Abstract
Osteonecrosis of the femoral head (ONFH) is a disorder that can cause collapse of the femoral head. The damage and dysfunction of femoral head microvascular endothelial cells are related to the pathogenesis of glucocorticoid-induced ONFH. Reports suggest that vitamin B2 can promote osteoblast differentiation and prevent low bone mineral density and prevent reperfusion oxidative injury. To explore the effect and possible molecular mechanism of vitamin B2 on the ONFH and Human Umbilical Vein Endothelial Cells (HUVECs), we performed a rat model of ONFH by dexamethasone. The rats were randomly divided into four groups: control group, vitamin B2 group, dexamethasone group, and dexamethasone combined with vitamin B2 treatment group. HUVECs were used to further prove the role and mechanism of vitamin B2 in vitro. In patients, according to immunohistochemical and qRT-PCR of the femoral head, the angiogenic capacity of the ONFH femoral head is compromised. In vivo, it showed that vitamin B2 could inhibit glucocorticoid-induced ONFH-like changes in rats by suppressing cell apoptosis, promoting the regeneration of blood vessels, and increasing bone mass. According to in vitro results, vitamin B2 could induce the migration of HUVECs, enhance the expression of angiogenesis-related factors, and inhibit glucocorticoid-induced apoptosis. The underlying mechanism may be that vitamin B2 activates the PI3K signaling pathway. Vitamin B2 alleviated dexamethasone-induced ONFH, and vitamin B2 could promote the proliferation and migration of HUVECs and inhibit their apoptosis by activating the PI3K/Akt signaling pathway. Vitamin B2 may be a potentially effective treatment for ONFH.
1. Introduction
Osteonecrosis of the femoral head (ONFH) is a common cause of hip disability among the middle-aged, with bilateral necrosis in 30 to 70 percent of cases, which severely affects patients' life and places a burden on public health finance [1, 2]. Clinically, glucocorticoid (GC) administration is considered the most common cause of ONFH, as 9 to 40 percent of patients treated with glucocorticoids experience ONFH [3, 4]. Specifically, a reduced number of blood vessels and reduced blood supply are considered important mechanisms of ONFH [5]. Presently, nonsurgical treatments for this disease include load protection, drug treatment, and physical therapy [6]. However, the effects of these conservative treatments are uncertain. Currently, surgery is still the main treatment option.
GC induced thrombosis, endothelial cell dysfunction and damage, and endothelial cell apoptosis, which leads to the development of ONFH [7]. Previous research has established that angiogenesis and osteogenesis are closely related [8]. Moreover, GC inhibits the activity of osteoblasts and osteocytes and decreases the number of blood vessels in bones, together leading to bone loss [9]. GC inhibited angiogenesis in bone and inhibited HIF-1α transcription and VEGF production in osteoblasts and osteocytes. GC inhibits the expression of VEGF, which is one of the key growth factors regulating vascular development and angiogenesis. VEGF directly regulates the differentiation and function of osteoblasts and osteoclasts [10, 11]. VEGF also exerts direct effects on osteoblasts via BMP produced by endothelial cells [12]. Therefore, drugs that can promote blood vessel formation would be promising for the treatment of ONFH.
Vitamin B2 in form of flavin adenine dinucleotide (FAD) and flavin mononucleotide (FMN) participates in redox reactions in the metabolic pathways that are involved in energy production [13, 14]. Perumal et al. demonstrated that lipid peroxidation and reperfusion oxidative injury of mitochondria were possibly prevented by vitamin B2 [15]. In a model of hepatocyte ischemia/reperfusion injury, vitamin B2 attenuates tissue oxidative/nitrosative stress and inflammatory infiltration and exerts antioxidants and effects after hepatic ischemia/reperfusion, reducing the damage of hepatocytes [16]. Moreover, vitamin B2 showed a protective effect in lipopolysaccharide-induced lung injury in rats [17]. Cheung et al. showed that vitamin B2 promotes the synthesis of normal extracellular matrix and reduces reactive oxygen species levels in keratoconus [18]. Vitamin B2 has an additive effect on osteoblast differentiation induced by ascorbic acid and β-glycerophosphate, in which the expression of osteoblast transcription factors (Runx2 and β-catenin) is upregulated [19]. Several lines of evidence suggest that adequate intake of vitamin B2 can promote osteoblast differentiation and prevent low BMD [20, 21]. Interruption of blood supply is a direct cause of ONFH disease, and vascular endothelial cell injury seriously affects the course of ONFH. It would be interesting if we could protect the vascular endothelial cells by medication and save the ONFH patient from surgery. Thus, we hypothesize that vitamin B2 can protect the head of femur from GC damage.
Due to the interconnection of angiogenesis and osteogenesis, as well as the physiological function of vitamin B2, we investigated whether vitamin B2 can rescue the fate of ONFH in GC-treated rats in vivo. At the same time, we also researched whether vitamin B2 inhibits apoptosis and increases angiogenesis with or without GC treatment HUVECs.
2. Materials and Methods
2.1. Processing of Human Samples
The study was approved by the Research Ethics Committee of the First Affiliated Hospital of Chongqing Medical University. Written informed consent was obtained from each donor and approved by the Institutional Review of the First Affiliated Hospital of Chongqing Medical University.
Our study included 5 ONFH patients and 5 femoral neck fracture (FNF) patients. The inclusion criteria of ONFH patients were as follows: (1) X-ray, magnetic resonance imaging (MRI), and pathological findings consistent with a diagnosis of ONFH; (2) levels 3 or 4 disease according to the association research circulation osseous (ARCO); and (3) patients on high doses (>3 g cumulative) of glucocorticoids. The inclusion criteria of FNF patients were X-ray, MRI, and histologic section diagnosis of femoral neck fracture and no history of medication or surgical treatment within one year. The exclusion criteria for both groups were as follows: patients with a severe endogenous disease such as Paget's disease, metabolic bone disease, metastatic bone cancer, or hyperparathyroidism. And all patients are in the same age group.
2.2. Cell Culture
Human umbilical vein endothelial cells (HUVECs) were purchased from the Cell Bank of the Chinese Academy of Sciences. HUVECs were cultured in Roswell Park Memorial Institute (RPMI) 1640 supplemented with 10% fetal bovine serum (Gibco, Grand Island, USA), penicillin (100 U/ml), and streptomycin (100 μg/ml) at 37°C in 5% CO2. Dexamethasone was administered at a concentration of 100 μM for the apoptosis experiment and 20 μM for the other cell experiments. The concentration of vitamin B2 administered to the cells was 20 μM.
2.3. In Vivo Studies
The animal experiments were performed in accordance with a previous study [22]. In brief, forty healthy 8-week-old female Sprague-Dawley (S-D) rats weighing 140 to 160 g were randomly divided into a control group, vitamin B2 group (VB2), dexamethasone group (DEX), and dexamethasone combined with vitamin B2 treatment group (DEX+VB2), with 10 rats in each group. The rats in the control group were intramuscularly injected with PBS. The rats in the vitamin B2 group were intramuscularly injected with vitamin B2 (3 mg/kg/day) on the first three days of each week for 4 weeks. The rats in the dexamethasone group were intramuscularly injected with dexamethasone (20 mg/kg/d) for the first three days of each week for the same time duration. In the DEX+VB2 group, the rats were intramuscularly injected with dexamethasone and vitamin B2 for the first three days of each week for the same duration. Two months after treatment, the rats were sacrificed, and the femoral heads were isolated for microcomputed tomography (micro-CT) examination and immunohistochemical staining. The investigators who performed micro-CT and immunohistochemistry were unaware of group assignments during the experiment and data analysis.
Micro-CT (Skyscan1174, Bruker, Belgium) scans of the rat femoral heads were obtained. At the end of the scanning, N-Recon software was used for three-dimensional reconstruction of the femoral head, and CT-AN software was used to analyze the osteogenic parameters BV/TV, Tb. N, Tb. Th, and Tb. Sp (BV/TV: bone volume per tissue volume; Tb. N: trabecular number; Tb. Th: trabecular thickness; Tb. Sp, trabecular separation).
2.4. Immunohistochemistry (IHC) and TUNEL Staining
Femoral head samples were obtained from patients at the Department of Orthopedics, the First Affiliated Hospital of Chongqing Medical University, and rats. We assessed angiogenic differentiation in necrotic areas of bone tissue of ONFH, and the control specimens were obtained from the same areas. In the IHC staining assay [23], paraffin sections were defatted with xylene, boiled in citrate buffer (pH 6.0) for 15 min, incubated with 3% H2O2 for 25 min at room temperature in the dark to block endogenous peroxidase, and blocked with normal goat serum. Then, the sections were then incubated with primary antibodies overnight at 4°C in a humidified chamber. After being washed, tissues were covered with secondary antibody (HRP labeled) and incubated for 50 min at room temperature. TUNEL staining was performed as previously described [24], the TDT enzyme, dUTP, and buffer in the TUNEL kit according mix at 1 : 5 : 50 ratio at 37°C for 60 min. Reagent streptavidin-HRP is added to cover the tissue and incubated at 37°C for 30minutes. Cell nuclei were stained with DAPI. Stained tissue was visualized under a Nikon E100 microscope (Nikon DS-U3, Japan).
2.5. CCK-8 Assay
Cell Counting Kit-8 (CCK-8, MCE, USA) was used to estimate cell proliferation. As previously reported [25], HUVECs were plated in 96-well plates at a density of 5 × 103 cells/well and processed in groups, with three duplicate wells in each group. When the growth and fusion degree reached 70-80 percent, the cells were treated according to these groups and incubated for 24, 48, or 72 h. The corresponding reagents were added according to the instructions of CCK-8 detection kit, and the absorbance (D) value of each well at 450 nm was detected on a microplate reader.
2.6. RNA Isolation and Quantitative PCR (qPCR)
Total RNA was purified from human femoral head tissue, rat femoral head tissue, and HUVECs using TRIzol (Invitrogen, USA) according to the manufacturer's instructions [26]. cDNA was generated from the total RNA extracted from tissues or cells with a reverse transcription reaction kit (TAKARA, Japan). The cDNA was used as a template for subsequent qPCR assays. The forward and reverse primers used in this study are shown in Table 1.
Table 1.
The quantitative real-time polymerase chain reaction primer sequences.
| Species | Genes | Forward primer | Reverse primer |
|---|---|---|---|
| Human | VEGF | GCCTTGCCTTGCTGCTCTACC | GGTCTCGATTGGATGGCAGTAGC |
| Human | CD31 | CGTCAAGCCTCAGCACCAGATG | GCACTCCTTCCACCAACACCTG |
| Human | ANG | CGCTGCCATTCTGACTCACATAGG | CGTACTCTCACGACAGTTGCCATC |
| Human | EMCN | TTCAGCAACCAGCCGGTCTTATTC | GGATCTGCCTTCCAGCACATTCG |
| Human | VWF | GCCGACTTCAACAGGAGCAAGG | GCAGCACCGTGACGTGGATG |
| Human | GAPDH | GGAGCGAGATCCCTCCAAAAT | GGCTGTTGTCATACTTCTCATGG |
| Rat | ALP | GTGCCCTGGCGACATGATACTG | TGCGGGACATAAGCGAGTTTCTG |
| Rat | BSP | AAGCGACGAGGAAGAGGAAGAGG | TTGGTGCTGGTGCCGTTGAC |
| Rat | OCN | GGACCCTCTCTCTGCTCACTCTG | ACCTTACTGCCCTCCTGCTTGG |
| Rat | RUNX2 | TCCGCCACCACTCACTACCAC | GGAACTGATAGGACGCTGACGAAG |
| Rat | VEGF | CACGACAGAAGGGGAGCAGAAAG | GGCACACAGGACGGCTTGAAG |
| Rat | CD31 | CAGAGCCAGCATTGTGACCAGTC | CAAGGCGGCAATGACCACTCC |
| Rat | ANG | TTCTTCGCTGCCATTCTGACTCAC | GCAGTTCCCGTCGTGTTCTGG |
| Rat | EMCN | GGCTGCTTCAAGTGACTGCTCTC | ATGTCTGGTGTTGTAGCCGATGC |
| Rat | VWF | CTGGTGGAGCCTCTGGTGGTAG | CACAAGCCTCCTCCGCAAACC |
| Rat | GAPDH | ATGCCATCACTGCCACTCA | CCTGCTTCACCACCTTCTTG |
2.7. Wound Healing Assay
A wound healing assay was performed as described previously [27]. The cells were plated in 6-well plates. When the cells reached 100% confluence, a scratch was made through the cell layer. The nonadherent cells were removed with PBS. The remaining adherent cells were incubated with serum-free medium in the presence or absence of vitamin B2 and dexamethasone to allow cell growth and wound healing. Every 12 h, the cells were photographed with a phase contrast microscope (Olympus, Tokyo, Japan).
2.8. Cell Apoptosis Assay
Cell apoptosis was analyzed by flow cytometry [28]. The cells were incubated in serum-free medium in the presence or absence of vitamin B2 and dexamethasone for 72 h. The cells were digested with 0.05% EDTA-free trypsin, collected, and washed with PBS twice. The cells were stained with staining Annexin V-FITC (BD Pharmingen, USA) and propidium iodide (BD Pharmingen, USA). The cells were incubated at room temperature for 20 min, and the cell apoptosis rate was assessed on flow cytometer.
2.9. Western Blot Assay
Protein expression levels were measured by Western blotting [29]. Briefly, protein was extracted with RIPA lysis buffer (Beyotime, China), and the total protein concentration was determined with a Bicinchoninic Acid (BCA) Protein Assay Kit (Beyotime, China). After electrophoresis, the proteins were transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore Corporation, USA), which was blocked in 5% fat-free milk powder (Boster Biological Technology, China) for 60 min at room temperature. The PVDF membrane was incubated with primary antibody overnight at 4°C. Next, the membrane was washed in TBST and incubated with secondary antibodies for 60 min. Primary antibodies against PI3K (1 : 1000, Abcam), p-PI3K (1 : 1000, Abcam), AKT (1 : 1000, Abcam), p-AKT (1 : 1000, Abcam), VEGF (1 : 1000, Wanleibio), VWF (1 : 1000, Wanleibio), CD31 (1 : 1000, Wanleibio), and GAPDH (Abcam, 1; 2000) were used.
2.10. Statistical Analysis
All experiments were performed in triplicate, and the experimental data are presented as mean ± standard deviation (SD). We applied one-way analysis of variance (ANOVA) and Student's t-test to analyze the data for each group. Differences were considered statistically significant when P values < 0.05.
3. Results
3.1. Diminished Angiogenesis Capacity in the Femoral Head of ONFH Patients
The general shapes of femoral heads are shown in Figure 1(a), the image on the left shows the femoral head of a femoral neck fracture (FNF) patient, and the image on the right shows the femoral head of a patient with ONFH. In Figure 1(b), the X-ray image shows a change in femoral head density and narrowing of the joint space. In Figure 1(c), the MRI image shows a subchondral fracture with low signal strength. Angiogenesis plays an important role in bone development and regeneration. Thus, we assessed angiogenic differentiation in necrotic areas of bone tissue of ONFH patients, and fracture specimens were obtained from the corresponding areas. Immunohistochemical images showed that the expression of VEGF, critical markers of angiogenesis, was notably decreased in ONFH bone tissue (Figure 1(d)). Meanwhile, the qPCR results showed that the mRNA levels of VEGF, CD31, VWF, ANGPT1, and EMCN were significantly decreased in ONFH bone tissue compared with FNF bone tissue (Figure 1(e)). In summary, these data suggested that vasculogenic capacity was impaired in the femoral heads of ONFH patients.
Figure 1.
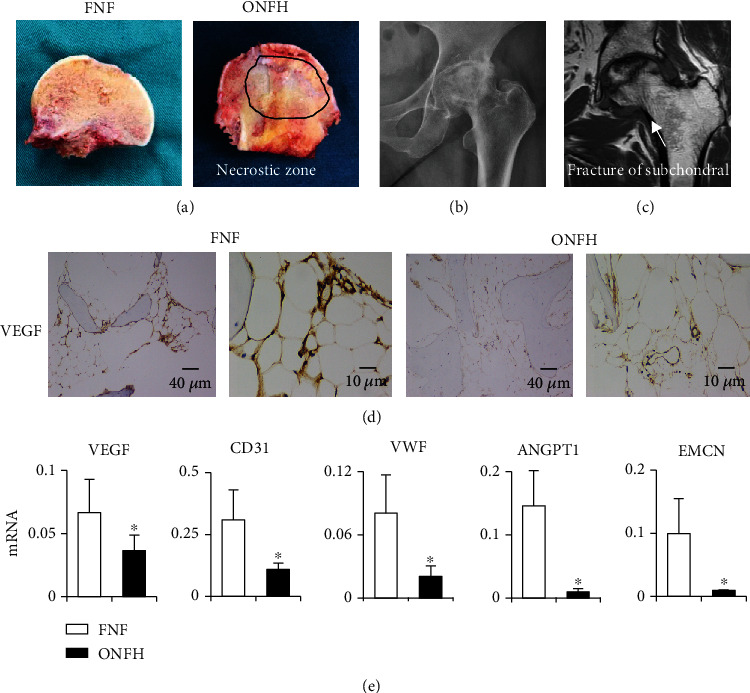
Diminished vasculogenic capacity in the femoral heads of ONFH patients. The general shapes of femoral heads are shown in (d), the left image is the femoral head of a femoral neck fracture (FNF) patient, and the image on the right is the femoral head of an osteonecrosis of the femoral head (ONFH) patient. (b and c) A radiograph and MRI image of a femoral head. (d) IHC staining of VEGF in femoral head tissue. (e) q-PCR analysis of VEGF, CD31, VWF, ANGPT1, and EMCN in the femoral head. The data are shown as mean ± SD for five separate experiments. ∗P < 0.05.
3.2. Vitamin B2 Facilitates Bone Formation of Femoral Head In Vivo
To assess the effect of vitamin B2 on bone mass and angiogenesis in vivo, the rats were treated according to the corresponding protocol for 8 weeks to establish an ONFH model [22]. Three-dimensional micro-CT images showed that the rat model of ONFH was successfully established. Analysis of the femoral head showed that 60% of the rats injected with dexamethasone developed trabecular changes, including decreased bone volume, trabecular number, and trabecular thickness, an increase in trabecular separation (Figure 2(a)). However, the trend of the dexamethasone-induced decrease in bone mass was rescued by vitamin B2 (Figure 2(b)). The immunohistochemical images showed that the expression of OCN significantly declined in the DEX group, showing that dexamethasone inhibits bone formation in the femoral head. In the DEX+VB2 group, the bone mass was recovered (Figure 2(c)). The qPCR results showed that the expression of ALP, OCN, BSP, and RUNX2 was significantly downregulated in the DEX group, and vitamin B2 restored the expression of ALP, OCN, BSP, and RUNX2 to some extent (Figure 2(d)). These data suggested that vitamin B2 probably limits the dexamethasone-induced impairment of osteogenesis in vivo.
Figure 2.
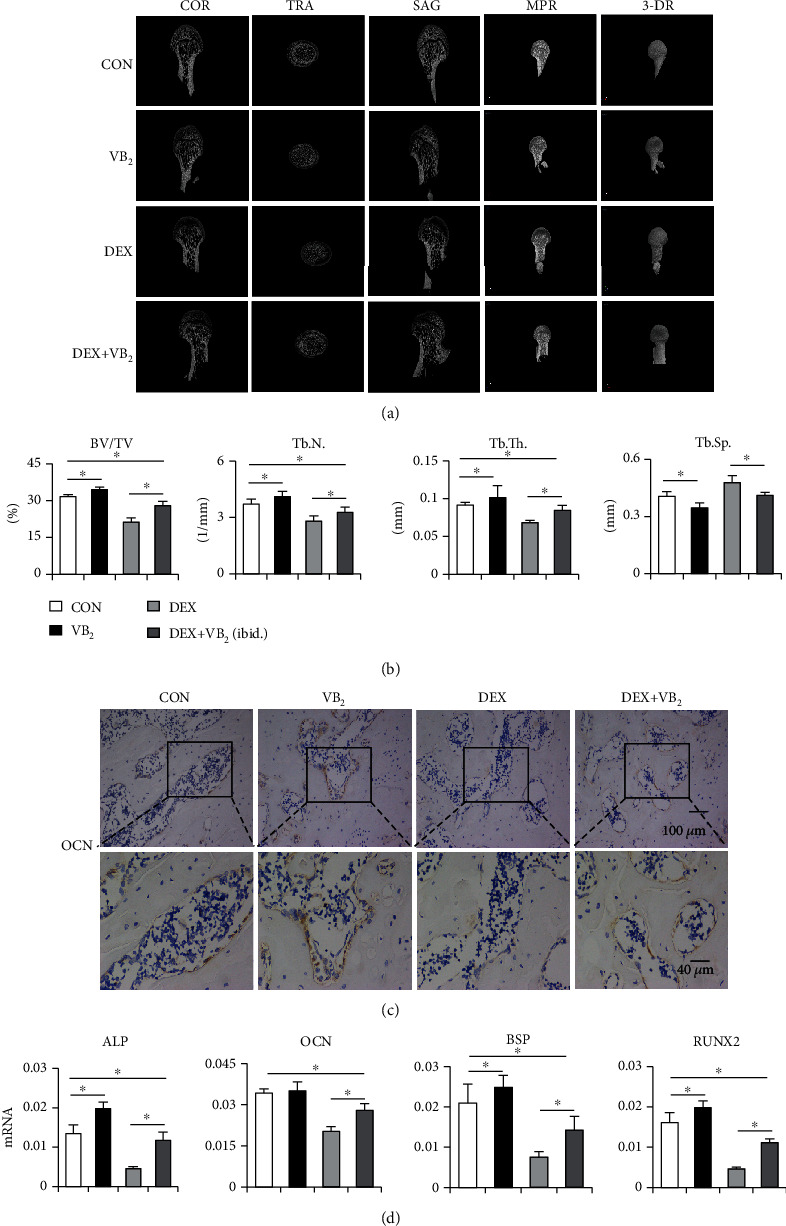
Vitamin B2 facilitates bone formation of femoral head in vivo. (a) Reconstructed COR, TRA, SAG, MPR, and 3-DR images in the femoral heads of each group were obtained using micro-CT (COR: coronal; TRA: transverse; SAG: sagittal; MPR: multiplanar reconstruction; 3-DR: three-dimensional reconstruction). (b) Quantitative analysis of the BV/TV, Tb. N, Tb. Th, and Tb. Sp of each group (BV/TV: bone volume per tissue volume; Tb. N; trabecular number; Tb. Th: trabecular thickness; Tb. Sp: trabecular separation). (c) Representative images of OCN staining in the femoral heads of each group. (d) q-PCR analysis of ALP, OCN, BSP, and RUNX2 in the femoral head. Each experiment was done in triplicate. The data are shown as mean ± SD for triplicate. ∗P < 0.05.
3.3. Vitamin B2 Facilitates Angiogenesis of Femoral Head In Vivo
Immunohistochemical staining showed that the expression of VEGF was significantly decreased in the DEX group and that dexamethasone dramatically inhibited the growth of vascular endothelial cells. In the DEX+VB2 group, the viability of vascular endothelial cells and the expression of VEGF was recovered (Figure 3(a)). Consistent with this finding, the qPCR results showed that the expression of VEGF, CD31, VWF, EMCN, and ANGPT1 was significantly downregulated in the DEX group and that vitamin B2 restored the expression of angiogenesis factors to some extent (Figure 3(b)) TUNEL staining revealed many more apoptotic cells in the DEX group than in the other groups and showed that vitamin B2 inhibited dexamethasone-induced apoptosis to some extent (Figure 3(c)). Collectively, these results confirmed that vitamin B2 probably restores angiogenesis after it is impaired by dexamethasone in vivo. We also observed the effect of dexamethasone on the apoptosis of cells in femoral head tissue.
Figure 3.
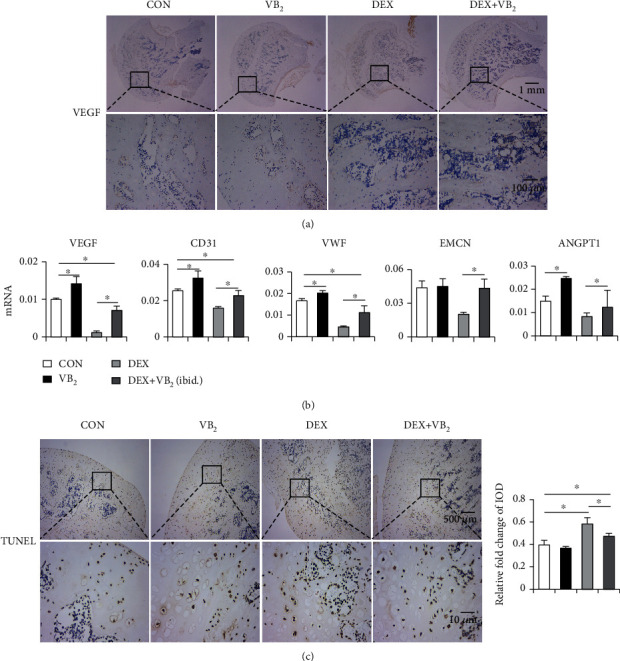
Vitamin B2 facilitates angiogenesis of femoral head in vivo. (a) Representative images of VEGF staining in the femoral heads of each group. (b) q-PCR analysis of VEGF, CD31, VWF, EMCN, and ANGPT1 in the femoral head. (c) Representative images of TUNEL staining in the femoral heads of each group. And quantification results were analyzed by ImageJ software. Each experiment was done in triplicate. ∗P < 0.05.
3.4. Vitamin B2 Promotes the Proliferation and Migration and Inhibits Apoptosis of HUVECs
CCK8 results showed that the proliferation ability of HUVECs was enhanced with increasing vitamin B2 concentration in a certain concentration range. When vitamin B2 treatment concentration was 20 μmol/L, the proliferation ability of HUVECs was strongest (Figure 4(a)). Dexamethasone significantly inhibited the proliferation ability of HUVECs, and the proliferation ability of HUVECs in the DEX+VB2 group was partially restored compared with the DEX group (Figure 4(b)). The above results suggested that dexamethasone inhibited the proliferation of HUVECs, and vitamin B2 could partially restore the dexamethasone-induced decrease in the proliferation of HUVECs. The wound healing assay showed that after 72 h, few cells in the DEX group migrated and that more cells migrated in the DEX+VB2 group than in the DEX group (Figures 4(c) and 4(d)). This result indicated that vitamin B2 prevented the inhibitory effect of dexamethasone on HUVEC migration. Next, a fluorescent conjugated Annexin V antibody was used to detect apoptotic cells due to its high affinity for anionic phospholipid phosphatidylserine on the outer surface of apoptotic cells. The results showed that apoptosis of HUVECs was triggered by dexamethasone and that vitamin B2 markedly reversed dexamethasone-induced apoptosis (Figures 4(e) and 4(f)).
Figure 4.
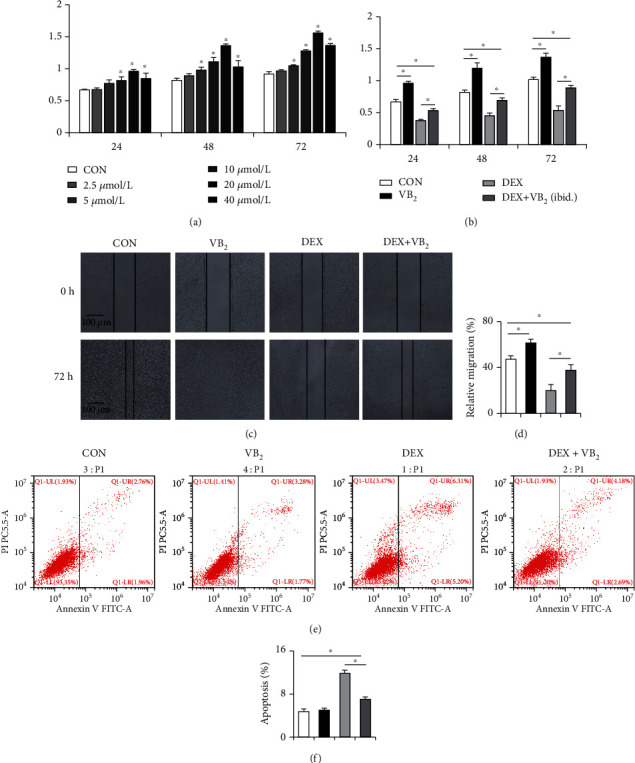
Vitamin B2 promotes the proliferation and migration and inhibits apoptosis of HUVECs. (a) The effect of different concentrations of vitamin B2 on HUVECs proliferation. (b) The change of proliferation ability of HUVECs in the presence or absence of vitamin B2 and dexamethasone. (c) Images of the wound healing assay at 72 h showing a larger number of migrated cells in the vitamin B2 coincubation group than the DEX group. (d) Quantitative analysis of the assay shown in (c). (e) The population of apoptotic cells was measured by Annexin V/PI staining and flow cytometric analysis. (f) Quantitative analysis of the data shown in (e). Each experiment was done in triplicate. ∗P < 0.05.
3.5. Mechanisms of Vitamin B2 Rescue the Impaired Angiogenic Function in ONFH
To explore potential mechanisms by which vitamin B2 rescues the impaired angiogenic function in ONFH, the qPCR and Western blot were performed. This effect of vitamin B2 was further confirmed by its ability to reverse the change in the expression of angiogenesis-related genes VEGF, CD31, ANGPT1, and VWF, which was downregulated by dexamethasone alone (Figure 5(a)). Consistent with the above results, Western blot analysis confirmed that vitamin B2 could restore the expression of VEGF, CD31, and VWF in dexamethasone-treated HUVECs (Figure 5(b)). The PI3K/AKT pathway is known to play a key role in numerous cellular functions including proliferation, migration, and metabolism [30]. The PI3K/AKT pathway induces VEGF mRNA transcription and VEGF protein expression which stimulates the formation of blood vessels [31]. The results confirmed that dexamethasone inhibited the levels of p-PI3K, p-Akt, and VEGF, and dexamethasone combined with vitamin B2 treatment group increased the expression of p-PI3K, p-Akt, and VEGF which resulted in the activation of the PI3K/Akt signaling pathway (Figure 5(c)). The Western blot results revealed that mechanistically, the PI3K/Akt pathway is important for migration, apoptosis, and expression of angiogenesis-related factors in HUVECs and that vitamin B2 is vital in activating this pathway.
Figure 5.
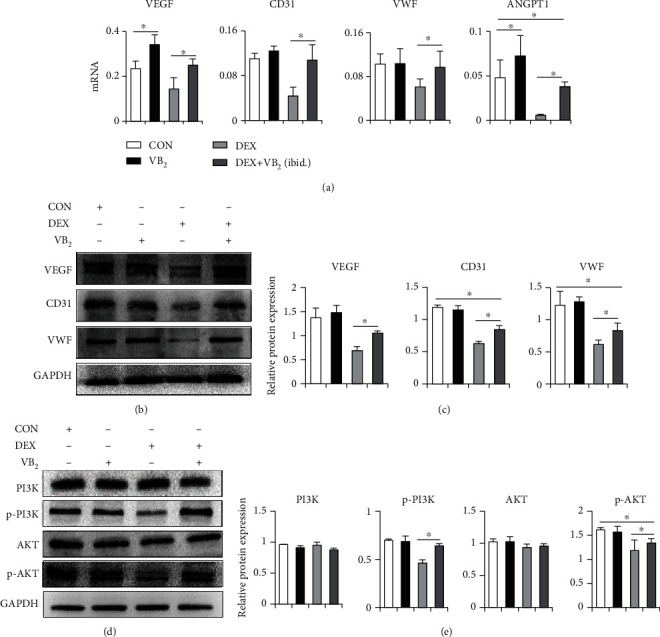
Mechanisms of vitamin B2 rescue the impaired angiogenic function in ONFH. (a) q-PCR analysis of VEGF, CD31, VWF, and ANGPT1 in HUVECs. (b) Western blot analysis of angiogenesis-associated proteins in HUVECs. (c) Statistical analysis was performed to measure the results in (b). (d) Western blot analysis of PI3K, p-PI3K, AKT, and p-AKT. (e) Statistical analysis was performed to measure the results in (d). Each experiment was done in triplicate. ∗P < 0.05.
4. Discussion
ONFH is a common refractory disease in orthopedics, which affects millions of people in China [32]. In the United States, its incidence is estimated at 15,000-20,000 new cases per year [33]. Osteonecrosis is characterized by bone cell death due to impaired blood supply to the bones, and osteonecrosis is most common in the femoral head [34]. The microenvironment of ONFH is closely related to cell proliferation, apoptosis, osteogenic differentiation, and angiogenesis [35]. Seguin et al. [36] showed that ONFH is strongly associated with regional endothelial dysfunction. And vascular endothelial cells play an important role in maintaining this microenvironment. Moreover, previous in vivo and in vitro experiments have provided large amounts of evidence that GC inhibits angiogenesis while also decreasing bone formation and increasing bone resorption [5]. In this study, vitamin B2 was found to reduce the negative effects of dexamethasone on angiogenesis-related cytokine expression, bone formation, and apoptosis in vivo experiments.
Numerous studies have suggested that vitamin B2 prevents oxidative damage by peroxidation and reperfusion and can promote osteoblast differentiation and treat osteoporosis [37]. Vitamin B2 is a water-soluble vitamin, and riboflavin transporter assists its entry into cells [38]. Vitamin B2 has an additive effect on ascorbic acid, and β-glycerophosphate induced osteogenic differentiation of MC3T3-E1 cells, which is characterized by enhanced alkaline phosphatase activity and promotion of osteogenic differentiation [19]. However, these studies have mainly focused on the effect of vitamin B2 on osteoporosis, and there are few studies related to ONFH.
At present, research on ONFH mainly focuses on the balance between osteoblast and osteoclast production [39]. However, femoral head tissue contains a variety of cells, such as mesenchymal stem cells, osteoblasts, and vascular endothelial cells. In the skeletal system, the vascular system is critical for bone development and remodeling and is essential for maintaining healthy bone tissue. Dysregulation of bone-vascular interactions underlies different pathologies [40, 41]. Previous studies have suggested that ONFH is related to angiogenesis-osteogenesis coupling [42, 43]. GC-induced ONFH is closely associated with dysfunction of bone microvascular endothelial cells (BMECs), and dysfunction of BMECs ultimately leads to reduced local blood flow to the femoral head and is thought to be the cause of GC-induced ONFH [44]. VEGF receptor 2 antibody can induce ONFH in the rat with a high incidence [45]. Xu et al. [6] demonstrated that enhancing VEGF-mediated angiogenesis and inhibiting apoptosis prevent bone loss in mouse ONFH. VEGF regulates the proliferation, migration, and vascularization of endothelial cells and is a key inducer of angiogenesis [46]. Maes et al. [47] suggested that VEGF binds angiogenesis and osteoblastic differentiation through its effects on endothelial cells and regulation of osteoblasts, both of which express VEGF receptors. In our study, mRNA and protein expression of both VEGF and CD31 vascular-related genes were substantially higher in the DEX+VB2 group compared to the DEX group, and VB2 alone also enhanced their expression, in vivo and in vitro. Furthermore, using immunohistochemical techniques, we observed more vessels with endothelial marker expression in rats with GC and VB2 administration, which further affirmed our hypothesis.
Endothelial cells can direct osteogenic differentiation of stem cells by activating AKT signaling pathway [48]. In our study, it was further demonstrated that dexamethasone can directly damage endothelial cells, inhibit cell migration, and promote apoptosis, which is consistent with the previous studies [44]. In addition, studies have confirmed that activation of the PI3K/Akt/VEGF signaling pathway promotes angiogenesis in renal cell carcinoma [49]. Li et al. [50] reported that microRNA-126 inhibited the function of HUVEC by inhibiting the expression of EGFL7 and downregulating the PI3K/AKT signaling pathway. And Chen et al. [51] demonstrated that tectorigenin increased the phosphorylation of PI3K and Akt in HUVEC and reduced H2O2-induced oxidative stress injury. It was found that in HUVEC cells, the protein levels of p-PI3K and p-AKT in the dexamethasone combined with vitamin B2 treatment group were significantly higher than those in the dexamethasone treatment group. The increase in phosphorylated proteins is not due to changes in total protein expression, but due to activation of kinases, which can increase the percentage of phosphorylated proteins. In vitro, these data revealed that the PI3K/Akt pathway is important for migration, apoptosis, and expression of angiogenesis-related cytokines in HUVECs and that vitamin B2 is vital in activating this pathway. Interesting, recent studies have found that ONFH is regulated by osteoclasts and microRNAs, not sure if the effects of vitamin B2 might modulate these activities [52, 53]. And more research will be conducted in the future.
5. Conclusions
In this study, an important role of vitamin B2 was found. In vivo, vitamin B2 increased bone mass in the femoral head, avoided femoral head collapse, decreased GC-induced bone loss, apoptosis of cells, and increased expression of angiogenesis-related genes. Although it does not fully restore the effects of GC on bone, vitamin B2 can attenuate the negative effects of dexamethasone on vascular endothelial cells, such as promoting the migration ability of endothelial cells, inhibiting apoptosis, and promoting the expression of angiogenesis-related factors. This study reveals the positive effect of vitamin B2 on angiogenesis and provides new ideas for clinical treatment of glucocorticoid-induced ONFH.
Acknowledgments
This study was performed with the help of the Orthopedics Department of the First Affiliated Hospital of Chongqing Medical University. This work was supported by the General Project of Technology Innovation Application Development of Chongqing Science and Technology Bureau (Grant No. cstc2019jscx-msxmX0245).
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Ethical Approval
The study was approved by the Research Ethics Committee of the First Affiliated Hospital of Chongqing Medical University.
Consent
Written informed consent was obtained from each donor and approved by the Institutional Review of the First Affiliated Hospital of Chongqing Medical University.
Conflicts of Interest
The authors declare no conflicts of interest in this work.
Authors' Contributions
MinKang Guo and Jian Zhang conducted the study. MinKang Guo carried out assays, collected all samples, and conducted the statistical analyses. Jian Zhang participated in research coordination and acted as a technical consultant. MinKang Guo drafted the paper. All authors read and approved the final version.
References
- 1.Arbab D., Konig D. P. Atraumatic femoral head necrosis in adults. Deutsches Arzteblatt International . 2016;113(3):31–38. doi: 10.3238/arztebl.2016.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grayson W. L., Bunnell B. A., Martin E., Frazier T., Hung B. P., Gimble J. M. Stromal cells and stem cells in clinical bone regeneration. Nature Reviews. Endocrinology . 2015;11(3):140–150. doi: 10.1038/nrendo.2014.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinstein S. R. Glucocorticoid-induced bone disease. The New England Journal of Medicine . 2011;365(1):62–70. doi: 10.1056/NEJMcp1012926. [DOI] [PubMed] [Google Scholar]
- 4.Cui L., Zhuang Q., Lin J., et al. Multicentric epidemiologic study on six thousand three hundred and ninety five cases of femoral head osteonecrosis in China. International Orthopaedics . 2016;40(2):267–276. doi: 10.1007/s00264-015-3061-7. [DOI] [PubMed] [Google Scholar]
- 5.Kerachian M. A., Seguin C., Harvey E. J. Glucocorticoids in osteonecrosis of the femoral head: a new understanding of the mechanisms of action. The Journal of Steroid Biochemistry and Molecular Biology . 2009;114(3-5):121–128. doi: 10.1016/j.jsbmb.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu T., Jin H., Lao Y., et al. Administration of erythropoietin prevents bone loss in osteonecrosis of the femoral head in mice. Molecular Medicine Reports . 2017;16(6):8755–8762. doi: 10.3892/mmr.2017.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Q., Jin L. V., Jin L. Role of coagulopathy in glucocorticoid-induced osteonecrosis of the femoral head. The Journal of International Medical Research . 2018;46(6):2141–2148. doi: 10.1177/0300060517700299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rather H. A., Jhala D., Vasita R. Dual functional approaches for osteogenesis coupled angiogenesis in bone tissue engineering. Materials Science and Engineering: C . 2019;103, article 109761 doi: 10.1016/j.msec.2019.109761. [DOI] [PubMed] [Google Scholar]
- 9.Lane N. E. Glucocorticoid-induced osteoporosis: new insights into the pathophysiology and treatments. Current Osteoporosis Reports . 2019;17(1):1–7. doi: 10.1007/s11914-019-00498-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu K., Olsen B. R. The roles of vascular endothelial growth factor in bone repair and regeneration. Bone . 2016;91:30–38. doi: 10.1016/j.bone.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu K., Olsen B. R. Vascular endothelial growth factor control mechanisms in skeletal growth and repair. Developmental Dynamics . 2017;246(4):227–234. doi: 10.1002/dvdy.24463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hankenson K. D., Gagne K., Shaughnessy M. Extracellular signaling molecules to promote fracture healing and bone regeneration. Advanced Drug Delivery Reviews . 2015;94:3–12. doi: 10.1016/j.addr.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Thakur K., Tomar S. K., Singh A. K., Mandal S., Arora S. Riboflavin and health: a review of recent human research. Critical Reviews in Food Science and Nutrition . 2016;55(17):3650–3660. doi: 10.1080/10408398.2016.1145104. [DOI] [PubMed] [Google Scholar]
- 14.Suwannasom N., Kao I., Pruß A., Georgieva R., Bäumler H. Riboflavin: the health benefits of a forgotten natural vitamin. International Journal of Molecular Sciences . 2020;21(3):p. 950. doi: 10.3390/ijms21030950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perumal S. S., Shanthi P., Sachdanandam P. Augmented efficacy of tamoxifen in rat breast tumorigenesis when gavaged along with riboflavin, niacin, and CoQ10: effects on lipid peroxidation and antioxidants in mitochondria. Chemico-Biological Interactions . 2005;152(1):49–58. doi: 10.1016/j.cbi.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Sanches S. C., Ramalho L., Mendes-Braz M., et al. Riboflavin (vitamin B-2) reduces hepatocellular injury following liver ischaemia and reperfusion in mice. Food and Chemical Toxicology . 2014;67:65–71. doi: 10.1016/j.fct.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Al-Harbi N. O., Imam F., Nadeem A., et al. Riboflavin attenuates lipopolysaccharide-induced lung injury in rats. Toxicology Mechanisms and Methods . 2015;25(5):417–423. doi: 10.3109/15376516.2015.1045662. [DOI] [PubMed] [Google Scholar]
- 18.Cheung I., McGhee C., Sherwin T. Beneficial effect of the antioxidant riboflavin on gene expression of extracellular matrix elements, antioxidants and oxidases in keratoconic stromal cells. Clinical & Experimental Optometry . 2014;97(4):349–355. doi: 10.1111/cxo.12138. [DOI] [PubMed] [Google Scholar]
- 19.Neto A. H. C., Yano C. L., Paredes-Gamero E. J., et al. Riboflavin and photoproducts in MC3T3-E1 differentiation. Toxicology In Vitro . 2010;24(7):1911–1919. doi: 10.1016/j.tiv.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 20.Clarke M., Ward M., Dickey W., et al. B-vitamin status in relation to bone mineral density in treated celiac disease patients. Scandinavian Journal of Gastroenterology . 2015;50(8):975–984. doi: 10.3109/00365521.2015.1015603. [DOI] [PubMed] [Google Scholar]
- 21.Yazdanpanah N., Uitterlinden A. G., Zillikens M. C., et al. Low dietary riboflavin but not folate predicts increased fracture risk in postmenopausal women homozygous for the MTHFR 677 T allele. Journal of Bone and Mineral Research . 2008;23(1):86–94. doi: 10.1359/jbmr.070812. [DOI] [PubMed] [Google Scholar]
- 22.Zhu W., Guo M., Yang W., et al. CD41-deficient exosomes from non-traumatic femoral head necrosis tissues impair osteogenic differentiation and migration of mesenchymal stem cells. Cell Death & Disease . 2020;11(4):p. 293. doi: 10.1038/s41419-020-2496-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao P., Zhu Z., Du C., et al. Silencing Smad7 potentiates BMP2-induced chondrogenic differentiation and inhibits endochondral ossification in human synovial-derived mesenchymal stromal cells. Stem Cell Research & Therapy . 2021;12(1):p. 132. doi: 10.1186/s13287-021-02202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He W., Ying-Fu L., Wang H., Peng Y. P. Delayed treatment of α5 GABAA receptor inverse agonist improves functional recovery by enhancing neurogenesis after cerebral ischemia-reperfusion injury in rat MCAO model. Scientific Reports . 2019;9(1):p. 2287. doi: 10.1038/s41598-019-38750-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu P., Zhou K., Lu S., Bai Y., Qi R., Zhang S. Modulation of aryl hydrocarbon receptor inhibits esophageal squamous cell carcinoma progression by repressing COX2/PGE2/STAT3 axis. Journal of Cell Communication and Signaling . 2020;14(2):175–192. doi: 10.1007/s12079-019-00535-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun Y., Kim E. J., Felt S. A., et al. A specific sequence in the genome of respiratory syncytial virus regulates the generation of copy-back defective viral genomes. PLoS Pathogens . 2019;15(4, article e1007707) doi: 10.1371/journal.ppat.1007707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J., Cheng P., Pavlyukov M. S., et al. Targeting NEK2 attenuates glioblastoma growth and radioresistance by destabilizing histone methyltransferase EZH2. The Journal of Clinical Investigation . 2020;130(11):p. 6187. doi: 10.1172/JCI144618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shang A., Gu C., Wang W., et al. Exosomal circPACRGL promotes progression of colorectal cancer via the miR-142-3p/miR-506-3p- TGF-β1 axis. Molecular Cancer . 2020;19(1):p. 117. doi: 10.1186/s12943-020-01235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kondo H., Ratcliffe C., Hooper S., et al. Single-cell resolved imaging reveals intra-tumor heterogeneity in glycolysis, transitions between metabolic states, and their regulatory mechanisms. Cell Reports . 2021;34(7, article 108750) doi: 10.1016/j.celrep.2021.108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bader A. G., Kang S., Zhao L., Vogt P. K. Oncogenic PI3K deregulates transcription and translation. Nature Reviews Cancer . 2005;5(12):921–929. doi: 10.1038/nrc1753. [DOI] [PubMed] [Google Scholar]
- 31.Karar J., Maity A. I3K/AKT/mTOR pathway in angiogenesis. Frontiers in Molecular Neuroscience . 2011;4:p. 51. doi: 10.3389/fnmol.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao D., Zhang F., Wang B., et al. Guidelines for clinical diagnosis and treatment of osteonecrosis of the femoral head in adults (2019 version) Journal of Orthopaedic Translation . 2020;21:100–110. doi: 10.1016/j.jot.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chughtai M., Piuzzi N. S., Khlopas A., Jones L. C., Goodman S. B., Mont M. A. An evidence-based guide to the treatment of osteonecrosis of the femoral head. The bone & joint journal . 2017;99(10):1267–1279. doi: 10.1302/0301-620X.99B10.BJJ-2017-0233.R2. [DOI] [PubMed] [Google Scholar]
- 34.Petek D., Hannouche D., Suva D. Osteonecrosis of the femoral head: pathophysiology and current concepts of treatment. EFORT Open Reviews . 2019;4(3):85–97. doi: 10.1302/2058-5241.4.180036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gillet C., Dalla Valle A., Gaspard N., et al. Osteonecrosis of the femoral head: lipotoxicity exacerbation in MSC and modifications of the bone marrow fluid. Endocrinology . 2017;158(3):490–502. doi: 10.1210/en.2016-1687. [DOI] [PubMed] [Google Scholar]
- 36.Seguin C., Kassis J., Busque L., et al. Non-traumatic necrosis of bone (osteonecrosis) is associated with endothelial cell activation but not thrombophilia. Rheumatology . 2008;47(8):1151–1155. doi: 10.1093/rheumatology/ken206. [DOI] [PubMed] [Google Scholar]
- 37.Saedisomeolia A., Ashoori M. Riboflavin in human health: a review of current evidences. Advances in Food and Nutrition Research . 2018;83:57–81. doi: 10.1016/bs.afnr.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Jaeger B., Bosch A. M. Clinical presentation and outcome of riboflavin transporter deficiency: mini review after five years of experience. Journal of Inherited Metabolic Disease . 2016;39(4):559–564. doi: 10.1007/s10545-016-9924-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weinstein R. S., Hogan E. A., Borrelli M. J., Liachenko S., O'Brien C. A., Manolagas S. C. The pathophysiological sequence of glucocorticoid-induced osteonecrosis of the femoral head in male mice. Endocrinology . 2017;158(11):3817–3831. doi: 10.1210/en.2017-00662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marrella A., Lee T. Y., Lee D. H., et al. Engineering vascularized and innervated bone biomaterials for improved skeletal tissue regeneration. Materials Today . 2018;21(4):362–376. doi: 10.1016/j.mattod.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pirosa A., Gottardi R., Alexander P. G., Tuan R. S. Engineering in-vitro stem cell-based vascularized bone models for drug screening and predictive toxicology. Stem Cell Research & Therapy . 2018;9(1):p. 112. doi: 10.1186/s13287-018-0847-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu S., Bennett S., Kuek V., et al. Endothelial cells produce angiocrine factors to regulate bone and cartilage via versatile mechanisms. Theranostics . 2020;10(13):5957–5965. doi: 10.7150/thno.45422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chim S. M., Tickner J., Chow S. T., et al. Angiogenic factors in bone local environment. Cytokine & Growth Factor Reviews . 2013;24(3):297–310. doi: 10.1016/j.cytogfr.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 44.Yu H., Liu P., Zuo W., et al. Decreased angiogenic and increased apoptotic activities of bone microvascular endothelial cells in patients with glucocorticoid-induced osteonecrosis of the femoral head. BMC Musculoskeletal Disorders . 2020;21(1):p. 277. doi: 10.1186/s12891-020-03225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao Y. S., Wang H. F., Ding H., Zhang C. Q. A novel rat model of osteonecrosis of the femoral head induced by periarticular injection of vascular endothelial growth factor receptor 2 antibody. Journal of Surgical Research . 2013;183(1):e1–e5. doi: 10.1016/j.jss.2013.01.046. [DOI] [PubMed] [Google Scholar]
- 46.Eshkar-Oren I., Viukov S. V., Salameh S., et al. The forming limb skeleton serves as a signaling center for limb vasculature patterning via regulation of Vegf. Development . 2009;136(8):1263–1272. doi: 10.1242/dev.034199. [DOI] [PubMed] [Google Scholar]
- 47.Maes C., Goossens S., Bartunkova S., et al. Increased skeletal VEGF enhances β-catenin activity and results in excessively ossified bones. The EMBO Journal . 2010;29(2):424–441. doi: 10.1038/emboj.2009.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsai T. L., Wang B., Squire M. W., Guo L. W., Li W. J. Endothelial cells direct human mesenchymal stem cells for osteo- and chondro-lineage differentiation through endothelin-1 and AKT signaling. Stem Cell Research & Therapy . 2015;6(1):1–14. doi: 10.1186/s13287-015-0065-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Di J., Gao K., Qu D., Wu Y., Yang J., Zheng J. Rap2B promotes angiogenesis via PI3K/AKT/VEGF signaling pathway in human renal cell carcinoma. Tumor Biology . 2017;39(7):p. 1010428317701653. doi: 10.1177/1010428317701653. [DOI] [PubMed] [Google Scholar]
- 50.Li Q., Cheng K., Wang A. Y., et al. MicroRNA-126 inhibits tube formation of HUVECs by interacting with EGFL7 and down-regulating PI3K/AKT signaling pathway. Biomedicine & Pharmacotherapy . 2019;116, article 109007 doi: 10.1016/j.biopha.2019.109007. [DOI] [PubMed] [Google Scholar]
- 51.Chen X., Zhang W., Sun L., Lian Y. Tectorigenin protect HUVECs from H2O2-induced oxidative stress injury by regulating PI3K/Akt pathway. Tissue and Cell . 2021;68, article 101475 doi: 10.1016/j.tice.2020.101475. [DOI] [PubMed] [Google Scholar]
- 52.Chen K., Liu Y., He J., et al. Steroid-induced osteonecrosis of the femoral head reveals enhanced reactive oxygen species and hyperactive osteoclasts. International Journal of Biological Sciences . 2020;16(11):1888–1900. doi: 10.7150/ijbs.40917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hong G., Han X., He W., et al. Analysis of circulating microRNAs aberrantly expressed in alcohol-induced osteonecrosis of femoral head. Scientific Reports . 2019;9(1):p. 18926. doi: 10.1038/s41598-019-55188-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


