Abstract
Background
Periprosthetic joint infection (PJI) can lead to a severe systemic inflammatory response and may result in systemic sepsis. However, little is known about how often systemic sepsis may occur in patients with PJI, and whether sepsis is associated with a greater likelihood of persistent or recurrent PJI.
Questions/purposes
(1) Among patients who present with acute or acute hematogenous PJI and who were treated with debridement, antibiotics, and implant retention (DAIR), what proportion have sepsis and what factors are associated with a presentation with sepsis? (2) For patients presenting with sepsis, what factors are associated with persistent or recurrent PJI?
Methods
In all, 320 patients who underwent DAIR for the treatment of acute postoperative or acute hematogenous PJI between January 2000 and December 2019 were included in this study. Exclusion criteria were patients with other known sources of infection, such as pneumonia or urinary tract infections, which could contribute to systemic sepsis (6% [18 of 320]), patients with chronic PJI, and those with less than 6 months of follow-up (21% [66 of 320]). Our final cohort consisted of 236 patients presenting with an acute postoperative or acute hematogenous PJI who underwent an irrigation and debridement procedure. Sepsis was defined by the criteria for systemic inflammatory response syndrome (SIRS) or bacteria-positive blood culture results. Inclusion of patients with positive blood culture by organisms that caused their joint infection was important as all patients presented with fulminant acute infection of a prosthetic joint. Data, including vital signs, surgical variables, and treatment outcomes, were collected retrospectively through a chart review of an electronic medical record system. The statistical analysis comparing patients with sepsis versus patients without sepsis consisted of logistic regression to identify factors associated with sepsis. After confirming its ability to identify patients with a higher association with the development of sepsis through area under the curve models, a nomogram was generated to standardize our results from the regression, which was supported by the area under the curve model, to help readers better identify patients who are more likely to develop sepsis.
Results
A total of 44% (103 of 236) of patients had infections that met the criteria for sepsis. After controlling for confounding variables, including congestive heart failure, anemia, serum C-reactive protein (CRP), and the male sex, it was revealed that serum CRP (odds ratio 1.07 [95% confidence interval 1.04 to 1.11]; p < 0.001) and male sex (OR 1.96 [95% CI 1.03 to 3.81]; p = 0.04) were associated with the development of systemic sepsis. For patients presenting with sepsis, persistent or recurrent PJI were associated with an increased CRP level (OR 1.06 [95% CI 1.02 to 1.11]; p = 0.01) and number of prior surgical procedures on the joint (OR 2.30 [95% CI 1.21 to 4.89]; p = 0.02).
Conclusion
Overall, our findings support that patients with systematic sepsis may benefit from two-stage revision rather than DAIR to decrease the bioburden more effectively, especially in those with methicillin-resistant Staphylococcus aureus and polymicrobial infections. High serum CRP levels and a history of prior surgical procedures on the involved joint should trigger prompt, aggressive surgical treatment if the patient’s overall clinical status can tolerate such an intervention.
Level of Evidence
Level III, therapeutic study.
Introduction
Periprosthetic joint infection (PJI) is a devastating complication of THA and TKA with an acute presentation consisting of a rapid-onset of local and systemic symptoms [9, 31, 35]. Debridement, antibiotics, and implant retention (DAIR) is the current preferred treatment for acute PJI with varying success, and PJI continues to have relatively high 5-year mortality rates, at approximately 21% [4, 8, 18, 20, 28, 32, 33]. It has been reported that 4% to 32% of patients with PJI present with positive blood culture [5, 12, 13, 26], and these patients have been associated with decreased treatment success [12-14]. Furthermore, patients presenting with bacteremia also demonstrate elevated serum C-reactive protein (CRP) levels and white blood cell count [12]. Sepsis occurs as a result of a dysregulated host response to an infection and is associated with acute organ dysfunction and a high risk of death [3]. Mortality rates in patients with systemic sepsis have been declining substantially over the past decade but continue to be high, at approximately 20% to 30% [10].
There is a paucity of data regarding the systemic repercussions of PJI focusing on the incidence of sepsis in patients presenting with acute postoperative or acute hematogenous PJI and whether this affects treatment outcomes. Appropriately controlling the source while minimizing surgical morbidity is paramount for improved outcomes [3] and requires a multidisciplinary team that includes orthopaedic surgeons, infectious disease specialists, and critical care specialists. Given the elevated mortality and morbidity associated with sepsis, identifying patients with acute PJI who are at increased risk of developing sepsis may justify early and aggressive treatment in an attempt to avoid the progression to sepsis. Furthermore, identifying factors associated with persistent or recurrent infection after DAIR would aid surgeons in determining when an explant and placement of antibiotic spacer would be a more suitable surgical strategy for patients presenting with sepsis.
Therefore, we asked: (1) Among patients who present with acute or acute hematogenous PJI and who were treated with DAIR, what proportion have sepsis and what factors are associated with a presentation with sepsis? (2) For patients presenting with sepsis, what factors are associated with persistent or recurrent PJI?
Patients and Methods
Study Design and Setting
We conducted a single-institution, retrospective comparative study at a primary urban care center. The DAIR procedures were completed by fellowship-trained adult reconstruction surgeons in a tertiary academic institution.
Participants
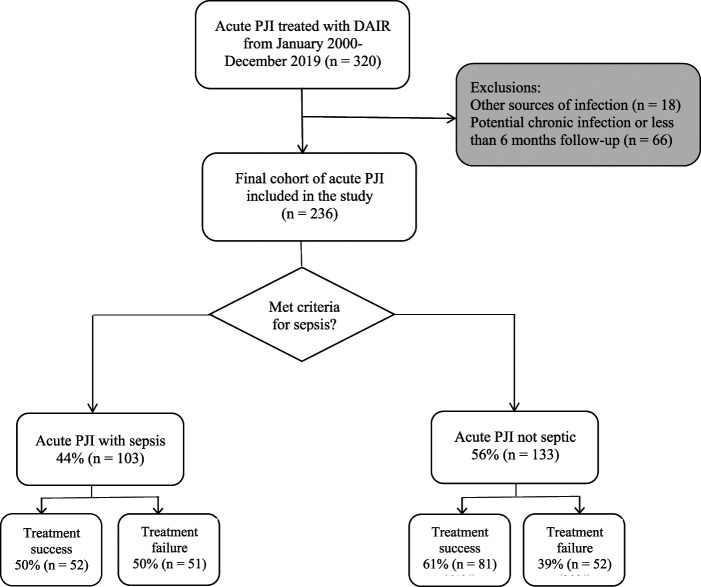
Patients who underwent DAIR for the treatment of acute postoperative or acute hematogenous PJI were eligible; we defined acute PJI according to the International Consensus Meeting criteria [22, 27]. We searched an institutional PJI database to identify patients who underwent DAIR for acute PJI between January 2000 and December 2019. In all, 320 patients were identified within this period. Acute postoperative infections were defined as infections within 6 weeks from the index THA or TKA, whereas acute hematogenous infections were defined as infections with less than 6 weeks of symptoms occurring more than 3 months after the index arthroplasty [22]. DAIR is typically indicated for patients with acute PJI who do not require more intensive surgical intervention, such as a two-stage revision arthroplasty. Most patients presenting with acute PJI had been treated with DAIR during our study’s timeframe. We followed Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines throughout data collection [6]. Exclusion criteria were patients with other known sources of infection, such as pneumonia or urinary tract infections, that could contribute to systemic sepsis (6% [18 of 320]), patients with chronic PJI, and those with less than 6 months of follow-up (21% [66 of 320]). This left 74% (236 of 320) for our final analysis of patients who presented with an acute postoperative or acute hematogenous PJI who underwent DAIR (Fig. 1). Patients with less than 6 months of follow-up were most likely lost to follow-up.
Fig. 1.
This flowchart depicts eligible patients with acute PJI treated with DAIR.
Sepsis is no longer defined solely as an inflammatory disorder triggered by an infection, but rather as organ dysfunction resulting from a dysregulated host response to infection [29]. In this study, sepsis was defined per Levy et al. [15] and involved a documented source of infection in addition to the presence of systemic inflammatory response syndrome. Systemic inflammatory response syndrome was defined as the presence of at least two of the following clinical criteria: core temperature above 38.3° C or below 36° C, heart rate above 90 beats per minute, tachypnea, and serum white blood cell count above 12,000 μ/L or below 4000 μ/L.
Patients’ Descriptive Data
Among patients presenting with systemic sepsis, 38% (39 of 103) were female with a mean age of 65 ± 12.3 years, mean BMI of 33.1 ± 7.9 kg/m2, and a median (range) follow-up of 29 months (6 to 188). For patients without systemic sepsis, 52% (69 of 133) were female, with a mean age of 66 ± 12.7 years, mean BMI of 32.4 ± 7.6 kg/m2, and a median follow-up of 25 months (6 to 183).
Data Sources
We reviewed emergency department admission records, inpatient records, operative reports, and laboratory data for every patient from an electronic medical record system as well as clinical notes from patient charts. We collected demographic and clinical variables, including sex, age, BMI, Charlson comorbidity index (CCI), Elixhauser comorbidity index, American Society of Anesthesiologists classification, and microbiology data on blood, synovial fluid, and intraoperative tissue cultures. Vital signs and laboratory data were also obtained from the first presentation to the hospital. Also, we documented information regarding the DAIR procedure, such as laterality and joint that was operated on (hip or knee). Lastly, we examined patient clinical outcomes, and we used the Musculoskeletal Infection Society definition of success to determine treatment success [7]. Specifically, this was defined as no recurrent or persistent infections and no reoperation. These clinical outcomes specifically focused on reoperations and mortality.
Bias
Several biases may apply to this study. First, the risk of selection bias is present given that only patients who received DAIR for an acute or acute hematogenous PJI were included. The decision between performing a DAIR versus a two-stage revision relies not only on the acuity of symptoms but also on baseline patient characteristics and infecting pathogen. Healthier patients with more virulent pathogens may have received a two-stage revision in lieu of DAIR and therefore were not included in the present study. Transfer bias may have also been present as our minimum follow-up threshold was set at 6 months. Although most recurrent or persistent PJIs after DAIR for an acute PJI occur in the early postoperative period, patients who developed recurrent infection outside of the 6 months window may not have been captured in this study. The study was also at risk for assessment bias. Relevant covariates that may have influenced the development of sepsis may not have been included in our data collection efforts or not present in the medical records. However, given the extensive clinical experience in treating PJI in our institution in addition to the thoroughness of the electronic medical records, we believe this risk is relatively low. Lastly, we attempted to control confounding biases by excluding patients with other potential sources of infection, such as pneumonia, which may have contributed to the development of sepsis.
Study Size
The study size was based off of a prospectively-generated PJI database that includes patients from 2000 to 2019. The database provides specific information concerning each patient’s case, allowing us to identify patients with acute and acute hematogenous PJI who were treated with DAIR.
Primary and Secondary Study Outcomes
Our primary goal was to identify factors associated with the development of sepsis in patients who presented to the hospital with acute or acute hematogenous PJI and were treated with DAIR. To achieve this, we conducted multivariable analysis, whose utility was supported by area under the curve models. Then, we generated a nomogram to standardize our results from the regression in a calculator to help readers better understand the risk of developing an acute infection.
Our secondary goal was to identify factors associated with persistent or recurrent PJI after treatment of acute or acute hematogenous PJI with DAIR. To achieve this, we conducted a multivariable analysis to identify the associated factors.
Ethical Approval
Ethical approval for this study was obtained from Thomas Jefferson University, Philadelphia, PA, USA (number 08R.207).
Statistical Analysis
For reporting demographic and clinical variables, we used descriptive statistics. For the univariable analysis, when data were normally distributed, we use the t-test; otherwise, we used a nonparametric Mann-Whitney test. For categorical variables, we used either the Fisher exact test or a chi-square test. Data are reported as the mean and SD for continuous variables and percentage of the total count for categorical variables. Because 39 comparisons were conducted in the univariable analysis, the alpha value was adjusted to be 0.05/39 for a new value of 0.001 to account for the family-wise error rate. A multivariable logistic regression analysis was performed to determine factors associated with the development of sepsis in a patient with acute PJI. Variables included in the regression analysis were those that were clinically relevant and demonstrated statistically significant differences in the univariable analysis. An area under the curve model (AUC) was developed to demonstrate the utility of the regression model. We determined that AUC values over 0.7 were a good model for use. Subsequently, we created a nomogram based on the odds ratios calculations from the logistic regression with a scoring system that assigned probability values of sepsis. This tool was made to standardize our results from the regression, which was supported by the area under the curve model, in a calculator to help readers better identify patients who are more likely to develop sepsis. A subgroup regression analysis including only patients with sepsis was performed to compare patients who had a persistent or recurrent infection after treatment with those who did not per the Musculoskeletal Infection Society infection management criteria [7]. We performed another multivariable logistic regression analysis to determine factors associated with persistent or recurrent infections in patients with PJI-related sepsis. Significance was determined at a p value < 0.05. All statistical analyses were performed using R Studio (Version 3.6.3).
Results
Proportion of Patients With Sepsis and Factors Associated With Sepsis Presentation
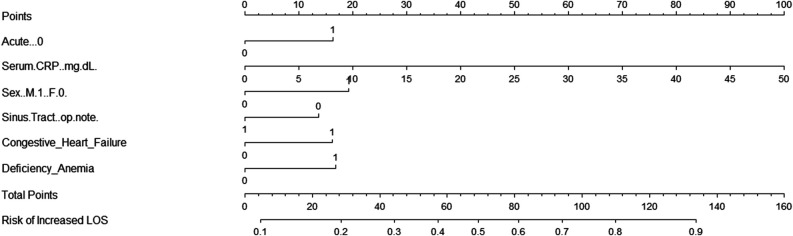
Among patients with acute PJI, 44% (103 of 236) had evidence of systemic sepsis on hospital presentation. The important factors associated with sepsis presentation were elevated serum CRP values and male sex. After controlling for potential confounding variables, such as acute versus acute hematogenous infection, serum CRP, male gender, anemia, and congestive heart failure, we found serum CRP (OR 1.07 [95% confidence interval 1.04 to 1.11]; p < 0.001) and male sex (OR 1.96 [95% CI 1.03 to 3.81]; p = 0.04) were associated with sepsis (Table 1). We used a logistic regression model to analyze factors associated with sepsis in patients presenting with acute PJI (AUC 0.76) (Supplementary Fig. 1; http://links.lww.com/CORR/A773). We constructed a nomogram with scoring points for each of the above variables and a corresponding percentage risk of developing sepsis based on the sum of all points (Fig. 2). The sepsis score ranges from 0 to 160 points. For example, a patient who has congestive heart failure receives a corresponding score of 16. All variables were added to generate a cumulative sepsis score. This total score is then used to identify the probability of a patient developing sepsis. The sepsis score produced by the nomogram identified patients with a high likelihood of developing sepsis in this study sample (OR 1.03 [95% CI 1.02 to 1.05]; p < 0.001) (Supplementary Table 1; http://links.lww.com/CORR/A774). For example, based on our nomogram, a patient presenting with an acute PJI and treated with DAIR, who has a score of 81, has a 60% chance of developing sepsis (Supplementary Fig. 2; http://links.lww.com/CORR/A775). The AUC model for the sepsis score demonstrated an AUC value of 0.75 [95% CI of 0.69 to 0.81], sensitivity of 0.60, and specificity of 0.78.
Table 1.
Logistic regression model analyzing factors associated with the development of sepsis in patients presenting with acute PJI
| Predictor | OR (95% CI) | p value |
| Acute hematogenous infection | 1.77 (0.84-3.75) | 0.14 |
| Serum CRP | 1.07 (1.04-1.11) | < 0.001 |
| Male sex | 1.96 (1.03-3.81) | 0.04 |
| Sinus tract | 0.62 (0.25-1.45) | 0.28 |
| Congestive heart failure | 1.73 (0.48-6.62) | 0.41 |
| Anemia | 1.77 (0.77-4.10) | 0.18 |
CRP = C-reactive protein
Fig. 2.
This nomogram depicts the scoring points for each of the variables included in the logistic regression model determining the ultimate risk of sepsis based on the sum of all points; CRP = C-reactive protein; LOS = length of stay.
Factors Associated With Persistent or Recurrent PJI After Treatment
After controlling for confounding variables, serum CRP (OR 1.06 [95% CI 1.02 to 1.11]; p = 0.009) and the total number of prior surgical procedures on the joint (OR 2.30 [95% CI 1.21 to 4.89]; p = 0.02) were found to be associated with a greater likelihood of experiencing persistent or recurrent PJI in patients with PJI-related systemic sepsis (Table 2). Additionally, following the Musculoskeletal Infection Society criteria for successful infection management, patients with sepsis had a lower likelihood of having no recurrent PJI, at 51% (53 of 103), compared with patients without sepsis, at 67% (89 of 133) (p = 0.02) (Table 3). Age, CCI scores, serum CRP level, and total number of prior operations on the joint had an AUC of 0.75 in the ability to identify patients with factors associated with sepsis in this group (Supplementary Fig. 3; http://links.lww.com/CORR/A776).
Table 2.
Logistic regression model analyzing risk factors for patients with PJI-related sepsis to fail treatment according to the Musculoskeletal Infection Society infection management criteria
| Predictor | OR (95% CI) | p value |
| Age | 1.02 (0.98-1.06) | 0.37 |
| CCI | 1.27 (0.97-1.74) | 0.10 |
| Serum CRP | 1.06 (1.02-1.11) | 0.009 |
| Number of prior procedures performed on the joint | 2.30 (1.21-4.89) | 0.02 |
CCI = Charlson comorbidity index; CRP = C-reactive protein.
Table 3.
Musculoskeletal Infection Society Periprosthetic Joint Infection Treatment Outcome Reporting Table for patients with sepsis and those without [21]
| Outcome | Without sepsis (n = 133) | With sepsis (n = 103) |
| Infection control | 67 (89) | 51 (53) |
| Tiers 1 and 2 | 60 (80) | 50 (51) |
| Tier 3A | 3 (4) | 2 (2) |
| Tier 4B | 4 (5) | 0 |
| Infection not controlled | 33 (44) | 49 (50) |
| Tier 3B | 7 (9) | 0 |
| Tier 3C | 2 (3) | 3 (3) |
| Tier 3D | 18 (24) | 33 (34) |
| Tier 3E | 4 (5) | 7 (7) |
| Tier 3F | 2 (2) | 1 (1) |
| Tier 4A | 1 (1) | 5 (5) |
Data presented as % (n); Tier 1: infection control with no continued antibiotic therapy; Tier 2: infection control with the patient on suppressive antibiotic therapy; Tier 3: need for reoperation; 3A: aseptic revision at > 1 year from the initiation of PJI treatment; 3B: septic revision at > 1 year from the initiation of PJI treatment (excluding amputation, resection arthroplasty, and arthrodesis); 3C: aseptic revision at ≤ 1 year from the initiation of PJI treatment; 3D: septic revision (including DAIR) at ≤ 1 year from the initiation of PJI treatment (excluding amputation, resection arthroplasty, and arthrodesis); 3E: amputation, resection arthroplasty, or arthrodesis; 3F: retained spacer; Tier 4: death; A: death ≤ 1 year from the initiation of PJI treatment; B: death > 1 year from the initiation of PJI treatment; Tiers 1, 2, 3A, and 4B represent successful treatment of PJI.
Infecting Organisms and Their Association With the Development of Sepsis
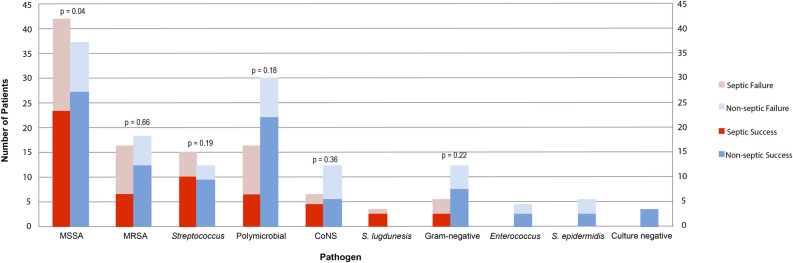
Intraoperative tissue cultures revealed that methicillin-susceptible Staphylococcus aureus (MSSA) was the most common pathogen causing acute PJI (Fig. 3). In patients with sepsis, MSSA was the infecting organism in 41% (42 of 103) of patients, and in those without sepsis, MSSA was responsible in 28% (37 of 133) (Table 4). MSSA demonstrated an increased proportion of persistent or recurrent infections in patients with systemic sepsis compared with those without systemic sepsis (p = 0.04). Methicillin-resistant Staphylococcus aureus (MRSA) and polymicrobial infections had the highest proportions of persistent or recurrent infections in the septic cohort. For the nonseptic cohort, coagulase-negative Staphylococcus and S. epidermidis had the highest proportion of persistent or recurrent infection, although the number of patients for these two pathogens were low (12 cases of coagulase-negative S. aureus and five patients of S. epidermidis). There were no enterococcal, S. epidermidis, or culture-negative PJIs in patients with sepsis. Forty-six patients had positive blood culture results, and all these infections met the criteria for sepsis. However, multivariable analysis did not find MSSA, MRSA, streptococcus, or polymicrobial infections to be associated with the development of sepsis (Table 5).
Fig. 3.
This graph represents infecting pathogens in patients with sepsis (red) and those without (blue), stratified by treatment success (bottom portion of the columns) and failure (top portion of the columns). A color image accompanies the online version of this article.
Table 4.
Microorganisms isolated in intraoperative tissue cultures in patients who do and do not meet sepsis criteria and the corresponding treatment success rate
| Septic | Nonseptic | |||
| Microorganism | Number of cases | Treatment success | Numbers of cases | Treatment success |
| MSSA | 41 (42) | 55 (23 of 42) | 28 (37) | 73 (27 of 37) |
| MRSA | 16 (16) | 38 (6 of 16) | 14 (18) | 67 (12 of 18) |
| Streptococcus | 15 (15) | 67 (10 of 15) | 9 (12) | 75 (9 of 12) |
| Polymicrobial | 16 (16) | 38 (6 of 16) | 23 (30) | 73 (22 of 30) |
| Coagulase-negative Staphylococcus | 6 (6) | 67 (4 of 6) | 9 (12) | 42 (5 of 12) |
| S. lugdunensis | 3 (3) | 67 (2 of 3) | 0 (0) | a |
| Gram-negative | 5 (5) | 40 (2 of 5) | 9 (12) | 58 (7 of 12) |
| Enterococcus | 0 (0) | a | 3 (4) | 50 (2 of 4) |
| S. epidermidis | 0 (0) | a | 5 (4) | 40 (2 of 5) |
| Culture negative | 0 (0) | a | 3 (2) | 100 (3 of 3) |
Data presented as % (n).
Success rates could not be calculated for organisms with 0 cases.
Table 5.
Multivariant regression analysis looking at specific organisms isolated in culture with sepsis as the dependent outcome
| Variable | Estimate | OR (95% CI) | p value |
| Acute infection | 0.74 | 2.10 (0.88-5.14) | 0.01 |
| Serum CRP | 0.06 | 1.07 (1.03-1.10) | < 0.001 |
| Male sex | 0.41 | 1.51 (0.70-3.32) | 0.30 |
| Sinus tract | -0.53 | 0.59 (0.19-1.63) | 0.32 |
| Congestive heart failure | 0.42 | 1.53 (0.41-6.07) | 0.53 |
| Anemia | 0.60 | 1.81 (0.72-4.69) | 0.21 |
| Organism | |||
| MRSA | Reference | ||
| MSSA | -0.001 | 0.99 (0.35-2.80) | 0.10 |
| Polymicrobial | -0.13 | 0.88 (0.29-2.70) | 0.82 |
| Streptococcus | -0.03 | 0.97 (0.26-3.51) | 0.96 |
CRP = C-reactive protein.
Discussion
Although studies have shown poor treatment outcomes with DAIR in patients presenting with positive blood culture results [12, 14] and patients with acute hematogenous PJI [13, 28], no previous studies that we know of have specifically reported on patients presenting with PJI-related systemic sepsis. Due to the lack of data regarding the proportion of patients presenting with sepsis as well as factors associated with sepsis, our study looked to answer these questions in a cohort of patients with acute PJI treated with DAIR procedures. Our findings highlight the potential need for more aggressive treatment, such as a two-stage revision, in patients presenting with systemic sepsis and factors associated with recurrent infection.
Limitations
This study has several limitations. The retrospective study design has constraints regarding the reliability of medical documentation and coding. To capture only acute PJIs, we included only patients treated with DAIR. It is possible that among patients treated initially with two-stage revision surgery, we missed some acute PJIs. However, by limiting our cohort to patients who underwent DAIR, we were able to analyze patients with similar clinical presentations rather than include patients with more severe infections. Conversely, the definition used for acute PJI was 6 weeks from the index surgery or 6 weeks of symptoms, which may have erroneously captured some patients with chronic PJI because of patient recall bias. Furthermore, selection bias may have occurred as patients treated with DAIR may have been the selectively sicker patients who would not withstand a two-stage revision. Additionally, healthier patients with more virulent pathogens who were treated with a two-stage revision were not included in our data. However, we believe that by including patients with acute infections or acute hematogenous infections with no other sort of identifiable cause of infection who underwent DAIR, we were able to generate a cohort that could provide us with cohesive data and analysis on sepsis in patients with acute PJI. The presentation of acute hematogenous infections in previously well-functioning prosthetic joints has shown to be similar to those with acute infections and as a result, they were analyzed together. Additionally, our minimum required follow-up was relatively short at 6 months. We believe, however, that in a study analyzing patients with acute infections treated with DAIR, this follow-up is sufficient to identify most recurrent or persistent infections and provide valuable data to the existing evidence. Not only is this a common bias to all similar studies published in the literature, but it is also a necessary measure given the low number of patients available for study in this very particular patient population. Although a multivariable analysis was used to identify factors associated with sepsis and recurrent infections after treatment, additional confounding variables such as variations in operative technique and antibiotic therapy were not considered. However, we believe that the core findings of the paper, such as the strong association of serum CRP levels with sepsis and recurrent infection, are unlikely to be influenced by incorporating additional variables. Lastly, the definitions used to categorize sepsis are constantly debated as the understanding of sepsis evolves [3]. For this study, with the data that were available, we elected to use the definition by Levy et al. [15], which may not represent the most updated clinical criteria.
Proportion of Patients With Sepsis and Factors Associated With Sepsis Presentation
Among patients presenting with acute postoperative or acute hematogenous PJI, nearly half had systemic sepsis. These patients were more likely to have a persistent or recurrent infection than patients without sepsis. Kuo et al. [13] reported on patients presenting with acute hematogenous PJI treated with DAIR and found similar results. These data are consistent with those in the present study, in which patients with systemic sepsis demonstrated higher proportions of recurrent or persistent infection after DAIR than did patients without sepsis. Although more aggressive initial treatment may be warranted for patients presenting with PJI-related systemic sepsis, given the high proportion of reinfection with DAIR, these patients often cannot physiologically withstand longer and more complex surgeries involving explantation and the placement of antibiotic spacers. In this scenario, a multidisciplinary approach involving critical care and anesthesiology teams is important to calculate the patient’s risk and to identify the treatment course that is most likely to reduce postoperative complications and reinfection.
Among patients presenting with acute PJI, elevated serum CRP levels and male sex were the only factors associated with the development of systemic sepsis. Klement et al. [11] determined serum CRP was associated with positive blood culture results. Additionally, although male sex has not been associated with treatment failure after DAIR, several studies have linked male sex with an increased PJI risk [11, 15, 21]. Males have also been reported to have a higher reported incidence of sepsis than females [1, 25]. Additionally, we constructed a nomogram that is useful to guide clinicians in determining appropriate treatment strategies based on the patient’s risk. The nomogram can identify patients using the scoring system who are not yet septic but present with a high probability of PJI-related sepsis. Due to their increased sepsis risk, these patients may benefit from earlier, more aggressive surgical treatment to avoid further clinical deterioration. Delaying surgical intervention in high-risk patients may allow sepsis development, which would not only decrease their chances of treatment success, but also limit surgical options given their increased clinical fragility.
Factors Associated With Persistent or Recurrent PJI After Treatment
Importantly, acute PJIs were more likely to present with a reinfection after treatment in patients with systemic sepsis than in those without, regardless of the infecting organism (except for coagulase-negative S. aureus). One possible reason for this is that patients with sepsis are unable to handle the bioburden associated with PJI. Although MSSA was the only pathogen to have an association with reinfection, the lower number of infections caused by the other pathogens may have been underpowered to detect a difference in reinfection between patients with sepsis and those without. Future studies may want to generate a larger patient cohort to conduct a more comprehensive analysis to determine the infecting organisms that may be more likely to cause reinfection in patients presenting with sepsis associated with PJI.
In our study, MRSA and polymicrobial infections showed the highest risk of persistent or recurrent infection among patients with sepsis. Several studies have demonstrated an increased failure risk with DAIR in patients with MRSA [4, 17, 24] as a result of its range of virulence factors; these studies favor a two-stage approach in these patients. Polymicrobial infections are also associated with an increased likelihood of treatment failure after DAIR [28, 33]. Because polymicrobial PJIs are more common in patients with rheumatoid arthritis [23], those with a higher comorbidity index [34], those older than 65 years, and those with persistent wound drainage [19], host risk factors may have a substantial influence on these infections. These are important factors to consider in our patient cohort, which demonstrated that several factors are associated with persistent or recurrent infections. As a result, our findings support the thinking that patients with systematic sepsis may benefit from two-stage revision rather than DAIR to decrease the bioburden more effectively, especially in those with MRSA and polymicrobial infections. Further studies should evaluate the role infecting organisms play in the development of systematic sepsis.
Higher serum CRP levels and an increasing number of previous operations on the affected joint were associated with treatment failure with DAIR in patients with sepsis. Serum CRP appears to be important for evaluating acute PJI cases with potential sepsis. An increasing number of previous procedures in the affected joint is associated not only with treatment failure [6], but also with an increased PJI risk [2, 30]. To avoid persistent or recurrent PJI and its associated pain and loss of function, high serum CRP levels and a history of prior surgical procedures on the involved joint should trigger prompt, aggressive surgical treatment if the patient’s overall clinical status can tolerate such an intervention.
Conclusion
Systemic sepsis is a severe PJI complication that occurs in more than 40% of patients presenting with acute hematogenous or postoperative PJI. These patients are associated with a higher proportion of persistent or recurrent infections after DAIR than patients with acute PJI without sepsis. Our clinically based nomogram can be used to calculate the sepsis risk in patients with acute PJI and as a guide for clinicians as they determine treatment strategies for these patients. Overall, our findings support that patients with systematic sepsis may benefit from two-stage revision rather than DAIR to decrease the bioburden more effectively, especially in those with MRSA and polymicrobial infections. Additionally, high serum CRP levels and a history of prior surgical procedures on the involved joint should trigger prompt, aggressive surgical treatment if the patient’s overall clinical status can tolerate such an intervention.
Footnotes
One of the authors (JP) certifies receipt of royalties in the amount of USD 100,001 to USD 1,000,000 from Corentec; consulting fees in the amount of less than USD 10,000 from Ethicon, Tenor, Jointstem, Peptilogics, and Fidia Pharm and in the amount of USD 10,000 to USD 100,000 from Zimmer Biomet, KCI / 3M (Acelity), Heraeus, and MicroGenDx. The author (JP) also has stock options with Parvizi Surgical Innovation and Subsidiaries, Hip Innovation Technology, Alphaeon/Strathsby Crown, Joint Purification Systems, Ceribell, Acumed, PRN-Veterinary, MD-Valuate, Intellijoint, MicroGenDx, Nanooxygenic, Sonata, and Molecular Surface Technologies.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Ethical approval for this study was obtained from Thomas Jefferson University, Philadelphia, PA, USA (number 08R.207).
This work was performed at Rothman Orthopaedic Institute at Thomas Jefferson University, Philadelphia, PA, USA.
Contributor Information
Leanne Ludwick, Email: leanne.ludwick@rothmanortho.com.
Marcelo Siqueira, Email: marcelobps1@gmail.com.
Noam Shohat, Email: noam.shohat@gmail.com.
Matthew B. Sherman, Email: matthew.sherman@rothmanortho.com.
Sydney Streicher, Email: sls196@jefferson.edu.
References
- 1.Annane D, Aegerter P, Jars-Guincestre MC, Guidet B, CUB-Réa Network. Current epidemiology of septic shock: the CUB-Réa Network. Am J Respir Crit Care Med. 2003;168:165-172. [DOI] [PubMed] [Google Scholar]
- 2.Berbari EF, Osmon DR, Lahr B, et al. The Mayo prosthetic joint infection risk score: implication for surgical site infection reporting and risk stratification. Infect Control Hosp Epidemiol . 2012;33:774-781. [DOI] [PubMed] [Google Scholar]
- 3.Cecconi M, Evans L, Levy M, Rhodes A. Sepsis and septic shock. Lancet. 2018;392:75-87. [DOI] [PubMed] [Google Scholar]
- 4.Cobo J, Garcia San Miguel L, Euba G, et al. Early prosthetic joint infection: outcomes with debridement and implant retention followed by antibiotic therapy. Clin Microbiol Infect. 2011;17:1632-1637. [DOI] [PubMed] [Google Scholar]
- 5.Coburn B, Morris AM, Tomlinson G, Detsky AS. Does this adult patient with suspected bacteremia require blood cultures? JAMA. 2012;308:502-511. [DOI] [PubMed] [Google Scholar]
- 6.Fillingham YA, Della Valle CJ, Suleiman LI, et al. Definition of successful infection management and guidelines for reporting of outcomes after surgical treatment of periprosthetic joint infection. J Bone Joint Surg Am. 2019;101:1-5. [DOI] [PubMed] [Google Scholar]
- 7.Flierl MA, Culp BM, Okroj KT, Springer BD, Levine BR, Della Valle CJ. Poor outcomes of irrigation and debridement in acute periprosthetic joint infection with antibiotic-impregnated calcium sulfate beads. J Arthroplasty. 2017;32:2505-2507. [DOI] [PubMed] [Google Scholar]
- 8.Izakovicova P, Borens O, Trampuz A. Periprosthetic joint infection: current concepts and outlook. EFORT Open Rev. 2019;4:482-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain S. Sepsis: an update on current practices in diagnosis and management. Am J Med Sci. 2018;356:277-286. [DOI] [PubMed] [Google Scholar]
- 10.Jämsen E, Huhtala H, Puolakka T, Moilanen T. Risk factors for infection after knee arthroplasty a register-based analysis of 43,149 cases. J Bone Joint Surg Am. 2009;91:38-47. [DOI] [PubMed] [Google Scholar]
- 11.Klement MR, Siddiqi A, Rock JM, Chen AF, Bolognesi MP, Seyler TM. Positive blood cultures in periprosthetic joint infection decrease rate of treatment success. J Arthroplasty. 2018;33:200-204.e1. [DOI] [PubMed] [Google Scholar]
- 12.Konigsberg BS, Valle CJD, Ting NT, Qiu F, Sporer SM. Acute hematogenous infection following total hip and knee arthroplasty. J Arthroplasty. 2014;29:469-472. [DOI] [PubMed] [Google Scholar]
- 13.Kuo FC, Goswami K, Klement MR, Shohat N, Parvizi J. Positive blood cultures decrease the treatment success in acute hematogenous periprosthetic joint infection treated with debridement, antibiotics, and implant retention. J Arthroplasty. 2019;34:3030-3034. [DOI] [PubMed] [Google Scholar]
- 14.Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Related Res. 2010; 468:52-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003; 31:1250-1256. [DOI] [PubMed] [Google Scholar]
- 16.Lora-Tamayo J, Murillo O, Iribarren JA, et al. A large multicenter study of methicillin-susceptible and methicillin-resistant Staphylococcus aureus prosthetic joint infections managed with implant retention. Clin Infect Dis. 2013;56:182-194. [DOI] [PubMed] [Google Scholar]
- 17.Lum ZC, Natsuhara KM, Shelton TJ, Giordani M, Pereira GC, Meehan JP. Mortality during total knee periprosthetic joint infection. J Arthroplasty. 2018;33:3783-3788. [DOI] [PubMed] [Google Scholar]
- 18.Marculescu CE, Cantey JR. Polymicrobial prosthetic joint infections: risk factors and outcome. Clin Orthop Relat Res. 2008;466:1397-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Natsuhara KM, Shelton TJ, Meehan JP, Lum ZC. Mortality during total hip periprosthetic joint infection. J Arthroplasty. 2019;34:S337-S342. [DOI] [PubMed] [Google Scholar]
- 20.Ong KL, Kurtz SM, Lau E, Bozic KJ, Berry DJ, Parvizi J. Prosthetic joint infection risk after total hip arthroplasty in the Medicare population. J Arthroplasty. 2009;24:105-109. [DOI] [PubMed] [Google Scholar]
- 21.Parvizi J, Tan TL, Goswami K, et al. The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty. 2018;33:1309-1314. [DOI] [PubMed] [Google Scholar]
- 22.Peel TN, Cheng AC, Buising KL, Choong PFM. Microbiological aetiology, epidemiology, and clinical profile of prosthetic joint infections: are current antibiotic prophylaxis guidelines effective? Antimicrob Agents Chemother. 2012;56:2386-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salgado CD, Dash S, Cantey JR, Marculescu CE. Higher risk of failure of methicillin-resistant Staphylococcus aureus prosthetic joint infections. Clin Orthop Relat Res . 2007;461:48-53. [DOI] [PubMed] [Google Scholar]
- 24.Sands KE, Bates DW, Lanken PN, et al. Epidemiology of sepsis syndrome in 8 academic medical centers. JAMA. 1997;278:234-240. [PubMed] [Google Scholar]
- 25.Shapiro NI, Wolfe RE, Wright SB, Moore R, Bates DW. Who needs a blood culture? A prospectively derived and validated prediction rule. J Emerg Med. 2008;35:255-264. [DOI] [PubMed] [Google Scholar]
- 26.Shohat N, Bauer T, Buttaro M, Budhiparama N, et al. Hip and knee section, what is the definition of a periprosthetic joint infection (PJI) of the knee and the hip? Can the same criteria be used for both joints? Proceedings of International Consensus on Orthopedic Infections. J Arthroplasty. 2019;34:S325-S327. [DOI] [PubMed] [Google Scholar]
- 27.Shohat N, Goswami K, Tan TL, Fillingham Y, Parvizi J. Increased failure after irrigation and debridement for acute hematogenous periprosthetic joint infection. J Bone Joint Surg Am. 2019;101:696-703. [DOI] [PubMed] [Google Scholar]
- 28.Singer M, Deutschman CS, Seymour C, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315:801-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan TL, Maltenfort MG, Chen AF, et al. Development and evaluation of a preoperative risk calculator for periprosthetic joint infection following total joint arthroplasty. J Bone Joint Surg Am. 2018;100:777-785. [DOI] [PubMed] [Google Scholar]
- 30.Tande AJ, Patel R. Prosthetic joint infection. Clin Microbiol Rev. 2014;27:302-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tornero E, Morata L, Martínez-Pastor JC, et al. KLIC-score for predicting early failure in prosthetic joint infections treated with debridement, implant retention and antibiotics. Clin Microbiol Infect. 2015;21:786.e9-786.e17. [DOI] [PubMed] [Google Scholar]
- 32.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495-1499. [DOI] [PubMed] [Google Scholar]
- 33.Westberg M, Grøgaard B, Snorrason F. Early prosthetic joint infections treated with debridement and implant retention. Acta Orthop. 2012;83:227-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zmistowski B, Fedorka CJ, Sheehan E, Deirmengian G, Austin MS, Parvizi J. Prosthetic joint infection caused by gram-negative organisms. J Arthroplasty. 2011;26:104-108. [DOI] [PubMed] [Google Scholar]
- 35.Zmistowski B, Karam JA, Durinka JB, Casper DS, Parvizi J. Periprosthetic joint infection increases the risk of one-year mortality. J Bone Joint Surg Am. 2013;95:2177-2184. [DOI] [PubMed] [Google Scholar]