Heterotrophic marine bacteria isolated from various habitats have been shown to produce siderophores by a universal siderophore production screening assay, the chrome azurol S (CAS) assay (31). Until now, the majority of work has focused on the CAS assay-detectable siderophores (5, 14, 19, 20, 27, 28, 32, 35) in response to Fe-limited stress. Meanwhile, little is known about the means by which marine bacteria which have no CAS assay-detectable siderophores obtain iron for growth.
Recent studies have reported that more than 99% of dissolved iron in the surface ocean is tightly bound to organic ligands (30, 40, 41). The nature of iron-bound organic ligands in seawater is still uncertain and little is known about their ecological significance. The high Fe-binding affinities of these unidentified compounds strongly suggest that they are siderophores biosynthesized by marine bacteria. Recently, iron uptake through bacterial siderophores by phytoplankton has been reported (18) and indicates the possibility that interactions through siderophores occur among bacteria and phytoplankton in the ocean. Thus, it suggests that siderophores may be one of the important factors that affect the “iron flow” (12, 33, 34) in the ocean among bacteria and/or between bacteria and other microorganisms. However, only a few oceanic siderophores have been structurally characterized (5, 20, 26, 27) compared to the abundance of bacterial species. Little is known on whether any other factors are necessary for bacterial siderophore production. Investigation of the conditions which affect bacterial siderophore production is essential to elucidate the iron acquisition mechanisms of marine bacteria and the environmental role of siderophores in the ocean.
In our current work, we focused on marine bacteria which have no CAS assay-detectable siderophores. We speculated that siderophores are a type of signal which affects iron uptake and growth of some bacteria in naturally iron-deficient ocean environments. The bacteria were isolated from different marine sponges and seawater, and these two habitats were chosen for the following reasons. (i) Marine sponges are animals which live symbiotically with bacteria and microalgae, and although many marine bacteria have been isolated from sponges (11), little is known about their physiological functions, including siderophore production and iron uptake activity. (ii) There is a lack of information regarding the influence of siderophore-mediated iron uptake by planktonic bacteria living in seawater. Using one isolated siderophore compound and three partially purified siderophores, we investigated the growth and iron uptake activity of more than 200 strains of non-siderophore-producing (Sid−) bacteria isolated from marine sponges and seawater. We observed that growth and siderophore production of some Sid− strains were stimulated by exogenous siderophores. One of the Sid− strains, alpha proteobacterium V0210, was specifically investigated in regard to its reactivity after the addition of four exogenous siderophore components. Our results indicate that siderophores are probably necessary signals for siderophore production in some marine bacteria under iron-limited conditions.
MATERIALS AND METHODS
Strains and culture conditions.
Bacteria were isolated from 17 different marine sponges collected in the Fiji Islands and from seawater collected in Okinawa, Japan. The sponge tissue was squeezed with a mixer. Sponge solutions and collected seawater were diluted from 10−1 to 10−4 in sterilized seawater, and then 100 μl of each solution was spread onto 3% NaCl supplemented with 1/10-diluted marine broth (Marine Broth 2216; Difco) agar plates [4 μM Fe(III)]. Morphologically different colonies were selected and inoculated onto fresh 1/10-diluted marine broth agar plates for further isolation. Marine bacteria were identified by growth on the plates containing 3% NaCl in comparison with no growth on the agar plates containing 0.15% NaCl for the same strain. All culturing was performed at 30°C.
The iron-deficient and low-nutrient seawater-based liquid medium (16) (IDSM) contained the following components (in grams/liter): NH4NO3, 1.0; NaCl, 30.0; MgSO4 · 7H2O, 0.5; KCl, 0.3; K2HPO4, 1.5; HEPES, 2.38; and CaCl2, 0.2; it also contained 0.1% glucose and 0.1 μM Fe(III). The medium was adjusted to pH 7.2 and was treated by Chelex-100 (Sigma) (10) to remove contaminating iron before the addition of FeCl3 solution. Liquid cultivation of bacteria was performed in 10 ml of IDSM with a shaker connected to an optical density-measuring device set to 600 nm (Bio-Photorecorder TN-2612; Advantec). The cultures were grown with shaking at 50 rpm and 30°C for a week, and the optical density at 600 nm was measured every 15 min. Glassware was acid washed in 6 N HCl for 24 h before use.
Bacterial DNA was extracted using a PureGene kit (Gentra Systems). A 16S ribosomal DNA (rDNA) V3 gene region fragment (169 to 194 bp in length) was amplified by PCR using a universal primer complementary to positions 517 to 534 (5′-ATTACCGCGGCTGCTGG-3′) and a bacterial primer complementary to positions 341 to 358 (5′-CCTACGGGAGGCAGCAG-3′) (22). The total 16S rDNA fragment (∼1,500 bp) was amplified by using two oligonucleotide primers, fD (5′-AGAGTTTGATCCTGGCTCAG-3′) and rD (5′-AAGGAGGTGATCCAGCC-3′) (37). PCR products were sequenced using a PE Biosystems 373 DNA sequencer, and homologies were searched for on DDBJ databases.
Siderophore detection.
The CAS assay (31) was used to detect siderophores. On CAS agar plates, siderophore-producing (Sid+) bacteria form colonies with an orange halo. The principle of this assay is based on a color change of CAS from blue to orange resulting from siderophoral removal of Fe from the dye. Siderophore halos were evaluated following 5 days of colony incubation at 30°C. The CAS solution assay (15, 31) was used to quantitate siderophore activity in culture supernatant extracts by measuring the decrease in the absorbance of blue color at 630 nm. Standard curves relating CAS reactivity to iron-binding ligands were determined using the fungal siderophore desferrioxamine (Desferal; CIBA GEIGY). The quantity of siderophores produced by the bacteria was reported in terms of iron-binding equivalents, expressed as micromoles of ligand per gram (dry weight) of bacteria (15). Hydroxamate and catechol functionality of 10-fold-concentrated siderophore extracts of bacterial isolates V0122, V0304, V0902, V1110, and V0210 were examined by the Csaky test (13) and the Arnow reaction (1), respectively. For these assays, hydroxylamine and 2,3-dihydroxybenzoic acid, respectively, were used as the standards.
Isolation of siderophores.
Culture broth of strain V0304 in IDSM liquid medium (16 liters) was centrifuged at 8,000 × g for 20 min after cultivation at 30°C for 72 h. The collected supernatant was batch loaded onto DIAION HP-20 resin (Mitsubushi Chemical Co.). After washing with acidic water (pH 2, adjusted with 6 N HCl), the Fe-binding fraction which was active in the CAS solution assay was obtained by elution with methanol. The methanol fraction was further chromatographed on a Sephadex LH-20 (Pharmacia) column (10 by 50 cm) using a 50% methanol-water solution as the mobile phase. CAS active fractions obtained by LH-20 column chromatography were collected and finally purified by reverse-phase high-pressure liquid chromatography (column, TSKgel ODS-80Ts, 7.8 by 300 mm) at 230 nm using 0.1% trifluoroacetic acid–40% acetonitrile–H2O as the mobile phase. The structure of the siderophore was identified by analysis of spectra obtained from nuclear magnetic resonance (NMR; Varian Unity INOVA 500 MHz) and mass spectroscopy (MS) (JEOL JMS-SX102 mass spectrometer) measurements.
To obtain siderophore extracts, bacterial cells grown in 200 ml of IDSM for 72 h at 30°C were harvested by centrifugation at 8,000 × g for 30 min. The supernatant was filtered through a 0.2-μm-pore-size membrane filter to completely remove the cells and was acidified to pH 3.0 with 6 N HCl. Supernatants were extracted three times with equal volumes of ethyl acetate for catechols and benzyl alcohol for hydroxamates (31). The concentrated organic extracts were dissolved in 1 ml of 0.01 M phosphate buffer (pH 7.0) or 0.01 M acetate buffer (pH 4.0). Partial purification of the siderophores was achieved by the fractionation of the organic extracts on a Sephadex LH-20 column in the respective buffers. Each eluted fraction was treated with Chelex-100 to remove the iron. The CAS assay-reactive fractions were pooled and concentrated 10-fold by lyophilization.
Cross-feeding assay.
Sid− strains were inoculated on IDSM agar plates containing 0.1 μM EDTA-Fe(III) complex. A sterilized paper disk (6-mm diameter) was treated with 100 μl of membrane-filtered (0.2 μm-pore-size) siderophore (1.0 nM) and siderophore extracts (10−3 μmol of ligand/g) dissolved in buffer solution. Colony formation around the paper disk on the IDSM agar plates was checked after 5 to 7 days of incubation. CAS agar plates were used for the estimation of siderophore productivity of Sid− bacteria. Siderophore or extract solution was spread on the CAS agar plate to have 10−3 μmol of ligand/g in the medium before Sid− strains were inoculated.
Paper electrophoresis.
The ionophoric mobility of siderophores was determined by paper electrophoresis (31) with Advantec EP-200 paper electrophoresis equipment using a volatile buffer at pH 5.6 (5.7 ml of glacial acetic acid and 24.3 ml of pyridine per liter). Ten microliters each of concentrated siderophore extracts (10 μmol of ligand/g) or 0.1 mM siderophore compound from V0304, adjusted to pH 5.6, was spotted on filter paper (51A; Advantec). Electrophoresis was run at approximately 30 V/cm for 1 to 2 h. The paper was then dried carefully to remove all traces of pyridine and acetic acid followed by spraying on both sides with CAS assay solution, and the appearance of spots was observed a few minutes later. Each spot was identified by measurement of the Rf value and compared to desferrioxamine as the standard. The Rf value (cm) represents the distance between CAS-stainable spots and that of the standard.
Outer membrane purification and SDS-PAGE.
All procedures were performed according to the method of Champomier-Verges et al. (6). After cultivating bacteria in iron-replete or iron-deficient media for 48 h, cells from 5-ml cultures were harvested by centrifugation and then resuspended in microcentrifuge tubes in 1 ml of 50 mM Tris-HCl–0.5 mM MgCl2 buffer, pH 7.4. The cells were disrupted by three cycles of 30-s sonication at 4°C using a microsonicator probe. The supernatant, following centrifugation at 15,000 × g (repeated twice), was transferred to a fresh tube and supplemented with Triton X to a final concentration of 2% (vol/vol). After vigorous mixing, the Triton X solution was kept on ice for 10 min and then centrifuged at 15,000 × g for 30 min. The obtained Triton X-insoluble material was rinsed quickly with 70% ethanol and dried. The samples were resuspended in 10 μl of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer and analyzed by electrophoresis on 12% polyacrylamide gels.
Measurement of iron quotas by 59Fe-labeling method.
Iron quotas (moles of Fe per cell) were measured using the radiotracer 59FeCl3 (specific activity, 10 to 15 mCi/mg; NEN Life Science Products, Inc.) following the method reported by Granger and Price (15). Cells were taken through one transfer in growth medium containing 1 or 10% of the total Fe as 59FeCl3 to ensure uniform labeling. Triplicates were then inoculated in fresh radioactive medium. Cells were collected by filtering the culture onto 0.2-μm-pore-size polycarbonate filters and washing with a titanium(III)-EDTA-citrate reducing solution to dissolve ferric species adsorbed to the cell surface (17). Particulate 59FeCl3 was measured by liquid scintillation counting on a Parkard CA 1900 counter. Cell densities were determined from measurements of absorbance at 600 nm during a 48-h incubation.
Determination of iron content in sponge tissue and seawater.
Collected squeezed sponge tissue solution (from a total of 10 g of sponge) or seawater (100 ml) was concentrated by lyophilization and resuspended in 2 ml of acid-treated distilled water. The concentration of iron in the above solutions was measured with an inductively coupled plasma spectrometry (ICPS) sequential plasma spectrometer (ICPS-1000IV; Shimazu) at the absorbance of an iron atom (259.940 nm and 239.562 nm, respectively). Iron standard solution (Fe100; WAKO Chemical Ltd.) [Fe(NO3)3 in 0.1 mol/1 · HNO3; 99 mg/liter] was used for the determination of a calibration curve.
Nucleotide sequence accession numbers.
The DDBJ GenBank accession numbers for the sequences of V0122, V0304, V0902, V1110, V0210, GMO7-1, GMO4-11, and GMO4-13 are U63999, AF064559, D88527, AB012864, AB012864, AB010390, U63938, and AF025569, respectively.
RESULTS
Siderophore production of marine bacteria.
Sponge-originated bacteria (230 strains) and seawater-originated planktonic bacteria (191 strains) were obtained by the isolation of different phenotypes on 1/10-diluted marine agar plates and tentatively identified by 16S rDNA V3 region sequence analysis (22). They were identified as marine species by their halophilic growth (NaCl, >3.0%). All of these strains were observed to have no growth on marine agar plates when iron was removed. Their siderophore production activity was investigated with CAS agar plates. Two hundred twenty-three strains (77 strains from sponges and 146 strains from seawater) from the total tested species were shown to be Sid− strains. Strains which neither grew nor formed a halo on the CAS agar plates containing 1 pM to 10 μM Fe(III) were defined as Sid− bacteria. One Sid− strain, V0210, and four Sid+ strains, V0122, V0304, V0902, and V1110, were isolated from different marine sponges. The ICPS measurements of these five sponge tissues showed that the iron content was 0.095, 0.102, 0.083, 0.09, and 0.104 μM, respectively. Seawater planktonic strains GMO7-1, GMO4-11, and GMO4-13 were also chosen for investigation. ICPS analysis indicated that the iron content in the seawater was 0.078 μM. GMO4-11 and GMO4-13 were Sid− species, and GMO7-1 was categorized as a Sid+ strain. The total 16S rDNA sequence of V0210 is 100% identical to that of alpha proteobacterium MBIC 3368 (AB012864 [DDBJ]). The total 16S rDNA sequences of V0122, V0304, V0902, and V1110 are 98, 98, 97, and 96% identical to those of an Agrobacterium sp. (U63999 [DDBJ]), a Vibrio sp. (AF064559 [DDBJ]), an alpha proteobacterium (AB012864 [DDBJ]), and a Marinobacter sp. (AB010390 [DDBJ]), respectively.
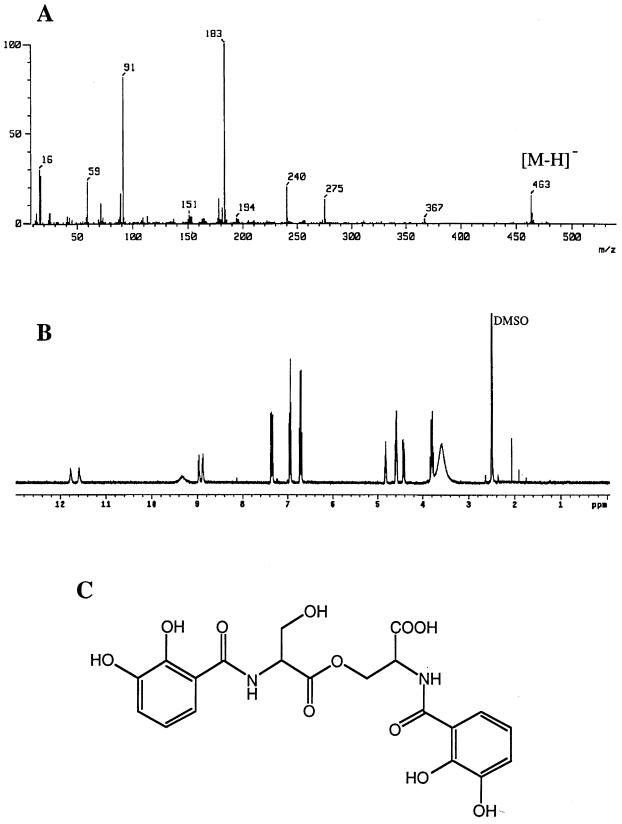
Siderophore components from Vibrio sp. strain V0304 were purified from IDSM [0.1 μM Fe(III)] liquid medium culture, and production was 0.862 μM (6 mg of Fe-binding ligand was obtained from 16 liters of culture supernatant) in this medium. Because the iron content in marine sponge tissues and seawater was close to 0.1 μM Fe, we decided to use the concentration of 0.1 μM Fe(III) as iron-limited conditions for all further cultivations. The chemical structure of the purified compound was determined by analysis of spectroscopic data. The 1H-NMR (Fig. 1B) and 13C-NMR (data not shown) spectra of this compound were identical to those of N,N′-bis (2,3-dihydroxybenzoyl)-O-serylserine (BDOS) (2). The fast atom bombardment (FAB)-MS showed a molecular ion at m/z 463 ([M − H]−) and a fragment ion originated from 2,3-dihydroxybenzoyl serine at m/z 240 (Fig. 1A) (3). The molecular formula of this compound was determined to be C20H20N2O11 by HR-FAB-MS [calculated for C20H21N2O11, m/z 465.1145; found, m/z 465.1145 (M + H)+] and 13C-NMR data. These results indicated that the structure of the siderophore biosynthesized by V0304 is BDOS (Fig. 1C). This compound has been reported to be a siderophore from Escherichia coli O111 (29) and has also been identified as an intermediate product of enterobactin biosynthesis (26). The siderophore components from V0122, V0902, and V1110 were partially purified, and each yield was evaluated to be approximately 0.13, 0.31, and 0.18 μmol of ligand/g, respectively, after cultivation in 200 ml of IDSM (Table 1). The yield of siderophore components biosynthesized by Sid+ planktonic Pseudomonas sp. strain GMO7-1 (AB010390 [DDBJ]) was 2.77 μmol of ligand/g.
FIG. 1.
Chemical features of an isolated siderophore from Vibrio sp. strain V0304. (A) FAB-MS (negative) spectrum of purified siderophore. The peak at m/z 463 is the molecular ion peak. The peak at m/z 240 is the fragment ion peak which originated from 2,3-dihydroxybenzoylserine. Peaks at m/z 91 and m/z 183 originated from the glycerol matrix (3). (B) 1H-NMR spectrum of the purified siderophore in dimethyl sulfoxide-d6 (DMSO) solution. (C) Chemical structure of BDOS.
TABLE 1.
Concentration of iron ligands detected with CAS assay in siderophore extracts of marine bacteria in IDSM [0.1 μM Fe(III)]
| Strain | Origin | Mean siderophore content ± SDa by CAS assay | Reference |
|---|---|---|---|
| Agrobacterium sp. strain V0122 | Sponge | 0.12 ± 0.21 | Present work |
| Vibrio sp. strain 0304 | Sponge | 0.864b | Present work |
| Alpha proteobacterium V0902 | Sponge | 0.31 ± 0.15 | 14 |
| Marinobacter sp. strain V1110 | Sponge | 0.18 ± 0.24 | Present work |
| Pseudomonas sp. strain GMO7-1 | Seawater | 2.77 ± 0.65 | Present work |
Values are given as micromoles of ligand per gram (dry weight) of cells (n = 3).
Value is given as a micromolar concentration.
Cross-feeding assay with Sid− marine bacteria.
The bacterial growth of 223 Sid− strains was investigated with the addition of 10 nM BDOS isolated from V0304 and with the addition of three siderophore extracts (10−3 μmol of ligand/g), from V0122, V0902, and V1110. One hundred thirty-four strains (49 from sponges and 85 from seawater) of the total tested Sid− strains showed signs of growth around paper disks to which BDOS or siderophore extract had been pre-added on iron-limited IDSM agar plates and also were observed to form colonies and halos on the CAS agar plates that were amended with siderophore extract. Sid− strain V0210 was observed to have stimulated colony and halo formation on the CAS agar plates with the addition of siderophore BDOS from V0304 or any of the three siderophore extracts from Sid+ strains V0122, V0902, and V1110. The siderophore component from Sid+ strains added to the CAS agar plate was 130 to 660 times less than that which induced a color change in the CAS assay. Thus, amended siderophore components did not induce a halo-like color change on the CAS agar plates. Obviously, the observed halo around V0210 colonies showed siderophore production from this bacterium.
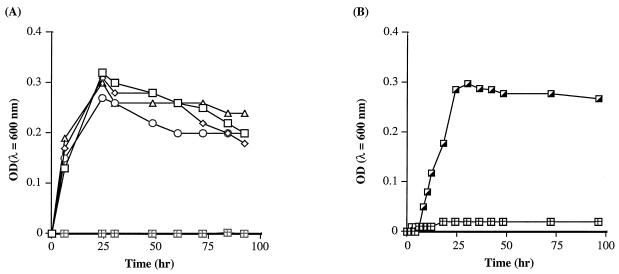
To confirm the influence of the exogenous siderophores on the growth of V0210 in liquid medium, V0210 was cultivated in IDSM containing 0.1 μM Fe(III) with or without the addition of the siderophore extracts from four Sid+ strains. The growth of V0210 began after 6 h of incubation with the addition of one of the siderophore extracts from V0122, V0304, V902, or V1110 (Fig. 2A) or siderophore compound BDOS (Fig. 2B). No growth was observed without the addition of exogenous siderophores. The same experiment was also performed in the presence of 1 pM to 10−1 mM fungal siderophore desferrioxamate and no stimulated bacterial growth of V0210 was observed (data not shown).
FIG. 2.
(A) Growth of alpha proteobacterium strain V0210 at 30°C in IDSM [0.1 μM Fe(III)] for 96 h with siderophore extracts (10−3 μmol of ligand/g) from Sid+ strains Agrobacterium sp. strain V0122 (□), Vibrio sp. strain V0304 (◊), alpha proteobacterium V0902 (○), and Marinobacter sp. strain V1110 (▵) or without any addition (⊞). (B) Growth of alpha proteobacterium strain V0210 at 30°C in IDSM for 96 h with 10 nM siderophore BDOS (┌) or without the addition of BDOS (⊞). Each point represents the mean coaggregration value from three separate experiments. OD, optical density.
Analysis of siderophore components by paper electrophoresis and chemical reactions for functionality.
To investigate whether the growth stimulation of V0210 was caused by direct utilization of the exogenous siderophore or by biosynthesis of its native siderophore, the iron chelator in the siderophore extract was evaluated by paper electrophoresis, the Csaky test, and the Arnow reaction.
Concentrated siderophore extracts (10 μmol of ligand/g) from V0122, V0902, and V1110 and 0.1 mM V0304 siderophore BDOS were analyzed by paper electrophoresis. Supernatant extracts of V0210 culture in IDSM combined with each of the above three siderophore extracts at 10−3 μmol of ligand/g or 10 nM BDOS were also analyzed by paper electrophoresis. Siderophore extracts from V0122, V0902, and V1110 and V0304 BDOS showed different Rf values (Table 2), which indicated that the iron-chelating compounds produced by these four Sid+ bacteria were different. CAS-stained spots were also observed from extracts of V0210 culture with the addition of each of the four siderophore extracts. The Rf values of all V0210 extracts were approximately 6.00 cm except in the case of no siderophore addition (Table 2). In that case the Rf value was completely different from those of the other Sid+ strains and indicated that the iron chelator in the V0210 extract was different from those produced by strains V0122, V0304, V0902, and V1110. In the case of the V0210 extract, only one spot was observed and cannot be due to the low amount of exogenous siderophore components which were added and which were below the detection limit for the paper electrophoresis assay. Therefore, we conclude that V0210 produced a siderophore.
TABLE 2.
Detection of siderophore extracts after cultivation in 200 ml of IDSM containing 0.1 μM Fe(III) with or without the addition of exogenous siderophores
| Strain | Rf valuea (cm) by paper electrophoresis | Mean siderophore content ± SDb by:
|
|
|---|---|---|---|
| Csaky test | Arnow reaction | ||
| V0122 | 6.22 ± 0.06 | ||
| V0902 | 5.85 ± 0.07 | ||
| V1110 | 6.80 ± 0.10 | ||
| BDOS from V0304 | 5.65 | 0.86d | |
| V0210 | None | ||
| V0210 (+ V0122 Sic) | 6.02 ± 0.03 | 0.27 ± 0.02 | |
| V0210 (+ V0304 Si) | 6.05 ± 0.02 | 0.30 ± 0.015 | |
| V0210 (+ V0902 Si) | 6.00 ± 0.05 | 0.29 ± 0.02 | |
| V0210 (+ V1110 Si) | 6.00 ± 0.03 | 0.24 ± 0.035 | |
| V0210 (+ BDOS) | 6.05 ± 0.03 | 0.31 ± 0.01 | |
Distance (Rf) shown here represents CAS-stained siderophore spots in comparison with 10 μl of 0.1 mM desferrioxamate sulfate and represents the mean ± the standard deviation of three experiments.
Values are given as micromoles of ligand per gram (dry weight) of cells for extracts and represent the means of three experiments.
Si, siderophore extract.
Value is given as a micromolar concentration.
To estimate the nature of the functional groups of iron chelators in siderophore extracts from the above strains, the Csaky test and the Arnow reaction were applied (Table 2). These two assays are well known for the detection of hydroxamate (Csaky test) or catechol (Arnow reaction) groups, which are typical functional groups that bind iron. The CAS-stainable compounds of V0122, V0902, and V1110 did not show positive reactions in either assay. In comparison, the positive reactivity of BDOS in the Arnow test, indicating catechol functionality, was different from the siderophore extract of V0210, which gave a positive result only in the Csaky assay, indicating hydroxamate functionality. The added siderophore component in the V0210 cultivation medium was 10−3 μmol of ligand/g, and this amount is below the detection limit of both assays. This revealed that a hydroxamate moiety came from the siderophore component produced by V0210.
Analysis of IROMP of V0210.
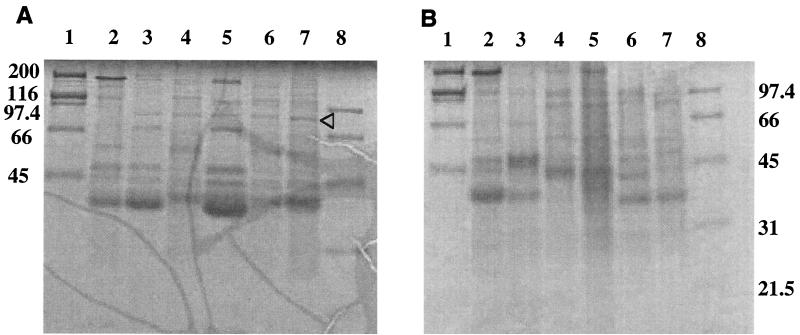
Fe(III)-siderophore complexes are known to bind to specific IROMP in gram-negative bacteria. The IROMP of V0210 with or without siderophore extracts was purified and analyzed under iron-replete and iron-limited conditions by SDS-PAGE (Fig. 3). An 85-kDa band was detected in the culture medium of V0210 iron-starved cells when siderophore extracts were added to IDSM (Fig. 3A, lanes 3 to 7), while it was not detected under conditions where siderophore extracts were not added (Fig. 3A, lane 2). No such band was induced in V0210 cells under iron-replete conditions even though the siderophore extracts were present (Fig. 3B).
FIG. 3.
IROMP patterns of alpha proteobacterium strain V0210 in iron-deficient IDSM [0.1 μM Fe(III)] (A) or in iron-replete IDSM [40 μM Fe(III)] (B) without (lanes 2) or with (lanes 3 to 7) siderophore extracts (10−3 μmol of ligand/g) from Sid+ strains V0122, V0304, V0902, V1110, and GM04-7 on an SDS–12% PAGE gel. Standard markers are shown in lanes 1 (high molecular mass) and 8 (low molecular mass). The arrowhead shows the 85-kDa outer membrane protein.
Iron uptake activity of Sid+ and Sid− strains.
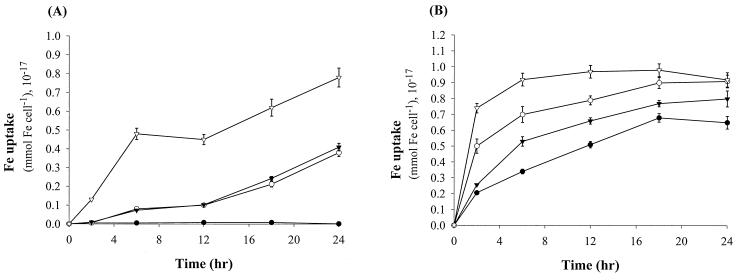
The iron quotas (moles of Fe per cell) of V0122, V0304, and V0210 with or without siderophores were measured by radiotracer 59Fe. Iron quotas of Sid+ strains V0122 and V0304 increased during a 24-h cultivation period (Fig. 4B). The iron quotas for V0122 and V0304 cells were 2.4 and 2.9 times higher, respectively, in the medium supplemented with their own siderophore component than in standard IDSM. In the meantime, the growth of these two strains was observed to be faster under the siderophore-enriched conditions (data not shown). On the other hand, measurement of the iron quota for V0210 was not possible due to lack of growth. The iron quotas for V0210 were obviously stimulated by the addition of the siderophore BDOS or the siderophore extract (Fig. 4A). The curve shows the same increased iron quotas for V0210 when either V0122 or V0304 siderophore was present. Growth and iron uptake of V0210 were very low during the first 2 h after the start of incubation and obviously increased after 6 h of incubation with the BDOS or V0122 siderophore extract. When the Csaky test-positive extract of V0210 was added to the medium, the iron uptake of V0210 started immediately and the quotas were shown to be 21.7 and 14.4 times higher than those supplemented with the exogenous siderophore extracts from V0122 and V0304 during a 2-h incubation (Fig. 4A).
FIG. 4.
(A) Iron uptake quotas (millimoles of Fe per cell) of Sid− strain alpha proteobacterium V0210 without (●) or with the addition of exogenous siderophore extract (10−3 μmol of ligand/g) from V0122 (○), with the addition of BDOS (10 nM) from V0304 (▾), or with the addition of the Csaky test-positive extract of V0210 (▿) after cultivation with the addition of BDOS in IDSM [0.1 μM Fe(III)] during a 24-h cultivation. (B) Iron uptake quotas of Sid+ strains Agrobacterium sp. strain V0122 and Vibrio sp. strain V0304 in IDSM [0.1 μM Fe(III)] without (● for V0122, ▾ for V0304) or with the supplement of their own siderophore extract (10−3 μmol of ligand/g) (○ for V0122) or siderophore BDOS (10 nM) (▿ for V0304) during a 24-h cultivation. Points represent the mean values of replicate samples for a single experiment.
DISCUSSION
Some heterotrophic marine bacteria have been reported to biosynthesize siderophores for their iron uptake under iron-deficient conditions (27), and siderophore production is thought to occur commonly in marine bacteria due to the low iron concentration in the ocean. In our present study, we investigated siderophore production by bacteria isolated from marine sponges and seawater. This is the first time we have compared the iron contents of five sponge tissues and reported the siderophore production of sponge-originated bacteria. After screening with the CAS assay, a universal siderophore production detection assay, we found that 60% of the total tested bacteria were Sid− strains under iron-limited conditions. The growth of 134 out of 233 Sid− strains was observed to be stimulated by both cross-streaking of Sid+ strains and the addition of a purified exogenous siderophore, BDOS (Fig. 1C), or by three siderophore extracts from Sid+ strains under iron-deficient conditions. All results indicated that the siderophore extracts from V0122, V0902, and V1110 affected Sid− strain V0210 in the same way as purified siderophore BDOS did. This suggests that active factors in these extracts are siderophores which have stimulatory functions on the growth of V0210. Although growth stimulation of some terrestrial bacteria has been reported by the addition of exogenous siderophores (6, 8, 21), it is not clear whether those bacteria might have been stimulated to synthesize their own siderophores. Paper electrophoresis of siderophore extracts from V0210 cultures showed that the CAS-positive component was produced only after cultivation with each of the exogenous siderophores from the four Sid+ strains, V0122, V0304, V0902, and V1110. The Rf value of the iron-binding component of V0210 was different from those of V0122, V0304, V0902, and V1110 as determined by paper electrophoresis. Only the siderophore extract from V0210 cultivated with the addition of exogenous siderophores gave a positive result in the Csaky assay (Table 2), while BDOS catechol resulted in positive reactivity in the Arnow assay. Thus, hydroxamate siderophore synthesis was initiated by V0210 only in the presence of an exogenous siderophore. This is the first time that the biosynthesis of a siderophore in response to an exogenous siderophore under iron-deficient conditions has been reported.
Studies on iron metabolism in terrestrial and pathogenic strains have shown that bacteria have established a variety of mechanisms by which to acquire chelated iron. In siderophore-producing bacteria, ferric iron is generally transported as an Fe(III)-siderophore complex that enters the periplasmic space of gram-negative bacteria through specific outer membrane receptors. These proteins are similar in size, ranging from 67 to 88 kDa, and are produced under iron-limited conditions (36). Outer membrane receptors recognize siderophore-iron complexes produced by other species as well as their own (7, 8, 9, 36). Expression of an 85-kDa outer membrane protein was observed in V0210 cells after cultivation with an exogenous siderophore under iron-limited conditions (Fig. 3). This outer membrane protein was not expressed without the addition of exogenous siderophore. Thus, expression of the 85-kDa outer membrane protein of V0210 might depend on a response to an exogenous siderophore, and the protein may play a significant role in the uptake of iron via its iron-siderophore complex.
Sid+ marine bacteria have been reported to acquire iron through siderophores as a main pathway, and some Sid− species takeup iron by the fungal siderophore desferrioxamine (15). Our results showed that Sid+ species V0122 and V0304 take up iron through their siderophores, which are results similar to those reported by Granger and Price (15). Desferrioxamine did not show any positive influence on either growth or iron uptake of strain V0210 (data not shown). Higher iron quotas and faster growth of V0210 under conditions of being supplemented with its Csaky test-positive extract than those with the addition of BDOS or V0122 siderophore extract (Fig. 4A) indicated that Sid− strain V0210 took up iron through induced original siderophores instead of utilizing exogenous species directly. This phenomenon differs from the concept that microorganisms utilize exogenous siderophores directly to acquire iron and suggests that multiple pathways may exist by which marine bacteria acquire iron.
Besides quotas “catching” iron for necessary growth, siderophores are thought to be a type of iron scavengers because they can move iron from weaker-associated ferric-siderophore complexes from other species. Our results indicate that siderophores are also important factors to stimulate growth and iron uptake of other species, besides their inhibition functions. This kind of function can also be observed in the other planktonic Sid− strains, GMO4-11 (Flavobacterium sp.) and GMO4-13 (Roseobacter sp.). The growth of these two Sid− strains could be stimulated by siderophore BDOS from V0304 (Vibrio sp.) and siderophore extract from GMO7-1 (Pseudomonas sp.). This indicates that siderophore stimulation activity exists not only between the bacteria from marine sponges but also between marine bacteria from different habitats. We have also shown production of a native siderophore by Sid− Pelagiobacter sp. strain V0110 (16). Its siderophore production was only observed with the addition of both an exogenous siderophore and an N-acyl-homoserine lactone which is a type of quorum-sensing chemical signal. The responses of V0110 and V0210 to exogenous siderophores indicate that siderophores may play a role that affects other species' siderophore biosynthesis and iron acquisition mechanisms in the marine environment.
ACKNOWLEDGMENTS
This work was performed as a part of The Industrial Science and Technology Project, Technological Development of Biological Resources in Bioconsortia, supported by the New Energy and Industrial Technology Development Organization, Japan.
We are grateful for the support of Y. Shizuri, Marine Biotechnology Institute, Shimizu Laboratories.
REFERENCES
- 1.Arnow L E. Colorimetric determination of the components of 3,4-hydroxyphenylalanine–tyrosine mixtures. Annu Rev Biochem. 1937;50:715–731. [Google Scholar]
- 2.Bergstrom C P, Liu M C, Bell C L. NMR studies of dimeric 2,3-dihydroxy-N-benzoyl serine. J Nat Prod. 1991;54:1003–1008. [Google Scholar]
- 3.Berner I, Greiner M, Metzger J, Jung G, Winkelmann G. Identification of enterobactin and linear dihydroxybenzoylserine compound by HPLC and ion spray mass spectrometry (LC/MS and MS/MS) Biol Metals. 1991;4:113–118. doi: 10.1007/BF01135388. [DOI] [PubMed] [Google Scholar]
- 4.Braun V, Hantke K, Koster W. Bacterial iron transport: mechanisms, genetics, and regulation. In: Sigel A, Sigel H, editors. Metal ions in biological systems. Vol. 35. New York, N.Y: Marcel Dekker Inc.; 1998. pp. 67–146. [PubMed] [Google Scholar]
- 5.Butler A. Acquisition and utilization of transition metal ions by marine organisms. Science. 1998;281:207–210. doi: 10.1126/science.281.5374.207. [DOI] [PubMed] [Google Scholar]
- 6.Champomier-Verges M-C, Stintzi A, Meyer J-M. Acquisition of iron by the non-siderophore-producing Pseudomonas fragi. Microbiology. 1996;142:1191–1199. doi: 10.1099/13500872-142-5-1191. [DOI] [PubMed] [Google Scholar]
- 7.Cox C D. Iron uptake with ferric pyochelin and ferric citrate by Pseudomonas aeruginosa. J Bacteriol. 1980;142:581–587. doi: 10.1128/jb.142.2.581-587.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crosa J H. Signal transduction and transcriptional and posttranscriptional control of iron-regulated genes in bacteria. Microbiol Mol Biol Rev. 1997;61:319–336. doi: 10.1128/mmbr.61.3.319-336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dean C R, Poole K. Cloning and characterization of the ferric enterobactin receptor gene (pfeA) of Pseudomonas aeruginosa. J Bacteriol. 1993;175:317–324. doi: 10.1128/jb.175.2.317-324.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dominigue P A G B, Mottle D W, Morck M R W, Brown J W, Costerton J W. A simplified rapid method for the removal of iron and other cations from complex media. J Microbiol Methods. 1990;12:13–22. [Google Scholar]
- 11.Fenical W. Chemical studies of marine bacteria: developing a new resource. Chem Rev. 1993;93:1673–1683. [Google Scholar]
- 12.Fuhrman J A, Sleeter T D, Carlson C A, Proctor L M. Dominance of bacterial biomass in the Sargasso Sea and its ecological implications. Mar Ecol Prog Ser. 1989;57:207–217. [Google Scholar]
- 13.Gillam A H, Lewis A G, Andersen R J. Quantitative determination of hydroxamic acids. Anal Chem. 1981;53:841–844. [Google Scholar]
- 14.Gonye E R, Carpenter E J. Production of iron-binding compounds by marine microorganisms. Limnol Oceanogr. 1974;19:840–841. [Google Scholar]
- 15.Granger J, Price N M. The importance of siderophores in iron nutrition of heterotrophic marine bacteria. Limnol Oceanogr. 1999;44:541–555. [Google Scholar]
- 16.Guan L L, Onuki H, Kamino K. Bacterial growth stimulation with exogenous siderophore and synthetic N-acyl homoserine lactone autoinducers under iron-limited and low-nutrient conditions. Appl Environ Microbiol. 2000;66:2797–2803. doi: 10.1128/aem.66.7.2797-2803.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hudson R J, Morel F M M. Distinguishing between extra- and intracellular iron in marine phytoplankton. Limnol Oceanogr. 1989;34:1113–1120. [Google Scholar]
- 18.Hutchins D A, Witter A E, Butler A, Luther G W., III Competition among marine phytoplankton for different chelated iron species. Nature. 1999;400:858–861. [Google Scholar]
- 19.Jalal M A F, et al. Structure of anguibactin, a unique plasmid related bacterial siderophore from the fish pathogen, Vibrio anguillarum. J Am Chem Soc. 1989;111:292–296. [Google Scholar]
- 20.Martinez J S, Zhang G P, Holt P D, Jung H-T, Carrano C J, Haygood M G, Butler A. Self-assembling amphiphilic siderophores from marine bacteria. Science. 2000;287:1245–1247. doi: 10.1126/science.287.5456.1245. [DOI] [PubMed] [Google Scholar]
- 21.Meyer J M, Stinizi A, De Vos D, Corneils P, Tappe R, Taraz K, Budzikiewicz H. Use of siderophores to type pseudomonas: the three Pseudomonas aeruginosa pyoverdine systems. Microbiology. 1997;143:35–43. doi: 10.1099/00221287-143-1-35. [DOI] [PubMed] [Google Scholar]
- 22.Muyzer G, De Waal E C, Uitterlinden A G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol. 1993;59:695–700. doi: 10.1128/aem.59.3.695-700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neilands J B. Siderophores: structure and function of microbial iron transport compounds. J Biol Chem. 1995;270:26723–26726. doi: 10.1074/jbc.270.45.26723. [DOI] [PubMed] [Google Scholar]
- 24.Payne S M. Detection, isolation, and characterization of siderophores. Methods Enzymol. 1994;235:329–344. doi: 10.1016/0076-6879(94)35151-1. [DOI] [PubMed] [Google Scholar]
- 25.Price N L, Morel F M M. Biological cycling of iron in the ocean. In: Sigel A, Sigel H, editors. Metal ions in biological systems. Vol. 35. New York, N.Y: Marcel Dekker Inc.; 1998. pp. 1–36. [Google Scholar]
- 26.Rastetter W H, Erickson T J, Venuti M C. Synthesis of iron chelators enterobactin, enantioenterobactin, and a chiral analogue. J Org Chem. 1981;46:3579–3590. [Google Scholar]
- 27.Reid R T, Butler A. Investigation of the mechanisms of iron acquisition by the marine bacterium Alteromonas luteoviolaceus: characterization of siderophore production. Limnol Oceanogr. 1991;36:1783–1792. [Google Scholar]
- 28.Reid R T, Live D H, Faulkner D J, Butler A. A siderophore from a marine bacterium with an exceptional ferric iron affinity constant. Nature. 1993;366:455–458. doi: 10.1038/366455a0. [DOI] [PubMed] [Google Scholar]
- 29.Rogers H J, Synge C, Kimber B, Bayley P M. Production of enterochelin by Escherichia coli 0111. Biochim Biophys Acta. 1977;497:548–557. doi: 10.1016/0304-4165(77)90211-2. [DOI] [PubMed] [Google Scholar]
- 30.Rue E L, Bruland K W. Complexation of iron(III) by natural organic ligands in the Central North Pacific as determined by a new ligand equilibration/adsorptive stripping voltametric method. Mar Chem. 1995;50:117–138. [Google Scholar]
- 31.Schwyn B, Neilands J B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi A, et al. Bisucaberin, a new siderophore, sensitizing tumor cells to macrophage-mediated cytolysis. 2. Physico-chemical properties and structure determination. J Antibiot. 1987;40:1671–1676. doi: 10.7164/antibiotics.40.1671. [DOI] [PubMed] [Google Scholar]
- 33.Tortell P D, Maldonado M T, Price N M. The role of heterotrophic bacteria in iron-limited ocean ecosystems. Nature. 1996;383:330–332. [Google Scholar]
- 34.Tortell P D, Maldonado M T, Granger J, Price N M. Marine bacteria and biogeochemical cycling of iron in the oceans. FEMS Microbiol Ecol. 1999;29:1–11. [Google Scholar]
- 35.Trick C G. Hydroxamate-siderophore production and utilization by marine eubacteria. Curr Microbiol. 1989;18:375–378. [Google Scholar]
- 36.van der Halm D. The physical chemistry of bacterial outer-membrane siderophore receptor proteins. In: Sigel A, Sigel H, editors. Metal ions in biological systems. Vol. 35. New York, N.Y: Marcel Dekker Inc.; 1998. pp. 355–401. [PubMed] [Google Scholar]
- 37.Weiburg W G, Barns S M, Pelletier D A, Lane D J. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winkelmann G, van der Helm D, Neilands J B, editors. Iron transport in microbes, plants and animals. Weinheim, Germany: VHC Press; 1987. [Google Scholar]
- 39.Winkelmann G. Specificity of iron transport in bacteria and fungi. In: Winkelmann G, editor. Handbook of microbial iron chelaters. Boca Raton, Fla: CRC Press; 1991. pp. 65–105. [Google Scholar]
- 40.Witter A E, Luther G W. Variation in Fe-organic complexation with depth in the Northwestern Atlantic Ocean as determined using a kinetic approach. Mar Chem. 1998;62:241–258. [Google Scholar]
- 41.Wu J, Luther G W. Complexation of Fe(III) by natural organic ligands in the Northwest Atlantic Ocean by a competitive ligand method and a kinetic approach. Mar Chem. 1995;50:159–177. [Google Scholar]